SUMMARY
Toll-like receptors (TLR) have a critical role in innate immunity against pathogens. We investigated the cytokine response to TLR stimulation in peripheral blood cells of subjects infected with hepatitis C virus (HCV) and / or human immunodeficiency virus (HIV) in the Women Interagency HIV Study (WIHS) cohort. Interleukin (IL)-6 in response to TLR3 and TLR4 ligands such as polyinosinic-polycytidylic acid and lipopolysaccharide was significantly compromised in HCV-infected women. High spontaneous secretion of IL-6 suggested pre-existing cell activation as a factor mediating reduced responses to TLR3 and TLR4 stimulation. To a lesser extent, tumour necrosis factor-α and IL-1β responses to TLR stimulation were also compromised. Monocytes, but not B cells or NK cells, were identified as the cell population spontaneously secreting cytokines and also as the cells responding to TLR stimulation. These results highlight a functional defect in antigen-presenting cells of women with HCV infection or co-infection. In women with existing HIV co-infection, decreased cytokine function of antigen-presenting cells suggests another mechanism contributing to immune dysfunction in addition to the HIV-associated CD4 defect.
Keywords: cytokines, hepatitis, hepatitis C virus, human immunodeficiency virus, inflammation, Toll-like receptors
INTRODUCTION
Infection with hepatitis C virus (HCV) often results in viral persistence and chronically infected individuals have an increased rate of liver cirrhosis and hepatocellular carcinoma. Th1-type immune responses and cytotoxic T lymphocyte activity appear associated with viral clearance [1–3]. Accordingly, T-cell responses to HCV proteins are readily detected during acute infection, but both CD4 and CD8 functions become significantly impaired in the chronic phase [reviewed in 4]. The scarcity of HCV-specific memory T-cell responses during chronic infection has been proposed to be the result of interference by HCV proteins which bypasses immune mechanisms protective to the host.
In human immunodeficiency virus (HIV)-infected individuals, HCV co-infection appears linked to increased morbidity and mortality [5–7]. We and others have previously reported more extensive T helper cell dysfunction in patients with HIV / HCV co-infection compared with individuals with either HIV or HCV mono-infection [8,9]. Although reduced CD4 cell counts in HIV-infected individuals have a significant impact on immune function, emerging investigations suggest that additional levels of dysfunction linked to HCV may also exist. In some studies, dendritic cells (DC) generated in vitro from peripheral blood of HCV-infected individuals appear impaired in their capacity for antigen presentation [10–12]. Although other studies found reduced percentages of myeloid and plasmacytoid DC, their capacity for IFN-α secretion and allogeneic antigen presentation was comparable with uninfected controls [13,14]. Thus, the potential effect of HCV infection on antigen presentation remains inconclusive. Emerging data [13,14] as well as our unpublished observations suggest that the T-cell impairment in patients with HCV mono-infection is specific for HCV, while memory responses to unrelated pathogens like CMV remain robust.
Several studies have demonstrated that HCV could infect and establish a reservoir in peripheral blood. Cells of the monocytic lineage appear permissive for HCV replication [15–18]. The effect of such extrahepatic infection remains unclear, although initial studies reported secretion of tumour necrosis factor (TNF)-α and interleukin (IL)-8 by in vitro infected monocytes [19] and high IL-8 levels in the serum of infected patients [20].
Toll-like receptors (TLR), primary components of innate immunity, play a critical role in defense against pathogens [reviewed in 21]. Both TLR3 [reviewed in 22] and TLR4 [23–26] have a recognized role in antiviral immunity. In this study, we investigated the ex vivo capacity of peripheral blood mononuclear cells (PBMC) from women with HCV mono-infection or co-infected with HIV to respond to TLR 3 and 4 ligands. The results highlight a state of functional activation associated with HCV infection reflected by a reduced response to TLR ligands and high spontaneous secretion of the pro-inflammatory cytokine IL-6 and to a lesser extent also TNF-α and IL-1β. Monocytes were found to be the only cell type secreting spontaneously pro-inflammatory cytokines as shown in depletion experiments and intracellular staining. The aberrant secretion of pro-inflammatory cytokines by monocytes in HCV infection does not appear to be driven directly by HCV viraemia.
METHODS
Subjects
This was a cross-sectional study nested within the Women’s Interagency HIV Study [WIHS, 27]. Women with confirmed HCV serological status enrolled in the Southern California WIHS were evaluated during a scheduled visit between March 2003 and December 2004. HCV serostatus was established at enrollment, 2–10 years prior to initiation of this study. None of the HCV+ women were receiving anti-HCV therapy. Cytokine responses to TLR stimulation were investigated in 43 women, of whom 14 were co-infected (HIV+ / HCV+), 16 were HIV+ only and 13 were HCV+ only. Healthy individuals (n = 20) confirmed to be seronegative for HIV and HCV were used as a control group. Written consent was obtained from all subjects, and the Institutional Review Board (IRB) of the University of Southern California approved the study.
