Abstract
The present studies aimed to understand the interrelationships between stress, hormones and heat shock proteins (HSPs) in the ovary. We examined (1) whether HSP70.2, HSP72 and HSP105/110 can be produced and accumulated in porcine ovarian tissue, (2) whether these HSPs could be indicators of stress, i.e. whether two kinds of stress (high temperatures and malnutrition/serum deprivation) can affect them, and (3) whether some hormonal regulators of ovarian functions (insulin-like growth factor (IGF)-I, leptin and follicle-stimulating hormone (FSH)) can affect these HSPs and response of ovaries to HSP-related stress. We analysed the expression of HSP70.2, HSP72 and HSP105/110 mRNA (by using real-time reverse transcriptase polymerase chain reaction) in porcine ovarian granulosa cells, as well as the accumulation of HSP70 protein (by using sodium dodecyl sulphate polyacrylamide gel electrophoresis–Western) in either whole ovarian follicles and granulose cells cultured at normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without IGF-I, leptin and FSH. Expression of mRNA for HSP70.2, HSP72 and HSP105/110 in ovarian granulosa cells and accumulation of HSP70 protein in whole ovarian follicles and granulosa cells were demonstrated. In all the groups, addition of either IGF-I, leptin and FSH reduced the expression of HSP70.2, HSP72 and HSP105/110 mRNA. Both high temperature, serum deprivation and their combination resulted in increase in mRNAs for all three analysed HSPs. Additions of either IGF-I, leptin and FSH prevented the stimulatory effect of both high temperature and serum deprivation on the transcription of HSP70.2, HSP72 and HSP105/110. In contrast, high temperature reduced accumulation of peptide HSP70 in both ovarian follicles and granulosa cell. Serum deprivation promoted accumulation of HSP70 in granulosa cells, but not in ovarian follicles. Addition of IGF-I, leptin and FSH was able to alter accumulation of HSP70 in both follicles and granulosa cells. The present observations suggest (1) that HSPs can be synthesised in ovarian follicular granulosa cells; (2) that hormones (IGF-I, leptin and FSH) can inhibit, whilst stressors (both high temperature and malnutrition/serum deprivation) can stimulate transcription of HSP70.2, HSP72 and HSP105/110 genes, whilst heat stress, but not malnutrition, can promote depletion of HSP70 in ovarian cells, and (3) that hormones (IGF-I, leptin and FSH) can prevent stress-related changes in HSPs. The application of HSPs as indicators and mediators of stress and hormones on ovarian functions, as well as use of hormones and HSPs as anti-stressor molecules, are discussed.
Keywords: Heat shock proteins (HSP70, HSP72, HSP105/110); IGF-I; Leptin; FSH; Ovarian follicles; Granulosa cells
Introduction
Reproductive processes, to be successful, should be synchronized with environment. Unfavourable conditions (for example, inadequate temperature, reduced delivery of nutrients) induce stress and suppress reproduction via stress-related substances, whilst conditions adequate for reproduction promote it via hormonal stimulators. Therefore, it is not to be excluded that hormones could potentially oppose and even prevent effects of stress on stress-related substances and reproductive processes.
As concerns the stress-related substances, in non-reproductive cells, elevated temperatures induce a number of anomalies in cellular function, including defects in protein synthesis, structure, cytoskeleton rearrangements, metabolism, cell membrane fluidity and cell proliferation (Sonna et al. 2002). These anomalies invoke changes in expression of numerous genes (Lanks 1986), including genes encoding heat shock proteins (HSPs; Trinklein et al. 2004; Akerfelt et al. 2007). HSPs belong to a large and diverse group of unrelated proteins known as chaperones that assist in correct non-covalent assembly and/or disassembly of other polypeptide-containing structures (Ellis 1997). HSPs can either prevent denaturation and incorrect assembly or folding of heat-denatured proteins, stabilise genetic variation in response to stress, increase cell proliferation and survival, block apoptosis, permit repair and thwart cell death. The most pronounced stress-related changes and involvement in different cell functions have been demonstrated for HSP70 (Queitsch et al. 2002; Beere 2004; Calderwood and Ciocca 2008; Jego et al. 2010).
Negative effects of suboptimal temperatures on reproductive processes are well documented (Putney et al. 1989; Edwards and Hansen 1997; Guzeloglu et al. 2001; Lawrence et al. 2004), but the mechanisms of such effect remain not fully known. It is demonstrated that high temperatures can affect reproductive processes via inhibition of both ovarian cell proliferation, apoptosis, induction of oversecretion of ovarian steroid hormones and alteration in response of cultured ovarian cells to hormones. Moreover, additions of hormones follicle-stimulating hormone (FSH), leptin and insulin-like growth factor (IGF)-I were able to prevent effect of heat stress on ovarian cell apoptosis, proliferation and secretory activity (Sirotkin 2010). Since HSP70 is present in the ovary (Driancourt et al. 1999; Guzeloglu et al. 2001; Narayansingh et al. 2004; Maniwa et al. 2005; Salvetti et al. 2008), it is not to be excluded that heat shock can affect reproduction, like other processes, via HSPs. Nevertheless, acute heat stress did not alter HSP90 level in bovine ovarian follicular fluid (Guzeloglu et al. 2001) and effect of high temperatures on ovarian HSPs, and involvement of HSPs in ovarian response to heat stress has not been demonstrated.
