Abstract
A post-natal increase in carotid body (CB) hypoxia responsiveness occurs at the level of carotid sinus nerve activity, intracellular calcium, cell membrane depolarization and hypoxic inhibition of O2-sensitive background K+ conductance. TASK-1, TASK-1/3 and TASK-3 are functionally expressed in CB glomus cells, with TASK-1/3 providing the major part of the O2-sensitive TASK-like background K+ conductance. Here we report the effects of graded hypoxia on TASK-like channel activity in CB glomus cells from rats aged 0-1, 6-7 and 16-18 days; the time frame of postnatal CB functional maturation. TASK was active in nearly all cell-attached patches and TASK activity during normoxia did not differ across ages. Hypoxia produced a progressive decrease in channel opening frequency with graded decreases in O2 level and also produced glomus cell depolarization, as assessed by the shift in reversal potential of TASK single channel current. Hypoxic inhibition of TASK activity was least at P0-P1 and increased with age mainly between 6-7 and 16-18 days. The O2-sensitive TASK activity was significantly greater in glomus cells from P16-P18 when compared to cells from P0-P1 day old rats. These results support the hypothesis that postnatal carotid body functional maturation is due, at least in part, to changes in the sensitivity of TASK to the hypoxic signals generated in glomus cells.
Keywords: carotid body, chemoreceptor, potassium channel, oxygen sensing, development
1. Introduction
Mammalian tissues depend on a steady supply of oxygen; in many species, including humans, even a transient interruption for several minutes may cause morbidity or even mortality. It is not surprising, therefore, that mammals have developed a robust, dynamic O2 chemoreceptor system with peripheral arterial O2 sensors, the carotid body (CB) chemoreceptors, providing continuous input to brainstem respiratory rhythm generation and cardiorespiratory control nuclei. The carotid bodies, located bilaterally at the bifurcation of the common carotid arteries, contain O2-sensing type-I or “glomus” cells, innervated by terminals of the carotid sinus nerves (CSN). The O2-sensitive glomus cells express multiple potassium channels that are inhibited upon exposure to hypoxia, causing glomus cell depolarization, release of neurotransmitters and excitation of CSN nerve terminals. This, in turn, stimulates brainstem cardiorespiratory centers to drive minute ventilation, to induce arousal from sleep and to evoke other homeostatic and defensive responses.
Hypoxia-induced depolarization of CB glomus cells is thought to be an essential step in O2 chemotransduction. Although the mechanisms are not fully understood, a widely accepted view is that the hypoxia-induced receptor potential (depolarization) in glomus cells is generated mainly by inhibition of a TASK-like background potassium conductance (Buckler, 1997; Buckler et al., 2000). We recently reported that the TASK-like K+ channels in rat CB glomus cells consist of TASK-1, TASK-3 and TASK-1/3 heteromers; the latter accounting for > 75% of the total TASK-like current in isolated glomus cells (Kim et al., 2009). In addition, TASK activity in glomus cells was reversibly inhibited, approximately 80%, by hypoxia (2-3% O2), consistent with the idea that TASK channels provide a major part of the O2-sensitive background K+ conductance. If TASK channels are a major driver of hypoxia induced glomus cell depolarization, as hypothesized, it may be predicted that the magnitude of inhibition of single TASK channels by graded reductions in O2 tension would be approximated by an exponential function, as observed for neural activity, [Ca2+]i responses, membrane potential and whole cell background K+ current (Biscoe and Duchen, 1990a, b; Buckler, 1997; Buckler and Vaughan-Jones, 1994). However, the response of single TASK channels to graded hypoxia has not been previously investigated.
Carotid chemoreceptor O2 responsiveness is low at birth and increases over time to adult-like levels in all mammalian species studied to date (See Carroll and Donnelly, 2008 for review). We previously reported that the intracellular calcium response ([Ca2+]i) and depolarization response to hypoxia, of enzymatically dissociated CB glomus cells, are also small in cells harvested from newborn rats and increase to reach adult-like levels during the same time frame as postnatal maturation of CB neural activity (Bamford et al., 1999; Wasicko et al., 1999). Previous work from our laboratory showed that the magnitude of inhibition of the whole-cell, O2-sensitive background current by hypoxia was less in glomus cells from newborns and approximately doubled between birth and 14 days of age, suggesting that O2 sensitivity of a background K+ conductance explains, at least in part, postnatal maturation of glomus cell O2 responsiveness (Wasicko et al., 2006). However, which specific K+ channel is responsible for this O2-sensitive background K current cannot be resolved from whole-cell studies. As TASK-1/3 has been identified as the major background K+ current in neonatal rat glomus cells, in the present study we sought to determine the response of single TASK channels to graded hypoxia and to determine whether single channel O2 responsiveness increases during the time frame of rat postnatal CB functional maturation (Kholwadwala and Donnelly, 1992). The results indicate that graded hypoxia inhibits the activity of single TASK channels in a graded manner that can be approximated by an exponential function and that single TASK channel inhibition by hypoxia is less in glomus cells from newborns and increases with age, mainly between 7 and 16 days.
2. Methods
2.1 Ethical approval
The use of animals in this study was approved by the Animal Care and Use Committee of Rosalind Franklin University.
