Abstract
Context
Etiologic factors and pathways leading to altered podocyte phenotype are clearly numerous and involve the activity of different cellular function.
Objective
To focus on recent discoveries in podocyte biology and genetics and their relevance to these human glomerular diseases, named podocytopathies.
Data Sources
Genetic mutations in genes encoding for proteins in the nucleus, slit diaphragm, podocyte cytoplasm, and cell membrane are responsible for podocyte phenotype and functional abnormalities. Podocyte injury may also derive from secondary stimuli, such as mechanical stress, infections, or use of certain medications. Podocytes can respond to injury in a limited number of ways, which include (1) effacement, (2) apoptosis, (3) arrest of development, and (4) dedifferentiation. Each of these pathways results in a specific glomerular morphology: minimal change nephropathy, focal segmental glomerulosclerosis, diffuse mesangial sclerosis, and collapsing glomerulopathy.
Conclusions
Based on current knowledge of podocyte biology, we organized etiologic factors and morphologic features in a taxonomy of podocytopathies, which provides a novel approach to the classification of these diseases. Current and experimental therapeutic approaches are also discussed.
In the past few years, an increased number of podocyte-expressed genes have been identified, stimulating a growing interest in studying their role in the development of proteinuria and glomerular disease. Substantial progress has been made not only in clarifying the mechanisms of podocyte injury as a major cause of proteinuria, but also in organizing this new knowledge and integrating it in new classification schemes where morphology, etiology, and mechanisms of podocyte injury are the variables that define each glomerulopathy. Here, we describe how one such schema relates to the pathogenesis of the various lesions.
Podocytes are highly specialized postmitotic cells with different structural and metabolic functions, including maintaining the filtration barrier and the normal structure of the glomerular tuft, remodeling the glomerular basement membrane (GBM), performing endocytosis of filtered proteins, and counteracting the intracapillary hydrostatic pressure.1
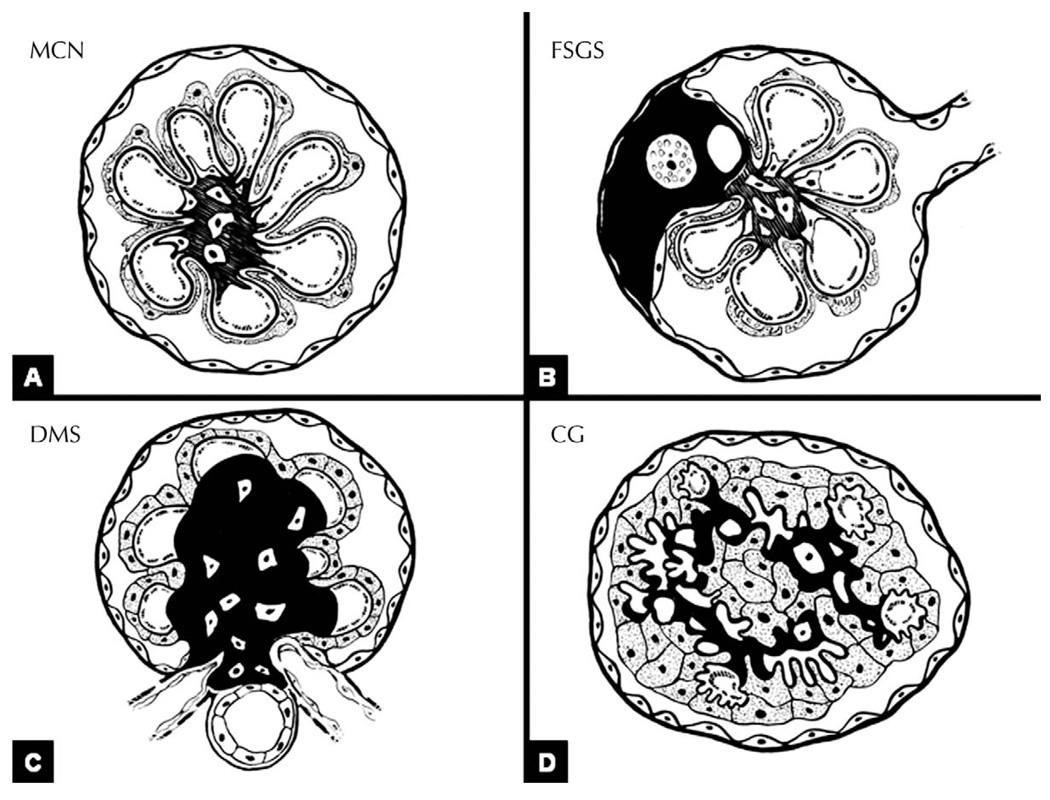
Podocytes are formed by a cell body containing the nucleus, primary processes, and foot processes, which adhere to the GBMs and are connected to each other by the slit diaphragms (SDs). The complex structure of the cell may be altered at any level: podocyte injury may derive from abnormalities of transcription factors in the nucleus, altered energy production by mitochondria in the cytoplasm, altered intracellular Ca+ homeostasis or aberrant lysosomal activity, abnormalities of proteins residing in the luminal (facing the urinary space) or abluminal (facing the GBM) surface of the cell membranes, and abnormalities of proteins that are part of the SD complex, and therefore participate in maintaining the integrity of the filtration barrier, or that are part of the actin-based cytoskeleton. In addition, podocyte pathologic changes may derive from abnormal immune system activation or direct viral infection of podocytes. Abnormal cell function may reflect primary, intrinsic damage of the podocytes, sometimes genetically determined, or may be the result of extrinsic factors that interfere with the normal molecular anatomy and biology of the cell. In response to these stimuli and according to the developmental state at the time of the injury, podocytes may react in different ways: (1) they can modify their phenotype and undergo foot process effacement, a phenomenon that is not associated with alteration of the number of podocytes per glomerulus; (2) they can undergo apoptosis, an event that may occur as result of intrinsic or extrinsic damage; (3) under certain circumstances, podocytes may undergo developmental arrest and resemble immature precursors, a phenomenon generally accompanied by mild proliferative activity; and (4) adult podocytes can dedifferentiate and reenter the cell cycle to actively proliferate and, under specific circumstances, express a dysregulated phenotype. A recently proposed taxonomy of podocytopathies identifies 4 patterns of glomerular injury that appear to result from these 4 pathways that injured podocytes may take: (1) minimal change nephropathy (MCN), if the number of podocytes per glomerulus is unchanged; (2) focal segmental glomerulosclerosis (FSGS), if there is podocytopenia; (3) diffuse mesangial sclerosis (DMS), where low proliferative index has been described; or (4) collapsing glomerulopathy (CG), if there is marked proliferation (Figures 1 and 2).1
Figure 1.
Injured podocytes may take 4 different pathways. Under certain circumstances, injured podocytes undergo foot process effacement without significant change in podocyte number. When this occurs, the glomeruli generally appear normal on light microscopy (LM), as is the case of minimal change nephropathy (MCN). If injured podocytes enter apoptotic pathways, the number of podocytes per glomerulus decreases (podocytopenia). Podocyte death leads to focal segmental glomerulosclerosis (FSGS). If the injury occurs early in life, it may lead to developmental arrest. In this case, glomeruli maintain an immature appearance, as reflected by mesangial expansion and crowding of podocytes, which may express for proliferative activity. This phenomenon translates into the morphologic pattern of diffuse mesangial sclerosis (DMS). Instead of engaging apoptotic pathways, specific injurious events may stimulate podocyte dedifferentiation and proliferation. Morphologically, this phenomenon translates into collapsing glomerulopathy (CG).
Figure 2.
Podocytopathies: morphologic patterns. A, No changes are seen in minimal change nephropathy (MCN), with the exception of foot process effacement on ultrastructural analysis. B, On the contrary, in focal segmental glomerulosclerosis (FSGS), by definition there is focal and segmental solidification of the glomerular tuft, accompanied by adhesion of the tuft to the Bowman capsule. Hyaline material and foam cells can be appreciated within the sclerosis. Parietal cells are bridging from the Bowman capsule toward the glomerular tuft. C, In diffuse mesangial sclerosis (DMS), the sclerosis is global and involves the mesangium. The overlying podocytes appear cuboidal rather than flat, resembling more immature stages. D, In collapsing glomerulopathy (CG), the capillary lumina are obliterated by the wrinkled and folded glomerular basement membranes (collapsed). In contrast to FSGS and DMS, there is no increased extracellular matrix. Podocytes are increased in number and form pseudocrescents, filling the urinary space. Figure modified with permission from the Clinical Journal of the American Society of Nephrology, Copyright 2007.1
We propose that the 4 pathways of response to podocyte injury can result from multiple pathogenic stimuli. Here, we describe how recent advances in podocyte biology present paradigms of podocytopathic injury. Each of these relates to specific functions of the podocyte.
MECHANISMS OF INJURY IN THE PODOCYTOPATHIES
Transcriptional Regulators in Podocyte Injury
Numerous transcription factors have been characterized in podocyte gene regulation. Some of these have been implicated in human diseases, whereas others are associated only with experimental animal models. Improved understanding of signal transduction processes in podocytes not only has increased our knowledge of the mechanisms of diseases but also identified new potential therapeutic strategies to reverse foot process effacement, limit apoptosis, and control podocyte differentiation state (see below).
Wilms tumor 1 (WT1), a zinc finger protein, is a transcription factor that regulates podocyte differentiation state and is expressed in all cell precursors during early phases of development, but it is expressed only by podocytes as the nephron matures. Various degrees of loss of podocyte WT1 protein content or WT1 gene mutation are noted in the different forms of genetically determined or acquired podocytopathies, including steroid-resistant FSGS,2 DMS,3 and CG.4 Data from experimental models suggest that WT1 is critical to the development of podocyte architecture and integrity of the filtration barrier, and WT1 mutations are associated with an immature podocyte phenotype. Two WT1-associated proteins, WT1-interacting protein (WTIP) and brain acid-soluble protein 1, function as cofactors in WT1 transcriptional activity. In particular, WTIP, mapped on chromosome 19q13.1, a region with genes linked to familial FSGS, can translocate from the nucleus to the SD with the probable purpose of modulating SD assembly during development and in adult life. It has been proposed that WTIP regulates podocyte phenotype by translating extracellular information into altered gene expression, by shuttling between cytoplasm and nucleus and, when restricted to the nucleus, by repressing WT1 transcriptional activity.5
PAX2, a protein that regulates early kidney development, also is linked closely to WT1, and its expression is downregulated by increased expression of WT1 as the glomerulus matures. On the other hand, WT1 is a direct target of PAX2 activity. A role for PAX2 in the development of nephrotic syndrome has been demonstrated in experimental models as well as in human disease.3,6 Podocyte-specific transgene expression results in glomerular collapse, confirming the observation that in human CG, PAX2 is reexpressed in relation to podocyte injury. A tamoxifen-inducible model of PAX2 reexpression reveals morphologic changes similar to those of human CG, with podocyte proliferation, and points toward a direct regulatory effect of both WT1 and nephrin expression as a cause of foot process effacement and proteinuria.7
A third important podocyte transcription factor is LIM homeobox transcription factor 1β (Lmx1b), which is mutated in patients with nail-patella syndrome. The Lmx1b protein contains 2 zinc-binding LIM domains at the NH2 terminus, which represent the interface for the interaction with other proteins, and a homeodomain in the middle, which is responsible for DNA binding. The mutations described so far delete or inactivate the homeodomain, so that the mutated protein is no longer able to recognize its target genes (reviewed in Rascle et al8). Lmx1b regulates the expression of COL4A3 and COL4A4 genes, as well as NPHS2, the gene encoding for podocin, and its mutated form is associated with abnormal deposition of collagen in the GBM, impaired podocyte differentiation, and development of mesangial and segmental sclerosis.9 Because of the variable disease manifestations of nail-patella syndrome, it has been suggested that regulatory proteins of Lmx1b, such as lbd1, act as modifiers of the transcriptional activity.10
Other relevant podocyte transcription factors have been studied only in experimental models of proteinuria and/or in vitro. For example, Pod1 (capsulin, epicardin), a basic helix-loop-helix protein expressed in developing and adult podocytes,11 modulates the expression of another transcription factor crucial to cell differentiation process, MafB (Maf-1, Kreisler) (reviewed in Rascle et al8). Inactivation of MafB results in downregulation of NPHS1, NPHS2, and CD2AP, genes that encode for critical proteins of the SD, and consequent foot process effacement.12
FOXC2 (Mfh2) plays a role in determining proliferation and differentiation of multiple tissues, including podocytes. Foxc2 knockout mice retain an immature phenotype and do not have organized foot processes and SD. As in Lmx1b and Pod1 knockout mice, in mice lacking FOXC2 synthesis of collagen type IV α3 and α4 chains, nephrin, MafB, and Cited2, a novel podocyte transcriptional coactivator, are downregulated (reviewed in Rascle et al8).
