Abstract
Chronic stress is implicated in diseases which differentially affect men and women. This study investigated how the activation of neuronal subpopulations contributes to changes in neuroendocrine regulation that predispose members of each sex to stress-related health challenges. Adult male and female rats were restrained in single (acute) or 14 consecutive daily (repeated) 30 min sessions; brain sections were immunohistochemically stained for Fos, arginine vasopressin (AVP) or glucocorticoid receptor (GR) within the paraventricular hypothalamic nucleus (PVH). Acute restraint increased the number of PVH cells expressing Fos, with greater increases in males than females. Habituated responses were seen following repeated stress in both sexes, with no sex differences between groups. No sex differences were found in the number of neurons co-expressing Fos and AVP. Absolute counts of cellular Fos and GR co-localization mirrored Fos expression. In contrast, when doubly-labeled cells were normalized to staining for Fos alone, females showed greater numbers of Fos- and GR-positive cells than males after both acute and repeated stress. These data demonstrate that sex-specific stress responses are evident at the level of neuronal activation, and may contribute to different consequences of chronic stress in females versus males. Females may be more sensitive to glucocorticoid negative feedback, suggesting that sex-dependent differences in the efficiency of initiating and terminating stress responses may exist. Understanding the neural and endocrine pathways that mediate these functions in males and females will inform targeted therapeutic strategies to alleviate stress and the sex-specific afflictions with which it is associated.
Keywords: HPA Axis, Paraventricular Nucleus, Fos, Vasopressin, Glucocorticoid Receptor
Introduction
“Stress” is a term that can be used to describe a wide variety of stimuli, both physiological and psychological, that can have major direct and indirect effects on bodily function [1]. Acute or short-term stress has been shown to induce an array of neurological and endocrine responses geared toward survival and the maintenance of homeostasis. Repeated or chronic stress, in contrast, often leads to an adaptation of these responses, yet has been linked with the onset or increased severity of numerous diseases including affective, immune and cardiovascular disorders (for recent review, see [2]). It is critically important, therefore, to understand how stress responses are initiated, and ultimately terminated, in order to effectively identify targets for the treatment of these stress-related illnesses which are becoming increasingly pervasive, contribute significantly to the rising costs of healthcare, and negatively impact quality of life in those who suffer from these disorders. Furthermore, differential responses to stress have been observed in males and females, and sex-specific outcomes have been noted in terms of stress-related disease incidence, but the mechanisms through which these outcomes are mediated remain unclear. The present studies were thus undertaken to more clearly define the central nervous system pathways and mechanisms that are activated by stress, with particular attention given to how these factors may differ in females compared to males since sex is one of the most important predictors of health. As a consequence of chronic stress, women tend to suffer more often from autoimmune illnesses while men are at a greater risk for developing coronary or infectious diseases [3]. With regard to psychiatric disorders, women more often experience anxiety, depression or panic disorders, while substance abuse and antisocial behaviors are more common in men [3]. Understanding the biological bases of these differences will inform future efforts to prevent or treat these sex-specific pathologies [4].
The hypothalamic-pituitary-adrenal (HPA) axis is a neuroendocrine control and regulatory mechanism through which the central nervous system can modulate peripheral hormone secretion and physiological function. Activated by stress, the HPA axis involves the secretion of corticotropin-releasing factor (CRF) from the paraventricular hypothalamic nucleus (PVH), which then stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland. ACTH, in turn, induces glucocorticoid (GC) release from the adrenal cortex. The PVH, therefore, is responsible for the generation of stress responses as it lies at the head of the HPA axis. Several studies have demonstrated that the PVH is activated by stress, as evidenced by enhanced expression of CRF mRNA or protein, c-fos mRNA, or Fos protein [5-8]. Furthermore, neuronal activation levels in the PVH have been shown to be high during acute stress, but decrease or habituate in response to repeated homotypic stress exposure [6, 9].
