Abstract
The role of neurotrophin-4/5 in the enhancement of axon regeneration in peripheral nerves produced by treadmill training was studied in mice. Common fibular nerves of animals of the H strain of thy-1-YFP mice, in which a subset of axons in peripheral nerves is marked by the presence of yellow fluorescent protein, were cut and surgically repaired using nerve grafts from non-fluorescent mice. Lengths of profiles of fluorescent regenerating axons were measured using optical sections made through whole mounts of harvested nerves. Measurements from mice that had undergone one hour of daily treadmill training at modest speed (10 m/min) were compared to those of untrained (control) mice. Modest treadmill training resulted in fluorescent axon profiles that were nearly twice as long as controls at one, two and four week survival times. Similar enhanced regeneration was found when cut nerves of wild type mice were repaired with grafts from neurotrophin-4/5 knockout mice or grafts made acellular by repeated freezing/thawing. No enhancement was produced by treadmill training in neurotrophin-4/5 knockout mice, irrespective of the nature of the graft used to repair the cut nerve. Much as had been observed previously for the effects of brief electrical stimulation, the effects of treadmill training on axon regeneration in cut peripheral nerves are independent of changes produced in the distal segment of the cut nerve and depend on the promotion of axon regeneration by changes in NT-4/5 expression by cells in the proximal nerve segment.
Keywords: exercise, neurotrophins, nerve grafts, transgenic mice
Introduction
Despite the considerable capacity for axon regeneration following peripheral nerve injury, functional recovery is poor (Brushart, 1998; Scholz et al., 2009). Even though modest injuries to nerves can result in adequate functional recovery, the outlook for such recovery after injuries that disrupt the endoneurial tubes that surround individual axons and their myelinating Schwann cells (Sunderland stage 5) is poor (Sunderland, 1970). Following injuries of this sort, axons distal to the site of injury undergo anterograde (Wallerian) degeneration. Regenerating neurites from the proximal segment of the injured nerve must enter a regeneration pathway in the distal segment of the injured nerve and regenerate in that pathway to reinnervate peripheral targets in muscle or skin. Two important factors contributing to poor functional outcomes following these types of injuries are the slow growth of regenerating axons in the regeneration pathway (Fawcett & Keynes, 1990) and the misdirection of regenerating axons to reinnervate functionally inappropriate targets (Brushart et al., 2002; Valero-Cabre & Navarro, 2002; English, 2005; de Ruiter et al., 2008; Robinson & Madison, 2009).
Gordon and co-workers have pioneered the use of brief electrical stimulation (ES) to enhance axon regeneration following peripheral nerve transection. If the proximal stump of a cut nerve is stimulated for as little as one hour at the time of its surgical repair, regenerating axons grow more than twice as far during the first two post-repair weeks (English et al., 2007) and the regeneration of axons of nearly twice as many motoneurons has been observed over the same period (Al-Majed et al., 2000; English, 2005; Hetzler et al., 2008). Similar enhancement of regeneration has been demonstrated by other groups using different model systems (Franz et al., 2008; Lu et al., 2008; Asensio-Pinilla et al., 2009; Huang et al., 2009). Brief ES results in an increased expression of brain derived neurotrophic factor (BDNF) and its receptor, trkB, in motoneurons (Al-Majed et al., 2000) and dorsal root ganglion neurons (English et al., 2007; Geremia et al., 2007), and in the enhanced expression of regeneration related-genes in motoneurons (Al-Majed et al., 2004; Sharma et al., 2010). Using a combination of transgenic and knockout mice, we have provided evidence that the efficacy of ES in enhancing axon regeneration in cut peripheral nerves is dependent on neurotrophin signaling originating in cells in the proximal nerve segment, especially neurotrophin-4/5 (NT-4/5) (English et al., 2007).
Recently we also showed that axon regeneration can be enhanced by modest treadmill training (Sabatier et al., 2008). The magnitude of this enhancement is comparable to that noted with ES but includes less misdirection of the regenerating axons to reinnervate functionally inappropriate targets (English et al., 2009). Using slightly different training paradigms and outcome measures, a similar observations have been made following crush injury in mice (Seo et al., 2006; Ilha et al., 2008) and after transection injury in rats (Asensio-Pinilla et al., 2009). Exercise is well known to produce an increase in BDNF content in both brain (Neeper et al., 1996; Adlard et al., 2004) and spinal motoneurons (Gomez-Pinilla et al., 2001; Perreau et al., 2005), and to increase axon outgrowth in cultured neurons (Molteni et al., 2004). However, whether our effective treadmill training paradigm requires the other trkB ligand, NT-4/5, to be effective is not known. Thus we wanted to use the mouse strategy described above to investigate a role for this neurotrophin in the enhancing effects of treadmill training. We report here that, much as noted previously for ES, treadmill training enhances axon regeneration in cut peripheral nerves in a manner that is independent of cells in the pathway surrounding the regenerating axons and is likely dependent on the presence of NT-4/5 in the regenerating axons.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Emory University and were in accordance with the Principles of the Use of Animals in Research of the Society for Neuroscience. All regeneration experiments were conducted using two month old males of the H strain of thy-1-YFP mice. In these mice, yellow fluorescent protein (YFP) is expressed under the control of the thy-1 promoter (Feng et al., 2000). In the H strain (thy-1-YFP-H), YFP is expressed in the axons of a subset of sensory and motor neurons (Groves et al., 2005) and marks axons completely (Feng et al., 2000). We assume that the behavior of the marked axons in these mice represents a reasonable sample of that of all of the axons in their peripheral nerves. A total of 44 nerves was studied in these experiments.