Another relevant transcription factor in nephrotic syndrome is MATH6 which, like WT1, is found in the meta-nephric-derived mesenchyme during development, but its expression is restricted to the podocyte during adult life. MATH6 may have a role in modulating cell differentiation state, as its expression is reduced in experimental CG.13
The role of hypoxia-inducible factors (HIFs) has been studied recently in podocytes. Hypoxia-inducible factors are heterodimeric proteins where expression of the α subunit (but not the β subunit) is sensitive to oxygen levels. In conditions of normoxia, the HIF-α subunit promotes an interaction with the von Hippel-Lindau protein, which leads to ubiquitination and consequent degradation of the HIF-α. Under hypoxic conditions, HIF-α subunit is stabilized and interacts with the p300/CBP transcriptional coactivator, which is involved in Smad/transforming growth factor β (Smad/TGF-β)–mediated signaling.14 Hypoxia-inducible factor activity varies from cell to cell and from inducing apoptosis to inducing proliferation. Target genes for HIF are those encoding for vascular endothelial growth factor (VEGF) and the chemokine receptor Cxcr4,15 and proteins involved in activation of proliferation (reviewed in Maxwell16).
Peroxisome proliferator-activated receptor γ (PPAR-γ) is a member of the nuclear hormone receptor superfamily containing ligand- and DNA-binding domains. When it is coupled in a heterodimeric form with retinoid X receptor, it translocates into the nucleus, where it targets specific genes. In particular, PPAR-γ has a role in cell cycle regulation inhibiting cell growth and promoting differentiation. In a puromicin aminonucleoside (PAN) model of glomerular injury, activation of PPAR-γ protects podocytes from apoptosis.17 Similarly, all-trans retinoid acid was effective in the treatment of a PAN model of podocyte injury.18 All-trans retinoic acid has been shown to promote podocyte differentiation in vivo and in vitro, and to reduce proliferation and increase expression of NPHS1 and NPHS2.19 The mechanism of action appears to be the binding and activation of retinoic acid receptors which, in response, undergo major conformational changes and orchestrate the transcription of specific gene networks through binding to specific DNA elements and recruiting cofactor complexes, such as p300/CBP.20
Very recently, the role of the Notch signaling pathway has also been investigated in human and murine podocytopathies.21,22 The signaling pathway has important regulatory functions and is activated by the proteolytic cleavage of Notch receptors by γ-secretase; the Notch intracellular domain can then translocate into the nucleus and bind to RBPJ-κ, a nuclear transcriptor repressor. Notch signaling is known to control podocyte differentiation during early stages of development, but its suppression is necessary to promote terminal differentiation. Ectopic activation of Notch during development or in adult life results in phenotype heterogeneity. In fact, ectopic expression of Notch intracellular domain in embryonic podocytes of transgenic mice leads to dedifferentiation and reentry of the cell cycle (proliferation), with a resulting pattern of glomerular injury resembling diffuse mesangial sclerosis.22 In contrast, when Notch intracellular domain is induced in adult mice, increased apoptosis is detected, and the resulting phenotype resembles segmental sclerosis. Notch expression has also been detected in human FSGS and diabetic nephropathy, two known examples of podocytopenia-associated glomerular injury.21 It has been suggested that Notch signaling may represent a common pathway of podocyte injury and a potential target for treatment.22
Altered Components of the SD Complex
The essential role of podocytes in the development of nephrotic syndrome was brought to the attention of nephrologists by the work of Nagata and others23,24 and the discovery by Kestila et al25 of NPHS1, the mutated gene responsible for congenital nephrotic syndrome of Finnish type, encoding for a critical protein of the SD, nephrin. This initial observation was followed by additional discoveries in the genetics of proteins that comprise the SD. Mutation of the gene encoding for podocin (NPHS2) was described in patients with steroid-resistant nephrotic syndrome.26 Podocin is a scaffolding protein that interacts with the cytoplasmic domain of nephrin and is essential for the structural integrity of the SD, as suggested by the downregulation of nephrin expression in mice lacking podocin. As demonstrated by more than one animal model where nephrin or podocin is missing, defects of SD composition do not interfere with foot process formation and interdigitation, suggesting that SD formation is a phenomenon secondary to foot process formation, but the maintenance of foot process structure is dependent on SD proteins (reviewed in Kriz27). In some cases it has been speculated that lack of any of these crucial components may lead to arrested development; in others, to lethal podocyte injury. In human disease, abnormalities of the SD complex result in extensive foot process effacement but may manifest with more than one morphologic pattern of injury, from no significant abnormalities on light microscopy, resembling MCN, to DMS or FSGS.1
More recently, the role of CD2-associated protein (CD2AP) has been investigated in experimental and human disease with proteinuria.28–30 CD2AP has different functions, including regulation of the actin-based cytoskeleton, cell motility, endocytosis, and mediated by TGF-β apoptosis and survival pathways. CD2AP directly interacts with other SD proteins (including podocin and nephrin), serving as a bridging protein between the SD and other cytoplasmic proteins.31 Mice null for CD2AP develop proteinuria and mesangial sclerosis and proliferation.30 Similarly, homozygous or heterozygous CD2AP mutations have been found in patients who have nephrotic syndrome and mesangial as well as segmental sclerosis.28,29
In addition to expression, localization and function of these proteins are important. Experimental evidence suggests that more than one mechanism may disrupt the integrity of the SD and podocyte phenotype, including (1) genetically determined mutations in genes directly encoding for SD proteins,25,26 (2) modulation of the conformation of the SD by WTIP through interaction with CD2AP,5 (3) nuclear relocation of SD proteins, such as nephrin and the CD2AP-binding protein dendrin, and induction of TGF-β–mediated apoptosis,32 and (4) modulation of the integrity of the SD by external forces challenging podocytes, as may occur in hyperfiltration-mediated FSGS33 (discussed below).
Leaking of proteins is inevitable once the SD is mislocalized. Not only is the functional gate missing from its usual location, but podocytes undergo effacement, which means that they lose their interdigitation and reduce their points of intercellular contact. Protein may then leak through the space between the cell bodies of different podocytes, as the absence of proteins such as nephrin reduces the number of cell-cell adhesions.34 Proteinuria also may occur without mislocalization of the SD and in the presence of perfectly normal foot processes by morphology, as shown in 3 different models of proteinuria, one due to injection of antibodies to VEGF or soluble VEGF receptor 1, the second due to genetic deletion of nephrin expression, and the third resulting from deletion of the α3 chain of type IV collagen in the GBM.35
Abnormal Assembly or Function of the Actin-Based Cytoskeleton
The α4-actinin gene, ACTN4, produces a protein that links the cell membrane adhesive structures to the cytoskeleton. Its mutation causes autosomal dominant familial FSGS.36 The mechanisms by which podocyte and glomerular damage occurs has been elucidated only partially. It has been suggested that the mutated proteins have an increased actin-binding activity and divert its normal localization.37 Podocyte cell lines generated from α4-actinin–deficient mice were less adherent than wild-type cells to GBM, and this phenomenon was related to reduced phosphorylation of β1-integrin.38 Interestingly, mutation of ACTN4 appears to induce disease relatively late in life. This observation suggests that a second “hit” is necessary to disrupt the cytoskeletal apparatus, which is otherwise balanced even in the presence of abnormal α4-actinin. In support of the second-hit hypothesis, mice with disrupted cytoskeleton, such as synaptopodin-null mice, have normal phenotype but develop FSGS if they are also heterozygous for CD2AP mutations.39
In addition to genetically determined podocyte dysfunction, other mechanisms of podocyte injury induce reorganization of the actin-based cytoskeleton. Podocytes are continuosly exposed to mechanical stress as they counterbalance the hydraulic pressure in glomerular capillaries. The wall tension in glomerular capillaries is transmitted to the podocyte cytoskeletal apparatus by cell–matrix contacts, including α3β1-integrin and the dystroglycan complex. In vitro experiments have demonstrated that podocytes in culture reorganize the actin-based cytoskeleton in response to mechanical stress,33 initiating mechanos-transduction. This event is accompanied by upregulation of proteins that modify the state of adhesion of podocytes from strong to intermediate.40 In vivo, diminished cell adhesion may lead to podocyte detachment and loss, resulting in podocytopenia and FSGS.
Expression and Localization of Membrane (Abluminal and Luminal Side) Proteins
A hallmark of diseases of the glomerular filter is podocyte detachment, resulting in the detection of podocytes in the urine.41 The preservation of appropriate podocyte adherence to the underlying GBM is critical for maintenance of normal cell function. This interaction is modulated by both intrinsic podocyte proteins and extracellular matrix constituents of the GBM. Integrin and dystroglycan heterodimers serve as a bridge between matrix proteins and the podocyte contractile apparatus. The α3β1 integrin complex is involved in signaling events, as demonstrated in α3β1 knockout mice, which develop aberrant cytoskeleton and foot processes.42 During effacement, cytoskeletal rearrangement moves the foot processes along the GBM. Migratory capacity also may be modulated by redistribution to the foot processes of proteins, such as the lysosomal protease, cathepsin L, which promotes detachment of podocytes mediated by effects on α3 integrin.43 The role of extracellular matrix in regulating cell adhesion and foot process conformation is suggested by experimental models as well as by the observation that patients with Alport disease eventually develop FSGS.
The α-β dystroglycan complex appears to be the major player in connecting the extracellular matrix of the GBM to the cytoskeleton. The dystroglycan complex is downregulated in steroid-sensitive MCN. In contrast, in steroid-resistant MCN and in FSGS, we have observed preserved dystroglycan expression in 90% of the cases studied (L. De Petris and L.B., unpublished data). These findings support the notion that FSGS and steroid-sensitive MCN do not represent different, temporally sequential phases of the same disease and that there is more than one pathway to effacement.
