Abstract
Oxidative stress causes damage at the cellular level and activates a number of signaling pathways. Earlier, we have demonstrated that pollutant-related oxidative stress upregulates heat-shock protein 90 alpha (HSP90α) against stress insult in hepatocytes of Mugil cephalus living in a polluted estuary. However, the impact of pollution-induced HSP90α upregulation on stress tolerance is not clear. Here we propose that the effect of stress resistance depends on the ability of HSP90α to modulate the signaling pathways involving proteins such as apoptosis signal-regulating kinase 1, c-Jun NH2-terminal protein kinase 1/2, signal transducers and activators of transcription, extracellular signal-regulated kinase 1/2, protein kinase B, nuclear factor-kappa binding, Ets-like protein 1, and B cell lymphoma-2. In order to investigate this, the activation of HSP90α-associated signaling molecules was examined by Western blotting and immunohistochemistry. The relationship between the protein expression patterns was identified by Spearman’s rank correlation analysis. The signaling proteins exhibited differential modulation as revealed from their expression patterns in pollutant-exposed fish hepatocytes, in comparison with the control fish hepatocytes. The results suggested that in spite of the prevalence of oxidative stress in pollutant-exposed fish hepatocytes, the stress-mediated induction of HSP90α enabled the hepatocytes to become stress tolerant and to survive by modulating the actions of key proteins and kinases in the signal transduction pathways.
Keywords: Apoptosis regulators, Hepatocytes, HSP90α, M. cephalus, STATs, Signal transduction, Stress tolerance, Survival kinases
Introduction
Exposure of cells to a myriad of stressors such as heat, heavy metals, toxins, anoxia, and reactive oxygen metabolites triggers the heat-shock response, which is a potent mechanism for resisting stress. Many proteins that mediate this response are conserved throughout cellular history (Georgopoulos and Welch 1993). The effector proteins of this response, stress proteins or heat-shock proteins, are remarkably conserved in the entire spectrum of organisms from bacteria to human cells. Heat-shock proteins (HSPs) play a number of roles in the cell and tissue physiology. The HSPs protect the proteome through their molecular chaperone function that permits them to recognize the damaged proteins and either channel such proteins into repair/folding pathways or to proteolysis. The 90-kDa heat-shock protein, HSP90, is an abundant molecular chaperone participating in the cytoprotection of eukaryotic cells. It accounts for 1–2% of total proteins under basal, non-stressed conditions and is upregulated in cells in response to stressors, during which its abundance increases up to 4–6% of cellular proteins (Pratt 1998). The inducible form of HSP90 is HSP90α that folds, stabilizes, and functionally regulates many cellular proteins in response to proteotoxic insults (Dias et al. 2005). It is also required for the conformational maturation and/or stability of a range of “client” proteins, including the key mediators of signal transduction processes (Flandrin et al. 2008). HSP90α plays a greater generic role in cell survival and is implicated as an inhibitor of programmed cell death (Calderwood et al. 2006).
Cells have developed sophisticated mechanisms apart from the induction of heat-shock proteins in order to maintain homeostasis and/or try to cope with the excess of reactive oxygen species (ROS) produced during oxidative stress. Cells respond to oxidant injury through the activation of multiple signal transduction pathways that coordinate the various cellular responses.
The mitogen-activated protein kinase (MAPK) cascade is an important signaling pathway playing major roles in the regulation of intracellular metabolism and gene expression. It has a critical role in the adaptive responses to thermal, osmotic, and oxygen stresses (Kultz and Avila 2001). Apoptosis signal-regulating kinase 1 (ASK1) is a 155-kDa ubiquitously expressed protein belonging to the member of mitogen-activated protein kinase kinase kinase, a serine–threonine protein kinase. It is activated in response to ROS, hydrogen peroxide, tumor necrosis factor (TNF), and other stress stimuli (Morita et al. 2001). ASK1, a critical mediator in stress-induced cell death, is reported to activate c-Jun NH2-terminal kinase 1/2 (JNK1/2)-mediated apoptotic pathway (Hatai et al. 2000; Tobiume et al. 2001). JNK1/2 is a SAPK that also belongs to the member of the MAPK superfamily responding to a variety of stress signals (Irving and Bamford 2002). It is the downstream target of ASK1 and its activation favors pathways that lead to apoptosis.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is the another important signaling mechanism that takes part in the regulation of cellular responses to a wide array of cytokines and growth factors. It transduces a multitude of signals for development and homeostasis in animals. This pathway transduces the signal carried by the extracellular polypeptides to the cell nucleus, where activated STAT proteins modify the gene expression.
Extracellular signal-regulated protein kinase (ERK) like JNK1/2 belongs to one of the subgroups of the MAPK superfamily. It is a serine/threonine kinase that mediates intracellular signal transduction in response to a variety of stimuli (Dou et al. 2005). Activation of this pathway has a role in cell proliferation, development, differentiation, and cellular survival (Cowan and Storey 2003). It has been shown that ERK1/2 is activated by hydrogen peroxide (H2O2), a reactive oxygen metabolite apart from mitogens and growth factors (Abe et al. 1998). Protein kinase B (Akt), also belonging to the serine–threonine-specific protein kinase family, is stimulated by a variety of receptors for growth factors, insulin, cytokines, and other signaling molecules. Akt is well characterized in growth and survival promoting cellular signals. Ets-like protein 1 (ELK1) is a member of the TCF subfamily of ETS-domain transcription factors (Sharrocks 2002; Shaw and Saxton 2003). It functions as a nuclear transcriptional activator through its association with the serum response factor (SRF) on SREs present in the promoters of immediate-early genes (Wasylyk et al. 1998). B cell lymphoma-2 (Bcl-2) is a 25-kDa anti-apoptotic protein that has physiological roles protecting against diverse cytotoxic insults. It is a prosurvival protein involved in the control of apoptosis. It functions as ion channels, preventing mitochondrial disruption (Green and Reed 1998). It maintains organelle integrity by preventing the release of cytochrome C from mitochondria. Nuclear factor-kappa binding (NF-κB) is a protein complex that controls the transcription of DNA. It is involved in the cellular response to stimuli such as stress, free radicals, cytokines, and ultraviolet radiation. Under non-stressed condition, this protein resides in the cytoplasm of most cells, associated with an inhibitory protein, inhibitor of NF-κB (IκB). NF-κB activation in response to a wide variety of stimuli occurs as a result of phosphorylation and proteolytic degradation of IκB by IκB kinase. This unmasks the nuclear localization signal of NF-κB, translocating it into the nucleus and regulating its gene expression.
Generally, ERK and Akt signaling pathways have been linked to NF-κB activation (Barradas et al. 1999; Faissner et al. 2006), and a negative correlation has been evidenced between activations of JNK1/2 and NF-κB (Zhang et al. 2004). Also, studies have demonstrated that ERK or Akt and JNK pathway activities oppose each other as a means of regulating apoptosis or survival (Friedman and Perrimon 2006).
We have previously shown that Mugil cephalus inhabiting the polluted Ennore estuary in south India is exposed to severe cytotoxic environmental contaminants which cause the metal-induced oxidative/nitrative stress (Padmini and Usha Rani 2009). However, hepatocytes of those fish, by upregulating HSP90α as an environmental adaptive strategy, resist stress (Padmini and Usha Rani 2010). Because HSP90α is involved in important cellular functions such as maintenance of cytoarchitecture, differentiation, cytoprotection, and cellular integrity through the activation of several signaling proteins and kinases (Sreedhar et al. 2004; Bagatell et al. 2005), this study investigated the hypothesis that oxidative stress tolerance and ability of fish to survive in a polluted estuary might be mediated by HSP90α and its associated signal transduction molecules. Hence, in order to establish the mechanism of stress tolerance, the potential role of signaling proteins associated with HSP90α was investigated by Western blotting and immunohistochemistry in response to stress exposure.
Materials and methods
Study sites
Two estuaries were chosen as the experimental sites for the present study. The Kovalam estuary (12°47′16 N, 80°14′58 E) is situated on the east coast of India and is about 35 km south of Chennai. It runs parallel to the sea coast and extends to a distance of 20 km. The temperature and salinity of this estuary ranged between 25°C and 28°C and 24–26 ppt, respectively. It was chosen as the control (unpolluted) site for the present investigation as it is surrounded by thick vegetation and it is free from industrial or urban pollution. The Ennore estuary (13°14′51 N, 80°19′31 E) also situated on the east coast of India and is about 15 km north of Chennai. It runs parallel to the sea coast and extends over a distance of 36 km. The temperature and salinity of this estuary ranged between 26°C and 30°C and 25–29 ppt, respectively. This estuary was chosen as the test (polluted) site as in its immediate coastal neighborhood situated are a number of industries which include petrochemicals, fertilizers, pesticides, oil refineries, rubber factories, and thermal power stations that discharge their effluents into this estuary. Contamination of the Ennore estuary by heavy metals including lead, cadmium, mercury, zinc, iron, etc. to a significant extent as compared to the Kovalam estuary has been confirmed by previous studies (Raghunathan and Srinivasan 1983; Padmini and Vijaya Geetha 2007).