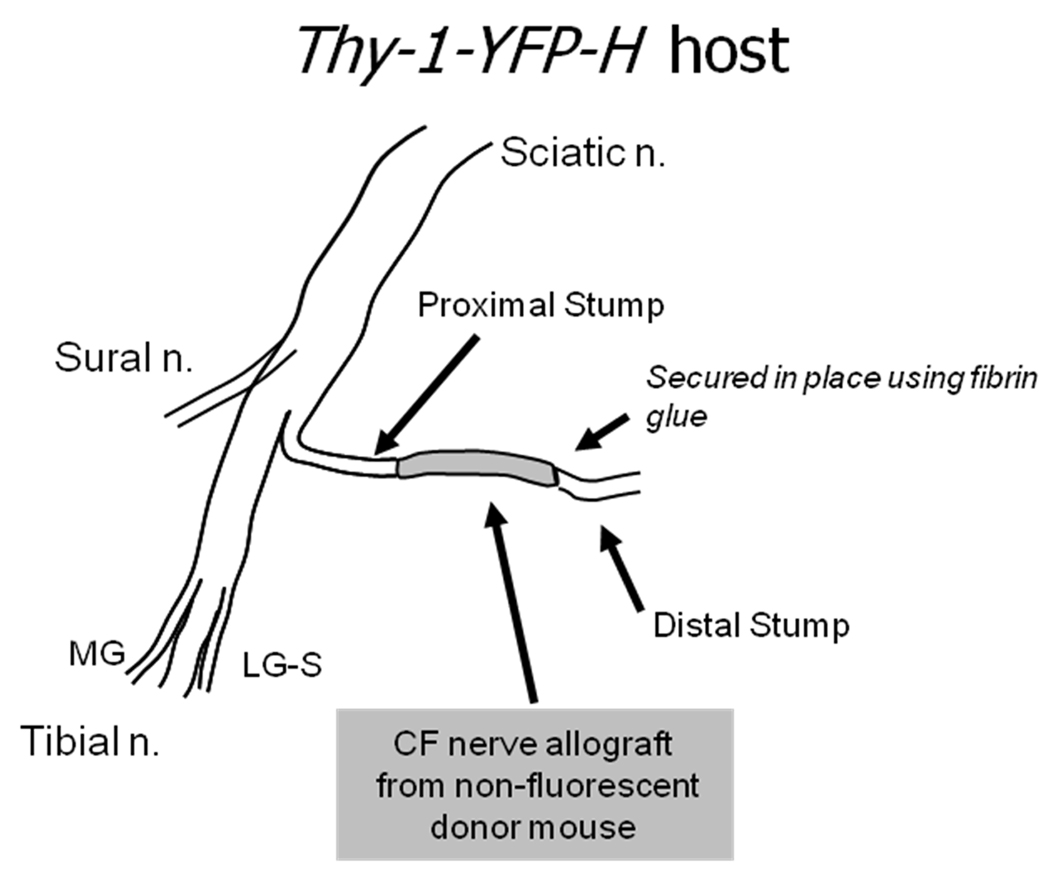
Experiments were conducted using the common fibular (CF) nerve as a model system (Fig. 1). The methods used are described in more detail elsewhere (Groves et al., 2005). Briefly, under pentobarbital (90 mg/kg, IP) anesthesia, the CF nerve was cut near its branching point from the sciatic nerve and repaired with a short (5–10 mm long) nerve graft obtained from the CF nerve of a strain-matched non-fluorescent mouse. The stumps of the cut nerve were aligned to the ends of the graft on a small piece of Gore-Tex that had been cut from a tube. The aligned segments of the cut nerve were then secured in place using fibrin glue (Menovsky & Beek, 2001; MacGillivray, 2003). Grafts were used for two purposes. First, they provide a dark background against which YFP positive regenerating axons can be visualized using fluorescence microscopy. Especially at short survival times, this avoids any problems with fluorescent products of anterograde (Wallerian) degeneration among regenerating axons. Second, the grafts were used experimentally to alter the environment or pathway through which regenerating axons grew. All mice were treated daily with oral meloxicam (Boehringer Ingelheim & Merial)(1 mg/Kg) during the first post-surgical week.
Figure 1.
Diagram of experimental design. One of the two main terminal branches of the sciatic nerve, the common fibular (CF) nerve, was cut in thy-1-YFP-H mice and repaired with a graft harvested from the common fibular nerve of a non-fluorescent, strain matched donor mouse. The two ends of the graft were aligned with the cut stumps of the host mouse and secured in place using fibrin glue.
To investigate the role of neurotrophin 4/5 (NT-4/5) in treadmill training enhancement of axon regeneration, we used NT-4/5 knockout mice, both as graft donors (eight nerves) and, after breeding with thy-1-YFP-H mice, as hosts (16 nerves). Founders of NT-4/5 knockout mice were obtained from The Jackson Laboratories (Bar Harbor, ME). These mice were developed originally on a strain 129 background, but in our laboratory they have been back crossed for at least six generations with mice of the C57B6 strain to insure that they are both immune- and strain-compatible with the background strain (C57B6) of thy-1-YFP-H mice (Evans et al., 1994). All knockout mice were genotyped using tail DNA before being used. Half of these nerves were studied in treadmill trained mice and half were studied in untrained controls.