A recent advance in familial FSGS has been the discovery of a mutant form of canonical transient receptor potential cation channel 6 (TRPC6), located in the luminal side of the podocyte cell membrane.44,45 The TRPC6 mutation causes a gain of function and increased intracellular calcium, but the mechanism by which these events lead to FSGS is unknown. It has been speculated that increased calcium concentration may modify the contractile apparatus. TRPC6 overexpression induces loss of stress fibers in cultured differentiated podocytes.46 TRPC6 is partially colocalized with nephrin and podocin, and it has been shown that following disruption of the SD complex, TRPC6 is overexpressed and mislocalized. These observations suggest that TRPC6 may be part of a complex signaling process that modulates the cytoskeleton as well as the SD. It has also been suggested that this complex contains Neph1, Neph2, nephrin, and CD2AP, as well as TRPC6, and acts as a sensor in monitoring glomerular pressure and filtration rate (reviewed in Huber et al47).
A role for calcium homeostasis in podocyte injury also has been supported by the observation that patients treated with high doses of bisphosphonates develop foot process effacement, which may manifest as part of MCN, FSGS, or CG.48,49 Bisphosphonates can be internalized by the cell and mediate various cellular activities, such as integrin signaling, endosomal trafficking, membrane raffling, and cytoskeletal organization.50
Other proteins located in the luminal/apical side of podocytes have been implicated in the mechanism of foot process effacement in experimental models. Proteins such as podocalyxin, the major sialoprotein of the cell membrane with a negative charge, appears to have a role in maintaining the SD structure. Disruption of podocalyxin or neutralization of its negative charge results in its dissociation from the cytoskeleton and subsequent foot process effacement.51
In most known podocytopathies, an intrinsic podocyte injury has been identified. What remains to be explained is the pathogenesis of the so-called idiopathic MCN, FSGS, or CG, where no specific intrinsic podocyte damage can be proved. It should be noted that the frequency of true idiopathic cases will continue to decrease as we learn more about the pathobiology of the podocytopathies. For example, it has been suggested that the immune system may play a major role in inducing podocyte injury and proteinuria. An interesting related observation is that B7-1, a transmembrane protein present in the B cell and other antigen-presenting cells and a costimulator that mediates outside-in signaling in B cells, is also expressed in podocytes. B7-1 expression is increased in nephrotic syndrome and following activation of the immune system by LPS in experimental models of proteinuria.52,53
Dysfunction of Cytoplasmic Proteins
Phospholipase Cε1 (PLCε1) is a signaling protein that promotes protein kinase C and enhancing calcium signaling. Its activity is regulated by many G-protein–coupled receptors. Its expression begins at the S-shaped body stage of nephron development and peaks at the capillary-loop stage. Lack of PLCε1 interferes with normal development and is associated with reduced nephrin and podocin expression. In childhood nephrotic syndrome secondary to mutation in PLCE1, the morphologic features at renal biopsy may vary from DMS in cases of truncating mutation, to FSGS with slightly later onset of the disease in cases of nontruncating mutation.54 Truncating mutations in the PLCE1 gene may be the most frequent genetic abnormality in children previously diagnosed as having “id-iopathic” DMS.55
Podocyte injury also can occur if mechanisms of energy production are affected. That is the case with the mito-chondriopathies, diseases that may affect one or more cell types and manifest as syndromic or nonsyndromic disorders. Mitochondrial injury has been described as a primary or secondary process in nephrotic syndrome. Primary mitochondrial dysfunction and respiratory chain deficiency were described in 2 boys and 1 girl with severe congenital nephrotic syndrome and DMS, in patients with mitochondrial tRNALeu and tRNATyr gene mutation and FSGS, and in patients with mutation in the genes encoding for enzymes of the CoQ10 biosynthetic pathway: COQ2, encoding the para-hydroxybenzoate-polyprenyl-transferase, and PDSS2, encoding for decaprenyl diphosphate synthase (reviewed in Barisoni et al56). Mutations in the gene encoding for CoQ6 have also been associated with FSGS and DMS (F. Hildebrandt, personal communication, June 2008). Secondary mitochondrial dysfunction has also been described in experimental FSGS induced by puromicin57 as well as in children with congenital nephrotic syndrome of Finnish type.58,59 Mitochondrial toxicity has been suggested as a mechanism leading to proteinuria in patients treated with high doses of pamidronate.60 The mechanism by which mitochondrial activity may mediate foot process effacement and FSGS is complex and still under investigation. On the one hand, mitochondria are crucial for cell motility via their interaction, mediated by the outer membrane protein zyxin, with the actin cytoskeleton. This observation suggests that intrinsic abnormalities of mitochondria may lead to effacement in a cytoskeleton-dependent manner.61 On the other hand, these organelles also are mediators of apoptotic stimuli, and cell death is the prerequisite for the development of segmental sclerosis. What remains to be explained is why some of these patients develop DMS or CG, where proliferation appears to be the defining variable for the diagnosis. One explanation may come from experimental models of ischemic myocardial injury, where mitochondria have been shown to promote proliferation rather than apoptosis. This pathway is mediated by the ion channels of the inner membrane, with activation of HIF-1 and increased expression of adaptive enzymes resulting in protection of the cells from death.62 As discussed above, HIF-1 is also implicated in promoting podocyte proliferation.15,16
Other syndromic genetic diseases associated with abnormalities that affect podocytes include lysosomal storage disorders, such as Fabry disease, due to α-galactosidase A (α-GAL A) deficiency, with glomerular lesions resembling FSGS; or action myoclonus-renal failure, due to a mutation in a gene encoding a lysosomal protein (SCARB2/LIMP-2), and presenting with glomerular lesions resembling CG.63 The mechanism by which accumulation of lysosomal inclusions in cells leads to FSGS needs to be further investigated. However, 2 major processes may be implicated: for example, defective activity of α-GAL A results in accumulation of globotriaosylceramide in endothelial cells of the microvasculature. This phenomenon may be associated with chronic ischemic changes, which may lead to podocyte damage and death. Alternatively, accumulation of globotriaosylceramide in podocytes may be directly lethal to this postmitotic cell. The mechanism by which other lysosomal defects lead to CG is still obscure, but it is possible that accumulation of lysosomal vacuoles not only in podocytes but also in endothelial cells may lead to secondary podocyte injury, ischemia induced.
PATHWAYS OF PODOCYTE INJURY
The pathways leading to altered podocyte phenotype are clearly numerous and involve the activity of different cellular apparati. There is also a long list of etiologic factors involved in stimulating any of these mechanisms. The next question to ask is how so many diverse stimuli translate into only 4 morphologic patterns of glomerular damage. The answer lies in the observation that podocytes can react to injurious stimuli in a very limited number of ways1 (Figure 1):
Effacement: Injured podocytes can simply undergo effacement with reorganization or redistribution of the cytoskeleton, SD, and other proteins. That is the case with MCN.
Apoptosis: Podocytes engage fatal pathways, detach, and die. Once podocytopenia is detectable, the glomerulus is committed to sclerosis (FSGS).
Arrested development: Podocytes fail to complete maturation, accompanied by preserved proliferative activity, typical of immature phases of nephrogenesis. The glomeruli also maintain an immature appearance with increased mesangial matrix (DMS).
Dedifferentiation: Injured podocytes regress to a more immature state and reengage the cell cycle; this phenomenon is associated with the morphologic picture of CG.
TAXONOMY OF THE PODOCYTOPATHIES
How does the above discussion translate into clinical practice? The difficult task for pathologists, nephrologists, and scientists is to put together all this information about podocyte mechanisms of injury with conventional pathologic diagnoses and patient care, with the final goal being to better target therapies for each form of nephrotic syndrome. In order to meet this goal, we have created a taxonomy of diseases with podocyte injury, termed podocytopathies (Table 1), organized along 2 axes: histopathology, defined by glomerular morphology and podocyte number, and etiology.1 We identified 4 major histopathologic entities, which are morphologically distinct (Figure 2, A through D; Table 2) but all have a variable degree of foot process effacement:
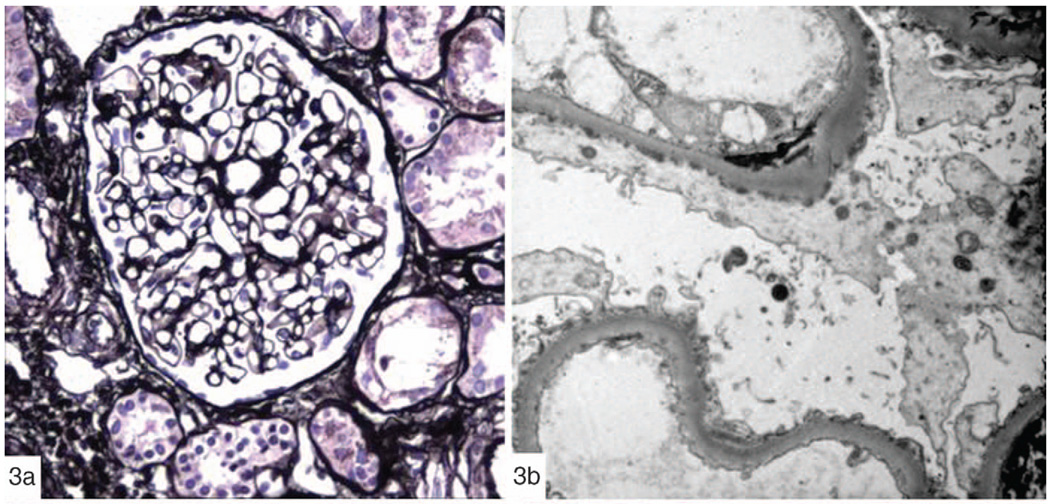
MCN, by definition with normal morphology on light microscopy and preserved podocyte number (Figures 2, A, and 3, a and b);
FSGS, defined by segmental solidification of the tuft, occasionally accompanied by hyalinosis, foam cells or adhesion to the Bowman capsule, and decreased number of podocytes (podocytopenia; Figures 2, B, and 4);
DMS, defined by the presence of mesangial sclerosis with or without mild mesangial proliferation, and mildly increased proliferative activity of podocytes (Figures 2, C, and 5);
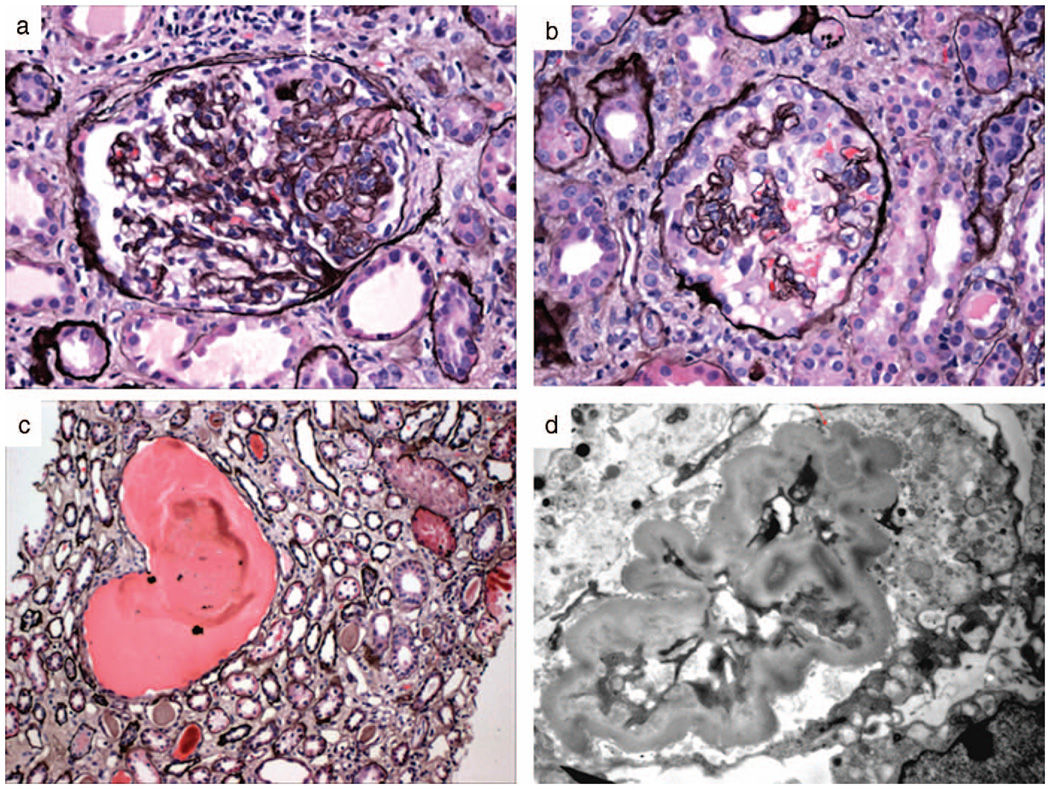
CG, where by definition the glomerular damage causes segmental or global capillary wall collapse and podocyte number is substantially increased with pseudocrescent formation (Figures 2, D, and 6, a through d).