2.2 Carotid body cell isolation
Rats (postnatal 0-18; 366 rats) were anesthetized with isoflurane, decapitated, and the heads placed in ice-cold buffered saline solution (118 mM NaCl, 23 mM NaHCO3, 3 mM KCl, 2 mM KH2PO4, 1.2 mM CaCl2, 1 mM MgCl2, 10 mM Glucose, pH 7.2). CBs from both sides were dissected and placed in ice-cold low-Ca2+, low-Mg2+ phosphate buffered saline solution (PBS: 137 mM NaCl, 2.8 mM KCl, 2 mM KH2PO4, 0.07 mM CaCl2, 0.05 mM MgCl2, pH 7.4). Each CB was cut into 3-4 pieces and placed in a solution containing trypsin (400 ug/mL) and collagenase (400 ug/mL) in low Ca2+/Mg2+ physiological buffer solution and incubated at 37°C for 20-25 minutes. CBs were gently triturated using a fire polished Pasteur pipette to mechanically dissociate the cells. Enzymatic digestion was continued for an additional few minutes when necessary. CB growth medium (Ham’s F-12, 10% fetal bovine serum, 23 mM glucose, 4 mM L-alanyl-glutamine (Glutamax-1), 10K units penicillin/streptomycin, and 300 ug/ml insulin) was added to stop enzyme activity. After brief trituration, the solution containing the digested CBs was centrifuged for 4 minutes at ~6000 rpm (~2000 x g) using a microcentrifuge. Supernatant was removed and warm CB growth media added to gently resuspend the pellet. This step was repeated to remove traces of enzymes. Suspended CB cells were placed on glass coverslips coated with poly-L-lysine, and incubated at 37°C for 50 minutes to allow settling and attachment of the cells. CB growth medium was further added to the plate containing the coverslips and then incubated at 37°C for additional 2 hours. Coverslips were then transferred to the recording chamber for electrophysiological experiments. Clusters of 2-5 cells were identified, and cells within this cluster that showed a granular cell surface were chosen for electrophysiological studies.
2.3 Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200, Axon Instruments, Union City, CA). Borosilicate glass pipettes with tip resistance of 3-4 megohms were used. Channel current was filtered at 3 kHz using 8-pole Bessel filter (−3 dB; Frequency Devices, Haverhill, MA) and transferred to a computer using the Digidata 1320 interface (Axon Instruments) at sampling rate of 20 kHz. Single-channel currents were analyzed with the pCLAMP program (Version 10). For single channel analysis, the minimum duration was set at 0.05 ms. The detection threshold was set at 50% (half way between open and closed state). Channel openings were analyzed to obtain channel activity (NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open). NPo was determined from ~30 s-1 min of current recording. The single channel current tracings shown in the figures were filtered at 1 kHz. In cell-attached recordings the bath solution contained (mM): 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, and 10 glucose (pH 7.3), and the pipette solution contained (mM): 140 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3). Because both 16-pS and 36-pS channels were present in the patches, amplitude threshold levels were set such that both 16-ps and 36-pS channels were detected as single and double openings. This method is appropriate, because we are interested in relative changes in channel activity.
2.4 Hypoxia studies
Cell-attached patches were formed on CB cells and perfused with a bicarbonate-buffered solution previously gassed with air/5% CO2 gas mixture for ~60 min. After steady state channel activity was obtained, the perfusion solution was switched to solution gassed (for at least 60 min) with the desired level of O2 pressure for ~2 min. The temperature of the perfusion solutions was kept at 34.0±0.5°C, and the rate of perfusion was ~2.2 ml/min. O2 pressure of the solutions was checked using an oxygen meter (ISO2, WPI, Sarasota, USA) that was calibrated to 0% with solution gassed with pure nitrogen for 60 min and to 21% with solution gassed with air for 60 min at 37°C.
2.5 Data analysis
Data were analyzed using ANOVA with repeated measures (oxygen level) using age as a grouping variable (SPSS, version 16.0). Post-hoc testing was based on unpaired t-test with Bonferroni correction to the critical p value. Post-hoc testing was not done on all data pairs, but done using specific, a priori hypotheses: comparison of raw NPo values between normoxia and degrees of hypoxia and comparison of normalized NPo values at each level of hypoxia at different age groups. Significance level was set at p<0.05. The portion of channel activity inhibited by hypoxia was analyzed using two-way ANOVA with repeated measures (oxygen level), with age as a grouping variable and Bonferroni post-hoc testing (GraphPad Prism, version 5.02).
The O2 level at half-maximal inhibition by hypoxia was determined for each individual single-channel recording by fitting the normalized channel activity-O2 (%) relationship using an exponential equation as follows: Y=Y0+(Plateau-Y0)*(1-exp(−K*X)), where X = O2 level, Y0 is the Y value when X = zero, Plateau is the Y value at maximum X and K is the rate constant (GraphPad Prism, version 5.02). The X value (O2 level) at which hypoxia-induced inhibition of channel activity was half-maximal was computed as ln(2)/K. In the P0-P1 age group, 16 of the 18 single channel recordings could be fit with an exponential curve using this equation. The remaining two cells exhibited only a minimal or erratic response to hypoxia which did not follow an exponential pattern. Therefore, data from these two experiments were excluded from the analysis. In the P6-P7 and P16-P18 age groups, all individual TASK channel recordings were successfully fit with an exponential curve using the above equation.
To determine whether the O2 level at half-maximal inhibition changed with age, the values from each individual channel recording for the three age groups were analyzed using one-way ANOVA. Because the variances differed significantly between ages (Bartlett’s test of equal variances), Kruskal-Wallis non-parametric ANOVA was used, with age as a grouping variable. Post-hoc testing was performed using Dunn’s Multiple Comparison Test (GraphPad Prism, version 5.02).