Ovarian cycle is associated with changes in both HSP-70, prostaglandins (Narayansingh et al. 2004) and steroid hormone receptors (Salvetti et al. 2008). Furthermore, in non-ovarian cells, HSP70 and HSP90 could be promoted by ovariectomy (Jayachandran and Miller 2002) or oestrogen administration (Bombardier et al. 2009), whilst HSP90 can be inhibitor of ovarian aromatase and oestrogen production (Driancourt et al. 1999). This demonstrates that HSPs can be controlled by hormones and vice versa. Nevertheless, effect of reproductive hormones on ovarian HSPs and their response to heat stress has not been previously examined.
Another stressor, malnutrition, can affect reproductive processes via changes in secretion of metabolic hormones. Food restriction reduces release of metabolic hormone leptin, a product of adipose and some other tissues, which can affect reproduction through the hypothalamohypophysial system and by direct action on gonads. Leptin is able to regulate growth of ovarian follicles, corpus luteum development, suppress ovarian cell apoptosis, activate ovarian cell proliferation and affect release of steroid hormones, oxytocin, prostaglandin and IGF-I and IGFBP-3 by ovarian cells (Spicer 2001; Smith et al. 2001; Barb et al. 2005; Sirotkin et al. 2005; Zieba et al. 2005). Furthermore, external factors, including malnutrition, can control reproductive processes affecting release of gonadotropins (FSH, luteinizing hormone), the most well-known promoters of ovarian cell proliferation and follicular growth, regulators of apoptosis and stimulators of release of ovarian steroid and peptide hormones (Hillier 1991; Erickson and Danforth 1995; Berisha and Schams 2005; Sirotkin et al. 2005). Both leptin (Spicer 2001; Sirotkin et al. 2005; Zieba et al. 2005) and gonadotropins (Erickson and Danforth 1995; Berisha and Schams 2005) can control ovarian functions through stimulation of local production of IGF-I, in which anti-apoptotic effect and stimulatory action on ovarian cell proliferation, follicullogenesis and hormone release are similar to action of leptin and gonadotropins (Sirotkin et al. 1998, 2005; Makarevich et al. 2000; Berisha and Schams 2005). Recently, it was shown that hormones FSH, leptin and IGF-I can prevent effect of malnutrition/serum deprivation on some porcine ovarian granulose cell functions (Sirotkin 2010). Malnutrition is an inhibitor of protein synthesis, which in turn can stabilise HSP70 (Theodorakis and Morimoto 1987). Therefore, it is not to be excluded that HSPs can be effectors and mediators of effect of metabolism and/or metabolism-dependent hormones on reproductive processes. Despite available evidence of importance of hormones in control of ovarian functions and in preventing effect of malnutrition on the ovary, it remains unknown whether this action can be mediated by HSPs.
Taken together, although presence of HSP70 or HSP70 mRNA in healthy rat (Narayansingh et al. 2004; Salvetti et al. 2008) and bovine (Maniwa et al. 2005) ovary has been reported, regulators and role of HSPs in reproductive system have not been studied. It remains unknown whether HSP70 is produced in ovaries in other species (for example, pigs); whether HSP72 and HSP105/110 genes are expressed in reproductive system at all; whether HSPs can be involved in response of reproductive system to heat stress, malnutrition, and hormonal regulators of reproductive processes and what could be interrelationships between stress, hormones and HSPs in reproductive system.
The general aim of the present studies is to understand the interrelationships between stress, hormones and HSPs in the ovary. In particular, we have tried to examine (1) whether HSP70.2, HSP72 and HSP105/110 can be produced and accumulated in porcine ovarian tissue; (2) whether these HSPs could be indicators of stress, i.e. whether two kinds of stress (high temperatures and malnutrition/serum deprivation) can affect them, and (3) whether some hormonal regulators of ovarian functions (IGF-I, leptin and FSH) can affect these HSPs and response of ovaries to HSP-related stress. For such studies, we have used an in vitro model—ovarian cells cultured in conditions of normal and increased temperature, in serum-supplemented medium (containing sufficient of nutrients) and in serum-free medium, with and without exogenous hormones.
Materials and methods
Experimental design
Two series of experiments were performed. In the 1st series of experiments, we examined the transcription of HSP70.2, HSP72 and HSP105/110 mRNAs in porcine ovarian granulosa cells cultured at normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without hormones IGF-I, leptin and FSH using real-time reverse transcriptase polymerase chain reaction (RT-PCR). The 2nd series of experiments was set up to verify and to update some results obtained during the 1st series of experiments concerning HSP70, by analysis of peptide HSP70 by using sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)–Western immunoblotting. In the 2nd series of experiments, we have analysed the accumulation of HSP70 in either whole porcine ovarian follicles or ovarian granulosa cells cultured at normal (37.5°C) and high (41.5°C) temperature. To examine the effect of malnutrition/serum deprivation, cells cultured at 37.5°C with and without serum were compared. To evaluate the effect of hormones, all the cells were cultured with and without hormones IGF-I, leptin and FSH. To examine the possible differences in response of different compartments of ovarian follicle, both cultured granulosa cells and whole follicles were analysed. Treatments performed in 1st and 2nd series of experiments are described below and summarized in Table 1.
Table 1.