Table 1.
Taxonomy of the Podocytopathies*
| Idiopathic Forms | Genetic Forms | Reactive Forms | |
|---|---|---|---|
| MCN | Idiopathic MCN Steroid sensitive Steroid resistant |
Nonsyndromic NPHS2 Syndromic DYSF (limb-girdle muscular dys- trophy 2B) |
Reactive MCN Hodgkin disease Immunogenic stimuli Medication associated: nonsteroidal anti-inflammatory agents, gold, penicillamine, lithium, interferon α and γ, biophospho- nates |
| FSGS | Idiopathic FSGS | Nonsyndromic NPHS1+ NPHS2 NPHS2 ACTN4 CD2AP TRPC6 WT1 mtDNA tRNALeu mtDNA tRNATyr PLCE1 Syndromic WT1 (Frasier) mtDNA tRNALeu (MELAS) PAX2 (renal-coloboma syndrome with oligomeganephronia) LMX1B (nail-patella) COQ2 COQ6‡ PDSS2 (Leigh) ITGB4 COL4A3, A4, A5 (Alport) GLA (Fabry) |
Postadaptive FSGS Reduced nephron mass: renal dysplasia, surgical renal mass re- duction, reflux nephropathy, chronic interstitial nephritis Initially normal nephron mass: obesity, increased muscle mass, sickle cell anemia, cyanotic congenital heart disease, hyperten- sion† Medication associated Cyclosporine, tacrolimus, interferon γ, lithium, biophosphonates |
| DMS | Idiopathic DMS | Nonsyndromic NPHS1 (CNF†) WT1 NPHS2 PLCE1 LAMB2 Syndromic LAMB2 (Pierson) WT1 (Denys-Drash) COQ6‡ |
|
| CG | Idiopathic CG | Nonsyndromic COQ2 Syndromic SCARB2/Limp2 (action myoclo- nus-renal failure) ZMPSTE24 (mandibuloacral dys- plasia) |
Infection Viruses (HIV-1, parvovirus B19, CMV) Others (Campylobacter enteritis, Loa loa filiariasis, visceral leish- maniasis, Mycobacterium tuberculosis) Disease associations Autoimmune diseases,§ Guillan-Barre syndrome, thrombotic mi- croangiopathy, hematologic malignancy‖ Medications Interferon γ, biophosphonates, valproic acid, calcineurin inhibi- tors Others Permeability factor Severe hyaline arteriopathy |
This table has been modified from its previous version reported in the Clinical Journal of the American Society of Nephrology, Copyright 2007.1 The primary podocytopathies are organized into 4 morphologic patterns and 3 etiologic categories. The morphologic patterns include minimal change nephropathy (MCN), focal segmental glomerulosclerosis (FSGS), diffuse mesangial sclerosis (DMS), and collapsing glomerulopathy (CG). Named syndromes are shown. The genes, encoded proteins, and syndrome abbreviations are as follows: NPHS2, podocin; DYSF, dysferlin; NPHS1, nephrin; ACTN4, α4-actinin; CD2AP, CD2-associated protein; TRPC6, transient receptor potential cation channel, member 6; WT1, Wilms tumor 1; PLCE1, phospholipase Cε1; MELAS, mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes; PAX2, paired homeobox protein 2; LMX1B, LIM homeobox transcription factor 1 β; COQ2, coenzyme Q synthetase 2; COQ6, coenzyme Q synthetase 6; PDSS2, decaprenyl diphosphate synthase; ITGB4, β4-integrin; COL4, type IV collagen; GLA, α-galactosidase A; LAMB2, laminin β2 chain; ZMPSTE24, zinc metalloproteinase gene. CNF indicates congenital nephrotic syndrome of Finnish type; HIV, human immunodeficiency virus; and CMV, cytomegalovirus.
Disease associations for which causation has not been clearly established.
COQ6 mutations have been discovered recently in 7 siblings from 3 different families. A total of 6 of the 7 patients had FSGS, and 1 had DMS. All patients had sensorineural deafness (F. Hildebrandt, personal communication, June 2008; manuscript in preparation).
Autoimmune diseases include adult Still disease, mixed connective tissue disorder, systemic lupus erythematosus, and lupuslike syndrome.
Hematologic malignancies include multiple myeloma, acute monoblastic leukemia, and hemophagocytic syndrome.
Table 2.
Major Morphologic Features of Podocytopathies*
| Light Microscopy |
|||
|---|---|---|---|
| Glomeruli | Tubulointerstitium | Electron Microscopy | |
| MCN | Normal morphology | Generally normal | Extensive foot process effacement Condensation of the actin-based cytoskeleton against the ablu- minal surface of podocytes Microvillous transformation of the luminal surface of podocytes |
| FSGS | Focal and segmental solidi- fication of the tuft Adhesion of the tuft to the Bowman capsule (sine- chae) Hyalinosis Foam cells Hypertrophy of podocytes Bridging of parietal cells |
Focal interstitial fibrosis Focal tubular atrophy Minimal inflammation, generally restricted to the areas of fibrosis |
Variable degree of foot process effacement Focal condensation of the actin-based cytoskeleton against the abluminal surface of podocytes Focal microvillous transformation of the luminal surface of podo- cytes Focal detachment from the underlying GBM with interposition of newly formed extracellular matrix |
| DMS | Diffuse and global in- creased mesangial matrix Mild mesangial cell hyper- cellularity Marked podocyte hypertro- phy Occasionally, slight imma- ture appearance of the glomeruli |
Focal interstitial fibrosis Focal tubular atrophy Minimal inflammation, generally restricted to the areas of fibrosis Microcysts can be seen |
Extensive foot process effacement Lack of primary processes Podocytes have a cuboidal (immature) appearance |
| CG | Segmental or global wrin- kling and folding of the GBM (collapse) Podocyte hypertrophy and hyperplasia (pseudocres- cents) |
Extensive interstitial fibrosis and inflammation Tubular atrophy Microcysts |
Extensive foot process effacement. Loss of primary processes Podocytes acquire a cuboidal appearance with pale cytoplasm Absent actin-based cytoskeleton Protein reabsorption droplets in podocyte cytoplasm Wrinkling and folding of the underlying GBM Focal detachment from the underlying GBM with interposition of newly formed extracellular matrix Loss of fenestration of endothelial cells In HIV-CG, tubuloreticular inclusions in endothelial cell cyto- plasm |
MCN indicates minimal change nephropathy; FSGS, focal segmental glomerulosclerosis; GBM, glomerular basement membrane; DMS, diffuse mesangial sclerosis; CG, collapsing glomerulopathy; and HIV, human immunodeficiency virus.
Figure 3.
Minimal change nephropathy. a, A normal glomerulus on light microscopy (silver, original magnification ×40). On electron microscopy (b), the glomerular basement membranes are normal in thickness and contour. Podocytes reveal extensive foot process effacement accompanied by condensation of the actin-based cytoskeleton against the sole of podocytes and microvillus transformation (uranyl acetate and lead citrate stain, original magnification ×10000).
Figure 4.
Focal segmental glomerulosclerosis. The glomerulus is partially obliterated by segmental sclerosis and hyalinosis. The tuft is adhering to the Bowman capsule, and no podocytes are present in the area where solidification of the tuft is seen (hematoxylin-eosin, original magnification.×40).
Figure 5.
Diffuse mesangial sclerosis. Two glomeruli reveal advanced global sclerosis with obliteration of the capillary lumina. Podocytes are hypertrophic and have a cuboidal appearance. Tubulointerstitial damage with focal dilatation of the tubules is also present (hematoxylin-eosin, original magnification ×40). Pictures were kindly provided by Helen Liapis, MD, Washington University, St Louis, Mo.
Figure 6.
Collapsing glomerulopathy. a, Segmental collapse with early podocyte hypertrophy and hyperplasia (white arrow). The remaining portion of the glomerulus appears intact (silver, original magnification ×40). b, Global collapse with almost complete obliteration of the capillary lumina by wrinkled glomerular basement membranes. Podocytes are markedly hypertrophic and hyperplastic and form pseudocrescents obliterating the urinary space (silver, original magnification ×40). c, Microcysts can be present in the interstitium and contain large proteinaceous casts (silver, original magnification ×20). d, On ultrastructural analysis, the folded glomerular basement membranes can be appreciated. The capillary lumen is obliterated. The overlying podocyte has a large, pale cytoplasm and lack of foot processes and primary processes. No condensation of the actin-based cytoskeleton is noted (uranyl acetate and lead citrate stain, original magnification ×10000).
Each of these morphologic entities needs then to be classified according to etiology as idiopathic, genetically transmitted, or reactive/secondary (Table 1). For proposed mechanisms of disease, see Figures 7 through 10.
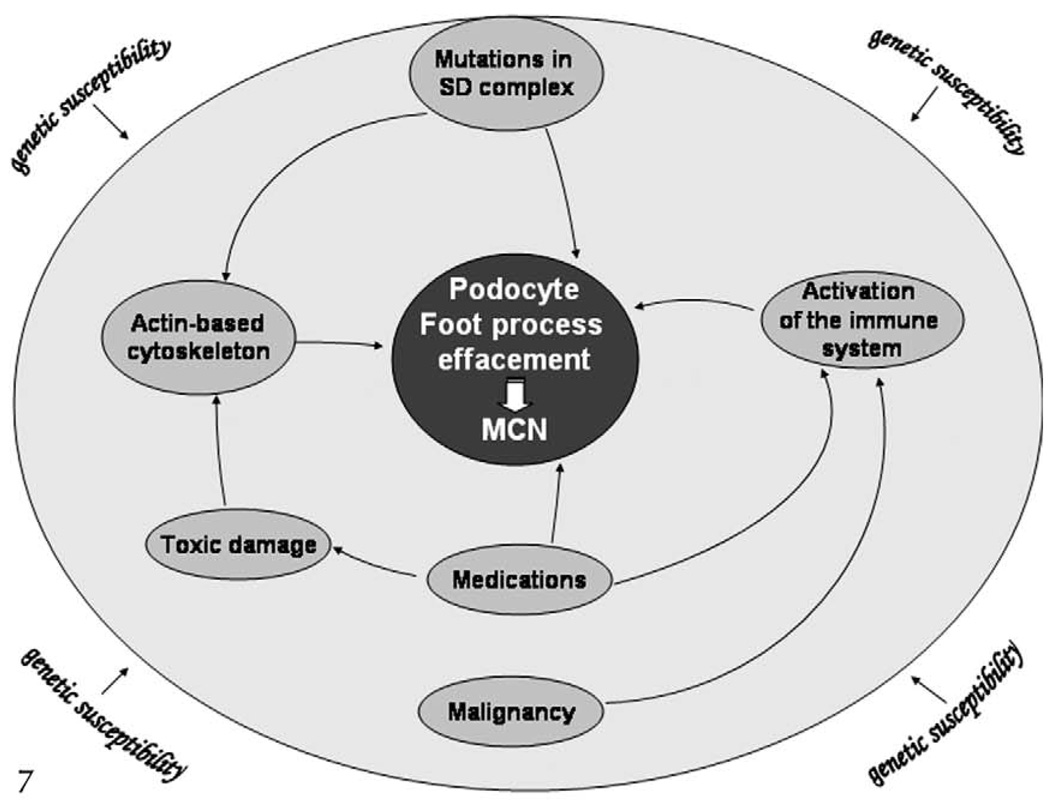
Figure 7.
