Abstract
The tetratricopeptide repeat (TPR) motif is one of many repeat motifs that form structural domains in proteins that can act as interaction scaffolds in the formation of multi-protein complexes involved in numerous cellular processes such as transcription, the cell cycle, protein translocation, protein degradation and host defence against invading pathogens. The crystal structures of many TPR domain-containing proteins have been determined, showing TPR motifs as two anti-parallel α-helices packed in tandem arrays to form a structure with an amphipathic groove which can bind a target peptide. This is however not the only mode of target recognition by TPR domains, with short amino acid insertions and alternative TPR motif conformations also shown to contribute to protein interactions, highlighting diversity in TPR domains and the versatility of this structure in mediating biological events.
Keywords: Tetratricopeptide repeat domains, Hsp70/Hsp90 chaperone machinery, Steroid receptors, p67phox, PEX5
Introduction
Numerous biological functions can only occur with the participation of a repeat protein, a molecule that is defined by possessing duplications of a basic sequence motif usually involved in the formation of a structural element of the protein (Andrade et al. 2001; Main et al. 2005). CDC23, CDC16, CDC27 and SSN6, all found in Saccharomyces cerevisiae, share sequence similarity with nuc2+ from Schizosaccharomyces pombe and are involved in the cell division cycle or RNA synthesis (Hirano et al. 1990; Sikorski et al. 1990; Tzamarias and Struhl 1995). Each of these proteins was found to possess a duplicated, degenerate 34 amino acid sequence termed the tetratricopeptide repeat (TPR) motif. The number of observed TPR units in proteins ranges from 3 to 16, and these are generally arranged as tandem arrays. Characterisation of a TPR-containing truncated form of nuc2+ suggested the assembled TPR domain functioned as a protein interaction module following the formation of large protein aggregates and potentially facilitated the assembly of multi-protein complexes (Hirano et al. 1990). Secondary structure predictions suggested each motif was divided into two anti-parallel α-helical sub-domains (A and B) of equivalent length. This was confirmed by crystallisation of the N-terminal TPR domain in protein phosphatase 5 (PP5) (Das et al. 1998). This structure showed the parallel packing of adjacent TPR motifs, which formed a regular series of anti-parallel α-helices reminiscent of 14-3-3 proteins, and generated a right-handed helical conformation that created a channel able to accommodate a polypeptide from another protein (Liu et al. 1995; Xiao et al. 1995). The discovery and characterisation of additional TPR-containing proteins from bacteria to humans has expanded the functional palette of TPR domains, such that they participate in diverse processes such as transcriptional control, mitochondrial and peroxisomal protein transport, protein kinase inhibition, NADPH oxidase activity, protein folding, immunity and viral replication (Goebl and Yanagida 1991; Lamb et al. 1995; Ponting 1996; Callahan et al. 1998; Cziepluch et al. 1998; Mamane et al. 2000).
The TPR motif is only one example of many repeat interaction motifs, which differ not only in sequence length but also in overall structure (Andrade et al. 2001; Main et al. 2005). β-Catenin possesses the armadillo repeat, which is ~42 residues long and consists of three α-helices arranged in a triangular bundle (Huber et al. 1997). Tandem armadillo units pack together to form a right-handed superhelix that creates a surface for protein–protein interactions, similar to TPR domains. The ankyrin repeat motif, found in inhibitors of cyclin-dependent kinase 4 (INK4) proteins, is 33 residues long, with each repeat folding into two anti-parallel α-helices followed by a loop, and consecutive repeats stack together to form an L-shaped domain with neighbouring loops forming a hairpin-like β-sheet (Li et al. 2006). HEAT motifs are also comprised of anti-parallel α-helices stacked in consecutive arrays and vary between 37 and 43 residues. Despite these differences, the common underlying feature of these repeats is that they mediate protein–protein interactions.
Recently resolved crystal structures of several TPR proteins have confirmed the general folding pattern observed for the tandem TPR motifs of PP5 and have revealed variations in structure, as well as adaptations, that provide TPR-containing proteins with alternative modes of interaction with different binding partners (Gregory and Michael 1999; D'Andrea and Regan 2003). In this review, we will focus on three groups of TPR proteins that incorporate TPR domains within unique structural arrangements to allow functional interactions with specific protein targets. Cyclophilin 40 (CyP40), FK506-binding protein 51 (FKBP51) and FKBP52, which belong to the family of immunophilin cochaperones, are found in steroid receptor complexes with heat shock protein 90 (Hsp90) and use a tandem array of three TPR units to bind the C-terminal EEVD peptide motif in Hsp90. p67phox, a cytosolic factor involved in NADPH oxidase activation, utilises a four-unit TPR domain to bind Rac.GTP. PEX5, a receptor for peroxisome-targeted proteins, contains seven TPR units and binds to the SKL peptide, the type 1 peroxisomal targeting signal. Figure 1 shows the domain architecture of the immunophilin and other TPR cochaperones with an established role in heat shock protein 70 (Hsp70) and/or Hsp90 chaperone function, together with the organisation of the p67phox and PEX5 TPR proteins. Collectively, these TPR-containing proteins help to elaborate the versatile nature of the TPR domain that allows recognition of diverse targets to mediate important processes and functions within the cell.
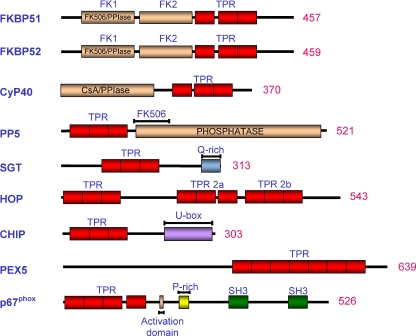
Fig. 1.
Schematic presentation of the domain structures of TPR-containing proteins associated with the Hsp70/Hsp90 chaperone machinery (FKBP51, FKBP52, CyP40, PP5, SGT, Hop and CHIP), peroxisomal protein movement (PEX5) and host defence (p67phox). TPR domains are depicted in red whilst other specialised functional domains are highlighted in other various colours and labelled accordingly. FKBP FK506-binding protein, PPIase peptidylprolyl isomerase, TPR tetratricopeptide repeat, CyP40 cyclophilin 40, CsA cyclosporin A, PP5 protein phosphatase 5, SGT small glutamine-rich TPR protein, Hop Hsp-organising protein, CHIP C-terminal of Hsp70-interacting protein, PEX5 peroxin 5, p67phox neutrophil cytosol factor 2
Functional domains of steroid receptor-associated TPR cochaperones
Folding of newly synthesised peptides to functionally mature proteins, such as steroid receptors, is important for cellular homeostasis. This process is actively regulated by Hsp70 and Hsp90 with the cooperation of other factors termed cochaperones in what is known as the Hsp70/Hsp90-based chaperone machinery (Pratt and Toft 2003). Cochaperones can regulate the nucleotide status, and thus function, of Hsp70 and Hsp90, and deliver non-native proteins to their respective polypeptide-binding domains for folding. Those cochaperones that regulate Hsp70 include Hsp40, Hsp70-interacting protein (Hip), Hsp-organising protein (Hop) and small glutamine-rich TPR protein (SGT), while Hsp90 is regulated by cochaperones that include Hop, p23, PP5, CyP40, FKBP51 and FKBP52. C-terminal of Hsp70-interacting protein (CHIP) is another cochaperone that regulates both Hsp70 and Hsp90.
Since the crystallisation of the PP5 TPR domain, the structures of several other steroid receptor-associated TPR-containing proteins have been solved. There are now full-length structures available for bovine CyP40, human FKBP52, PP5 and Hop, human and squirrel monkey FKBP51, and mouse CHIP, as well as the structure of the human SGT TPR domain. It is known that TPR domains in these proteins can mediate interactions with Hsp70 and/or Hsp90 (Angeletti et al. 2002; Smith 2004), but in addition to their Hsp-recognition domains, each also possesses other localised functional domains important for their own conformation and/or the regulation of associated proteins.
CyP40, FKBP51 and FKBP52
CyP40 and the two FKBPs have a similar structural arrangement with each possessing an N-terminal binding site for the immunosuppressants cyclosporin A or FK506, respectively, and a C-terminal TPR domain (Taylor et al. 2001; Sinars et al. 2003; Wu et al. 2004). The cyclophilin domain of CyP40 is similar to other single-domain cyclophilins (Kallen et al. 1998). In FKBP51 and FKBP52, FK506 binds to the first of two FKBP domains, termed FK1, while the second domain, called FK2, lacks drug-binding activity. Bound immunosuppressants inhibit the peptidyl prolyl isomerase (PPIase) activity of the cyclophilin and FK1 domains, which may be important for target protein regulation by direct or indirect association.
PP5
PP5 is a phosphatase that dephosphorylates serine and threonine residues on target proteins (Barford 1996; Cohen 1997). Crystallisation of the full-length phosphatase in the absence of ligands or binding partners revealed the structural organisation of the autoinhibited form of PP5 (Yang et al. 2005). The TPR domain in PP5 is oriented to the N terminus and is linked to a C-terminal phosphatase catalytic domain followed by a short C-terminal subdomain. In this inactive conformation, the TPR domain engages with the catalytic domain in such a way to restrict target protein access to the enzymatic site, and this structure is stabilised by the C-terminal subdomain. Suppression of catalytic activity can be abolished by an allosteric conformational change that disrupts the TPR–catalytic domain interface, and this can be induced upon binding of polyunsaturated fatty acids or Hsp90 to the TPR domain (Chen and Cohen 1997; Skinner et al. 1997; Ramsey and Chinkers 2002).