Study animal and sampling
Mugil cephalus (grey mullet), a natural inhabitant of the estuaries, using Food and Agriculture Organization (FAO) species identification sheets (Fischer and Bianchi 1984) was chosen as the experimental animal for the study. Grey mullets of average length of 30–32 cm were collected from multiple locations of both the control and pollutant-exposed estuaries using baited minnow traps throughout the year during the period December 2007–March 2010. Collected fish were placed immediately in insulated containers filled with aerated estuarine water at ambient temperature (25–30°C) and salinity (24–29 ppt). Fish were maintained in the above specified conditions for 4–5 h until the isolation of hepatocytes.
Hepatocyte isolation and cell viability assessment
Hepatocytes were isolated by following Krumschnabel et al. (1994) and Buckley et al. (2004) with slight modification as described previously (Padmini and Usha Rani 2008). The cell viability of hepatocyte preparations was assessed using trypan blue staining. As demonstrated earlier, cells from the pollutant-exposed site showed a significant decrease in their viability as compared to their control counterparts (Padmini and Usha Rani 2008). Hence, cells with viability ≥94% and ≥82% from control and pollutant-exposed fish, respectively, were chosen for this study.
Whole cell extract preparation and subcellular fractionation
Whole cell extract was prepared by suspending the cells in cell lysis buffer containing 20 mM Tris pH 7.5, 1% Triton X-100, 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM ethylene glycol-bis (2-aminoethyl)-tetraacetic acid (EGTA), 150 mM sodium chloride, 0.1% sodium dodecyl sulfate, 1 mM phenylmethyl sulfonylfluoride (PMSF), 5 mM sodium pyrophosphate, 2 mM sodium orthovanadate, and protease inhibitors (2 μg/ml pepstatin A, 1 μg/ml leupeptin). The cell suspension was incubated for 30 min at 4°C, with occasional shaking, or it was sonicated and centrifuged at 16,000×g for 10 min in a 4°C refrigerated microcentrifuge to remove the cellular debris. The supernatant was the whole cell lysate.
For examining the subcellular localization of signaling proteins, cytosolic fraction was prepared by suspending the cells in 20 mM Tris pH 7.5, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1% Triton X-100, 1 mM dithiothreitol (DTT), 0.5 mM PMSF, and 1 μg/ml leupeptin. The cell suspension was incubated for 10 min at 4°C followed by vortexing for 10 s. The sample was centrifuged at 13,000×g for 30 s in a 4°C refrigerated microcentrifuge. The supernatant was used as the cytosol fraction. The resulting pellets were resuspended in 20 mM Tris pH 7.5, 1% Triton X-100, 10% glycerol, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 0.4 M Nacl, 1 mM DTT, and 1 μg/ml leupeptin and agitated on ice for 20 min. The supernatant obtained by centrifugation at 13,000×g for 20 min at 4°C was used as the nuclear extract. The protein concentration of the extracts was determined by the classical method of Bradford (1976) with Coomassie brilliant blue G-250, using bovine serum albumin as a standard.
Protein immunoblotting analysis of HSP90α and signaling proteins
Western blotting protocol for the analysis of expression pattern of HSP90α and signaling proteins (ASK1, JNK1/2, STAT1α, STAT3, ERK1/2, Akt, ELK1, Bcl-2, and NF-κB) was performed according to the method of Towbin et al. (1979). The whole procedure for all the proteins was performed simultaneously to analyze and compare their coexpression patterns in response to pollutant stress.
Equal amounts of protein (50 μg/lane) of whole cell lysate (HSP90α, ASK1, JNK1/2, ERK1/2, Akt, ELK1, and Bcl-2) and cytoplasmic and nuclear fractions (STAT1α, STAT3, and NF-κB) were separated by 10% SDS-PAGE using dual cool mini-vertical PAGE electrophoretic system according to the protocol described by Laemmli (1970). Separated proteins were transferred to BioTrace polyvinylidene fluoride (PVDF) membrane of 0.45 μm pore size (Pall Corporation, East Hills, New York, USA) at 15 V for 90 min using alkaline buffer (48 mM Tris, 39 mM glycine, 0.037% w/v SDS, 20% v/v methanol, pH 8.3) and semi-dry blotting units (Cleaver Scientific Ltd., Rugby, UK). The PVDF blots were blocked with TBST (10 mM Tris, 150 mM NaCl, 1.54 mM sodium azide, 0.05% v/v Tween-20, pH 7.5) containing 5% w/v skimmed milk powder for 3 h, followed by two 5-min washes with TBST-3X and three 5-min washes with TBST-1X buffer. The blots were stained with antibodies against HSP90α (rabbit polyclonal (SPS-771); 1:1,000 dilution), ASK1 (rabbit polyclonal (AAP-480); 1:700 dilution), phospho JNK1/2 (rabbit polyclonal (KAP-SA011); 1:500 dilution), STAT1α (rabbit polyclonal (KAP-TF001); 1:1,000 dilution), STAT3 (rabbit polyclonal (905-554); 1:1,000 dilution), ERK1/2 (rabbit polyclonal (KAP-MA001); 1:1,000 dilution), phospho Akt (rabbit polyclonal (905-773-100); 1:1,000 dilution), phospho ELK1 (rabbit polyclonal (KAP-MA035); 1:1,000 dilution), Bcl-2 (anti-human Bcl-2 oncoprotein (AM287-5M); used without further dilution, protein concentration 10–15 mg/ml), and NF-κB (rabbit polyclonal (KAP-TF112); 1:500 dilution), with an anti-β-actin antibody (rabbit polyclonal (CSA-400); 1:1,000 dilution) being used to assess equal loading for overnight at 4°C. The blots were then washed as described above and incubated for 1 h in secondary anti-rabbit IgG antibodies coupled to alkaline phosphatase (GeNei, Bangalore, India) diluted 1:1,000 in TBST buffer. The final detection of immunoblotted proteins was accomplished by exposing the blots to a solution of BCIP-NBT substrate for 1–15 min. The band intensities were scanned with the HP scanners (hp psc 1310 series) and quantified using TotalLab image analysis software (Nonlinear dynamics, All Saints, UK). The results were expressed in terms of relative levels of Western blotted proteins which indicated the ratio obtained from the density of the band of interested protein expression standardized with that of the β-actin protein expression level.
Immunohistochemical analysis of signaling proteins
Fish liver tissue samples were fixed in 10% phosphate-buffered formaldehyde solution and embedded in paraffin. The formalin-fixed and paraffin-embedded tissue blocks of fish liver were then cut into 7-μm-thick sections, mounted onto poly-l-lysine-coated slides and stored under dry conditions until histologic analysis. The sections were deparaffinized in xylene, rehydrated in ethanol, and incubated in 3% H2O2 in absolute methanol for 5 min in order to inhibit endogenous peroxidase activity. The sections were then rinsed in 0.05 M tris-buffered saline (TBS), pH 7.6, for 5 min. Antigen retrieval was performed by heat-treating sections in 0.01 mol/l citrate buffer (pH 6.0) at 95°C in a microwave oven for 5 min (3 cycles). To reduce non-specific binding, slides were incubated in 10% normal goat serum for 10 min at room temperature before 1-h incubation with antibodies against phospho JNK1/2 (1: 200 dilution), ERK1/2 (1:500 dilution), phospho Akt (1:200 dilution), phospho ELK1 (1:200 dilution), and Bcl-2 (used without further dilution, protein concentration 10–15 mg/ml) in a humidified chamber at 4°C. After rinsing with TBS, the procedure for the detection of these proteins includes sequential application of biotinylated secondary link antibodies and streptavidin–peroxidase conjugate and incubation at 4°C for 30 min in each of them. Peroxidase activity was detected using 0.1% H2O2 in 3, 3′-diaminobenzidine solution applied to the tissue sections for 5 min. It was then counterstained with hematoxylin for 5 s before rinsing, dehydrating, and mounting with coverslips using xylene and DPX mountant. The immunohistochemical images acquired with Zeiss microscopes (Carl Zeiss Microimaging Inc, Thornwood, USA) were subjected to image analysis for the measurement of average staining intensity of signaling molecule to confirm the results.