3. Results
3.1 General protocol for studying TASK-like activity in response to hypoxia in CB cells
CB glomus cells express channels with properties similar to TASK-1, TASK-3 and TASK-1/3 heteromer that are sensitive to hypoxia (Kim et al., 2009). In this study, the inhibitory effect of a range of hypoxic levels was further investigated using cell-attached patches to isolate and record TASK channels. When the pipette potential was set at 0 mV, single channel openings with a mean open time duration of ~1 ms and amplitude levels of ~1-pA and ~2.2-pA were nearly always present. Our recent study has shown that these single channels represent TASK-1, TASK-3 and TASK-1/3, based on their single channel conductance levels (~16-pS and ~36-pS), and their sensitivity to pHo, Mg2+o, ruthenium red and methanandamide (Kim et al., 2009). Under this experimental condition, BK was observed in a few patches at a very low frequency, and did not interfere with recording of TASK.
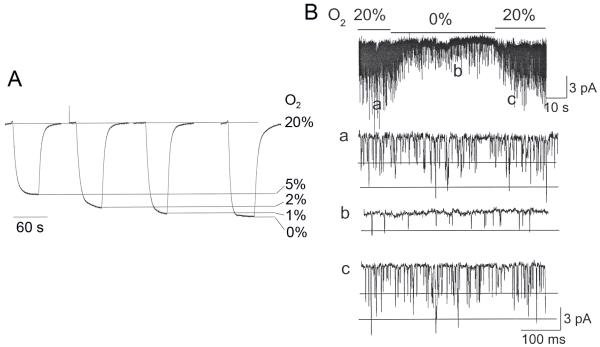
The CB cells in the recording chamber were continuously perfused by gravity with solution bubbled with a gas mixture containing 5% CO2 and 95% air. The heater located just before the recording chamber kept the temperature of the solution in the recording chamber at a constant level of 34.0±0.5°C. After initially forming the cell-attached patch on a CB cell, the perfusion of the recording chamber was continued for ~1-2 min to be sure that channel activity remained relatively constant. The perfusion solution was then switched from that containing normal O2 level (~20%) to one with lower O2 level (~5%, ~2%, ~0% determined by the gas bubbling the reservoir), and channel activity was recorded for ~2 min before switching to another solution. As expected, the actual O2 level (expressed as %) within the recording chamber near the centre of the glass coverslip where the cells are usually plated was slightly higher than the designated values, as determined using an O2 meter (Fig. 1A). To obtain the lowest O2 concentration possible, we added glucose oxidase (22 units/ml) and catalase (135 units/ml) to the perfusion solution gassed with 0% O2 / 5% CO2 (Englander et al., 1987). The actual percent O2 levels measured using the O2 meter were (range of three determinations) 19.2-20.8%, 4.9-5.3%, 2.0-2.3%, 0.8-1.3% and 0.3-0.5% for gas tanks with O2 levels of 20%, 5%, 2%, 0% and 0% with the two enzymes, respectively. To present the results more simply, the measured O2 levels were rounded to the nearest whole number and are presented as 20%, 5%, 2%, 1% and 0%. When switching of solutions was made, the change in O2 level occurred very rapidly, and the new level of O2 reached a steady state within ~30 sec. Fig. 1B illustrates a typical response of TASK when O2 level in the perfusion solution was briefly reduced from 20% to 0%, and shows that both inhibition and recovery occurs relatively rapidly within 30 s. Therefore, TASK activity was determined ~60 sec after switching to the new solution. At the end of each experiment, cells were perfused with control solution (20% O2), and only those that showed full recovery of TASK activity were used for analysis. The activity of all TASK-like channels was determined, as described in the methods.
Figure 1.
(A) O2 measurement in the recording chamber during perfusion using an O2 meter, and (B) a typical time course of TASK response to hypoxia (0% O2) in a cell-attached patch from a CB cell isolated from 16 day-old rat. Expanded current tracings show TASK activity before, during and after perfusion with 0% O2 (solution bubbled with 0%O2/5%CO2/95%N2 gas mixture with glucose oxidase and catalase added)
3.2 Responses of TASK-like channels to hypoxia in three age groups:
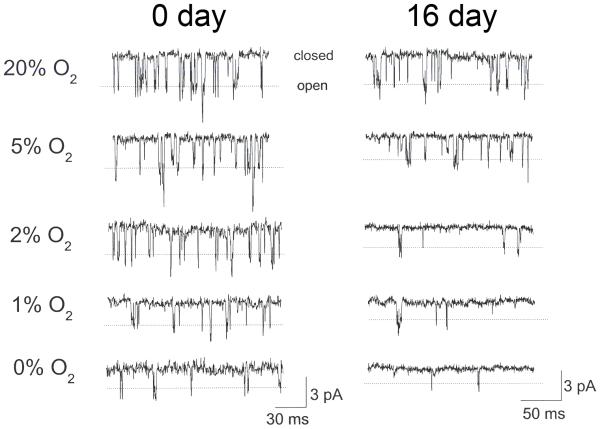
The first experiment tested the hypothesis that increased sensitivity of the CB to hypoxia during early postnatal development is associated with altered TASK sensitivity to hypoxia. Three age groups consisting of 0-1 day, 6-7 day and 16-18 day rats were chosen, as this represents the critical period of postnatal CB development (Kholwadwala and Donnelly, 1992; Wasicko et al., 1999). TASK single channels were recorded from cell-attached patches in response to hypoxia ranging in O2 level from ~0% to 20%. Thus, at a pipette potential of 0 mV, TASK channel openings produced an inward current. Similar single channel recordings were also obtained from CB cells from 6-7 day old rats (not shown). TASK was active in nearly all cell-attached patches obtained from cells that were chosen based on clustering and granular appearance of cell surface. With the pipette tip size used in this study, mainly one to two open levels were obtained. As described earlier, both the small conductance (~16-pS) and large conductance (36-pS) channels were present, representing TASK-1 and TASK-1/3 (and TASK-3), respectively. The dotted lines in figure 2 indicate the open levels of TASK-3 and TASK-1/3 that have indistinguishable single channel conductance levels (Kang et al., 2004; Kim et al., 2009).
