Abstract
Retrovirus infection is initiated by receptor-dependent fusion of the envelope to the cell membrane. The modular organization of the envelope protein of C type retroviruses has been exploited to investigate how binding of the surface subunit (SU) to receptor triggers fusion mediated by the transmembrane (TM) subunit. We show that deletion of the receptor-binding domain (RBD) from SU of Friend murine leukemia virus (Fr-MLV) abolishes infection that is restored by supplying RBD as a soluble protein. Infection by this mechanism remains dependent on receptor expression. When membrane attachment of the virus lacking RBD is reestablished by inserting the hormone erythropoietin, infection remains dependent on the RBD/receptor complex. However, infection increases 50-fold to 5 × 105 units/ml on cells that also express the erythropoietin receptor. Soluble RBD from Fr-MLV also restores infection by amphotropic and xenotropic MLVs in which RBD is deleted. These experiments demonstrate that RBD has two functions: mediating virus attachment and activating the fusion mechanism. In addition, they indicate that receptor engagement triggers fusion by promoting a subgroup-independent functional interaction between RBD and the remainder of SU and/or TM.
Retrovirus infection is initiated by fusion of the virus envelope to the host cell membrane, resulting in delivery of the virion core into the cytoplasm. Fusion is triggered when the viral envelope proteins bind to receptors expressed on the host cell membrane. The retroviral envelope protein is a trimer of heterodimers formed between the surface (SU) and the transmembrane (TM) subunits (1, 2). At present, it is unclear how virus binding to receptor, which is mediated by SU, is coupled to fusion, which is mediated by TM. There is currently no evidence that the SU/receptor complex participates in membrane fusion, nor that the receptor makes direct contact with TM. Therefore, critical intra- and possibly interenvelope interactions must occur that link receptor binding to the fusion mechanism.
To investigate how this functional linking occurs, we have exploited the modular organization of the mammalian C-type retroviral envelope proteins (Fig. 1A). Sequence-based alignment of these proteins reveals a proline-rich region in the middle of SU that separates the N-terminal segment, which varies among murine leukemia virus (MLV) subgroups, from the C-terminal segment, which is relatively conserved (3–5). Chimeric envelope proteins, created by combining segments from viruses that use different receptors, are often functional and use the receptor specified by the N-terminal segment (4, 6, 7). In the SU of Friend MLV (Fr-MLV), the N-terminal 236 residues before the proline-rich region form a discrete domain (receptor-binding domain, RBD) that binds to receptor with high affinity and 1:1 stoichiometry (4, 8, 9). From functional studies informed by the atomic structure (Fig. 1B) (10), a pocket has been identified at the top of Fr-MLV RBD that is required for receptor binding and likely forms a critical contact with receptor (11). Near the base of RBD, a conserved histidine residue has been identified that mediates a critical postbinding step in infection (12). Lavillette et al. (13) confirmed that virions in which this histidine residue has been deleted can bind, but not fuse, to receptor-bearing cells. They made the striking observation that infection by this virus was completed by adding supernatant containing soluble RBD (13). We have used this observation as the basis for an assay by using purified RBD to further investigate the mechanism of leukemia virus infection. Our findings suggest that receptor binding promotes the formation of a critical contact between RBD and the remainder of SU/TM that is required for infection. They also provide the basis for targeting MLV infection by using nonviral receptors.
Figure 1.
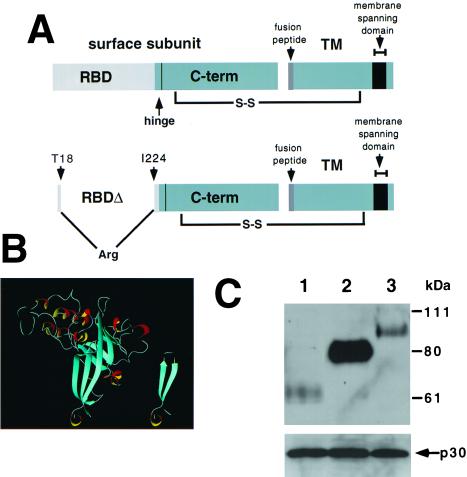
(A) Schematic diagram of RBD in Fr-MLV envelope glycoprotein. The top diagram illustrates that RBD is composed of the N-terminal portion of the SU and is connected to the C-terminal portion of SU by a proline-rich “hinge.” The SU and TM are linked by a disulfide bond. The bottom diagram illustrates the location of the deletion (residues 19–223) and insertion of an arginine residue introduced to obtain Fr-MLV (env ΔRBD). (B) Proposed structure of Fr-MLV Env ΔRBD. Ribbon diagram of the RBD of the Fr-MLV surface glycoprotein (Left). It is composed of a barrel-like structure (blue) similar to an Ig-fold and a series of loops (white) and helices (red/yellow) at the top that contact receptor (10, 11). The N- and C-terminal β-strands are adjacent and together form the base of RBD. Env ΔRBD was prepared by deleting residues 19–223 and connecting these two β-strands by inserting an arginine residue between T18 and I224 at the top of the stalk. The env ΔRBD structure was modeled in the Swiss Protein Data Bank, selecting a structure that minimized steric clashes, and was visualized by using molmol (Right). (C) Incorporation of MLV envelope proteins into virions. Immunoblot of a nitrocellulose filter prepared from lysates of purified virions after SDS/PAGE. The top filter was probed with goat anti-MLV gp70 and HRP mouse anti-goat antibodies. Lane 1: lysate from Fr-MLV (env ΔRBD). Lane 2: lysate from wild-type Fr-MLV. Lane 3: lysate from Fr-MLV (Epo-env). To control for MLV particle production, aliquots of the same lysates were examined for the presence of the p30 capsid protein after SDS/PAGE by using goat anti-MLV and HRP mouse anti-goat antibodies (below).