Statistical analysis
Statistical analysis by Mann–Whitney U test was used to ascertain the significance of variations between the control and pollutant-exposed fish hepatocytes. All data were presented as mean. The relationship between variables was identified by Spearman’s rank correlation analysis. Differences were considered significant at p < 0.05.
Results
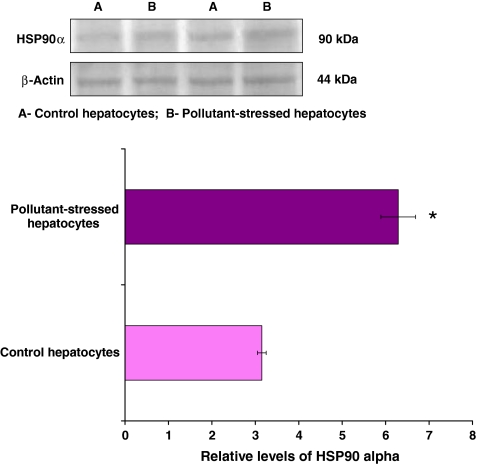
Levels of HSP90α protein increase in hepatocytes of pollutant-exposed fish
To examine the function of HSP90α in response to pollutant stress, its expression pattern was studied by Western blotting. As described earlier (Padmini and Usha Rani 2010), HSP90α protein levels were differentially expressed in control and pollutant-exposed fish hepatocytes (Fig. 1). Oxidative stress mediated by the redox cycling property of environmental contaminants induces HSP90α synthesis (Padmini and Usha Rani 2008). Therefore, the protein amount of HSP90α significantly increased (p < 0.05) in pollutant-exposed fish hepatocytes when compared to their control counterparts.
Fig. 1.
Expression pattern of HSP90α in hepatocytes of M. cephalus inhabiting the control and pollutant-stressed estuaries. Representative immunoblot is shown. β-Actin has been used as the loading control. A and B represent control and pollutant-stressed hepatocytes, respectively, and the bar graph shows the densitometric evaluation. Values are expressed as means ± SE (n = 16 fish per estuary). *p < 0.05, significant when compared with control hepatocytes
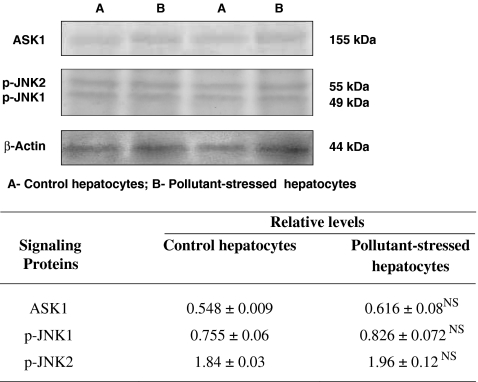
HSP90α induction inhibits apoptotic pathways by downregulating ASK1–JNK1/2 activation
HSP90α is regarded as a protein with a potentially anti-apoptotic function (Bagatell et al. 2005). Protein immunoblotting analysis demonstrated modulation in the expression patterns of ASK1 and JNK1/2 during HSP90α induction. A significant induction of HSP90α was associated with an increase in the expression of ASK1 and JNK1/2 that was not significant in pollutant-exposed fish hepatocytes as compared to their control counterparts. The insignificant expression of ASK1 might be attributed to the protein–protein interactions of ASK1 by HSP90α. As JNK1/2 is the downstream target of ASK1, the inhibition of ASK1 decreased the kinase activity of JNK1/2 (Fig. 2). Immunohistochemistry also revealed a difference that was not statistically significant in the expression of JNK1/2 in the control and pollutant-exposed fish hepatocytes (Fig. 4). Hence, HSP90α upregulation played a key role in modulating the ASK1 expression to the extent of inhibiting the JNK1/2 expression and thereby downregulated apoptosis in the pollutant-exposed fish hepatocytes. The results were very well in accordance with the Spearman’s rank correlation analysis which demonstrated a nil linear relationship between the expression patterns of HSP90α and ASK1/JNK1/2 and a significant positive correlation between the activation of ASK1 and JNK1/2 (Table 1).
Fig. 2.
Expression pattern of apoptotic signaling kinases ASK1 and JNK1/2 in hepatocytes of M. cephalus inhabiting the control and pollutant-stressed estuaries. Representative immunoblots are shown. β-Actin has been used as the loading control. A and B represent the control and pollutant-stressed hepatocytes, respectively and the table demonstrates the relative levels of the protein determined by densitometric analysis. Data are expressed as means ± SE (n = 16 fish per estuary). NS, statistically not significant when compared with control hepatocytes
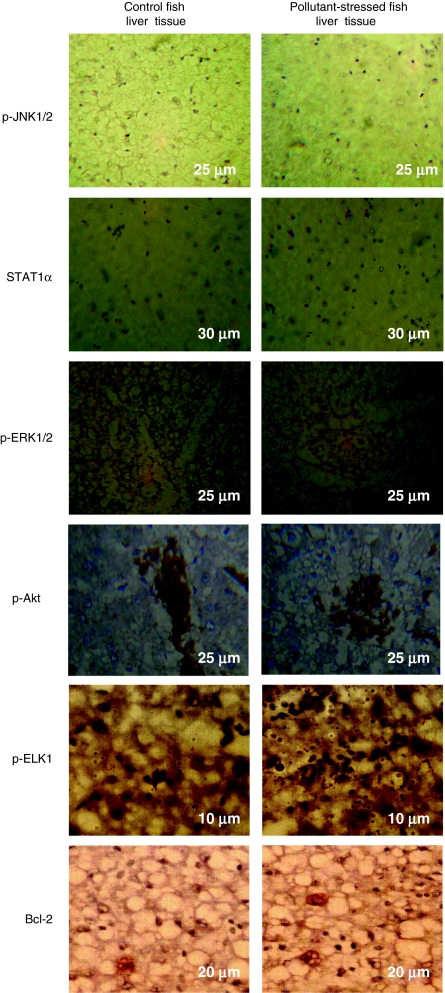
Fig. 4.
Immunohistochemical stainings of signaling proteins like p-JNK1/2, STAT1α, ERK1/2, p-Akt, p-ELK1, and Bcl-2 in the liver tissue of M. cephalus inhabiting the control and pollutant-stressed estuaries. Liver tissue of fish from the control estuary shows light immunostaining, whereas liver tissue of fish from the pollutant-stressed estuary shows intense immunostaining of all signaling molecules except for p-JNK1/2, the results being confirmed from average staining intensity of signaling proteins. The measurements in the micrographs represent scale bar size
Table 1.
