Abstract
The medullary ventral respiratory column (VRC) of neurons is essential for respiratory motor pattern generation; however, the functional connections among these cells are not well understood. A rostral extension of the VRC, including the retrotrapezoid nucleus/parafacial region (RTN-pF), contains neurons responsive to local perturbations of CO2/pH. We addressed the hypothesis that both local RTN-pF interactions and functional connections from more caudal VRC compartments—extending from the Bötzinger and pre-Bötzinger complexes to the ventral respiratory group (Böt-VRG)—influence the respiratory modulation of RTN-pF neurons and their responses to central chemoreceptor and baroreflex activation. Spike trains from 294 RTN-pF and 490 Böt-VRG neurons were monitored with multielectrode arrays along with phrenic nerve activity in 14 decerebrate, vagotomized cats. Overall, 214 RTN-pF and 398 Böt-VRG neurons were respiratory modulated; 124 and 95, respectively, were cardiac modulated. Subsets of these neurons were tested with sequential, selective, transient stimulation of central chemoreceptors and arterial baroreceptors; each cell's response was evaluated and categorized according to the change in firing rate (if any) following the stimulus. Cross-correlation analysis was applied to 2,884 RTN-pF↔RTN-pF and 8,490 Böt-VRG↔RTN-pF neuron pairs. In total, 174 RTN-pF neurons (59.5%) had significant features in short-time scale correlations with other RTN-pF neurons. Of these, 49 neurons triggered cross-correlograms with offset peaks or troughs (n = 99) indicative of paucisynaptic excitation or inhibition of the target. Forty-nine Böt-VRG neurons (10.0%) were triggers in 74 Böt-VRG→RTN-pF correlograms with offset features, suggesting that Böt-VRG trigger neurons influence RTN-pF target neurons. The results support the hypothesis that local RTN-pF neuron interactions and inputs from Böt-VRG neurons jointly contribute to respiratory modulation of RTN-pF neuronal discharge patterns and promotion or limitation of their responses to central chemoreceptor and baroreceptor stimulation.
Keywords: Bötzinger, breathing, cross-correlation, brainstem, circuits
contemporary views consider the region of the retrotrapezoid nucleus (RTN)/parafacial-area (RTN-pF) to be a rostral extension of the classically defined ventral respiratory column (VRC), which encompasses the Bötzinger (Böt) and pre-Bötzinger (pre-Böt) complexes along with the rostral and caudal ventral respiratory groups (collectively referred to as Böt-VRG). These compartments are key components of the neural network responsible for the generation and modulation of the respiratory rhythm (Abdala et al. 2009a; Alheid et al. 2002; Feldman and Del Negro 2006; Molkov et al. 2010; Onimaru and Homma 2006; Rybak et al. 2007; Smith et al. 1991, 2007).
Some RTN neurons have respiratory-modulated firing patterns (Connelly et al. 1990; Pearce et al. 1989). Anatomical projections to the RTN-pF from medullary areas involved in respiratory control have been demonstrated (Smith et al. 1989). Lesions within the Böt-VRG reduce or eliminate the respiratory modulation of a subset of RTN-region neurons, an effect that has been attributed to loss of inhibitory influences from the respiratory central pattern generator (Guyenet et al. 2005a).
Several lines of evidence support the hypothesis that the RTN-pF is an essential site of central chemoreception (Guyenet 2008; Loeschcke 1982): 1) the region contains chemoresponsive neurons (Nattie et al. 1993); 2) lesions of neurons in the region reduce or eliminate phrenic activity, alter cycle frequency (St. John et al. 1989), and decrease CO2 sensitivity (Nattie et al. 1991); 3) RTN neurons have properties indicative of intrinsic chemosensitivity (Guyenet et al. 2005b; Mulkey et al. 2004; Nattie and Li 1995; Takakura et al. 2006); 4) RTN neurons respond to ATP released by nearby chemosensitive astrocytes (Gourine et al. 2010); and 5) specific deletion of glutamatergic Phox2b-expressing neurons in the RTN-pF region causes a lack of CO2 sensitivity and fatal central apnea (Dubreuil et al. 2008). RTN neurons influenced by both central and peripheral chemoreceptor inputs have also been identified (Takakura et al. 2006); however, pathways contributing to these neuronal response profiles are not known. Functional inputs, whether locally within the RTN-pF region or coming from Böt-VRG neurons, may contribute to the network mechanisms for respiratory modulation and the mediation of chemoresponsiveness. These potential circuit routes remain largely unexplored.
The present study was motivated by the aforementioned gaps in knowledge and sought evidence for the hypothesis that both local RTN-pF neuron interactions and influences from the Böt-VRG contribute to respiratory modulation of RTN-pF region neurons and affect or mediate their central chemoreceptor responses. Identification of the functional connectivity of the respiratory network will enhance our understanding of circuit level mechanisms responsible for the regulation of breathing and its dynamic coordination with other autonomic activities.
Preliminary accounts of some of the results have been reported (Ott et al. 2007, 2008).
METHODS
General methods and surgical preparation.
All experiments were performed according to protocols approved by the University of South Florida's Institutional Animal Care and Use Committee with strict adherence to all American Association for Accreditation of Laboratory Animal Care International (AAALAC), National Institutes of Health (NIH), and National Research Council guidelines.
Methods have been previously described in detail (Nuding et al. 2009; Segers et al. 2008). Briefly, data were obtained from 14 adult cats (2.9–5.2 kg) of either sex. Animals were initially anesthetized with 5% isoflurane mixed with air and maintained with 0.5–3.0% isoflurane until decerebration. The level of anesthesia was periodically evaluated by testing the corneal reflex and toe pinch. A urinary catheter was inserted to monitor renal function; rectal temperature was measured and maintained at 38.0 ± 0.5°C. The trachea was cannulated, and the animal was artificially ventilated with air. The trachea was periodically suctioned, and the lungs were hyperinflated to counteract atelectasis. Arterial blood pressure, end-tidal CO2, and tracheal pressure were monitored continuously; arterial Po2, Pco2, and pH were measured periodically. These parameters were maintained within normal limits.
An embolectomy catheter was placed into the left femoral artery and advanced into the descending aorta rostral to the renal arteries (Arita et al. 1988; Li et al. 1999). A concentric catheter was inserted into the left axillary artery and advanced to the bifurcation of the vertebral artery (Nuding et al. 2009); preceding branches of the axillary artery were ligated (Kuwana and Natsui 1987). The left and right C5 phrenic nerve rootlets were isolated, cut peripherally, and placed on bipolar silver electrodes in a pool of warm mineral oil. The left and right vago-sympathetic nerve (CN X) trunks were isolated in the neck and sectioned to remove vagal sensory feedback from pulmonary stretch receptors. Animals were then neuromuscularly blocked by pancuronium bromide (initial bolus of 0.1 mg/kg followed by 0.2 mg·kg−1·h−1 iv), and the brain stem was immediately transected at the midcollicular level (Kirsten and St. John 1978). Following the decerebration, isoflurane was discontinued and the air flow rate was increased to facilitate its elimination (Sasano et al. 2001); sufficient CO2 was added to the inhaled gas mixture to prevent hyperventilation during this blow-off period (Vesely et al. 2003). An occipital craniotomy was performed, and the caudal portion of the cerebellum was removed to expose the dorsal surface of the brain stem.
Neuronal recordings and postexperimental processing.
Neuronal activity was extracellularly recorded using two multielectrode arrays, with 24 (4 × 6 electrode arrangement; Supplemental Fig. S1A) and 32 (2 × 16) individually adjustable high impedance tungsten microelectrodes (1 μm tip diameter; 10–12 MΩ). Electrode placement was guided by anatomical landmarks (obex, brain stem midline), appropriate stereotaxic coordinates derived from Berman (1968), and results of previous studies (Baekey et al. 2004; Connelly et al. 1992; Schwarzacher et al. 1995). Tips of the 24-electrode array were coated with a fluorescent dye (di-I) before recording (DiCarlo et al. 1996) and placed in the rostral region of the medulla to monitor RTN-pF neurons. The 32-electrode array was placed in the region of the Böt-VRG. A schematic of dimensions and a photograph detailing the footprint configuration of the RTN-pF array are included in Supplemental Fig. S1.
Neuronal signals were amplified, filtered (0.3–10 kHz band pass) and recorded on an instrumentation recorder (25-kHz sampling frequency with 16-bit accuracy per channel) together with systemic arterial blood pressure, end-tidal CO2, stimulus event markers, efferent nerve activity, and tracheal pressure. Filtered phrenic nerve activity (10 Hz-10 kHz band pass) was used as an indication of respiratory drive, to assess stimulus effectiveness and to identify the phases of breathing. Control neuronal activity was recorded for 30 min before any stimuli were presented. Signals from single neurons were isolated using interactive spike sorting software (O'Connor et al. 2005). X-scope, a utility program designed in our laboratory (Lindsey et al. 1992), provided a graphical representation of analog signals and the times of action potentials in simultaneously monitored neurons. Stereotaxic coordinates of recording sites were mapped into the three-dimensional space of a computer-based brain stem atlas derived from The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates (Berman 1968) with permission of the University of Wisconsin Press, as described in Segers et al. (2008).
Histological confirmation of electrode location.
At the end of each experiment, animals were overdosed with Beuthanasia (0.97 mg/kg; Schering-Plough Animal Health) and perfused using a 10% neutral-buffered formalin solution. Alternate frozen sections (40 μm) were stained with cresyl violet and examined using bright field optics. Unstained sections were examined for fluorescent electrode tracks using a Typhoon 9410 multiple mode imager. Images were aligned and stacked using the image processing program ImageJ. Histological data were used to corroborate stereotaxic recording sites by comparing anatomical landmarks delineated by coordinates from Berman (1968).
Neuron characterization: respiratory and cardiac modulation of firing rates.