Materials and Methods
Cell Lines and Viruses.
Human 293 cells were grown in DMEM supplemented with 10% FCS/4 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin at 37°C in 5% CO2. Preparation of human 293 cells expressing mouse cationic amino acid transporter 1 (mCAT1) has been described previously (11). 293-derived cell lines expressing the erythropoietin receptor (EpoR) were prepared by introducing the EpoR expression plasmid (14) into both human 293 and 293 mCAT1 cells and selecting in media containing Zeocin (Invitrogen) (0.4 mg/ml).
Recombinant Virus Production and Infection.
To obtain virus, 80% confluent plates of human 293 cells were transfected with 20 μg of pMD.old.gagpol, encoding MLV gag/pol, 20 μg of pBABE-lacZ, which contains Escherichia coli lacZ, and 20 μg of the expression construct encoding the desired MLV envelope protein, as described previously (15). The next day, cells were washed to remove DNA and refed with fresh medium. After an additional 24 h, the medium was collected and filtered through a 0.45-μm membrane. Virus infection was measured by assaying for acquired β-galactosidase activity on indicator cells 2 days after overnight exposure to the filtered supernatant in the presence of 8 μg/ml polybrene. In some experiments, the titer of virus was determined by endpoint dilution.
An aliquot of the virus-containing supernatant was used to measure incorporation of envelope protein into virions. Virions were separated from cell debris by centrifugation over a sucrose cushion (20% sucrose in 20 mM Hepes, pH 7.4/100 mM NaCl), and lysates were prepared and analyzed after SDS/PAGE by immunoblot by using goat anti-MLV SU antibody [as previously described (11)].
Plasmid Construction.
An expression plasmid encoding the Fr-MLV env ΔRBD was constructed by PCR by using the plasmid pCMV-Frgp85, encoding Fr-MLV57 envelope, as template. The primers, 5′-AGCTTACCAAGAGCAACTAGACC-3′ (−231 base pairs relative to the initiator ATG) and 5′-GCGCGGATCCTGGTCACTTCCCAGGTAATGTT-3′ (+156 base pairs relative to the ATG), containing an artificial BamHI site (underlined), were used to amplify the DNA fragment upstream of the deletion. The PCR product was digested with BamHI. The pCMV-Frgp85 vector was digested with BamHI to release the 830-bp DNA fragment between −60 and +770 base pairs. The BamHI-digested PCR fragment was ligated to the BamHI-digested pCMV-Frgp85 vector to create pFr-MLV env ΔRBD, which lacks sequences encoding amino acid residues 19–223 from envelope.
The expression plasmid pFr-MLV Epo envelope (Epo-env) was constructed by a two-step protocol. In the first step, the EcoRV fragment in pCMV-Frgp85 (+783 base pairs relative to the ATG of envelope and pCDNA3 vector polylinker) was deleted, forming pFrgp85ΔEcoRV. A BstEII site was introduced into this plasmid by PCR-based change of nucleotide A148 to G and nucleotide G151 to A (silent changes) to create pFrgp85ΔEcoRV-BstEII. The coding region of secreted human EPO was amplified from pSG5-EPO/WT (16) by PCR by using the primers 5′-GGCCGGTAACCGCCCCACCACGCCTCAT-3′ and 5′-GCCGGATCCTGTCCCCTGTCCTGCAGGC-3′ that contain BstEII and BamHI sites, respectively (underlined). The final construct was produced by ligation of the HindIII-BstEII fragment of pFrgp85ΔEcoRV-BstEII, the BstEII-BamHI fragment of the pSG5-EPO/WT PCR product, and pCMV-Frgp85 digested with BamHI and HindIII.