HIV and HCV viral loads
Plasma HIV-RNA levels at the time of cytokine testing were determined using the Nucleic Acid Sequence Based Amplification assay (NucliSens; bioMerieux, Inc., Durham, NC, USA) lower detection limit 25 copies / mL. HCV-RNA was detected by real-time PCR assay using the Cobas Amplicor HCV Monitor 2.0 (Roche Diagnostics®, Basel, Switzerland), with a dynamic range of 10–200 000 000 IU / mL.
PBMC stimulation
Fresh PBMC were cultured in serum-free medium (X-vivo 20; BioWhittaker, Walkersville, MD, USA) at 2 × 105 cells / well for 24 h in 96-well round-bottom plates. Supernatants from cultures with medium only were used to evaluate spontaneously secreted cytokines. Parallel cultures received TLR3 and TLR4 stimulants [polyinosinic-polycytidylic acid (pIC), 200 µg /mL or lipopolysaccharide (LPS), 20 ng /mL respectively, both from Sigma, St. Louis, MO, USA]. These concentrations of pIC and LPS were established as optimal in preliminary experiments. Supernatants were recovered at 16 h of culture and stored frozen until batch analysis.
Cytometric bead array assay for IL-1β, IL-6, IL-10, IL-12 and TNF-α
The cytometric bead array (CBA) assay was performed using undiluted tissue culture supernatants. For samples with high levels of cytokines, supernatants were retested at 1:5 dilution as described by the supplier (BD Biosciences, San Jose, CA, USA). For this assay, soluble cytokines were captured on microparticles and then measured using a fluorescence-based detection system and flow cytometry analysis as described previously [28,29]. A series of 10 dilutions using cytokine standards were run in each assay for generation of standard curves. Samples were analysed in a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) using the BD CBA Analysis Software, (Becton Dickinson). Spontaneous secretion of each cytokine was defined as the mean ± 3 SD of background release in PBMC from healthy controls (n = 20). The lower limits for IL-1β, IL-6, IL-10, IL-12 and TNF-α using this criterion were 12, 73, 3, 2.5 and 24 pg /mL respectively.
Depletion of monocytes, B cells and NK cells
Specific cell populations were removed by immunomagnetic separation. Briefly, PBMC were incubated for 30 min at 4 °C with mouse monoclonal antibodies specific for CD14, CD19 or CD56 for removal of monocytes, B cells or NK cells respectively. Following washing, anti-mouse Ig-coated magnetic beads (Dynabeads; Dynal, Oslo, Norway) were added at a 10:1 ratio. Bound cells were retained on a magnetic field. The preparations after two-rounds of depletion contained <2% of the target population as monitored by flow cytometry. Depleted preparations were recounted and stimulated with TLR ligands or left in medium only for spontaneous release.
IL-6 and TNF-α detection by intracellular staining
Peripheral blood mononuclear cells were stimulated for 5 h with TLR ligands or without stimuli for direct ex vivo detection of cytokines. Frozen PBMC were used for the detection of TNF-α. For analysis of both cytokines, Brefeldin A (1 µm; BD PharMingen, San Jose, CA, USA) was added during the last 4 h of incubation. Cells were stained with APC-labelled anti-CD14, FITC-labelled anti-CD19 monoclonal antibody (MAb) and CyC5-labelled anti-CD56. After fixation / permeabilization, cells were incubated with PE-labelled anti-human MAb to IL-6 or TNF-α, or an isotype control in wash / permeabilization buffer according to the manufacturer’s instructions (all reagents from BD PharMingen). Stained preparations were analysed on a FACSCalibur flow cytometer (Becton Dickinson). Fifty thousand eventswere scored for each sample.
Statistical analyses
Student’s t-test was used for the comparison of continuous variables between two groups. Analysis of variance (ANOVA) was used for comparison of continuous variables between three or more groups. Further comparisons between any two groups within these multiple groups were conducted using Scheffe’s method. Proportions between categorical groups were compared using chi-squared test or Fisher’s exact test. Wilcoxon Rank Sum test was used for the comparison of IL-6 ratios. Two-tailed P-value <0.05 was considered statistically significant.
RESULTS
Characteristics of the study populations
In general, the study groups were similar except that a history of injection drug use was more common in the 27 women with HCV infection (92% in the 13 mono-infected and 86% in the 14 co-infected) compared with the 16 women with HIV mono-infection (0%, P < 0.0001, Table 1).As expected, CD4 counts were significantly lower in women with HIV infection, either as mono-infection (mean 493 cells / µL) or as co-infection (448 cells / µL), compared with women with HCV mono-infection (1045 cells / µL, P < 0.0001).
Table 1.