All neurons were characterized as either respiratory modulated or nonrespiratory modulated using two complementary statistical tests: ANOVA using a subjects-by-treatments experimental design (Netick and Orem 1981; Orem and Netick 1982) and a nonparametric sign test (Morris et al. 1996). Neurons were classified as respiratory modulated if either test rejected the null hypothesis (P < 0.05); neurons with no preferred phase of maximum activity were considered nonrespiratory modulated (NRM).
Standard and normalized respiratory cycle-triggered histograms (rCTH) were constructed for each recorded neuron by comparing the cell's activity with phrenic nerve activity during the control period to provide an estimate of the average firing rate of each cell throughout the respiratory cycle. The normalized rCTH was computed using a spike train in which the durations of the inspiratory and expiratory phases were normalized to the average phase lengths; individual spike times within each phase were proportionately shifted to fit the normalized phase. The rCTHs were used to classify respiratory-modulated neurons as inspiratory (I), expiratory (E), or phase-spanning (IE or EI) according to the part of the cycle during which the cell was most active (Cohen 1968). If the peak firing rate occurred during the first or second half of the phase, I and E cells were further classified as decrementing (Dec) or augmenting (Aug), respectively. The abrupt rise in pulse pressure associated with systole was used as a reference point to calculate cardiac cycle-triggered histograms for each neuron. Spike trains were evaluated for significant arterial pulse pressure modulation of firing rate using an ANOVA as described in Dick and Morris (2004). Respiratory and cardiac cycle-triggered histograms results were used as physiologically relevant attributes in classifying neurons.
Protocol for the stimulation of chemoreceptors and baroreceptors.
Central chemoreceptors were selectively stimulated by 30-s injections of 1.0 ml of a CO2-saturated 0.9% saline solution into the vertebral artery (Nuding et al. 2009). Each stimulus challenge was presented at least five times; trials were separated by 4.5-min intervals to allow phrenic nerve activity to return to prestimulus levels. Injections of 1.0 ml sterile 0.9% saline separated by 1.5-min intervals were used as a negative control in some experiments to verify that changes in blood pressure and/or efferent phrenic output during central chemoreceptor stimulation were not solely due to volume effects. Control saline injections did not evoke significant changes in phrenic nerve frequency or amplitude, evidence that the effects of the CO2-saturated saline injections were not due to the saline solution itself. Arterial baroreceptors were stimulated by 30-s inflations of the embolectomy catheter to transiently elevate blood pressure 20–25 mmHg (Lindsey et al. 1998). Baroreceptor stimulus challenges were repeated at least four times and separated by 1.5-min intervals to allow the mean systemic arterial pressure to return to prestimulus levels. Animals were ventilated with room air to avoid paradoxical stimulation of chemoreceptors by exposure to 100% O2 (Dean et al. 2004).
Measurement of responses to chemoreceptor and baroreceptor stimulation.
Every spike train was evaluated for firing rate changes during each effective stimulus challenge. Stimulus effectiveness was confirmed by measures of the peak amplitude of the integrated phrenic nerve signal; effective reflexes were identified by a change (±2 standard deviations) in the peak integrated phrenic nerve amplitude from the mean of prestimulus values (Nuding et al. 2009). A peristimulus time histogram (PSTH) was constructed for each neuron to aid assessment of changes in firing rate following stimulus onset relative to the immediately preceding control period. Cumulative sum (CUSUM) histograms (Ellaway 1978) were calculated from the PSTHs; changes in activity that exceeded 3 standard deviations (Davey et al. 1986) were confirmed using a bootstrap-based statistical method (as described in Nuding et al. 2009). Response classification consisted of five categories: increase (↑), decrease (↓), biphasic response [increase-decrease (↑↓) or decrease-increase (↓↑)], or no change (→).
Cross-correlation analysis.
Features detected in cross-correlation histograms (CCHs) permit model-based inferences about several simple classes of functional connectivity between the neurons (Aertsen and Gerstein 1985; Kirkwood 1979; Moore et al. 1970). Central peaks or troughs can be simply interpreted as evidence of a common (unobserved) shared trigger neuron influencing the cells' firing rates with similar or opposite effects, respectively. An offset feature located entirely on one side of the trigger event origin can be simply interpreted as evidence of a paucisynaptic functional connection between the two cells; an offset peak is evidence for excitation of the target by the trigger neuron, whereas an offset trough suggests an inhibitory process. Alternatively, an offset feature may also be due to a common shared input that influences both cells with different time delays. Correlograms were calculated using the entire recording for all pairs of simultaneously monitored neurons and evaluated for evidence of features that departed significantly from background firing rates in shift-predictor control correlograms calculated for each pair (Nuding et al. 2009). Peaks and troughs ≥3 standard deviations from the mean of control activity were considered significant. Detectability indexes (equal to the ratio of the maximum amplitude of feature departure from background activity, divided by the standard deviation of the correlogram noise) were calculated (Aertsen and Gerstein 1985; Melssen and Epping 1987). Autocorrelation histograms (ACHs) were calculated as an indicator that each spike train represented the activity of a single neuron; spike event times derived from multiple neurons would include short intervals not constrained by a refractory period. ACHs also aided in the interpretation of the CCHs (Moore et al. 1970; Perkel et al. 1967a, b).
RESULTS
Data were acquired from 18 recording sessions in 14 decerebrate, vagotomized, neuromuscularly blocked, and ventilated cats. The use of arrays of microelectrodes, each with individual depth adjustment, permitted the monitoring and testing of many neurons at the same time under identical environmental conditions. The spike trains of 784 neurons were monitored extracellularly; ≤75 single neurons were simultaneously recorded in a single animal.
The results presented herein on local RTN-pF neuron interactions and evidence of functional inputs from Böt-VRG to RTN-pF neurons are part of a larger study on functional connectivity among medullary VRC neurons. The data are organized into three main sections: 1) characterization of neuronal firing patterns with regard to respiratory and cardiac-pulse modulations, 2) classification of neuronal subsets' response profiles to selective stimulation of central chemoreceptors and/or arterial baroreceptors, and 3) evaluation of short-time scale correlation feature sets reflecting underlying parallel circuit mechanisms that could contribute to the pattern shaping of neuronal modulations and the mediation of responses to central chemoreceptor and baroreceptor perturbations.
Recording locations and discharge patterns of neurons.
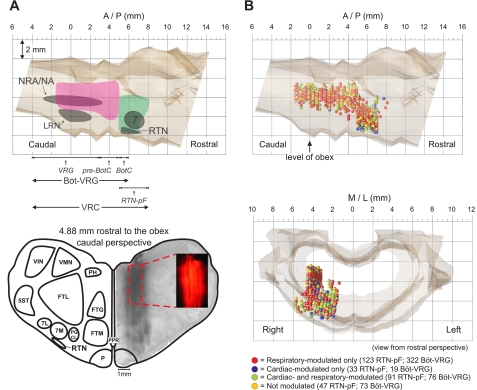
Figure 1A (top) illustrates the rostro-caudal and dorso-ventral extents of the recording sites; anatomical and respiratory-related areas associated with the Böt-VRG and RTN-pF regions are also indicated. One electrode array was used to record RTN-pF neurons (n = 294) in an area ranging from 5.0 to 8.1 mm rostral to the obex, 1.4 to 5.9 mm lateral to the midline, and 3.3 to 7.8 mm below the dorsal surface of the medulla. Böt-VRG neurons (n = 490) were monitored by an additional array within a region extending from 1.5 mm caudal to 5.1 mm rostral to the obex, 3.3 to 4.6 mm lateral to the midline, and 2.5 to 6.2 mm below the dorsal surface of the medulla. Twelve neurons recorded in regions of array overlap were classified as Böt-VRG cells. Figure 1A (bottom) shows the location of four fluorescent dye-labeled electrode tracks relative to surrounding anatomical structures and their straight course throughout the dorso-ventral penetration; the inset details a segment of three of the tracks.
Fig. 1.
Recording sites. A, top: sagittal view showing the rostro-caudal and dorso-ventral extents of our medullary recording sites [pink, the Bötzinger (BötC) and pre-Bötzinger (pre-BötC) complexes along with the rostral and caudal ventral respiratory groups (Böt-VRG); green, retrotrapezoid nucleus/parafacial-area (RTN-pF)]. Approximate rostro-caudal extents of respiratory-related areas are shown across the bottom. A, bottom: caudal view of fluorescent dye-labeled electrode tracks constructed from a stack of 6 alternate 40-μm sections; inset shows detail of 3 electrode tracks from a single section. Note the electrodes' straight course throughout the dorso-ventral penetration. B, top: sagittal view of neuronal stereotaxic recording sites (n = 294 RTN-pF; n = 490 Böt-VRG) colored according to significant modulation of firing rate discharge pattern. Neurons recorded at identical coordinates are shown with a downward vertical displacement to allow illustration of each cell's discharge modulation. B, bottom: rostral view of neuronal stereotaxic recording sites plotted in the brain stem atlas. 5ST, spinal trigeminal tract; 7, facial nucleus; 7M, medial division of facial nucleus; 7L, lateral division of facial nucleus; FTG, gigantocellular tegmental field; FTM, magnocellular tegmental field; LRN, lateral reticular nucleus; NRA/NA, nuclei retroambigualis and ambiguus; P, pyramidal tract; PGCL, lateral paragigantocellular reticular nucleus; PH, nucleus praepositus hypoglossi; PPR, postpyramidal nucleus of the raphé; VIN, inferior vestibular nucleus; VMN, medial vestibular nucleus; VRG, ventral respiratory group.