The expression construct encoding amphotropic env ΔRBD (A-MLV env ΔRBD) was created from pSV-MLV-A (17), a simian virus 40 large T based expression plasmid encoding the amphotropic 4070 MLV envelope. This plasmid was used as template to prepare two PCR fragments. The sequence located 5′ of the deletion was amplified by PCR by using the primers, 5′-GGGCAATTACCTGCATAGACGAA-3′ and 5′-GCGCAGATCTTACATTAAAGACCTGATGGGGGCT-3′ and digested with NheI and BglII. The sequence 3′ of the deletion was amplified by using the primers 5′-GCGCAGATCTCTGACCCGGCAGGTCCTTAATGT-3′ and 5′-GTAAGCTTATGTTGGGAAGTG-3′ and was digested with BglII and HindIII. The final construct, pA-MLV env ΔRBD, was created by a three-part ligation of the two PCR-derived fragments that flank the deletion and the vector backbone prepared by NheI and HindIII digestion of pSV-MLV-A. In the plasmid pA-MLV env ΔRBD, the sequences encoding residues 11–178 of the A-MLV 4070 envelope are deleted and replaced with the arginine codon, AGA, that is part of the BglII site.
To create the plasmid pA-MLV Epo-env, the Epo coding region was amplified by PCR from pSG5-EPO/WT by using primers, 5′-GCGCAGATCTGCCCCACCACGCCTCATC-3′ and 5′-GCGCAGATCTGTCCCCTGTCCTGCAGGC-3′. After digestion with BglII, this fragment was inserted into the unique BglII site of pA-MLV env ΔRBD and correctly oriented.
The plasmid pX-MLV env ΔRBD was prepared by PCR by using the envelope encoding sequence of a xenotropic New Zealand Black MLV provirus. The proviral sequences located 5′ of the deletion were amplified by PCR by using the T7 primer in the vector backbone (sense) and 5′-GCGCTCGAGGTAACTCTCCAAGTAACATT-3′ (antisense), incorporating a XhoI site (underlined). The sequences flanking the 3′ portion of the deletion were amplified by PCR by using the primers SP6 (antisense) and 5′-GCGCTCGAGGACCCGCCAGGTCCTCAATGT-3′ (sense), also incorporating a XhoI site. The 5′ PCR product was digested with HindIII and XhoI and the 3′ PCR product was digested with XhoI and XbaI, and these DNAs were inserted by three-part ligation into pCDNA3 (Invitrogen) digested with HindIII and XbaI. In the final construct, pX-MLV env ΔRBD, the sequences encoding the residues N18-L192 in RBD, were deleted and replaced with TCGAGG encoding a serine and an arginine residue.
The EpoR expression plasmid was prepared by inserting the KpnI fragment from PXM-190 (14) into pCDNA3.1Zeocin (Invitrogen).
All plasmid constructs were validated by DNA sequencing.
Purification of RBD Proteins.
The production and purification of the RBDs of Friend MLV (Fr-RBD) and A-MLV (A-RBD) have been described previously, and xenotropic MLV (X-MLV) RBD (residues 1–204) (X-RBD) was produced by using the same protocol (9). The yield of purified RBDs was 1 mg/liter of original culture medium. N-terminal protein sequencing (Biopolymer Facility, Harvard Medical School) verified that X-RBD was correctly processed.
Results
The RBD of Fr-MLV is formed by a series of loops that connect two adjacent helices that abut a barrel-shaped stalk composed of nine β-strands (Fig. 1B Left). The first and last of the nine β-strands are juxtaposed in an antiparallel orientation, forming the base of the stalk (10). The portion of RBD between the adjacent residues, T18, on the first β-strand, and I224, on the ninth β-strand (which includes the receptor-binding pocket), was deleted and replaced with a single arginine residue linking the two β-strands (Fig. 1A). The structure of ΔRBD was modeled by using the Swiss Protein Data Bank (Fig. 1B Right). The envelope protein containing this deletion (env ΔRBD) was introduced into MLV containing E. coli lacZ. The incorporation of the ΔRBD envelope protein into virions was reduced compared with wild-type envelope protein (Fig. 1C). No infection by Fr-MLV (env ΔRBD) was observed on a permissive human 293-derived cell line that expresses the Fr-MLV receptor, mCAT1.