Demographic and Clinical characteristics of the study populations
| Characteristics | Group | HCV+/HIV+ | HIV+ | HCV+ | P-value |
|---|---|---|---|---|---|
| n | 14 | 16 | 13 | ||
| Injection drug use | Ever | 12 (86) | 0 (0) | 12 (92) | <0.0001 |
| HAART-last 6 months | Yes | 8 (57) | 13 (81) | NS | |
| Length of HAART† (years) | 3.6 | 4.1 | NS | ||
| CD4 (cells/µL)† | 448 | 493 | 1045* | <0.0001 | |
| Nadir CD4† | 239 | 250 | 788* | <0.0001 | |
| ALT (U/L)† | 57 | 30 | 52 | NS | |
| HIV (Log10 RNA copies/mL)† | 1.97 | 1.49 | NS | ||
| HCV (Log10 IU/mL)† | 4.78 | 4.16 | NS |
Data are no. (%) of women, unless otherwise indicated.
HCV, hepatitis C virus; HIV, human immunodeficiency virus; NS, not significant.
Significantly higher than the other two groups.
Group mean.
High spontaneous cytokine secretion
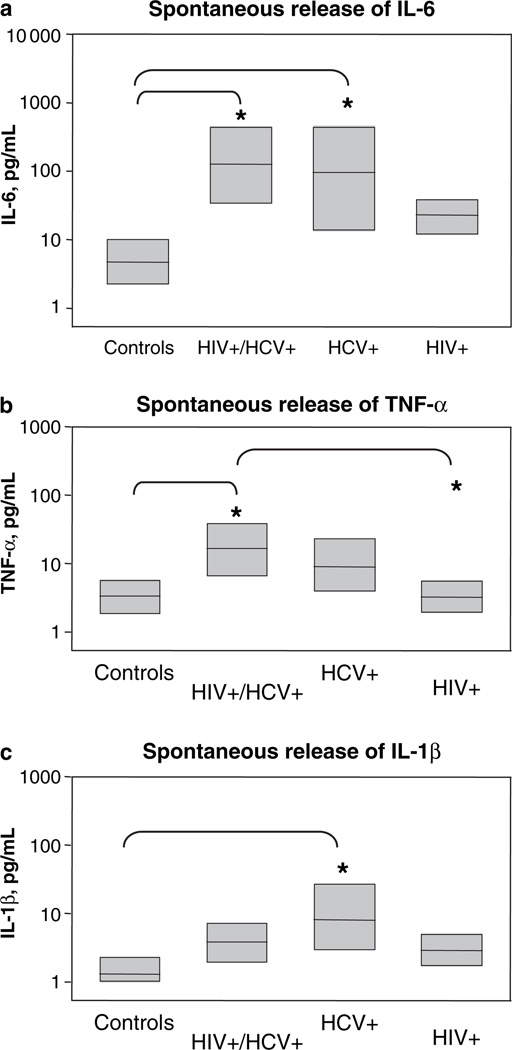
Compared with healthy controls, HCV (54%) and HIV / HCV positive women (64%) had high levels of spontaneous IL-6 release (P < 0.05, Fig. 1a). Spontaneous secretion of IL-6 in women with HIV mono-infection was not statistically different from the value observed in healthy controls (34.1 and 8.1 pg /mL respectively). Spontaneous TNF-α was significantly higher among HIV / HCV-positive women (Fig. 1b, 16 pg / mL) compared with both HIV mono-infected (3.3 pg / mL) and controls (3.2 pg / mL, P < 0.05 for both comparisons). Compared with controls, levels of spontaneous IL-1β were also significantly enhanced in HCV-positive women (Fig. 1c, 7.8 and 1.4 pg /mL respectively, P < 0.05). However, IL-6 was the most abundantly spontaneously secreted cytokine among HIV / HCV-positive women, with overall levels about 10-fold higher than TNF-α and 50-fold higher than IL-1β. Spontaneous secretion of IL-10 was observed at low levels in only four women, but overall there were no differences between the groups. No spontaneous secretion was observed for IL-12.
Fig. 1.
Altered cytokine response in hepatitis C virus infection. Spontaneous release of (a) interleukin (IL)-6, (b) tumour necrosis factor (TNF)-α and (c) IL-1β. Peripheral blood mononuclear cells from healthy controls or infected patients were cultured in medium only. Supernatants recovered at 16 h of culture were tested for released cytokines. Data shown represent total values of secreted IL-6, TNF-α and IL-1β in pg /mL. Group values that were significantly different (*P < 0.05) are indicated in each plot.