Mechanisms of disease: minimal change nephropathy (MCN). Minimal change nephropathy may result from specific mutations in genes encoding for slit diaphragm (SD) proteins, activation of the immune system, or modification of the actin-based cytoskeleton. It is possible that genetic susceptibility may play a role in the disease.
Figure 10.
Mechanisms of disease: collapsing glomerulopathy (CG). Collapsing glomerulopathy derives from various and heterogeneous etiologic factors that may act through few common pathways here represented. Some forms are clearly linked to a specific genetic mutation; however, it has been recognized that there is probably a genetic susceptibility to develop CG once the patient encounters any of the potential injurious stimuli. Genetic variation in MYH9 has been recently identified as a risk factor for certain forms of CG in African Americans.109 Figure modified with permission from the Clinical Journal of the American Society of Nephrology, Copyright 2007.65
The relationship between DMS and CG is interesting. In both cases there is podocyte proliferation, although in DMS the proliferative activity is lower. It has been noted that some cases of DMS also have morphologic features resembling pseudocrescents.64
The taxonomy of the podocytopathies differs from previous classification schemes by several points. Previous morphologic classifications were limited to only some of these diseases but did not include, for example, MCN and DMS. Moreover, they did not take into consideration recent discoveries in podocyte biology and mechanisms of foot process effacement, as well as etiology. Differently from the Columbia classification, in our taxonomy CG is not listed as part of the spectrum of FSGS, but as an independent disease. The rationale behind this distinction is based on 2 considerations. First is the morphologic definition of these 2 entities itself, with FSGS defined by solidification of the tuft with increased extracellular matrix, and CG characterized by wrinkled and folded GBM covered by pseudocrescents. Second, the pathogenetic mechanism(s) lead to podocytopenia in one case and cell proliferation in the other (reviewed in Barsoni et al1 and Albaqumi and Barisoni65). The taxonomy also does not include the cellular lesion and tip lesion.
The Cellular Lesion
Cellular lesion is an uncommon disease, and it is not fully characterized. We know that it presents with nephrotic syndrome and has a clinical prognosis intermediate between FSGS and CG,66,67 but very little is known about its etiology and pathomechanism. Although morphologically there is foot process effacement, the hallmark of the disease is endocapillary proliferation, mostly due to endothelial cell hypertrophy and hyperplasia. It is intriguing to speculate that the cellular lesion may possibly represent an “endotheliopathy” with a secondary podocyte injury, or a mixed endotheliopathy and podocytopathy, probably mediated by mechanism of cross-talk between endothelium and podocytes. Another example in the literature of simultaneous endothelial and podocyte damage presenting as proteinuria is preeclampsia, mediated by a soluble VEGF receptor, which acts as an inhibitor of endogenous VEGF, normally synthesized in podocytes and critical for maintenance of endothelial fenestration (reviewed in Stillman and Karumanchi68). The role of VEGF in mediating endothelial and podocyte injury has been further elucidated recently by the observation that cancer patients treated with anti-VEGF antibodies may develop severe glomerular lesions with endothelial cell and podocyte injury and severe proteinuria.69 These findings are reproducible in animal models involving either administration of anti-VEGF antibodies70 or genetic suppression of VEGF expression exclusively in podocytes.69 Some of the patients with preeclampsia also have FSGS-like lesions, which appear to be reversible after pregnancy.71 In contrast, patients with primary/idiopathic cellular lesions have a worse prognosis with progression of the disease,67 perhaps due to continued exposure to the as-yet unidentified injurious stimulus. Although not much can be said about the pathogenesis, it is possible that in the future a fifth subcategory of podocytopathy will be created to include diseases with dual damage across the basement membrane and manifesting simultaneous endothelial and podocyte injury.
The Tip Lesion
The case of tip lesion is slightly different. Morphologically, it is defined by the presence of foam cells within the tip of the glomerular tuft, podocyte hypertrophy (but not hyperplasia) with bridging of podocytes toward the area where the parietal epithelium meets the tubular epithelium, and extensive foot process effacement on ultrastructural analysis. The nature of this morphologic entity and its relationship with primary podocyte injury remains to be investigated. Howie and colleagues72 have shown that abnormalities of the glomerular tuft on the site of the tubular origin, including foam cells occluding the capillary lumina and demonstrated by immunohistochemistry to be monocytes/macrophages, precede prolapse of podocytes into the proximal tubule origin and adhesion formation. Why this occurs at the tip of the glomerular tuft is still unknown. In addition, tip lesions are not considered a specific independent entity, but have been described in association with numerous diseases, including primary podocytopathies. Some authors believe it is a nonspecific glomerular reaction to proteinuria.73–75 According to the unifying hypothesis of Howie et al,76 enlargement of the tuft may be the primary cause of the tip lesion, leading to mechanical damage of podocytes and consequent prolapse into the tubular origin.76
Podocytopathies in Kidney Transplants
Most podocytopathies can recur after transplantation. Proteinuria and foot process effacement are not unusual, regardless of the original disease in the native kidney, but a distinction should be made between de novo and recurrent podocyte injury and between early versus late onset of proteinuria. In patients undergoing transplantation after chronic kidney failure secondary to FSGS, proteinuria and segmental sclerosis can recur within the first 4 to 8 weeks after transplantation in approximately 30% of patients. Recurrence is more common in whites and Hispanics than in African Americans.77 A circulating factor78 or the presence of immunologic factors79 has been proposed as the mechanism of podocyte injury. This hypothesis is reinforced by the observation that after transplantation, recurrent proteinuria is often sensitive to plasmapheresis and/or cyclophosphamide. Unexpectedly, a small percentage of patients with known podocin deficiency also have recurrent podocyte injury after transplantation, despite the complete absence of anti-podocin antibodies.80 There is no clear explanation for posttransplant recurrence of proteinuria in patients with homozygous or heterozygous NPHS2 mutations. It has been suggested that proteinuria may be explained by a multifactorial pathogenesis, with susceptibility to the disease given by podocin but really produced by plasma mechanisms.81 No recurrence of DMS is known in patients with WT1 mutations. The causes of recurrent CG have not been addressed in the literature, but it is reasonable to speculate that, similarly to FSGS, a plasma component or an abnormal immune response inducing proliferative pathways may be implicated. Although a specific circulating factor has not yet been identified, rats injected with serum from patients with CG develop proteinuria and glomerular disease.82 Given the known causative relationship between CG and viral infection, it is also possible that latent viruses (such as parvovirus B19, for example) can be reactivated upon immunosuppression.
Podocytopathies can occur as de novo diseases. For example, a single kidney is susceptible to hyperfiltration and, with time, mechanical stress can compromise podocyte integrity and lead to FSGS. The possibility of a preexisting condition in the donor kidney that predisposes to or accelerates podocyte injury should also be taken into consideration during evaluation of potential donors. De novo CG also has been described as idiopathic83 or in association with diseases that are causative for glomerular collapse in native kidneys. Immunocompromised patients are obviously more susceptible to infections such as CMV or parvovirus B19.84 In addition, obliterative vascular changes secondary to transplant arteriopathy, vascular rejection, or calcineurin inhibitor–induced thrombotic microangiopathy can cause collapsing pattern of glomerular injury.85
The same consideration and precautions used in the treatment of native kidney podocytopathies should be used when approaching these diseases in allografts, and etiology and pathogenetic mechanisms should be taken into serious consideration before approaching already immunocompromised patients.
THERAPEUTIC APPROACH TO PODOCYTOPATHIES
Conventional Therapies
Conventional treatment approaches to the podocytopathies should take into account available genetic testing (Table 3). Current approaches are outlined in Table 4. At present, these therapies have been adopted based largely on empiric evidence of efficacy. Only cyclosporine has been shown efficacious in FSGS in a randomized controlled trial. Further, we have an extremely limited understanding of how these therapies might exert favorable effects on the podocytes.
Table 3.
Genetic Podocytopathies: Genetic Testing Available From CLIA-Certified Laboratories*
| Gene | Protein | Locus | CLIA-Certified Laboratory |
Contact | Methodology |
|---|---|---|---|---|---|
| NPHS1 | Nephrin | 19q13.1 | Athena Diagnostics, Boston, Mass |
www.athenadiagnostics.com | Sequence all exons |
| NPHS2 | Podocin | 1q25.2 | Athena Diagnostics | www.athenadiagnostics.com | Sequence all exons |
| WT1 | Wilms tumor 1 | 11p13 | Athena Diagnostics; University of Colora- do, DNA Diagnostic Laboratory, Denver |
www.athenadiagnostics.com; elaine.spector@uchsc.edu | Sequence all exons |
| ACTN4 | α4-actinin | 19q13.2 | Center for Nephrology and Metabolic Dis- orders, Weisswasser, Germany |
labor@moldiag.de | Sequence all exons |
| TRPC6 | Transient receptor potential, cation channel, subfam- ily C, member 6 |
11q22.1 | Center for Nephrology and Metabolic Dis- orders |
labor@moldiag.de | Sequence all exons |
| CD2AP | CD2-associated protein |
6p12.3 | Center for Nephrology and Metabolic Dis- orders |
labor@moldiag.de | Sequence all exons |
| Mitochondrial tRNALeu |
NA | NA | Athena Diagnostics | www.athenadiagnostics.com | 11 Mitochondrial DNA sin- gle-nucleotide polymor- phisms associated with neuromuscular disorders, including MELAS |
Academic laboratories carrying out genetic testing, as well as Clinical Laboratory Improvement Amendments (CLIA)–certified laboratories, can be found at www.genetests.org (last accessed May 30, 2008). Permission to publish contact e-mail addresses has been granted by all principal investigators and laboratories. NA indicates not applicable; MELAS, mitochondrial encephalopathy, lactic acidosis, and stroke.
Table 4.