Spearman’s rank correlation coefficients between HSP90α and signaling proteins and their proposed cellular targets in the whole hepatocyte lysates of pollutant-stressed fish
| Variable 1 | Variable 2 | rs | p |
|---|---|---|---|
| HSP90α | ASK1 | 0.106 | NS |
| p-JNK1/2 | 0.106 | NS | |
| STAT1α | 0.868 | 0.05* | |
| STAT3 | 0.829 | 0.05* | |
| p-ERK1 | 0.894 | 0.05* | |
| p-Akt | 0.953 | 0.05* | |
| p-ERK1 | p-Elk-1 | 0.988 | 0.05* |
| NF-κB | 0.959 | 0.05* | |
| p-JNK1/2 | −0.026 | NS | |
| p-Akt | Bcl-2 | 1.000 | 0.05* |
| NF-κB | 0.829 | 0.05* | |
| ASK1 | 0.026 | NS | |
| p-JNK1/2 | ASK1 | 0.982 | 0.05* |
| Elk-1 | 0.021 | NS | |
| Bcl-2 | 0.018 | NS | |
| NF-κB | −0.058 | NS | |
| STAT1α/STAT3 | NF-κB | 0.934/0.874 | 0.05* |
The correlation coefficient (Spearman’s rho—rs) values were significant and showed a perfect positive correlation at *p < 0.05. Certain rs values were statistically not significant (NS) demonstrating a nil linear correlation between the variables
Upregulation of HSP90α activates prosurvival pathways
HSP90α synthesis induces STAT activation in stressed fish hepatocytes
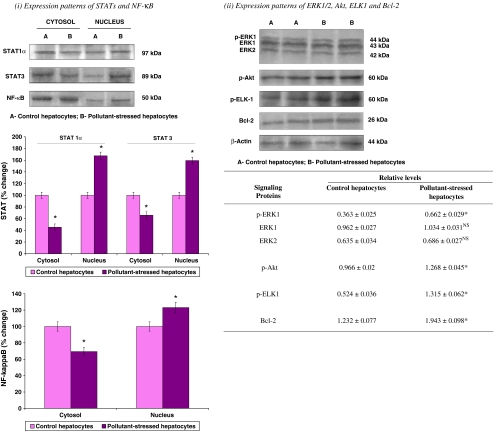
Western blotting studies revealed modulation of STAT levels during the HSP90α induction in the pollutant-stressed fish hepatocytes. STAT1α and STAT3 showed an enhanced activation as characterized by their translocation from the cytosol to the nucleus in the pollutant-stressed fish hepatocytes as compared to their control counterparts. In the control fish hepatocytes, STAT1α and STAT3 were detected mainly in the cytosol and their expression was less in the nuclear fraction (Fig. 3). However, in pollutant-stressed hepatocytes, STAT1α and STAT3 levels were considerably decreased in the cytosolic fraction, but increased in the nucleus (Fig. 3). In support of the immunoblotting results, the immunohistochemical analysis revealed a positive STAT immunostaining in the tissues of both groups. Liver tissue of fish from the control site exhibited a diffuse cytoplasmic and nuclear distribution of STAT. However, an intense nuclear localization of STAT (activation) was observed in pollutant-stressed fish liver confirming the response of liver to pollutant stress (Fig. 4). Spearman’s rank correlation analysis in the whole hepatocyte lysates of pollutant-stressed fish also supported this finding by showing a significant and positive correlation between the HSP90α induction and STAT activation (Table 1), suggesting a central role of HSP90α in the activation of STAT signaling.
Fig. 3.
Expression pattern of prosurvival signaling kinases such as (i) STATs (1α and 3), and NF-κB in cytoplasmic and nuclear fractions (ii) ERK1/2, Akt, ELK1, and Bcl-2 in whole cell lysates of hepatocytes of M. cephalus inhabiting control and pollutant-stressed estuaries. Their representative immunoblots are shown. For STATs and NF-κB, the bar graph shows the densitometric evaluation. The lanes on the blot correspond with the bars on the histogram. Band intensities of STATs and NF-κB protein were quantified and expressed as percentage (100%) of control hepatocytes. Values are expressed as means ± SD (n=5 fish per estuary). *p<0.05, significant when compared with control hepatocytes. For ERK1/2, Akt, ELK1, and Bcl-2, β-actin has been used as the loading control. The table represents the relative levels of the protein determined by densitometric evaluation. Data are expressed as means ± SE (n=16 fish per estuary). *p<0.05, significant; NS – statistically not significant, when compared with control hepatocytes
ERK1/2 and Akt activations parallel HSP90α induction
ERK1/2 and Akt can be activated in response to oxidative stress and can play an important role in promoting cell survival (Ikeyama et al. 2002). Hence, in the current study, ERK1/2 and Akt expressions were analyzed to study their influence on the survival in control and pollutant-stressed fish hepatocytes in response to the environmental pollutant-mediated oxidative stress. ERK1/2 and Akt activities were assessed by immunoblotting using anti-ERK1/2 and anti-phospho Akt antibodies. The results revealed a significant increase in the levels of phosphorylated ERK1 in the pollutant-stressed fish hepatocytes as compared to their control counterparts. The total protein level of ERK1/2 remained unchanged in both the control and pollutant-stressed fish hepatocytes (Fig. 3). Similarly, an enhanced Akt activation characterized by an increase in its phosphorylated form was also observed in the hepatocytes of pollutant-stressed fish (Fig. 3). The activation of these survival proteins was further confirmed by immunohistochemistry. Liver tissue of both the control and pollutant-stressed fish demonstrated the activation of ERK1/2 and Akt. However, the immunohistochemical stainings of active ERK1/2 and Akt visualized in pollutant-stressed fish liver tissue were more characterized by their intense immunostaining as compared to moderate and light immunostaining observed in the control fish liver tissue (Fig. 4). Spearman’s rank correlation analysis also supported the latter finding by showing a significant and positive correlation between HSP90α induction and ERK1/2 or Akt activation (Table 1).
ERK1/2 activation upregulates ELK1 expression pattern
As ELK1 is considered as one of the important cellular targets of ERK1/2 (Kim-Kaneyama et al. 2000), its expression pattern was also examined as part of our analysis. A significant upregulation of p-ELK1 was observed during p-ERK1 induction in the pollutant-stressed fish hepatocytes (Fig. 3). Demonstration of p-ELK1 expression by immunohistochemistry also confirmed the Western blotting results showing an intense brown staining in the pollutant-stressed fish hepatocytes as compared to the moderate immunostaining observed in their control counterparts (Fig. 4). Spearman’s rank correlation analysis also agreed well with the current findings where a significant and positive correlation was observed between p-ERK1 and p-ELK1 levels (Table 1).
Active Akt induces Bcl-2 overexpression
Akt is a critical mediator of cell survival and is demonstrated to exert a cellular protective effect in response to stress (Franke et al. 1997; Zhang et al. 2005). To examine this observation with regard to its role in cell survival, the expression pattern of an anti-apoptotic protein, Bcl-2, which is believed to have an impact through Akt, was studied. A significant upregulation in Bcl-2 levels was observed during the Akt activation (Fig. 3). Also, a positive and intense immunohistochemical staining of Bcl-2 in the pollutant-stressed fish liver tissue as compared to the moderate Bcl-2 immunostaining in their control counterpart confirmed the results (Fig. 4). Furthermore, a positive correlation (p < 0.05) observed between the Akt activation and Bcl-2 levels by Spearman’s rank correlation analysis strengthened these findings (Table 1).
NF-κB translocates from the cytosol to the nucleus in response to pollutant stress
Activation of the transcription factor NF-κB has an essential role in protecting the cells from apoptosis induced by a variety of stress stimuli, including TNFα, γ-irradiation, and certain chemotherapeutic agents (Baeuerle and Henkel 1994; Baichwal and Baeuerle 1997). Because NF-κB is also known to be activated during oxidative stress (Dias et al. 2005; Wu et al. 2009), in the current study, its expression pattern was analyzed to determine its influence on the survival of pollutant-stressed hepatocytes. Together with ERK1/2, JNK1/2, and Akt, NF-κB activation was determined by using the immunoblotting in the hepatocyte cytosolic and nuclear fractions. Significant activation of ERK1/2 and Akt and statistically nonsignificant induction of JNK1/2 were associated with an enhanced NF-κB activation in the pollutant-stressed fish hepatocytes. In these hepatocytes as compared to their control counterparts, NF-κB levels significantly decreased in the cytoplasm, whereas its levels increased in the nucleus (Fig. 3). Spearman’s rank correlation analysis also confirmed the latter finding suggesting a positive correlation between p-ERK1–NF-κB and p-Akt–NF-κB and a nil linear correlation between the activation of p-JNK1/2–NF-κB (Table 1).
Discussion
Prevalence of oxidative stress has been demonstrated in hepatocytes of fish inhabiting the polluted Ennore estuary (Padmini and Usha Rani 2009). The stress phenomenon characterized by an oxidant–antioxidant imbalance constitutes a major threat to organisms living in an aerobic environment. The cellular response to stress is complex often involving multiple signaling pathways that act in concert to influence cell survival. Many of the signaling pathways triggered in response to different types of stresses play important roles in protecting cells against the deleterious effect of ROS and promoting cell survival. The outcome of exposure of cells to stress under such conditions of the prevalence of survival pathways generally results in the development of a state of tolerance to subsequent exposure to stress (Gabai and Sherman 2002). The key cellular responses to oxidative stress associated with survival mechanisms include the induction of the stress proteins (HSPs), activation of MAPKs, and modulation of expression of apoptosis regulators like Bcl-2 and NF-κB.
Induction of HSP90α synthesis by pollutant stress
HSP90α is a homologous molecular chaperone that plays an important protective role in protein folding processes in response to stress stimuli (Yu et al. 2008). As demonstrated earlier (Padmini and Usha Rani 2010), the results showed a significant upregulation of HSP90α protein in the pollutant-stressed fish hepatocytes (Fig. 1). The current study result is in accordance with the finding by Currie and Tufts (1997) who have demonstrated HSP70 induction in rainbow trout red blood cells in response to heat shock and anoxia. Iwama et al. (1998) and Vijayan et al. (1998) have also demonstrated induction of HSPs in response to aquatic contaminants. Studies that reported the induction of various HSP families in fish following exposure to various environmental stressors demonstrated a protective role of HSPs, also suggesting the use of the HSP response as an indicator of stress status in fish (Sherry 2003; Sreedhar and Csermely 2004).