Compromised IL-6 responses to TLR stimulation
Contrasting the spontaneous secretion, IL-6 response to TLR3 (pIC) stimulation was significantly reduced in HCV-infected women (Table 2). Moreover, most HCV-mono-infected and HIV-co-infected women presented both high spontaneous cytokine secretion and low TLR-mediated cytokine responses. Similar results were obtained in cultures stimulated with the TLR4 ligand LPS, indicating that aberrant pro-inflammatory cytokine secretion in these patients comprises both TLR3 and TLR4 responses. Overall, only one HCV-positive patient (4%) had responses comparable with healthy controls for all cytokines.
Table 2.
Analysis of cytokine secretion in response to TLR stimulation by infection status
| Stimuli | Cytokines | n | Infection | Geometric Mean |
95% CI | P-value* | ||
|---|---|---|---|---|---|---|---|---|
| HIV | HCV | Control | ||||||
| pIC | IL-1β | 14 | HCV/HIV | 130.8 | 48.2–354.8 | < 0.05 | NS | NS |
| 10 | HIV | 1739.9 | 962.8–3144.3 | <0.05 | NS | |||
| 13 | HCV | 222.6 | 89.9–551.2 | NS | ||||
| 20 | Control | 442.6 | 230.9–848.2 | |||||
| IL-6 | 14 | HCV/HIV | 6046.7 | 3529.9–10358.0 | NS | NS | <0.05 | |
| 10 | HIV | 12367.0 | 11574.4–13214.0 | NS | NS | |||
| 13 | HCV | 6184.4 | 4709.7–8120.9 | <0.05 | ||||
| 20 | Control | 13041.3 | 10719.2–15866.5 | |||||
| TNF-α | 14 | HCV/HIV | 814.3 | 359.7–1843.1 | <0.05 | NS | NS | |
| 10 | HIV | 3187.9 | 2415.5–4207.3 | <0.05 | NS | |||
| 13 | HCV | 610.4 | 311.2–1196.9 | NS | ||||
| 20 | Control | 1783.1 | 1329.2–2391.9 | |||||
| IL-10 | 14 | HCV/HIV | 61.5 | 21.4–176.1 | <0.05 | NS | NS | |
| 10 | HIV | 535.8 | 346.0–829.7 | <0.05 | NS | |||
| 13 | HCV | 64.7 | 20.1–208.4 | NS | ||||
| 20 | Control | 141.3 | 70.8–282.0 | |||||
| LPS | IL-1β | 14 | HCV/HIV | 349.4 | 128.4–951.2 | <0.05 | NS | NS |
| 16 | HIV | 1970.5 | 1327.6–2924.9 | <0.05 | NS | |||
| 13 | HCV | 355.0 | 142.9–882.1 | NS | ||||
| 20 | Control | 664.9 | 363.9–1214.9 | |||||
| IL-6 | 14 | HCV/HIV | 7956.2 | 5898.6–10731.7 | NS | NS | NS | |
| 16 | HIV | 10836.6 | 9900.4–11861.4 | <0.05 | NS | |||
| 13 | HCV | 5531.1 | 3529.2–8668.5 | <0.05 | ||||
| 20 | Control | 10719.4 | 7888.8–14565.6 | |||||
| TNF-α | 14 | HCV/HIV | 1385.2 | 628.7–3051.7 | NS | NS | NS | |
| 16 | HIV | 1990.3 | 1194.7–3315.9 | <0.05 | NS | |||
| 13 | HCV | 488.1 | 210.3–1133.0 | NS | ||||
| 20 | Control | 1671.2 | 1079.8–2586.5 | |||||
| IL-10 | 14 | HCV/HIV | 79.4 | 26.1–241.2 | <0.05 | NS | NS | |
| 10 | HIV | 515.7 | 358.8–741.3 | <0.05 | <0.05 | |||
| 13 | HCV | 52.3 | 16.6–165.0 | NS | ||||
| 20 | Control | 86.8 | 39.9–188.8 | |||||
pIC, polyinosinic-polycytidylic acid; LPS, lipopolysaccharide; IL, interleukin; TNF, tumour necrosis factor; HCV, hepatitis C virus; HIV, human immunodeficiency virus; NS, not significant.
Statistical significance when comparing the infection group in a row with the infection group in a column.
Other cytokine responses to TLR stimulation
Levels of IL-1β and TNF-α in response to TLR3 (pIC) or TLR4 (LPS) stimulation were also reduced among women with HCV infection, either as mono-infection or in HIV co-infection (Table 2). Despite the overall trend, IL-1β among HCV-infected women (mono- or co-infected) was significantly reduced only compared with HIV+ women (but not healthy controls) for both TLR3 and TLR4 stimulation. A similar pattern was observed for TNF-α, except that the response to TLR4 ligand among co-infected women was not statistically different from all other groups. Levels of the regulatory cytokine IL-10 were likewise reduced among women with HCV infection compared with women with HIV mono-infection but not different from healthy controls, although IL-10 values were overall lower than those of IL-6, IL-1β and TNF-α. IL-12 was seldom detected and there were no differences between the groups (data not shown).