Figure 2.
TASK single channel activity in a cell-attached patch from a CB cell isolated from 0-day and 16-day old rats in response to different levels of O2. Pipette potential was 0 mV. Dotted lines indicate the open level of the 36-pS channel (TASK-3 and TASK-1/3). Smaller conductance channels (16-pS) that represent TASK-1 are also present.
Using the same size of pipette, the level of TASK activity during normoxia was not different across ages (ANOVA, F=0.084, df=2, p=0.9), suggesting the product of channel number x activity was not different across age groups. As can be seen from the single channel openings in Fig. 2, hypoxia produced a progressive decrease in channel opening frequency in CB cells with decreasing O2 levels. Interestingly, hypoxia also produced a decrease in single channel amplitude, particularly at low O2 levels (0-2%). This could be an effect of hypoxia on cell membrane potential, resulting in altered potential gradient across the membrane, or an effect on the single channel conductance of TASK itself. Checking the single channel conductance levels from +40 mV to +120 mV (pipette potential set at −20, 0, +20, +40 and +60 mV) showed that the current-voltage slope of the 36-pS channel (TASK-1/3 and TASK-3) was unchanged by hypoxia (36±1 pS; n=3), indicating that hypoxia caused depolarization of CB cells resulting in a reduction in single channel current amplitude.
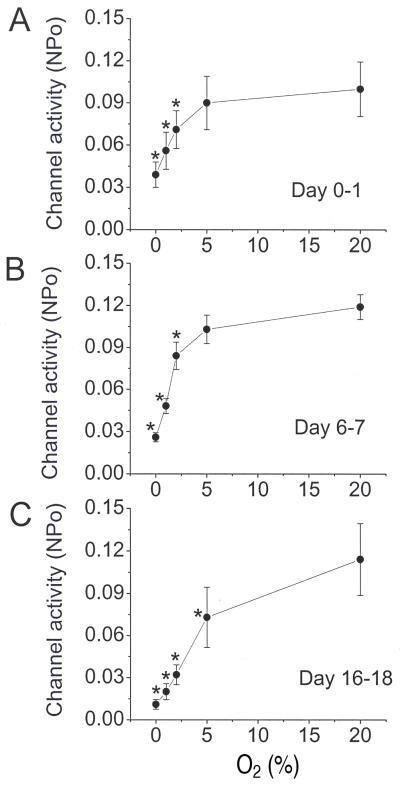
Averaged channel activity (NPo) determined from 13-18 patches was plotted as a function of O2 level for the three age groups (Fig. 3A-C). At each age, oxygen level had a significant effect on channel activity (ANOVA, F=13.1, df=4, p<0.001 for P0-P1, F=44.3, df=4, p<0.001 for P6-P7 and F=13.7, df=4, p<0.001 for P16-P18). Post-hoc comparison of NPo between normoxia and hypoxia at each age group showed a significant decrease in NPo at all levels of hypoxia, except between 20% and 5% O2 for the P0-P1 and P6-P7 age groups, which fell slightly below significance (p=0.1)(Fig. 3).
Figure 3.
TASK activity in cell-attached patches at five different O2 levels in the three age groups. (A-C) Channel activity (NPo) is plotted as function of O2 level. Each point represents the mean±SE of 13-18 determinations. (*) = significant difference from the control (20% O2) value (p<0.05)
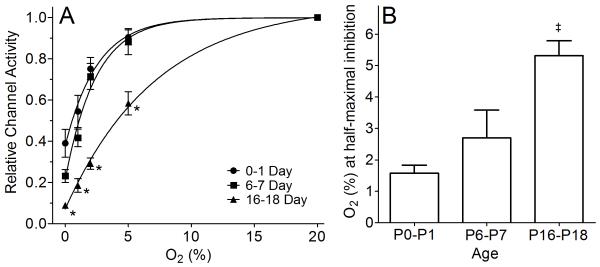
Due to the variability in channel activity in normoxia (20% O2), data were normalized to the value obtained in normoxia for each CB cell, and then replotted (Fig. 4A). Hypoxia significantly affected normalized channel activity (F=178, df=4, p<0.001) and this was different among age groups (F=6.1, df=8, p<0.001). Comparison among age groups indicated that P0-P1 day old were not significantly different than the P6-P7 age group (p=0.25), but P16-P18 was significantly different than both the P0-P1 (p<0.001) and P6-P7 (p<0.001). Comparison of normalized channel activity demonstrated a significant reduction in normalized channel activity at each level of oxygen for the P16-P18 compared to the P0-P1 age group (Fig 4A).
Figure 4.
Relative TASK activity at different O2 levels and O2 (%) at half-maximal inhibition in three age groups. (A) The channel activity from Figure 3A-C was normalized to that observed at 20% O2 for each age group, and the graphs re-plotted and combined into one graph. All data points at 0-5% levels in 16-18 day group were significantly different (p<0.05) from those of 0-1 and 6-7 day groups (indicated by *). TASK activity in 0-1 day and 6-7 day groups were not significantly different (p>0.05). The data points were fitted to an exponential function (see text). (B) Average O2 level at half-maximal inhibition in three age groups (mean±SE). (‡) = 16-18 day group significantly different (p<0.05) from the 0-1 day and 6-7 day group. O2 level at half-maximal inhibition did not differ significantly (p>0.05) for the 0-1 day vs. 6-7 day age groups.
