Abstract
Protein kinase and phosphatase signaling cascade, coupled with other post-translational modifications, orchestrates temporal order of various events during cell division. Among the many mitotic kinases, Polo-like kinase 1 (PLK1) as a key regulator, participates in regulating mitosis from mitotic entry to cytokinesis. The advancement in optical reporter engineering and the recent development of specific chemical probes enable us to visualize spatiotemporal gradient of kinase activity at nano-scale. One of such tools is FRET-based optic sensor that allows us to delineate the PLK1 activity in space and time. In this review, we address the interrelationships between PLK1 and other protein kinases/phosphatases, as well as the crosstalk between PLK1 phosphorylation and ubiquitination during cell division. In particular, we discuss the molecular mechanisms and steps underlying PLK1 kinase priming, activation and turn-off during cell division.
Keywords: Polo-like kinase 1, Protein kinase and phosphatase signaling cascade, Ubiquitination, Crosstalk, Mitosis
1. Introduction
The accurate and equal distribution of duplicated genomes to two daughter cells is the fundamental mission of mitosis. Errors in mitosis result in genetic instability, which is a common feature of cancer. Therefore, molecular delineation of mitotic regulators has become one of the most active fields in biological researches in the last two decades.
The feasibility and ease of genetic screens in yeast and Drosophila lead to the identification of many of the key mitotic regulators, among which include polo kinase [1,2]. Subsequent researches revealed that polo kinase is well conserved in different species during evolution: Cdc5 in budding yeast, Polo in Drosophila, Plx1 in Xenopus and Polo-like kinases in mammals. In human cells there are 4 Polo-like kinases: PLK1, PLK2, PLK3 and PLK4. PLK1 is most closely related to yeast Cdc5 and Drosophila Polo, and has been well characterized as a key mitotic regulator [3]; PLK2 and PLK3 are suggested to be involved in G1-S checkpoint [4,5]; while PLK4 has a pivotal role in controlling centriole duplication in interphase [6,7]. Here we focus on PLK1, as it is the most prominent form of Polo kinase in human cells.
PLK1, as well as its orthologs, has an amino-terminal Ser/Thr kinase domain followed by carboxy-terminal Polo-box domains (PBD). Like many other protein kinases, the activity of PLK1 is controlled by phosphorylation within the T-loop region, which connects protein kinase subdomains VII and VIII [8]. In particular, mutation of T210 inside the T-loop to negatively charged Asp residue enhances the activity of PLK1. Moreover, sequence alignment indicates that T210 is highly conserved in all the Polo kinase subfamily [3], which suggests the possibility of existing a conserved regulatory mechanism in Polo kinase activation. The carboxyl-terminal PBD bestows the cell another potent means of spatially regulating PLK1 via phosphorylation-regulated protein–protein interactions. Milestone studies on PBD were performed by Elia and colleagues in 2003 [9,10]. They elegantly characterized PBD as a phospho-peptide binding domain with the optimal binding motif of S-[pT/pS]-[P/X], further crystallization study indicated that the phospho-peptide binds along a conserved, positively charged cleft located at the edge of the Polo-box interface. This feature enables PLK1 parking inside the cell in response to another kinase (such as CDK1) phosphorylation.
Mounting evidence demonstrates that PLK1 plays a pivotal role in regulating cell cycle division in addition to CDK1. It was reported that PLK1 is involved in activation of Cdc25 [11], as well as MYT1 inhibition [12] and Wee1 degradation [13]. Consistently, cells that lack of PLK1 display delay of mitotic entry [14]. Depletion of PLK1 by small interference RNA [14] or inhibition of PLK1 activity by chemical inhibitors [15] both lead to the formation of monopolar spindle and checkpoint-dependent mitotic arrest. Recently, by taking advantage of the chemical inhibitor, researchers have been able to uncover the novel roles of PLK1 in anaphase and telophase, including spindle elongation and cleavage furrow ingression [16–19]. An outstanding question is how PLK1 accomplishes these tasks efficiently and correctly. Accumulating evidence suggests that PLK1 cooperates with other mitotic kinases or other signaling proteins to fulfill its calling. In this review, we brief the relationship between PLK1 and the other mitotic kinases, with respect to the coordination between PLK1 and phosphatases. In addition, we discuss the crosstalk between PLK1 and ubiquitination pathway. Lastly, we highlight other signaling molecules, especially kinases of Aurora family, in coordinating the activity of PLK1 at the early stages of mitosis.
2. Coordination between PLK1 and other mitotic kinases
Protein phosphorylation is one of the fundamental regulating mechanisms in mitosis. Instead of single site modification, many mitotic proteins are modified at multiple sites by different kinases. The coordinated action of mitotic kinases increases the complexity and flexibility of mitotic orchestra.