Monocytes involved in spontaneous secretion of pro-inflammatory cytokines
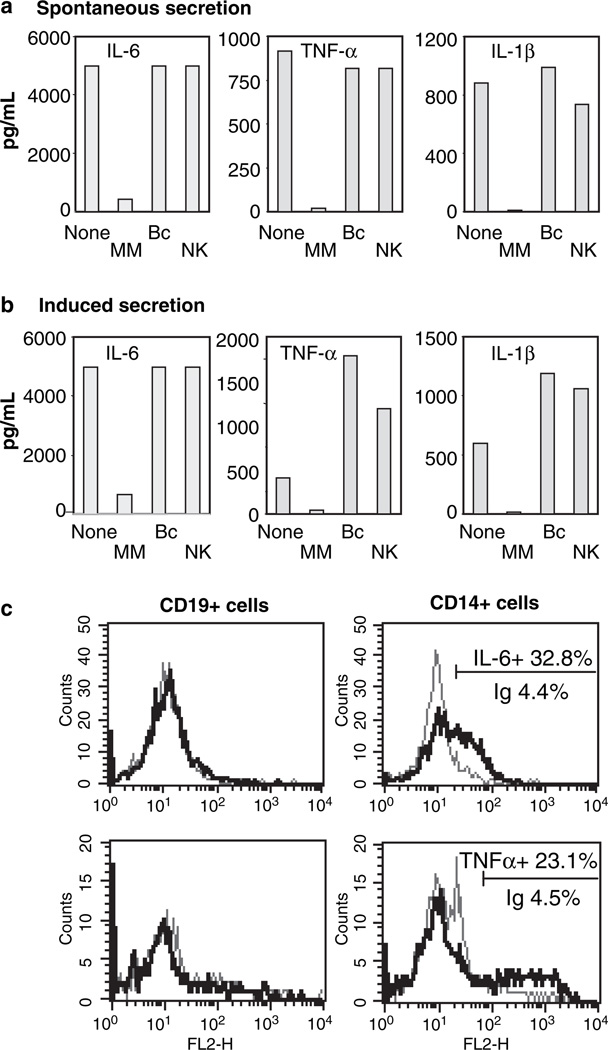
Following depletion of monocytes / macrophages (CD14+ cells), B cells (CD19+) or NK cells (CD56+), only depletion with anti-CD14 antibody resulted in nearly abolished secretion of pro-inflammatory cytokines both for spontaneous and TLR-mediated secretion (shown for IL-6, TNF-α and IL-1β in Fig. 2a,b). This observation was further confirmed by intracellular staining for IL-6 and TNF-α (Fig. 2c). Flow cytometry data shown are PBMC from a woman with HIV / HCV co-infection indicating a positive IL-6 signal in 32.8% of CD14+ cells (4.4% for the corresponding isotype control, Fig. 2c upper row). No IL-6 signal was detected in CD19+ or CD56+ cells. Staining for TNF-α indicated a positive signal in 23.1% of CD14+ cells compared to 4.5% in the corresponding isotype control (Fig. 2c, lower row). No TNF-α signal was detected in CD19+ (panels to the left) or CD56+ cells. This pattern of cytokine secretion by CD14+ cells but not by CD19+ or CD56+ cells was identical in a total of six patients in independent experiments.
Fig. 2.
Monocytes secrete cytokines both spontaneously and in response to Toll-like receptor stimulation. (a) Untreated peripheral blood mononuclear cells (PBMC) (None) or PBMC depleted from monocytes (CD14+), B cells (CD19+) or NK cells (CD56+) by immune-magnetic separation were cultured in medium alone. (b) PBMC untreated or population-depleted were stimulated with lipopolysaccharide for 16 h. Secreted cytokines in the supernatants were evaluated by cytometric bead array assay. Data shown [from a patient with hepatitis C virus (HCV) mono-infection] is representative of identical results from four separate patients. (c) Intracellular staining for interleukin (IL)-6 (upper row) in B cells (CD19+) and monocytes (CD14+) from a patient with human immunodeficiency virus/HCV co-infection. Staining in NK cells (CD56+) was also negative (data not shown). The grey line depicts the flow cytometric profile with an isotype control antibody (4.4% of CD14+ cells) and the black line indicates IL-6 staining (32.8% of the CD14+ population). Staining for tumour necrosis factor (TNF)-α was positive in 23.1% of CD14+ cells (4.5% for the isotype control). No TNF-α signal was detected in CD56+ (NK) cells (data not shown).
DISCUSSION
This study detected a reduced IL-6 response to TLR ligands in patients with HCV infection irrespective of HIV status. High spontaneous secretion of IL-6 was observed among HCV-infected women. Noteworthy, levels of spontaneous secretion in co-infected women were similar to the levels observed in women with HCV mono-infection, suggesting that the cytokine dysregulation is linked to HCV infection. Although the impaired TLR-mediated IL-6 response was more noticeable in patients with high spontaneous secretion, absolute levels of stimulated IL-6 secretion were overall reduced in HCV infection.