HSP90α induction inhibits pollutant stress-mediated ASK1–JNK1/2 apoptotic signaling
HSP90α is a chaperone essential for the maturation and stabilization of multiple client proteins. It is therefore associated with important cellular functions including cell proliferation and survival through chaperoning key proteins of the associated signaling pathways (Schoof et al. 2009). HSP90α blocks cell apoptosis by inhibiting the pro-apoptotic kinase, ASK1 (Zhang et al. 2005). A direct association of HSP90α with ASK1 is reported to be critical for the regulation of ASK1 activity (Sato et al. 2000; Basso et al. 2002). In accordance with these, the results of the current study indicated that HSP90α inhibited oxidative stress-induced ASK1 activation in the pollutant-stressed fish hepatocytes. HSP90α overexpression contributes to retain ASK1 in an inactive state, thereby inhibiting the stress-induced ASK1 signaling and hepatocyte apoptosis. JNK is the downstream target of ASK1, and its activation in response to stress stimuli is generally involved in the stress response (Deng et al. 2003). Oxidative stress can upregulate JNK by activating its upstream kinase such as ASK1 (Shen and Liu 2006). However, JNK activity is regulated during inhibition of ASK1 by its interacting partners (Saitoh et al. 1998; Kim et al. 2001). These reports are again in accordance with the results of the current study where a statistically insignificant increase in JNK1/2 activation was observed in the pollutant-stressed fish hepatocytes as compared to their counterparts. HSP90α upregulated under stress associate with ASK1 and terminate ASK1-mediated JNK1/2 signaling cascades (Basso et al. 2002; Zhang et al. 2005). HSP90α overexpression in the current study might be therefore responsible in part for the observed inhibition of the activities of the signaling molecules ASK1 and JNK1/2.
HSP90α upregulation favors prosurvival pathways via STAT, ERK1/2, Akt, ELK1, Bcl-2, and NF-κB signaling
STAT activation and signaling facilitate hepatocyte survival
JAK1/2, the upstream activator of STAT, is one of the important client proteins of HSP90 (Shang and Tomasi 2006). Hence, JAK1/2 has been described to interact with HSP90 and could be involved in a sustained JAK–STAT signaling. Recently, HSP90 has also been reported to interact with STATs and this interaction is required for STAT phosphorylation, which further led to hypothesize that STAT1α and STAT3 may also act as a client of HSP90 (Shang and Tomasi 2006). In accordance with these reports, the current study demonstrates that the upregulation of HSP90α and the activation of STAT are correlated, and HSP90α may play a role in mediating STAT activation. In line with these findings, it is demonstrated that inhibition of HSP90 could be associated with the interruption of permanent STAT activation (Schoof et al. 2009). Treatment of classical Hodgkin lymphoma cell line with 17-allylamino-17-demethoxygeldanamycin has led to reduced cell proliferation and a complete inhibition of STAT phosphorylations probably as a result of decreased expression of JAK protein. A decrease of STAT phosphorylations along with decreased cell proliferation of L428 cells is also evident after knock-down of HSP90 (Schoof et al. 2009). This study suggests that HSP90 is important for mediating STAT activation. Furthermore, it is reported that constitutive activation of NF-κB may promote the expression of IL-13 and therefore activation of STATs (Hinz et al. 2002). The present study also demonstrated a positive (p < 0.05) correlation between activation of STAT and NF-κB (Table 1), characterized by their translocations from the cytosol to the nucleus in the pollutant-stressed fish hepatocytes.
HSP90α controls cell survival via ERK1/2 and Akt signaling
HSP90α plays an important role in facilitating the proper folding, maturation, and activity of its client proteins (Neckers 2002). Hence, disruption of HSP90α function inhibits the interaction of this chaperone with its client proteins (Beck et al. 2009; Shen et al. 2009). Also, loss of protection provided by HSP90 facilitates their degradation (Dou et al. 2005). HSP90α has been reported to regulate Raf, the upstream kinase of ERK1/2 in the Ras–Raf–MEK–ERK pathway (Schulte et al. 1995). It is demonstrated to act as a molecular chaperone in late-phase activation of ERK1/2 stimulated by oxidative stress in vascular smooth muscle cells (Liu et al. 2007). Dou et al. (2005) have also reported inhibition of ERK1/2 activation during disruption of HSP90 function (Piatelli et al. 2002). Similarly, Akt is a well-characterized client protein of HSP90 (Meares et al. 2004). It is complexed with HSP90 and this interaction is necessary for this protein to perform various processes in cellular signaling (Sato et al. 2000; Fontana et al. 2002; Fujita et al. 2002). Consequently, many reports have verified that Akt is downregulated, and Akt-dependent survival activities are suppressed following inhibition of HSP90 with geldanamycin (Basso et al. 2002; Doong et al. 2003; Meares et al. 2004). In accordance with these reports, a significant activation of ERK1/2 and Akt observed during HSP90α upregulation in the pollutant-stressed fish hepatocytes in the current study demonstrated the positive regulatory role of HSP90α on these prosurvival kinases. Our findings are supported by Dou et al. (2005) who have also reported that the extent of phosphorylation of ERK in primary neurons decreases after treatment with geldanamycin, while the total ERK protein does not change. This indirectly suggests the regulation of ERK activity by HSP90. A similar observation is reported by using transfection experiments where Akt activation parallels HSP90 induction and the formation of the Akt-HSP90 complex stabilizes Akt, protecting the cells from undergoing apoptosis (Sato et al. 2000).
HSP90α-mediated ERK1/2 and Akt activations provide tolerance against pollutant stress
ERK1/2 and Akt of the Ras/Raf/MAP kinase cascade and PI3K pathway are the two key prosurvival kinases implicated in influencing the cell survival in response to stress (Helmreich 2001). They play important roles in regulating cell proliferation and preventing apoptosis (Kandel and Hay 1999). A direct correlation between resistance to oxidative stress and activation of ERK1/2 or Akt has also been put forth by Ikeyama et al. (2002). Consistent with this, the current study demonstrated an activation of ERK1/2 and Akt in the pollutant-stressed fish hepatocytes as compared to their control counterparts and revealed the prevalence of prosurvival mechanisms against environmental pollutant-mediated oxidative stress. Reinforcing the view of the current finding, it has been shown that ERK and PI3K/Akt signaling pathways contribute to the survival of cells under H2O2 treatment (Guyton et al. 1996; Wang et al. 2000). Ikeyama et al. (2002) have also demonstrated loss of tolerance for oxidative stress with aging that is being linked with the diminished ERK and Akt activities.
ELK1 activation promotes survival of hepatocytes subjected to pollutant stress
Regulation of cell growth is dependent on a number of genes including proto-oncogene, growth factor, growth factor receptor, and immediate-early transcription factor gene (Demir and Kurnaz 2008). ELK1 is known to be involved in the regulation of immediate-early genes such as c-fos upon mitogen activation and, thus commonly, is implied in cell proliferation (Demir and Kurnaz 2008). It plays an important role in transducing extracellular signals to a nuclear response by acting as targets for the MAPK signaling pathways such as ERK1/2 and JNK1/2 (Sharrocks 2002). The current study revealed enhanced ELK1 activation in the pollutant-stressed fish hepatocytes as compared to their control counterparts. Immunohistochemical analysis also demonstrated that in addition to its nuclear localization, ELK1 was distributed throughout the cytoplasm. This is in agreement with a previous reported study on ELK1 in neuronal cells (Barrett et al. 2006). With regard to ELK1 regulation by ERK1/2 and JNK1/2, in the current study, a positive correlation was observed between ERK1/2–ELK1 activation and a negative correlation between JNK1/2–ELK1 activation. This is further supported by a report that ERK1/2 activation by mitogenic stimulation phosphorylates ELK1, thus increasing its affinity for the SRF and enhancing transcription of growth-related proteins (Aplin et al. 2001). Also, the induction of c-fos expression through JNK-mediated ELK1 phosphorylation, which is demonstrated only in the presence of an insignificant increase in the levels of ERK1/2 (Cavigelli et al. 1995), corroborates the current study results.