2.1. Cdk1 orchestrates the spatial–temporal dynamics of PLK1
The function of PLK1 in early mitosis as an activator of Cdk1 has been reported previously [20], although the necessity of PLK1 in mitotic entry has been doubt as cell can still enter mitosis in PLK1 deficient cells [21]. Conversely, accumulating evidence suggests Cdk1 acts as a prime kinase and regulates the spatial–temporal dynamics of PLK1 at different stages of mitosis.
In 1998, Lee and colleagues first proposed that the polo-box domain played a critical role in PLK1 function by directing its subcellular localization [22]. Using human wild type and mutant forms of PLK1 to complement cdc5-1 defect yeast strain, they identified a single mutation W414F that compromised the ability to restore yeast growth, and this mutant disrupted the spatial distribution of PLK1. The following researches revealed that the dynamic distribution of PLK1 is directly mediated by its polo-box domain [23,24]. Since then, the mystery of the dynamic distribution of PLK1 in mitosis starts to be uncovered. Another breakthrough in PLK1 researches came in 2003, two exciting studies from Michael Yaffe group illustrate the common phosphor-peptide-binding properties of the PBDs conserved in human, Xenopus and yeast PLK1. The X-ray structure of a human PLK1 PBD-phosphopeptide complex clearly indicated that W414 is in a position that crucial for ligand binding, which perfectly explained the phenotype that W414F abrogates cellular localizations of PLK [9,10].
The significance of these discoveries is to provide a structural basis accounting for PLK1 docking at various subcellular structures and its substrates, implying the existence of spatial cue to “prime” kinase such as Cdk1 in coordinating PLK1 functions. In fact, several exciting studies provide supporting evidence of the “priming” model. The first example is the Golgi protein Nir2 that is first phosphorylated by Cdk1 at the onset of mitosis. Then the “primed” Nir2 binds to PLK1 through the PBD to participate in the execution of cytokinesis [25]. Based on this mechanism, several substrates or interacting proteins of PLK1 has been identified by using far western and other techniques, including Bub1, BubR1, PICH, Cep55, Rock2,Nedd1, NudC, PBIP1, etc. [26–34]. It is worth noting that the optimal binding motif for PBD, S-[pT/pS]-[P/X], is very similar to the Cdk1 phosphorylation cassette.
Although PLK1 may create its own docking site in some cases, it is emerging that Cdk1 plays a crucial role in coordinating spatial and temporal activity of PLK1 inside the mitotic cells. Specifically, in early mitosis, Cdk1 phosphorylates Bub1 at T609, BubR1 at T605, and Incenp at T388. These primed sites can then recruit PLK1 to kinetochores. However, it has remained to be elucidated how those sites contribute to PLK1 targeting to different structure in mitosis. In anaphase, Cdk1 is also involved in regulating PRC1-mediated PLK1 docking, though the mechanism is quite different. Cdk1 phosphorylates PRC1 at T470, T481, thus inhibits PLK1 mediated phosphorylation of PRC1 at T578, T602. When Cyclin B is degraded and Cdk1 activity falls at the onset of anaphase, these Cdk1 sites dephosphorylated and then PLK1 can create its own docking sites on PRC1 [35]. Other PLK1 binding proteins (MKlp1,MKlp2, etc.) in anaphase all have potential Cdk1 phosphorylation sites, implying that it may be a general mechanism that orchestrates PLK1 activity in late stages of mitosis.
2.2. PLK1 coordinates with BubR1 to execute its function on kinetochore
BubR1 kinase is long known as a component of spindle assembly checkpoint. Till recently, the regulatory role of BubR1 in chromosome-spindle attachments has been reported [36]. In 2007, three groups independently discovered that PLK1 phosphorylated BubR1 to facilitate kinetochore–microtubule interactions and chromosome alignments [27,37,38]. As mentioned in the previous section, the substrate recognition process is also mediated by Cdk1 prime phosphorylation of BubR1 at T620 (in human) and T605 (in Xenopus), and then this site is recognized by polo-box domain of PLK1. Subsequently PLK1 may phosphorylate multiple sites on BubR1, including S676, T792 and T1008. Phosphorylation of S676 is positively correlated with tension across kinetochore pairs, suggesting S676 is a contributor to the 3F3/2 epitope that created by PLK1 [37]; Phosphorylation of the last two sites greatly enhances the kinase activity of BubR1 [27,38], which may regulate kinetochore–microtubule attachment and chromosome congression.