Hop
Hop plays a dual role in mature steroid receptor complex assembly by recruiting Hsp90 to preformed Hsp70–receptor complexes and inhibiting the ATPase of Hsp90 for client loading onto the chaperone for subsequent folding (Chen et al. 1996b; Dittmar et al. 1996; Chen and Smith 1998; Kosano et al. 1998; Prodromou et al. 1999; Siligardi et al. 2004). Hop has an N-terminal TPR domain (TPR1) followed by an aspartic acid/proline (DP)-rich region, and two more adjacent TPR domains (TPR2a and TPR2b) followed by a second DP-rich region.
CHIP
The cochaperones described above are involved in maintaining an activatable conformation of Hsp70/Hsp90-dependent ‘clients’, but TPR proteins also function to mediate the degradation of misfolded proteins, indicating a role in quality control (Cyr et al. 2002). Selection of proteins for degradation is mediated by E3 ubiquitin ligases, and CHIP is a member of this enzymatic class (Jiang et al. 2001; Murata et al. 2001). CHIP has an N-terminal TPR domain and a C-terminal U-box domain that mediates its ligase activity, which promotes ubiquitylation of target substrates prior to their degradation by the proteasome.
SGT
Human SGT binds to viral protein U (Vpu) and Group specific Antigen, two proteins associated with human immunodeficiency virus-1, and the rat homologue was identified as an interactor of the non-structural protein NS-1 of the parvovirus H-1. The central TPR domain in SGT is flanked by an N-terminal dimerisation domain and a C-terminal glutamine-rich domain involved in SGT association with type 1 glucose transporter (Callahan et al. 1998; Cziepluch et al. 1998; Liou and Wang 2005).
TPR cochaperone regulation of steroid receptor function
Steroid receptor-associated TPR-containing cochaperones play varying roles in regulating hormone activity. These are intimately associated with the assembly of mature steroid receptor complexes, modulation of receptor responsiveness to hormone and degradation. Their functional roles have been extensively discussed in other reviews (Ratajczak et al. 2003; Pratt et al. 2004; Riggs et al. 2004; Smith 2004; Davies and Sánchez 2005; Golden et al. 2008; Hinds and Sánchez 2008) and as such are only described briefly below.
Regulation of Hsp70 and Hsp90 ATPases
Both Hsp70 and Hsp90 require ATP for their functional association with substrates (Pratt and Toft 2003). In the case of a steroid receptor, Hip binding to the N-terminal ATPase domain of Hsp70, possibly through a unique TPR binding site located within this region (see below), stabilises the Hsp70–receptor complex (Höhfeld et al. 1995; Frydman and Höhfeld 1997) in a step that may be important for recognition by Hop and loading of the receptor onto Hsp90 for further processing. Hop contains three distinct TPR domains (TPR1, TPR2a, TPR2b) (Fig. 1), with TPR1 and TPR2a providing anchor points for the C-terminal EEVD peptides of Hsp70 and Hsp90, respectively. These specific interactions, coupled with domain–domain interactions, also involving its TPR domains, allow Hop to play a key role in coordinating the actions of Hsp70 and Hsp90 (Chen et al. 1996b; Chen and Smith 1998; Prodromou et al. 1999; Scheufler et al. 2000; Odunuga et al. 2003; Carrigan et al. 2006; Ramsey et al. 2009). While the TPR acceptor site for Hop in the C-terminal region of Hsp90 serves to anchor the cochaperone, studies have shown that Sti1, the yeast homologue of Hop, markedly inhibits the ATPase activity of yeast Hsp90 through secondary interactions that block the ATP-binding pocket in the Hsp90 N-terminal domain (Prodromou et al. 1999). By directly competing with Sti1 for binding to Hsp90, the CyP40 yeast homologue Cpr6 can negate the Sti1-mediated blockade of Hsp90 ATPase activity following TPR protein exchange (Prodromou et al. 1999). In contrast, in vitro studies with human Hop determined that the cochaperone had no influence on the weak basal ATPase activity of human Hsp90, but significantly inhibited the increased rate of ATP hydrolysis by Hsp90 in response to interaction with the ligand-binding domain of the glucocorticoid receptor, an established Hsp90 ‘client’ protein (McLaughlin et al. 2002). On the other hand, FKBP52, which like CyP40 binds competitively with Hop to the C-terminal TPR interaction site of Hsp90, was shown to enhance Hsp90 ATPase activity stimulated by the glucocorticoid receptor (McLaughlin et al. 2002). This control over ATP utilisation is important for the functional activity of newly synthesised substrates, but ATPase regulation is also required for the degradation of improperly folded substrates. CHIP can bind Hsp70 and inhibit Hsp40-stimulated Hsp70 ATPase activity, and has been reported to deplete cellular glucocorticoid receptor levels (Ballinger et al. 1999; Connell et al. 2001). Therefore, CHIP can be regarded as a degradatory cochaperone of Hsp70 and Hsp90. SGT negatively regulates Hsp70 such that the chaperone has a reduced ability to refold denatured luciferase (Angeletti et al. 2002).
Receptor preferences
Mature steroid receptor–Hsp90 complexes typically contain PP5 or one of three TPR immunophilins: CyP40, FKBP51 or FKBP52 (Smith 2004). Each of these proteins compete for a common interaction site at the C-terminal end of Hsp90 via their TPR domains (Ratajczak and Carrello 1996; Young et al. 1998; Carrello et al. 1999) and at equilibrium one could expect their associations with receptor-Hsp90 complexes to be determined by relative cellular abundance of the cochaperones, regardless of receptor. However, representation of each in any one receptor–Hsp90 complex is disproportionate and may be governed by which receptor is bound to Hsp90, with glucocorticoid receptor preferring to associate with PP5 and FKBP51, progesterone receptor with FKBP51 and estrogen receptor with CyP40 (Ratajczak et al. 1990; Silverstein et al. 1997; Barent et al. 1998). Several studies have shown association of receptor with a particular immunophilin or PP5 to be functionally relevant in regulating receptor activity (Chen et al. 1996a; Denny et al. 2000; Riggs et al. 2003; Cheung-Flynn et al. 2005; Tranguch et al. 2005; Yang et al. 2006; Gallo et al. 2007; Yong et al. 2007). SGT may also have a preference for particular steroid receptors since it was shown that SGT overexpression selectively reduced the hormone responsiveness of androgen receptor compared to estrogen receptor α (Buchanan et al. 2007).
Hormone-binding affinity
Insensitivity to cortisol has been observed in New World primates, and this has been partly attributed to the overexpression of FKBP51 in glucocorticoid receptor complexes lowering receptor affinity for glucocorticoid (Reynolds et al. 1999; Denny et al. 2000; Scammell et al. 2001; Westberry et al. 2006). Hormone responsiveness of the progesterone receptor was similarly attenuated by FKBP51 overexpression (Hubler et al. 2003). The inhibitory effect of this cochaperone on the function of glucocorticoid and progesterone receptors was critically dependent on an intact FKBP51 Hsp90-binding TPR domain (Hubler et al. 2003; Denny et al. 2005; Wochnik et al. 2005). On the other hand, recent studies have shown that increased expression of FKBP51 promotes the assembly of mature androgen receptor–Hsp90 complexes leading to an increased capacity for androgen binding without affecting receptor hormone-binding affinity (Ni et al. 2010). This regulation of the androgen receptor ligand-binding domain requires the interaction of the FKBP51 TPR domain with Hsp90 (Ni et al. 2010). In contrast to the negative regulation of glucocorticoid and progesterone receptor function by FKBP51, FKBP52 selectively potentiates the activity of glucocorticoid, progesterone and androgen receptors through a mechanism that is again dependent on its ability to bind Hsp90 through its TPR domain (Riggs et al. 2003; Cheung-Flynn et al. 2005; Tranguch et al. 2005; Yang et al. 2006; Yong et al. 2007). More recent work in the Smith laboratory, specifically investigating the FKBP52 FK1 domain and the FK1-FK2 linker, has further defined the mechanism/s that regulate steroid receptor signalling. A hairpin loop (116AGS119PPKIP123) overhanging the catalytic site in FK1 of FKBP52 differs from the corresponding sequence in FKBP51 and confers steroid receptor potentiating activity by FKBP52 (Riggs et al. 2007). A proline substitution for Leu119 in FKBP51 allows the mutated immunophilin to mimic the role of FKBP52. In the two FKBPs, FK1 is linked to FK2 by a seven- to nine-residue loop, which in FKBP52 contains a casein kinase II phosphorylation site that when phosphorylated, blocks FKBP52–Hsp90 interaction (Miyata et al. 1997). A comparative analysis of the dissimilar loop sequences in FKBP51 and FKBP52 failed to explain the differential influences of these immunophilins on steroid receptor activity, but phosphorylation of the FKBP52 linker was shown to abrogate receptor potentiation (Cox et al. 2007).
Dynein binding and receptor nucleocytoplasmic trafficking
Dynein is an important microtubule-associated motor protein that facilitates movement of the glucocorticoid receptor to the nucleus from the cytoplasm. CyP40, FKBP52 and PP5 have been reported to bind dynein, and in FKBP52 this interaction has been localised to the PPIase domain, but is independent of PPIase activity (Galigniana et al. 2001; Davies et al. 2002; Galigniana et al. 2002). The steroid receptor repertoire that is regulated by this FKBP52-mediated translocation has been further expanded to include the mineralocorticoid receptor (Gallo et al. 2007; Galigniana et al. 2010). The above studies have investigated the role of TPR cochaperones in hormone-induced movement of steroid receptors, but a recent study has shown TPR cochaperones to be selectively involved in the localisation of hormone-free glucocorticoid and progesterone receptors, and that localisation is mediated by the receptor ligand-binding domain (Banerjee et al. 2008). Figure 2a, b provides a structural comparison between CyP40, FKBP51 and FKBP52, immunophilin cochaperones characterised by N-terminal PPIase and C-terminal TPR protein interaction domains.