Bcl-2 upregulation is required for the inhibition of hepatocyte apoptosis
Bcl-2, an apoptosis suppressing protein, can inhibit apoptosis induced by a variety of stimuli including oxidative stress (Kaufmann et al. 2003). This protein, under non-stressful conditions, forms complexes with a pro-apoptotic protein, BAD. Upon growth or survival stimuli, the interaction between these proteins is regulated primarily by Akt (Datta et al. 1997). Akt phosphorylates BAD on a serine residue, which causes its release from the complex with Bcl-2, allowing the latter to perform its anti-apoptotic function (Franke and Cantley 1997). Recent studies have provided evidence suggesting that Bcl-2 exerts its anti-apoptotic function through the suppression of the JNK signaling pathway (Park et al. 1997). Park et al. (1996) have also demonstrated that Bcl-2 overexpression promotes survival by blocking JNK activation caused by withdrawal of nerve growth factor in PC12 cells. Hence, the current findings were in agreement with those mentioned above supporting that Bcl-2 upregulation was observed during increased Akt activation in pollutant-stressed fish hepatocytes demonstrating a significant and positive correlation between them. Also, the current study revealed marginal induction of JNK1/2 during Bcl-2 upregulation that supported the inverse relationship of Bcl-2 and JNK1/2 expression. This has been reinforced by other reports which show that the stimulation of cardiac myocytes with 0.2 mM H2O2 induces apoptosis with a marked downregulation of Bcl-2 protein (Markou et al. 2009). Dunschede et al. (2008) have reported that Bcl-2 upregulation may protect against the postischemic burst of ROS and therefore suppresses apoptotic-related cell death. Oxidative stress-dependent upregulation of Bcl-2 expression has also been demonstrated in the central nervous system of aged rats (Kaufmann et al. 2003). A cooperative control between Akt activation and Bcl-2 expression has been reported in capillary endothelial cells (Flusberg et al. 2001). Pugazhenthi et al. (2000) have also defined upregulation of Bcl-2 expression as a novel anti-apoptotic function of Akt signaling. Several studies have also demonstrated the protective actions of Bcl-2 through inhibition of the JNK pathway, further suggesting that Bcl-2 acts downstream of JNK to regulate apoptosis (Goillot et al. 1997; Park et al. 1997; Wang et al. 1998).
NF-κB activation influences survival of stressed fish hepatocytes
NF-κB is a MAPK-regulated transcription factor known to be activated by oxidative stress. Under normal growth conditions, NF-κB is present in the cytoplasm in an inactive state and translocates from the cytosol to the nucleus upon activation by a wide variety of stimuli including the proinflammatory cytokine TNFα, oxidative stress, and bacterial and viral proteins (Ahn and Aggarwal 2005). Bellas et al. (1997) have suggested that active NF-κB has an important role in the control of cell proliferation and survival. Inhibition of this protein activation results in increased susceptibility to apoptosis leading to cell death. During environmental contaminant-mediated oxidative stress, enhanced activation of NF-κB characterized by its translocation from the cytosol to the nucleus was observed suggesting the prevalence of prosurvival mechanism mediated by NF-κB in pollutant-stressed fish hepatocytes. The latter finding was similar to those reported in the hyperoxia model of oxidative stress, where a significant increase in NF-κB activation has been demonstrated (Li et al. 1997).
NF-κB in the cytosol is kept non-active through its interaction with one or more members of the IκB family of inhibitory proteins. Conversion of NF-κB to an active form requires the release of IκB. This is accomplished through the phosphorylation of IκB leading to its ubiquitination and degradation. The ERK signaling pathway has been associated with NF-κB activation through the finding that the ERK-regulated kinase p90-RSK can phosphorylate IκB, leading to its inactivation in response to mitogenic stimulation (Schouten et al. 1997). The same pathway also downregulates the expression of PAR-4, an inhibitor of NF-κB activation (Barradas et al. 1999). Akt activates NF-κB via regulating IKK, thus resulting in NF-κB-mediated transcription of prosurvival genes (Faissner et al. 2006). In this regard, the current study is in agreement with the reported observations by others and demonstrated a significant and positive correlation between ERK1/2 or Akt and NF-κB activation, further suggesting the role of ERK1/2 and Akt activation signals in cell survival through NF-κB activation (Table 1). However, on the other hand, with regard to JNK1/2, a negative correlation was observed between the activation of JNK1/2 and NF-κB (Table 1) in pollutant-stressed fish hepatocytes. This might be because of the marginal induction of JNK1/2 that was observed during the NF-κB activation. But the latter finding also suggested the preponderance of survival mechanism as it is in accordance with a recent finding which has reported that suppressed NF-κB and sustained JNK activation favor cellular events that lead to apoptosis and cell death (Zhang et al. 2004). Also, a negative correlation between ERK1/2 and JNK1/2 as observed in the current study was demonstrated to be critical for hepatocyte resistance to cell death, suggesting the possibility that the protective action of ERK1/2 was mediated by downregulation of JNK signaling (Singh and Czaja 2007). The findings of the latter authors were further explained by the ability of ERK1/2 to induce expression of specific phosphatases that inactivate JNK with low levels of oxidative stress. Junttila et al. (2008) have also depicted the function of the ERK pathway as survival promoting, in essence by opposing the pro-apoptotic activity of the stress-activated JNK pathways. During apoptosis, the ERK-mediated survival signaling is inhibited through JNK-induced PP2A-mediated inhibition of MEK1, 2 (the upstream activator of ERK1/2). Similarly, active Akt in association with HSP90 is reported to inhibit pro-apoptotic kinase ASK1 activity. HSP90–Akt binds to and phosphorylates ASK1 at serine 83 to maintain ASK1 in an inactive state. Inhibition of HSP90 or PI3K–Akt signaling disrupts the HSP90–Akt–ASK1 complex leading to activation of ASK1 signaling (Zhang et al. 2005). Consistent with these reports, the current study had shown a marginal induction of ASK1 during HSP90α induction, and Akt activation confirmed the inhibition of ASK1-mediated JNK activation. Hence, increased ERK1/2 phosphorylation and Akt-mediated NF-κB activation, together with slight induction of JNK1/2, influenced the survival of stressed fish hepatocytes.
Conclusion
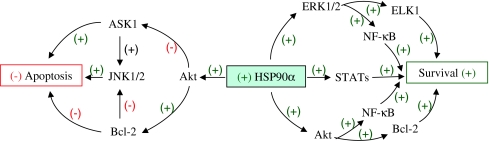
In conclusion, under normal physiological conditions, MAPK cascades and other signaling pathways would function independently with no crosstalk between them, whereas an interplay between those pathways would be induced during situation of stress when signal strength exceeds the capacity of the pathway. The crosstalk observed between cellular mechanisms and signaling pathways in the current study in stressed fish hepatocytes might be due to an increase in the specific cellular responses against pollutant stress. Induction of HSP90α and activation of cell signaling pathways (STAT, ERK1/2, Akt, and NF-κB) regulated by HSP90α mediated the hepatocellular resistance to oxidative stress induced by environmental pollutants, facilitating their survival. At the same time, the HSP90α–Akt pathway apparently acted to downregulate ASK1, thus allowing the inhibition of ASK1-mediated JNK1/2 apoptotic signaling (Fig. 5). Thus, a crosstalk between anti- and pro-apoptotic signaling pathways regulated by HSP90α modulate the balance between survival and death, ultimately favoring the shift towards oxidative stress tolerance and cellular survival. Hence, as a further advancement in signal transduction toxicology, it is conceivable from the current study that HSP90α induction in fish hepatocytes in response to pollutant stress might have provided an adaptative strategy by favoring the signal transduction mechanisms that worked towards survival (STAT, ERK1/2-ELK1, Akt-Bcl-2, and NF-κB signaling) and against apoptosis (ASK1-JNK1/2 signaling).
Fig. 5.
Schema flowchart illustrating the role of HSP90α in the mechanisms of cellular survival. HSP90α induction in response to environmental pollutant-mediated oxidative stress promotes the activation of its client proteins such as STATs of the JAK/STAT pathway, ERK1/2 of the Ras/Raf/MAPK pathway, and Akt of the PI3K pathway. STATs upon activation during HSP90α induction favor survival after translocation to the nucleus. ERK1/2 and Akt activations under similar conditions promote cellular survival via their downstream effectors like Elk-1 activations and Bcl-2 upregulation, respectively. Activation of NF-κB by both the survival kinases also provides anti-apoptotic effect facilitating cellular survival. HSP90α-mediated Akt activation at the same time also acts to downregulate ASK1 allowing inhibition of ASK1-mediated JNK1/2 apoptotic signaling. Thus, HSP90α by modulating a series of signaling cascades protects against apoptosis and promotes hepatocyte survival
Acknowledgments
The Department of Science and Technology (DST) project (SP/SO/AS-10/2003) funded by the Ministry of Science and Technology, Government of India is acknowledged. The Council of Scientific and Industrial Research (CSIR) is also acknowledged for providing the Senior Research Fellowship (SRF) to M. Usha Rani.