Recently, another interesting study suggested that BubR1 localizes to centrosome and the non-catalytic domain locates at its N-termini inhibits PLK1 activity thus prevents centrosome amplification in interphase [39]. This discovery suggests that the interrelationship between PLK1 and BubR1 varies in different phases of cell cycle, and the underlying mechanism is still waiting to be explored.
3. Crosstalk between PLK1 and phosphatases
Recently, more and more crosstalks between protein kinases and phosphatases have been discovered in cell cycle control, which increases the hierarchy and accuracy of the regulatory cascade. As an important cell cycle regulator, the intimacy between PLK1 and phosphatase Cdc25C has been discovered years ago. Xenopus PLK1 or Plx1 was first purified from Xenopus egg extracts as a Cdc25C interacting protein, and recombinant Plx1 can phosphorylate Cdc25C in vitro [11]. Subsequent work suggested that Plx1-mediated phosphorylation is essential for activation of Cdc25C and participates in initiation of G2/M transition [40]. In human cells PLK1 promotes nuclear translocation of Cdc25C by phosphorylating S198 in the nuclear export signal of Cdc25C [41]. However, the situation in human is more complicated, as several isoforms of PLKs and Cdc25 exist (reviewed in [42–44]).
Another interesting finding recently is the interrelationship between PLK1 and Cdc14 family (Cdc14A and Cdc14B). Our recent finding indicated that hCdc14A was a novel binding protein of PLK1; moreover, PLK1 can phosphorylate hCdc14A at S351 and S363; this modification relieved the intro-molecular inhibition of hCdc14A and enhanced its phosphatase activity in vitro and in vivo [45]. It has been demonstrated that hCdc14A can dephosphorylate Cdh1 therefore activate APC/C-Cdh1, which suggests PLK1-hCdc14A pathway may be involved in activation of APC/C machinery in late mitosis. Later, researches in Van Vugt group supported this finding [46]. Using live cell imaging technique, they found that a depletion of PLK1 did not affect APC/C-Cdc20-mediated Cyclin B degradation, however the degradation of the two APC/C-Cdh1 substrates, Aurora A and Cdc20, were greatly delayed in PLK1 depleted cells. Moreover, exogenous express phospho-mimicking mutant of hCdc14A in these cells can restore the APC/C-Cdh1-mediated Aurora A degradation, strongly indicating that PLK1 stimulates ACP/C-Cdh1 through activating hCdc14A.
It remains elusive whether hCdc14A regulates PLK1 activity conversely, however, another report from Pagano group convincingly demonstrated that Cdc14B indirectly influenced PLK1 stability in DNA damage response [47]. Cdc14B resides in interphase nucleolus, and then translocates to the nucleus following doxorubicin or ionizing radiation induced DNA damage, where reactivates APC/C-Cdh1. APC/C-Cdh1 then degrades PLK1 through its D-box located at 337–340 amino acids [48]. This down-regulation of PLK1 is required for an efficient DNA damage-induced G2 checkpoint.
4. Crosstalk between PLK1 and ubiquitination pathway
Besides phospho-regulation, another pivotal controlling mechanism of the cell cycle is ubiquitination-mediated protein destruction. In mitosis, the most important ubiquitin E3 ligases include SKP1/Cul1/F-box protein complex (SCF) and anaphase-promoting complex/cyclosome (APC/C), which destroy different regulators at different stages of mitosis. Interestingly, the ubiquitination pathway collaborates with the kinase–phosphatase cascade to achieve an accurate regulation of mitosis.
As a key mitotic kinase, PLK1 is involved in the crosstalk between phosphorylation pathway and ubiquitination pathway. After years of researches, it is emerging that the cooperation between PLK1 and SCFβTrCP plays an important role in SCF-mediated protein degradation in early stages of mitosis. Several substrates have been identified including Claspin, Wee1, Emi1, etc. All these substrates have one common feature, which is that they all harbor a canonical DSGxxS degron sequence. Once phosphorylated by PLK1, this motif can serve as the βTrCP-binding site and promote SCF-mediated protein degradation. One example is the degradation of Claspin during recovery from DNA damage checkpoint. The DNA damage checkpoints protect genomic integrity by restraining mitotic onset in response to genotoxic stress. Claspin harbors a canonical DSGxxS degron at 29–34 amino acids. This motif can be phosphorylated by PLK1 and thus promotes the recognition and degradation by SCFβTrCP [49–51]. Finally, the decrease of Claspin in turns terminates Claspin-mediated activation of Chk1 and turns off the DNA damage checkpoint. Similarly, the early mitotic inhibitor 1 (Emi1), which inhibits the activity of APC/C in early mitosis, is also degraded in a PLK1 and SCFβTrCP coordinated way [52,53].
