Abstract
O2-[4-(3-Pyridyl)-4-oxobut-1-yl]thymidine (O2-POB-dThd) is the most persistent adduct detected in the lung and liver of rats treated with tobacco specific nitrosamines; N′-nitrosonornicotine (NNN), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). It is an important biomarker to assess the human exposure to these carcinogens. The only synthetic method reported for O2-POB-dThd requires repeated HPLC purifications and could only be used to prepare an analytical standard due to very low yield (0.4%). We have developed for the first time a regioselective and efficient method for the total synthesis of O2-POB-dThd and its site-specifically adducted oligonucleotides. The main step in the synthesis of O2-POB-dThd was achieved by novel method. The treatment of O2-5'-anhydrothymidine with sodium salt of 4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol gave exclusively the O2-alkylated adduct, which was deprotected in one step to furnish the desired O2-POB-dThd in excellent yield. The product was characterized by NMR (1H, 13C), high-resolution MS and HPLC analysis. This work provided for the first time a reliable method for large scale total synthesis of O2-POB-dThd that allowed for solid state site-specifically adducted oligomer synthesis. The O2-POB-dThd was converted to its phosphoramidite and subsequently used for the synthesis of oligodeoxynucleotides by standard methods. The oligomers were characterized by MS and HPLC analysis. These oligomers will facilitate elucidation of mutagenic potential of O2-POB-dThd adduct, which will provide further insight into the role of tobacco specific nitrosamines in inducing cancers in smokers.
Introduction
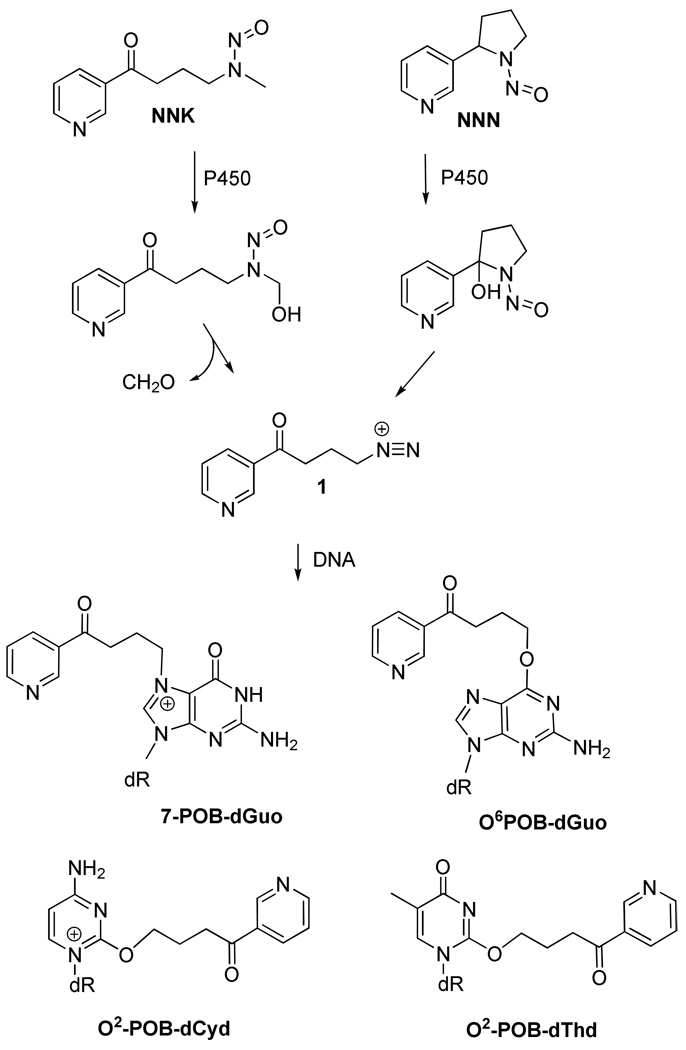
The tobacco specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK1) and N′-nitrosonornicotine (NNN) are the most potent and well established pulmonary carcinogens in rodents (1). They are present in both unburned tobacco and its smoke in substantial amounts and are suggested as important etiological factors for cancer development among tobacco smokers (2). Both NNK and NNN require metabolic activation by cytochrome P450 to exhibit their mutagenicity and possible carcinogenicity (1). Cytochrome P450 mediated bioactivation of both NNK (through α-hydroxylation followed by elimination of formaldehyde) and NNN (through 2′-hydroxylation followed by ring opening) resulted in the formation of a reactive diazonium ion 1, which alkylate DNA to produce pyridyloxobutyl (POB)-DNA adducts (Scheme 1). The formation of these DNA adducts is believed to be an important mechanism of carcinogenesis in both rodents (3–7) and possibly smokers (8, 9), since pyridoxobutylated DNA has been detected in rat esophagus cultured with NNN, and in animals treated with NNK and its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) (1, 9, 10). In addition, POB-DNA adducts were also detected in the lung tissue from smokers (9), thus elucidation of the role of POB-DNA adducts in the human lung carcinogenesis is critical.
Scheme 1.
Formation of POB-DNA adducts from NNK and NNN
The four POB-DNA adducts identified both in vitro and in vivo are O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxyguanosine (O6-POB-dGuo) (11–13), 7-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxyguanosine (7-POB-dGuo) (14), O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxycytidine (O2-POB-dCyd) (15), and O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (O2-POB-dThd) (15). Among them the O2-POB-dThd is recently identified as the most persistent of the POB adducts formed in the lung and liver of male F344 rats treated with NNK and NNAL (16) and it may play a role in tobacco induced cancers. It is an important biomarker to assess the human exposure to these carcinogens and would also serve as a useful precursor for the synthesis of site-specifically modified oligomers required for mutagenicity studies. Although, O2-POB-dThd may play an important role in mutagenesis and tumorigenesis, limited data is available, primarily due to the lack of suitable methods available for a larger scale synthesis of O2-POB-dThd required for these studies. The only direct synthesis reported is rather laborious, requires repeated HPLC purifications, is low yielding (0.4% yield) and could only be used to prepare an analytical standard (17). We report here a highly regioselective and efficient method developed for the total synthesis of O2-POB-dThd, and the methodology leading successfully to site-specifically adducted oligomer synthesis.