In eight experiments, cut CF nerves were repaired unilaterally using acellular nerve grafts. Four of these nerves were studied in treadmill trained mice, four were studied in untrained controls. Segments of CF nerves from non-fluorescent donor mice were removed from anesthetized animals, placed on a small piece of aluminum foil, and then frozen rapidly in liquid nitrogen. Once frozen, the nerve segment was then rapidly thawed by immersion in a saline bath at 37°C. This freeze thaw cycle was repeated three times, after which the nerve segment was used to repair the cut CF nerve of a host mouse, as described above. We (English et al., 2007) and others (Nadim et al., 1990; Anderson et al., 1991; Haninec et al., 2000; Krekoski et al., 2001) have shown that this procedure results in nerve grafts in which Schwann cells have been killed but endoneurial tubes and their associated Schwann cell basal laminae are preserved.
Altogether, 28 nerves were studied in treadmill trained male mice. Treadmill training was begun on the third day after transection and surgical repair of the CF nerve. Mice were placed on a motor driven treadmill at a belt speed of 10 m/min and were treadmill trained continuously for one hour. Animals generally required little incentive to walk at this modest speed. Training was conducted five days per week.
After survival periods of one, two, or four weeks, mice were euthanized with pentobarbital (150 mg per kilogram IP), and perfused transcardially, first with normal saline and then followed by periodate-lysate-paraformaldehyde fixative (McLean & Nakane, 1974). The repaired nerves including the entire graft were removed from the animals and placed on a microscope slide in the same orientation as in the animal. This whole mount was then cover slipped using Vectashield (Vector Labs, Burlingame, CA). Cover slips were sealed at their edges using clear nail polish. Stacks of optical sections were made at relatively low magnification through the full thickness of the nerve at 10 µm intervals using a laser scanning confocal microscope (Zeiss LSM510). Capture of several stacks of overlapping microscope fields was required to include all of the regenerating axons. Each of the images in each stack was aligned with and connected to the corresponding slice of contiguous stacks, in register, using Adobe Photoshop. The net result of this image stitching was a single large stack of connected optical sections encompassing the entire length and thickness of the repaired nerve. Lengths of profiles of individual fluorescent axons were measured throughout their courses in these stacks, from the proximal surgical repair site to their growth cones.
Measurements of axon profile lengths in each nerve were expressed as cumulative histograms (bin size equal 100 µm) and then averaged within different treatment groups. Significance of differences between pairs of average distributions was evaluated using the Mann-Whitney U-test. This non-parametric method evaluates the probability that two frequency distributions are samples drawn from the same population. In addition, the median axon profile length was determined for each nerve studied and averages were computed for each treatment group. Significance of differences between groups was evaluated using analysis of variance, with appropriate post-hoc (Fisher's least significant differences (LSD)) paired testing.
Results
The effects of treadmill training on axon regeneration
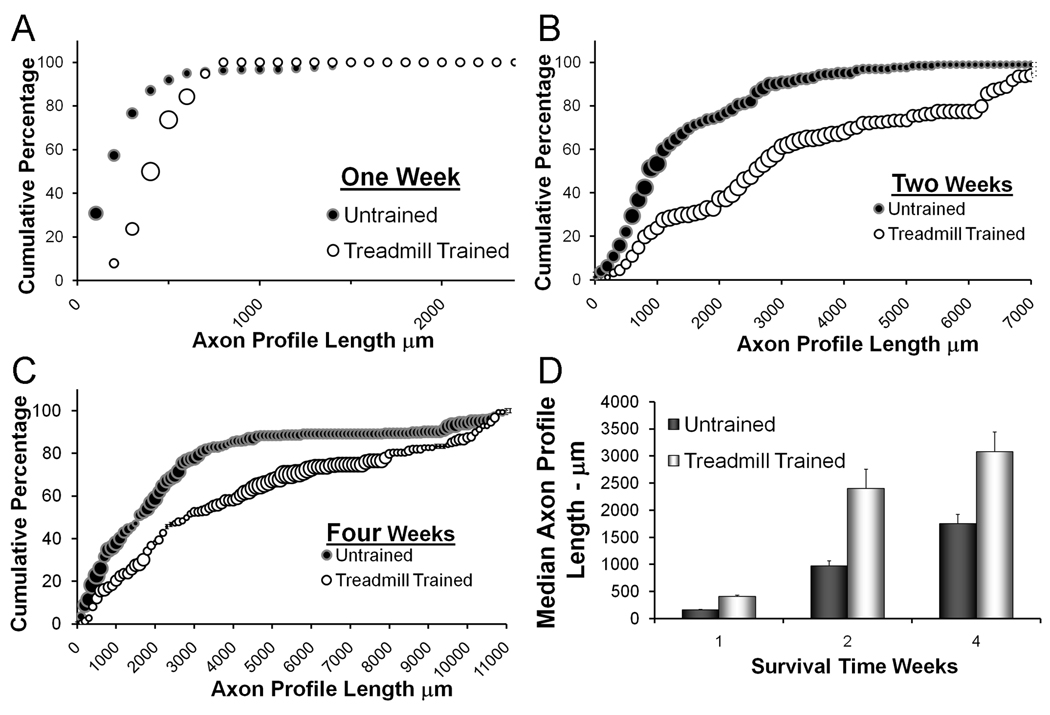
The profiles of YFP positive regenerating axons were reconstructed from stacks of optical sections made through the cut and repaired nerves. Fluorescent axons are very bright in these sections and their profiles can be followed easily from the proximal surgical repair site to their termination as growth cones. Measurements of the lengths of regenerating axon profiles were studied as frequency distributions. The effects of treadmill training were studied by comparison of these distributions between trained and untrained mice at three different survival times. These are shown in Figure 2A–C. Examples of regenerating axons in trained and untrained mice and lengths of regenerating axon profiles at the two week survival time have been published elsewhere (Sabatier et al., 2008). Data for trained mice at the one and four week survival times are new. Each data point in these graphs (trained and untrained) represents an average of data from four nerves. The size of each symbol represents the SEM about that average. At each time, the distributions of axon profile lengths measured in nerves from treadmill trained mice were shifted to the right relative to the distribution obtained from measurements from untrained mice. At all three times studied, this shift was statistically significant (U-Test, p< 0.01 for all). The mean number of regenerating axons measured did not differ between treatment groups (data not shown), but average median axon profile lengths (Fig. 2D) were significantly longer in the treadmill trained mice than in untrained controls (ANOVA, F=4.72, DF= 10, LSD test, p < 0.05) at all three times. Thus, modest treadmill training applied for two weeks resulted in enhanced axon regeneration and this enhancement persists past the termination of the training period.