Another mitotic E3 ligase APC/C, is also received regulation from PLK1. Using recombinant APC components, Todokoro group provide evidence that Cdc16, Cdc27 and Tsg24 can be phosphorylated by activated PLK1, and these phosphorylation stimulated E3 ligase activity of APC/C [54,55]. Interestingly, Gorbsky group reported that the 3F3/2 antibody, which recognized phospho-epitopes that created mainly by PLK1 on unattached kinetochores, immunoprecipitated APC/C components Cdc16, Cdc27 and APC1 from mitotic HeLa cell lysates specifically [56], suggesting either these APC/C components are substrates of PLK1, or PLK1-mediated phosphorylation promotes interactions between 3F3/2 epitopes and APC/C.
5. Dynamic regulation of PLK1 activity
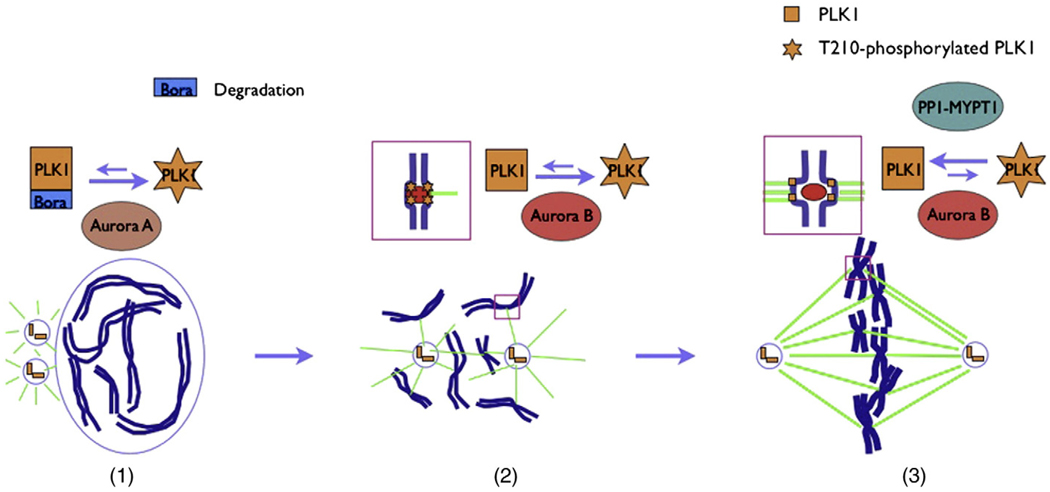
PLK1 functions in multiple stages of mitosis, and overactivation of PLK1 is linked to the developments of many cancers. Therefore, the cell unites several regulatory pathways and develops a highly coordinated and multi-layer way to control the spatial and temporal dynamics of PLK1 activity (Fig. 1).
Fig. 1.
Initial activation and afterwards maintenance of PLK1 activity by Aurora kinases. (1) During unperturbed G2-M transition or recovery from DNA damage checkpoint, Aurora A is first activated by unknown signaling pathway, and then collaborates with Bora to phosphorylate T210 of PLK1 at late G2 phase centrosomes. Once activated, PLK1 phosphorylates the degron on Bora and promotes SCFβTrCP-mediated degradation of Bora to terminate the activation loop. (2) During prophase and prometaphase, the bi-orientation is not generated and the tension across kinetochore pairs is low, which lead to the spatially nearness of PLK1 and Aurora B. Aurora B then phosphorylates PLK1 at T210 and increases its kinase activity. (3) Once the kinetochores are firmly and correctly captured, stretch on kinetochore pairs results in spatially separation of PLK1 and Aurora B. Under the influence of the kinetochore-resided protein phosphatase (MYPT-PP1C), PLK1 becomes dephosphorylated and its activity returns to the basal level.
The first layer of regulation depends on the intrinsic property of the C-termini Polo-box domain. Using several deletion mutations, Erikson group functionally dissected the role of Polo-box domain of PLK1. Surprisingly, they found the kinase activity of PLK1 with C-terminal deleted were significantly higher than wild type. Based on this observation, they proposed that the C-terminal Polo-box domain harbored an inhibitory role in regulating PLK1 activity. Indeed, the C-terminal PLK1 interacted with unphosphorylated PLK1 in G2 arrested cells, but not phosphorylated PLK1 from mitotic cells [24], suggesting the intramolecular binding restrains the activity of PLK1 and this inhibition can be removed by phosphorylation.