Experimental design
| Series no. | Parameters of experiments | |||
|---|---|---|---|---|
| Object and method of analysis | Temperature (°C) | Presence of serum | Addition of hormones | |
| 1 | HSP70, HSP72 and HSP105 mRNA in ovarian granulosa cells, RT-PCR | 37.5 | Serum | None |
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 41.5 | Serum | None | ||
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 37.5 | No serum | None | ||
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 41.5 | No serum | None | ||
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 2 | HSP70 in ovarian granulosa cells and ovarian follicles, SDS-PAGE–Western blotting | 37.5 | Serum | None |
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 41.5 | Serum | None | ||
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
| 37.5 | No serum | None | ||
| IGF-I | ||||
| Leptin | ||||
| FSH | ||||
Preparation, culture and processing of granulosa cells
Granulosa cells were collected from the ovaries of sexually immature non-cycling Slovakian white gilts, 200 days of age (to avoid effect of ovarian cycle-related changes), after slaughter at a local abattoir. They were processed and precultured as described previously (Sirotkin 1996; Sirotkin and Makarevich 1999) in DMEM/F-12 1:1 mixture supplemented with 10% bovine foetal serum and 1% antibiotic–antimycotic solution (all from Sigma, St. Louis, MO, USA). Granulosa cells (1 × 106 cells/ml) intended for real-time RT-PCR or Western immunoblotting were cultured in 2 ml culture medium in Falcon 24 well plates (Becton Dickinson, Lincoln Park, NJ, USA). First the cells were precultured in medium at 37°C under 5% CO2 in humidified air. After 2 days of preculture, when cells attached to the bottom of the wells, the medium was replaced with medium of the same composition. Cells were cultured during 2 days in fresh medium supplemented or not supplemented with foetal calf serum containing necessary cell nutrients (10%, Sigma) at temperatures 37.5°C (optimal and physiologically relevant temperature for cultured mammalian ovarian cells) or 41.5°C (supraoptimal temperature inducing heat stress and production of HSPs, but not cell death, Lanks 1986; Trinklein et al. 2004; Akerfelt et al. 2007) with or without hormones. Culture medium for different experimental groups was supplemented with biological grade recombinant human leptin (Sigma, St. Louis, MO, USA, 100 ng/ml medium), immunological grade recombinant IGF-I (Calbiochem, Lucerne, Switzerland, 100 ng/ml) or biological grade porcine FSH (Sigma, 100 ng/ml). These doses were comparable with amounts of corresponding hormones in the blood and/or with doses used in previous in vitro experiments (Sirotkin et al. 1998, 2001; Schams et al. 1999; Berisha and Schams 2005). All hormones were dissolved in medium immediately before experiment. Control groups contained cells cultured without exogenous hormones. Cells were collected after 48 h of culture with and without treatments (the appropriate time of maximal response to hormones, as was determined in our previous experiments, Sirotkin 1996; Sirotkin et al. 1998, 2001, 2005; Sirotkin and Makarevich 1999; Makarevich et al. 2000). Immediately after culture, total RNA was isolated from cells intended for quantitative real-time RT-PCR using Trizol (Invitrogen). Cells intended for gel electrophoresis and Western immunoblotting were lyzed in ice-cold lysis buffer (1% Triton X-100, 0.5% Igepal NP-40, 5 mM EDTA, 20 μg/ml phenylmethylsulphonyl fluoride, 10 μg/ml aprotonin, 10 μg/ml leupeptin, 5 μg/ml pepstatin, 10 mM sodium orthovanadate in phosphate-buffered saline, pH 7.5, all from Sigma, 50 μg/well) and frozen at −18°C. After culture, cell number and viability were determined by Trypan blue staining and counting by haemocytometer. No statistically significant differences in these indices between the groups were observed.
Isolation, culture and processing of ovarian follicles
Non-cycling Slovakian white gilts, 180 days of age and without visible reproductive abnormalities, were killed at a local slaughterhouse. Ovarian follicles (2.5–3.5 mm diameter) were collected, processed and cultured 2 days in Falcon 24 well plates (Becton Dickinson), 1 follicle per well/2 ml culture medium DME/F-12 1:1 mixture supplemented with 1% antibiotic–antimycotic solution, with or without 10% heat-inactivated foetal calf serum (all from Sigma), by 37.5°C and 41.5°C, and with and without hormones at doses listed above as described previously (Sirotkin et al. 1998). Immediately after culture, follicles were weighed and stored at −18°C. Thereafter the frozen follicles were lysed by three repeated cycles of thawing, pipetting and freezing (5 min each) followed by lysis in lysis buffer as was described for granulosa cells and subjected to SDS-PAGE–Western blotting. The number and viability of cells within the follicles were not determined, although markers of cell proliferation, proliferating cell nuclear antigen and ERK1,2-related mitogen-activated protein kinase were detected by Western blotting of follicular lysates after culture (not shown).