Figure 2.
The effects of treadmill training on axon regeneration in cut and repaired nerves are shown. Panels A–C contain cumulative frequency histograms of axon profile lengths measured at different times after nerve repair. The distributions shown in each graph are of the lengths of YFP+ axon profiles measured in treadmill trained mice (white circles) or in sedentary controls (black circles). Each point in each histogram represents the average of four nerves. The sizes of the bubbles in these graphs are proportional to the SEM of each point (N=4). In Panel D, the average median axon profile lengths (±SEM) are shown for the same groups of mice.
Effects of altering the environment surrounding regenerating axons
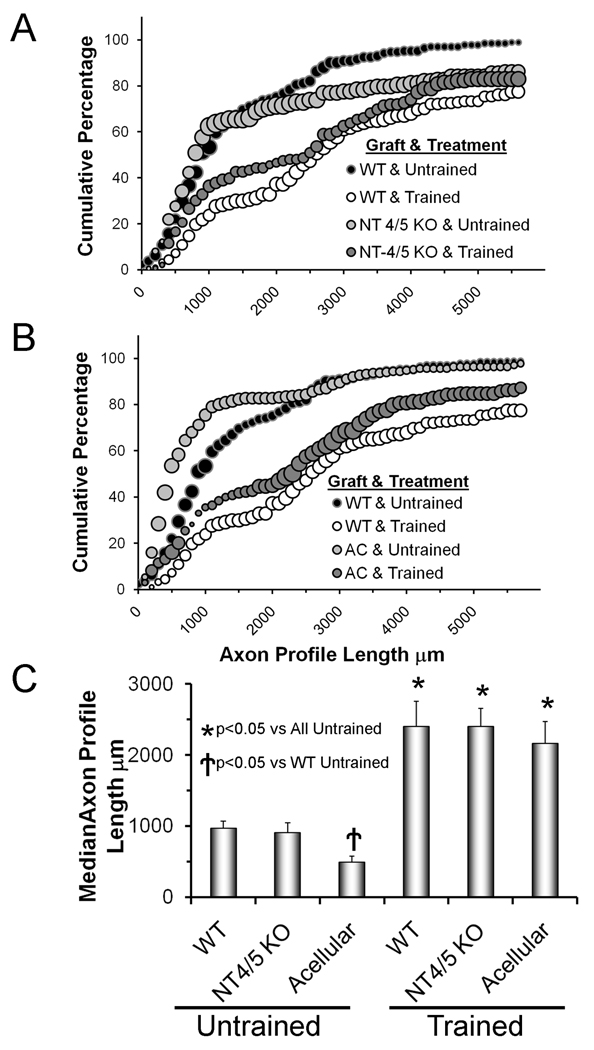
We studied the effects of treadmill training when regenerating axons were constrained to grow into an experimentally manipulated environment. We showed previously (English et al., 2005) that regenerating axons grew very poorly through grafts from NT- 4/5 knockout mice. The original background strain of the NT-4/5 knockout mice is different (129) from that of the thy-1-YFP-H (host) mice that we use (C57B6). In our previous experiments with NT-4/5 knockout mice we used direct descendents of the founders that we obtained from the Jackson Laboratories because differences in major histocompatability complex antigens between these strains that might give rise to graft rejection are minor (Evans et al., 1994). However, in an attempt to eliminate any effects of these strain differences that are not immunological, all of the NT-4/5 knockout mice used in the present study were back crossed extensively to C57B6 mice. The distribution of lengths of profiles of axons of thy-1-YFP-H host mice that had regenerated into grafts from these mixed-strain NT-4/5 knockout mice is shown in figure 3A. In marked contrast to our previous findings, we found that regenerating axon profile lengths were slightly longer under these circumstances than in controls (nerves in untrained wild type mice repaired with grafts from wild type mice). For axon profile lengths ≤4000 µm, no significant differences were noted (U-test, p=0.13), while for axon profile lengths >4000 µm, a significant (U-test, p<0.01) shift toward longer lengths was noted. Overall median axon profile lengths are not significantly different (ANOVA F=25.57, DF=5, LSD, p=0.37) from that of controls (Fig. 3C). Thus, although we cannot rule out entirely that any effects we observed previously were due to the lack of NT-4/5 in the grafts from strain 129-based NT-4/5 knockout mice, it is clear that differences in background strain are profound.
Figure 3.