Meanwhile, as mentioned in the introduction section, phosphorylation of T210 in the T-loop is crucial for activating PLK1 in mitosis, and substitution of Thr-210 with Asp elevates the kinase activity [57]. In addition, phosphorylation at T210 is inhibited in response to DNA damage, which is consistent with the role of PLK1 in recovery from DNA damage checkpoint [58]. These discoveries support the notion that the existence of a second layer of regulation, phosphorylation, in controlling PLK1 activity. Though this hypothesis has been proposed for several years, the specific kinase(s) that takes charge of phosphorylating PLK1 at T210 has not been reported until recently. In 2008, two independent works demonstrated that Aurora A kinase played a central role in activating PLK1 at the initial stage of mitosis. Focused on the genes whose transcription was increased in G2, Guowei Fang group identified a regulator for G2-M transition, hBora, depletion of which delayed mitotic entry in synchronized HeLa cells. Subsequent work indicated that PLK1 was a major hBora-interacting protein, moreover, PLK1 and Bora immunoprecipitated with each other mostly in G2 phase, just before PLK1 activation. Most significantly, depletion of Bora affected the activation of PLK1 in vivo, while in vitro Aurora A directly phosphorylated PLK1 at T210 and this phosphorylation was enhanced by Bora. The authors proposed that binding of Bora to PLK1 overrided the autoinhibition by PBD and increased the accessibility of PLK1-T210 to Aurora A. Therefore, the synergistic action of Bora and Aurora A controls the PLK1 activity during G2-M transition [59]. Almost simultaneously, Medema group confirmed that Aurora A phosphorylated PLK1 T210 in vitro with the help of Bora. Moreover, they adopted a fluorescence resonance energy transfer (FRET)-based optic sensor to visualize the activation process of PLK1 during G2-M transition, and found that in the Aurora A depleted cells, the activation of PLK1 was greatly delayed [60]. These two reports convincingly established the activation role of Aurora A toward PLK1 during G2-M transition, and considering the spatial distribution of these two proteins, this activation process mainly occurs at centrosomes. Considering that PLK1 distributed both on mitotic centrosomes and kinetochores, it is still elusive how PLK1 activity on mitotic kinetochore is orchestrated.
Protein sequence analysis shows that a degron locates at 496–501 amino acids in human Bora, and this site is conserved in both rat and Xenopus. As mentioned in the previous section, the known substrates that are degraded in a PLK1 and SCFβTrCP dependent way all harbor the degron sequence. Therefore, it is likely that Bora is a substrate of SCFβTrCP. Indeed, researches from Guowei Fang group demonstrated that Bora was degraded when the cell entered mitosis, and the degradation was executed by SCFβTrCP-mediated ubiquitination. More interestingly, degradation of Bora was also PLK1 phosphorylation dependent [61]. These results yield a question: how is PLK1 activity maintained after Bora degradation in later stages of mitosis?
Aurora A and Aurora B are paralogues with a high sequence identity in their catalytic domains, while the distinct N-terminus specifies their different spatial distribution during mitosis [62]. Recently, it is reported that a single residue change transforms Aurora A into functional Aurora B, suggesting the functional similarity between these kinases [63]. In reminiscent of the role of Aurora A in activating PLK1, and the fact that in the absence of Bora or Aurora A, PLK1 activation is delayed but still occurs [60], it is high likely that Aurora B is also involved in regulating PLK1 activity, especially on mitotic kinetochores. Indeed, our preliminary results indicate that Aurora B can also phosphorylate PLK1 at T210 in vitro. More importantly, using targeted FRET-based optic sensor for PLK1 and T210 phospho-antibody staining, we found that Aurora B phosphorylation towards PLK1 is tension-dependent (Fig. 1): lack of tension between kinetochore pairs facilitates Aurora B-mediated phosphorylation of PLK1, when tension is generated, Aurora B is spatially separated from PLK1 and accordingly the phosphorylation level of PLK1 decreases due to the presence of phosphatases (Kai Yuan et al. submitted for publication).
The central role of the residue T210 in controlling PLK1 activation and the functions of Aurora family in this process have been well established, meanwhile, the mechanisms that cease the PLK1 activity is emerging. Recently, Matsumura group reported Myosin phosphatase-targeting subunit 1 (MYPT1), which acted in a complex with protein phosphatase catalytic subunit PP1C, antagonized PLK1 activity during mitosis. They demonstrated that MYPT1 was prime phosphorylated by Cdk1 thus generated a binding motif for PLK1. Interestingly, depletion of both MYPT1 and PLK1 restored the mitotic defects that caused by depleting PLK1 alone. In addition, they found the phosphorylation level on T210 of PLK1 increased in the absence of MYPT1 [64]. These results indicate that protein phosphatase 1 (MYPT1-PP1C) counteracts Aurora kinases in controlling T210 phosphorylation status of PLK1, hence achieves a dynamic regulation of PLK1 activity.