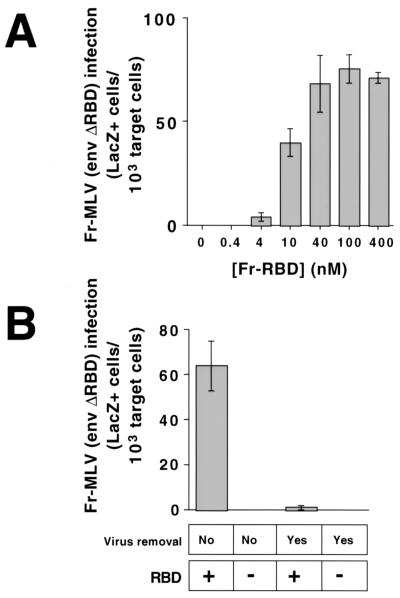
Addition of purified RBD to the culture medium restored Fr-MLV (env ΔRBD) infection of 293 mCAT1 cells (Fig. 2A). Fr-MLV (env ΔRBD) infection increased as a function of the concentration of RBD in the culture medium, reaching saturation at 40 nM. In a subsequent experiment with this concentration of RBD, the titer of Fr-MLV (env ΔRBD), determined by endpoint dilution, was 1.6 × 104 units/ml. No infection by Fr-MLV (env ΔRBD) was observed on human 293 cells that do not express mCAT1, even when the RBD concentration was increased to 4 μM, and RBD did not restore infection by MLV particles that lack env ΔRBD. Infection was reduced more than 100-fold if Fr-MLV (env ΔRBD) was removed before RBD was added (Fig. 2B). These findings strongly suggest that during infection, soluble RBD is in contact with both mCAT1 on the cell membrane and env ΔRBD on the virus membrane.
Figure 2.
Rescue of Fr-MLV (env ΔRBD) infection by soluble RBD on 293 mCAT1 cells. (A) In each well of a 6-well plate, 5 × 105 human 293-derived cells expressing the Fr-MLV receptor, mCAT1, were exposed to Fr-MLV (env ΔRBD) containing E. coli lacZ in the presence of increasing concentrations of purified Fr-RBD (0–400 nM). A 1:10 dilution of viral supernatant (1 ml/well), RBD, and polybrene (8 μg/ml) was added to cells simultaneously, and the plates were incubated overnight at 37°C before refeeding with fresh media. After an additional 24 h of incubation, cells were assayed for acquired β-galactosidase activity. The experiments were performed in triplicate, and standard errors are indicated. (B) Six-well plates of 293 mCAT1 cells were exposed to 1 ml per well of Fr-MLV (env ΔRBD) for 3 h on ice. Virus-containing media were then replaced with fresh media on half of the wells, and RBD (450 nM) was either added or not to each well. After 48 h, cells were fixed and stained, and β-galactosidase-positive cells were counted. The experiments were performed in triplicate, and standard errors are indicated.
The molecular basis for the putative interaction between RBD and env ΔRBD was examined further by studying infection by A-MLV. A-MLV is closely related to Fr-MLV but binds to a distinct receptor (Pit2) (18, 19) that is expressed on human 293 cells. Deletion of RBD from A-MLV (residues 11–178) abrogated infection that was restored by addition of purified RBD (40 nM) from Fr-MLV to the culture medium, but only on the 293-derived cell line that expresses mCAT1 (Table 1). The concentration dependence of A-MLV (env ΔRBD) infection on Fr-RBD was identical to Fr-MLV (env ΔRBD), and the titer in the presence of excess Fr-RBD (40 nM) was comparable (1 × 104 units/ml). Similar findings were obtained in studies of X-MLV lacking RBD (env ΔRBD, residues 18–192). Surprisingly, addition of purified RBDs from A-MLV envelope (A-RBD) or from X-MLV envelope (X-RBD) failed to support infection of either Fr-, A-, or X-MLV (env ΔRBD), even at 100 nM. The functional integrity of A- and X-RBD was verified by binding studies (data not shown). Also, A-RBD rescued infection by A-MLV in which histidine 5 in SU had been deleted, confirming the findings of Lavillette et al. (13) (data not shown). These findings demonstrate that the ability of soluble Fr-RBD to support MLV (env ΔRBD) infection is not duplicated by either A- or X-RBD, despite the expression of their cognate receptors, hPit2 and hSYG1, on human 293 cells.
Table 1.
Effect of three different soluble RBDs on MLV (env ΔRBD) infection (units/ml × 104 +/− SE)
| Virus | Soluble RBD (100 nM)
|
||
|---|---|---|---|
| A-RBD | Fr-RBD | X-RBD | |
| A-MLV (env ΔRBD) | 0 | 1.17 (±0.11) | 0 |
| Fr-MLV (env ΔRBD) | 0 | 1.39 (±0.19) | 0 |
| X-MLV (env ΔRBD) | 0 | 1.09 (±0.12) | 0 |
Human 293 mCAT1 cells were challenged with A-, Fr-, or X-MLV (env ΔRBD) encoding lacZ in the absence or in the presence of soluble A-, Fr-, or X-RBD (100 nM). Two days later, cells were fixed and lacZ+ cells were counted. Data are presented as infectious units/ml (×104) with one standard error indicated. No lacZ+ cells were observed in the absence of soluble RBD.