The effects of manipulating the environment surrounding regenerating axons on enhancement of axon regeneration produced by treadmill training are shown. Panels A and B are cumulative histograms of axon profile lengths measured two weeks after transection and surgical repair of the common fibular nerve. Each point in each histogram represents the average of four nerves. The sizes of the bubbles in this graph are proportional to the SEM of each point (N=4). Black bubbles represent the distributions of axon profile lengths in untrained mice in which the cut nerve was repaired with a graft from a wild type donor mouse. The white bubbles represent the distribution of axon profile lengths measured in treadmill trained mice in which the cut nerve was repaired with a graft from a wild type donor mouse. These are the same data as shown in figure 2B. In panel A, data are presented in a similar format (light grey bubbles = untrained, dark grey bubbles = treadmill trained) from mice in which the cut CF nerve was repaired with a graft obtained from an NT-4/5 knockout mouse. In panel B, data are presented in a similar format (light grey bubbles = untrained, dark grey bubbles = treadmill trained) from mice in which the CF nerve had been cut and repaired with acellular nerve grafts. The graft type used and the treatment applied are shown in the legend. In panel C, average (±SEM) median axon profile lengths are shown for all six groups.
Poor growth of regenerating axons through acellular grafts is well-documented (Nadim et al., 1990; Sondell et al., 1998; Ghalib et al., 2001; Krekoski et al., 2001; English et al., 2007). This poor growth during the first two weeks following nerve transection and repair is noted in our data by a statistically significant (U-Test, p< 0.01) shift to the left in the distribution of axon profile lengths (Fig. 3B) and significantly (ANOVA F=17.61, DF=5, LSD, p< 0.004) smaller average median axon profile lengths (Fig. 3C).
If animals whose cut nerves were repaired in this manner are treated with modest daily treadmill training a different outcome is obtained. The distributions of axon profile lengths measured in these mice two weeks following transection and surgical repair of the CF nerve (Fig 3A: NT-4/5 KO & Trained; Fig. 3B: AC & Trained) are shifted significantly (U-Test, p< 0.01) to the right of both their appropriately matched untrained controls (Fig 3A: NT-4/5 KO & Untrained; Fig. 3B: AC & Untrained) and to that of untrained animals whose cut nerves had been repaired with grafts from wild type donor mice (Fig. 3A, B: WT & Untrained). These differences are noted in the much larger average median axon profile lengths in all of the treadmill trained mice (Fig. 3C). Among these groups of mice, only very small (and not statistically significant) differences in average median axon profile lengths were found (Fig. 3C). These findings are strong evidence that the effects of treadmill training on enhancing axon regeneration occur independent of the environment surrounding the regenerating axons.
Treadmill training and axon regeneration in the NT-4/5 knockout mouse
Enhancement of axon regeneration produced by brief electrical stimulation has been shown to depend on the availability of NT-4/5 in cells of the proximal nerve segment (English et al., 2007). To investigate whether the same dependence might underlie the effects of treadmill training, we studied axon regeneration in NT-4/5 knockout mice. We bred thy-1-YFP-H mice with mixed strain NT-4/5 knockout mice, and then back-crossed offspring until homozygous knockout mice carrying the transgene were obtained. We then cut and repaired CF nerves in these mice using grafts from non-fluorescent wild type or mixed strain NT-4/5 knockout mice. Half of these mice were subjected to two weeks of daily treadmill training and half served as untrained controls. Four nerves were studied in each of these treatment groups.
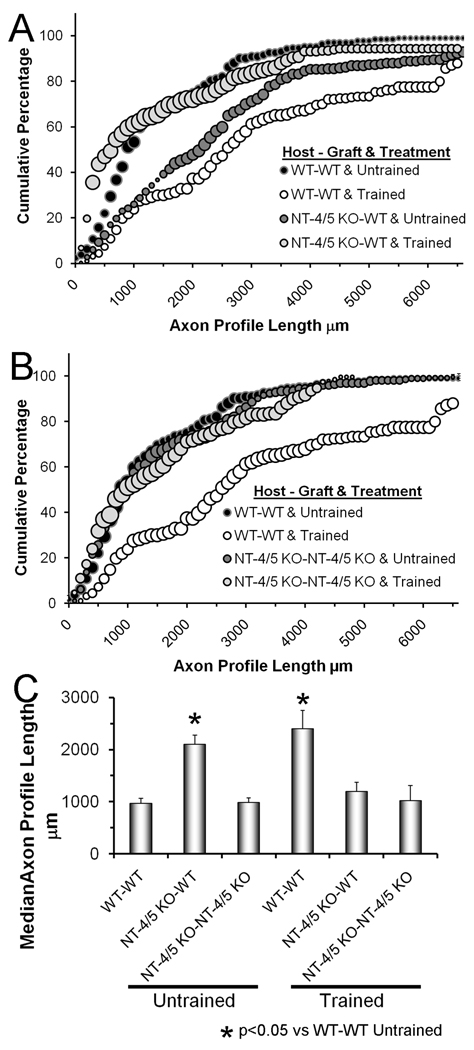
The distributions of regenerating axon profile lengths in NT-4/5 knockout mice are shown as cumulative histograms in Figure 4 A & B. In panel A data from mice repaired with grafts from wild type mice are shown. Data from mice in which cut nerves were repaired with grafts from NT-4/5 knockout mice are summarized in panel B. Median axon profile lengths measured in these groups of mice are shown in figure 4C. If regenerating axons from NT-4/5 knockout mice are constrained to grow through grafts from wild type mice, axon regeneration is enhanced. The distribution of axon profile lengths is shifted significantly (U-test, p< 0.05) to the right of untrained wild type mice whose nerves were repaired with grafts from wild type donors (Fig.4A: compare dark grey circles to black circles). The extent of this shift is similar to that observed in wild type mice that were trained for two weeks (Fig. 4A: compare dark grey circles to white circles). This is noted also in comparing median axon profile lengths in these groups (ANOVA, F=5.52, DF=6, p<0.01)(Fig. 4C). However, if cut nerves from NT-4/5 knockout mice are repaired with grafts from wild type mice and then subjected to two weeks of treadmill training, there is no significant difference in the distributions of axon profile lengths from those of untrained wild type mice whose nerves were repaired with grafts from wild type donors (Fig. 4A: compare light grey circles to black circles, Fig. 4C: medians). If cut nerves from NT-4/5 knockout mice are repaired with grafts from NT-4/5 knockout mice, regeneration is enhanced in neither untrained mice nor treadmill trained mice (Fig. 4B,C). Thus, the enhancement of axon regeneration produced by treadmill training is dependent on the availability of NT-4/5 in the proximal stump.