The recent researches of Sgo1 shed new light on PLK1 activity regulation. Hongtao Yu group reported that depletion of protein phosphatase 2A (PP2A) abolished centromeric localization of Sgo1. However, if PLK1 was codepleted, Sgo1 remained on centromeres [65]. In Drosophila, Polo phosphorylates MEI-S332 and removes it from centromeres [66]. These results suggest that it is likely the balance between PLK1 phosphorylation and PP2A dephosphorylation determines centromeric localization of Sgo1 [67], although more direct evidence is needed to further support this hypothesis.
6. Conclusion
After years of extensive study, the work of characterizing functions of a single mitotic regulator has been almost completed, while exploring the synergistic actions of several proteins or several signaling pathways is coming into spotlight. As a key protein kinase in the regulatory hierarchy of mitosis, PLK1 has been proven to regulate almost every aspect of mitosis. To fulfill its function, PLK1 collaborates with other mitotic kinases and phosphatases, as well as ubiquitination signaling pathways. One fascinating aspect of PLK1 is that the C-terminal PBD bestows it a versatile way for localization and substrate recognition, in response to Cdk1. However, not all the reported binding partners and substrates of PLK1 share the same mechanism. Further characterizations and comparisons are still needed. Another breakthrough recently in PLK1 researches is, the kinase that takes charge of the initial activation of PLK1 has been identified as Aurora A. Our subsequent work emphasized that, after initiation by Aurora A, the activity of PLK1 on kinetochore is more likely to be regulated by Aurora B. How kinases of Aurora family cooperate in dynamic orchestrating PLK1 activity remains to be resolved. There is no doubt that molecular delineation how protein phosphatases coordinate with these kinases to achieve equilibrium in mitotic orchestra remains to be a hotspot in future investigation.
Acknowledgements
This work was supported in part by National Institutes of Health Grants DK-56292, CA118948 and CA132389 and NIH, National Center for Research Resources (NCRR), Grant UL1 RR025008 from the Clinical and Translational Science Award program. This work was also supported by Chinese Natural Science Foundation Grants 90508002 and 90913016; Chinese Academy of Science Grants KSCX1-YW-R-65, KSCX2-YW-H-10, KSCX2-YW-R-195; Chinese 973 Project Grants 2006CB943603, 2007CB914503, and 2010CB912103; and Anhui Province Key Project Grant 08040102005. YH is an awardee of China Scholarship Council.
References
- 1.Sunkel CE, Glover DM. J. Cell Sci. 1988;89(Pt 1):25. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Hartwell LH, Mortimer RK, Culotti J, Culotti M. Genetics. 1973;74(2):267. doi: 10.1093/genetics/74.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KS, Erikson RL. Mol. Cell. Biol. 1997;17(6):3408. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimmerman WC, Erikson RL. Proc. Natl Acad. Sci. U. S. A. 2007;104(6):1847. doi: 10.1073/pnas.0610856104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthew EM, Yen TJ, Dicker DT, Dorsey JF, Yang W, Navaraj A, El-Deiry WS. Cell Cycle. 2007;6(20):2571. doi: 10.4161/cc.6.20.5079. [DOI] [PubMed] [Google Scholar]
- 6.Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Dev. Cell. 2007;13(2):190. doi: 10.1016/j.devcel.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. Nat. Cell Biol. 2005;7(11):1140. doi: 10.1038/ncb1320. [DOI] [PubMed] [Google Scholar]
- 8.Marshall CJ. Nature. 1994;367(6465):686. doi: 10.1038/367686a0. [DOI] [PubMed] [Google Scholar]
- 9.Elia AE, Rellos P, Haire LF, Chao JW, Ivins FJ, Hoepker K, Mohammad D, Cantley LC, Smerdon SJ, Yaffe MB. Cell. 2003;115(1):83. doi: 10.1016/s0092-8674(03)00725-6. [DOI] [PubMed] [Google Scholar]