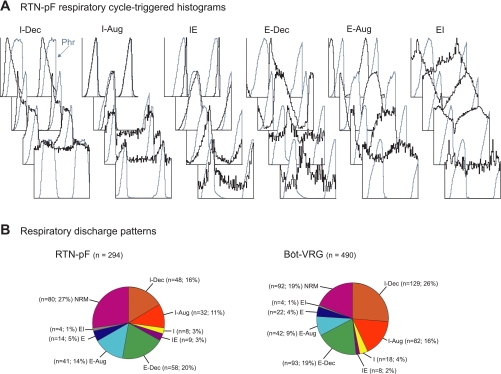
Colored spheres in Fig. 1B indicate the recording sites of neurons and their classification according to the presence (or absence) of respiratory and/or cardiac modulation of their firing rates. Neurons recorded at the same coordinates are depicted as vertically displaced spheres to allow visualization of each cell's discharge modulation. Similar percentages of RTN-pF and Böt-VRG neurons were respiratory modulated (73% and 81%, respectively); however, a greater proportion of RTN-pF cells were cardiac modulated (42% vs. 19%). Correspondingly, the proportion of RTN-pF neurons that were both respiratory and cardiac modulated was almost double that found in the Böt-VRG cell group (31% vs. 16%). The majority (86%) of respiratory-modulated RTN-pF neurons were active throughout the respiratory cycle. Figure 2A depicts examples of respiratory cycle-triggered histograms showing the variety of respiratory-modulated discharge patterns exhibited by RTN-pF neurons. Numbers and proportions of RTN-pF and Böt-VRG neurons were similar among the discharge pattern categories (Fig. 2B).
Fig. 2.
Respiratory modulation. A: normalized RTN-pF respiratory cycle-triggered histograms (rCTHs) grouped by the half of the respiratory phase or transition in which the neuron fires with peak average activity. Respiratory phases were defined by efferent phrenic nerve activity (blue trace). The number of respiratory cycles averaged, bin widths, and maximum firing rates for each rCTH are as follows (top to bottom). Inspiratory (I) + decrementing (Dec) (I-Dec) neurons: 905 cycles, 35.0 ms, 65.2 spikes/s; 759 cycles, 35.0 ms, 39.5 spikes/s; 759 cycles, 35.0 ms, 25.0 spikes/s; 1,299 cycles, 25.0 ms, 20.7 spikes/s. I + augmenting (Aug) (I-Aug) neurons: 759 cycles, 35.0 ms, 68.2 spikes/s; 591 cycles, 30.7 ms, 28.0 spikes/s; 295 cycles, 75.0 ms, 9.0 spikes/s; 295 cycles, 75.0 ms, 10.9 spikes/s. Phase-spanning (IE) neurons: 202 cycles, 30.0 ms, 12.2 spikes/s; 403 cycles, 80.0 ms, 20.8 spikes/s; 1,060 cycles, 15.0 ms, 12.8 spikes/s; 418 cycles, 70.0 ms, 3.4 spikes/s. Expiratory (E)-Dec neurons: 1,060 cycles, 15.0 ms, 23.3 spikes/s; 759 cycles, 35.0 ms, 11.4 spikes/s; 418 cycles, 70.0 ms, 2.6 spikes/s; 418 cycles, 70.0 ms, 0.9 spikes/s. E-Aug neurons: 759 cycles, 35.0 ms, 57.4 spikes/s; 177 cycles, 105.0 ms, 48.5 spikes/s; 418 cycles, 70.0 ms, 4.4 spikes/s; 591 cycles, 30.7 ms, 15.7 spikes/s. Phase-spanning (EI) neurons: 1,060 cycles, 15.0 ms, 29.4 spikes/s; 401 cycles, 60.0 ms, 20.7 spikes/s; 401 cycles, 60.0 ms, 45.5 spikes/s; 591 cycles, 30.7 ms, 5.6 spikes/s. B: respiratory discharge patterns of all recorded RTN-pF (left) and Böt-VRG (right) neurons.
Responses to central chemoreceptor stimulation.
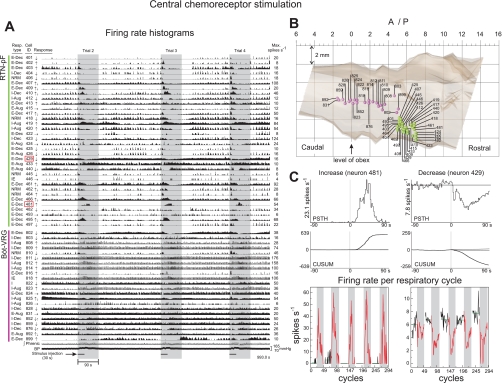
Significant changes in phrenic nerve activity in response to selective stimulation of central chemoreceptors were identified in seven animals. Neuronal firing rate data acquired during one subset of trials (3 of the 5 trials are shown for detail) together with integrated efferent phrenic activity and arterial blood pressure are shown in Fig. 3A. The respiratory pattern, identification number (400s: RTN-pF, 800s: Böt-VRG), central chemoreceptor response profile, and maximum firing rate are shown for each neuron; colored spheres (Fig. 3B) mark stereotaxic coordinates of recording sites. Vertical gray panels in Fig. 3A delineate the 90-s time window during which firing rates were compared with corresponding control activity to measure significant firing rate changes. Window duration extended beyond the 30-s stimulus injection period to ensure that responses with varying time lags would be detected. We note the diverse set of repeatable neuronal firing rate profiles (i.e., response latency and duration) seen in the firing rate histograms shown in Fig. 3A, even among cells with the same respiratory-modulated discharge pattern (e.g., E-Dec neurons 429 and 481; details in Fig. 3C).
Fig. 3.
Central chemoreceptor stimulation. A: firing rate histograms for 56 of 75 simultaneously monitored RTN-pF and Böt-VRG neuron spike trains during sequential stimulation of central chemoreceptors. For each trace, the respiratory-modulated pattern, cell identification number, response to central chemoreceptor stimulus, and maximum firing rate are shown. Vertical gray panels delineate 3 of 5 central chemoreceptor stimulus evaluation periods: 30-s stimulus injection plus a 60-s poststimulus period. Response profiles of neurons outlined with red boxes are detailed in C. B: colored spheres (green: RTN-pF, pink: Böt-VRG) mark coordinates of recording sites for single neurons illustrated in A. C: peristimulus time histograms (PSTH) and corresponding cumulative sum histograms (CUSUM) depict the increase (left) and decrease (right) responses of neurons 481 and 429, respectively. The PSTH plots the average firing rate of the cell 90 s before and after stimulus onset (applied at time 0) over all 5 presented challenges. The CUSUM reflects the data trends depicted in the PSTH. The bottom graph is a display of the firing rate per respiratory cycle for five paired control (white panels) and stimulus periods (gray panels) used to calculate statistics. See methods.
Methods used to classify neuronal responses are illustrated in Fig. 3C. The PSTH (top; averaged from all 5 trials) and CUSUM (middle) display a significant firing rate increase (e.g., neuron 481) and decrease (e.g., neuron 429) in response to stimulation. The bottom plot is a graphical display of mean firing rates per respiratory cycle for paired control (white panels) and stimulus (gray) periods. Firing rates during response (red lines) and preceding control periods (black) were assessed by a bootstrap-based statistical method to detect changes that reached significance. Similar percentages of the 144 RTN-pF and 232 Böt-VRG neurons tested with central chemoreceptor perturbation responded with a significant change in firing rate (63% and 66%, respectively; Table 1). However, the direction of rate change differed between regions: 61% of chemoresponsive RTN-pF neurons initially decreased in rate, whereas more chemoresponsive Böt-VRG cells initially increased (69%).
Table 1.
Responses of single neurons to central chemoreceptor stimulation
| Location and Numbers of Neurons |
||
|---|---|---|
| Response to Central Chemoreceptor Stimulation | RTN-pF | Böt-VRG |
| ↑ | 25 | 101 |
| ↑↓ | 10 | 5 |
| ↓ | 52 | 42 |
| ↓↑ | 3 | 6 |
| → | 54 | 78 |
| Totals | 144 | 232 |
RTN-pF, retrotrapezoid nucleus/parafacial-area. Böt-VRG, Bötzinger and pre-Bötzinger complexes along with the rostral and caudal ventral respiratory groups. ↑, Increased neuronal firing rate; ↓, decreased firing rate; →, no significant change in firing rate to presented stimulus. For both RTN-pF and Böt-VRG, 376 neurons tested for response in seven animals.
Responses to arterial baroreceptor stimulation.
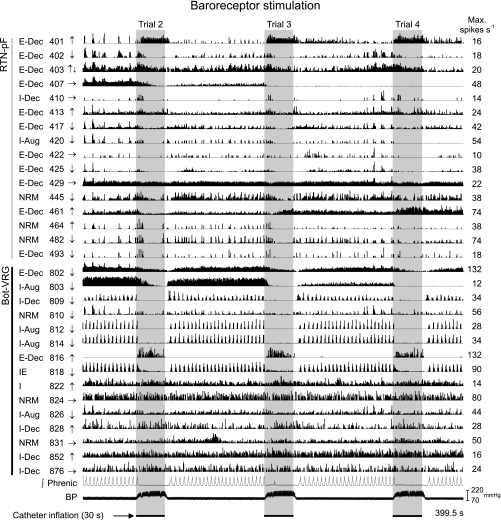
Arterial baroreceptors were stimulated in a total of seven animals. Firing rate histograms from neurons recorded during sequential stimulation of arterial baroreceptors are shown together with integrated efferent phrenic nerve activity and arterial blood pressure in Fig. 4 (same recording as Fig. 3). Vertical gray panels delineate periods of embolectomy catheter inflation (3 of the 5 trials are shown for greater detail). For all baroreceptor stimulus trials, a transient decline in the amplitude of the phrenic nerve signal was elicited; for this recording, the elevation in blood pressure evoked an abrupt decline of the phrenic nerve signal to apnea following each stimulus presentation. Upon deflation of the catheter, the phrenic nerve signal returned to prestimulus levels. Similar percentages of RTN-pF and Böt-VRG neurons tested with baroreceptor stimulation responded with a change in firing rate (43% and 44%, respectively; Table 2); most of these cells responded with a decreased discharge rate.
Fig. 4.
Baroreceptor stimulation. Firing rate histograms for 31 of 75 simultaneously monitored RTN-pF and Böt-VRG neuron spike trains during sequential stimulation of arterial baroreceptors (3 of 5 trials shown for greater detail). For each trace, the respiratory-modulated pattern, cell identification number, response to arterial baroreceptor activation, and peak firing rate are shown. Vertical gray panels delineate baroreceptor stimulus trials: 30-s embolectomy catheter inflation period.