Figure 4.
The effects of treadmill training on axon regeneration in NT-4/5 knockout mice are shown. Panels A and B are cumulative histograms of axon profile lengths measured two weeks after transection and surgical repair of the common fibular nerve. Each point in each histogram represents the average of four nerves. The sizes of the bubbles in this graph are proportional to the SEM of each point (N=4). Black bubbles represent the distributions of axon profile lengths in untrained mice in which the cut nerve was repaired with a graft from a wild type donor mouse. The white bubbles represent the distribution of axon profile lengths measured in treadmill trained mice in which the cut nerve was repaired with a graft from a wild type donor mouse. These are the same data as shown in figure 3 A & B. In panel A, data are presented in a similar format (light grey bubbles = untrained, dark grey bubbles = treadmill trained) from NT-4/5 knockout mice in which the cut CF nerve was repaired with a graft obtained from a wild type donor mouse. In panel B, data are presented in a similar format (light grey bubbles = untrained, dark grey bubbles = treadmill trained) from NT-4/5 knockout mice in which the cut CF nerve was repaired with a graft obtained from an NT-4/5 knockout donor mouse. The Host-Graft combination and the treatment applied are shown in the legend. In panel C, average (±SEM) median axon profile lengths are shown for all six groups.
Discussion
A common reason given for the poor functional outcomes following peripheral nerve injury in humans is the relatively slow growth of regenerating axons (Fawcett & Keynes, 1990; Scholz et al., 2009). Using a mouse model of axon regeneration in injured peripheral nerves, we have shown that modest treadmill training following transection and conventional surgical repair of peripheral nerves results in a significant enhancement of axon regeneration (Sabatier et al., 2008). We have combined that model system with mice null for the gene for NT-4/5 to investigate the role played by this neurotrophin in the enhancement. Our principal findings are that treadmill training enhances axon regeneration in cut peripheral nerves irrespective of the environment through which they grow and that NT-4/5 in the proximal stumps of cut nerves is implicated in the treadmill training-induced enhancement of axon regeneration.
During axon regeneration in cut peripheral nerves, regenerating neurites form from axons in the proximal stump, migrate past the injury site, and if they can invade endoneurial tubes, they elongate in the distal stump. Because transformed Schwann cells in the distal stump begin to express the trkB ligands, BDNF and NT-4/5, within the first week following nerve transection (Funakoshi et al., 1993), it is widely held that regenerating neurite elongation is promoted by these neurotrophins. The growth of regenerating axons is very poor if the Schwann cells surrounding them are devoid of one or more of these neurotrophins or their receptor (Boyd & Gordon, 2002; Gordon et al., 2003) or if the Schwann cells are destroyed (Nadim et al., 1990; Sondell et al., 1998; Ghalib et al., 2001; Krekoski et al., 2001; English et al., 2007). Brief topical application of either recombinant human BDNF or NT-4/5 to transected nerves overcomes these deficits (English et al., 2005). In this study we observed that if modest daily treadmill training is applied following nerve transection and repair, regenerating axons grow robustly through grafts in which either no NT-4/5 is expressed by transformed Schwann cells or no Schwann cells exist. The lengths of these regenerating axons are similar to those measured in treadmill trained mice in which regenerating axons grew through grafts from wild type mice, suggesting that the effects of the treadmill training occurred independent of the Schwann cell environment of the regenerating axons. The enhancing effects of treadmill training are thus dependent on cells in the proximal stump.
The results of our experiments studying axon regeneration in the NT-4/5 knockout mouse are interpreted as evidence that the enhancing effects of treadmill training on axon regeneration include NT-4/5 in the proximal stump. A population of primary afferent neurons does not survive to adulthood in the NT-4/5 knockout mouse, and some of these NT-4/5 dependent neurons are present in lumbar dorsal root ganglia (Liu et al., 1995; Liebl et al., 2000). One might assume that in the NT-4/5 knockout mouse, only neurons whose axons do not require NT-4/5 to stimulate their growth would have survived. Such an hypothesis might be used to explain why, when cut nerves in NT-4/5 knockout mice are repaired with nerve grafts from NT-4/5 knockout mice, axon regeneration is similar to wild type controls. Other growth promoting molecules would be adequate. However, if cut nerves in NT-4/5 knockout mice are repaired with grafts from wild type mice, axon regeneration is enhanced relative to controls. The simplest interpretation of this finding is that even though regenerating axons in the NT-4/5 knockout mouse have never interacted with NT-4/5, they must be capable of responding to NT-4/5 in their environment, presumably produced by transformed wild type Schwann cells in the grafts. In either of these host-graft scenarios, treadmill training was ineffective in enhancing axon regeneration, suggesting that NT-4/5 in the proximal stump is one molecule responsible for the enhancing effects of treadmill training.