Experimental Procedures
General
Melting points were recorded on a Fischer-Johns melting point apparatus and are uncorrected. NMR spectra were collected with Bruker Avance II500 MHz instrument and the chemical shifts are given as δ values with reference to tetramethylsilane (TMS) as internal standard. HMBC and HSQC experiments were performed in methanol-d4 on a Bruker Avance II 500 MHz instrument equipped with BBO-probe. The standard Bruker pulse sequences (hmbcgplpndprqf and hsqcetgp) were used for HMBC and HSQC experiment, respectively. High-resolution MS (EI) were determined at the Chemistry Instrumentation Center, State University of New York at Buffalo. Thin-layer chromatography (TLC) was developed on aluminum-supported pre-coated silica gel plates (EM industries, Gibbstown, NJ). All column chromatography was performed on silica gel (70–230 mesh).
3′,5′-O-[1,1,3,3-tetrakis(isopropyl)-1,3-disiloxanediyl]-thymidine (2)
To a suspension of thymidine (3.5 g, 14.5 mmol) in anhydrous pyridine (100 mL) at 0° C was added 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane (5.0 mL, 15.6 mmol) dropwise under nitrogen atmosphere and the solution was stirred overnight at room temperature. The pyridine was removed under reduced pressure, the residue was diluted with CH2Cl2, and washed successively with 10% HCl, saturated sodium bicarbonate, and brine. The organic layer was dried over MgSO4, filtered, and the solvent was removed to give the crude product which was purified by silica gel column chromatography (eluant: CH2Cl2/MeOH : 99/1) to yield 2 (6.2 g, 86%) as a white solid. Mp 168–170 °C; 1H NMR (500 MHz, acetone-d6) δ 1.06–1.15 (m, 28H, 4 × CH(CH3)2), 1.82 (s, 3H, CH3), 2.41–2.54 (m, 2H, H2′), 3.81–3.84 (m, 1H, H4′), 4.08–4.10 (m, 2H, H5′), 4.70 (q, J = 7.5 Hz, 1H, H3′), 6.12 (dd, J = 7.5 and 3.5 Hz, 1H, H1′), 7.46 (s, 1H, pyrimidyl H6), 9.96 (s, 1H, NH); HRMS (ESI) calcd for C22H40N2O6Si2.H+, 485.2398; found, 485.2493.
N3-[3-{2-(3-pyridyl)-1,3-dithian-2-yl}propyl]-3′,5′-O-[1,1,3,3-tetrakis(isopropyl)-1,3-disiloxanediyl]thymidine (3)
To a solution of 2 (3.75 g, 7.49 mmol) and triphenylphosphine (2.95 g, 11.2 mmol) in anhydrous THF under nitrogen atmosphere was added 4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol (2.1 g, 8.2 mmol) dropwise and the reaction mixture was stirred for 30 min at room temperature. Diethyl azodicarboxylate (0.98 g, 5.6 mmol) was then added dropwise and the mixture was stirred overnight. The solvent was removed under reduced pressure and the crude product thus obtained was purified by silica gel column chromatography (eluant: hexanes/ethyl acetate: 70/30) to yield 3.8 g (66%) of 3 as a colorless oil. 1H NMR (500 MHz, acetone-d6) δ 1.07–1.16 (m, 28 H, 4 × CH(CH3)2), 1.57–1.63 (m, 2H, CH2), 1.82 (s, 3H, CH3), 2.06–2.08 (m, 2H, CH2), 2.09–2.14 (m, 2H, CH2), 2.41–2.49 (m, 2H, H2′), 2.63–2.69 (m, 2H, SCH2), 2.76–2.82 (m, 2H, SCH2), 3.75–3.84 (m, 3H, H4′ and OCH2), 4.08 (dd, J = 12.5 and 3.5 Hz, 2H, H5b′), 4.12 (dd, J = 12.5 and 4.5 Hz, 2H, H5a′), 4.67 (q, J = 8.0 Hz, 1H, H3′), 6.11 (dd, J = 7.0 and 4.0 Hz, 1H, H1′), 7.40 (dd, J = 8.0 and 5.0 Hz, 1H, H5), 7.47 (s, 1H, H6-pyrimidyl), 8.20 (m, 1H, H4), 8.50 (dd, J = 5.0 and 2.0 Hz, 1H, H6-pyridyl), 9.04 (d, J = 2.0 Hz, 1H, H2); 13C NMR (125 MHz, DMSO-d6) δ 16.7, 22.0, 24.5, 26.8, 29.4, 39.0, 39.9, 41.2, 55.5, 61.1, 69.3, 84.1, 84.6, 108.4, 122.8, 133.7, 135.9, 137.2, 147.9, 149.9, 150.1, 162.4; HRMS (ESI) calcd for C34H55N3O6S2Si2.H+, 722.3144; found, 722.3157.