Conventional Therapy for the Podocytopathies*
| Reactive Forms |
||||
|---|---|---|---|---|
| Idiopathic Forms | Genetic Forms | Others | Medication Associated | |
| MCN | First: daily prednisone Second: CSA or tacrolimus Third: mycophenolate mofetil |
PLCE1 mutation: glucorticoids† | Treat underlying disease | Stop medication Consider glucorticoids |
| FSGS | Nephrotic proteinuria First: daily prednisone or al- ternate day prednisone (fewer data) Second: CSA/tacrolimus Third: mycophenolate mofetil Subnephrotic proteinuria As above or ACEI or ARB |
NPHS2 mutation Avoid glucorticoids Cautious trial CSA,† or ACEI† or ARB† Other mutations ACEI† or ARB† or possibly CSA† CoQ10† in CoQ2 nephropathy and Leigh syndrome |
Postadaptive FSGS ACEI or ARB† Obesity-associated: weight loss Treat underlying disease |
Stop medication Consider glucorcorti- coids |
| DMS | ACEI/ARB† | ACEI† or ARB† | ||
| CG | First†: daily prednisone or daily prednisone + CSA Second†: CSA or tacrolimus Third†: mycophenolate mofetil or sirolimus |
CoQ10† in CoQ2 nephropathy | HIV-1 associated HAART therapy—ACEI or ARB† Parvovirus B19-associated IVIG only if immunosup- pressed† |
Stop medication Consider glucorti- coids† |
Therapies without symbols are supported by randomized controlled trials (cyclosporine for focal segmental glomerulosclerosis [FSGS] is the only therapy with this level of support) or by nonrandomized controlled trials, uncontrolled trials, or observational studies (all other therapies). MCN indicates minimal change nephropathy; CSA, cyclosporine; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DMS, diffuse mesangial sclerosis; CG, collapsing glomerulopathy; HAART, highly active antiretroviral therapy; IVIG, intravenous immunoglobulin; and HIV, human immunodeficiency virus.
Therapies that are supported by opinion from one or more expert clinicians.
Novel Therapies
Given the limited efficacy of conventional therapies, there is currently intense interest in novel approaches. Sirolimus has been used in FSGS, with 1 group reporting favorable results86 and 2 groups cautioning about possible exacerbation of glomerular dysfunction.87,88 Pirfenidone, an antifibrotic agent being developed for idiopathic pulmonary fibrosis, has been shown to slow progressive loss of renal function in patients with advanced FSGS.89 Table 5 lists 6 clinical trials that are currently enrolling subjects.
Table 5.
Active Clinical Trials for the Podocytopathies*
| Trial Identifier | Disease Entities | Therapy | Trial Design | Sponsor | Sites |
|---|---|---|---|---|---|
| NCT00065611 | MCN, FSGS, CG |
Pulse dexamethasone | Open-label, randomized to 2 doses |
NIDDK, Bethesda, Md | NIH Clinical Center, Bethes- da, Md; Mayo Clinic, Rochester, Minn |
| NCT00098020 | MCN, FSGS, DMS, CG |
Isotretinoin | Open label, randomized to 2 doses |
NIDDK | NIH Clinical Center, Bethes- da, Md |
| NCT00135811 | FSGS, CG | 1. CSA 2. Pulse dexametha- sone + mycophen- olate mofetil |
RCT | NIDDK | Multicenter (United States): data coordinating center, Cleveland Clinic, Cleve- land, Ohio |
| NCT0019927 | Proteinuric ne- phropathies |
Fluvastatin | RCT, active control |
Mario Negri Institute, Bergamo, Italy |
Multicenter (Italy): Mario Ne- gri Insititute |
| NCT0040483 | FSGS, CG | 1. Prednisolone + mycophenolate 2. Prednisolone + chlorambucil |
RCT | University of Hong Kong, Hong Kong, China |
University of Hong Kong |
| NCT00464321 | FSGS, CG | Anti-TGF- β1,2,3 hu- manized monocle- nal antibody (GC1008) |
Phase I, single dose |
Genzyme, San Fran- cisco, Calif |
Multicenter (USA and Eu- rope), Genzyme, Boston, Mass |
| NCT00550342 | FSGS, primary and recur- rent; CG |
Rituximab (anti-CD20 monoclonal anti- body) |
Open label, 4 doses |
NIDDK | Indiana University, Indianapo- lis |
| NCT003020536 | FSGS | Tacrolimus | Randomized, open label, active control |
Nanjing University School of Medi- Cine, China |
Nanjing, China |
Data are excerpted from trials listed at www.clinicaltrials.gov, limited to trials that are actively recruiting patients. Web site accessed May 30, 2008. MCN indicates minimal change nephropathy; FSGS, focal segmental glomerulosclerosis; DMS, diffuse mesangial sclerosis; CG, collapsing glomerulopathy; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; CSA, cyclosporine; and RCT, randomized controlled trial.
Experimental Therapeutic Approaches
Therapies to Promote Podocyte Differentiation and Phenotype Stabilization and Prevent Podoycte Apoptosis
These therapies might offer benefit in MCN, FSGS, and DMS.
Glucocorticoids were introduced into the therapy of podocyte disorders largely on empiric grounds and have been assumed to act via suppression of the immune system, possibly by reducing putative soluble factors that are posited to affect podocytes. Recent in vitro data suggest that these agents have favorable direct effects on podocytes, stabilizing actin filaments,90 increasing the expression of podocyte proteins, including nephrin and p21, reducing expression of interleukin 6,91 and preventing apoptosis in response to PAN.92 There remains uncertainty as to the best way to administer glucocorticoids (daily, alternate day, or intermittent pulse therapy), the appropriate duration of therapy, and the benefit of combining glucorticoids with a second immunosuppressive agent (eg, cy closporine), particularly in unfavorable histologies, such as collapsing glomerulopathy.
Retinoids are critical to fetal kidney development and have been shown to partially reverse podocyte injury in several animal models of podocyte injury. These effects include preventing apoptosis and promoting a differentiated phenotype, including PAN nephrosis,18 anti-GBM nephritis,19 a human immunodeficiency virus–transgenic mouse model,93 and cultured podocytes.19,94 These encouraging in vitro and in vivo studies have prompted the clinical trial using retinoids that is listed in Table 5.
The statin class of HMG-CoA reductase inhibitors has recently been shown to have direct effects on podocytes, including restoring expression of nephrin and podocin via inhibiting RhoA activation and stabilizing actin filaments in PAN nephrosis,95 as well as reducing oxidative stress in Zucker obese rats.96 Table 5 lists 2 clinical studies in nondiabetic nephropathies.
PPAR-γ agonists have potential as therapies for podocytopathies, as shown by piaglitazone-induced suppression of apoptosis and TGF-β1 expression in PAN-treated podocytes.17 A recently completed, unpublished phase 1 trial examined the effects of rosiglitzaone in children with FSGS (ClinicalTrial.gov identifier: NCT-00193648; listed at www.clinicaltrials.gov, Web site accessed May 30, 2008).
Protein misfolding is a feature of podocytopathies associated with certain genetic mutations of podocyte proteins, including nephrin and podocin, as well as a feature of a more generalized process of cellular stress. Mizoribine is an immunosuppressant agent that has been used in renal transplantation, vasculitis, and lupus nephritis, among other conditions. Puromicin aminonucleoside induces endoplasmic reticulum stress in mice, leading to impaired delivery of nephrin to the podocyte SD; mizoribine has been shown to restore posttranslational processing of nephrin by improving intracellular energy balance, thereby normalizing nephrin trafficking.97
An ever-expanding list of proteins, including cytokines, growth factors, adipokines, and the like, have been shown to protect or to injure podocytes, arriving via the circulation or from intraglomerular cells. On the protective side, leptin infusion prevented the glomerular damage and podocyte loss that are present in a mouse model of lipoatrophy,98 and bone morphogenetic protein 7 (BMP7) reduced podocyte loss associated with diabetes in vivo99 and podocyte damage associated with hyperglycemia in vitro.100 Recently, activated protein C has been shown to inhibit podocyte apoptosis in diabetic mice, acting to suppress the mitochondrial apoptosis pathway.101 On the injury side, small molecule therapies targeting endothelin102 and insulin-like growth factor–mediated phosphatidylinositol-3-kinase activation103 have demonstrated protective effects on podocyte injury models. Monoclonal antibodies are available against many potentially pathogenic proteins; antibodies against basic fibroblast growth factor reduce proteinuria and podocyte desmin staining in puromcyin aminonucleoside nephrosis.104 Antibodies against TGF-β and connective tissue growth factor may hold promise in the primary podocytopathies.
Studies in diabetic nephropathy models have suggested that certain agents prevent podocyte loss; these interventions might also benefit primary podocytopathies. In streptozotocin-treated rats, therapy with the antioxidant lipoic acid reduced podocyte loss as assessed in tissue sections.105 In a clinical pilot study, the anti-platelet drug dilazep reduced microalbuminuria by 40% and urinary podocyte excretion by 70%.106
Therapies to Promote Podocyte Replacement
These approaches might benefit FSGS and perhaps DMS. Fibroblast growth factor induces podocyte cell cycle reentry in vivo, and the cells undergo nuclear division; there remains a block to cyokinesis, and the cells do not undergo cell division.107 Further understanding of the mechanisms underlying pathologic cell division in collapsing glomerulopathy may lead to therapeutic interventions to restore podocyte numbers in the setting of absolute or relative podocyte deficiency.
Therapies to Reduce Podocyte Proliferation
These therapies might offer benefit in CG, particularly when used in conjunction with therapies to promote podocyte differentiation. In particular, preclinical studies using cyclin-dependent kinase inhibitors and glycogen synthetase kinase-3 inhibitors may demonstrate suppressed podocyte proliferation.108
CONCLUSION
Progress has been made during the last decade to better understand podocyte pathobiology and its relation to proteinuric diseases. The proposed taxonomy of the podocytopathies is an attempt to integrate new knowledge on podocyte mechanism of injury with pathologic diagnosis and clinical syndromes. The next critical step, in addition to continuing the investigation on the mechanism of these diseases, should be dedicated to identifying patient-specific molecular signatures and new therapeutic interventions to target each specific mechanism of podocyte damage. Critical for this process is close collaboration among pathologists, nephrologists, and scientists.
Figure 8.
Mechanisms of disease: focal segmental glomerulosclerosis (FSGS). Focal segmental gloemrulosclerosis may result from specific genetic mutations affecting the slit diaphragm (SD), the actin-based cytoskeleton, protein located in the cytoplasm, in mitochondria, in the luminal or abluminal cell membrane, and in the nucleus (transcriptor factors). Focal segmental glomerulosclerosis can also result from mechanical stress on podocytes or toxic effect secondary to use of certain medications. It is possible that certain patients are genetically more susceptible to podocyte depletion under these circumstances. In fact, recently, genetic variation in MYH9 has been identified as a risk factor for idiopathic and hypertension-associated FSGS in African Americans.109
Figure 9.