Fig. 2.
Ribbon representations of molecular structures of TPR-containing proteins. a CyP40 and b FKBP51, FKBP52. The CsA-binding domain (CyP40) and FK regions (FKBP51 and FKBP52) are shown in green. Core TPR domains for CyP40, FKBP51 and FKBP52 are depicted in red, with the final extended helices, at the C-terminal ends of each protein, shown in yellow. c p67phox. The N-terminal TPR domain of p67phox (red) is shown in complex with the small GTPase Rac (yellow). The β-hairpin insertion is green, while the C-terminal extended loop (blue) is accommodated in the binding groove formed by the TPRs. d PEX5. The C-terminal TPR domain of PEX5 is shown in complex with a PTS1 peptide (black) as viewed down the long axis of the peptide. The PEX5 TPR domain corresponds to two separate TPR helical bundles comprised of TPRs 1–3 (red) and 5–7 (green), while the extended TPR4 helix is blue. Remaining structural regions are yellow. All structures were derived from the RCSB Protein Data Bank with ViewerLite 5.0
HSP interaction determinants in TPR cochaperones
Deletion studies were the first to demonstrate that TPR domains mediated binding to Hsp90 (Radanyi et al. 1994; Chen et al. 1996a; Ratajczak and Carrello 1996; Barent et al. 1998). Determination of the TPR domain structure of PP5 revealed the packing of adjacent TPR units generated an exposed groove capable of accepting a target protein peptide (Das et al. 1998). Although TPR motifs are highly degenerate, they do display a consistent pattern of key residues important for structural integrity. The two α-helical sub-domains in each TPR motif are arranged such that the groove is mainly composed of residues from the A helix of each repeat, while B helix residues are buried to form the structural backbone of the superhelix, and this groove forms a critical Hsp recognition surface.
In a PP5 mutagenesis study, Russell and co-workers carefully selected A helix residues with side chains extended into the groove and identified four basic residues important for PP5–Hsp90 interaction (Russell et al. 1999). These amino acids are highly conserved in other Hsp90-binding TPR proteins, and mutation of aligned residues in CyP40 confirmed their importance in Hsp90 recognition (Ward et al. 2002). The key recognition sequence for the TPR domain in these proteins is the EEVD peptide located at the extreme C terminus of Hsp90 (Chen et al. 1998; Young et al. 1998; Carrello et al. 1999), which is conserved in Hsp70. Crystallisation of individual Hop TPR domains with Hsp70 and Hsp90 N-terminally extended EEVD peptides has defined the mechanism of TPR domain–peptide interaction (Scheufler et al. 2000). The TPR1 domain of Hop binds to Hsp70, while the TPR2a domain mediates Hsp90 recognition (Chen et al. 1996b; Lassle et al. 1997). The groove in each TPR domain accommodates their respective peptide in an extended conformation where the ultimate aspartate residue is tightly held by electrostatic interactions with TPR residue side chains in a two-carboxylate clamp. Additional EEVD contacts involve hydrogen bonding, while amino acids upstream of the EEVD enhance the affinity of the peptides for TPR domains and mediate specificity of Hsp70 and Hsp90 to TPR1 and TPR2a, respectively. Notably, Hop TPR2a provides an example of where an additional sequence within the TPR domain does not disrupt the overall structure. TPR2a contains an insertion between units 2 and 3 that extends the helices by a single turn but does not impact Hsp90 peptide recognition (Scheufler et al. 2000).
The Hsp90 dimerisation domain, located in the C-terminal region upstream of the MEEVD peptide, contributes to TPR cochaperone recognition (Chen et al. 1996b) and contains the putative binding site for novobiocin, a coumarin-based Hsp90 inhibitor (Marcu et al. 2000). In vitro studies demonstrated that novobiocin had a differential effect on Hsp90–immunophilin cochaperone interaction, suggesting that the TPR cochaperones modulate Hsp90 function through distinct contacts within the Hsp90 C-terminal domain (Allan et al. 2006).
Although EEVD interactions with the TPR domain groove are critical for Hsp binding, regions outside of the TPR domains are also important in mediating recognition. TPR domains are typically followed by a seventh α-helix that packs against and extends beyond the TPR domain and has been shown to be involved in binding Hsp90 in addition to the TPR domain. FKBP51 and FKBP52 have different affinities for Hsp90 and are assembled differentially with specific receptor complexes, and these differences map in part to sequences C-terminal of their respective TPR domains (Barent et al. 1998; Pirkl and Buchner 2001; Cheung-Flynn et al. 2003). The charge-Y motif was identified and found to be essential for FKBP–Hsp90 interaction, which was also confirmed for CyP40, but sequences further downstream in FKBP51 and FKBP52 differentially regulated Hsp90 binding (Ratajczak and Carrello 1996; Cheung-Flynn et al. 2003; Allan et al. 2006). The acidic linker flanking the N terminus of the CyP40 TPR domain was also shown to be important for efficient interaction (Ratajczak and Carrello 1996; Mok et al. 2006). Although an interaction partner for Hop TPR2b has yet to be identified, mutations in TPR2b reduced Hop interaction with both Hsp70 and Hsp90, while mutations in the C-terminal DP-rich region inhibited Hop binding to Hsp70 (Chen and Smith 1998; Nelson et al. 2003).
Interestingly, the charge-Y motif in FKBP52 and CyP40 overlaps a calmodulin consensus sequence. Indeed, early studies provided evidence of Ca2+-dependent calmodulin interaction with both immunophilins prompting suggestions of a regulatory role for Ca2+/calmodulin in FKBP52 and CyP40 function (Callebaut et al. 1992; Massol et al. 1992; Ratajczak et al. 1995). More recent data, which showed that GST-fusion proteins incorporating the putative calmodulin-binding domains failed to retain calmodulin, run counter to this proposal (Cheung-Flynn et al. 2003; Shimamoto et al. 2010). However, it has been shown that S100A1 and S100A2, belonging to a subfamily of the EF-hand type calcium-sensing proteins (Donato 1999), form Ca2+-sensitive interactions with the TPR domains of FKBP52 and CyP40 resulting in a competitive inhibition of Hsp90 binding (Shimamoto et al. 2010). S100 proteins, then, may function as regulators of Hsp90-TPR protein chaperone machinery.
Alternative HSP recognition by TPR cochaperones
Like Hop, CHIP binds to both Hsp70 and Hsp90 (Ballinger et al. 1999; Connell et al. 2001), but CHIP interacts with either of these major chaperones through a single TPR domain. Recent elucidation of the binding of Hsp90 C-terminal peptide (NH2-DDTSRMEEVD) with the CHIP TPR domain has revealed that the peptide sequence is not accommodated in an extended conformation as for Hop, but turns at the methionine residue and becomes buried within a hydrophobic pocket (Zhang et al. 2005). This pocket can accommodate either the methionine or isoleucine that lies immediately upstream of the EEVD sequence in Hsp90 and Hsp70, respectively, and the peptide is twisted, negating the role of upstream residues in conferring the same specificity seen in binding Hop TPR domains. SGT also recognises Hsp70 and Hsp90 via its single TPR domain, but possibly through a different mechanism to that described for CHIP as SGT lacks the residues that form the hydrophobic pocket which allows the respective C-terminal peptides in the chaperones to twist (Dutta and Tan 2008).
Hydrophobic pockets themselves may also be important structural features within TPR domains that confer Hsp specificity, as the crystal structure of Hop TPR2a with the non-cognate Hsp70 peptide shows the hydrophobic pocket to be less accommodating for the Ile (–5) residue in the extended Hsp70 peptide than Met (–5) in the extended Hsp90 peptide, with the notable feature of a lack of bending by the Hsp70 peptide, such as with CHIP, to perhaps enhance affinity for TPR2A (Kajander et al. 2009).
GCUNC-45 is a TPR protein that positively regulates progesterone receptor function (Chadli et al. 2006). Hsp90-binding experiments in the presence of Hop revealed a novel GCUNC-45 TPR recognition site in the N-terminal domain of Hsp90, which also bound FKBP52 (Chadli et al. 2008). Further analysis defined a non-contiguous EEVD-like motif, centred in and around the Hsp90 N-terminal ATP-binding pocket, arranged in a structural conformation that can recognise TPR domains. Nucleotide binding negatively regulates the interaction. These authors also alluded to CyP40 binding to the N-terminal interaction motif, although Onuoha and co-workers have recently confirmed CyP40 interaction only with the C-terminal domain of Hsp90 (Onuoha et al. 2008). An EEVD-like motif interaction with a TPR domain has also been described for androgen receptor recognition by SGT, where binding is mediated by the first two TPR motifs of the SGT TPR domain and the hinge region located between the DNA-binding and ligand-binding domains in the receptor (Buchanan et al. 2007).
Hip has similarly been reported to bind the Hsp70 N-terminal ATPase domain via its TPR domain (Höhfeld et al. 1995). Through this interaction, Hip, originally identified in progesterone receptor complex assembly (Smith 1993; Prapapanich et al. 1996), can stabilise substrate-Hsp70 binding and competitively counteract the destabilising effects of the non-TPR cochaperone BAG1 (Gebauer et al. 1997; Höhfeld and Jentsch 1997; Takayama et al. 1997; Bimston et al. 1998). The Hip–Hsp70 interaction also allows for the simultaneous association of Hip with Hsp70–Hop complexes (Prapapanich et al. 1996; Gebauer et al. 1997). By analogy with the mode of GCUNC-45 interaction with Hsp90, there is the possibility that Hip targets a similar TPR recognition site in the N-terminal region of Hsp70. However, Hip is unique among the steroid receptor-associated TPR proteins in terms of Hsp recognition in that it binds Hsp70 independently of EEVD interactions (Höhfeld et al. 1995), and that efficient binding may be due to a greater requirement for additional Hsp-interaction determinants, such as the adjacent highly charged region and a C-terminal DP-repeat domain (Prapapanich et al. 1998). It is possible the mechanism of Hsp70 recognition by Hip is utilised by other steroid–receptor TPR cochaperones to interact with binding partners in distinct cellular pathways. Dutta and Tan (2008) reported the SGT TPR domain is sufficient to bind Vpu and identified the sequence 31KILRQ35 in Vpu as being important for this interaction.