N3-[4-(3-pyridyl)-4-oxobut-1-yl]-3′,5′-O-[1,1,3,3-tetrakis(isopropyl)-1,3-disiloxanediyl]thymidine (4)
A solution of N-chlorosuccinimide (0.36 g, 2.72 mmol) and AgNO3 (0.52 g, 3.06 mmol) in acetonitrile/water (1/1, 4 mL) was added to a solution of 3 (0.50 g, 0.69 mmol) in acetonitrile (10 mL). The reaction mixture was stirred at room temperature for 30 min and then treated with saturated Na2SO3, saturated Na2CO3 and brine. The product was extracted with ethyl acetate and dried over MgSO4, filtered and concentrated in vacuo to give a residue, which was purified by silica gel column chromatography with hexanes/ethyl acetate (1:1) as eluant to yield 4 (0.31 g, 70%) as a colorless oil. 1H NMR (500 MHz, acetone-d6) δ 1.06–1.15 (m, 28 H, 4 × CH(CH3)2), 1.81 (s, 3H, CH3), 2.05–2.07 (m, 2H, CH2), 2.40–2.51 (m, 2H, H2′), 3.14 (dt, J = 6.5 and 3.5 Hz, 2H, CH2CO), 3.81–3.84 (m, 1H, H4′), 4.01 (t, J = 6.5 Hz, 2H, OCH2), 4.08 (dd, J = 12.5 and 3.0 Hz, 1H, H5a′), 4.12 (dd, J = 12.5 and 4.0 Hz, 1H, H5b′), 4.66 (q, 1H, J = 8.0 Hz, H3′), 6.12 (dd, J = 7.5 and 3.5 Hz, 1H, H1′), 7.48 (s, 1H, H6, pyrimidyl), 7.52 (dd, J = 8.0 and 5.0 Hz, 1H, H5-pyridyl), 8.27 (dt, J = 8.0 and 2.0 Hz, 1H, H4-pyridyl), 8.77 (dd, J = 5.0 and 2.0 Hz, 1H, H6-pyridyl), 9.12 (d, J = 2.0 Hz, 1H, H2); HRMS (ESI) calcd for C31H49N3O7Si2.H+, 632.3182; found, 632.3189.
N3-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (5)
Tetrabutyl ammonium fluoride (1.0 M solution in THF, 1.14 mL, 1.14 mmol) was added dropwise to a solution of 4 (180 mg, 0.285 mmol) in anhydrous THF (10 mL). The reaction mixture was stirred at room temperature for 1.5 h, the solvent was removed by rotoevaporation in vacuo and the product was purified by silica gel column chromatography (eluant: CH2Cl2/MeOH : 95/5) to give 78 mg (71%) of 5 as a colorless viscous oil. 1H NMR (500 MHz, DMSO-d6) δ 1.80 (s, 3H, CH3), 1.91 (m, 2H, CH2), 2.04–2.12 (m, 2H, H2′), 3.11 (t, J = 7.0 Hz, 2H, CH2CO), 3.54–3.63 (m, 2H, H5′), 3.78 (d, J = 3.5 Hz, 1H, H4′), 3.90 (t, J = 7.0 Hz, 2H, OCH2), 4.24 (br s, 1H, H3′), 6.16 (t, J = 6.5 Hz, 1H, H1′) 7.56 (dd, J = 8.0 and 4.5 Hz, 1H, H5-pyridyl), 7.77 (s, 1H, H6-pyrimidyl), 8.25 (d, J = 8.0 Hz, 1H, H4-pyridyl), 8.79 (d, J = 4.5 Hz, 1H, H6-pyridyl), 9.08 (s, 1H, H2-pyridyl); 13C NMR (125 MHz, CD3OD) δ 11.7 (CH3), 21.2(CH2-CH2-CH2), 35.6 (CH2-CO), 39.9 (C2′), 40.3 (NCH2), 61.3 (C4′), 70.6 (C3′), 85.8 (C1′), 87.5 (C5′), 110.3 (C5), 123.9 (pyridyl C5), 132.7 (pyridyl C3), 135.1 (pyridyl C4), 136.0 (pyrimidyl C6), 148.6 (pyridyl C6), 151.1 (pyridyl C2), 152.3 (pyrimidyl C2), 164.2 (pyrimidyl C=O), 188.5 (CH2-C=O); HRMS (EI) calcd for C19H23N3O6.H+, 390.1742; found, 390.1749.
O2-[3-{2-(3-pyridyl)-1,3-dithian-2-yl]thymidine (7)
To a solution of 4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol (0.59 g, 2.45 mmol) in anhydrous THF under nitrogen atmosphere was added sodium (225 mg, 9.8 mmol) and stirred for 30 min. O2-5'-anhydrothymidine 6 (0.5 g, 2.23 m mol) was then added and the reaction mixture was stirred at 30° C overnight. The solvent was removed under reduced pressure and the crude product was extracted with ethyl acetate, washed with brine and dried over anhydrous MgSO4. The product was purified by silica gel column chromatography (eluant: CH2Cl2/MeOH: 95/5) to yield 305 mg (65%) of 7 as a colorless oil. 1H NMR (500 MHz, DMSO-d6) δ 1.60–1.64 (m, 2H), 1.79 (s,3H), 1.84–1.91 (m, 2H), 2.14–2.17 (m, 2H), 2.23–2.26 (m, 2H), 2.58–2.63 (m, 2H), 2.87–2.91(m, 2H), 3.55–3.58 (dd, J = 3.5 and 4.0 Hz, 1H), 3.61–3.64 (dd, J = 3.5 and 4.0 Hz, 1H), 3.81–3.83 (dd, J = 4.0 and 3.5 Hz, 1H), 4.20–4.26 (m, 3H), 6.03–6.06 (t, 1H, 6.5 Hz), 7.45–7.47 (dd, J = 4.5 and 3.5 Hz, 1H),7.81 (s, 1H), 8.13–8.15 (m, 1H,), 8.50–8.51 (d, J = 1.5 Hz, 1H), 8.96–8.97 (d, 1H, J = 2.5 Hz,); HRMS (EI) calcd for C22H29 N3O5S2.H+, 480.1621; found, 480.1618.