Real-time RT-PCR
A total RNA from ovarial granulosa cells was isolated using Trizol reagent according manufacturer’s instructions (Invitrogen). All RNA samples were treated with the RNase-free DNase I amplification grade (Invitrogen) before reverse transcription to destroy contaminating DNA, if present. Purity of extracted total RNA was determined as the 260:280-nm ratio, and the integrity was checked by electrophoresis in 1% agarose gel. The cDNA was synthesised using oligo (dT)16 primer and 0.5 μg of total RNA from each sample in 20 μl reactions consisting of 1× PCR buffer, 5 mM MgCl2, 4 mM dNTP, 2.5 μM oligo (dT)16, 20 U RNase inhibitor (Applied Biosystems) and 50 U M-MuLV reverse transcriptase (Applied Biosystems). The reaction was performed at 25°C for 5 min, 42°C for 15 min, 99°C for 5 min and 4°C for 5 min. A qPCR was performed in 15 μl parallel reactions containing 2 μl cDNA, ABsolute qPCR SYBR Green Mix (ABgene Ltd., UK) and 5 pmol each of corresponding primers for Hsp70.2 (5′–3′, cgacctcaacaagagcatca; aagatctgcgtctgcttggt, sequence account no. NM_213766), Hsp72 (5′–3′, actccctggaggcctatgtt; tgagca ccacaactgctacc, sequence account no. M29506) and Hsp105/110 (5′–3′, tggactgtca gcatcagagg; gtcagcatctggggttttgt, sequence account no. NM_001097504) in Rotor-Gene 6000 real-time PCR system (Corbett Research). Quantification of HSP genes expression was performed using primers for the following housekeeping genes: 18S rRNA (5′–3′, Atccctgagaagttccagca; acacgttccacctcatcctc, account no. NM_213940), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Duvigneau et al. 2003) and cyclophilin (Verfaillie et al. 2001). All samples were amplified in triplicate from the same RNA preparation, and the mean value was considered. A melting curve analysis was performed within temperature range 72–95°C to check the specificity of PCR products. Standard curves were generated for all genes using a serial dilution of template cDNA from control granulosa cells. A relative quantification of HSP genes expression to housekeeping genes was calculated using the threshold (CT) values and PCR reaction efficiencies according to Pfaffl (2001).
Western blotting
The separation of HSP70 performed using SDS-PAGE and its subsequent visualization by Western immunoblotting using mouse monoclonal antibody against HSP70 and housekeeping protein GAPDH (1:250 dilution; all from Santa Cruz, Santa Cruz, CA, USA), secondary HRP-conjugated anti-mouse IG antibodies (Sevac. Prague, Czech Republic), ECL detection reagents and ECL Hyper-film (Amersham International) was performed as described previously (Sirotkin and Makarevich 1999). The primary antisera against HSP70 and GAPDH were specific for mouse, rat, human, porcine and bovine cells. Incubation medium without cells, or samples processed in the absence of primary antibody, were used as negative controls. The molecular weights of fractions were evaluated using a molecular weight calibration set (18, 24, 45 and 67 kDa; ICN Biomedicals, Inc., Irvine, CA, USA). Band intensity was evaluated by densitometry analysis (not shown here).
Statistics
Each experiment was performed three times using ovaries obtained from 15 to 20 animals per experiment. Each in vitro experimental group was represented by four culture wells with granulosa cells (2 × 106 cells/well) or six wells with ovarian follicles (one follicle per well). The data shown are means of values obtained on three separate experiments performed in different days using separate pools of ovaries. The samples intended for real-time RT-PCR were analysed separately. The corresponding samples intended for SDS-PAGE–Western blotting (total 12 samples of granulose cells or 18 samples of follicles per treatment obtained in three experiments) were pooled before processing. Significant differences between the experiments were determined using two-ways analysis of variance, followed by Student’s t test to detect significant differences between treatment and control groups by using Sigma Plot/SigmaStat 11.0 statistical software (Systat Software, GmbH, Erkrath, Germany). Differences from control at P < 0.05 were considered as significant.
Results
1st series of experiments: effect of hormones, high temperature and serum deprivation on the expression of HSP70.2, HSP72 and HSP105/110 mRNA in porcine ovarian granulosa cells (data of real-time RT-PCR)
A quantitative real-time RT-PCR demonstrated the expression of mRNA for HSP70.2, HSP72 and HSP105/110 genes in porcine ovarian granulosa cells. Significant influence of hormonal treatments, high temperature and serum deprivation on the expression of above genes was found. The relative amount of HSPs mRNA was normalized to three commonly used housekeeping genes, GAPDH, 18S rRNA and cyclophilin. In order to choose the best housekeeping gene with stable expression under our experimental conditions, mRNA transcription levels for all samples were measured (not shown). Based on the results of geNorm analysis of obtained experimental data (Vandesompele et al. 2002), we have chosen the cyclophilin as the most suitable housekeeping gene for relative quantification of HSPs mRNA expression in our experiments (data not shown).