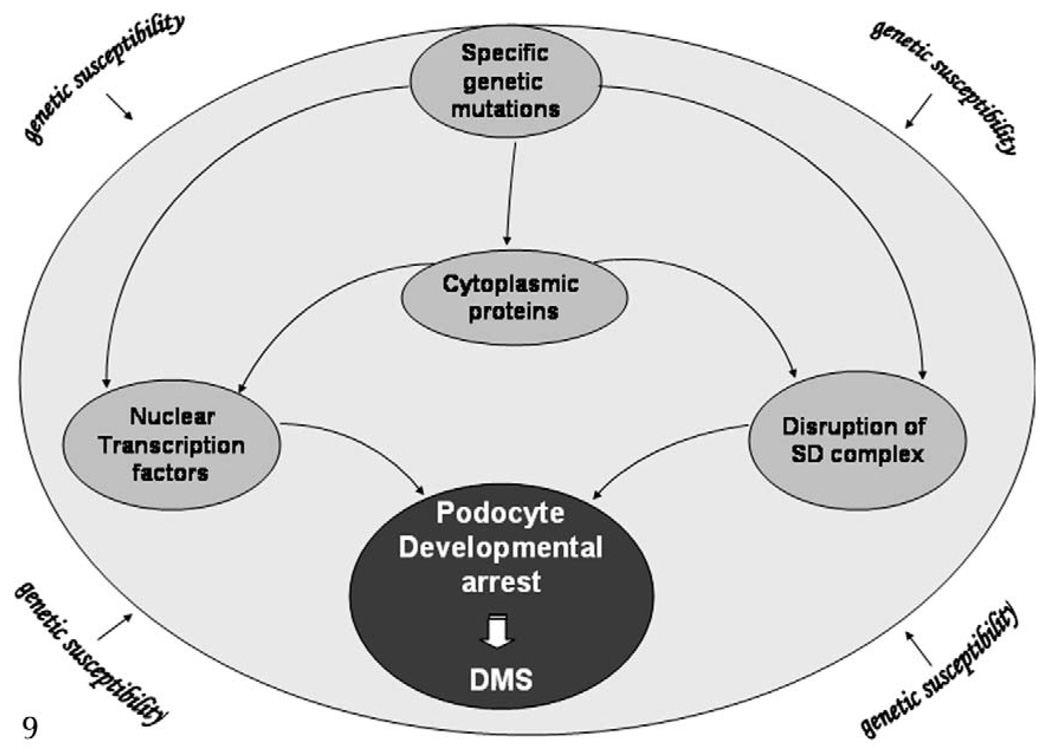
Mechanisms of disease: diffuse mesangial sclerosis (DMS). Diffuse mesangial sclerosis is often observed in the presence of specific mutations affecting nuclear transcriptor factors, slit diaphragm (SD), or cytoplasmic proteins.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Barisoni L, Schnaper HW, Kopp JB. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007;2:529–542. doi: 10.2215/CJN.04121206. [DOI] [PubMed] [Google Scholar]
- 2.Niaudet P, Gubler MC. WT1 and glomerular diseases. Pediatr Nephrol. 2006;21:1653–1660. doi: 10.1007/s00467-006-0208-1. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Jeanpierre C, Dressler GR, Lacoste M, Niaudet P, Gubler MC. WT1 and PAX-2 podocyte expression in Denys-Drash syndrome and isolated diffuse mesangial sclerosis. Am J Pathol. 1999;154:181–192. doi: 10.1016/S0002-9440(10)65264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 1999;10:51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 5.Srichai MB, Konieczkowski M, Padiyar A, et al. A WT1 co-regulator controls podocyte phenotype by shuttling between adhesion structures and nucleus. J Biol Chem. 2004;279:14398–14408. doi: 10.1074/jbc.M314155200. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Gubler MC, Beaufils H. Dysregulation of podocyte phenotype in idiopathic collapsing glomerulopathy and HIV-associated nephropathy. Nephron. 2002;91:416–423. doi: 10.1159/000064281. [DOI] [PubMed] [Google Scholar]
- 7.Wagner KD, Wagner N, Guo JK, et al. An inducible mouse model for PAX2-dependent glomerular disease: insights into a complex pathogenesis. Curr Biol. 2006;16:793–800. doi: 10.1016/j.cub.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 8.Rascle A, Suleiman H, Neumann T, Witzgall R. Role of transcription factors in podocytes. Nephron Exp Nephrol. 2007;106:e60–e66. doi: 10.1159/000101794. [DOI] [PubMed] [Google Scholar]
- 9.Rohr C, Prestel J, Heidet L, et al. The LIM-homeodomain transcription factor Lmx1b plays a crucial role in podocytes. J Clin Invest. 2002;109:1073–1082. doi: 10.1172/JCI13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suleiman H, Heudobler D, Raschta AS, et al. The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev Biol. 2007;304:701–712. doi: 10.1016/j.ydbio.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Quaggin SE, Schwartz L, Cui S, et al. The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development. 1999;126:5771–5783. doi: 10.1242/dev.126.24.5771. [DOI] [PubMed] [Google Scholar]
- 12.Sadl V, Jin F, Yu J, et al. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002;249:16–29. doi: 10.1006/dbio.2002.0751. [DOI] [PubMed] [Google Scholar]
- 13.Ross MD, Martinka S, Mukherjee A, Sedor JR, Vinson C, Bruggeman LA. Math6 expression during kidney development and altered expression in a mouse model of glomerulosclerosis. Dev Dyn. 2006;235:3102–3109. doi: 10.1002/dvdy.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi YF, Fong CC, Zhang Q, et al. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007;581:203–210. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Ding M, Cui S, Li C, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell P. HIF-1: an oxygen response system with special relevance to the kidney. J Am Soc Nephrol. 2003;14:2712–2722. doi: 10.1097/01.asn.0000092792.97122.e0. [DOI] [PubMed] [Google Scholar]
- 17.Kanjanabuch T, Ma LJ, Chen J, et al. PPAR-gamma agonist protects podocytes from injury. Kidney Int. 2007;71:1232–1239. doi: 10.1038/sj.ki.5002248. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Manzano V, Mampaso F, Sepulveda-Munoz JC, et al. Retinoids as a potential treatment for experimental puromycin-induced nephrosis. Br J Pharmacol. 2003;139:823–831. doi: 10.1038/sj.bjp.0705311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaughan MR, Pippin JW, Griffin SV, et al. ATRA induces podocyte differentiation and alters nephrin and podocin expression in vitro and in vivo. Kidney Int. 2005;68:133–144. doi: 10.1111/j.1523-1755.2005.00387.x. [DOI] [PubMed] [Google Scholar]
- 20.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Niranjan T, Bielesz B, Gruenwald A, et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat Med. 2008;14:290–298. doi: 10.1038/nm1731. [DOI] [PubMed] [Google Scholar]
- 22.Waters AM, Wu MY, Onay T, et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J Am Soc Nephrol. 2008;19:1139–1157. doi: 10.1681/ASN.2007050596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata M, Kriz W. Glomerular damage after uninephrectomy in young rats, II: mechanical stress on podocytes as a pathway to sclerosis. Kidney Int. 1992;42:148–160. doi: 10.1038/ki.1992.272. [DOI] [PubMed] [Google Scholar]
- 24.Nagata M, Scharer K, Kriz W. Glomerular damage after uninephrectomy in young rats. I. Hypertrophy and distortion of capillary architecture. Kidney Int. 1992;42:136–147. doi: 10.1038/ki.1992.271. [DOI] [PubMed] [Google Scholar]
- 25.Kestila M, Lenkkeri U, Mannikko M, et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 26.Boute N, Gribouval O, Roselli S, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. doi: 10.1038/74166. [DOI] [PubMed] [Google Scholar]
- 27.Kriz W. Ontogenetic development of the filtration barrier. Nephron Exp Nephrol. 2007;106:e44–e50. doi: 10.1159/000101792. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Wu H, Green G, et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003;300:1298–1300. doi: 10.1126/science.1081068. [DOI] [PubMed] [Google Scholar]
- 29.Lowik MM, Groenen PJ, Pronk I, et al. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AP mutation. Kidney Int. 2007;72:1198–1203. doi: 10.1038/sj.ki.5002469. [DOI] [PubMed] [Google Scholar]
- 30.Shih NY, Li J, Karpitskii V, et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]
- 31.Shih NY, Li J, Cotran R, Mundel P, Miner JH, Shaw AS. CD2AP localizes to the slit diaphragm and binds to nephrin via a novel C-terminal domain. Am J Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asanuma K, Campbell KN, Kim K, Faul C, Mundel P. Nuclear relocation of the nephrin and CD2AP-binding protein dendrin promotes apoptosis of podocytes. Proc Natl Acad Sci U S A. 2007;104:10134–10139. doi: 10.1073/pnas.0700917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endlich N, Kress KR, Reiser J, et al. Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol. 2001;12:413–422. doi: 10.1681/ASN.V123413. [DOI] [PubMed] [Google Scholar]
- 34.Khoshnoodi J, Sigmundsson K, Ofverstedt LG, et al. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol. 2003;163:2337–2346. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalluri R. Proteinuria with and without renal glomerular podocyte effacement. J Am Soc Nephrol. 2006;17:2383–2389. doi: 10.1681/ASN.2006060628. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan JM, Kim SH, North KN, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 37.Weins A, Schlondorff JS, Nakamura F, et al. Disease-associated mutant alpha-actinin-4 reveals a mechanism for regulating its F-actin-binding affinity. Proc Natl Acad Sci U S A. 2007;104:16080–16085. doi: 10.1073/pnas.0702451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dandapani SV, Sugimoto H, Matthews BD, et al. Alpha-actinin-4 is required for normal podocyte adhesion. J Biol Chem. 2007;282:467–477. doi: 10.1074/jbc.M605024200. [DOI] [PubMed] [Google Scholar]
- 39.Yanagida-Asanuma E, Asanuma K, Kim K, et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV. Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol. 2003;285:F40–F44. doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kreidberg JA, Donovan MJ, Goldstein SL, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 43.Reiser J, Oh J, Shirato I, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 44.Reiser J, Polu KR, Moller CC, et al. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winn MP, Conlon PJ, Lynn KL, et al. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 46.Moller CC, Wei C, Altintas MM, et al. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 47.Huber TB, Schermer B, Benzing T. Podocin organizes ion channel-lipid supercomplexes: implications for mechanosensation at the slit diaphragm. Nephron Exp Nephrol. 2007;106:e27–e31. doi: 10.1159/000101789. [DOI] [PubMed] [Google Scholar]
- 48.Barri YM, Munshi NC, Sukumalchantra S, et al. Podocyte injury associated glomerulopathies induced by pamidronate. Kidney Int. 2004;65:634–641. doi: 10.1111/j.1523-1755.2004.00426.x. [DOI] [PubMed] [Google Scholar]
- 49.Markowitz GS, Appel GB, Fine PL, et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol. 2001;12:1164–1172. doi: 10.1681/ASN.V1261164. [DOI] [PubMed] [Google Scholar]
- 50.Rogers MJ, Gordon S, Benford HL, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000;88:2961–2978. doi: 10.1002/1097-0142(20000615)88:12+<2961::aid-cncr12>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- 51.Takeda T, McQuistan T, Orlando RA, Farquhar MG. Loss of glomerular foot processes is associated with uncoupling of podocalyxin from the actin cytoskeleton. J Clin Invest. 2001;108:289–301. doi: 10.1172/JCI12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reiser J, Mundel P. Danger signaling by glomerular podocytes defines a novel function of inducible B7-1 in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol. 2004;15:2246–2248. doi: 10.1097/01.ASN.0000136312.46464.33. [DOI] [PubMed] [Google Scholar]
- 53.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinkes B, Wiggins RC, Gbadegesin R, et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 55.Gbadegesin R, Hinkes BG, Hoskins BE, et al. Mutations in PLCE1 are a major cause of isolated diffuse mesangial sclerosis (IDMS) Nephrol Dial Transplant. 2008;23:1291–1297. doi: 10.1093/ndt/gfm759. [DOI] [PubMed] [Google Scholar]
- 56.Barisoni L, Diomedi-Camassei F, Santorelli FM, et al. Collapsing glomerulopathy associated with inherited mitochondrial injury. Kidney Int. 2008;74:237–243. doi: 10.1038/sj.ki.5002767. [DOI] [PubMed] [Google Scholar]
- 57.Hagiwara M, Yamagata K, Capaldi RA, Koyama A. Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney Int. 2006;69:1146–1152. doi: 10.1038/sj.ki.5000207. [DOI] [PubMed] [Google Scholar]