O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (8)
A solution of N-chlorosuccinimide (0.18 g, 1.36 mmol) and AgNO3 (0.26 g, 1.53 mmol) in acetonitrile/water (4/1, 4 mL) was added to a solution of 7 (0.25 g, 0.35 mmol) in acetonitrile (10 mL). The reaction mixture was stirred at room temperature for 30 min. and then treated with saturated Na2SO3, saturated Na2CO3 and brine. The product was extracted with ethyl acetate and dried over MgSO4, filtered and concentrated in vacuo to give a residue, which was purified by silica gel column chromatography with CH2Cl2/MeOH (95/5) as eluant to yield 8 (0.15 g, 75%) as a white solid [mp 168–170° C]. 1H NMR (500 MHz, DMSO-d6) δ 1.79 (s, 3H, CH3), 2.09 (q, 2H, J=6.80 Hz), 2.18 (dd, 2H, J = 6.8 and 6.42 Hz), 3.25 (t, 2H, J = 7.05 Hz,), 3.58–3.65(m, 2H), 3.79 (q, 1H), 4.24 (m, 1H), 4.37 (t, 2H, J = 6.5Hz), 5.06 (t, 1H, J = 5.15Hz), 5.27 (d, 1H, J = 4.40 Hz), 6.10 (t, 1H, J = 6.50 Hz), 7.57 (ddd, 1H, J = 8.01, 4.87, 0.7 Hz), 7.83 (d, 1H, J = 1.08 Hz), 8.31 (d, 1H, J = 8.01 and 2.01 Hz), 8.80 (dd, 1H, J = 4.87 and 1.87 Hz), 9.15 (d, 1H, J = 1.58 Hz); 13C NMR (500 MHz, CD3OD) δ 12.20 (CH3), 21.2(OCH2-CH2-CH2), 34.73 (OCH2-CH2-CH2), 40.64 (C2′), 61.08 (C5′), 68.35 (OCH2-CH2-CH2), 70.33 (C3′), 86.50 (C1′), 88.00 (C4′), 116.13 (pyrimidyl C5), 123.99 (pyridyl C5), 132.49 (pyridyl C3), 135.29(pyrimidyl C6), 136.08(pyridyl C4), 148.64 (pyridyl C2), 152.46 (pyridyl C6), 155.31 (pyrimidyl C2), 173.55 (pyrimidyl C=O), 198.24 (CO-CH2); HRMS (EI) calcd for C19H23N3O6.H+, 390.1660; found, 390.1659.
O2-[3-{2-(3-pyridyl)-1,3-dithian-2-yl]-3′,5′-O-[4,4’-dimethoxytrityl]thymidine (9)
To a solution of 7 (300 mg, 0.62 mmol) in anhydrous pyridine (10 ml) was added 4,4′-dimethoxyltritylchloride (1.0 g, 2.8 mmol) and the mixture was stirred at RT for 4 h under nitrogen atmosphere. Pyridine was removed under reduced pressure and the crude mixture was purified by silica gel column chromatography (eluant: CH2Cl2/MeOH/Et3N:98/2/0.2) to give 240 mg (62%) of 9 as a white foam. 1H NMR (500 MHz, DMSO-d6) δ 1.47 (s, 3H, CH3), 1.60–1.64 (m, 2H), 1.85–1.89 (m, 2H), 2.09–2.37 (m, 4H), 2.57–2.64 (m, 2H), 2.86–2.90 (m, 2H), 3.17–3.18 (d,2H, J = 5.0 Hz.), 3.61 (m, 1H), 3.74 (s, 6H, 2 × OCH3), 3.94–3.95 (m, 1H), 4.22–4.24 (t, J = 6.5 Hz, 2H), 6.06–6.09 (t, J = 6.5 Hz, 1H, H1′), 6.88–6.90 (d, 4H, J = 7.5 Hz, Tr), 7.22–7.26 (m, 5H), 7.29–7.32 (t, 2H, J = 7.5 Hz), 7.37–7.39 (d, J = 7.5, 2H), 7.43–7.45 (dd, J = 4.5 Hz, 1H), 7.61 (S, 1H, pyrimidyl), 8.12–8.14 (m, 1H, pyridyl), 8.50 (dd, J = 1.5 Hz, 1H, pyridyl), 8.96–8.97 (d, J = 2.5 Hz, 1H, pyridyl); δ HRMS (ESI) calcd for C43H47N3O7S2.H+, 782.2928; found, 782.2956.
5′-O-(4,4′-Dimethoxytrityl)-O2-{3-[2-(3-pyridyl)-1,3-dithian-2-yl]propyl}thymidine-3′-O-(2-cyanoethyl)-N,N-diisopropylamide-O-phosphite (10)
To a solution of 9 (0.36 g, 0.46 mmol) in dry THF (7 ml) at 0 °C, was added N,N-diisopropylethylamine (240 mg, 1.84 mmol) followed by cyanoethyl N,N-diisopropylchlorophosphoramidite (218 mg, 0.92 mmol) and the reaction mixture was stirred at RT for 1.5 h. The mixture was concentrated under reduced pressure, dissolved in ethyl acetate and washed with brine. The organic phase was dried, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluant: hexanes/ethyl acetate/Et3N, 2:8:1) to yield is 0.49 g (53%) of 10 as a white foam.