Cells that remain active in the repeated stress condition tend to be localized within a more ventral subregion of the PVH, compared to the more dorsally situated PVH neurons which are seen to be activated during acute stress [10]. This suggests that distinct subsets of neurons in the PVH may exist, and may be differentially responsible for initiating stress responses and maintaining them in the repeated condition. We therefore investigated Fos expression in the PVH in response to acute and repeated restraint stress, and further determined whether the activated neurons in each case express distinct neuropeptide phenotypes that would allow us to discriminate their function.
Arginine vasopressin (AVP) is an osmoregulatory neuroendocrine hormone which is normally secreted by magnocellular neurons in the PVH. During prolonged stress, however, AVP is also increasingly expressed and co-released from parvocellular PVH neurons, and travels to the pituitary to act as a co-secretagogue with CRF to enhance ACTH release [11]. AVP is believed to play an important role in sustaining pituitary responsiveness during chronic stress [12], and CRF and AVP are both essential for coordinating the behavioral and metabolic responses to stress [13]. Circulating GCs bind to glucocorticoid receptors (GRs), low-affinity corticosteroid receptors that are expressed at high levels throughout the brain and pituitary [14]. A member of the nuclear hormone receptor family of ligand-activated transcription factors [15], GRs are active when GC concentrations are high, and serve to mobilize energy resources and terminate stress responses through negative feedback at the level of the hypothalamus [13]. GRs also facilitate recovery from stress by interfering with transcription and repressing cellular activities that are induced by stress such as increased CRF and AVP synthesis [13]. In addition, GCs are capable of binding to GRs in extrahypothalamic brain regions that can subsequently modify the activity of the HPA axis [16]. The secretion of GCs into the circulation is primarily mediated by the HPA axis [16]; inhibition of this axis serves to minimize the amount of tissue exposure to GCs, thus reducing the catabolic, lipogenic, anti-reproductive and immunosuppressive effects of these hormones [16]. Lastly, GR promotes memory storage in preparation for future stressful events [13].
Given the highly significant roles of AVP and GR in HPA axis regulation, we examined the PVH in male and female rats under control, acute stress and repeated stress conditions to determine if stress-sensitive neurons would also express these markers. Our hypothesis was that the activation and phenotype of hypothalamic neurons that function in the initiation and termination of stress responses would differ by stress condition and sex. We further anticipated that the mechanisms through which sex-specific responses to stress are mediated would include neuroendocrine and feedback control systems involved in fluid and energy homeostasis, as these mechanisms are critical for the survival of females and their offspring.
Methods
Experimental animals
Young adult (3-4 months of age) male and female Sprague Dawley rats (Harlan, Houston, TX) were used in the present experiments. All rats were individually housed in standard cages in a temperature-controlled animal facility maintained on a 12:12 hour light:dark cycle, with food and water provided ad libitum. Following shipment, the rats were allowed at least one week of acclimatization to the facility before experimentation was initiated. Rats were randomly assigned to Control, Acute restraint or Repeated restraint groups (n=5/group). Animal care and use were in accordance with the Guidelines of the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee.
Restraint stress
Emotional stress was applied in the form of acute or repeated restraint, which consisted of placing the rats in a plastic restraining device (Kent Scientific, Torrington, CT) for 30 min. Repeatedly stressed rats were restrained in their home cages for 30 min daily over 14 consecutive days. Acutely stressed rats were exposed to open restrainers for 30 min/d over the first 13 days, then restrained for 30 min on the 14th day only. Unstressed control rats were exposed to open restraining devices on each of 14 consecutive days, but were never restrained. All restraint and exposure occurred near the beginning of the light cycle, between the hours of 0900 and 1100.
Perfusion
Rats were transcardially perfused 2 hours after the termination of restraint or exposure on the final day. Rats were weighed and deeply anesthetized by i.p. injection of 100 mg/kg sodium pentobarbital (Nembutal®; McKesson, Washington Courthouse, OH). Perfusion through the ascending aorta was done using ~100 mL of ice-cold 0.9% saline, followed by 400-500 mL of ice-cold 4% paraformaldehyde (JTBaker, Inc., Pittsburg, NJ) at pH 9.5 in 0.1 M borate buffer. Brain tissues were then collected and post-fixed for 5 hours at 4°C, followed by cryoprotection overnight at 4°C in 10% sucrose in KPBS.