- 10.Elia AE, Cantley LC, Yaffe MB. Science. 2003;299(5610):1228. doi: 10.1126/science.1079079. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai A, Dunphy WG. Science. 1996;273(5280):1377. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 12.Inoue D, Sagata N. EMBO J. 2005;24(5):1057. doi: 10.1038/sj.emboj.7600567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe N, Arai H, Iwasaki J, Shiina M, Ogata K, Hunter T, Osada H. Proc. Natl Acad. Sci. U. S. A. 2005;102(33):11663. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumara I, Gimenez-Abian JF, Gerlich D, Hirota T, Kraft C, de la Torre C, Ellenberg J, Peters JM. Curr. Biol. 2004;14(19):1712. doi: 10.1016/j.cub.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 15.Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, Gurtler U, Garin-Chesa P, Lieb S, Quant J, Grauert M, Adolf GR, Kraut N, Peters JM, Rettig WJ. Curr. Biol. 2007;17(4):316. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 16.Brennan IM, Peters U, Kapoor TM, Straight AF. PLoS ONE. 2007;2(5):e409. doi: 10.1371/journal.pone.0000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burkard ME, Randall CL, Larochelle S, Zhang C, Shokat KM, Fisher RP, Jallepalli PV. Proc. Natl Acad. Sci. U. S. A. 2007;104(11):4383. doi: 10.1073/pnas.0701140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petronczki M, Glotzer M, Kraut N, Peters JM. Dev. Cell. 2007;12(5):713. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Santamaria A, Neef R, Eberspacher U, Eis K, Husemann M, Mumberg D, Prechtl S, Schulze V, Siemeister G, Wortmann L, Barr FA, Nigg EA. Mol. Biol. Cell. 2007;18(10):4024. doi: 10.1091/mbc.E07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Nature. 2001;410(6825):215. doi: 10.1038/35065617. [DOI] [PubMed] [Google Scholar]
- 21.Petronczki M, Lenart P, Peters JM. Dev. Cell. 2008;14(5):646. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Lee KS, Grenfell TZ, Yarm FR, Erikson RL. Proc. Natl Acad. Sci. U. S. A. 1998;95(16):9301. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seong YS, Kamijo K, Lee JS, Fernandez E, Kuriyama R, Miki T, Lee KS. J. Biol. Chem. 2002;277(35):32282. doi: 10.1074/jbc.M202602200. [DOI] [PubMed] [Google Scholar]
- 24.Jang YJ, Lin CY, Ma S, Erikson RL. Proc. Natl Acad. Sci. U. S. A. 2002;99(4):1984. doi: 10.1073/pnas.042689299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Litvak V, Argov R, Dahan N, Ramachandran S, Amarilio R, Shainskaya A, Lev S. Mol. Cell. 2004;14(3):319. doi: 10.1016/s1097-2765(04)00214-x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Chen Q, Feng J, Hou J, Yang F, Liu J, Jiang Q, Zhang C. J. Cell Sci. 2009;122(Pt 13):2240. doi: 10.1242/jcs.042747. [DOI] [PubMed] [Google Scholar]
- 27.Wong OK, Fang G. J. Cell Biol. 2007;179(4):611. doi: 10.1083/jcb.200708044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowery DM, Clauser KR, Hjerrild M, Lim D, Alexander J, Kishi K, Ong SE, Gammeltoft S, Carr SA, Yaffe MB. EMBO J. 2007;26(9):2262. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumann C, Korner R, Hofmann K, Nigg EA. Cell. 2007;128(1):101. doi: 10.1016/j.cell.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Qi W, Tang Z, Yu H. Mol. Biol. Cell. 2006;17(8):3705. doi: 10.1091/mbc.E06-03-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishino M, Kurasawa Y, Evans R, Lin SH, Brinkley BR, Yu-Lee LY. Curr. Biol. 2006;16(14):1414. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 32.Kang YH, Park JE, Yu LR, Soung NK, Yun SM, Bang JK, Seong YS, Yu H, Garfield S, Veenstra TD, Lee KS. Mol. Cell. 2006;24(3):409. doi: 10.1016/j.molcel.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Goto H, Kiyono T, Tomono Y, Kawajiri A, Urano T, Furukawa K, Nigg EA, Inagaki M. Nat. Cell Biol. 2006;8(2):180. doi: 10.1038/ncb1350. [DOI] [PubMed] [Google Scholar]
- 34.Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK. Dev. Cell. 2005;9(4):477. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Neef R, Gruneberg U, Kopajtich R, Li X, Nigg EA, Sillje H, Barr FA. Nat. Cell Biol. 2007;9(4):436. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 36.Lampson MA, Kapoor TM. Nat. Cell Biol. 2005;7(1):93. doi: 10.1038/ncb1208. [DOI] [PubMed] [Google Scholar]
- 37.Elowe S, Hummer S, Uldschmid A, Li X, Nigg EA. Genes Dev. 2007;21(17):2205. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsumura S, Toyoshima F, Nishida E. J. Biol. Chem. 2007;282(20):15217. doi: 10.1074/jbc.M611053200. [DOI] [PubMed] [Google Scholar]