- 58.Holthofer H, Kretzler M, Haltia A, et al. Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J. 1999;13:523–532. doi: 10.1096/fasebj.13.3.523. [DOI] [PubMed] [Google Scholar]
- 59.Solin ML, Pitkanen S, Taanman JW, Holthofer H. Mitochondrial dysfunction in congenital nephrotic syndrome. Lab Invest. 2000;80:1227–1232. doi: 10.1038/labinvest.3780130. [DOI] [PubMed] [Google Scholar]
- 60.Sauter M, Julg B, Porubsky S, et al. Nephrotic-range proteinuria following pamidronate therapy in a patient with metastatic breast cancer: mitochondrial toxicity as a pathogenetic concept? Am J Kidney Dis. 2006;47:1075–1080. doi: 10.1053/j.ajkd.2006.02.189. [DOI] [PubMed] [Google Scholar]
- 61.Moeller MJ, Holzman LB. Imaging podocyte dynamics. Nephron Exp Nephrol. 2006;103:e69–e74. doi: 10.1159/000090619. [DOI] [PubMed] [Google Scholar]
- 62.O’Rourke B, Cortassa S, Aon MA. Mitochondrial ion channels: gatekeepers of life and death. Physiology (Bethesda) 2005;20:303–315. doi: 10.1152/physiol.00020.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berkovic SF, Dibbens LM, Oshlack A, et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:673–684. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liapis H. Childhood nephrotic syndrome: from generic to molecular diagnosis. Pediatr Dev Pathol. 2008;11:154–163. doi: 10.2350/07-11-0375.1. [DOI] [PubMed] [Google Scholar]
- 65.Albaqumi M, Barisoni L. Current views on collapsing glomerulopathy. J Am Soc Nephrol. 2008;19:1276–1281. doi: 10.1681/ASN.2007080926. [DOI] [PubMed] [Google Scholar]
- 66.Stokes MB, Valeri AM, Markowitz GS, D’Agati VD. Cellular focal segmental glomerulosclerosis: clinical and pathologic features. Kidney Int. 2006;70:1783–1792. doi: 10.1038/sj.ki.5001903. [DOI] [PubMed] [Google Scholar]
- 67.Thomas DB, Franceschini N, Hogan SL, et al. Clinical and pathologic characteristics of focal segmental glomerulosclerosis pathologic variants. Kidney Int. 2006;69:920–926. doi: 10.1038/sj.ki.5000160. [DOI] [PubMed] [Google Scholar]
- 68.Stillman IE, Karumanchi SA. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 69.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagai Y, Arai H, Washizawa Y, et al. FSGS-like lesions in pre-eclampsia. Clin Nephrol. 1991;36:134–140. [PubMed] [Google Scholar]
- 72.Howie AJ, Lee SJ, Sparke J. Pathogenesis of segmental glomerular changes at the tubular origin, as in the glomerular tip lesion. J Pathol. 1995;177:191–199. doi: 10.1002/path.1711770213. [DOI] [PubMed] [Google Scholar]
- 73.Haas M. The glomerular tip lesion: what does it really mean? Kidney Int. 2005;67:1188–1189. doi: 10.1111/j.1523-1755.2005.00188.x. [DOI] [PubMed] [Google Scholar]
- 74.Haas M, Yousefzadeh N. Glomerular tip lesion in minimal change nephropathy: a study of autopsies before 1950. Am J Kidney Dis. 2002;39:1168–1175. doi: 10.1053/ajkd.2002.33386. [DOI] [PubMed] [Google Scholar]
- 75.Howie AJ, Pankhurst T, Sarioglu S, Turhan N, Adu D. Evolution of nephrotic-associated focal segmental glomerulosclerosis and relation to the glomerular tip lesion. Kidney Int. 2005;67:987–1001. doi: 10.1111/j.1523-1755.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 76.Howie AJ, Ferreira MA, Majumdar A, Lipkin GW. Glomerular prolapse as precursor of one type of segmental sclerosing lesions. J Pathol. 2000;190:478–483. doi: 10.1002/(SICI)1096-9896(200003)190:4<478::AID-PATH539>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 77.Fine RN. Recurrence of nephrotic syndrome/focal segmental glomerulosclerosis following renal transplantation in children. Pediatr Nephrol. 2007;22:496–502. doi: 10.1007/s00467-006-0361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Savin VJ, McCarthy ET, Sharma M. Permeability factors in focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:147–160. doi: 10.1053/snep.2003.50024. [DOI] [PubMed] [Google Scholar]
- 79.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 80.Bertelli R, Ginevri F, Caridi G, et al. Recurrence of focal segmental glomerulosclerosis after renal transplantation in patients with mutations of podocin. Am J Kidney Dis. 2003;41:1314–1321. doi: 10.1016/s0272-6386(03)00364-0. [DOI] [PubMed] [Google Scholar]
- 81.Caridi G, Dagnino M, Sanna-Cherchi S, Perfumo F, Ghiggeri GM. Podocinrelated mechanisms in posttransplant [corrected] recurrence of focal segmental glomerulosclerosis [corrected] Transplant Proc. 2006;38:3486–3490. doi: 10.1016/j.transproceed.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Avila-Casado Mdel C, Perez-Torres I, Auron A, Soto V, Fortoul TI, Herrera-Acosta J. Proteinuria in rats induced by serum from patients with collapsing glomerulopathy. Kidney Int. 2004;66:133–143. doi: 10.1111/j.1523-1755.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- 83.Meehan SM, Pascual M, Williams WW, et al. De novo collapsing glomerulopathy in renal allografts. Transplantation. 1998;65:1192–1197. doi: 10.1097/00007890-199805150-00009. [DOI] [PubMed] [Google Scholar]
- 84.Moudgil A, Nast CC, Bagga A, et al. Association of parvovirus B19 infection with idiopathic collapsing glomerulopathy. Kidney Int. 2001;59:2126–2133. doi: 10.1046/j.1523-1755.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 85.Nadasdy T, Allen C, Zand MS. Zonal distribution of glomerular collapse in renal allografts: possible role of vascular changes. Hum Pathol. 2002;33:437–441. doi: 10.1053/hupa.2002.124333. [DOI] [PubMed] [Google Scholar]
- 86.Tumlin JA, Miller D, Near M, Selvaraj S, Hennigar R, Guasch A. A prospective, open-label trial of sirolimus in the treatment of focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2006;1:109–116. doi: 10.2215/CJN.00120605. [DOI] [PubMed] [Google Scholar]
- 87.Cho ME, Hurley JK, Kopp JB. Sirolimus therapy of focal segmental glomerulosclerosis is associated with nephrotoxicity. Am J Kidney Dis. 2007;49:310–317. doi: 10.1053/j.ajkd.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Fervenza FC, Fitzpatrick PM, Mertz J, et al. Acute rapamycin nephrotoxicity in native kidneys of patients with chronic glomerulopathies. Nephrol Dial Transplant. 2004;19:1288–1292. doi: 10.1093/ndt/gfh079. [DOI] [PubMed] [Google Scholar]
- 89.Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- 90.Ransom RF, Lam NG, Hallett MA, Atkinson SJ, Smoyer WE. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 2005;68:2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 91.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int. 2006;70:1038–1045. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 92.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16:2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 93.He JC, Lu TC, Fleet M, et al. Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway. J Am Soc Nephrol. 2007;18:93–102. doi: 10.1681/ASN.2006070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takano Y, Yamauchi K, Hiramatsu N, et al. Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol. 2007;292:F1573–F1582. doi: 10.1152/ajprenal.00423.2006. [DOI] [PubMed] [Google Scholar]
- 95.Shibata S, Nagase M, Fujita T. Fluvastatin ameliorates podocyte injury in proteinuric rats via modulation of excessive Rho signaling. J Am Soc Nephrol. 2006;17:754–764. doi: 10.1681/ASN.2005050571. [DOI] [PubMed] [Google Scholar]
- 96.Whaley-Connell A, DeMarco VG, Lastra G, et al. Insulin resistance, oxidative stress, and podocyte injury: role of rosuvastatin modulation of filtration barrier injury. Am J Nephrol. 2008;28:67–75. doi: 10.1159/000109394. [DOI] [PubMed] [Google Scholar]
- 97.Nakajo A, Khoshnoodi J, Takenaka H, et al. Mizoribine corrects defective nephrin biogenesis by restoring intracellular energy balance. J Am Soc Nephrol. 2007;18:2554–2564. doi: 10.1681/ASN.2006070732. [DOI] [PubMed] [Google Scholar]
- 98.Suganami T, Mukoyama M, Mori K, et al. Prevention and reversal of renal injury by leptin in a new mouse model of diabetic nephropathy. FASEB J. 2005;19:127–129. doi: 10.1096/fj.04-2183fje. [DOI] [PubMed] [Google Scholar]
- 99.Wang S, de Caestecker M, Kopp J, Mitu G, Lapage J, Hirschberg R. Renal bone morphogenetic protein-7 protects against diabetic nephropathy. J Am Soc Nephrol. 2006;17:2504–2512. doi: 10.1681/ASN.2006030278. [DOI] [PubMed] [Google Scholar]
- 100.Mitu GM, Wang S, Hirschberg R. BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am J Physiol Renal Physiol. 2007;293:F1641–F1648. doi: 10.1152/ajprenal.00179.2007. [DOI] [PubMed] [Google Scholar]
- 101.Isermann B, Vinnikov IA, Madhusudhan T, et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–1358. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- 102.Ortmann J, Amann K, Brandes RP, et al. Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition. Hypertension. 2004;44:974–981. doi: 10.1161/01.HYP.0000149249.09147.b4. [DOI] [PubMed] [Google Scholar]
- 103.Bridgewater DJ, Ho J, Sauro V, Matsell DG. Insulin-like growth factors inhibit podocyte apoptosis through the PI3 kinase pathway. Kidney Int. 2005;67:1308–1314. doi: 10.1111/j.1523-1755.2005.00208.x. [DOI] [PubMed] [Google Scholar]
- 104.Sasaki T, Jyo Y, Tanda N, et al. Changes in glomerular epithelial cells induced by FGF2 and FGF2 neutralizing antibody in puromycin aminonucleoside nephropathy. Kidney Int. 1997;51:301–309. doi: 10.1038/ki.1997.37. [DOI] [PubMed] [Google Scholar]
- 105.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC., III Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC Nephrol. 2006;7:6. doi: 10.1186/1471-2369-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nakamura T, Ushiyama C, Shimada N, et al. Effect of the antiplatelet drug dilazep dihydrochloride on urinary podocytes in patients in the early stage of diabetic nephropathy. Diabetes Care. 2000;23:1168–1171. doi: 10.2337/diacare.23.8.1168. [DOI] [PubMed] [Google Scholar]
- 107.Kriz W, Hahnel B, Rosener S, Elger M. Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int. 1995;48:1435–1450. doi: 10.1038/ki.1995.433. [DOI] [PubMed] [Google Scholar]
- 108.Obligado SH, Ibraghimov-Beskrovnaya O, Zuk A, Meijer L, Nelson PJ. CDK/GSK-3 inhibitors as therapeutic agents for parenchymal renal diseases. Kidney Int. 2008;73:684–690. doi: 10.1038/sj.ki.5002731. [DOI] [PubMed] [Google Scholar]
- 109.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]