Tissue Processing
The following day, brains were removed from cryoprotectant, mounted on a tabletop freezing microtome (Model SM 2000R; Leica Microsystems, Bannockburn, IL), and serial frozen sections taken in the coronal plane from a block of brain tissue containing the hypothalamic paraventricular nucleus. Five 1:5 series at 30μm intervals were collected into antifreeze (30% ethylene glycol, 20% glycerol) and stored at −20°C until used for immunohistochemical analyses.
Immunohistochemistry
Brain tissue sections were immunohistochemically stained for peptides and receptors known to play key roles in the stress response. First, Fos immunoreactivity was measured using a nickel-intensified avidin-biotin-immunoperoxidase technique. Sections were washed in KPBS, then placed in 0.3% hydrogen peroxide to quench endogenous peroxidases and 1% sodium borohydride to reduce free aldehydes. After being washed thoroughly, the tissue was placed in primary antiserum (rabbit anti-Fos; Oncogene Science, Cambridge, MA) diluted 1:50,000 in KPBS containing 0.3% Triton X-100 and 2% normal goat serum and incubated at 4°C overnight with gentle agitation. On the following day, sections were incubated in secondary antibody (biotinylated goat anti-rabbit IgG, 1:200 dilution; Vector Laboratories, Burlingame, CA) for 1h. An avidin-biotin-complexing solution (Vectastain Elite kit; Vector Laboratories, Burlingame, CA) was then applied for 1h, and a nickel-enhanced glucose oxidase method using diaminobenzidine (DAB) as a chromogen was utilized to visualize specific binding [17].
Subsequently, dual localization of Fos with other markers was accomplished using the same immunoperoxidase method as described above but with sequential staining for the second marker done without nickel enhancement. Cells expressing both markers were therefore visible as having black nuclei (Fos) and a brown cytoplasm (second marker). Due to the exothermic nature of the DAB reaction, the concentration of the Fos primary in these analyses was changed to a 1:10,000 dilution and the reaction carried out on ice in order to optimize our double staining. Fos was co-localized with arginine vasopressin (AVP) and the glucocorticoid receptor (GR), using primary antisera at 1:10,000 dilutions (Abcam, Inc., Cambridge, MA), to determine the number of stress-activated cells that also expressed these markers. In all cases, stained sections were mounted on gelatin-coated slides and allowed to dry overnight before being defatted through a graded ethanol series and xylene and coverslipped. Lastly, one series from each rat was stained with 0.25% thionin for Nissl material. These slides were used to evaluate the basic architecture of the brain regions to be examined, and served as a reference during analysis of staining for other markers.
Quantification of Staining
Light microscopy (AxioScop; Carl Zeiss, Inc., Thornwood, NY) and simple cell counting methods were used to measure immunoreactivity in both single- and dual-staining experiments. Initial counts were made at 20× magnification for Fos-positive and doubly-labeled cells, with a higher magnification (40×) and different focal planes used for verification of double labeling. Cell counts were performed both manually and with the assistance of a digital microimaging system coupled to computer software (AxioCam with AxioVision software; Carl Zeiss, Inc., Thornwood, NY), and quantified in five sections through the PVH. Proper identification of the anatomical region of interest was assured through use of adjacent Nissl series as well as the The Rat Brain atlas of Paxinos and Watson (4th edition, 1998) [18]. Specifically, immunopositive cells were counted bilaterally; the number of cells expressing each marker per side was then summed, and group means, standard deviations and standard errors were calculated.
Statistical Analysis
The mean number of immunopositive cells was compared and evaluated with a one-way analysis of variance (ANOVA) to determine overall effects, followed by Tukey-Kramer post hoc tests to determine individual differences (JMP program, version 7; SAS Institute, Inc., Cary, NC). Significance was determined at the p<0.05 level.