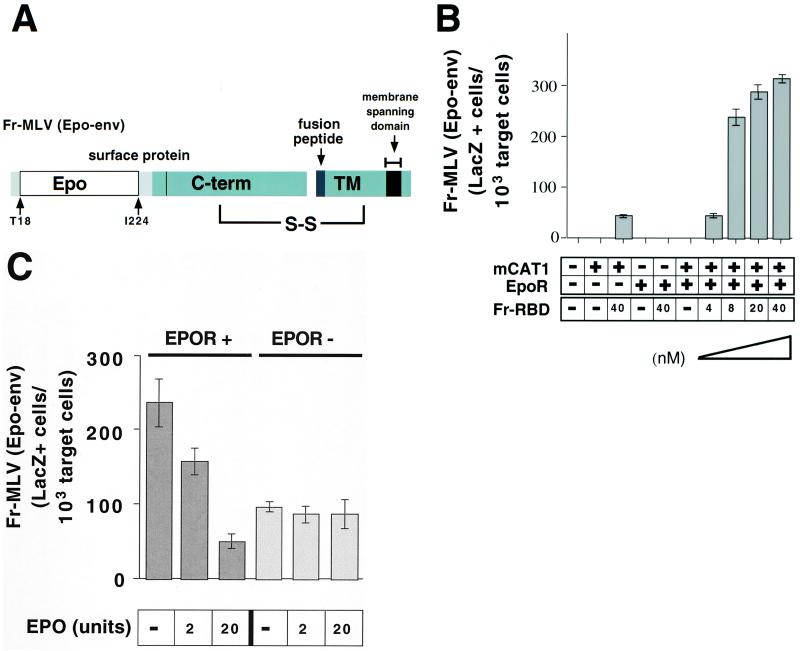
The titer of Fr-MLV (env ΔRBD) infection in the presence of Fr-RBD was only 1% of the titer of wild-type Fr-MLV (106 units/ml) prepared by using the same protocol. This reduction in infection may be caused by: (i) the reduced incorporation of env ΔRBD into virions; (ii) the disruption of critical intramolecular interactions in env ΔRBD; and/or (iii) the diminished probability that virions will encounter soluble Fr-RBD bound to receptor, because they are unable to attach to the membrane. To distinguish among these hypotheses, we reestablished binding of Fr-MLV (env ΔRBD) to the cell membrane by inserting the hormone Epo in place of RBD and tested for infection on cells that express the EpoR. The chimeric Epo-env was prepared by inserting a portion of the cDNA encoding Epo (amino acids 32–177, lacking the signal peptide) into the plasmid encoding Fr-MLV (env ΔRBD) between T18 and I224 (Fig. 3A). Like env ΔRBD, the incorporation of Epo-env into virions was markedly reduced compared with wild type (Fig. 1C). No infection by Fr-MLV (Epo-env) was observed on 293 cells or on 293-derived cell lines that express either EpoR and/or mCAT1 (Fig. 3B). On addition of Fr-RBD (40 nM) to the culture medium, infection was observed on cells that express mCAT1. No RBD-dependent infection by Fr-MLV (Epo-env) was observed on cells that express EpoR alone, but there was striking infection of cells that express both EpoR and mCAT1. The titer of Fr-MLV (Epo-env) is 50-fold greater on cells that express EpoR and mCAT1 (5 × 105 units/ml) than on cells that express mCAT1 alone (1 × 104 units/ml) and is comparable to wild-type Fr-MLV (1 × 106 units/ml) prepared by using the same protocol. The EpoR-dependent increase in infection was inhibited in a concentration-dependent manner by addition of Epo to the culture medium, consistent with binding of Epo-env to EpoR on the cell membrane (Fig. 3C). Also, when Fr-MLV (Epo-env) was removed before addition of RBD to the culture medium, infection decreased to 1% on 293 mCAT1 cells, but only by half on 293 mCAT1/EpoR cells (data not shown). Fr-RBD, but not A-RBD (40 nM), also rescued infection by A-MLV in which Epo was inserted in place of RBD (A-MLV Epo-env). Fr-RBD-dependent restoration of A-MLV (Epo-env) infection was mCAT1 dependent, saturated at 40 nM, and was markedly increased by EpoR expression (data not shown). These findings suggest that it is primarily the lack of membrane binding (hypothesis iii) and not the reduction in envelope expression (hypothesis i) nor the loss of covalent attachment of RBD to envelope (hypothesis ii) that limits the efficiency of Fr-MLV (env ΔRBD) infection mediated by soluble Fr-RBD.