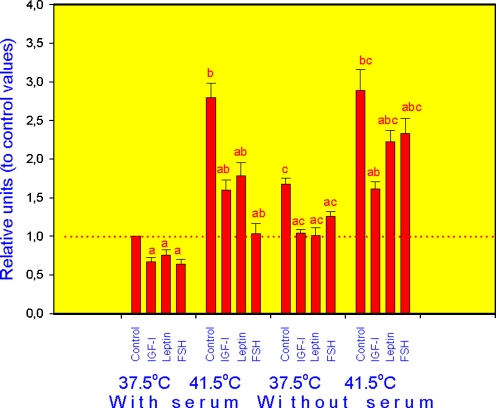
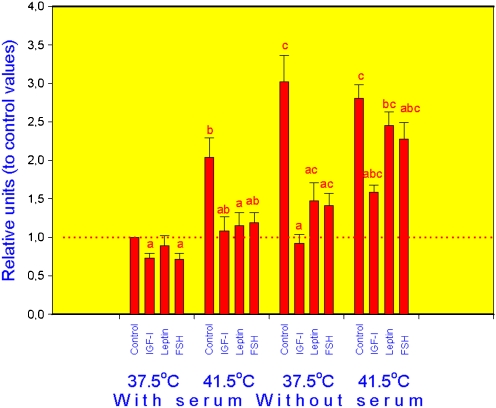
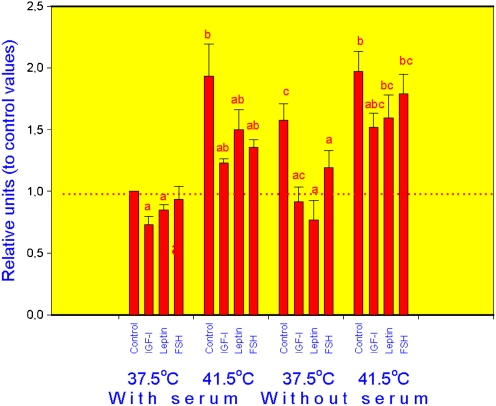
In all groups (cells incubated at normal, or high temperature, with or without serum), addition of hormones significantly reduced the expression of HSP70.2 (Fig. 1), HSP72 (Fig. 2) and HSP105/110 (Fig. 3) mRNA in comparison with cells cultured without exogenous hormones, although in cells cultured at 37.5°C with serum leptin effect on HSP72 and FSH action on HSP105 were not expressed. In control cells (cultured without hormones), both increase of temperature to 41.5°C and serum deprivation as well as their combination resulted in dramatic increase of mRNA amount for all three analysed HSPs. Addition of either IGF-I, leptin and FSH to culture medium fully or partially prevented the stimulatory effect of both high temperature and serum deprivation on the expression of HSP70.2 (Fig. 1), HSP72 (Fig. 2) and HSP105/110 (Fig. 3).
Fig. 1.
Expression of HSP70 mRNA in porcine ovarian granulosa cells cultured in conditions of normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without hormones IGF-I, leptin and FSH. Values are means ± SED. a Effect of hormone treatment: significant (p < 0.05) differences between control cells (cultured without hormones) and cells cultured with hormones in similar conditions, b effect of high temperature: significant (p < 0.05) differences between corresponding groups of cells cultured in conditions of normal and high temperature, c effect of serum deprivation: significant (p < 0.05) differences between corresponding groups of cells cultured with and without serum. Data of RT-PCR
Fig. 2.
Expression of HSP72 mRNA in porcine ovarian granulosa cells cultured in conditions of normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without hormones IGF-I, leptin and FSH. Legend as in Fig. 1
Fig. 3.
Expression of HSP105 mRNA in porcine ovarian granulosa cells cultured in conditions of normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without hormones IGF-I, leptin and FSH
2nd series of experiments: effect of hormones, high temperature and serum deprivation on the accumulation of HSP70 in porcine ovarian follicles and granulosa cells (data of SDS-PAGE–Western blotting)
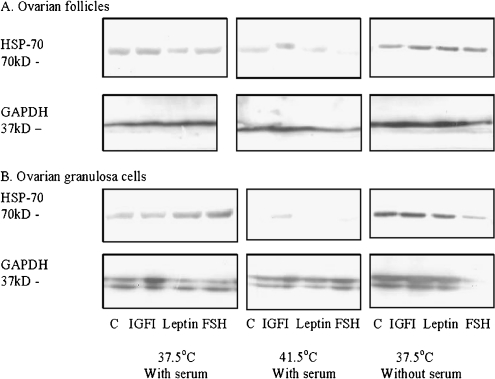
Single fraction of HSP70 with MW approximately 70 kDa was observed in lysates of both whole ovarian follicles (Fig. 4a) and ovarian granulosa cells (Fig. 4b). Housekeeping protein GAPDH was present in ovarian follicles as single fraction and in granulosa cells as double fraction with MW approximately 37 kD. Addition of hormones affected the expression of HSP70, but not GAPDH fraction, although the pattern of effect of hormonal additions on HSP70 depended on temperature and presence of serum, as well as on the kind of cultured cells.
Fig. 4.
Accumulation of HSP70 in porcine ovarian follicles (a) and ovarian granulosa cells (b) cultured in conditions of normal (37.5°C) and high (41.5°C) temperature, with and without blood serum and with and without hormones IGF-I, leptin and FSH. Data of SDS-PAGE–Western blotting. Analysed substances (HSP70 and housekeeping protein GAPDH) and their molecular weights (in kilodaltons) are indicated
In control follicles (cultured at 37.5°C in presence of serum), leptin, but not IGF-I or FSH, reduced HSP70 accumulation. By high temperature, no significant effect of hormones has been found. All three tested hormones increased HSP70 accumulation in follicles cultured in serum-free medium. In control follicles (cultured without hormones), high temperature, but not serum deprivation, reduced accumulation of HSP70 (Fig. 4a).
In cultured granulosa cells, HSP70 increased after administration of both leptin and FSH (in normal conditions), or IGF-I (culture by high temperature) or did not increase after any hormonal treatment (culture in serum-free medium). High temperature resulted reduction (up to full disappearance) of HSP70, whilst serum deprivation promoted accumulation of HSP70 in both control and hormone-treated granulosa cells (Fig. 4b).