Table 2.
Responses of single neurons to arterial baroreceptor stimulation
| Location and Numbers of Neurons |
||
|---|---|---|
| Response to Baroreceptor Stimulation | RTN-pF | Böt-VRG |
| ↑ | 19 | 26 |
| ↑↓ | 1 | 3 |
| ↓ | 44 | 83 |
| ↓↑ | 0 | 2 |
| → | 86 | 147 |
| Totals | 150 | 261 |
For both RTN-pF and Böt-VRG, 411 neurons tested for response in seven animals.
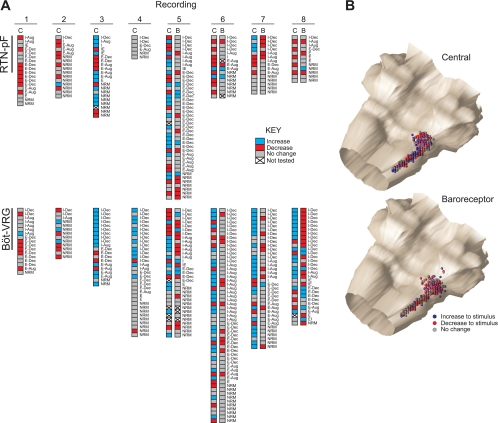
Changes in blood pressure were observed during central chemoreceptor stimulation. To evaluate the contribution of the concurrent change in blood pressure to the cell's response to chemoreceptor activation, we compared the responses of 230 neurons to sequentially applied central chemoreceptor and arterial baroreceptor challenges; the majority of these cells (n = 199) reacted differently to the two stimuli (see dually tested neuronal response profiles in recordings 5–8 of Fig. 5). For example, the response profile of RTN-pF neuron 401 (marked with an asterisk in Fig. 5, recording 5) consisted of a decrease to central chemoreceptor stimulation (see Fig. 3A) and an increase to baroreceptor activation (see Fig. 4), suggesting that the response of cell 401 to central chemoreceptor activation was not solely a consequence of the increase in blood pressure elicited by the chemoreceptor stimulus.
Fig. 5.
Response profiles of neurons. A: RTN-pF (top) and Böt-VRG (bottom) neuron response profile diagrams from recordings (arranged in columns) in which arterial baroreceptor (B) and/or central chemoreceptor (C) stimulation was applied. Each set of boxes represents a single neuron. Because of signal loss, some cells could not be evaluated for response to all presented stimuli (*denotes cell 401 response profile; see text). B: isometric views of colored spheres indicating the stereotaxic coordinates of the neurons in A and their initial responses to stimulation of central chemoreceptors (top) and baroreceptors (bottom).
Neuron response profiles mapped to recording site coordinates.
Neuronal response profiles (detailed in Fig. 5A) summarize the initial responses of 376 neurons tested with central chemoreceptor stimulation. Responses to additional perturbations of arterial baroreceptors are shown along with the cell's respiratory-modulated discharge pattern. The stereotaxic coordinates of neuronal recording sites for each stimulus protocol (Fig. 5B) show each cell represented as a sphere with its initial response to the particular stimulus correspondingly colored. Because of signal loss, some neurons could not be tested with both stimuli.
Evidence for functional connectivity among RTN-pF neurons.
Cross-correlation analysis was applied to 2,884 pairs of RTN-pF neurons. Roughly equal numbers of offset peaks and troughs (n = 49 and 50, respectively) were identified in correlograms triggered by 49 (16.7%) of the RTN-pF neurons (Table 3); 28 of these reference neurons were correlated with more than one target neuron, a result consistent with local divergent connectivity.
Table 3.
Significant offset feature correlations detected in the analysis of 2,884 RTN-pF↔RTN-pF neuron pairs
| RTN-pF |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-Dec |
I-Aug |
I |
IE |
E-Dec |
E-Aug |
E |
EI |
NRM |
||||||||||
| RTN-pF | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T |
| I-Dec | 5 | 3 | 1 | — | — | — | — | — | — | — | 1 | — | — | — | — | — | 2 | 1 |
| 94* | 96* | 14* | 25* | 188* | 124* | 28* | 16* | 225* | ||||||||||
| I-Aug | — | — | 1 | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | — |
| 31* | 4* | 22* | 160* | 79* | 18* | 10* | 137* | |||||||||||
| I | — | — | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | 1 |
| 2* | 3* | 11* | 16* | 11* | 2* | 37* | ||||||||||||
| IE | — | 1 | — | 1 | 1 | — | — | — | — | — | 1 | 3 | — | 1 | — | — | 1 | 2 |
| 1* | 49* | 28* | 7* | 4* | 49* | |||||||||||||
| E-Dec | 4 | 4 | 1 | 2 | 1 | — | — | — | 4 | 3 | 3 | 5 | — | 1 | 1 | 1 | 1 | 2 |
| 293* | 179* | 38* | 18* | 279* | ||||||||||||||
| E-Aug | 1 | — | — | — | — | — | — | — | — | 3 | 2 | — | — | — | — | 1 | 1 | — |
| 46* | 20* | 12* | 214* | |||||||||||||||
| E | — | — | — | — | — | — | — | — | 1 | — | — | 1 | — | — | — | — | — | 2 |
| 7* | 5* | 56* | ||||||||||||||||
| EI | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — |
| 1* | 15* | |||||||||||||||||
| NRM | 1 | — | 2 | 1 | — | 1 | — | — | — | 1 | 6 | 6 | — | — | 1 | 1 | 5 | 1 |
| 210* | ||||||||||||||||||
Mean detectability index (DI) and time-lag from origin (means ± SD) for offset peaks: 7.6 ± 7.3, 27.6 ± 37.1 ms; offset troughs: 6.2 ± 3.4, 36.5 ± 40.0 ms. Detected peaks (P) and troughs (T) simply interpreted as evidence for a functional connection from one RTN-pF neuron to another. Correlated neuron pairs are organized so that offset correlogram features have positive time-lags.
Total number of pairs composed of neurons with the discharge patterns indicated by the row and column labels; numbers were summed to calculate the total number of RTN-pF pairs analyzed. These numbers were used to calculate the percentages of neurons correlated [e.g., of the 279 pairs composed of an E-Dec and a nonrespiratory modulated (NRM) neuron, an E-Dec → NRM connection may be inferred for 3 pairs (1.1%; 1 peak and 2 troughs) and an NRM → E-Dec connection for 1 pair (0.4%; 1 trough)].
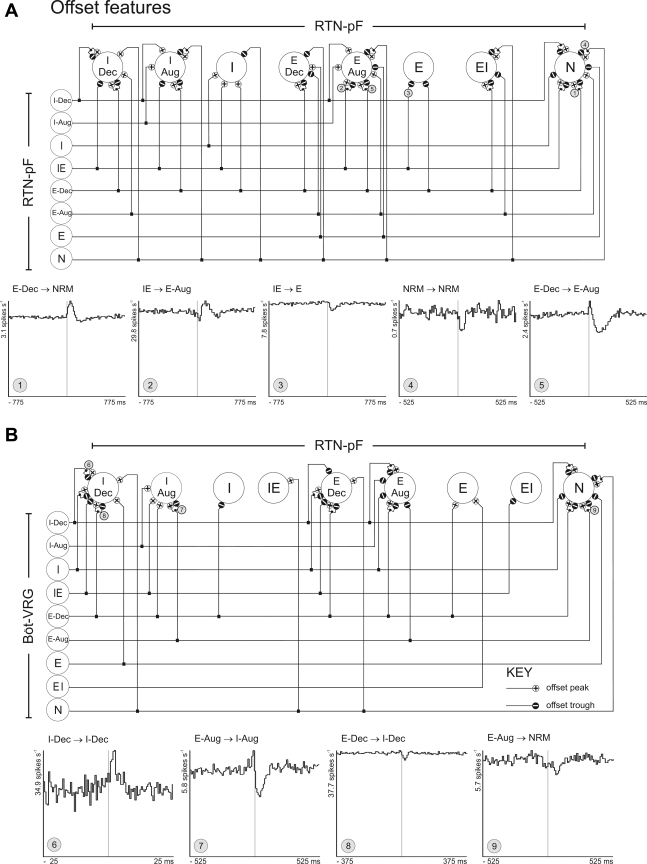
Correlations were detected among cells with similar as well as different respiratory-modulated discharge patterns (including cells with no respiratory modulation). The cross-correlation feature summary diagram in Fig. 6A is a graphic representation detailing the offset peaks and troughs detected in correlograms calculated from all RTN-pF pairs. Trigger neuron respiratory-modulated discharge patterns are arranged along the left side of the diagram; target neurons are shown across the top. The connecting lines delineate the observed features for that neuronal pair type; small circles attached to the target cell represent a feature detected in at least one CCH. Circled numbers correspond to representative CCHs shown here.
Fig. 6.
Intra-RTN-pF and Böt-VRG→RTN-pF functional connectivity. Cross-correlation feature summary diagram (CFSD) illustrating the offset peaks (+) and troughs (−) detected in cross-correlation histograms (CCHs) calculated among RTN-pF neuron pairs (A) and Böt-VRG trigger neuron-to-RTN-pF target neurons (B). Cross-correlograms labeled with circled numbers have features corresponding to similarly labeled correlational linkages represented in the CFSD. 1: peak, detectability index (DI) = 15.26, 162,941 trigger spikes, and 36,042 target spikes; 2: peak, DI = 6.15, 91,386 trigger spikes, and 397,751 target spikes; 3: trough, DI = 7.63, 91,386 trigger spikes, and 97,841 target spikes; 4: trough, DI = 4.49, 91,786 trigger spikes, and 12,201 target spikes; 5: trough, DI = 13.97, 162,941 trigger spikes, and 28,460 target spikes. 6: peak, DI = 9.21, 72,867 trigger spikes, and 118,307 target spikes; 7: trough, DI = 8.24, 16,508 trigger spikes, and 36,032 target spikes; 8: trough, DI = 6.39, 35,709 trigger spikes, and 375,817 target spikes; 9: trough, DI = 4.02, 16,508 trigger spikes, and 30,857 target spikes.