Abbreviations
- Akt
Protein kinase B
- ASK1
Apoptosis signal-regulating kinase 1
- BAD
Bcl-2-associated death promoter
- Bcl-2
B cell lymphoma-2
- ELK1
Ets-like protein 1
- ERK1/2
Extracellular signal-regulated kinase 1/2
- ETS
E twenty-six (ETS)-domain transcription factor
- H2O2
Hydrogen peroxide
- HSP90α
Heat-shock protein 90 alpha
- IκB
Inhibitor of NF-κB
- JAK
Janus kinase
- JNK1/2
c-Jun NH2-terminal protein kinase 1/2
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor-kappa binding
- ROS
Reactive oxygen species
- SAPK
Stress-activated protein kinase
- SRE
Serum response element
- SRF
Serum response factor
- STATs
Signal transducers and activators of transcription
- TCF
Ternary complex factor
- TNF
Tumor necrosis factor
Contributor Information
Ekambaram Padmini, Phone: +91-44-26213748, FAX: +91-44-25280473, Email: dstpadmini@rediffmail.com.
Munuswamy Usha Rani, Phone: +91-44-26213748, FAX: +91-44-25280473, Email: musharani.2007@rediffmail.com.
References
- Abe MK, Kartha S, Karpova AY, Li J, Liu PT, Kuo WL, Hershenson MB. Hydrogen peroxide activates extracellular signal-regulated kinase via protein kinase C, Raf-1, and MEK1. Am J Respir Cell Mol Biol. 1998;18:562–569. doi: 10.1165/ajrcmb.18.4.2958. [DOI] [PubMed] [Google Scholar]
- Ahn KS, Aggarwal BB. Transcription factor NF-κB: a sensor for smoke and stress signals. Ann NY Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273–281. doi: 10.1083/jcb.153.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. HSP90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplastin. Int J Cancer. 2005;113:179–188. doi: 10.1002/ijc.20611. [DOI] [PubMed] [Google Scholar]
- Baichwal VR, Baeuerle PA. Apoptosis: activate NF-κB or die? Curr Biol. 1997;7:R94–R96. doi: 10.1016/S0960-9822(06)00046-7. [DOI] [PubMed] [Google Scholar]
- Barradas M, Monjas A, Diaz-Meco MT, Serrano M, Moscat J. The downregulation of the pro-apoptotic protein Par-4 is critical for Ras-induced survival and tumor progression. EMBO J. 1999;18:6362–6369. doi: 10.1093/emboj/18.22.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LE, Bockstaele EJ, Sul JY, Takano H, Haydon PG, Eberwine JH. Elk-1 associates with the mitochondrial permeability transition pore complex in neurons. Proc Natl Acad Sci USA. 2006;103:5155–5160. doi: 10.1073/pnas.0510477103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (HSP90) and Cdc37 and is destabilized by inhibitors of HSP90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, Feron O, Calderon PB. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem Pharmacol. 2009;77:375–383. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Bellas RE, FitzGerald MJ, Fausto N, Sonenshein GE. Inhibition of NF-κB activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;151:891–896. [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckley BA, Place SP, Hofmann GE. Regulation of heat shock genes in isolated hepatocytes from an Antarctic fish, Trematomus bernacchii. J Exp Biol. 2004;207:3649–3656. doi: 10.1242/jeb.01219. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Cavigelli M, Dolfi F, Claret FX, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206:1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Currie S, Tufts BL. Synthesis of stress protein 70 (HSP70) in rainbow trout (Oncorhynchus mykiss) red blood cells. J Exp Biol. 1997;200:607–614. doi: 10.1242/jeb.200.3.607. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Demir O, Kurnaz IA. Wildtype Elk-1, but not a SUMOylation mutant, represses egr-1 expression in SH-SY5Y neuroblastomas. Neurosci Lett. 2008;437:20–24. doi: 10.1016/j.neulet.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/S0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gallego JG. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J Nutr. 2005;135:2299–2304. doi: 10.1093/jn/135.10.2299. [DOI] [PubMed] [Google Scholar]
- Doong H, Rizzo K, Fang S, Kulpa V, Weissman AM, Kohn EC. CAIR-1/BAG-3 abrogates heat shock protein-70 chaperone complex-mediated protein degradation: accumulation of polyubiquitinated Hsp90 client proteins. J Biol Chem. 2003;278:28490–28500. doi: 10.1074/jbc.M209682200. [DOI] [PubMed] [Google Scholar]
- Dou F, Yuan LD, Zhu JJ. Heat shock protein 90 indirectly regulates ERK activity by affecting Raf protein metabolism. Chin J Biochem Biophys. 2005;37:501–505. doi: 10.1111/j.1745-7270.2005.00069.x. [DOI] [PubMed] [Google Scholar]
- Dunschede F, Tybl E, Kiemer AK, Dutkowski P, Erbes K, Kircher A, Gockel I, Zechner U, Schad A, Lang H, Junginger T, Kempski O. Bcl-2 upregulation after 3-nitropropionic acid preconditioning in warm rat liver ischemia. Shock. 2008;30:699–704. doi: 10.1097/SHK.0b013e31816f6562. [DOI] [PubMed] [Google Scholar]
- Faissner A, Heck N, Dobbertin A, Garwood J. DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol. 2006;557:25–53. doi: 10.1007/0-387-30128-3_3. [DOI] [PubMed] [Google Scholar]
- Fischer W, Bianchi G. FAO species identification sheets for fishery purposes. Western Indian Ocean (Fishing area 51) Rome: FAO; 1984. [Google Scholar]
- Flandrin P, Guyotat D, Duval A, Cornillon J, Tavernier E, Nadal N, Campos L. Significance of heat-shock protein (HSP) 90 expression in acute myeloid leukemia cells. Cell Stress Chaperones. 2008;13:357–364. doi: 10.1007/s12192-008-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flusberg DA, Numaguchi Y, Ingber DE. Cooperative control of Akt phosphorylation, Bcl-2 expression, and apoptosis by cytoskeletal microfilaments and microtubules in capillary endothelial cells. Mol Biol Cell. 2001;12:3087–3094. doi: 10.1091/mbc.12.10.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, Sessa WC. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–873. doi: 10.1161/01.RES.0000016837.26733.BE. [DOI] [PubMed] [Google Scholar]
- Franke TF, Cantley LC. A Bad kinase makes good. Nature. 1997;390:116–117. doi: 10.1038/36442. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKT ion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/S0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Friedman A, Perrimon N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signaling. Nature. 2006;444:230–234. doi: 10.1038/nature05280. [DOI] [PubMed] [Google Scholar]
- Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of HSP90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277:10346–10353. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- Gabai VL, Sherman MY. Interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol. 2002;92:1743–1748. doi: 10.1152/japplphysiol.01101.2001. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Goillot E, Raingeaud J, Ranger A, Tepper RI, Davis RJ, Harlow E, Sanchez I. Mitogen-activated protein kinase-mediated Fas apoptotic signaling pathway. Proc Natl Acad Sci USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.7.3604. [DOI] [PubMed] [Google Scholar]
- Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. J Biol Chem. 2000;275:26576–26581. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Helmreich EJM (2001) Components of signaling networks: linkers and regulators. In: The biochemistry of cell signaling. Oxford University Press, New York
- Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S, Dorken B, Zenke M, Stein H, Scheidereit C. Nuclear factor kappaB-dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5a activity. J Exp Med. 2002;196:605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. FASEB J. 2002;16:114–116. doi: 10.1096/fj.01-0409fje. [DOI] [PubMed] [Google Scholar]
- Irving EA, Bamford M. Role of mitogen- and stress-activated kinases in ischemic injury. J Cereb Blood Flow Metab. 2002;22:631–647. doi: 10.1097/00004647-200206000-00001. [DOI] [PubMed] [Google Scholar]
- Iwama GK, Thomas PT, Forsyth RB, Vijayan MM. Heat shock protein expression in fish. Rev Fish Biol Fisher. 1998;8:35–56. doi: 10.1023/A:1008812500650. [DOI] [Google Scholar]
- Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C. Characterization of the signal that directs Bcl-XL, but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Kaneyama J, Nose K, Shibanuma M. Significance of nuclear relocalization of ERK1/2 in reactivation of c-fos transcription and DNA synthesis in senescent fibroblasts. J Biol Chem. 2000;275:20685–20692. doi: 10.1074/jbc.M908723199. [DOI] [PubMed] [Google Scholar]