Discussion
Presence of mRNAs for HSP70.2 (corresponding to HSPA2 gene), HSP72 (corresponding to HSPA1A gene) and HSP105/110 (corresponding to HSPH1 gene), as well as of heat shock protein with immunological properties and MW of 70 kDa, suggests that HSP70 and, maybe, HSP72 and HSP105 can be produced in porcine granulosa cells. This is the first demonstration of synthesis (mRNA) of HSPs in porcine ovarian cells, as well as the confirmation of accumulation of HSP70 in porcine ovary observed previously in other species (Driancourt et al. 1999; Guzeloglu et al. 2001; Narayansingh et al. 2004; Maniwa et al. 2005; Salvetti et al. 2008). The presence of HSP in both granulosa cells and whole follicle confirm previous indications (Driancourt et al. 1999) that HSP is produced in both granulosa and theca compartments but predominantly in granulosa cells. Nevertheless, substantial differences in response of HSP70 in whole follicles and in granulosa cells to stresses and hormonal treatments observed in the 2nd series of our experiments indicate existence of different mechanisms of regulation of this HSP in granulosa cells and other parts of ovarian follicle.
Results of the 1st series of experiments demonstrate that IGF-I, leptin and FSH can reduce transcription and/or accumulation of mRNA of all three analysed HSPs. On the other hand, in the 2nd series of experiments, these hormones did not reduce, but in some cases increased accumulation of HSP70 in both ovarian follicles and their granulosa cells. This is the first direct evidence for hormonal control of ovarian HSPs, although increase in HSP70 mRNA accumulation in rat corpus luteum regressed after prostaglandin treatment has been reported previously (Narayansingh et al. 2004). The explanation causes and physiological significance of HSPs changes observed in our experiments requires further studies, but it could be suggested that all tested hormones are able to reduce both synthesis and degradation or utilisation of HSP by the cells. Some of the effects of hormones could be primary, but other effects could be secondary. For example, both FSH (Erickson and Danforth 1995; Berisha and Schams 2005) and leptin (Sirotkin et al. 2005) can affect ovarian function through stimulation of ovarian IGF-I release. Either IGF-I, leptin and FSH are known stimulators of basic ovarian functions including secretion of oestrogen, possible regulators of HSPs (see “Introduction”). Therefore, it is possible that hormone-induced activation of ovarian functions is associated with decrease in synthesis and use of HSP by ovarian cells. The obtained data demonstrated hormonal regulation of HSPs in ovarian cells.
Furthermore, the obtained results demonstrated that two kind of stress, heat stress and malnutrition (deprivation of serum containing the main cell nutrients), promote accumulation of mRNA for HSP70, HSP72 and HSP105 (1st series of experiments), whilst heat stress (but not malnutrition) reduced accumulation of HSP70 itself in ovarian cells (2nd series of experiments). This effect is in line with previous reports (Theodorakis and Morimoto 1987; Trinklein et al. 2004; Akerfelt et al. 2007) that heat stress can induce production of HSPs by non-ovarian cells, but not with reports on lack of effect of heat stress on ovarian HSP90 (Guzeloglu et al. 2001). The ability of high temperatures to induce both HSP mRNAs accumulation and HSP depletion suggest that thermal stress can promote both HSP synthesis and metabolisation by ovarian cells. On the other hand, it is not to be excluded that different actions of heating on HSPs and their RNAs might be explained by different effect on HSPs and their RNAs, changes in their localisation, time required for cellular accumulation of RNAs and their peptide, release of HSPs from the cells in response to stress and/or by negative feedback mechanisms affecting HSPs or their RNAs. For example, heat shock can affect not (or not only) total intracellular HSP and its mRNA but rather their migration, localisation and interrelationships with their targets within the cells. Furthermore, heat stress cannot affect basal HSP70 synthesis, production and accumulation but increase the stability of its mRNA in non-ovarian cells (Theodorakis and Morimoto 1987). Finally, heat stress can promote not only RNA and synthesis of HSPs but also HSP release from the non-ovarian cells (Calderwood et al. 2007), which can result accumulation of HSP RNA but depletion of intracellular HSP level. Just such phenomenon was observed in our experiments.
This is the first evidence that malnutrition (serum deprivation) can affect HSP and their mRNAs. In contrast to high temperatures, malnutrition was able to promote accumulation of both HSP and HSP mRNA. It suggests that nutritional stress, in contrast to thermal stress, can promote HSP synthesis and accumulation but not utilisation, release etc.
The role of HSPs in response of ovaries to stress remains to be elucidated. A likely possibility is the protective role of HSP during stress. The ability of HSPs to increase the resistance of non-ovarian cells to stress and eliminate its negative physiological consequences has been reported (Queitsch et al. 2002; Sonna et al. 2002; Beere 2004; Akerfelt et al. 2007; Jego et al. 2010). Furthermore, the ability of IGF-I, leptin and FSH to prevent stress-induced events (Sirotkin 2010) and to promote HSP70 accumulation suggests that hormones neutralise negative effect of stress via HSP, which have a protective role. On the other hand, the ability of stress to inhibit both ovarian functions (Putney et al. 1989; Edwards and Hansen 1997; Lawrence et al. 2004) and HSPs mRNAs and the ability of hormones both to reduce HSPs mRNA and to prevent stress-induced changes in ovarian cell functions (Sirotkin 2010) suggest that HSPs can mediate negative effect of stress on reproduction. This hypothesis is less probably due to ability of HSPs not to promote but to prevent effect of different kinds of stress (Queitsch et al. 2002; Akerfelt et al. 2007; Jego et al. 2010). The physiological role of HSP in the ovary requires further study. Nevertheless, the available data suggest that HSPs could be practically used as indicators, markers and maybe protectors against stress induced by inadequate environmental conditions (heat shock, malnutrition and deficiency of hormonal promoters) of both non-ovarian and ovarian cells.