Other targets of CyP40, FKBP51, FKBP52 and PP5
–CyP40
c-Myb is a transcription factor involved in haematopoiesis that is regulated by association of its N-terminal DNA-binding region with its C-terminal negative regulatory domain (Mucenski et al. 1991; Dash et al. 1996; Ness 1996). CyP40 inhibited DNA binding of c-Myb, and this inhibition required both the N-terminal PPIase and C-terminal TPR domains in CyP40 (Leverson and Ness 1998). The effect of CyP40 is mediated through a direct interaction of the TPR domain with the c-Myb DNA-binding domain and was negated by cyclosporin A, suggesting a role for CyP40 PPIase function in c-Myb regulation. The c-Myb oncogenic derivative v-Myb possesses a mutated CyP40-binding site that abrogated CyP40 interaction and thus its activity was not inhibited, suggesting v-Myb is able to evade a negative regulatory mechanism mediated by CyP40, implicating CyP40 in the regulation of transcription, transformation and differentiation.
–FKBP51
In response to artery injury, TNFα is released with subsequent activation of the IKK kinase complex. This activated complex then phosphorylates IκB, resulting in its degradation and the release, translocation and activation of the NF-κB transcription factor (Shishodia and Aggarwal 2002). The PPIase activity of FKBP51 is important for the function of IKK by regulation of the complex’s α-subunit (Bouwmeester et al. 2004). In vascular smooth muscle cells, rapamycin was shown to inhibit TNFα-induced NF-κB activity by abrogating FKBP51 PPIase activity and thus IKK-dependent effects on NF-κB signalling (Giordano et al. 2006).
–FKBP52
FKBP52 has been reported to have a similar effect on transcriptional regulation as that described for CyP40. FKBP52 was found to bind a proline-rich region in interferon regulatory factor-4 (IRF-4) via its TPR domain, while FKBP52 PPIase activity induced a structural modification in IRF-4 that resulted in impaired DNA binding and transcriptional activation (Mamane et al. 2000).
The phosphorylation status of FKBP52 may play an important role in the expression of viral vectors. Tyrosine dephosphorylation of FKBP52 by T-cell protein tyrosine phosphatase and serine/threonine dephosphorylation by PP5 has been reported to alleviate phosphorylated FKBP52-mediated inhibition of the expression of an adeno-associated virus (AAV) genome which is used for transgene expression in human gene therapy (Qing et al. 2001; Zhao et al. 2007).
–PP5
Cell homeostasis requires a delicate balance of phosphorylating and dephosphorylating events. PP5 is a regulator of a wide range of cellular functions by virtue of its TPR-mediated interaction interface, phosphatase activity and presence in nearly all mammalian tissues. Numerous studies have demonstrated a role for PP5 in processes such as proliferation, differentiation and DNA damage repair through association with transcription factors and involvement in a multitude of phosphorylation cascades. The reader is referred to more extensive reviews of PP5 and its biological functions (Golden et al. 2008; Hinds and Sánchez 2008).
Although the list of reported targets other than Hsp90 for the TPR immunophilins is limited, the unique interaction between the Hip TPR domain and Hsp70 and the current lack of a mechanistic basis for this interaction raises the possibility of alternative target recognition patterns. This is certainly the case for TPR proteins involved in host defence via NADPH oxidase and the localisation of peroxisomal enzymes. Elucidation of crystal structures revealed TPR domain insertions can form structures other than α-helices and be involved in binding to associated proteins and that the TPR domain itself can exist in a conformation other than a right-handed helix.
p67phox
NADPH oxidase is a membrane-associated enzyme complex that generates reactive oxygen species, forming the primary defence mechanism protecting against microbial infection. In resting neutrophils, the NADPH oxidase component p67phox acts as a connector between p40phox and p47phox in a cytosolic trimeric complex. Stimulation of neutrophils leads to conformational changes in the trimeric complex and phosphorylation of the components. This is followed by translocation to and association with the membrane-bound cytochrome b558, which is composed of p22phox and gp91phox, and the active GTPase Rac (Sumimoto et al. 2005).
Rac recognition by p67phox is crucial for the assembly and activation of NADPH oxidase (Abo et al. 1991; Knaus et al. 1991; Diekmann et al. 1994), and the Rac-binding site is located in the N-terminal TPR domain of p67phox (Diekmann et al. 1994; Ahmed et al. 1998; Han et al. 1998; Koga et al. 1999). Two crystal structures for the TPR domain in p67phox have been determined, one for an inactive p67phox in complex with Rac and a slightly longer active form of p67phox lacking Rac (Lapouge et al. 2000; Grizot et al. 2001). The N-terminal domain in each structure is very similar, suggesting the binding of Rac does not require or induce structural rearrangements within the TPR domain (Grizot et al. 2001). The TPR domain of p67phox has four TPR motifs (Ponting 1996) arranged in a similar manner to that seen in Hsp-binding TPR domains (Smith 2004). TPR4 is followed by another helix that packs against TPR4, forming into a nine helical bundle that exhibits a right-handed superhelical twist with a binding groove, which accommodates an 18-residue loop that lies downstream of the final helix (Fig. 2c). Between TPR3 and TPR4, there is a 20 amino acid insertion that forms two short anti-parallel β-strands. This is a notable feature for two reasons. Firstly, it does not disrupt the superhelical structure of the TPR domain by aligning along one edge of the TPR bundle, and secondly, this β-hairpin insertion forms part of the Rac-binding site along with the loops that link TPR1–TPR3 (Lapouge et al. 2000; Grizot et al. 2001). This alternative mode of recognition provides evidence that precludes the groove formed by the superhelix as a general target-binding interface.
The role of this β-hairpin insertion in Rac recognition may be to enhance the affinity of p67phox for the GTPase. Rac is a positive regulator of thyroid hormone-dependent KCNH2 potassium channel stimulation (Storey et al. 2002), and Gentile and co-workers have recently shown that PP5 is important for binding Rac and mediating its signalling (Gentile et al. 2006). Modelling comparisons of the TPR domain of PP5 and the first three TPR motifs of p67phox revealed a similar domain structure and aided in identifying two key contact lysines in PP5 critical for Rac recognition. In the autoinhibited form of PP5 (Yang et al. 2005), these lysines locate to the opposite side of the TPR domain–catalytic site interface and are thus accessible for Rac binding. It is interesting that PP5 can bind Rac since the TPR domain of the phosphatase lacks the β-hairpin important for p67phox recognition of Rac. This suggests the β-hairpin functions as a secondary interaction site for Rac in p67phox and enhances the affinity for the GTPase. It also raises the possibility of other TPR-containing proteins binding Rac and mediating Rac signalling. Alternatively, Rac itself may adopt a different mode of binding with PP5. Recent studies have confirmed a role for the PP5 TPR domain in specific and direct interaction with Rac, allowing Rac control over PP5 phosphatase activity and localisation to the cellular membrane (Chatterjee et al. 2010).
Another interesting feature of p67phox and PP5 is the involvement of their TPR regions in intramolecular interactions. As stated earlier, the PP5 TPR domain engages with the enymatic site to curb target protein binding for dephosphorylation. A recent report has shown p67phox to exist as a multi-domain protein with flexibility between each domain, with results suggesting an intramolecular interaction may exist between the TPR region and the N-terminal SH3 domain in p67phox which masks a C-terminal phosphorylation site in the full-length p67phox (Dang et al. 2003; Durand et al. 2010). However, it is not known if the TPR domain itself or the region encompassing residues 155–199 is the actual region for the intramolecular interaction in p67phox (Dang et al. 2003). It is possible that, although Rac does not induce TPR domain rearrangements in p67phox upon binding, there may be a degree of relaxation in this intramolecular interaction following Rac interaction that can expose the C-terminal phosphorylation site.
PEX5/Pex5p
PEX5 is a seven TPR unit-containing receptor that functions to translocate newly synthesised and sometimes functional peroxisomal enzymes to their correct subcellular compartments within the peroxisomal matrix. Via its C-terminal TPR domain, PEX5 recognises protein targets displaying an accessible C-terminal signal peptide, –SKL or conserved variants thereof, also called the type 1 peroxisomal targeting signal (PTS1) motif. The N-terminal part of PEX5 hosts most of the interaction sites for other peroxins that are important for docking of the target-bound receptor at the peroxisomal membrane and subsequent translocation and recycling events, supporting a model for the involvement of PEX5 in all the major steps of target translocation through the formation of multi-protein complexes. This region of PEX5 may also function to import PEX5-dependent targets by a PTS1-independent mechanism (Brocard and Hartig 2006; Stanley and Wilmanns 2006; Stanley et al. 2007).
Although reports of TPR crystal structures show the TPR units to pack in tandem clusters, forming a single domain, two reports of the TPR domain of PEX5 show two TPR clusters derived from TPR motifs 1–3 to 5–7 arranged into a ring-like structure with the non-canonical TPR4 acting as a bridge between the two clusters, allowing them to form a single binding site (Gatto et al. 2000; Stanley et al. 2006) (Fig. 2d). TPR7 is followed by a ‘7C-loop’, which in turn links to a C-terminal helical bundle that may act as a secondary binding site to the TPR domain and perform a peroxisomal protein sorting role (Stanley et al. 2006).