Evidence for Böt-VRG → RTN-pF functional connectivity.
Böt-VRG↔RTN-pF neuron pairs (n = 8,490) were evaluated for offset features indicative of paucisynaptic functional neuronal connectivity from Böt-VRG to RTN-pF neurons. Forty-nine Böt-VRG neurons (10%) triggered 74 Böt-VRG→RTN-pF correlograms that exhibited offset peaks or troughs indicative of excitatory or inhibitory actions on their respective RTN-pF targets (n = 54, 18%; Table 4). Some examples and a correlation feature summary diagram are shown in Fig. 6B. More offset troughs were detected than offset peaks (n = 45 vs. 29, respectively). Twenty Böt-VRG reference neurons were correlated with more than one RTN-pF target neuron.
Table 4.
Böt-VRG→RTN-pF significant offset feature correlations detected in the analysis of 8,490 Böt-VRG↔RTN-pF neuron pairs
| RTN-pF |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I-Dec |
I-Aug |
I |
IE |
E-Dec |
E-Aug |
E |
EI |
NRM |
||||||||||
| Böt-VRG | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T | P | T |
| I-Dec | 2 | 2 | — | — | — | — | — | — | — | 1 | 1 | 1 | — | — | — | — | 1 | 1 |
| 396* | 258* | 36* | 61* | 368* | 285* | 94* | 27* | 612* | ||||||||||
| I-Aug | — | — | 1 | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — |
| 223* | 191* | 33* | 45* | 300* | 193* | 42* | 25* | 367* | ||||||||||
| I | 1 | 2 | — | — | — | — | — | — | 1 | 1 | — | 4 | — | — | — | — | — | 1 |
| 58* | 15* | 22* | 4* | 42* | 33* | 25* | 2* | 52* | ||||||||||
| IE | — | 2 | 1 | — | — | — | — | — | — | 2 | — | — | — | — | — | 1 | — | — |
| 19* | 13* | 4* | 3* | 41* | 17* | 12* | 4* | 33* | ||||||||||
| E-Dec | 1 | 2 | 1 | — | — | 1 | — | — | 1 | 1 | 1 | 4 | 1 | — | — | — | 2 | 5 |
| 296* | 172* | 31* | 50* | 348* | 213* | 73* | 30* | 410* | ||||||||||
| E-Aug | — | — | 2 | 2 | — | — | — | — | — | — | — | 1 | — | — | — | — | 2 | 5 |
| 137* | 82* | 14* | 25* | 98* | 101* | 34* | 13* | 209* | ||||||||||
| E | 1 | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 1 |
| 60* | 27* | 19* | 7* | 43* | 33* | 30* | 4* | 65* | ||||||||||
| EI | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — |
| 10* | 10* | 0* | 2* | 6* | 10* | 4* | 1* | 15* | ||||||||||
| NRM | 3 | — | — | — | — | — | 1 | — | 1 | — | — | — | — | — | — | — | 3 | 4 |
| 357* | 180* | 24* | 49* | 405* | 256* | 36* | 14* | 532* | ||||||||||
Mean DI and time-lag from origin (means ± SD) for offset peaks: 5.9 ± 2.4, 57.8 ± 63.7 ms; offset troughs: 5.9 ± 5.4, 37.1 ± 33.3 ms. Detected peaks and troughs simply interpreted as evidence for a functional connection from Böt-VRG→RTN-pF neurons. Correlated neuron pairs are organized so that offset correlogram features have positive time-lags.
Total number of pairs composed of neurons with the discharge patterns indicated by the row and column labels; numbers were summed to calculate the total number of Böt-VRG↔RTN-pF pairs analyzed. These numbers were used to calculate the percentages of neurons correlated [e.g., of the 348 pairs composed of an E-Dec Böt-VRG and an E-Dec RTN-pF neuron, a functional connection may be inferred for 2 pairs (0.6%; 1 peak and 1 trough)].
Central features indicative of inputs shared by RTN-pF neurons.
A total of 137 RTN-pF neurons (47%) were elements of RTN-pF neuron pairs with central peaks (n = 161) or troughs (n = 43) indicative of a shared input of like or opposite sign, respectively. Examples and a cross-correlation feature summary diagram are shown in Supplemental Fig. S2. In 14 instances, offset features in additional correlograms were consistent with an RTN-pF neuron having divergent connections and serving as a source of the shared influence on the RTN-pF neuron pairs; 11 instances involving Böt-VRG cells as the putative shared input source were also detected.
Central features were found in correlograms of RTN-pF neuron pairs that shared the same respiratory-modulated pattern and those that did not. Analysis of 241 inspiratory neuron pairs yielded 13 peaks and 6 troughs; 37 peaks and 10 troughs were found among 583 E cell pairs. Among 635 pairs composed of an I and an E neuron, features included 28 peaks and 10 troughs. Central features from 210 pairs of NRM cells included 15 central peaks and 2 central troughs. Pairs including at least one NRM cell provided evidence for shared inputs with every category of RTN-pF neuron. Overall, 174 RTN-pF neurons (59.5%) had short-time scale central and/or offset feature correlations with other RTN-pF neurons.
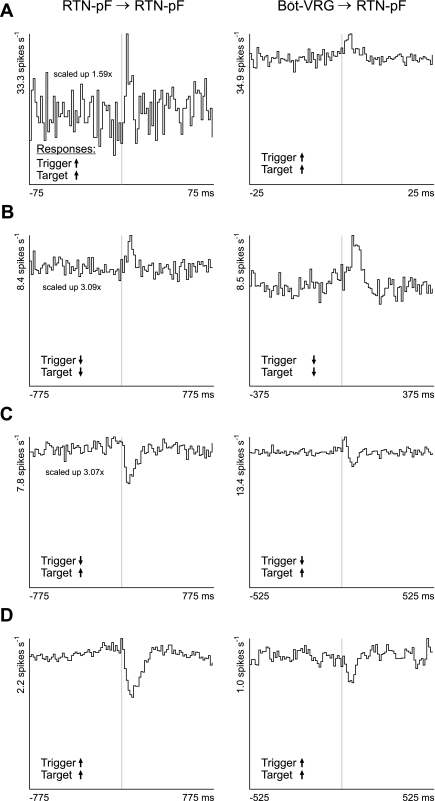
Interactions among chemoresponsive neurons.
Correlogram features between pairs of chemoresponsive cells provided evidence of local RTN-pF interactions and functional inputs from Böt-VRG neurons. Offset cross-correlogram features and responses to central chemoreceptor stimulation for RTN-pF→RTN-pF and Böt-VRG→RTN-pF neuron pairs are detailed in Table 5, which also summarizes offset features for neuron pairs challenged with baroreceptor activation. Examples of offset feature correlograms for pairs of chemoresponsive neurons are shown in Fig. 7. The change in firing rate of each trigger and target neuron in response to central chemoreceptor stimulation is indicated by the arrow in the lower left corner of that pair's histogram. Mechanisms by which these inferred functional connections and the response of the trigger cell to stimulation may work together to affect the response of the RTN-pF target cell are presented in the discussion.
Table 5.
Responses and offset correlation features for tested neuron pairs
| Stimulation of Central Chemoreceptors |
Stimulation of Baroreceptors |
||||
|---|---|---|---|---|---|
| Response |
Offset Correlogram Feature |
Offset Correlogram Feature |
|||
| RTN-pF Trigger | RTN-pF Target | Peaks | Troughs | Peaks | Troughs |
| ↑ | 2 | 4 | 1 | 1 | |
| ↑ | ↓ | 2 | 1 | 1 | 1 |
| → | 2 | 0 | 3 | 2 | |
| ↑ | 4 | 14 | 1 | 0 | |
| ↓ | ↓ | 5 | 4 | 3 | 0 |
| → | 3 | 3 | 3 | 2 | |
| ↑ | 2 | 7 | 5 | 2 | |
| → | ↓ | 0 | 0 | 1 | 7 |
| → | 1 | 1 | 17 | 9 | |
| Böt-VRG Trigger | RTN-pF Target | ||||
| ↑ | 1 | 1 | 0 | 0 | |
| ↑ | ↓ | 2 | 1 | 0 | 3 |
| → | 2 | 0 | 3 | 2 | |
| ↑ | 0 | 2 | 0 | 1 | |
| ↓ | ↓ | 7 | 6 | 2 | 3 |
| → | 2 | 6 | 2 | 2 | |
| ↑ | 1 | 4 | 0 | 1 | |
| → | ↓ | 0 | 0 | 4 | 7 |
| → | 4 | 1 | 5 | 13 | |
Offset peaks and troughs simply interpreted as evidence for a functional connection among RTN-pF neurons (top) and from Böt-VRG→RTN-pF neurons (bottom) tested with either central chemoreceptor or baroreceptor stimulation. Arrows indicate initial firing rate changes in trigger (left) and target (right) neurons in response to presented stimulus. Numbers of offset peaks and troughs detected are tabulated (e.g., 2 peaks and 4 troughs were detected in correlograms in which both the trigger and target cells were RTN-pF neurons that responded with an increased firing rate to central chemoreceptor stimulation; 1 peak and 1 trough were detected for RTN-pF pairs whose constituent neurons responded to baroreceptor stimulation with an increased firing rate).
Fig. 7.