Toll-like receptors, primary components of innate immunity, play a critical role in defence against pathogens [reviewed in 21]. Specifically, TLR3 has a well-recognized role in antiviral immunity [reviewed in 22]. Also TLR4 has been implicated before in responses to viral infections [23,24]. In humans, respiratory syncytial virus [25] and coxsackie virus B4 [26] stimulate IL-6 secretion via TLR4 signalling. Moreover, the HCV NS5A protein has been recently reported to activate TLR4 but not TLR3 in human B cells [30]. A recent microarray analysis indicated compromised expression of mRNA for TLRs in HCV patients with poor treatment response [31]. However, this defect appears to involve primarily the receptor for single-stranded RNA TLR7 and only marginally other TLRs.
Our depletion studies demonstrate that cells expressing the monocytic marker CD14 mediated the spontaneous secretion of IL-1β, IL-6 and TNF-α, as well as the TLR-mediated cytokine secretion. Cells of the monocyte / macrophage lineage are characteristically very potent producers of cytokines and have a relevant role in amplification and modulation of innate responses by their release of cytokines. Although abnormal function of B cells and NK cells have been associated with HCV infection [reviewed in 32], our depletion experiments found no evidence of contribution by NK cells or B cells in either spontaneous or induced secretion of the cytokines investigated. Intracellular staining further confirmed the sole involvement of monocytes / macrophages in this response. Compromised IFN-α and IL-6 responses to a combination of TLR ligands in plasmacytoid but not myeloid DC from HCV-infected subjects was reported recently [33]. Although we did not investigate specifically DC, removal of monocytes / macrophages from PBMC was sufficient to nearly ablate spontaneous secretion of IL-6, TNF-α and IL-1β.
Several reasons may account for monocyte activation ex vivo and the resulting spontaneous cytokine secretion. Monocytes may be directly infected by HCV and several studies have detected negative strand HCV RNA in monocytes indicating HCV replication, albeit to low efficiency [15–18]. Infection of monocytes by HCV may account for a defective cytokine response by the infected cells. It is also plausible that non-infected monocytes are affected by infected ones in a bystander fashion because of release of viral proteins. For example, HCV core and NS3 proteins were reported to induce secretion of TNF-α and IL-10 by normal monocytes in vitro [11]. Our study detected significantly higher levels of spontaneous TNF-α secretion in a substantial number of co-infected women, but differing from Dolganiuc’s observations, no evidence of spontaneous IL-10 secretion was found in our experimental system. Recently, secretion of TNF-α and IL-8 was detected in cultures containing HCV-infected monocytes [19]. Yet, the question whether the cytokine defect was mediated in a direct or a bystander fashion remains to be investigated. In other studies, HCV core and NS3 proteins mediated NFkB and inflammatory activation in vitro by using the TLR-2 pathway [34]. Such a mechanism involving key elements of intracellular activation is in agreement with our observation of enhanced spontaneous cytokine secretion but impaired TLR-mediated cytokine response. In contrast, recent studies characterized two additional mechanisms of interference with innate immunity involving disruption of retinoic acid-inducible gene I (RIG-I) and cleavage of the TLR3 adaptor protein TRIF, both effects mediated by HCV NS3 / 4A [35,36]. In both cases, the end result is inhibition of interferon regulatory factor 3 (IRF-3) and NFkB in cells expressing NS3 / 4A. The apparently contradictory observations of our ex vivo studies with these in vitro investigations may be explained by a dual model of viral interference, where infected cells are subjected to disruption of RIG-I and TRIF signalling, but bystander uninfected cells are activated by circulating viral products.
Monocyte / macrophage activation ex vivo could also be a consequence of tissue injury, for example in liver. High serum levels of IL-6 in chronic HCV infection have been reported [37–39], as well as IL-6 mRNA in liver tissue of infected patients [40]. Elevated circulating levels of IL-10, IL-12 and TNF-α have been reported previously [39,41]. However, to our knowledge, a direct contribution to IL-6 production by monocytes / macrophages in peripheral blood of HCV-infected patients has not been investigated. Interestingly, a recent study indicated that IL-6 secretion could be induced by the HCV core protein [42]. Although the possibility that IL-6 being stimulated by endogenous core protein seems plausible in our patients, the question requires further investigation because a direct correlation between spontaneous secretion of IL-6 and levels of viraemia could not be established in the current groups.