Determination of the PEX5 receptor structure in complex with a pentapeptide containing a canonical PTS1 has revealed not only an alternative TPR domain conformation but also a novel mode of TPR recognition (Gatto et al. 2000). The TPR cluster derived from TPRs 5 to 7 interacted with the backbone of the peptide similar to the interaction between Hop and –EEVD peptides (Scheufler et al. 2000). Recognition of the peptide by the N-terminal TPR cluster, derived from TPRs 1 to 3, is performed by the intra-repeat loops that face the ligand interface in the C-terminal TPR cluster, thus the peptide is completely encompassed by the ring-like TPR domain (Gatto et al. 2000). A closer structural investigation of Trypanosoma brucei Pex5 indicated recognition of the C-terminal leucine of the PTS1 motif is specific and conserved, and other interaction points occur between the PTS1 peptide main chain and up to five invariant asparagine side chains in the receptor (Sampathkumar et al. 2008).
The conformation of the PEX5 receptor when bound to either a PTS1-containing peptide or a full-length binding partner, such as sterol carrier protein 2 (SCP2), is similar, but analysis of the ligand-free receptor has revealed that structural changes occur within the TPR domain of the receptor which are induced by ligand binding, indicating the PTS1 signal is sufficient to induce this conformational transition. In the presence of a target, the conformation of the TPR domain changes from an ‘open’ to a ‘closed’ structure with the 7C-loop, which has a role in target recognition, associating with TPR1 and establishing the closed conformation (Stanley et al. 2006). A partially resolved structure for TPR4 suggested that it might form a continuous helix rather than the usual TPR fold, and this segment was originally thought to act as a hinge allowing the two TPR clusters to form the target binding site (Gatto et al. 2000). The recent molecular structure of the PEX5/SCP2 complex has questioned this role for TPR4 and suggested that the closing of the TPR domain upon SCP2 binding is a result of folding changes in TPRs 5 and 6 (Stanley et al. 2006).
Despite the unique conformation of the TPR domain in PEX5, the non-canonical structure and flexible nature of the TPR motif is not novel. Kumar and co-workers described the structure of the first TPR cluster in the T. brucei PEX5 homologue to have an alternative fold for the TPR3 helix (Kumar et al. 2001). Rather than forming the typical anti-parallel hairpin, the motif has ‘sprung out’ to form an extended continuous helix, which was also reported for TPR2 of the tetragonal crystal form of CyP40 (Taylor et al. 2001). An independent study has provided evidence that the tetragonal crystal form of CyP40 may not exist in solution and that it more likely adopts the monoclinic form where each TPR unit forms the hairpin (Taylor et al. 2001; Onuoha et al. 2008), but this does not rule out the fact that continuous helical structures from the same polypeptide can exist in different conformations. Mouse CHIP was reported to exist as a dimer with the two monomers having different conformations. In the elongated monomer, the seventh helix that follows the TPR domain is a straight ~50 residue helix, while this helix in the compact monomer is broken into two perpendicular helices of equivalent length joined by a short loop, with these two distinct regions forming part of the dimer interface (Zhang et al. 2005). However, this may simply represent a snap shot in time in the life of the CHIP protein, with the suggestion that this flexible region in both monomers in human CHIP may be continuously alternating between folded and extended helices in solution (Graf et al. 2010). This rearrangement of the helix that packs against and then extends beyond the third TPR motif may be more pronounced for FKBP51 with influences on Hsp90 interaction. Analysis of residues downstream of the charge-Y motif in FKBP51 and FKBP52 revealed this segment to enhance and moderate their interaction with Hsp90, respectively (Cheung-Flynn et al. 2003). The authors proposed these differences may be due to a fold-back or ‘jack-knife’ (Kumar et al. 2001) rearrangement of the final helix in FKBP51 to become part of the core TPR domain. It is possible an initial association with Hsp90 may induce the conformational change in the helix, enhancing FKBP51 interaction with the chaperone, but this alteration is not induced in FKBP52. It has been suggested that TPR motifs that are able to extend and fold back may lack a residue with an αL conformation in intra-TPR helix loops, which may allow for alternative TPR motif arrangements (Sampathkumar et al. 2008).
The functional role of the TPR domain in PEX5 appears to exclude an important docking event in the peroxisomal protein translocation process, with the TPR domain being reported to have no role in recognition of the peroxisome membrane-associated protein Pex14 (Gatto et al. 2000; Stanley et al. 2006). This is further supported by an extended, partially unfolded Pex5 N-terminal region being retained upon cargo binding and Pex14 interaction compared to the free receptor, where Pex14 binds the N terminus of Pex5 (Shiozawa et al. 2009). However, this does not exclude the possibility of the Pex5 TPR domain still acting as a scaffold for other translocation machinery components at the membrane once the receptor has bound to Pex14 (Gatto et al. 2000).
Conclusions
The TPR motif is a helical structural unit that, when packed in tandem with additional TPR units, plays a role in protein–protein interactions leading to the formation of multi-protein complexes eliciting varying biological roles. Although accommodation of a target peptide into a channel formed by a TPR domain is a well-defined mode of interaction, it is however not the only mode of target recognition by a TPR domain, which not only expands the plethora of TPR domain-interacting partners but also the cellular processes TPR domains are involved in, including processes linked to disease. Studies have identified mutations within PEX5 TPR motifs in some patients diagnosed with peroxisomal biogenesis disorder (Carvalho et al. 2007; Ebberink et al. 2009), and it would be interesting to see if TPR domain mutations are implicated in steroid-based disease and host defence.
The multiple TPR cochaperones of the Hsp70/Hsp90 molecular chaperone system, together with p67phox and PEX5, have served to illustrate the diverse activities mediated by TPR proteins with a varied number of tandem TPR structural motifs. Much of the focus of this review has centred on the regulation of steroid receptor function by TPR cochaperones within receptor–Hsp90 complexes. While steroid receptors are the best characterised Hsp90 client proteins, there is increasing recognition that the TPR cochaperones may also play important roles in pathways associated with other Hsp90 substrates. For example, CyP40 has recently been reported to regulate microRNA activity in plants, possibly by chaperoning Argonaute 1 (Smith et al. 2009). The observed functional dependence of the mammalian counterpart, Argonaute 2, on Hsp90 provides an avenue for regulatory control of Argonaute–Hsp90 complexes by CyP40 and other TPR cochaperones (Maniataki and Mourelatos 2005; Pare et al. 2009).
The apparent specificity of EEVD peptide binding to the TPR pocket of HSP70/Hsp90 cochaperones offers the potential for molecular therapies targeting individual TPR cochaperones. In the case of the immunophilins, CyP40, FKBP51 and FKBP52, there is the prospect of cyclosporin A and FK506 modulation of Hsp90 client protein function in clinically relevant pathways, including the inhibition of androgen receptor-dependent signalling in prostate cancer (Periyasamy et al. 2010). In this regard, the use of small molecules tailored to block the immunophilin drug-binding pocket without immunosuppressive effects appears particularly attractive.
Further structural studies are required to enhance our understanding of how TPR cochaperones regulate Hsp70/Hsp90 function at the molecular level. There is now compelling evidence that structural features within Hsp70, Hsp90 and Hop, lying outside of the critical EEVD peptide motifs and TPR domains, contribute significantly to functionally relevant protein–protein interactions. Three-dimensional structures derived from the assembly of full-length proteins for Hop with both Hsp70 and Hsp90 are likely to reveal the core elements involved in the coordinate control by Hop of Hsp70 and Hsp90 function.
Acknowledgements
Research in the authors’ laboratory is supported by the National Health & Medical Research Council of Australia and the National Breast Cancer Foundation. The authors are also grateful to Carmel Cluning and Danny Mok for assistance in preparing the manuscript.