Each of the three curves could be well fitted to a exponential function of the form: Y=Y0+(Plateau-Y0)*(1-exp(−K*x)). Based on fitting this exponential function to the averaged data in figure 4A, the calculated O2 levels at which half-maximal inhibition of TASK activity occurred were 1.76, 1.68 and 5.04% for P0-P1, P6-P7 and P16-P18 respectively. In a separate analysis, the relative TASK activity - O2 (%) curve for each individual TASK channel recording was fit using the above exponential equation, in order to determine the O2 level at half-maximal inhibition, on a cell-by-cell basis. Using this approach, the averaged O2 levels at half-maximal inhibition of TASK activity by hypoxia, grouped by age, were 1.58±0.25, 2.69± 0.89 and 5.31±0.47% (mean±SE) for age groups P0-P1 (n=16), P6-P7 (n=15) and P16-P18 (n=13) (p<0.0003, non-parametric ANOVA) (Fig 4B). Post hoc testing showed that O2 level at half-maximal TASK activity inhibition by hypoxia in the P16-P18 age group was significantly different (p<0.05) from the two younger age groups. Values in the P0-P1 age group did not differ from P6-P7 (p>0.05) (Fig 4B).
These results show a strong increase in O2 sensitivity of TASK beginning after the first week of life, and strongly suggest that the increased sensitivity of the CB to hypoxia that occurs during early postnatal development is partly due to increased TASK sensitivity to hypoxia.
3.3 Membrane depolarization by hypoxia in 0-1 and 16-18 day groups
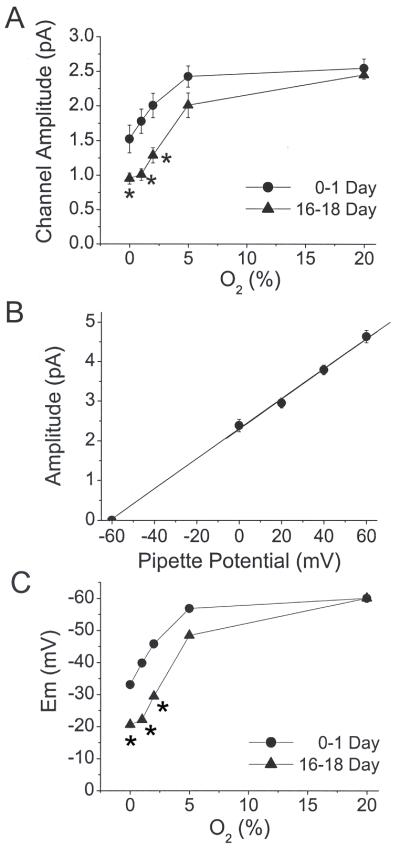
Hypoxia causes depolarization of rat CB glomus cells, at least in part, by inhibiting the background K+ current (Buckler, 1997; Buckler et al., 2000). Because TASK is an O2-sensitive background K+ channel in rat CB glomus cells, it is anticipated that the inhibition of TASK by hypoxia would be temporally associated with cell depolarization. Hypoxia may also depolarize glomus cells by other mechanisms such as inhibition of Kv and BK channels. Because the current amplitude of TASK single channels is dependent on the membrane potential, one can estimate the degree of cell depolarization from the change in TASK amplitude. Amplitude of TASK-3 and TASK-1/3-like channels was determined at each level of hypoxia and plotted in Fig. 5A. The decrease in amplitude produced by hypoxia was generally greater in CB cells from 16-18 day than 0-1 day. We estimated the degree of cell depolarization from the change in the amplitude of TASK single channel openings that occurs in response to hypoxia. To do this, a current-voltage relationship of TASK in CB cells was obtained using the 36-pS channel representing TASK-3 or TASK-1/3. A cell-attached patch was formed and the TASK amplitude plotted as a function of pipette potential ranging from −60 to +60 mV (Fig. 5B). A linear current-voltage relationship was present, and the current reversed at the pipette potential of ~−60 mV.
Figure 5.
Estimation of cell depolarization produced by hypoxia. (A) Single channel amplitude levels of the high conductance (TASK-1/3-like) channel were determined at each O2 level in the 0-1 day and 16-18 day groups. Each point is the mean±SE of 4-5 determinations. (*) = significant difference (p<0.05). (B) To correlate change in single channel amplitude with change in membrane potential, amplitude levels of the high conductance channel (TASK-1/3) was determined at various pipette potential and plotted. (C) From this linear relationship in (B), the mean zero-current potential (Em) was calculated and plotted.
The mean shift in reversal potential, an estimate of change in membrane potential, at each level of hypoxia was calculated from the linear current-voltage relationship. The results show that the estimated degree of depolarization in response to a given level of hypoxia (2% and lower) is significantly greater in CB cells isolated from 16-18 day than 0-1 day rats (p<0.05) (Fig. 5C). According to our estimates, ~20 mV depolarization is produced in CB cells from 0-1 day rats, whereas >30 mV depolarization is produced in the 16-18 day in response to 2% O2. Similarly, the estimated depolarization in response to 0% O2 is twice as large in CB cells from older rats (16-18 day) compared to newborn rats (Fig. 5C). The stronger depolarization observed in CB cells from older rats is likely to be contributed, in part, by the greater inhibition of TASK.