Correlation features among central chemoresponsive neurons provide evidence for functional connectivity proposed to influence responses of RTN-pF target neurons. Left: cross-correlation histograms calculated from pairs of chemoresponsive RTN-pF neurons. Right: CCHs from chemoresponsive neuron pairs consisting of a Böt-VRG trigger neuron and RTN-pF target neuron. Central chemoreceptor responses of trigger and target neurons are delineated with up (increase) and down (decrease) black arrows in the lower left corner of each CCH. A: trigger and target neurons both increase firing rate in response to central chemoreceptor perturbation. CCH A left: exhibits an offset peak, DI = 3.84, 1.5 ms binwidth, 1,687 trigger spikes, and 89,177 target spikes; CCH A right: offset peak, DI = 9.21, 0.5 ms binwidth, 72,867 trigger spikes, and 118,307 target spikes. B: trigger and target neurons both decrease firing rate. CCH B left: offset peak, DI = 3.49, 15.5 ms binwidth, 33,461 trigger spikes, and 53,671 target spikes; CCH B right: offset peak, DI = 6.82, 7.5 ms binwidth, 4,263 trigger spikes, and 33,461 target spikes. C: Trigger and target neurons whose firing rates decrease and increase, respectively, in response to stimulation. CCH C left: offset trough, DI = 7.63, 15.5 ms binwidth, 91,386 trigger spikes, and 97,841 target spikes; CCH C right: offset trough, DI = 7.89, 10.5 ms binwidth, 49,233 trigger spikes, and 119,144 target spikes. D: trigger and target neurons both respond to stimulation by increasing their firing rates. CCH D left: offset trough: DI = 11.71, 15.5 ms binwidth, 71,132 trigger spikes, and 28,640 target spikes. CCH D right: offset trough, DI = 5.98, 10.5 ms binwidth, 114,866 trigger spikes, and 12,201 target spikes.
DISCUSSION
The results of this study provide the first evidence, based on pair-wise neuron correlation analysis, of functional circuit pathways contributing to the network mechanisms for respiratory modulation of RTN-pF neurons and their responses to central chemoreceptor and baroreceptor perturbations. The relative percentages of respiratory-modulated discharge patterns for neurons located within the RTN-pF were similar to those in the Böt-VRG. This observation supports the hypothesis that the RTN-pF region, as a rostral extension of the VRC, contains elements of distributed circuits that shape the modulation of neuronal firing rates as well as influence (via multiple mechanisms) chemoresponsive neuronal response profiles.
Cross-correlograms exhibited offset peaks and troughs indicative of paucisynaptic influences of an RTN-pF or Böt-VRG trigger neuron upon an RTN-pF target cell. Central correlogram features detected among RTN-pF neuron pairs indicate that nearly half of the sampled RTN-pF neurons shared a functional influence with at least one other RTN-pF cell. Most of these shared inputs had a similar effect upon the firing rates of the two target cells. The present results further suggest that local RTN-pF neurons and divergent functional connectivity from the Böt-VRG are among the sources of this shared modulation. In several cases, our multiarray technology permitted simultaneous recordings from putative RTN-pF or Böt-VRG sources of usually unobserved input shared by the target cells (e.g., correlograms for A→B and A→C have offset peaks, whereas a central peak is evident in the B→C correlogram).
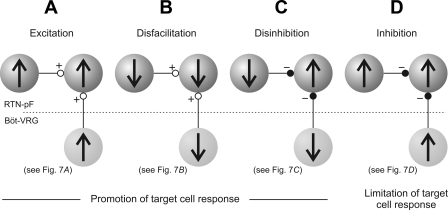
Local circuit interactions identified with cross-correlation analysis among chemoresponsive RTN-pF neurons and correlational linkages with chemoresponsive Böt-VRG trigger neurons are indicative of multiple integrative mechanisms acting within the RTN-pF region. Four circuit mechanisms inferred from the data presented in Fig. 7 are diagrammed in Fig. 8. In Fig. 7A, the trigger and target neurons of each pair respond to central chemoreceptor stimulation with an increase in firing rate; the offset peak in each cross-correlogram is evidence for a paucisynaptic excitatory functional connection. Taken together, these results provide an example of promotion of target cell response via excitation (Fig. 8A). The firing rates of each cell in the pairs shown in Fig. 7B decreased with chemoreceptor stimulation. As in the first example, the offset peaks in the correlograms can be simply interpreted as an excitatory effect of the trigger neuron upon its target, but in this case the target cell's decreased response activity can be attributed to the reduction in excitatory drive from the trigger cell during chemoreceptor stimulation (disfacilitation; Fig. 8B). Functional inhibitory connections detected among chemoresponsive neuron pairs whose constituent neurons exhibited opposite (Fig. 7C) or similar (Fig. 7D) changes in firing rate are consistent with mechanisms of disinhibition, in which the reduction of inhibitory input promotes an increase in target neuron response activity (Fig. 8C), and inhibition, which, in this case, may provide a limiting of target cell response activity (Fig. 8D). These data, together with the observation of diverse time-varying firing rate profiles in simultaneously recorded chemoresponsive RTN-pF cells (e.g., Fig. 3A), strongly suggest more than a simple relay of central chemoreceptor information and support a model with cascading interactions that sequentially promote and limit reflex effects of the target cell.
Fig. 8.
Ball-and-stick diagrams illustrate potential mechanisms underlying the mediation of chemoresponsiveness of RTN-pF target neurons, both locally within RTN-pF region (top; dark gray spheres) as well as inputs coming from Böt-VRG neurons (bottom; light gray). Direction of change in neuronal firing rate in response to central chemoreceptor stimulation is represented by up (increase) or down (decrease) black arrows. Inferred excitatory and inhibitory influences are indicated by open (+) and closed (−) circles, respectively. A: excitation. B: disfacilitation. C: disinhibition. D: inhibition.
Similarly, offset features provide evidence for shaping of RTN-pF respiratory-modulated neuronal discharge patterns via local RTN-pF interactions and both excitatory and inhibitory influences of Böt-VRG cells. Simple inferences of functional connectivity based on cross-correlational data (Fig. 6) provide evidence of interactions among neurons from both regions with a variety of respiratory modulated patterns that could affect the shape of the target neuron's firing pattern. For example, an RTN-pF decrementing inspiratory (I-Dec) neuron's pattern of activity could be promoted by local sources with similar respiratory modulated patterns (Fig. 6A) and/or functional inputs from Böt-VRG I-Dec neurons (e.g., Fig. 6B; CCH 6). Functional routes contributing to the network mechanisms for respiratory modulation also include excitatory and/or inhibitory influences from local RTN-pF cells and Böt-VRG neurons with dissimilar respiratory modulated patterns (Fig. 6A and B; CCH 8).
Relationship to prior work.
Previous studies (Connelly et al. 1990; Nattie et al. 1993) recorded respiratory- and nonrespiratory-modulated neurons within the RTN and immediately surrounding areas in the cat. We found a higher percentage of respiratory cells than did Nattie et al. (1993), who recorded activity from neurons located in an area including and just rostral to the RTN in the decerebrate cat (73% vs. 37%). This difference may be due to our larger region of interest as well as our use of two statistical tests to verify the presence of significant respiratory modulation (see methods) as opposed to a single measure of “respiratoriness.” In the same study, Nattie's group evaluated neuronal responses to increased alveolar Pco2, reporting that 24 of 46 neurons responded to hypercapnia, most with an increase in firing rate (79%). We found a similar percentage of RTN-pF neurons responsive to central chemoreceptor stimulation; however, most of these neurons responded with a rate decrease. This observation could be attributed to different methods of chemosensory challenge: an inhaled hypercapnic gas simultaneously activates both peripheral and central chemoreceptors, whereas a local injection of CO2-saturated saline solution in the vertebral artery allows for the selective stimulation of central brain stem chemoreceptors. Over the course of this study, subsets of neurons from both regions were tested using these two methods and the distinct protocols elicited dissimilar responses from a majority of single cells (M. M. Ott, unpublished observation).
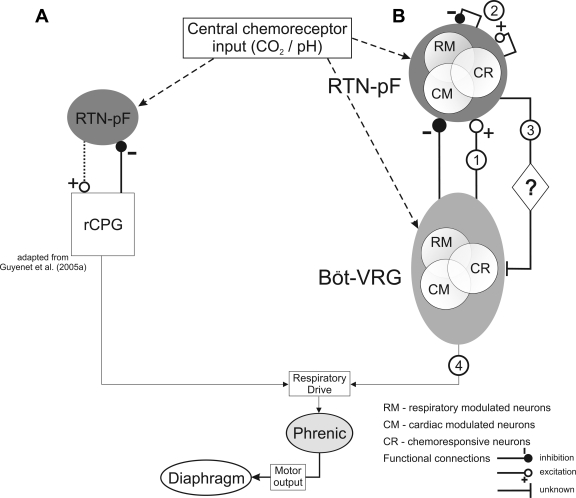
A recent model developed by Guyenet and colleagues (Fig. 9A) proposes that the respiratory central pattern generator (rCPG; located in the vicinity of the pre-Böt complex) contributes to the respiratory-modulated discharge patterns of RTN neurons through inhibitory inputs and assumes that RTN neurons are the major source of chemoreceptor drive for inspiratory activity within the rCPG (Guyenet et al. 2005a; Mulkey et al. 2004; Takakura et al. 2006). We found evidence for predominantly inhibitory but also excitatory functional influences upon RTN-pF cells of almost every respiratory (Fig. 6B and Table 4) and central chemoresponse category (Table 5) from Böt-VRG neurons of similar as well as dissimilar categories. Thus our data support Guyenet's model and extend it to include excitatory inputs from Böt-VRG neurons that influence neuronal chemoreflex responses as well as respiratory-modulated firing patterns (Fig. 9B, circle 1). In addition, our model incorporates functional interactions among RTN-pF cells (Fig. 9B, circle 2).
Fig. 9.
Models depict functional interactions among RTN-pF and Böt-VRG neuron populations. A: a recent model adapted from Guyenet et al. (2005a) proposes that Böt-VRG neurons contribute to the respiratory-modulated discharge patterns of RTN neurons predominately through inhibitory inputs and assumes that RTN neurons are the major source of chemoreceptor drive for inspiratory activity within the respiratory central pattern generator (rCPG). B: our model confirms the previous findings of Guyenet and colleagues and extends it to include excitatory inputs from Böt-VRG neurons that influence neuronal chemoreflex responses as well as respiratory modulated firing patterns (circle 1). Illustration of network interactions inferred from correlogram features suggests that multiple functional routes (including local RTN-pF interactions; circle 2) could contribute to the network mechanisms responsible for the mediation of a target neuron's response to central chemoreceptor stimulation. (See discussion text for future directions regarding circles 3 and 4).