Earlier studies by our group and others provided evidence of lymphocyte activation in peripheral blood of patients with HIV [43–45] or HIV / HCV co-infection [46]. It seems plausible that spontaneous secretion of cytokines by monocytes contributes to the activation of lymphocytes observed ex vivo and further studies are needed to characterize this possible relationship. It also seems likely that activated monocytes may function inefficiently as mediators of inflammatory responses during antigen presentation and this may play a role in the reduced cell-mediated responses reported previously among HIV / HCV co-infected patients [8,9]. Because of the immunodeficiency linked to reduced CD4 numbers in HIV infection, such a defect becomes evident in HIV / HCV co-infected individuals but not in those with HCV mono-infection that have normal CD4 numbers. Further and expanded investigations are needed to clarify the role of IL-6 and other inflammatory cytokines in disorders associated with chronic HCV infection, both as mono-infection and in co-infection with HIV.
ACKNOWLEDGMENTS
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centres (Principal Investigators) at New York City / Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County / Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993 and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-CH-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). This study was also supported by NIAID ROI-AI 52065 (AK). We thank Dr. Eva Operskalski for critical reading of the manuscript.
Abbreviations
- CBA
cytometric bead array
- DC
dendritic cells
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- LPS
lipopolysaccharide
- MAb
monoclonal antibody
- PBMC
peripheral blood mononuclear cells
- pIC
polyinosinic-polycytidylic acid
- TLR
Toll-like receptors
- TNF
tumour necrosis factor
- WIHS
Women Interagency HIV Study
REFERENCES
- 1.Gerlach JT, Diepolder HM, Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 2.Jackson M, Smith B, Bevitt DJ, et al. Comparison of cytotoxic T-lymphocyte responses to hepatitis C virus core protein in uninfected and infected individuals. J Med Virol. 1999;58:239–246. doi: 10.1002/(sici)1096-9071(199907)58:3<239::aid-jmv9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 3.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 4.Cerny A, Chisari FV. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology. 1999;30:595–601. doi: 10.1002/hep.510300312. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DL, Shih JW, Alter HJ, et al. Effect of human immunodeficiency virus on hepatitis C virus infection among injecting drug users. J Infect Dis. 1996;174:690–695. doi: 10.1093/infdis/174.4.690. [DOI] [PubMed] [Google Scholar]
- 6.Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- 7.Daar ES, Lynn H, Donfield S, et al. Hepatitis C virus load is associated with human immunodeficiency virus type 1 disease progression in hemophiliacs. J Infect Dis. 2001;183:589–595. doi: 10.1086/318539. [DOI] [PubMed] [Google Scholar]
- 8.Lauer GM, Nguyen TN, Day CL, et al. Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J Virol. 2002;76:2817–2826. doi: 10.1128/JVI.76.6.2817-2826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villacres MC, Literat O, Degiacomo M, et al. Reduced type 1 and type 2 cytokines in antiviral memory T helper function among women coinfected with HIV and HCV. J Clin Immunol. 2005;25:134–141. doi: 10.1007/s10875-005-2819-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 11.Dolganiuc A, Kodys K, Kopasz A, et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J Immunol. 2003;170:5615–5624. doi: 10.4049/jimmunol.170.11.5615. [DOI] [PubMed] [Google Scholar]
- 12.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 14.Longman RS, Talal AH, Jacobson IM, Rice CM, Albert ML. Normal functional capacity in circulating myeloid and plasmacytoid dendritic cells in patients with chronic hepatitis C. J Infect Dis. 2005;192:497–503. doi: 10.1086/431523. [DOI] [PubMed] [Google Scholar]
- 15.Lerat H, Rumin S, Habersetzer F, et al. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood. 1998;91:3841–3849. [PubMed] [Google Scholar]
- 16.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. The presence of active hepatitis C virus replication in lymphoid tissue in patients coinfected with human immunodeficiency virus type 1. J Infect Dis. 1998;178:1189–1192. doi: 10.1086/515682. [DOI] [PubMed] [Google Scholar]
- 17.Navas S, Martin J, Quiroga JA, Castillo I, Carreno V. Genetic diversity and tissue compartmentalization of the hepatitis C virus genome in blood mononuclear cells, liver, and serum from chronic hepatitis C patients. J Virol. 1998;72:1640–1646. doi: 10.1128/jvi.72.2.1640-1646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskus T, Radkowski M, Wang LF, Nowicki M, Rakela J. Uneven distribution of hepatitis C virus quasispecies in tissues from subjects with end-stage liver disease: confounding effect of viral adsorption and mounting evidence for the presence of low-level extrahepatic replication. J Virol. 2000;74:1014–1017. doi: 10.1128/jvi.74.2.1014-1017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radkowski M, Bednarska A, Horban A, et al. Infection of primary human macrophages with hepatitis C virus in vitro: induction of tumour necrosis factor-alpha and interleukin 8. J Gen Virol. 2004;85:47–59. doi: 10.1099/vir.0.19491-0. [DOI] [PubMed] [Google Scholar]