Synthesis of oligodeoxynucleotide
Oligodeoxynucleotides were synthesized at the 1 µmol scale under phosphoramidite conditions using the ultramild base protection scheme as describe by Glen Research. Under these conditions, the following protected phosphoramidites were used: N6-phenoxyacetyl-dAdo, N4-acetyl-dCyd, and N2-4-(iso-propyl)-phenoxyacetyl-dGuo. In addition, the capping agent used was phenoxyacetic anhydride in place of acetic anhydride. Using these changes allowed the oligodeoxynucleotide to be deprotected with 1 mL concentrated NH4OH at room temperature for 2 h, instead of the 12 h incubation at 55 °C. The solvent was evaporated, the residue was dissolved in 100 mM triethylamine/acetate solution (pH 7) and the oligodeoxynucleotides having a terminal DMTr group were isolated by the HPLC condition 1 described below. The DMTr-group was removed by treatment with 1 mL of 80% acetic acid for 30 min. The solvent was evaporated and the oligodeoxynucleotide purified by HPLC condition 2. The dithiane protection group was removed by incubation of 2 µM oligodeoxynucleotide with 40 µM N-chlorosuccinimide in 100 µL 50% CH3CN in H2O for 20 min at room temperature. The solution was cooled on ice until HPLC purification using the gradient described using condition 2.
The oligodeoxynucleotides were characterized by MALDI-TOF MS as well as enzymatic digestion followed by HPLC analysis to identify the presence of O2-POB-dThd (See supplementary data). Mass spectra of the modified oligodeoxynucleotides were obtained using a MALDI-TOF mass spectrophotometer (Voyager DE-PRO, Perseptive Biosystems/Applied Biosystems). The system was operated in the linear positive ion mode using a matrix mixture of 0.25 M 3-hydroxypicolinic acid, 25 mM ammonium phosphate, 0.1% CF3CO2H (v/v), and 30% CH3CN (v/v).
HPLC apparatus and conditions
The HPLC system consisted of Waters Model 680 gradient controller, two Model 510 pumps, and a Model 440 absorbance detector with a 256 nm filter. The analog output was saved with a Peaksimple Model 202 analog to digital converter. The purifications were performed with a Clarity 5μ Oligo-RP 100 × 10 mm column (Phenomenex). Two gradient conditions were employed using 100 mM triethylamine-acetate (pH 7.0) as buffer A and methanol as buffer B at 4 mL/min. Condition 1 was a linear 10% to 80% methanol gradient over 20 min. Condition 2 was a linear 10% to 35% methanol gradient over 25 min.
Results and Discussion
The O-alkylation of thymidine with diazoalkanes has been reported to give a mixture of O2- and O4-alkylated products (18). In our quest for the efficient synthesis of O2-POB-dThd, we required selective O-alkylation at the O2-position. First, we adapted the Mitsunobu reaction with appropriately protected thymidine to achieve an exclusive pyridyloxobutylation at O2-position. The synthetic strategy is outlined in Scheme 2. The 3′- and 5′-hydroxyl groups of thymidine were first protected with 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane to obtain compound 2. This type of protection has earlier been established by our group and others for the protection of 3′- and 5′-hydroxyl groups of deoxyguanosine moiety (14, 19). Treatment of 2 with 3-{2-(3-pyridyl)-1,3-dithian-2-yl}propanol (12), prepared according to literature methods (20, 21), in the presence of triphenylphosphine and diethyl azodicarboxylate gave exclusively the N-alkylated product 3 in 70% yield. The 1H NMR and the mass spectral data, however, were inconclusive in determining whether compound 3 was an O- or N-alkylated product. The dithiane protection group in 3 was then removed following a literature method (21, 22), by reacting with N-chlorosuccinimide in the presence of silver nitrate to yield O2-pyridyloxobutyl derivative 4, which was desilylated by treating with tetrabutylammonium fluoride to furnish the fully deprotected product 5 in 71% yield. The product was purified by a silica gel column chromatography and characterized based on NMR experiments and high-resolution MS. At this stage, though 1H NMR, MS, HPLC retention time and UV (Figures in Supporting Information) were still inconclusive in deciding the N- vs. O-alkylation and seemed in accordance with that reported in literature values (23); the comparison of the 13C NMR spectral data, however, clearly indicated the product to be the N-alkylated compound. The C1 (butyl) appeared at δ 40.3 as compared to δ 67.2 as reported, indicating the formation of N-alkylated product.
Scheme 2.
Synthesis of N3-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (5).
Reagents and conditions: a, [(i-Pr)2SiCl]2O; b, PPh3 diethyl azodicarboxylate; c N-chlorosuccinimide/AgNO3; d, Bu4NF4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol
We then changed our synthetic strategy to achieve an exclusive pyridyloxobutylation at O2-position by the treatment of O2,5'-anhydrothymidine (6) with the sodium salt of 4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol to obtain O2-[3-{2-(3-pyridyl)-1,3-dithian-2-yl]thymidine (7) (Scheme 3). Briefly, 4-(1,3-dithian-2-yl)-4-(3-pyridyl)butan-1-ol was converted to its sodium salt by treatment with sodium in anhydrous THF, followed by an in situ nucleophilic addition to O2,5'-anhydrothymidine (6) by stirring overnight to yield compound 7 in 65% yield. The structure of 7 was assigned based on the 1H NMR and MS spectra. The dithiane protection group in 7 was removed by reacting with N-chlorosuccinimide in the presence of silver nitrate, following a literature method (21, 22), to yield the desired O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (O2-POB-dThd) (8) in 75% yield. The product was purified by a silica gel column chromatography and characterized based on NMR experiments and high-resolution MS.
Scheme 3.
Synthesis of O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine (8).