Figure 3.
(A) Schematic diagram of chimeric erythropoietin/MLV envelope protein (Epo-env). The diagram demonstrates the location of erythropoietin inserted in the Fr-MLV envelope protein (Fig. 1B). (B) Rescue of Fr-MLV (Epo-Env) infection by purified RBD. 293 cells or 293-derived cell lines expressing EpoR and/or mCAT1 were treated with a 1:10 dilution of viral supernatant containing Fr-MLV (Epo-env) in the presence or absence of Fr-RBD (40 nM). On cells that expressed both EpoR and mCAT1, Fr-MLV (Epo-env) infection as a function of RBD concentration at 0, 4, 8, 20, and 40 nM RBD was examined. Cells were assayed for acquired β-galactosidase expression 48 h postinfection. Experiments were performed in triplicate, and standard errors are indicated. (C) Competitive inhibition of Fr-MLV (Epo-env) infection by erythropoietin. Either human 293-derived cell lines expressing mCAT1 (EpoR−) or mCAT1 and EpoR (EpoR+) were exposed to Fr-MLV (Epo-env) in the presence of RBD (40 nM) and increasing concentrations of erythropoietin (0–20 units). Infection was measured by staining for β-galactosidase expression 48 h later.
Discussion
In previous experiments, we demonstrated that Fr-MLV infection is initiated by binding of the envelope glycoprotein to the cationic amino acid transporter, mCAT1, on the plasma membrane of susceptible murine cells (9, 20). Virus binding to mCAT1 is mediated by a discreet domain composed of the first 236 residues of the envelope SU subunit (445 residues) (Fig. 1A). The remainder of SU is composed of a second domain that, unlike RBD, is relatively conserved among the mammalian C-type retroviruses that use distinct receptors. This domain is linked to RBD by a short proline-rich “hinge” (21–23) and to the TM subunit by a disulfide bond (24).
To investigate the role of the receptor/RBD interaction in infection, we studied a Fr-MLV that is defective because of deletion of RBD from the envelope protein. We observed that infection by this virus is restored in the presence of purified Fr-RBD. This observation demonstrates that RBD is not required to process or assemble a fusion-competent Fr-MLV envelope protein. It also indicates that the virus receptor, mCAT1, does not promote infection by relieving the suppressive effect of Fr-RBD on the fusion mechanism.
No infection of Fr-MLV (env ΔRBD) was observed on cells that lacked mCAT1, even in the presence of RBD at a concentration 100-fold greater (4 μM) than required for maximal infection in the presence of receptor. In addition, no infection was observed when membrane binding of Fr-MLV (env ΔRBD) was reestablished by insertion of Epo and infection was tested on cells that express EpoR. Therefore, at present, conditions that support Fr-MLV infection in the absence of its receptor, mCAT1, have not been identified.
At saturating concentrations of Fr-RBD, the titer of Fr-MLV (env ΔRBD) on 293 mCAT1 cells was only 1% of wild-type virus. However, the titer was increased 50-fold by inserting Epo and providing soluble Fr-RBD to cells that express both EpoR and mCAT1. The increase in infection caused by insertion of Epo was not observed on cells that lack EpoR and was competitively inhibited by exogenous Epo. This indicates that the efficiency of RBD-dependent infection is increased by attachment of Fr-MLV (Epo-env) to the Epo receptor on the membrane of the target cell. Insertion of Epo did cause a small increase in the incorporation of Epo-env into virions compared with env ΔRBD. However, this increase cannot explain the 50-fold enhancement of infection because, in previous studies, changes in envelope incorporation over a 10-fold range had no demonstrable effect on MLV infection (11, 25).
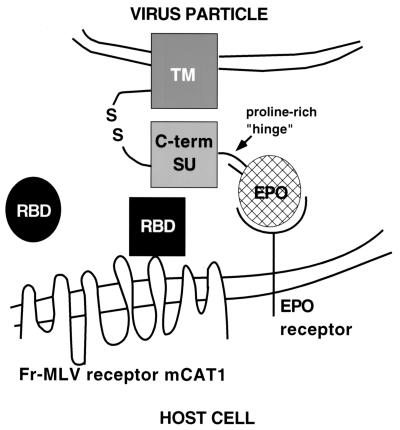
These experiments demonstrate that the fusion mechanism is triggered only after RBD is bound to receptor. In the simplest model, when RBD binds to receptor, it undergoes a conformational change that establishes or alters its interaction with the C-terminal domain of SU/TM in a way that triggers fusion. It is unlikely that mCAT1 makes direct contact with the C-terminal portion of SU/TM, because the mCAT1/Fr-RBD complex supports infection by A- and X-MLVs (env ΔRBD) that normally bind to other receptors. This model is summarized in the diagram in Fig. 4. If this model is correct, the flexibility of the proline-rich segment may accommodate changes in intra- and/or intertrimer contacts between RBD and SU/TM that are induced by receptor binding. Binding studies using purified proteins will be required to verify this model.