- Krumschnabel G, Schwarzbaum PJ, Wieser W. Coupling of energy supply and energy demand in isolated goldfish hepatocytes. Physiol Zool. 1994;67:438–448. [Google Scholar]
- Kultz D, Avila K. Mitogen-activated protein kinases are in vivo transducers of osmosensory signals in fish gill cells. Comp Biochem Physiol B. 2001;129:821–829. doi: 10.1016/S1096-4959(01)00395-5. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Mantell LL, Kazzaz JA, Fein AM, Horowitz S. Nuclear factor-κB is activated by hyperoxia but does not protect from cell death. J Biol Chem. 1997;272:20646–20649. doi: 10.1074/jbc.272.33.20646. [DOI] [PubMed] [Google Scholar]
- Liu Dh, Hy Y, Cy C, Zp G, By Z, Hl H, Df L. Heat shock protein 90 acts as a molecular chaperone in late-phase activation of extracellular signal-regulated kinase 1/2 stimulated by oxidative stress in vascular smooth muscle cells. Acta Pharmacol Sin. 2007;28:1907–1913. doi: 10.1111/j.1745-7254.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- Markou T, Dowling AA, Kelly T, Lazou A. Regulation of Bcl-2 phosphorylation in response to oxidative stress in cardiac myocytes. Free Radic Res. 2009;43:809–816. doi: 10.1080/10715760903071649. [DOI] [PubMed] [Google Scholar]
- Meares GP, Zmijewska AA, Jope RS. Heat shock protein-90 dampens and directs signaling stimulated by insulin-like growth factor-1 and insulin. FEBS Lett. 2004;574:181–186. doi: 10.1016/j.febslet.2004.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Saitoh M, Tobiume K, Matsuura H, Enomoto S, Nishitoh H, Ichijo H. Negative feedback regulation of ASK1 by protein phosphatase 5 (PP5) in response to oxidative stress. EMBO J. 2001;20:6028–6036. doi: 10.1093/emboj/20.21.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer therapeutic agents. Trends Mol Med. 2002;8:555–561. doi: 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- Padmini E, Usha Rani M. Impact of seasonal variation on HSP70 expression quantitated in stressed fish hepatocytes. Comp Biochem Physiol B. 2008;151:278–285. doi: 10.1016/j.cbpb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Padmini E, Usha Rani M. Evaluation of oxidative stress biomarkers in hepatocytes of grey mullet inhabiting natural and polluted estuaries. Sci Total Environ. 2009;407:4533–4541. doi: 10.1016/j.scitotenv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Padmini E, Usha Rani M. Thioredoxin and HSP90α modulate ASK1-JNK1/2 signaling in stressed hepatocytes of Mugil cephalus. Comp Biochem Physiol C. 2010;151:187–193. doi: 10.1016/j.cbpc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Padmini E, Vijaya Geetha B. A comparative seasonal pollution assessment study on Ennore estuary with respect to metal accumulation in the grey mullet, Mugil cephalus. Oceanol Hydrobiol Stud. 2007;35:1–13. [Google Scholar]
- Park DS, Stefanis L, Yan CYI, Farinelli SE, Greene LA. Ordering the cell death pathway: differential effects of Bcl-2, an interleukin-1-converting enzyme family protease inhibitor, and other survival agents on JNK activation in serum/nerve growth factor-deprived PC12 cells. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- Park J, Kim I, Oh YJ, Kw L, Han PL, Choi EJ. Activation of c-Jun N-terminal kinase antagonizes an anti-apoptotic action of Bcl-2. J Biol Chem. 1997;272:16725–16728. doi: 10.1074/jbc.272.27.16725. [DOI] [PubMed] [Google Scholar]
- Piatelli MJ, Doughty C, Chiles TC. Requirement for a hsp90 chaperone-dependent MEK1/2-ERK pathway for B cell antigen receptor-induced cyclin D2 expression in mature B lymphocytes. J Biol Chem. 2002;277:12144–12150. doi: 10.1074/jbc.M200102200. [DOI] [PubMed] [Google Scholar]
- Pratt WB. The HSP90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–431. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Raghunathan MB, Srinivasan M (1983) Zooplankton dynamics and hydrographic features of Ennore estuary, Madras. Records of the Zoological survey of India, Calcutta, India
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK)1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to HSP90. Proc Natl Acad Sci USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof N, Fv B, Trumper L, Kube D. HSP90 is essential for Jak–STAT signaling in classical Hodgkin lymphoma cells. Cell Commun Signal. 2009;7:17–21. doi: 10.1186/1478-811X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten GJ, Vertegaal ACO, Whiteside ST, Israel A, Toebes M, Dorsman JC, Eb AJ, Zantema A. IκBα is a target for the mitogen-activated 90 kDa ribosomal S6 kinase. EMBO J. 1997;16:3133–3144. doi: 10.1093/emboj/16.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1–Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1–Ras association. J Biol Chem. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Shang L, Tomasi TB. The heat shock protein 90-CDC37 chaperone complex is required for signaling by types I and II interferons. J Biol Chem. 2006;281:1876–1884. doi: 10.1074/jbc.M509901200. [DOI] [PubMed] [Google Scholar]
- Sharrocks AD. Complexities in ETS-domain transcription factor function and regulation: lessons from the TCF (ternary complex factor) subfamily. The Colworth Medal Lecture. Biochem Soc Trans. 2002;30:1–9. doi: 10.1042/BST0300001. [DOI] [PubMed] [Google Scholar]
- Shaw PE, Saxton J. Ternary complex factors: prime nuclear targets for mitogen-activated protein kinases. Int J Biochem Cell Biol. 2003;35:1210–1226. doi: 10.1016/S1357-2725(03)00031-1. [DOI] [PubMed] [Google Scholar]
- Shen HM, Liu Z-g. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Shen S, Zhang P, Lovchik MA, Li Y, Tang L, Chen Z, Zeng R, Ma D, Yuan J, Yu Q. Cyclodepsipeptide toxin promotes the degradation of Hsp90 client proteins through chaperone-mediated autophagy. J Cell Biol. 2009;185:629–639. doi: 10.1083/jcb.200810183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry JP. The role of biomarkers in the health assessment of aquatic ecosystems. Aquat Ecosys Health Manag. 2003;6:423–440. doi: 10.1080/714044172. [DOI] [Google Scholar]
- Singh R, Czaja MJ. Regulation of hepatocyte apoptosis by oxidative stress. J Gastroenterol Hepatol. 2007;1:S45–S48. doi: 10.1111/j.1440-1746.2006.04646.x. [DOI] [PubMed] [Google Scholar]
- Sreedhar AS, Csermely P. Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy. A comprehensive review. Pharmacol Ther. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Sreedhar AS, Kalmar E, Csermely P, Shen YF. HSP90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–15. doi: 10.1016/S0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan MM, Pereira C, Kruzynski G, Iwama GK. Sublethal concentrations of contaminant induce the expression of hepatic heat shock protein 70 in two salmonids. Aquat Toxicol. 1998;40:101–108. doi: 10.1016/S0166-445X(97)00057-X. [DOI] [Google Scholar]
- Wang X, Martindale JL, Liu Y, Holbrook NJ. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signaling pathways on cell survival. Biochem J. 1998;333:291–300. doi: 10.1042/bj3330291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McCullough KD, Franke TF, Holbrook NJ. Epidermal growth factor receptor-dependent Akt activation by oxidative stress enhances cell survival. J Biol Chem. 2000;275:14624–14631. doi: 10.1074/jbc.275.19.14624. [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras–MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/S0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- Wu M, Bian Q, Liu Y, Fernandes AF, Taylor A, Pereira P, Shang F. Sustained oxidative stress inhibits NF-kappaB activation partially via inactivating the proteasome. Free Radic Biol Med. 2009;46:62–69. doi: 10.1016/j.freeradbiomed.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Bao E, Yan J, Lei L. Expression and localization of HSPs in the heart and blood vessel of heat-stressed broilers. Cell Stress chaperones. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-κB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-α-induced apoptosis in human cancer cells. Carcinogenesis. 2004;25:2191–2199. doi: 10.1093/carcin/bgh234. [DOI] [PubMed] [Google Scholar]
- Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. HSP90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]