Reagents and conditions: a, Na/THF; b, NCS
1H NMR spectrum of 8 was in accordance with that reported in the literature (23). Only partial 13C-NMR assignment of O2-POB-dThd was reported in the literature; the pyridyl, butyl C4, and 2′-deoxyribosyl 1′ and 3′ carbons were not reported (23) probably due to insufficient quantity of the sample. We recorded the 13C NMR spectrum of 8 in methanol-d4 and all the carbon atoms were assigned with the aids of HSQC and HMBC experiments. Collectively, these NMR data supported the formation of O2-alkylated adduct 8 (Figs. S8–S11, in the Supporting Information). The HSQC experiments indicated that the H1′ proton on the sugar ring interacts with the thymine C2, C5 and C6 carbons but the interaction does not extend to the C4 carbon on the pyrimidyl ring. Additionally, the H6 proton interacts with methyl, C6, C5, C4, C2 and C1′ carbons. These interactions clearly establish the chemical shift assignment of the C2 and C4 carbons at the pyrimidyl ring. Further analysis of the HMBC data, indicated that the OCH2 proton from the POB group interacts not only with C2 and C4 carbons of the pyrimidyl ring but also with C1′ carbon of the sugar ring, an interaction that is only possible when the butyl substitution is at the O2-position. Furthermore, the C1 (butyl) appeared at δ 68.35 as compared to δ 67.2 as reported in literature (23), clearly indicating the formation of an O-alkylated product. Based on these data the O2-alkylation assignment is well supported. Further confirmation was obtained by the observed similar UV spectrum and identical retention time of our adduct 8 and that reported earlier (~74 min.) (23) using same HPLC conditions.
The synthesis of a dimethoxytrityl phosphoramidite of the protected O2-POB-dThd is shown in Scheme 4. The incorporation of 8 into an oligodeoxynucleotide required retaining of the dithiane protection. Therefore, 5′-O-(4,4′-dimethoxytrityl) derivative 9 was prepared by treatment of 7 with 4,4′-dimethyltrityl chloride (DMTrCl) in pyridine. Reaction of 9 with 2-cyanoethyl-N,N,N′,N′-tetraisopropylphosphoramidite in the presence of Et3N furnished 3′-[O-(2-cyanoethyl)-diisopropylphosphoramidite] derivative 10 (Scheme 4) in good yield. The compounds were characterized on the basis of their 1H NMR spectra and MS analysis. The crude phosphoramidite 10 (~90% pure; 1H NMR spectrum in Supporting Information) was used as such for oligo syntheses.
Scheme 4.
Synthesis of O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine phosphoramidite 10 and its incorporation into oligodeoxynucleotides.
Reagents and conditions: a, DMTr-Cl/pyridine; b, 2-cyanoethyl-N,N-diisopropylchlorophosphoramidite; c, phosphoramidite DNA synthesis followed by NH4OH deprotection; d, NCS
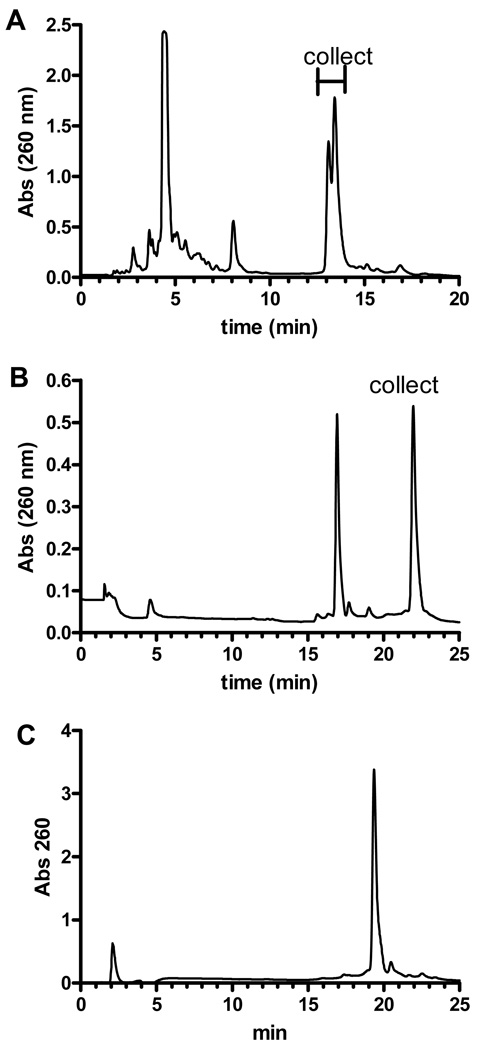
Phosphoramidite 10 was incorporated into the 5'-DMTr-oligodeoxynucleotide (11) by standard phosphoramidite methods using the ultramild base protection methods described by Glen Research (Table 1). The oligodeoxynucleotides were removed from the resin and the bases deprotected by treatment with concentrated NH4OH for 2 h at room temperature. The purification and MS analysis of 12a is shown in Figures 1 and 2, respectively. The DMTr-protected oligodeoxynucleotide 11a was purified by reverse-phase HPLC as shown in Figure 1A. The two eluting peaks indicated that the oligodeoxynucleotide was not pure. They were collected in a single fraction, the solvent evaporated and treated with 80% HOAc to remove the DMTr-group. The oligodeoxynucleotide 11a was purified by reverse-phase HPLC as shown in Figure 1B. The peaks were analyzed by MALDI-TOF MS. The early eluting peak (17 min.) had a m/z of 3698 as shown in Figure 2A, consistent with ammonolysis or hydrolysis of the O2-alkylthymine. The late eluting peak had a m/z value of 3937 (Figure 2B) consistent with the dithiane-protected oligodeoxynucleotide. The dithiane protection was then removed by NCS treatment and purified by HPLC (Figure 1C). The shorter retention time of 19 min compared with 22 min is consistent with removal of the hydrophobic dithiane group. The oligodeoxynucleotide was analyzed by MALDI-TOF mass spectrometric analysis which afforded a m/z of 3848 (Figure 2C) consistent with the fully deprotected O2-POB-dThd–adducted oligo. The enzymatic hydrolysis, followed by HPLC analysis also clearly indicated the presence of O2-POB-dThd. The oligonucleotides 12b and 12c were prepared and characterized using a similar methodology.