Advantages and limitations of the experimental approach.
Our approach and equipment permitted both the mapping of extended pair-wise correlational linkages among neurons monitored simultaneously at multiple sites and the assessment of their responses to stimulation of multiple sensory modalities under the same state- and history-dependent conditions, eliminating potential confounds associated with sequential recordings of single neurons. Because we were interested in the response of the isolated brain stem respiratory circuit network, we used a decerebrate, vagotomized, and paralyzed preparation to preclude the effects of anesthetics, suprapontine influences, and afferent feedback upon the medullary respiratory network and chemo- and baroreflex responses. Respiratory frequency, tidal volume, and ventilatory responses to hypoxia and hypercapnia in the decerebrate cat have been shown to be very similar to those in the intact awake animal (Tenney and Ou 1977).
Our stimulation protocol enabled the investigation of the independent effect of central chemoreceptor perturbation. We used the CO2-saturated saline injection method to selectively activate central chemoreceptors, thereby avoiding simultaneous peripheral chemoreceptor stimulation that occurs when inhaled gas mixtures are used to produce systemic hypercapnia or hypoxia. Over the course of this study, we observed that both medullary neurons and efferent phrenic nerve activity responded differently to a hypercapnic gas mixture than to injection of CO2-saturated saline into the vertebral artery (M. M. Ott, unpublished observation).
Central chemoreceptor activation may elicit a reflex increase in blood pressure in addition to changes in the respiratory motor pattern. In some experiments, we assessed the potential influence of the chemoreceptor-evoked baroreflex effects by elevating the blood pressure via occlusion of the descending aorta, although this approach may not replicate all the effects of concurrent activation of both systems. The majority of neurons tested with both sequential central chemoreceptor and baroreceptor activation had dissimilar responses (Fig. 5A), suggesting that responses evoked during central chemoreceptor stimulation were not exclusively due to baroreflex activation (Chang et al. 2000; Lindsey et al. 1998; Nuding et al. 2009).
Previous studies from our laboratory have shown that the cross-correlation technique is sufficiently sensitive to detect evidence of excitatory and inhibitory connections among a variety of respiratory and nonrespiratory modulated brain stem neurons. Notably, many interactions first identified with cross-correlation in those studies were subsequently confirmed by spike-triggered averaging of synaptic potentials or supported by other approaches (reviewed in Bianchi et al. 1995; Segers et al. 2008). The multisite recordings in this study enabled the detection of extended correlational linkages among many neurons. The acquired sets of correlogram features permitted inferences of plausible connections to produce “the simplest neuronal model that replicates the experimentally observed features of measurements made” (Aertsen et al. 1989).
Future directions.
Remaining questions for future research include the possible influences of chemoresponsive RTN-pF cells upon the Böt-VRG (Fig. 9B, circle 3) and how they may contribute to the motor output of the brain stem respiratory network (Fig. 9B, circle 4). Functional roles of RTN-pF cells include the possibility that a subset of these neurons regulates both inspiratory and expiratory motor outputs given that the majority of respiratory-modulated RTN-pF cells were active throughout the respiratory cycle. The respiratory modulation of RTN-pF chemoresponsive neurons may function to gate the delivery of excitation and inhibition during the correct phase of the respiratory cycle to facilitate appropriate changes in tidal volume and respiratory frequency in a state-dependent manner. For example, the increased activity of some inspiratory RTN-pF neurons in response to increased central chemoreceptor activation could increase phrenic amplitude by promoting Böt-VRG inspiratory activity and/or decrease respiratory frequency via inhibition of Böt-VRG expiratory cells and the resultant delay of inspiratory to expiratory phase switching. Synchronization among these respiratory-modulated neurons may assist in matching ventilation with perfusion, thereby contributing to optimum cardiorespiratory performance (Dick et al. 2008). Ongoing investigations of RTN-pF→Böt-VRG neuron connectivity (Ott et al. 2007, 2008) should provide further insight into the functional roles of RTN-pF neurons in respiratory motor pattern generation and modulation.
Our findings regarding the juxtaposition of RTN-pF respiratory-, cardiac-, and dually modulated neurons (subsets of which are also chemo- and baroresponsive), along with the detection of their functional interactions, further support the involvement of RTN-pF neurons in cardiorespiratory integration and modulation of both rhythms. Moreover, we encountered neurons that exhibited a cardiac-related, slower-than-respiration modulation of neuronal firing rate (Fig. 3A; e.g., neuron 407) time-locked to concurrent ∼0.1 Hz Mayer waves observed in the blood pressure (Julien 2006; Morris et al. 2010). Further characterizations of Mayer wave-related oscillations (MWROs) in RTN-pF neuron firing rates in response to central chemoreceptor and baroreflex stimulation are needed to extend the seminal observations of Cherniack et al. (1969), who found that hypercapnia and central chemoreceptor stimulation enhance or evoke Mayer waves in systemic arterial blood pressure. Coupled with inferences of connectivity, MWRO neuronal characterizations will assist in investigating the network mechanisms involved in their modulation associated with sympathetic activation. The present observations are consistent with the hypothesis that RTN-pF neurons are elements of a distributed network responsible for generation of MWROs and the coordination of respiratory and vasomotor rhythms (Montano et al. 1996).
Pontine-ventral respiratory column connectivity is necessary for the regulation and maintenance of the normal (eupneic) respiratory rhythm (Dick et al. 2008). Recent studies have used transections through the pontomedullary junction and rostral-to-caudal sectioning of the neuraxis to investigate the origin of respiratory rhythmogenesis (Abdala et al. 2009a, b; Baekey et al. 2008; Smith et al. 2007). A major insight gained from these studies is a rostral-to-caudal stacking of respiratory circuit “building blocks” with distinct functions. These compartments collectively form an adaptive hierarchical network structure that enables the emergence of distinct rhythm generating mechanisms upon sequential sectioning (Smith et al. 2009). Transection studies may disrupt the linkages among RTN-pF and Böt-VRG neurons reported here. Extending the present analyses to identify functional connections from RTN-pF neurons to Böt-VRG circuits will further enhance the conceptual framework that guides the design and interpretation of perturbative studies on network mechanisms for the generation and modulation of the motor patterns for breathing and respiratory-related behaviors.
GRANTS
This study was supported by NIH grant R37 NS19814.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Russell O'Connor, Peter Barnhill, Kimberly Ruff, Kathryn Ross, and Andrew Ross for excellent technical assistance.
REFERENCES
- Abdala et al., 2009a. Abdala APL, Rybak IA, Smith JC, Paton JFR. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol 587: 3539–3559, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdala et al., 2009b. Abdala APL, Rybak IA, Smith JC, Zoccal DB, Machado BH, St-John WM, Paton JFR. Multiple pontomedullary mechanisms of respiratory rhythmogenesis. Respir Physiol Neurobiol 168: 19–25, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aertsen and Gerstein, 1985. Aertsen AM, Gerstein GL. Evaluation of neuronal connectivity: sensitivity of cross-correlation. Brain Res 340: 341–354, 1985 [DOI] [PubMed] [Google Scholar]
- Aertsen et al., 1989. Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity”. J Neurophysiol 61: 900–917, 1989 [DOI] [PubMed] [Google Scholar]
- Alheid et al., 2002. Alheid GF, Gray PA, Jiang MC, Feldman JL, McCrimmon DR. Parvalbumin in respiratory neurons of the ventrolateral medulla of the adult rat. J Neurocytol 31: 693–717, 2002 [DOI] [PubMed] [Google Scholar]
- Arita et al., 1988. Arita H, Kogo N, Ichikawa K. Locations of medullary neurons with non-phasic discharges excited by stimulation of central and/or peripheral chemoreceptors and by activation of nociceptors in cat. Brain Res 442: 1–10, 1988 [DOI] [PubMed] [Google Scholar]
- Baekey et al., 2008. Baekey DM, Dick TE, Paton JFR. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008 [DOI] [PubMed] [Google Scholar]
- Baekey et al., 2004. Baekey DM, Morris KF, Nuding SC, Segers LS, Lindsey BG, Shannon R. Ventrolateral medullary respiratory network participation in the expiration reflex in the cat. J Appl Physiol 96: 2057–2072, 2004 [DOI] [PubMed] [Google Scholar]
- Berman, 1968. Berman AL. The Brain Stem of the Cat: A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison, WI: University of Wisconsin Press, 1968 [Google Scholar]
- Bianchi et al., 1995. Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45, 1995 [DOI] [PubMed] [Google Scholar]
- Chang et al., 2000. Chang EY, Morris KF, Shannon R, Lindsey BG. Repeated sequences of interspike intervals in baroresponsive respiratory related neuronal assemblies of the cat brainstem. J Neurophysiol 84: 1136–1148, 2000 [DOI] [PubMed] [Google Scholar]
- Cherniack et al., 1969. Cherniack NS, Edelman NH, Fishman AP. Pattern of discharge of respiratory neurons during systemic vasomotor waves. Am J Physiol 217: 1375–1383, 1969 [DOI] [PubMed] [Google Scholar]
- Cohen, 1968. Cohen MI. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol 31: 142–165, 1968 [DOI] [PubMed] [Google Scholar]
- Connelly et al., 1992. Connelly CA, Dobbins EG, Feldman JL. Pre-Botzinger complex in cats: respiratory neuronal discharge patterns. Brain Res 590: 337–340, 1992 [DOI] [PubMed] [Google Scholar]
- Connelly et al., 1990. Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol Lung Cell Mol Physiol 258: L33–L44, 1990 [DOI] [PubMed] [Google Scholar]