References
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, Segal AW, Lim L. Cryptic Rac-binding and p21Cdc42HS/Rac-activated kinase phosphorylation sites of NADPH oxidase component p67phox. J Biol Chem. 1998;273:15693–15701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- Allan RK, Mok D, Ward BK, Ratajczak T. Modulation of chaperone function and cochaperone interaction by novobiocin in the C-terminal domain of Hsp90: evidence that coumarin antibiotics disrupt Hsp90 dimerization. J Biol Chem. 2006;281:7161–7171. doi: 10.1074/jbc.M512406200. [DOI] [PubMed] [Google Scholar]
- Andrade MA, Perez-Iratxeta C, Ponting CP. Protein repeats: structures, functions, and evolution. J Struct Biol. 2001;134:117–131. doi: 10.1006/jsbi.2001.4392. [DOI] [PubMed] [Google Scholar]
- Angeletti PC, Walker D, Panganiban AT. Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity. Cell Stress Chaperones. 2002;7:258–268. doi: 10.1379/1466-1268(2002)007<0258:sgrpvp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin L-Y, Patterson C. Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions. Mol Cell Biol. 1999;19:4535–4545. doi: 10.1128/mcb.19.6.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Periyasamy S, Wolf IM, Hinds TD, Yong W, Shou W, Sanchez ER. Control of glucocorticoid and progesterone receptor subcellular localization by the ligand-binding domain is mediated by distinct interactions with tetratricopeptide repeat proteins. Biochemistry. 2008;47:10471–10480. doi: 10.1021/bi8011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barent RL, Nair SC, Carr DC, Ruan Y, Rimerman RA, Fulton J, Zhang Y, Smith DF. Analysis of FKBP51/FKBP52 chimeras and mutants for Hsp90 binding and association with progesterone receptor complexes. Mol Endocrinol. 1998;12:342–354. doi: 10.1210/mend.12.3.0075. [DOI] [PubMed] [Google Scholar]
- Barford D. Molecular mechanisms of the protein serine/threonine phosphatases. Trends Biochem Sci. 1996;21:407–412. doi: 10.1016/s0968-0004(96)10060-8. [DOI] [PubMed] [Google Scholar]
- Bimston D, Song J, Winchester D, Takayama S, Reed JC, Morimoto RI. BAG-1, a negative regulator of Hsp70 chaperone activity, uncouples nucleotide hydrolysis from substrate release. EMBO J. 1998;17:6871–6878. doi: 10.1093/emboj/17.23.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand P-O, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon A-M, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin A-C, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G. A physical and functional map of the human TNF-a/NF-kB signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- Brocard C, Hartig A. Peroxisome targeting signal 1: is it really a simple tripeptide? Biochim Biophys Acta, Mol Cell Res. 2006;1763:1565–1573. doi: 10.1016/j.bbamcr.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Buchanan G, Ricciardelli C, Harris JM, Prescott J, Yu ZC-L, Jia L, Butler LM, Marshall VR, Scher HI, Gerald WL, Coetzee GA, Tilley WD. Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein a. Cancer Res. 2007;67:10087–10096. doi: 10.1158/0008-5472.CAN-07-1646. [DOI] [PubMed] [Google Scholar]
- Callahan MA, Handley MA, Lee Y-H, Talbot KJ, Harper JW, Panganiban AT. Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family. J Virol. 1998;72:5189–5197. doi: 10.1128/jvi.72.6.5189-5197.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, Renoir JM, Lebeau MC, Massol N, Burny A, Baulieu EE, Mornon JP. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. PNAS. 1992;89:6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrello A, Ingley E, Minchin RF, Tsai S, Ratajczak T. The common tetratricopeptide repeat acceptor site for steroid receptor-associated immunophilins and Hop is located in the dimerization domain of Hsp90. J Biol Chem. 1999;274:2682–2689. doi: 10.1074/jbc.274.5.2682. [DOI] [PubMed] [Google Scholar]
- Carrigan PE, Sikkink LA, Smith DF, Ramirez-Alvarado M. Domain:domain interactions within Hop, the Hsp70/Hsp90 organizing protein, are required for protein stability and structure. Protein Sci. 2006;15:522–532. doi: 10.1110/ps.051810106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Grou CP, Pinto MP, Alencastre IS, Costa-Rodrigues J, Fransen M, Sá-Miranda C, Azevedo JE. Functional characterization of two missense mutations in Pex5p—C11S and N526K. Biochim Biophys Acta, Mol Cell Res. 2007;1773:1141–1148. doi: 10.1016/j.bbamcr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Chadli A, Graham JD, Abel MG, Jackson TA, Gordon DF, Wood WM, Felts SJ, Horwitz KB, Toft D. GCUNC-45 is a novel regulator for the progesterone receptor/Hsp90 chaperoning pathway. Mol Cell Biol. 2006;26:1722–1730. doi: 10.1128/MCB.26.5.1722-1730.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadli A, Bruinsma ES, Stensgard B, Toft D. Analysis of Hsp90 cochaperone interactions reveals a novel mechanism for TPR protein recognition. Biochemistry. 2008;47:2850–2857. doi: 10.1021/bi7023332. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Wang L, Armstrong DL, Rossie S. Activated Rac1 GTPase translocates protein phosphatase 5 to the cell membrane and stimulates phosphatase activity in vitro. J Biol Chem. 2010;285:3872–3882. doi: 10.1074/jbc.M109.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MX, Cohen PTW. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- Chen S, Smith DF. Hop as an adaptor in the heat shock protein 70 (Hsp70) and Hsp90 chaperone machinery. J Biol Chem. 1998;273:35194–35200. doi: 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- Chen M-S, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- Chen S, Prapapanich V, Rimerman RA, Honore B, Smith DF. Interactions of p60, a mediator of progesterone receptor assembly, with heat shock proteins Hsp90 and Hsp70. Mol Endocrinol. 1996;10:682–693. doi: 10.1210/mend.10.6.8776728. [DOI] [PubMed] [Google Scholar]
- Chen S, Sullivan WP, Toft DO, Smith DF. Differential interactions of p23 and the TPR-containing proteins Hop, Cyp40, FKBP52 and FKBP51 with Hsp90 mutants. Cell Stress Chaperones. 1998;3:118–129. doi: 10.1379/1466-1268(1998)003<0118:diopat>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung-Flynn J, Roberts PJ, Riggs DL, Smith DF. C-terminal sequences outside the tetratricopeptide repeat domain of FKBP51 and FKBP52 cause differential binding to Hsp90. J Biol Chem. 2003;278:17388–17394. doi: 10.1074/jbc.M300955200. [DOI] [PubMed] [Google Scholar]
- Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. doi: 10.1210/me.2005-0071. [DOI] [PubMed] [Google Scholar]
- Cohen PTW. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Sci. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3:93. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- Cox MB, Riggs DL, Hessling M, Schumacher F, Buchner J, Smith DF. FK506-binding protein 52 phosphorylation: a potential mechanism for regulating steroid hormone receptor activity. Mol Endocrinol. 2007;21:2956–2967. doi: 10.1210/me.2006-0547. [DOI] [PubMed] [Google Scholar]
- Cyr DM, Höhfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- Cziepluch C, Kordes E, Poirey R, Grewenig A, Rommelaere J, Jauniaux J-C. Identification of a novel cellular TPR-containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1. J Virol. 1998;72:4149–4156. doi: 10.1128/jvi.72.5.4149-4156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea LD, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655–662. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Dang PM-C, Morel F, Gougerot-Pocidalo M-A, Benna JE. Phosphorylation of the NADPH oxidase component p67phox by ERK2 and P38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003;42:4520–4526. doi: 10.1021/bi0205754. [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PTW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein–protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash AB, Orrico FC, Ness SA. The EVES motif mediates both intermolecular and intramolecular regulation of c-Myb. Genes Dev. 1996;10:1858–1869. doi: 10.1101/gad.10.15.1858. [DOI] [PubMed] [Google Scholar]
- Davies TH, Sánchez ER. FKBP52. Int J Biochem Cell Biol. 2005;37:42–47. doi: 10.1016/j.biocel.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Davies TH, Ning Y-M, Sanchez ER. A new first step in activation of steroid receptors. Hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem. 2002;277:4597–4600. doi: 10.1074/jbc.C100531200. [DOI] [PubMed] [Google Scholar]
- Denny WB, Valentine DL, Reynolds PD, Smith DF, Scammell JG. Squirrel monkey immunophilin FKBP51 is a potent inhibitor of glucocorticoid receptor binding. Endocrinology. 2000;141:4107–4113. doi: 10.1210/endo.141.11.7785. [DOI] [PubMed] [Google Scholar]
- Denny WB, Prapapanich V, Smith DF, Scammell JG. Structure–function analysis of Squirrel monkey FK506-binding protein 51, a potent inhibitor of glucocorticoid receptor activity. Endocrinology. 2005;146:3194–3201. doi: 10.1210/en.2005-0027. [DOI] [PubMed] [Google Scholar]
- Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- Dittmar KD, Hutchison KA, Owens-Grillo JK, Pratt WB. Reconstitution of the steroid receptor·Hsp90 heterocomplex assembly system of rabbit reticulocyte lysate. J Biol Chem. 1996;271:12833–12839. doi: 10.1074/jbc.271.22.12833. [DOI] [PubMed] [Google Scholar]
- Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta, Mol Cell Res. 1999;1450:191–231. doi: 10.1016/s0167-4889(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Durand D, Vivès C, Cannella D, Pérez J, Pebay-Peyroula E, Vachette P, Fieschi F. NADPH oxidase activator p67phox behaves in solution as a multidomain protein with semi-flexible linkers. J Struct Biol. 2010;169:45–53. doi: 10.1016/j.jsb.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Dutta S, Tan Y-J. Structural and functional characterization of human SGT and its interaction with Vpu of the human immunodeficiency virus type 1. Biochemistry. 2008;47:10123–10131. doi: 10.1021/bi800758a. [DOI] [PubMed] [Google Scholar]
- Ebberink MS, Mooyer PAW, Koster J, Dekker CJM, Eyskens FJM, Dionisi-Vici C, Clayton PT, Barth PG, Wanders RJA, Waterham HR. Genotype–phenotype correlation in PEX5-deficient peroxisome biogenesis defective cell lines. Hum Mutat. 2009;30:93–98. doi: 10.1002/humu.20833. [DOI] [PubMed] [Google Scholar]