3.4 O2-sensitive TASK current in three age groups
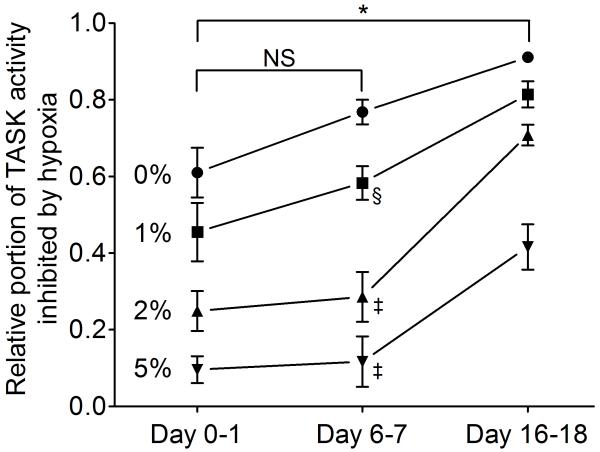
To show the O2 sensitivity of TASK activity at different ages and at different O2 levels, we calculated the portion of total TASK activity that is inhibited at each hypoxia level and at each age (Fig. 6). The results show that the portion of TASK activity inhibited by hypoxia was significantly different among age groups (F=24, df=2, p<0.0001). Thus, ~91% of total TASK activity is inhibited by nearly anoxic (O% O2+enzymes) solution at 16-18 days, whereas ~60% is inhibited in 0-1 day and ~76% is inhibited at 6-7 days. For milder hypoxia (2-5% O2), the difference in O2sensitivity between 6-7 and 16-18 days becomes even greater. This plot also shows that the O2 sensitivity at 6-7 days tends to be higher than that of 0-1 days, although the difference did not reach statistical significance due to large variability among cells.
Figure 6.
Relative portion of TASK activity inhibited by hypoxia in CB cells from three age groups. O2-sensitive components of the TASK activity were calculated from data shown in Fig. 4 and re-plotted. (*) = significant difference between 0-1 day and 16-18 day group at each level of O2 (p<0.001). (§) = significant difference from day 16-18 (p<0.05). (‡) = significant difference from day 16-18 (p<0.001). NS = not significant (p>0.05).
Could the age-related difference in O2 sensitivity of TASK be due to altered expression levels of TASK? The basal TASK activity in normoxia (20% O2) in the CB cells of three age groups were not significantly different (see Fig. 3A-C; p>0.05%), suggesting that the TASK-like current does not change during early development. These results indicate that the increased O2sensitivity of TASK in 16-18 day old compared to 0-7 day old rats is due to a greater O2 sensitivity of TASK in CB glomus cells from older rats and not due to a change in channel density. Thus, our results are consistent with the idea that the strength of signaling between hypoxia and TASK increases during postnatal development.
4. Discussion
The present study examines the effects of graded hypoxia on TASK activity in CB glomus cells at three ages during the critical period of rat carotid chemoreceptor functional maturation. The main finding is that hypoxic inhibition of rat glomus cell TASK activity, at the single channel level, increases with postnatal age, mainly between 6-7 days and 16-18 days of age. In addition, graded reductions in oxygen tension cause graded inhibition of single TASK channel activity, the pattern of inhibition can be approximated by an exponential function, hypoxia decreases TASK open probability, and maturation of the inhibitory effects of hypoxia is greatest with submaximal stimulus levels.
4.1 Graded response to hypoxia
It is well known that graded reductions in PO2 result in a graded increase in CB neural output (carotid sinus nerve activity) that is best fit by a hyperbolic function (Biscoe et al., 1970). When [Ca2+]i is used as a response marker, enzymatically dissociated glomus cells, both isolated and in clusters, exhibit the same characteristic hyperbolic relationship between PO2 and the [Ca2+]i response to hypoxia (Biscoe and Duchen, 1990b; Buckler and Vaughan-Jones, 1994). Similarly, when dissociated CB glomus cells are studied under conditions that isolate the O2-sensitive background current, the relationship between PO2 and the O2-sensitive leak conductance is well-approximated by a hyperbolic function that mirrors the hyperbolic PO2-[Ca2+]I relationship (Buckler, 1997). Our results extend these observations to the level of TASK single channel activity, showing an exponential relationship between O2 level and TASK activity (Figs. 3, 4A) that is nearly identical to the glomus cell PO2-whole-cell, leak conductance relationship reported by Buckler (Buckler, 1997) and to previously reported PO2-[Ca2+]i curves (Biscoe and Duchen, 1990b; Buckler and Vaughan-Jones, 1994; Wasicko et al., 1999).
The observations that TASK provides the major background K+ current in glomus cells (Kim et al., 2009) and that, in the present study, TASK single channel activity shows an exponential relationship with oxygen level ~ identical to that of whole glomus cells (Buckler, 1997; Wasicko et al., 2006) strongly suggest that TASK channels carry the O2-sensitive background current of CB glomus cells and play a role in mediating the hypoxia-induced receptor potential. The exponential shape of the response likely reflects the response characteristics of the O2 sensor(s) or signaling, as there is no inherent reason, related to channel properties, that the relationship between PO2 and TASK activity should be exponential in nature.
Normally, TASK current would be outward in CB cells under physiological conditions. In the above experiments, inward TASK current was recorded at the pipette potential of 0 mV. Under our experimental conditions (fast perfusion flow rate and at 34°C), outward TASK current obtained by setting the pipette potential at −120 mV showed increased basal membrane noise and low single channel conductance. Furthermore, BK channels were activated at depolarized membrane potentials. It was, therefore, not feasible to accurately determine the O2-sensitivity of TASK under depolarized conditions (producing outward current). However, because TASK is relatively voltage-independent, any change in TASK inward current is similarly reflected in the outward current. Thus, our earlier studies show that a ~50% decrease in inward TASK current produced by low pHo is associated with a ~50% decrease in outward TASK current (Kim et al., 1999, 2000). Therefore, the measurement of TASK activity at the pipette potential of 0 mV should still be valid for assessing TASK function at different levels of O2 tension.