Table 1.
Sequences and mass spectral characterization of oligodeoxynucleotides containing modified thymidine residues.
| m/z [M+H]+ | |||
|---|---|---|---|
| Id | Sequencea | Expected | Observed |
| 11a | GTG CGY GTT TGT | 3936 | 3937 |
| 12a | GTG CGX GTT TGT | 3846 | 3848 |
| 11b | CTGCGACAYCTGCGTCTGCGGTGC | 7575 | 7582 |
| 12b | CTGCGACAXCTGCGTCTGCGGTGC | 7485 | 7485 |
| 11c | CTGCGACGYCTGCGTCTGCGGTGC | 7591 | 7598 |
| 12c | CTGCGACGXCTGCGTCTGCGGTGC | 7501 | 7501 |
Y = 7, X = O2-POB-dThd (8)
Figure 1.
Purification of oligodeoxynucleotide 12a, containing O2-POB-dThyd. A, reverse-phase HPLC chromatogram of DMTr-11a. The gradient was 10 to 80% MeoH in 100 mM triethylamine acetate (pH 7). B, Reverse-phase HPLC of 11a. The gradient was 10 to 35% methanol over 25 min. C, Reverse-phase HPLC of 12a. The gradient was 10 to 35% methanol over 25 min. The absorbance of the eluant was monitored at 254 nm.
Figure 2.
The MALDI-TOF MS. A. Peak at 17 min of Figure 1B. B, Peak at 22 min of Figure 1B. C, Peak at 19 min of Figure 1C. The spectra were obtained in the linear positive ion mode using a matrix mixture of 0.25 M 3-hydroxypicolinic acid, 25 mM ammonium phosphate, 0.1% CF3CO2H (v/v), and 30% CH3CN (v/v).
In conclusion, a highly facile synthesis of O2-POB-dThd was established. This adduct is a useful biomarker to assess the risk of lung and liver cancers in smokers. The synthetic strategy developed for O2-POB-dThd was successfully extended for the synthesis of corresponding phosphoramidite and site-specifically adducted oligonucleotides. The availability of these oligomers will facilitate studies of mechanistic aspects of adduction and mutagenic potential of O2-POB-dThd adduct, providing further insight into the role of tobacco specific nitrosamines and metabolites in inducing cancers in smokers.
Supplementary Material
ACKNOWLEDGEMENT
The authors thank Solution Phase NMR Facility at Core Research Facilities of the Penn State College of Medicine for recording of NMR spectra and the Macromolecular Core facility for synthesis of the oligodeoxynucleotides.
FUNDING SUPPORT. This study was supported by NCI Contract # NO2-CB-81013-74, Pennsylvania Department of Health using Tobacco Settlement Funds, and the Penn State Hershey Cancer Institute of the Penn State Hershey College of Medicine. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Abbreviations: DMTr, 4,4'-dimethoxyltrityl; DIEA, N,N-diisopropyl-N-ethylamine; NCS, N-chlorosuccinimide; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol; NNN, N′-nitrosonornicotine; O2-POB-dThd, O2-[4-(3-pyridyl)-4-oxobut-1-yl]thymidine; O6-POB-dGuo, O6-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxyguanosine; 7-POB-dGuo, 7-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxyguanosine; O2-POB-dCyd, O2-[4-(3-pyridyl)-4-oxobut-1-yl]-2'-deoxycytidine; HMBC, Heteronuclear Multiple Bond Correlation; HSQC, Heteronuclear Single-Quantum Coherence.
SUPPORTING INFORMATION AVAILABLE. 1H NMR and/or 13C NMR spectra for compounds 2–5 and 7–10, HMBC and HSQC spectra for compound 5, HPLC trace and UV spectrum of 5, a table containing 1H and 13C NMR assignment of 8. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS, Hoffmann D. The relevance of tobacco-specific nitrosamines to human cancer. Cancer Surv. 1989;8:273–294. [PubMed] [Google Scholar]
- 3.Trushin N, Rivenson A, Hecht SS. Evidence supporting the role of DNA pyridyloxobutylation in rat nasal carcinogenesis by tobacco-specific nitrosamines. Cancer Res. 1994;54:1205–1211. [PubMed] [Google Scholar]
- 4.Morse MA, Wang CX, Stoner GD, Mandal S, Conran PB, Amin SG, Hecht SS, Chung FL. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced DNA adduct formation and tumorigenicity in the lung of F344 rats by dietary phenethyl isothiocyanate. Cancer Res. 1989;49:549–553. [PubMed] [Google Scholar]
- 5.Staretz ME, Foiles PG, Miglietta LM, Hecht SS. Evidence for an important role of DNA pyridyloxobutylation in rat lung carcinogenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone: effects of dose and phenethyl isothiocyanate. Cancer Res. 1997;57:259–266. [PubMed] [Google Scholar]
- 6.Sticha KR, Kenney PM, Boysen G, Liang H, Su X, Wang M, Upadhyaya P, Hecht SS. Effects of benzyl isothiocyanate and phenethyl isothiocyanate on DNA adduct formation by a mixture of benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Carcinogenesis. 2002;23:1433–1439. doi: 10.1093/carcin/23.9.1433. [DOI] [PubMed] [Google Scholar]
- 7.Boysen G, Kenney PM, Upadhyaya P, Wang M, Hecht SS. Effects of benzyl isothiocyanate and 2-phenethyl isothiocyanate on benzo[a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism in F-344 rats. Carcinogenesis. 2003;24:517–525. doi: 10.1093/carcin/24.3.517. [DOI] [PubMed] [Google Scholar]