- Frydman J, Höhfeld J. Chaperones get in touch: the Hip–Hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/s0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Radanyi C, Renoir J-M, Housley PR, Pratt WB. Evidence that the peptidylprolyl isomerase domain of the Hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem. 2001;276:14884–14889. doi: 10.1074/jbc.M010809200. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJM, Chinkers M, Radanyi C, Renoir J-M, Zhang M, Pratt WB. Binding of Hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G. The Hsp90–FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol. 2010;30:1285–1298. doi: 10.1128/MCB.01190-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LI, Ghini AA, Piwien Pilipuk G, Galigniana MD. Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry. 2007;46:14044–14057. doi: 10.1021/bi701372c. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Geisbrecht BV, Gould SJ, Berg JM. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nat Struct Biol. 2000;9:788–788. doi: 10.1038/81930. [DOI] [PubMed] [Google Scholar]
- Gebauer M, Zeiner M, Gehring U. Proteins interacting with the molecular chaperone Hsp70/Hsc70: physical associations and effects on refolding activity. FEBS Lett. 1997;417:109–113. doi: 10.1016/s0014-5793(97)01267-2. [DOI] [PubMed] [Google Scholar]
- Gentile S, Darden T, Erxleben C, Romeo C, Russo A, Martin N, Rossie S, Armstrong DL. Rac GTPase signaling through the PP5 protein phosphatase. PNAS. 2006;103:5202–5206. doi: 10.1073/pnas.0600080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Avellino R, Ferraro P, Romano S, Corcione N, Romano MF. Rapamycin antagonizes NF-kB nuclear translocation activated by TNF-a in primary vascular smooth muscle cells and enhances apoptosis. Am J Physiol Heart Circ Physiol. 2006;290:H2459–H2465. doi: 10.1152/ajpheart.00750.2005. [DOI] [PubMed] [Google Scholar]
- Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991;16:173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Golden T, Swingle M, Honkanen R. The role of serine/threonine protein phosphatase type 5 (PP5) in the regulation of stress-induced signaling networks and cancer. Cancer Metastasis Rev. 2008;27:169–178. doi: 10.1007/s10555-008-9125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf C, Stankiewicz M, Nikolay R, Mayer MP. Insights into the conformational dynamics of the E3 Ubiquitin ligase CHIP in complex with chaperones and E2 enzymes. Biochemistry. 2010;49:2121–2129. doi: 10.1021/bi901829f. [DOI] [PubMed] [Google Scholar]
- Gregory LB, Michael L. The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. BioEssays. 1999;21:932–939. doi: 10.1002/(SICI)1521-1878(199911)21:11<932::AID-BIES5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Grizot S, Fieschi F, Dagher M-C, Pebay-Peyroula E. The active N-terminal region of p67phox : structure at 1.8 Å resolution and biochemical characterizations of the A128V mutant implicated in chronic granulomatous disease. J Biol Chem. 2001;276:21627–21631. doi: 10.1074/jbc.M100893200. [DOI] [PubMed] [Google Scholar]
- Han C-H, Freeman JLR, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase: identification of an activation domain in p67phox. J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- Hinds TD, Jr, Sánchez ER. Protein phosphatase 5. Int J Biochem Cell Biol. 2008;40:2358–2362. doi: 10.1016/j.biocel.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Kinoshita N, Morikawa K, Yanagida M. Snap helix with knob and hole: essential repeats in S. pombe nuclear protein nuc2+ Cell. 1990;60:319–328. doi: 10.1016/0092-8674(90)90746-2. [DOI] [PubMed] [Google Scholar]
- Höhfeld J, Jentsch S. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 1997;16:6209–6216. doi: 10.1093/emboj/16.20.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J, Minami Y, Hartl F-U. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell. 1995;83:589–598. doi: 10.1016/0092-8674(95)90099-3. [DOI] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI. Three-dimensional structure of the armadillo repeat region of b-catenin. Cell. 1997;90:871–882. doi: 10.1016/s0092-8674(00)80352-9. [DOI] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Höhfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase. Identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276:42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- Kajander T, Sachs JN, Goldman A, Regan L. Electrostatic interactions of Hsp-organizing protein tetratricopeptide domains with Hsp70 and Hsp90: computational analysis and protein engineering. J Biol Chem. 2009;284:25364–25374. doi: 10.1074/jbc.M109.033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Mikol V, Taylor P, Walkinshaw DM. X-ray structures and analysis of 11 cyclosporin derivatives complexed with cyclophilin A. J Mol Biol. 1998;283:435–449. doi: 10.1006/jmbi.1998.2108. [DOI] [PubMed] [Google Scholar]
- Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- Kosano H, Stensgard B, Charlesworth MC, McMahon N, Toft D. The assembly of progesterone receptor–Hsp90 complexes using purified proteins. J Biol Chem. 1998;273:32973–32979. doi: 10.1074/jbc.273.49.32973. [DOI] [PubMed] [Google Scholar]
- Kumar A, Roach C, Hirsh IS, Turley S, deWalque S, Michels PAM, Hol WGJ. An unexpected extended conformation for the third TPR motif of the peroxin PEX5 from Trypanosoma brucei. J Mol Biol. 2001;307:271–282. doi: 10.1006/jmbi.2000.4465. [DOI] [PubMed] [Google Scholar]
- Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- Lapouge K, Smith SJM, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Structure of the TPR domain of p67phox in complex with Rac·GTP. Mol Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- Lassle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1. Characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- Leverson JD, Ness SA. Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol Cell. 1998;1:203–211. doi: 10.1016/s1097-2765(00)80021-0. [DOI] [PubMed] [Google Scholar]
- Li J, Mahajan A, Tsai M-D. Ankyrin repeat: a unique motif mediating protein–protein interactions. Biochemistry. 2006;45:15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- Liou S-T, Wang C. Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch Biochem Biophys. 2005;435:253–263. doi: 10.1016/j.abb.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Liu D, Bienkowska J, Petosa C, Collier RJ, Fu H, Liddington R. Crystal structure of the zeta isoform of the 14-3-3 protein. Nature. 1995;376:191–194. doi: 10.1038/376191a0. [DOI] [PubMed] [Google Scholar]
- Main ERG, Lowe AR, Mochrie SGJ, Jackson SE, Regan L. A recurring theme in protein engineering: the design, stability and folding of repeat proteins. Curr Opin Struct Biol. 2005;15:464–471. doi: 10.1016/j.sbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Sharma S, Petropoulos L, Lin R, Hiscott J. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity. 2000;12:129–140. doi: 10.1016/s1074-7613(00)80166-1. [DOI] [PubMed] [Google Scholar]
- Maniataki E, Mourelatos Z. Human mitochondrial tRNAMet is exported to the cytoplasm and associates with the Argonaute 2 protein. RNA. 2005;11:849–852. doi: 10.1261/rna.2210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- Massol N, Lebeau M-C, Renoir J-M, Faber LE, Baulieu E-E. Rabbit FKBP59-heat shock protein binding immunophillin (HBI) is a calmodulin binding protein. Biochem Biophys Res Commun. 1992;187:1330–1335. doi: 10.1016/0006-291x(92)90448-t. [DOI] [PubMed] [Google Scholar]
- McLaughlin SH, Smith HW, Jackson SE. Stimulation of the weak ATPase activity of human Hsp90 by a client protein. J Mol Biol. 2002;315:787–798. doi: 10.1006/jmbi.2001.5245. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Chambraud B, Radanyi C, Leclerc J, Lebeau M-C, Renoir J-M, Shirai R, Catelli M-G, Yahara I, Baulieu E-E. Phosphorylation of the immunosuppressant FK506-binding protein FKBP52 by casein kinase II: regulation of Hsp90-binding activity of FKBP52. PNAS. 1997;94:14500–14505. doi: 10.1073/pnas.94.26.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok D, Allan RK, Carrello A, Wangoo K, Walkinshaw MD, Ratajczak T. The chaperone function of cyclophilin 40 maps to a cleft between the prolyl isomerase and tetratricopeptide repeat domains. FEBS Lett. 2006;580:2761–2768. doi: 10.1016/j.febslet.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, McLain K, Kier AB, Swerdlow SH, Schreiner CM, Miller TA, Pietryga DW, Scott WJ, Potter SS. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- Murata S, Minami Y, Minami M, Chiba T, Tanaka K. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep. 2001;2:1133–1138. doi: 10.1093/embo-reports/kve246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson GM, Huffman H, Smith DF. Comparison of the carboxy-terminal DP-repeat region in the co-chaperones Hop and Hip. Cell Stress Chaperones. 2003;8:125–133. doi: 10.1379/1466-1268(2003)008<0125:cotcdr>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness SA. The Myb oncoprotein: regulating a regulator. Biochim Biophys Acta Rev Cancer. 1996;1288:F123–F139. doi: 10.1016/s0304-419x(96)00027-3. [DOI] [PubMed] [Google Scholar]
- Ni L, Yang C-S, Gioeli D, Frierson H, Toft DO, Paschal BM. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 2010;30:1243–1253. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunuga OO, Hornby JA, Bies C, Zimmermann R, Pugh DJ, Blatch GL. Tetratricopeptide repeat motif-mediated Hsc70-mSTI1 interaction. J Biol Chem. 2003;278:6896–6904. doi: 10.1074/jbc.M206867200. [DOI] [PubMed] [Google Scholar]
- Onuoha SC, Coulstock ET, Grossmann JG, Jackson SE. Structural studies on the co-chaperone Hop and its complexes with Hsp90. J Mol Biol. 2008;379:732–744. doi: 10.1016/j.jmb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Pare JM, Tahbaz N, Lopez-Orozco J, LaPointe P, Lasko P, Hobman TC. Hsp90 regulates the function of Argonaute 2 and its recruitment to stress granules and P-bodies. Mol Biol Cell. 2009;20:3273–3284. doi: 10.1091/mbc.E09-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periyasamy S, Hinds T, Jr, Shemshedini L, Shou W, Sanchez ER. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene. 2010;29:1691–1701. doi: 10.1038/onc.2009.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkl F, Buchner J. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and CyP40. J Mol Biol. 2001;308:795–806. doi: 10.1006/jmbi.2001.4595. [DOI] [PubMed] [Google Scholar]