The results presented above are strikingly similar to those we have published on the role of NT-4/5 in enhancing axon regeneration using brief electrical stimulation (ES). Gordon and colleagues (Al-Majed et al., 2000) have shown that application of as little as one hour of ES at the time of repair of a peripheral nerve results in an enhancement of axon regeneration. Using the same combination of transgenic and knockout mice, we showed that this enhancement was independent of the environment of the regenerating axons and dependent on NT-4/5 in the cells of the proximal stump (English et al., 2007). Since blocking neural transmission proximal to the site of ES also eliminates its enhancement of axon regeneration (Al-Majed et al., 2000), we proposed that the most likely source of NT-4/5 in the proximal stump is in the regenerating axons themselves (English et al., 2007). We thus concurred with Gordon and colleagues (Al-Majed et al., 2000) that the effectiveness of ES may be the result of an autocrine/paracrine neurotrophin stimulation of axon elongation. We postulated that this stimulation involved neuronal NT-4/5 (English et al., 2007).
In the present study, neurons whose axons are regenerating are presumed to have been activated by the spinal circuitry related to the generation of treadmill locomotion, so that we cannot infer the source of origin of enhancement of axon regeneration as simply as with ES. Although it is well established that treadmill exercise results in an increased expression of BDNF in spinal motoneurons (Gomez-Pinilla et al., 2001; Ying et al., 2008), any effects of exercise on its synthesis by non-neuronal cells in the proximal stump are not known. Similar measurements of the effects of treadmill training on the expression of NT-4/5 in neurons or non-neuronal cells are not yet available. Until we are able to knock out NT-4/5 or/and BDNF in a cell type specific manner we will not be able to determine the extent to which Schwann cells, neurons, or other cell types in the proximal stump might mediate the effect of treadmill training.
We believe that the results presented above are further evidence for the existence of two distinct neurotrophin signaling mechanisms regulating axon regeneration. Elongation of regenerating neurites could be stimulated by the release of neurotrophins from Schwann cells in the environment surrounding them (Funakoshi et al., 1993), a classical retrograde signaling pathway. Alternatively, neurotrophins could be released by cells in the proximal nerve segment, either the regenerating axons themselves or surrounding non-neuronal cells. This would then lead to autocrine/paracrine enhancement of axon regeneration. Under most circumstances, the former mechanism seems to predominate, but the latter mechanism can be invoked by ES or treadmill training.
We have confined most of our observations to NT-4/5 because the NT-4/5 knockout mouse is viable and fertile and can be used in our experiments. Mice homozygous null for BDNF die as neonates (Ernfors et al., 1994) so that they cannot be used in our experiments. If cut nerves in thy-1-YFP-H mice are repaired with grafts from heterozygous BDNF knockout mice, axon regeneration is not different from controls (English et al., 2005). It is possible that BDNF in cells in the proximal stumps of cut nerves plays a role similar to or even more important than that proposed for NT-4/5 in enhancing axon regeneration following either ES or treadmill training. This hypothesis is especially attractive given the large increase in BDNF expression found in neurons following treadmill training.
Acknowledgements
This work was completed with support from grant NS057190 from the USPHS. Manning J Sabatier was supported by the USPHS NIH Institutional Research and Academic Career Development grant, K12 GM00680-05. Thanks to W. L. Gore & Associates, Inc., for the gift of the Gore-Tex tubing. Thanks to Saiyaka Heno and Hasan Saiyed who helped with some of the treadmill training. Special thanks to Dr. Jennifer Wilhelm for her insightful comments on the manuscript.
Abbreviations
- BDNF
brain derived neurotrophic factor
- CF
common fibular
- ES
brief electrical stimulation
- IP
intraperitoneal
- LSD
Fisher's least significant difference
- NT-4/5
neurotrophin-4/5
- p75NTR
common neurotrophin receptor
- trkB
neurotrophic tyrosine kinase, receptor, type 2
- U-test
Mann Whitney U test
- YFP
yellow fluorescent protein
Literature Cited
- Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004;24:379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PN, Nadim W, Turmaine M. Schwann cell migration through freeze-killed peripheral nerve grafts without accompanying axons. Acta Neuropathol (Berl) 1991;82:193–199. doi: 10.1007/BF00294445. [DOI] [PubMed] [Google Scholar]
- Asensio-Pinilla E, Udina E, Jaramillo J, Navarro X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp Neurol. 2009;219:258–265. doi: 10.1016/j.expneurol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Boyd JG, Gordon T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur J Neurosci. 2002;15:613–626. doi: 10.1046/j.1460-9568.2002.01891.x. [DOI] [PubMed] [Google Scholar]
- Brushart TM. Nerve repair and grafting. In: Green D, Hotchkiss R, Pederson W, editors. Green's operative hand surgery. New York: Churchill Livingstone; 1998. pp. 1381–1403. [Google Scholar]
- Brushart TM, Hoffman PN, Royall RM, Murinson BB, Witzel C, Gordon T. Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J Neurosci. 2002;22:6631–6638. doi: 10.1523/JNEUROSCI.22-15-06631.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter GC, Malessy MJ, Alaid AO, Spinner RJ, Engelstad JK, Sorenson EJ, Kaufman KR, Dyck PJ, Windebank AJ. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exp Neurol. 2008;211:339–350. doi: 10.1016/j.expneurol.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW. Enhancing axon regeneration in peripheral nerves also increases functionally inappropriate reinnervation of targets. J. Comp. Neurol. 2005;490:427–441. doi: 10.1002/cne.20678. [DOI] [PubMed] [Google Scholar]
- English AW, Meador W, Carrasco DI. Neurotrophin 4/5 is required for the early growth of regenerating axons in peripheral nerves. Eur J Neurosci. 2005;21:2624–2634. doi: 10.1111/j.1460-9568.2005.04124.x. [DOI] [PubMed] [Google Scholar]