- 39.Izumi H, Matsumoto Y, Ikeuchi T, Saya H, Kajii T, Matsuura S. Oncogene. 2009 doi: 10.1038/onc.2009.141. [DOI] [PubMed] [Google Scholar]
- 40.Qian YW, Erikson E, Taieb FE, Maller JL. Mol. Biol. Cell. 2001;12(6):1791. doi: 10.1091/mbc.12.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toyoshima-Morimoto F, Taniguchi E, Nishida E. EMBO Rep. 2002;3(4):341. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myer DL, Bahassi el M, Stambrook PJ. Oncogene. 2005;24(2):299. doi: 10.1038/sj.onc.1208278. [DOI] [PubMed] [Google Scholar]
- 43.Karlsson-Rosenthal C, Millar JB. Trends Cell Biol. 2006;16(6):285. doi: 10.1016/j.tcb.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 44.van de Weerdt BC, Medema RH. Cell Cycle. 2006;5(8):853. doi: 10.4161/cc.5.8.2692. [DOI] [PubMed] [Google Scholar]
- 45.Yuan K, Hu H, Guo Z, Fu G, Shaw AP, Hu R, Yao X. J. Biol. Chem. 2007;282(37):27414. doi: 10.1074/jbc.M703555200. [DOI] [PubMed] [Google Scholar]
- 46.van Leuken R, Clijsters L, van Zon W, Lim D, Yao X, Wolthuis RM, Yaffe MB, Medema RH, van Vugt MA. PLoS ONE. 2009;4(4):e5282. doi: 10.1371/journal.pone.0005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassermann F, Frescas D, Guardavaccaro D, Busino L, Peschiaroli A, Pagano M. Cell. 2008;134(2):256. doi: 10.1016/j.cell.2008.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindon C, Pines J. J. Cell Biol. 2004;164(2):233. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mailand N, Bekker-Jensen S, Bartek J, Lukas J. Mol. Cell. 2006;23(3):307. doi: 10.1016/j.molcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 50.Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A, Medema RH, Freire R. Curr. Biol. 2006;16(19):1950. doi: 10.1016/j.cub.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 51.Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, Sherman NE, Pagano M. Mol. Cell. 2006;23(3):319. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Hansen DV, Loktev AV, Ban KH, Jackson PK. Mol. Biol. Cell. 2004;15(12):5623. doi: 10.1091/mbc.E04-07-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moshe Y, Boulaire J, Pagano M, Hershko A. Proc. Natl Acad. Sci. U. S. A. 2004;101(21):7937. doi: 10.1073/pnas.0402442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kotani S, Tugendreich S, Fujii M, Jorgensen PM, Watanabe N, Hoog C, Hieter P, Todokoro K. Mol. Cell. 1998;1(3):371. doi: 10.1016/s1097-2765(00)80037-4. [DOI] [PubMed] [Google Scholar]
- 55.Golan A, Yudkovsky Y, Hershko A. J. Biol. Chem. 2002;277(18):15552. doi: 10.1074/jbc.M111476200. [DOI] [PubMed] [Google Scholar]
- 56.Daum JR, Tugendreich S, Topper LM, Jorgensen PM, Hoog C, Hieter P, Gorbsky GJ. Curr. Biol. 2000;10(23):R850. doi: 10.1016/s0960-9822(00)00836-8. [DOI] [PubMed] [Google Scholar]
- 57.Jang YJ, Ma S, Terada Y, Erikson RL. J. Biol. Chem. 2002;277(46):44115. doi: 10.1074/jbc.M202172200. [DOI] [PubMed] [Google Scholar]
- 58.Tsvetkov L, Stern DF. Cell Cycle. 2005;4(1):166. doi: 10.4161/cc.4.1.1348. [DOI] [PubMed] [Google Scholar]
- 59.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Science. 2008;320(5883):1655. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Nature. 2008;455(7209):119. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 61.Seki A, Coppinger JA, Du H, Jang CY, Yates JR, III, Fang G. J. Cell Biol. 2008;181(1):65. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ke YW, Dou Z, Zhang J, Yao XB. Cell Res. 2003;13(2):69. doi: 10.1038/sj.cr.7290152. [DOI] [PubMed] [Google Scholar]
- 63.Hans F, Skoufias DA, Dimitrov S, Margolis RL. Mol. Biol. Cell. 2009;20(15):3491. doi: 10.1091/mbc.E09-05-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamashiro S, Yamakita Y, Totsukawa G, Goto H, Kaibuchi K, Ito M, Hartshorne DJ, Matsumura F. Dev. Cell. 2008;14(5):787. doi: 10.1016/j.devcel.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H. Dev. Cell. 2006;10(5):575. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Clarke AS, Tang TT, Ooi DL, Orr-Weaver TL. Dev. Cell. 2005;8(1):53. doi: 10.1016/j.devcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Dai W, Wang X. Cell Div. 2006;1:9. doi: 10.1186/1747-1028-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]