As was mentioned above, the available data suggest the almost opposite effect of stress and hormones treatments on ovarian functions and HSPs production observed in our experiments. Moreover, hormones in our experiments were able to prevent effect of stress on HSPs. It suggests that stress and hormones are antagonists in control of not only ovarian functions but also in HSP synthesis. These facts indicate that IGF-I, leptin and FSH could neutralise effect of stress on HSP and on reproductive processes. This ability of hormones could be practically used not only for promotion of ovarian functions but also for elimination of negative effects of stress induced by inadequate temperature and nutrition on reproductive processes. It was hypothesised above that HSPs could be used as markers of biological effects of these factors.
Taken together, our observations enable us to formulate some hypotheses concerning physiological role and possible application of HSPs detected in the ovary. It might be suggested that HSPs can be indicators of stress and efficiency of hormones action and that hormones can be used for neutralisation of effect of stress on reproductive processes. Nevertheless, these hypotheses should be supported by demonstration the involvement of different members of HSPs family in control of reproductive processes. Furthermore, it remains to be understood what for HSP-related processes (transcription, translation, folding, cell cycle, apoptosis, HSP synthesis, storage, reception, action, metabolism etc.) are regulated by stressor and hormones and by what mechanism. Although understanding interrelationships between stress, hormones and HSPs in the ovary requires further studies, the present observations (1) show that HSP70, HSP72 and HSP105/110 can be synthesised in porcine ovarian follicular granulosa cells and (2) demonstrate for the first time that hormones (IGF-I, leptin and FSH) can inhibit and stressors (high temperature and malnutrition/serum deprivation) can stimulate synthesis of HSP70, HSP72, HSP105/110 in ovarian cells. In addition, (3) heat stress, but not malnutrition, can promote depletion of HSP70 in ovarian cells. Finally, it was demonstrated (4) that hormones (IGF-I, leptin and FSH) can prevent stress-related changes in both synthesis and accumulation of HSPs.
Acknowledgements
The authors thank Ing. Ž. Kuklová and K. Tothová for technical assistance. The present studies were supported by Ministry of Agriculture of Slovak Republic (RVVU 07-02).
References
- Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann NY Acad Sci. 2007;1391:1–13. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- Barb CR, Hausman GJ, Czaja K. Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol. 2005;29:186–192. doi: 10.1016/j.domaniend.2005.02.024. [DOI] [PubMed] [Google Scholar]
- Beere HM. ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- Berisha B, Schams D. Ovarian function in ruminants. Domest Anim Endocrinol. 2005;29:305–317. doi: 10.1016/j.domaniend.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Bombardier E, Vigna C, Iqbal S, Tiidus PM, Tupling AR. Effects of ovarian sex hormones and downhill running on fiber-type-specific HSP70 expression in rat soleus. J Appl Physiol. 2009;106:2009–2015. doi: 10.1152/japplphysiol.91573.2008. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- Driancourt MA, Guet P, Reynaud K, Chadli A, Catelli MG. Presence of an aromatase inhibitor, possibly heat shock protein 90, in dominant follicles of cattle. J Reprod Fertil. 1999;115:45–58. doi: 10.1530/jrf.0.1150045. [DOI] [PubMed] [Google Scholar]
- Duvigneau JC, Hartl RT, Teinfalt M, Gemeiner M. Delay in processing porcine whole blood affects cytokine expression. J Immunol Methods. 2003;272:11–21. doi: 10.1016/S0022-1759(02)00372-1. [DOI] [PubMed] [Google Scholar]
- Edwards JL, Hansen PJ. Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev. 1997;46:138–145. doi: 10.1002/(SICI)1098-2795(199702)46:2<138::AID-MRD4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Ellis RJ. Do molecular chaperones have to be proteins? Biochem Biophys Res Commun. 1997;238:687–692. doi: 10.1006/bbrc.1997.7339. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Danforth DR. Ovarian control of follicle development. Am J Obstet Gynecol Ź. 1995;172:736–747. doi: 10.1016/0002-9378(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Guzeloglu A, Ambrose JD, Kassa T, Diaz T, Thatcher MJ, Thatcher WW. Long-term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim Reprod Sci. 2001;66:15–34. doi: 10.1016/S0378-4320(01)00082-3. [DOI] [PubMed] [Google Scholar]
- Hillier SG. Cellular basis of follicular endocrine function. In: Hillier SG, editor. Ovarian endocrinology. Oxford: Blackwell Science; 1991. pp. 73–105. [Google Scholar]
- Jayachandran M, Miller VM. Ovariectomy upregulates expression of estrogen receptors, NOS, and HSPs in porcine platelets. Am J Physiol Heart Circ Physiol. 2002;283:H220–H226. doi: 10.1152/ajpheart.00950.2001. [DOI] [PubMed] [Google Scholar]