4.2 Developmental changes in TASK inhibition by hypoxia
We previously reported that rat glomus cell capacitance did not change significantly with age, during the time frame of functional maturation, being 5.9±0.6 pF in cells from 1 to 3 day old and 5.3±0.4 pF in cells from 11 to 14 day old rats (Wasicko et al., 2006), suggesting that the cell surface area does not change with development. In the present study, TASK activity during normoxia was similar in all three age groups and did not change significantly with age. Thus, developmental changes in glomus cell hypoxia responsiveness related to TASK appear to be due to effects of hypoxia on channel activity and are unlikely to be related to the level of channel expression.
Numerous studies in multiple mammalian species have documented that CB responsiveness to hypoxia is low in newborns and increases with age. This holds true, in rat CB, at the level of CSN nerve activity, catecholamine secretion, intracellular calcium response ([Ca2+]i), cell membrane depolarization by hypoxia and hypoxic inhibition of O2-sensitive background K+ conductance (Donnelly and Doyle, 1994; Kholwadwala and Donnelly, 1992; Wasicko et al., 2006; Wasicko et al., 1999). The present study extends these observations to the level of single channel TASK activity, showing greater hypoxic inhibition of TASK activity in glomus cells from 16-18 day old rats compared to 0-1 day or 6-7 days (Fig. 4A).
The use of an exponential function fitted to the individual single-channel activity data during hypoxia challenge allowed calculation of O2 level at half-maximal inhibition on a cell-by-cell basis. The exponential function was successfully applied to data from 44 of 46 recordings. In two experiments, both in the P0-P1 age group, channel activity was not inhibited by 0% O2 and therefore the data could not be fitted using the exponential function. Elimination of these two cells would be unlikely to affect results of the analysis shown in figure 4B; the effect would be a slight rightward shift in the O2 level at half-maximal inhibition, which would tend to reduce differences with the other age groups. In spite of this, the O2 level at half-maximal inhibition for the P16-P-18 age group remained significantly different from the other two age groups (Fig. 4B).
In a previous study we investigated the effects of graded hypoxia in dissociated rat CB glomus cells from rats 1-3 days vs. 11-14 days old, estimating the average current at −50 mV under conditions that isolate the O2-sensitive background K+ current (Wasicko et al., 2006). The results indicated that graded reductions in PO2 resulted in graded reduction in the background K+ current in CB glomus cells. The hypoxic inhibition was 3-4 times greater in glomus cells from mature (11-14 days) rats compared to newborns, within an estimated PO2 range of 0 to 10 mmHg (Fig. 5 in Wasicko et al., 2006). It was noted that the developmentally regulated rat glomus cell O2 sensitive background K+ current was remarkably similar to the TASK-like background current in glomus cells described by Buckler (Buckler, 1997). The present results, showing that single channel TASK activity is significantly more inhibited in glomus cells from rats at 16-18 days compared to 0-1 day strongly suggests that developmental changes in the magnitude of hypoxic inhibition of TASK-1/3 or TASK-3 activity account for our previous findings.
In the present study, we found no change in TASK O2 sensitivity between birth and one week of age; the developmental increase in TASK inhibition by hypoxia occurred between 6-7 and 16-18 days of age (Fig. 4). Our previous study of hypoxic inhibition of glomus cell background K+ conductance during postnatal development, did not include an intermediate age group such as 6-7 days (Wasicko et al., 2006). However, our previous study of [Ca2+]i responses to graded hypoxia, in dissociated glomus cells from rats 1, 3, 7, 11, 14 and 21 days, also found only a minor increase in [Ca2+]i responses between 1 and 7 days, with the largest increase occurring between 7 and 11-14 days of age (Wasicko et al., 1999). Thus, the developmental profile of TASK channel O2 responsiveness is quite similar to the maturation profile for glomus cell [Ca2+]i responses to graded hypoxia. The observation that postnatal development of graded hypoxic inhibition of TASK activity and glomus cell [Ca2+]i responses to hypoxia follow a similar developmental time course is consistent with the hypothesis that a developmental increase in TASK inhibition by hypoxia accounts, at least in part, for glomus cell functional maturation.
It is difficult to speculate about mechanism underlying the developmental increase in TASK hypoxic inhibition with age, as little is known about the structural details of the channels at various ages, the mechanism of TASK O2 sensitivity or signaling mechanisms. Given that TASK channels are not intrinsically O2-sensitive (Buckler et al., 2000; Lee et al., 2006), the developmental increase in graded inhibition by hypoxia likely reflects a developmental increase in the response of O2 sensor(s) and/or coupling of O2 sensors to TASK channels via signaling pathways. At present, the coupling pathway has not been elucidated but ATP acting directly or through modulation of AMP-kinase have been implicated (Evans et al., 2009; Varas et al., 2007).
4.3 Membrane depolarization by hypoxia
Previous studies from our laboratory using perforated-patch, whole-cell recordings reported that rat glomus cells depolarized ~ 8 mV in cells from 1-3 day old rats vs. ~18 mV in cells from 11-14 day old rats when exposed ~2 mmHg O2 tension (Wasicko et al., 2006). Thus, the magnitude of hypoxia-induced depolarization in neonatal rat glomus cells approximately doubled within the first two weeks after birth. Similarly, our rough estimation of hypoxia-induced membrane depolarization in the present study (Fig. 5) indicates, for the 2% O2 challenge, that the magnitude of depolarization in cells from 0-1 day was about 20 mV compared to ~ 40 mV in glomus cells from 16-18 day old rats (Fig. 5C).