Results
Body weight
The body weights of all rats were recorded at the time of perfusion. While males were significantly larger than females in all treatment groups, as would be expected, no significant differences were seen between treatment groups within either sex: Control males = 358 ± 8g, Acute males = 345 ± 7g, Repeated males = 332 ± 7g, Control females = 255 ± 8g, Acute females = 250 ± 7g, Repeated females = 241 ± 6g.
Immunohistochemical staining for neuroendocrine markers
Single and dual immunostaining for Fos, AVP and GR yielded numerous cells that were subsequently visualized and quantified throughout the rostrocaudal extent of the PVH (Fig. 1). Localization of a nuclear Fos signal within neurons of the PVH was achieved in both sexes and all treatment groups. Similarly, cytoplasmic staining for AVP or GR was done in conjunction with the Fos labeling, allowing doubly-labeled cells to be seen and counted in tissue sections from all animals.
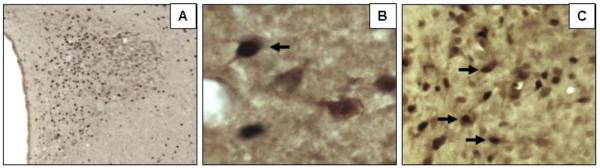
Figure 1. Immunohistochemical staining for Fos, AVP and GR.
Neuronal markers were localized to PVH cells using single- or dual-label immunohistochemical staining, and an immunoperoxidase method with or without nickel enhancement. Numerous stress-sensitive neurons are seen in a representative photomicrograph (5× magnification) of the PVH from an acutely stressed male rat stained for Fos immunoreactivity in panel A. Co-localization of Fos with AVP was also demonstrated (panel B), in which dual staining for Fos (black nuclei) and AVP (brown cytoplasm) was performed. A single cell expressing both markers can be seen (black arrow) in this image, which was captured at 40× magnification. As shown in panel C, many cells were seen to co-express Fos and GR in the PVH, some of which are indicated by the black arrows in this image (20× magnification).
Fos expression in the PVH of stressed male and female rats
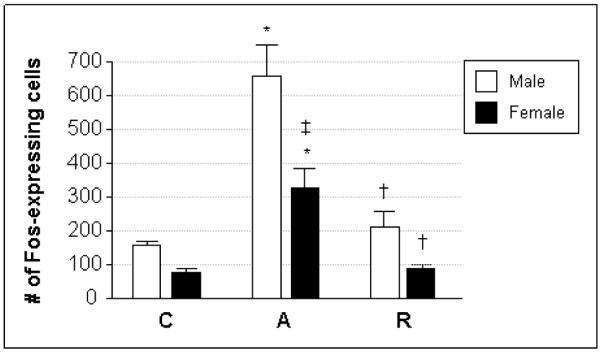
The profile of neuronal activation in the PVH was similar in male and female rats exposed to acute or repeated restraint stress; significant sex differences were seen, however, in the acute stress paradigm (Fig. 2). The number of cells showing Fos immunoreactivity in the PVH was low in control males (159 ± 3), but increased significantly in response to acute restraint stress (657 ± 87). With repeated stress exposure, a habituation was seen in the number of activated cells (210 ± 47) such that Fos expression was restored to control levels in this group. Female rats displayed qualitatively similar responses to stress as the males, with acute stress increasing the number of Fos-positive cells in the PVH (329 ± 64) over that seen in controls (76 ± 13) and repeated restraint leading to a complete habituation of the Fos response (88 ± 11). It should be noted that the absolute level of Fos expression following acute restraint was significantly less in females compared to males. No sex differences were found in the number of neurons expressing Fos in the control or repeated stress conditions. The overall effect of stress treatment on Fos expression was significant (F=19.97; p<0.0001).
Figure 2. Quantification of stress-activated neurons in the PVH.