- English AW, Mulligan A, Cucoranu D, Sabatier MJ. Treadmill training enhances axon regeneration in cut peripheral nerves without effecting topographic specificity of reinnervating motoneurons. J. Comp. Neurol. 2009;517:245–255. doi: 10.1002/cne.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AW, Mulligan A, Meador W, Sabatier MJ, Schwartz G. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007;67:158–172. doi: 10.1002/dneu.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee K, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Evans PJ, Midha R, Mackinnon SE. The peripheral nerve allograft: a comprehensive review of regeneration and neuroimmunology. Progr. Neurobiol. 1994;43:187–233. doi: 10.1016/0301-0082(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Ann. Rev. Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Franz CK, Rutishauser U, Rafuse VF. Intrinsic neuronal properties control selective targeting of regenerating motoneurons. Brain. 2008;131:1492–1505. doi: 10.1093/brain/awn039. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geremia NM, Gordon T, Brushart TM, Al-Majed AA, Verge VM. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp Neurol. 2007;205:347–359. doi: 10.1016/j.expneurol.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Ghalib N, Houst'ava L, Haninec P, Dubovy P. Morphometric analysis of early regeneration of motor axons through motor and cutaneous nerve grafts. Ann Anat. 2001;183:363–368. doi: 10.1016/S0940-9602(01)80183-7. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- Groves ML, McKeon R, Werner E, Nagarsheth M, Meador W, English AW. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp. Neurol. 2005;195:278–292. doi: 10.1016/j.expneurol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Haninec P, Dubovy P, Houst'ava L, Stejskal L. Acellular nerve graft re-seeded by Schwann cells migrating from the nerve stump can stimulate spinal motoneurons for functional reinnervation of the rat muscle. Ann Anat. 2000;182:123–131. doi: 10.1016/S0940-9602(00)80069-2. [DOI] [PubMed] [Google Scholar]
- Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. Otolaryngol Head Neck Surg. 2008;139:62–67. doi: 10.1016/j.otohns.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Huang J, Hu X, Lu L, Ye Z, Wang Y, Luo Z. Electrical stimulation accelerates motor functional recovery in autograft-repaired 10 mm femoral nerve gap in rats. J Neurotrauma. 2009;26:1805–1813. doi: 10.1089/neu.2008.0732. [DOI] [PubMed] [Google Scholar]
- Ilha J, Araujo RT, Malysz T, Hermel EE, Rigon P, Xavier LL, Achaval M. Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair. 2008;22:355–366. doi: 10.1177/1545968307313502. [DOI] [PubMed] [Google Scholar]
- Krekoski CA, Neubauer D, Zuo J, Muir D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J Neurosci. 2001;21:6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl D, Klesse L, Tessarollo L, Wohlman T, Parada L. Loss of brain-derived neurotrophic factor-dependent neural crest-derived sensory neurons in neurotrophin-4 mutant mice. Proc Natl Acad Sci U S A. 2000;97:2297–2302. doi: 10.1073/pnas.040562597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Enfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Lu MC, Ho CY, Hsu SF, Lee HC, Lin JH, Yao CH, Chen YS. Effects of electrical stimulation at different frequencies on regeneration of transected peripheral nerve. Neurorehabil Neural Repair. 2008;22:367–373. doi: 10.1177/1545968307313507. [DOI] [PubMed] [Google Scholar]
- MacGillivray TE. Fibrin sealants and glues. J Card Surg. 2003;18:480–485. doi: 10.1046/j.0886-0440.2003.02073.x. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysate-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J. Histochem. Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Menovsky T, Beek JF. Laser, fibrin glue, or suture repair of peripheral nerves: a comparative, functional, and morphometric study in the rat sciatic nerve. J. Neurosurg. 2001;95:694–699. doi: 10.3171/jns.2001.95.4.0694. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim W, Anderson PN, Turmaine M. The role of Schwann cells and basal lamina tubes in the regeneration of axons through long lengths of freeze-killed nerve grafts. Neuropathol Appl Neurobiol. 1990;16:411–421. doi: 10.1111/j.1365-2990.1990.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- Perreau VM, Adlard PA, Anderson AJ, Cotman CW. Exercise-induced gene expression changes in the rat spinal cord. Gene Expr. 2005;12:107–121. doi: 10.3727/000000005783992115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson GA, Madison RD. Influence of terminal nerve branch size on motor neuron regeneration accuracy. Exp Neurol. 2009;215:228–235. doi: 10.1016/j.expneurol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Sabatier M, Redmon N, Schwartz G, English A. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz T, Krichevsky A, Sumarto A, Jaffurs D, Wirth GA, Paydar K, Evans GR. Peripheral nerve injuries: an international survey of current treatments and future perspectives. J Reconstr Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- Seo TB, Han IS, Yoon JH, Hong KE, Yoon SJ, Namgung U. Involvement of Cdc2 in axonal regeneration enhanced by exercise training in rats. Med Sci Sports Exerc. 2006;38:1267–1276. doi: 10.1249/01.mss.0000227311.00976.68. [DOI] [PubMed] [Google Scholar]
- Sharma N, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation and testosterone differentially enhance expression of regeneration-associated genes. Exp Neurol. 2010;223:183–191. doi: 10.1016/j.expneurol.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Sunderland S. Anatomical features of nerve trunks in relation to nerve injury and nerve repair. Clin Neurosurg. 1970;17:38–62. doi: 10.1093/neurosurgery/17.cn_suppl_1.38. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Navarro X. Functional impact of axonal misdirection after peripheral nerve injuries followed by graft or tube repair. J Neurotrauma. 2002;19:1475–1485. doi: 10.1089/089771502320914705. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Zhong H, Zdunowski S, Edgerton VR, Gomez-Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]