- Jego G, Hazoumé A, Seigneuric R, Garrido C (2010) Targeting heat shock proteins in cancer. Cancer Lett. PubMed PMID: 21078542. [DOI] [PubMed]
- Lanks KW. Modulators of the eukaryotic heat shock response. Exp Cell Res. 1986;165:1–10. doi: 10.1016/0014-4827(86)90528-8. [DOI] [PubMed] [Google Scholar]
- Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL. Retinol improves development of bovine oocytes compromised by heat stress during maturation. J Dairy Sci. 2004;87:2449–2454. doi: 10.3168/jds.S0022-0302(04)73368-8. [DOI] [PubMed] [Google Scholar]
- Makarevich A, Sirotkin A, Chrenek P, Bulla J, Hetenyi L. The role of IGF-I, cAMP/protein kinase A and MAP kinase in the control of steroid secretion, cyclic nucleotide production, granulosa cell proliferation and preimplantation embryo development in rabbits. J Steroid Biochem Mol Biol. 2000;73:123–133. doi: 10.1016/S0960-0760(00)00067-4. [DOI] [PubMed] [Google Scholar]
- Maniwa J, Izumi S, Isobe N, Terada T. Studies on substantially increased proteins in follicular fluid of bovine ovarian follicular cysts using 2-D PAGE and MALDI-TOF MS. Reprod Biol Endocrinol. 2005;3:23. doi: 10.1186/1477-7827-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayansingh RM, Senchyna M, Vijayan MM, Carlson JC. Expression of prostaglandin G/H synthase (PGHS) and heat shock protein-70 (HSP-70) in the corpus luteum (CL) of prostaglandin F2 alpha-treated immature superovulated rats. Can J Physiol Pharmacol. 2004;82:363–371. doi: 10.1139/y04-032. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS. Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci. 1989;19:37–51. doi: 10.1016/0378-4320(89)90045-6. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Salvetti NR, Baravalle C, Mira GA, Gimeno EJ, Dallard BE, Rey F, Ortega HH. Heat shock protein 70 and sex steroid receptors in the follicular structures of induced ovarian cysts. Reprod Domest Anim. 2008;44:805–814. doi: 10.1111/j.1439-0531.2008.01086.x. [DOI] [PubMed] [Google Scholar]
- Schams D, Berisha B, Kosmann M, Einspanier R, Amselgruber WM (1999) Possible role of growth hormone, IGFs, and IGF-binding proteins in the regulation of ovarian function in large farm animals. Domest Anim Endocrinol. 17:279–285 [DOI] [PubMed]
- Sirotkin AV. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J Exp Biol. 2010;213:2125–2130. doi: 10.1242/jeb.040626. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV (1996) Inter-relationships between nonapeptide hormones and cyclic nucleotides within cultured porcine granulosa cells. J Endocrinol. 150:343–348 [DOI] [PubMed]
- Sirotkin AV, Makarevich AV (1999) GH regulates secretory activity and apoptosis in cultured bovine granulosa cells through the activation of the cAMP/protein dinase A system. J Endocrinol. 163:317–327 [DOI] [PubMed]
- Sirotkin AV, Makarevich AV, Corkins MR, Kotwica J, Bulla J (2001) The transfection-induced overexpression of IGF-binding protein-4 affects the secretory activity of porcine ovarian granulosa cells and their response to hormones and IGF-I. J Mol Endocrinol. 26:241–248 [DOI] [PubMed]
- Sirotkin AV, Makarevich AV, Kotwica J, Marnet P-G, Kwon HB, Hetenyi L. Isolated porcine ovarian follicles as a model for the study of hormone and growth factor action on ovarian secretory activity. J Endocrinol. 1998;159:313–321. doi: 10.1677/joe.0.1590313. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV, Mlyncek M, Kotwica J, Makarevich AV, Florkovicova I, Hetenyi L. Leptin directly controls secretory activity of human ovarian granulosa cells: possible inter-relationship with the IGF/IGFBP system. Horm Res. 2005;64:198–202. doi: 10.1159/000089009. [DOI] [PubMed] [Google Scholar]
- Smith GD, Jackson LM, Foster DL. Leptin regulation of reproductive function and fertility. Theriogenology. 2001;57:73–86. doi: 10.1016/S0093-691X(01)00658-6. [DOI] [PubMed] [Google Scholar]
- Sonna LA, Fujita J, Gaffin SL, Lilly CM. Effects of heat and cold stress on mammalian gene expression. J Appl Physiol. 2002;92:1725–1742. doi: 10.1152/japplphysiol.01143.2001. [DOI] [PubMed] [Google Scholar]
- Spicer LJ. Leptin: a possible metabolic signal affecting reproduction. Domest Anim Endocrinol. 2001;21:251–270. doi: 10.1016/S0739-7240(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, Morimoto RI. Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987;7:4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinklein ND, Murray JI, Hartman SJ, Botsein D, Myers RM. The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell. 2004;15:1254–1261. doi: 10.1091/mbc.E03-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, Preter K, Pattyn F, Poppe B, Roy N, Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie T, Cox E, To LT, Vanrompay D, Bouchaut H, Buys N, Goddeeris BM. Comparative analysis of porcine cytokine production by mRNA and protein detection. Vet Immunol Immunopathol. 2001;81:97–112. doi: 10.1016/S0165-2427(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Zieba DA, Amstalden M, Williams GL. Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest Anim Endocrinol. 2005;29:166–185. doi: 10.1016/j.domaniend.2005.02.019. [DOI] [PubMed] [Google Scholar]