- Ponting CP. Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci. 1996;5:2353–2357. doi: 10.1002/pro.5560051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Nair SC, Rimerman RA, Smith DF. Molecular cloning of human p48, a transient component of progesterone receptor complexes and an Hsp70-binding protein. Mol Endocrinol. 1996;10:420–431. doi: 10.1210/mend.10.4.8721986. [DOI] [PubMed] [Google Scholar]
- Prapapanich V, Chen S, Smith DF. Mutation of Hip’s carboxy-terminal region inhibits a transitional stage of progesterone receptor assembly. Mol Cell Biol. 1998;18:944–952. doi: 10.1128/mcb.18.2.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the Hsp90/Hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Galigniana MD, Harrell JM, DeFranco DB. Role of Hsp90 and the Hsp90-binding immunophilins in signalling protein movement. Cell Signal. 2004;16:857–872. doi: 10.1016/j.cellsig.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Prodromou C, Siligardi G, O'Brien R, Woolfson DN, Regan L, Panaretou B, Ladbury JE, Piper PW, Pearl LH. Regulation of Hsp90 ATPase activity by tetratricopeptide repeat (TPR)-domain co-chaperones. EMBO J. 1999;18:754–762. doi: 10.1093/emboj/18.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radanyi C, Chambraud B, Baulieu EE. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. PNAS. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey AJ, Chinkers M. Identification of potential physiological activators of protein phosphatase 5. Biochemistry. 2002;41:5625–5632. doi: 10.1021/bi016090h. [DOI] [PubMed] [Google Scholar]
- Ramsey AJ, Russell LC, Chinkers M. C-terminal sequences of Hsp70 and Hsp90 as non-specific anchors for tetratricopeptide repeat (TPR) proteins. Biochem J. 2009;423:411–419. doi: 10.1042/BJ20090543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A. Cyclophilin 40 (CyP-40), mapping of its Hsp90 binding domain and evidence that FKBP52 competes with CyP-40 for Hsp90 binding. J Biol Chem. 1996;271:2961–2965. doi: 10.1074/jbc.271.6.2961. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Hlaing J, Brockway MJ, Hahnel R. Isolation of untransformed bovine estrogen receptor without molybdate stabilization. J Steroid Biochem. 1990;35:543–553. doi: 10.1016/0022-4731(90)90197-z. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Carrello A, Minchin RF. Biochemical and calmodulin binding properties of estrogen receptor binding cyclophilin expressed in Escherichia coli. Biochem Biophys Res Commun. 1995;209:117–125. doi: 10.1006/bbrc.1995.1478. [DOI] [PubMed] [Google Scholar]
- Ratajczak T, Ward BK, Minchin RF. Immunophilin chaperones in steroid receptor signalling. Curr Top Med Chem. 2003;3:1348–1357. doi: 10.2174/1568026033451934. [DOI] [PubMed] [Google Scholar]
- Reynolds PD, Ruan Y, Smith DF, Scammell JG. Glucocorticoid resistance in the squirrel monkey is associated with overexpression of the immunophilin FKBP51. J Clin Endocrinol Metab. 1999;84:663–669. doi: 10.1210/jcem.84.2.5429. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Roberts PJ, Chirillo SC, Cheung-Flynn J, Prapapanich V, Ratajczak T, Gaber R, Picard D, Smith DF. The Hsp90-binding peptidylprolyl isomerase FKBP52 potentiates glucocorticoid signaling in vivo. EMBO J. 2003;22:1158–1167. doi: 10.1093/emboj/cdg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Cheung-Flynn J, Prapapanich V, Carrigan PE, Smith DF. Functional specificity of co-chaperone interactions with Hsp90 client proteins. Crit Rev Biochem Mol Biol. 2004;39:279–295. doi: 10.1080/10409230490892513. [DOI] [PubMed] [Google Scholar]
- Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LC, Whitt SR, Chen M-S, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem. 1999;274:20060–20063. doi: 10.1074/jbc.274.29.20060. [DOI] [PubMed] [Google Scholar]
- Sampathkumar P, Roach C, Michels PAM, Hol WGJ. Structural insights into the recognition of peroxisomal targeting signal 1 by Trypanosoma brucei Peroxin 5. J Mol Biol. 2008;381:867–880. doi: 10.1016/j.jmb.2008.05.089. [DOI] [PubMed] [Google Scholar]
- Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain–peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Shimamoto S, Kubota Y, Tokumitsu H, Kobayashi R. S100 proteins regulate the interaction of Hsp90 with Cyclophilin 40 and FKBP52 through their tetratricopeptide repeats. FEBS Lett. 2010;584:1119–1125. doi: 10.1016/j.febslet.2010.02.055. [DOI] [PubMed] [Google Scholar]
- Shiozawa K, Konarev PV, Neufeld C, Wilmanns M, Svergun DI. Solution structure of human Pex5·Pex14·PTS1 protein complexes obtained by small angle X-ray scattering. J Biol Chem. 2009;284:25334–25342. doi: 10.1074/jbc.M109.002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. Nuclear factor-κB activation: a question of life or death. J Biochem Mol Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Boguski MS, Goebl M, Hieter P. A repeating amino acid motif in CDC23 defines a family of proteins and a new relationship among genes required for mitosis and RNA synthesis. Cell. 1990;60:307–317. doi: 10.1016/0092-8674(90)90745-z. [DOI] [PubMed] [Google Scholar]
- Siligardi G, Hu B, Panaretou B, Piper PW, Pearl LH, Prodromou C. Co-chaperone regulation of conformational switching in the Hsp90 ATPase cycle. J Biol Chem. 2004;279:51989–51998. doi: 10.1074/jbc.M410562200. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen M-S, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor·Hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. PNAS. 2003;100:868–873. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner J, Sinclair C, Romeo C, Armstrong D, Charbonneau H, Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain to its identification as a homolog of protein phosphatase 5. J Biol Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- Smith DF. Dynamics of heat shock protein 90-progesterone receptor binding and the disactivation loop model for steroid receptor complexes. Mol Endocrinol. 1993;7:1418–1429. doi: 10.1210/mend.7.11.7906860. [DOI] [PubMed] [Google Scholar]
- Smith DF. Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones. 2004;9:109–121. doi: 10.1379/CSC-31.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Willmann MR, Wu G, Berardini TZ, Möller B, Weijers D, Poethig RS. Cyclophilin 40 is required for microRNA activity in Arabidopsis. PNAS. 2009;106:5424–5429. doi: 10.1073/pnas.0812729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WA, Wilmanns M. Dynamic architecture of the peroxisomal import receptor Pex5p. Biochim Biophys Acta, Mol Cell Res. 2006;1763:1592–1598. doi: 10.1016/j.bbamcr.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Stanley WA, Filipp FV, Kursula P, Schüller N, Erdmann R, Schliebs W, Sattler M, Wilmanns M. Recognition of a functional peroxisome type 1 target by the dynamic import receptor Pex5p. Mol Cell. 2006;24:653–663. doi: 10.1016/j.molcel.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley WA, Fodor K, Marti-Renom MA, Schliebs W, Wilmanns M. Protein translocation into peroxisomes by ring-shaped import receptors. FEBS Lett. 2007;581:4795–4802. doi: 10.1016/j.febslet.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Storey NM, O'Bryan JP, Armstrong DL. Rac and Rho mediate opposing hormonal regulation of the Ether-A-Go-Go-related potassium channel. Curr Biol. 2002;12:27–33. doi: 10.1016/s0960-9822(01)00625-x. [DOI] [PubMed] [Google Scholar]
- Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC. BAG-1 modulates the chaperone activity of Hsp70/Hsc70. EMBO J. 1997;16:4887–4896. doi: 10.1093/emboj/16.16.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Dornan J, Carrello A, Minchin RF, Ratajczak T, Walkinshaw MD. Two structures of cyclophilin 40: folding and fidelity in the TPR domains. Structure. 2001;9:431–438. doi: 10.1016/s0969-2126(01)00603-7. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. From the cover: cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. PNAS. 2005;102:14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzamarias D, Struhl K. Distinct TPR motifs of Cyc8 are involved in recruiting the Cyc8–Tup1 corepressor complex to differentially regulated promoters. Genes Dev. 1995;9:821–831. doi: 10.1101/gad.9.7.821. [DOI] [PubMed] [Google Scholar]
- Ward BK, Allan RK, Mok D, Temple SE, Taylor P, Dornan J, Mark PJ, Shaw DJ, Kumar P, Walkinshaw MD, Ratajczak T. A structure-based mutational analysis of cyclophilin 40 identifies key residues in the core tetratricopeptide repeat domain that mediate binding to Hsp90. J Biol Chem. 2002;277:40799–40809. doi: 10.1074/jbc.M207097200. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100:34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem. 2005;280:4609–4616. doi: 10.1074/jbc.M407498200. [DOI] [PubMed] [Google Scholar]
- Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, Ye S, Bartlam M, Shen B, Rao Z. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. PNAS. 2004;101:8348–8353. doi: 10.1073/pnas.0305969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, Gamblin SJ. Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature. 1995;376:188–191. doi: 10.1038/376188a0. [DOI] [PubMed] [Google Scholar]
- Yang J, Roe SM, Cliff MJ, Williams MA, Ladbury JE, Cohen PTW, Barford D. Molecular basis for TPR domain-mediated regulation of protein phosphatase 5. EMBO J. 2005;24:1–10. doi: 10.1038/sj.emboj.7600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20:2682–2694. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sanchez ER, Shou W. Essential role for co-chaperone FKBP52 but not FKBP51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. doi: 10.1074/jbc.M609360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ET, Saario J, Kacherovsky N, Chao A, Sloan JS, Dombek KM. Characterization of a p53-related activation domain in Adr1p that is sufficient for ADR1-dependent gene expression. J Biol Chem. 1998;273:32080–32087. doi: 10.1074/jbc.273.48.32080. [DOI] [PubMed] [Google Scholar]
- Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH. Chaperoned ubiquitylation—crystal structures of the CHIP U Box E3 ubiquitin ligase and a CHIP–Ubc13–Uev1a complex. Mol Cell. 2005;20:525–538. doi: 10.1016/j.molcel.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wu J, Zhong L, Srivastava A. Adeno-associated virus 2-mediated gene transfer: role of a cellular serine/threonine protein phosphatase in augmenting transduction efficiency. Gene Ther. 2007;14:545–550. doi: 10.1038/sj.gt.3302886. [DOI] [PubMed] [Google Scholar]