Figure 4.
Schematic diagram of the proposed mechanism of RBD-dependent rescue of Fr-MLV (Epo-env) infection. The viral membrane containing the chimeric Epo-env is at the top. The cellular membrane containing the viral receptor (mCAT1) and the EpoR is at the bottom. Soluble RBD is depicted as a circle that, on receptor contact, undergoes a conformational change (receptor-bound RBD is now a square) that is required for activation of infection through direct interaction with the envelope protein.
An additional or alternative function of mCAT1 may be to deliver the Fr-RBD/env ΔRBD complex to a cellular compartment that favors fusion. Mothes et al. (26) recently provided evidence against pH-dependent fusion of MLV within endosomes. Lu and Silver (27) have reported that 30% of mCAT1 is associated with low-density lipid rafts that are enriched in cholesterol, and that cholesterol depletion of cells by cyclodextrin reduced ecotropic MLV infection. Therefore, the receptor may reside in a specific lipid environment that favors fusion.
Previous studies have shown that MLVs that express chimeric envelopes (eco-/ampho-, ampho-/eco-, xeno-/ampho-, and ampho-/xenotropic) joined near the junction of RBD and the proline-rich region are infectious (6, 28). Therefore, although the RBDs of ecotropic, A-, and X-MLVs bind to distinct receptors, it is probable that each activates fusion by using a common mechanism. The observation that Fr-RBD can restore infection by A- and X-MLV lacking RBD is consistent with this conclusion. Also, these results are consistent with the findings of Lavillette et al. (13) that Fr-RBD rescued infection by both ecotropic and A-MLV in which the histidine in the conserved SPHQV sequence near the N terminus of SU was deleted. Soluble A- and X-RBD may be unable to restore MLV (env ΔRBD) infection because the affinity of each for SU/TM may be too low to establish functional contact in trans. Alternatively, if the density of A-RBD (hPit2) and X-RBD (hSYG1) receptors on the plasma membrane is significantly lower than that of mCAT1, the covalent bond between RBD and the remainder of the envelope may be required to assemble a fusion pore. The failure of RBD to rescue MLV (env ΔRBD) infection also may occur if hPit2 and hSYG1 are rapidly down-regulated from the cell surface. Down-regulation of hPit2 by cells exposed to virus has been reported (29).
The organization of MLV SU, in which RBD is attached to a conserved C-terminal portion by a “hinge” region, is consistent with the modular organization of other viral envelope proteins. In this scheme, the C-terminal portion of SU and TM may have evolved from an ancestral membrane fusion protein similar to the fusion proteins of paramyxoviruses that are also cleaved and remain covalently linked by a disulfide bond (30). If true, the function of RBD in MLV infection is analogous to the function of the hemagluttinin-neuraminidase (HN) in triggering F2-mediated fusion of paramyxovirus (31, 32). However, unlike HN, which is a distinct protein with its own transmembrane domain, RBD is attached by the “hinge” region to the C-terminal portion of SU which, along with TM, comprise the two subunits of the disulfide-linked fusion protein. Similarly, HIV gp120 is organized into an inner domain that contacts TM and an outer domain that contacts receptor (33).
Infection of Fr-MLV (Epo-env) was increased 50-fold on cells that expressed the erythropoietin receptor, likely because binding of Epo-env to EpoR increased the proximity of the virus to RBD bound to mCAT1 on the cell membrane. This observation suggests it may be feasible to adapt this protocol to target infection by MLV (Epo-env) to erythroid progenitor cells. Also, given the modular organization of envelope, it is likely that other ligands can be inserted in place of RBD to permit virion attachment and RBD-triggered infection of other cell types. This approach is unlikely to be limited by the availability of mCAT1, which has a broad distribution of tissue expression (34).
Acknowledgments
We are grateful to Shuang Xu for technical assistance, H. Franklin Bunn (Brigham and Women's Hospital, Boston, MA), Alan D'Andrea (Dana–Farber Cancer Institute, Boston, MA), and Jeng-Shin Lee (Harvard Medical School, Boston, MA) for plasmids, and members of the Cunningham laboratory for support and advice. This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant 2R01CA/AI61246–06.