- 8.Foiles PG, Akerkar SA, Carmella SG, Kagan M, Stoner GD, Resau JH, Hecht SS. Mass spectrometric analysis of tobacco-specific nitrosamine-DNA adducts in smokers and nonsmokers. Chem. Res. Toxicol. 1991;4:364–368. doi: 10.1021/tx00021a017. [DOI] [PubMed] [Google Scholar]
- 9.Schlobe D, Holze D, Richter E, Tricker AR. Determination of tobacco-specific nitrosamine hemoglobin and lung DNA adducts. Proc. Am. Assoc. Cancer Res. 2002;43:346. [Google Scholar]
- 10.Thomson NM, Kenney PM, Peterson LA. The pyridyloxobutyl DNA adduct, O6-[4-oxo-4-(3-pyridyl)butyl]guanine, is detected in tissues from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-treated A/J mice. Chem. Res. Toxicol. 2003;16:1–6. doi: 10.1021/tx025585k. [DOI] [PubMed] [Google Scholar]
- 11.Mijal RS, Loktionova NA, Vu CC, Pegg AE, Peterson LA. O6-pyridyloxobutylguanine adducts contribute to the mutagenic properties of pyridyloxobutylating agents. Chem. Res. Toxicol. 2005;18:1619–1625. doi: 10.1021/tx050139t. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Spratt TE, Liu XK, Hecht SS, Pegg AE, Peterson LA. Pyridyloxobutyl adduct O6-[4-oxo-4-(3-pyridyl)butyl]guanine is present in 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone-treated DNA and is a substrate for O6-alkylguanine-DNA alkyltransferase. Chem. Res. Toxicol. 1997;10:562–567. doi: 10.1021/tx9602067. [DOI] [PubMed] [Google Scholar]
- 13.Pauly GT, Peterson LA, Moschel RC. Mutagenesis by O(6)-[4-oxo-4-(3-pyridyl)butyl]guanine in Escherichia coli and human cells. Chem. Res. Toxicol. 2002;15:165–169. doi: 10.1021/tx0101245. [DOI] [PubMed] [Google Scholar]
- 14.El-Bayoumy K, Sharma AK, Lin JM, Krzeminski J, Boyiri T, King LC, Lambert G, Padgett W, Nesnow S, Amin S. Identification of 5-(deoxyguanosin-N2-yl)-1,2-dihydroxy-1,2-dihydro-6-aminochrysene as the major DNA lesion in the mammary gland of rats treated with the environmental pollutant 6-nitrochrysene. Chem. Res. Toxicol. 2004;17:1591–1599. doi: 10.1021/tx049849+. [DOI] [PubMed] [Google Scholar]
- 15.Wang M, Cheng G, Sturla SJ, Shi Y, McIntee EJ, Villalta PW, Upadhyaya P, Hecht SS. Identification of adducts formed by pyridyloxobutylation of deoxyguanosine and DNA by 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone, a chemically activated form of tobacco specific carcinogens. Chem. Res. Toxicol. 2003;16:616–626. doi: 10.1021/tx034003b. [DOI] [PubMed] [Google Scholar]
- 16.Lao Y, Yu N, Kassie F, Villalta PW, Hecht SS. Formation and accumulation of pyridyloxobutyl DNA adducts in F344 rats chronically treated with 4-(methylnitrosamino)-1- (3-pyridyl)-1-butanone and enantiomers of its metabolite, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol. Chem. Res. Toxicol. 2007;20:235–245. doi: 10.1021/tx060207r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sturla SJ, Scott J, Lao Y, Hecht SS, Villalta PW. Mass spectrometric analysis of relative levels of pyridyloxobutylation adducts formed in the reaction of DNA with a chemically activated form of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem. Res. Toxicol. 2005;18:1048–1055. doi: 10.1021/tx050028u. [DOI] [PubMed] [Google Scholar]
- 18.Drosdziok W, Lutze C, Kruger K, Glusenkamp K-H, Rajewsky MF. 3H-labelled alkyl-nucleotides, -nucleosides and -bases for the immunoanalytical quantification of DNA damage and repair. J. Label. Compd. Radiopharm. 2003;46:815–835. [Google Scholar]
- 19.Wang Z, Rizzo CJ. Synthesis of the C8-deoxyguanosine adduct of the food mutagen IQ. Org. Lett. 2001;3:565–568. doi: 10.1021/ol006968h. [DOI] [PubMed] [Google Scholar]
- 20.Brown TJ, Chapman RF, Cook DC, Hart TW, McLay IM, Jordan R, Mason JS, Palfreyman MN, Walsh RJ, Withnall MT, et al. Synthesis and biological activity of trans(+)-N-methyl-2-(3-pyridyl)-2-tetrahydrothiopyrancarbothioamide 1-oxide (RP 49356) and analogues: a new class of potassium channel opener. J. Med. Chem. 1992;35:3613–3624. doi: 10.1021/jm00098a004. [DOI] [PubMed] [Google Scholar]
- 21.Lin JM, Desai DH, Morse MA, Amin S, Hecht SS. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone pulmonary metabolism and tumorigenicity in mice by analogues of the investigational chemotherapeutic drug 4-ipomeanol. Chem. Res. Toxicol. 1992;5:674–679. doi: 10.1021/tx00029a013. [DOI] [PubMed] [Google Scholar]
- 22.Corey EJ, Crouse D. Application of bisthio carbanions to the elaboration of 2-cyclohexenone systems. J. Org. Chem. 1968;33:298–300. [Google Scholar]
- 23.Hecht SS, Villalta PW, Sturla SJ, Cheng G, Yu N, Upadhyaya P, Wang M. Identification of O2-substituted pyrimidine adducts formed in reactions of 4-(acetoxymethylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(acetoxymethylnitros-amino)-1-(3-pyridyl)-1-butanol with DNA. Chem. Res. Toxicol. 2004;17:588–597. doi: 10.1021/tx034263t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.