- 20.Polyak SJ, Khabar KS, Rezeiq M, Gretch DR. Elevated levels of interleukin-8 in serum are associated with hepatitis C virus infection and resistance to interferon therapy. J Virol. 2001;75:6209–6211. doi: 10.1128/JVI.75.13.6209-6211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 22.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462–468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Burzyn D, Rassa JC, Kim D, Nepomnaschy I, Ross SR, Piazzon I. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J Virol. 2004;78:576–584. doi: 10.1128/JVI.78.2.576-584.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rassa JC, Meyers JL, Zhang Y, Kudaravalli R, Ross SR. Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proc Natl Acad Sci USA. 2002;99:2281–2286. doi: 10.1073/pnas.042355399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurt-Jones EA, Popova L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 26.Triantafilou K, Triantafilou M. Coxsackievirus B4-induced cytokine production in pancreatic cells is mediated through toll-like receptor 4. J Virol. 2004;78:11313–11320. doi: 10.1128/JVI.78.20.11313-11320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;20049:117–125. [PubMed] [Google Scholar]
- 28.Cook EB, Stahl JL, Lowe L, et al. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J Immunol Methods. 2001;254:109–118. doi: 10.1016/s0022-1759(01)00407-0. [DOI] [PubMed] [Google Scholar]
- 29.Oliver KG, Kettman JR, Fulton RJ. Multiplexed analysis of human cytokines by use of the FlowMetrix system. Clin Chem. 1988;44:2057–2060. [PubMed] [Google Scholar]
- 30.Machida K, Cheng KT, Sung VM, Levine AM, Foung S, Lai MM. Hepatitis C virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon and interleukin-6. J Virol. 2006;80:866–874. doi: 10.1128/JVI.80.2.866-874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor MW, Tsukahara T, Brodsky L, et al. Changes in gene expression during pegylated interferon and ribavirin therapy of chronic hepatitis C virus distinguish responders from non-responders to antiviral therapy. J Virol. 2007;81:3391–3401. doi: 10.1128/JVI.02640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavio N, Lai MM. The hepatitis C virus persistence: how to evade the immune system? J Biosci. 2003;28:287–304. doi: 10.1007/BF02970148. [DOI] [PubMed] [Google Scholar]
- 33.Yankers NL, Rodriguez B, Milkovich KA, et al. TLR ligand-dependent activation of naïve CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 34.Dolganiuc A, Oak S, Kodys K, et al. Hepatitis C core and nonstructural 3 proteins trigger toll-like receptor 2-mediated pathways and inflammatory activation. Gastroenterology. 2004;127:1513–1524. doi: 10.1053/j.gastro.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 35.Foy E, Li K, Sumpter R, Jr, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci USA. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3 / 4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci USA. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malaguarnera M, Di F, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211–215. doi: 10.1007/BF02936370. [DOI] [PubMed] [Google Scholar]
- 38.Spanakis NE, Garinis GA, Alexopoulos EC, et al. Cytokine serum levels in patients with chronic HCV infection. J Clin Lab Anal. 2002;16:40–46. doi: 10.1002/jcla.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuman MG, Benhamou JP, Malkiewicz IM, et al. Cytokines as predictors for sustained response and as markers for immuno-modulation in patients with chronic hepatitis C. Clin Biochem. 2001;34:173–182. doi: 10.1016/s0009-9120(01)00212-0. [DOI] [PubMed] [Google Scholar]
- 40.Oyanagi Y, Takahashi T, Matsui S, et al. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19:464–472. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 41.Nelson DR, Lim HL, Marousis CG, et al. Activation of tumor necrosis factor-α system in chronic hepatitis C virus infection. Digest Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 42.Feldmann G, Nischalke HD, Nattermann J, et al. Induction of interleukin-6 by hepatitis C virus core protein in Hepatitis C-associated mixed cryoglobulinemia and B-cell non-Hodgkin’s lymphoma. Clin Cancer Res. 2006;12:4491–4498. doi: 10.1158/1078-0432.CCR-06-0154. [DOI] [PubMed] [Google Scholar]
- 43.Landay A, Benning L, Bremer J, et al. Correlates of immune activation marker changes in human immunodeficiency virus (HIV)-seropositive and high-risk HIV-seronegative women who use illicit drugs. J Infect Dis. 2003;188:209–218. doi: 10.1086/376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 45.Leng Q, Borkow G, Weisman Z, Stein M, Kalinkovich A, Bentwich Z. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27:389–397. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 46.Al-Harthi L, Voris J, Du W, et al. Evaluating the impact of hepatitis C virus (HCV) on highly active antiretroviral therapy-mediated immune responses in HCV / HIV-coinfected women: role of HCV on expression of primed / memory T cells. J Infect Dis. 2006;193:1202–1210. doi: 10.1086/500843. [DOI] [PMC free article] [PubMed] [Google Scholar]