Abbreviations
- env
envelope
- Fr-MLV
Friend murine leukemia virus
- A-MLV
amphotropic MLV
- X-MLV
xenotropic MLV
- RBD
receptor-binding domain
- mCAT1
mouse cationic amino acid transporter 1
- Epo
erythropoietin
- EpoR
erythropoietin receptor
- Epo-env
Epo envelope protein
- SU
surface subunit
- TM
transmembrane subunit
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fass D, Harrison S C, Kim P S. Nat Struct Biol. 1996;3:465–468. doi: 10.1038/nsb0596-465. [DOI] [PubMed] [Google Scholar]
- 2.Pinter A, Fleissner E. Virology. 1977;83:417–422. doi: 10.1016/0042-6822(77)90187-8. [DOI] [PubMed] [Google Scholar]
- 3.Heard J M, Danos O. J Virol. 1991;65:4026–4032. doi: 10.1128/jvi.65.8.4026-4032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Battini J-L, Heard J M, Danos O. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacKrell A J, Soong N W, Curtis C M, Anderson W F. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peredo C, O'Reilly L, Gray K, Roth M J. J Virol. 1996;70:3142–3152. doi: 10.1128/jvi.70.5.3142-3152.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L, Hofmann T, Chian Y, Anderson W F. Somatic Cell Mol Genet. 1995;21:205–214. doi: 10.1007/BF02254771. [DOI] [PubMed] [Google Scholar]
- 8.Battini J-L, Danos O, Heard J M. J Virol. 1995;69:713–719. doi: 10.1128/jvi.69.2.713-719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey R A, Hamson C A, Healey J J, Cunningham J M. J Virol. 1997;71:8096–8102. doi: 10.1128/jvi.71.11.8096-8102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 11.Davey R A, Zuo Y, Cunningham J M. J Virol. 1999;73:3758–3763. doi: 10.1128/jvi.73.5.3758-3763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae Y, Kingsman S M, Kingsman A J. J Virol. 1997;71:2092–2099. doi: 10.1128/jvi.71.3.2092-2099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavillette D, Ruggieri A, Russell S J, Cosset F L. J Virol. 2000;74:295–304. doi: 10.1128/jvi.74.1.295-304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Andrea A D, Lodish H F, Wong G G. Cell. 1989;57:277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- 15.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman M S, Kingsman A J. Nucleic Acids Res. 1995;23:629–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu A, Belanger A, Yoon H W, Bunn H F. J Biol Chem. 1998;273:11173–11176. doi: 10.1074/jbc.273.18.11173. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz D, Goff S, Bank A. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 18.Miller D G, Edwards R H, Miller A D. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O'Hara B. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albritton L M, Tseng L, Scadden D, Cunningham J M. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 21.Weimin Wu B, Cannon P M, Gordon E M, Hall F L, Anderson W F. J Virol. 1998;72:5383–5391. doi: 10.1128/jvi.72.7.5383-5391.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavillette D, Maurice M, Roche C, Russell S J, Sitbon M, Cosset F L. J Virol. 1998;72:9955–9965. doi: 10.1128/jvi.72.12.9955-9965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayman S C, Park H, Saxon M, Pinter A. J Virol. 1999;73:1802–1808. doi: 10.1128/jvi.73.3.1802-1808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinter A, Kopelman R, Li Z, Kayman S C, Sanders D A. J Virol. 1997;71:8073–8077. doi: 10.1128/jvi.71.10.8073-8077.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachrach E, Narin M, Pelegrin M, Karavanas G, Piechaczyk M. J Virol. 2000;74:8480–8486. doi: 10.1128/jvi.74.18.8480-8486.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mothes W, Boerger A L, Narayan S, Cunningham J M, Young J A T. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 27.Lu X, Silver J. Virology. 2000;276:251–258. doi: 10.1006/viro.2000.0555. [DOI] [PubMed] [Google Scholar]
- 28.O'Reilly L, Roth M J. J Virol. 2000;74:899–913. doi: 10.1128/jvi.74.2.899-913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jobbagy Z, Garfield S, Baptiste L, Eiden M V, Anderson W F. J Virol. 2000;74:2847–2854. doi: 10.1128/jvi.74.6.2847-2854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid A, Choppin P W. Virology. 1977;80:54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal P B, Zhang X, Formanowski F, Fitz W, Wong C H, Meier-Ewert H, Skehel J J, Wiley D C. Nature (London) 1998;396:92–96. doi: 10.1038/23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patterson R G, Hiebert S W, Lamb R A. Proc Natl Acad Sci USA. 1995;82:7520–7524. doi: 10.1073/pnas.82.22.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Nature (London) 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J W, Closs E I, Albritton L M, Cunningham J M. Nature (London) 1991;352:725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]