It should be noted that our estimate of glomus cell membrane depolarization (Fig. 5) assumes that intracellular [K+] is ~ 150 mM and that intracellular [K+] does not change significantly during hypoxia exposure. However, in a series of experiments by Zhang et al., in which rat glomus cells were impaled with two microelectrodes for simultaneous recordings of intracellular K+ activity and membrane potential, intracellular K+ activity averaged ~ 54 mM (range 14 – 104 mM) (Zhang et al., 1995). Since our estimate of resting potential depends on the point of K+ current reversal, an intracellular K+ concentration lower than 150mM would shift the apparent resting potential in the negative direction. Thus, the true resting potential may be positive to our estimated values, but this error magnitude would likely be constant over age groups. In addition, intracellular K+ may change during hypoxia (Zhang et al., 1995) which would similarly influence our estimate of intracellular potential. However, these errors may be relatively minor since results previously obtained in our laboratory using perforated patch whole-cell recordings, in which the concentration of small intracellular cations was likely clamped to that present in the pipette, are consistent with the present measurements. Thus, it seems reasonable to conclude that hypoxic inhibition of TASK channels is capable of causing a large magnitude depolarization and that the depolarizing effect of hypoxia due to TASK inhibition approximately doubles between newborn and two weeks of age.
4.4 Role of TASK channels in CB glomus cells
Although the present results suggest a major role for TASK channels in carotid body function and maturational changes in carotid body function, other oxygen-sensitive pathways may also play a role. Genetically engineered mice, lacking TASK-1, TASK-3 and TASK1/TASK3, continue to respond to hypoxia with an increase in ventilation (Mulkey et al., 2007; Trapp et al., 2008) and their carotid bodies respond to hypoxia with an enhanced catecholamine secretion (Ortega-Saenz et al., 2010). However, it should be pointed out that the oxygen sensing pathway and channels involved in the transduction cascade have not been identified in these knockout models, and until they have been identified, it is difficult to speculate whether the continued ability to sense hypoxia is due to compensatory changes in channel expression or the manifestation of redundant sensing pathways which are normally secondary to the TASK pathway.
Acknowledgements
This work was funded by NIH grant HL054621.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bamford OS, Sterni LM, Wasicko MJ, Montrose MH, Carroll JL. Postnatal maturation of carotid body and type I cell chemoreception in the rat. Am J Physiol. 1999;276:L875–884. doi: 10.1152/ajplung.1999.276.5.L875. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR. Cellular basis of transduction in carotid chemoreceptors. Am J Physiol. 1990a;258:L271–278. doi: 10.1152/ajplung.1990.258.6.L271. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Duchen MR. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990b;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Purves MJ, Sampson SR. The frequency of nerve impulses in single carotid body chemoreceptor afferent fibres recorded in vivo with intact circulation. J Physiol. 1970;208:121–131. doi: 10.1113/jphysiol.1970.sp009109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498( Pt 3):649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JL, Donnelly DF. Postnatal development of Carotid Chemoreceptor Function. In: Marcus CL, Carroll JL, Donnelly DF, editors. Sleep and Breathing in Children: Developmental Changes in Breathing During Sleep. Informa Healthcare; New York: 2008. pp. 47–82. [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol. 1994;475:267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englander SW, Calhoun DB, Englander JJ. Biochemistry without oxygen. Anal Biochem. 1987;161:300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Evans AM, Hardie DG, Peers C, Wyatt CN, Viollet B, Kumar P, Dallas ML, Ross F, Ikematsu N, Jordan HL, Barr BL, Rafferty JN, Ogunbayo O. Ion channel regulation by AMPK: the route of hypoxia-response coupling in thecarotid body and pulmonary artery. Ann N Y Acad Sci. 2009;1177:89–100. doi: 10.1111/j.1749-6632.2009.05041.x. [DOI] [PubMed] [Google Scholar]
- Kang D, Han J, Talley EM, Bayliss DA, Kim D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004;554:64–77. doi: 10.1113/jphysiol.2003.054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholwadwala D, Donnelly DF. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cavanaugh EJ, Kim I, Carroll JL. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–2975. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K(+) channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999;277:H1669–1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006;18:499–507. doi: 10.1016/j.cellsig.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci. 2007;27:14049–14058. doi: 10.1523/JNEUROSCI.4254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Saenz P, Levitsky KL, Marcos-Almaraz MT, Bonilla-Henao V, Pascual A, Lopez-Barneo J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–392. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Aller MI, Wisden W, Gourine AV. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–8850. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas R, Wyatt CN, Buckler KJ. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol. 2007;583:521–536. doi: 10.1113/jphysiol.2007.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicko MJ, Breitwieser GE, Kim I, Carroll JL. Postnatal development of carotid body glomus cell response to hypoxia. Respir Physiol Neurobiol. 2006;154:356–371. doi: 10.1016/j.resp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Wasicko MJ, Sterni LM, Bamford OS, Montrose MH, Carroll JL. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J Physiol. 1999;514(Pt 2):493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Pang L, Eyzaguirre C. Effects of hypoxia on the intracellular K+ of clustered and isolated glomus cells of mice and rats. Brain Res. 1995;676:413–420. doi: 10.1016/0006-8993(95)00158-m. [DOI] [PubMed] [Google Scholar]