The number of cells expressing Fos, an immediate-early gene marker of activation, was quantified in the PVH of male (white bars) and female (black bars) rats subjected to acute (A) or repeated (R) restraint stress, and compared to unstressed controls (C). The data are presented as mean ± SEM. Acute stress significantly increased Fos immunoreactivity in the PVH of both sexes, although to a lesser degree in females compared to males. Both males and females showed habituated levels of Fos expression following repeated stress exposure, resulting in cell counts that were not different from control in either case. Symbols designate significant differences (p ≤ 0.05) compared to C (*), A (†), or between males and females within a treatment group (‡).
Stress-induced activation of AVP-expressing neurons
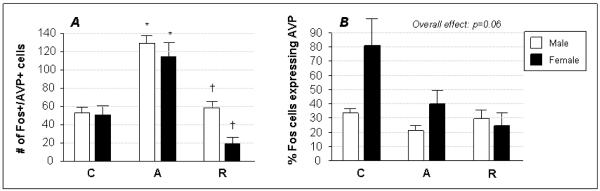
Stress-sensitive PVH neurons that also express AVP were identified by dual localization of Fos and AVP in individual cells, which were seen in all groups. The absolute number of Fos- and AVP-expressing neurons was increased by acute stress and decreased following repeated stress exposure in both males and females, resulting in an overall treatment effect of stress (F=20.13; p<0.0001), but no sex differences were seen within any of the stress treatment groups (Fig. 3A). Normalization of the data so that cells expressing both Fos and AVP are given as a percentage of the number of neurons staining positively for Fos alone, however, yielded some interesting trends (Fig. 3B). The overall effect of stress when the data are presented in this way was non-significant (F=2.42; p=0.06), but a strong trend toward a decrease in AVP neuronal activation after acute stress (21 ± 4) compared to control (34 ± 3) was seen in the males (p=0.04 by t-test). Likewise, a trend (p=0.09 by t-test) was observed between males and females during acute stress, with females tending to show a greater relative recruitment of AVP-expressing neurons.
Figure 3. Co-localization of Fos with AVP in the PVH.
Neurons co-expressing Fos and AVP were quantified in the PVH of control (C), acutely restrained (A) and repeatedly restrained (R) rats. Data from males (white bars) and females (black bars) are given as mean ± SEM. Acute stress increased the absolute number of doubly-labeled cells, compared to control, in both sexes (panel A), an effect that was seen to habituate in animals subjected to repeated stress. When these data were expressed as the percentage of Fos-positive neurons also staining for AVP (panel B), no significant differences were seen across stress or sex groups, although females tended to activate relatively more AVP-positive neurons under control and acute stress conditions compared to males. Symbols designate significant differences (p ≤ 0.05) compared to C (*) or A (†). No significant differences were seen between males and females in any treatment group.
Sex differences in the recruitment of GR-expressing neurons during stress
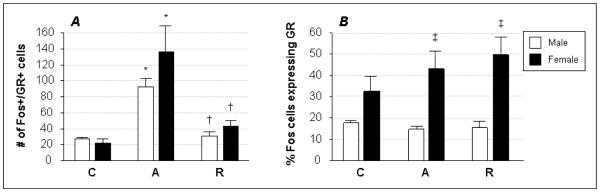
The activation of neurons staining positively for GR was seen in all control and stressed rat groups, with an overall significant effect of stress on Fos and GR co-expression (F=11.41; p<0.0001) (Fig. 4). Acute restraint increased the absolute number of Fos- and GR-labeled PVH cells in both males and females, and both sexes again had numbers of dually-stained neurons that were similar to control levels after repeated stress exposure (Fig. 4A). No sex differences were seen for any treatment group, but when the number of cells expressing both markers was normalized to the total number of Fos-expressing cells, significant sex-dependent responses then became apparent (Fig. 4B). Male rats showed equivalent numbers of Fos- and GR-expressing cells regardless of whether they were stressed or not, while females showed a trend (non-significant) toward increasing recruitment of GR-expressing neurons as their exposure to stress increased. In addition, females in the acute and repeated restraint groups displayed significantly greater numbers of neurons co-expressing Fos and GR than their male counterparts in the same treatment groups (43 ± 9 and 50 ± 8 compared to 15 ± 2 and 15 ± 3, respectively).