- Davey et al., 1986. Davey NJ, Ellaway PH, Stein RB. Statistical limits for detecting change in the cumulative sum derivative of the peristimulus time histogram. J Neurosci Methods 17: 153–166, 1986 [DOI] [PubMed] [Google Scholar]
- Dean et al., 2004. Dean JB, Mulkey DK, Henderson RA, Potter SJ, Putnam RW. Hyperoxia, reactive oxygen species, and hyperventilation: oxygen sensitivity of brain stem neurons. J Appl Physiol 96: 784–791, 2004 [DOI] [PubMed] [Google Scholar]
- DiCarlo et al., 1996. DiCarlo JJ, Lane JW, Hsiao SS, Johnson KO. Marking microelectrode penetrations with fluorescent dyes. J Neurosci Methods 64: 75–81, 1996 [DOI] [PubMed] [Google Scholar]
- Dick and Morris, 2004. Dick TE, Morris KF. Quantitative analysis of cardiovascular modulation in respiratory neural activity. J Physiol 556: 959–970, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick et al., 2008. Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Pontine respiratory-modulated activity before and after vagotomy in decerebrate cats. J Physiol 586: 4265–4282, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil et al., 2008. Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C. A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105: 1067–1072, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway, 1978. Ellaway PH. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45: 302–304, 1978 [DOI] [PubMed] [Google Scholar]
- Feldman and Del Negro, 2006. Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci 7: 232–241, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine et al., 2010. Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet, 2008. Guyenet PG. The 2008 Carl Ludwig Lecture: retrotrapezoid nucleus, CO2 homeostasis, and breathing automaticity. J Appl Physiol 105: 404–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al., 2005a. Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet et al., 2005b. Guyenet PG, Stornetta RL, Bayliss DA, Mulkey DK. Retrotrapezoid nucleus: a litmus test for the identification of central chemoreceptors. Exp Physiol 90: 247–253, 2005b [DOI] [PubMed] [Google Scholar]
- Julien, 2006. Julien C. The enigma of Mayer waves: facts and models. Cardiovasc Res 70: 12–21, 2006 [DOI] [PubMed] [Google Scholar]
- Kirkwood, 1979. Kirkwood PA. On the use and interpretation of cross-correlation measurements in the mammalian central nervous system. J Neurosci Methods 1: 107–132, 1979 [DOI] [PubMed] [Google Scholar]
- Kirsten and St. John, 1978. Kirsten EB, St. John WM. A feline decerebration technique with low mortality and long-term homeostasis. J Pharmacol Methods 1: 263–268, 1978 [Google Scholar]
- Kuwana and Natsui, 1987. Kuwana S, Natsui T. Effect of hypercapnic blood injection into the vertebral artery on the phrenic nerve activity in cats. Jpn J Physiol 37: 155–159, 1987 [DOI] [PubMed] [Google Scholar]
- Li et al., 1999. Li Z, Morris KF, Baekey DM, Shannon R, Lindsey BG. Responses of simultaneously recorded respiratory-related medullary neurons to stimulation of multiple sensory modalities. J Neurophysiol 82: 176–187, 1999 [DOI] [PubMed] [Google Scholar]
- Lindsey et al., 1998. Lindsey BG, Arata A, Morris KF, Hernandez YM, Shannon R. Medullary raphe neurones and baroreceptor modulation of the respiratory motor pattern in the cat. J Physiol 512: 863–882, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey et al., 1992. Lindsey BG, Hernandez YM, Morris KF, Shannon R, Gerstein GL. Dynamic reconfiguration of brain stem neural assemblies: respiratory phase-dependent synchrony versus modulation of firing rates. J Neurophysiol 67: 923–930, 1992 [DOI] [PubMed] [Google Scholar]
- Loeschcke, 1982. Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol 332: 1–24, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melssen and Epping, 1987. Melssen WJ, Epping WJ. Detection and estimation of neural connectivity based on crosscorrelation analysis. Biol Cybern 57: 403–414, 1987 [DOI] [PubMed] [Google Scholar]
- Molkov et al., 2010. Molkov YI, Abdala APL, Bacak BJ, Smith JC, Paton JFR, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CPG. J Neurophysiol 104: 2713–2729, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano et al., 1996. Montano N, Gnecchi-Ruscone T, Porta A, Lombardi F, Malliani A, Barman SM. Presence of vasomotor and respiratory rhythms in the discharge of single medullary neurons involved in the regulation of cardiovascular system. J Auton Nerv Syst 57: 116–122, 1996 [DOI] [PubMed] [Google Scholar]
- Moore et al., 1970. Moore GP, Segundo JP, Perkel DH, Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J 10: 876–899, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris et al., 1996. Morris KF, Arata A, Shannon R, Lindsey BG. Inspiratory drive and phase duration during carotid chemoreceptor stimulation in the cat: medullary neurone correlations. J Physiol 491: 241–259, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris et al., 2010. Morris KF, Nuding SC, Segers LS, Baekey DM, Shannon R, Lindsey BG, Dick TE. Respiratory and Mayer wave related discharge patterns of raphe and pontine neurons change with vagotomy. J Appl Physiol 109: 189–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey et al., 2004. Mulkey D, Stornetta R, Weston M, Simmons J, Parker A, Bayliss D, Guyenet P. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004 [DOI] [PubMed] [Google Scholar]
- Nattie et al., 1993. Nattie EE, Fung ML, Li A, St. John WM. Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol 94: 35–50, 1993 [DOI] [PubMed] [Google Scholar]
- Nattie and Li, 1995. Nattie EE, Li A. Retrotrapezoid nucleus (RTN) metabotropic glutamate receptors and long-term stimulation of ventilatory output. RTN glutamate receptors and breathing. Adv Exp Med Biol 393: 39–45, 1995 [DOI] [PubMed] [Google Scholar]
- Nattie et al., 1991. Nattie EE, Li A, St. John WM. Lesions in retrotrapezoid nucleus decrease ventilatory output in anesthetized or decerebrate cats. J Appl Physiol 71: 1364–1375, 1991 [DOI] [PubMed] [Google Scholar]
- Netick and Orem, 1981. Netick A, Orem J. Erroneous classification of neuronal activity by the respiratory modulation index. Neurosci Lett 21: 301–306, 1981 [DOI] [PubMed] [Google Scholar]
- Nuding et al., 2009. Nuding SC, Segers LS, Shannon R, O'Connor R, Morris KF, Lindsey BG. Central and peripheral chemoreceptors evoke distinct responses in simultaneously recorded neurons of the raphé-pontomedullary respiratory network. Philos Trans R Soc Lond B Biol Sci 364: 2501–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor et al., 2005. O'Connor RE, Barnhill PR, Nuding SC, Morris KF, Lindsey BG. Open source spike sorting software for large multi-electrode systems. Program No. 689.3 Neuroscience 2005 Abstracts. Washington, DC: Society for Neuroscience, 2005. Online [Google Scholar]
- Onimaru and Homma, 2006. Onimaru H, Homma I. The parafacial respiratory group/pre-Botzinger complex is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol 100: 2094–2098, 2006 [DOI] [PubMed] [Google Scholar]
- Orem and Netick, 1982. Orem J, Netick A. Characteristics of midbrain respiratory neurons in sleep and wakefulness in the cat. Brain Res 244: 231–241, 1982 [DOI] [PubMed] [Google Scholar]
- Ott et al., 2008. Ott MM, Nuding SC, Morris KF, Lindsey BG. Correlational linkage maps of rostral medullary neurons and the ventral respiratory column suggest multiple network sites for motor pattern modulation. Program No. 476.4 Neuroscience 2008 Abstracts. Washington, DC: Society for Neuroscience, 2008. Online [Google Scholar]
- Ott et al., 2007. Ott MM, Nuding SC, Morris KF, Lindsey BG. Functional interactions and responses of retrotrapezoid nucleus and ventral respiratory column neurons to central chemoreceptor and baroreceptor stimulation. Program No. 297.9 Neuroscience 2007 Abstracts. San Diego, CA: Society for Neuroscience, 2007. Online [Google Scholar]
- Pearce et al., 1989. Pearce RA, Stornetta RL, Guyenet PG. Retrotrapezoid nucleus in the rat. Neurosci Lett 101: 138–142, 1989 [DOI] [PubMed] [Google Scholar]
- Perkel et al., 1967a. Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. I: The single spike train. Biophys J 7: 391–418, 1967a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel et al., 1967b. Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II: Simultaneous spike trains. Biophys J 7: 419–440, 1967b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak et al., 2007. Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC, Paul Cisek TD, John FK. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. In: Progress in Brain Research. Elsevier, 2007, p. 201–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano et al., 2001. Sasano H, Vesely A, Iscoe S, Tesler J, Fisher J. A simple apparatus for accelerating recovery from inhaled volitile anesthetics. Anesth Analg 93: 1188–1191, 2001 [DOI] [PubMed] [Google Scholar]
- Schwarzacher et al., 1995. Schwarzacher SW, Smith JC, Richter DW. Pre-Botzinger complex in the cat. J Neurophysiol 73: 1452–1461, 1995 [DOI] [PubMed] [Google Scholar]
- Segers et al., 2008. Segers LS, Nuding SC, Dick TE, Shannon R, Baekey DM, Solomon IC, Morris KF, Lindsey BG. Functional connectivity in the pontomedullary respiratory network. J Neurophysiol 100: 1749–1769, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 2007. Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 2009. Smith JC, Abdala APL, Rybak IA, Paton JFR. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 1991. Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 1989. Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J Comp Neurol 281: 69–96, 1989 [DOI] [PubMed] [Google Scholar]
- St. John et al., 1989. St. John WM, Hwang Q, Nattie EE, Zhou D. Functions of the retrofacial nucleus in chemosensitivity and ventilatory neurogenesis. Respir Physiol 76: 159–171, 1989 [DOI] [PubMed] [Google Scholar]
- Takakura et al., 2006. Takakura AC, Moreira TS, Colombari E, West GH, Stornetta R, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney and Ou, 1977. Tenney SM, Ou LC. Ventilatory response of decorticate and decerebrate cats to hypoxia and CO2. Respir Physiol 29: 81–92, 1977 [DOI] [PubMed] [Google Scholar]
- Vesely et al., 2003. Vesely A, Fisher JA, Sasano N, Preiss D, Somogyi R, El-Beheiry H, Prabhu A, Sasano H. Isocapnic hyperpnoea accelerates recovery from isoflurane anaesthesia. Br J Anaesth 91: 787–792, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.