Figure 4. Fos and GR co-expression in the PVH.
Brain sections from male (white bars) and female (black bars) rats were immunohistochemically stained to co-localize Fos within GR-expressing neurons in the PVH after acute (A) or repeated (R) restraint stress compared to controls (C). Quantification of the data, presented as mean ± SEM, showed a significant increase in the number of activated GR-positive cells following acute stress, compared to control, in both sexes (panel A). A habituation of this response was seen in females as well as males following repeated stress. When the number of doubly-stained cells was considered as a percentage of neurons staining positively for Fos alone (panel B), no effects of stress were seen in either sex, but females recruited significantly more GR-expressing neurons after both acute and repeated stress exposure compared to males. Symbols designate significant differences (p ≤ 0.05) compared to C (*), A (†), or between males and females within treatment groups (‡).
Discussion
Numerous studies have demonstrated that males and females differ in their responses to stress [3, 4, 19-21], and further evidence in support of these findings comes from the results of the present work. We have identified changes in neuronal activation and the recruitment of specific cell types within the PVH that may be differentially involved in the initiation and termination of stress responses through their ability to modulate HPA axis function. Hyperactivity of the HPA axis is frequently seen in patients suffering from major depression and has also been associated with increased susceptibility to infectious diseases, whereas HPA axis hypoactivity has been correlated with autoimmune diseases [19]. Therefore, understanding the biological mechanisms that underlie these differences and contribute to dysregulation of the HPA axis will provide information about the pathology and progression of stress-related disorders, and will aid the development of preventative and therapeutic strategies to alleviate these maladies [4]. In addition, sex has a significant influence over the normal functioning of the brain, with males and females showing differences in neurotransmitter levels, pain perception, emotion, memory, and vision, as well as in stress hormone action and the manifestation of various disease states [20]. The neurobiological correlates of stress identified in this study may affect the ability of males and females to cope with stressful situations, and may inform new approaches for the treatment of sex-specific illnesses that result from chronic stress exposure.
Neuronal activation in response to stress
As shown previously [10], neurons expressing Fos as a consequence of stress were primarily localized within the medial parvocellular component of the PVH in both sexes. Acute restraint led to an increased number of Fos-positive nuclei throughout this region, while the habituated response seen following repeated restraint exposure involved Fos-expressing cells that were more restricted to the ventral subregion of the medial parvocellular PVH. Therefore, while the entire PVH was quantified in our experiment, results on the numbers of cells doubly-labeled for Fos and AVP or Fos and GR, below, are driven by the pattern of Fos expression in this nucleus.
The response to acute stress was diminished in females, compared to males, in terms of the absolute number of PVH neurons expressing Fos as a consequence of a single exposure to restraint. This could suggest that females have a reduced sensitivity to stress, and recruit fewer neurons than males in response to the same stimulus at the time of initial exposure. Alternatively, given that the female adrenal gland has been shown to exhibit enhanced sensitivity to ACTH [21], perhaps a lesser level of PVH activation is sufficient to produce the appropriate neuroendocrine response needed to deal effectively with the stressor. A final possibility is that males and females may excite the same proportion of PVH neurons, but that the absolute number of activated cells is reduced in the females due to a smaller brain size or PVH nucleus volume. This possibility cannot be discounted at this time, as volumetric analysis of the PVH was not performed in this study. However, our data do demonstrate that the level of activation in acutely stressed females (433% of control) is similar to that in acutely stressed males (413% of control).
Body weight
Body weights at the time of perfusion were not significantly different across treatment groups within either sex. There was a trend, however, toward decreasing body weights with increasing numbers of stress exposures. When compared by t-test, the p value was 0.055 between control males and males subjected to repeated restraint. Therefore, while these data do not demonstrate a significant effect of continued stress, they do suggest, as least, that the animals may have been subjectively experiencing stress throughout the study even though our findings show clearly habituated responses to repeated restraint in terms of neuronal activation.
The role of AVP in stress responses
The number of stress-sensitive PVH neurons co-expressing AVP was increased in acutely stressed rats compared to unstressed controls, and the number of activated, AVP-expressing cells habituated with repeated compared to acute stress exposure. These effects are likely a function of changing levels of Fos expression in each stress condition, and do not reflect changes in AVP expression per se, as absolute counts of Fos-positive and Fos-and AVP-positive cells followed the same profile. No sex differences were observed in any of the treatment groups, but expressing the number of neurons doubly-labeled for Fos and AVP as a percentage of the total number of Fos-positive cells gave rise to the suggestion of possible sex differences in both control and acutely stressed groups. While the results were statistically non-significant, females tended to display greater numbers of activated AVP neurons under basal (unstressed) conditions and after acute restraint. Male rats, in contrast, appear to de-recruit AVP-expressing cells during acute exposure to restraint stress. These findings, although somewhat less than convincing, do not entirely preclude the possibility that females may require fewer PVH neurons to be activated in order to mount an effective response to an acute stressor, due to their increased utilization of AVP as a co-secretagogue for ACTH. This is particularly interesting in light of the fact that other researchers have suggested a greater role for AVP during situations of chronic, and not acute, stress [22-26]. Our data allow for additional alternative interpretations, as well. First, the derecruitment of AVP neurons by females with increasing exposure to a homotypic stressor may indicate that other neuronal types or mechanisms play a more critical role in modulating the HPA axis and are becoming activated at the expense of this cell type. Second, as we have not quantified the number of AVP-expressing neurons that were Fos-negative, there is still room for the idea of an enhanced role for AVP during repeated stress. Finally, repeated exposures to corticosterone during stress applications could subsequently and progressively prevent the activation of AVP neurons, protecting the organism from excessive responses to that stressor. Future studies will be needed to further define the mechanisms at play in this system.
GR expression and implications for GC feedback
Similar to the results seen in Fos- and AVP-expressing cells, the number of neurons co-expressing Fos and GR was elevated by acute restraint, habituated in response to repeated stress, and was not different between males and females in any treatment group. Once again, however, consideration of the number of cells expressing Fos and GR as a percentage of those expressing Fos alone yielded some interesting sex differences that were largely significant in this case. In all stress treatment groups, females had increased numbers of Fos- and GR-expressing PVH neurons compared to males, with the acutely and repeatedly stressed females showing statistically greater numbers of doubly-stained cells. Although not significant, the trend was for females to activate increasing numbers of GR-positive neurons as a function of increasing exposures to stress. This suggests that females may be more sensitive to GC negative feedback, which may serve to dampen their responses to stress or terminate those responses more quickly. If, as stated above, females also show enhanced release of GCs from the adrenal gland after stimulation by ACTH, then they may also be able to respond to increases in circulating GCs at a more rapid pace than males.
It is possible that the increased number of GR-expressing cells that also stain positively for Fos in acutely restrained animals of either sex reflects a kind of compensatory mechanism. In this role, GR could allow for rapid termination of the stress response, which would be appropriate under the conditions of our paradigm in which the stress is emotional (i.e., not posing an immediate survival threat to the organism) and short-lived. Perhaps more interesting is the fact that females display a larger percentage of activated GR neurons than males, again suggesting that they may be more sensitive to negative feedback. Convincing evidence for sex differences in negative feedback sensitivity comes from Louvart et al. [27], in which females exposed to footshock showed enhanced corticosterone negative feedback to subsequent restraint, compared to males. In addition, Kudielka and Kirshbaum [3] discuss the ability of estrogen to regulate the HPA axis and its sensitivity to negative feedback. Lastly, Weiser and Handa [28] demonstrate that individuals with affective disorders have impaired glucocorticoid negative feedback, which is mediated at least in part by estradiol acting through the estrogen receptor α in the PVH.
Steroid hormone regulation in neuroendocrine responses to stress
Basal sex differences in HPA axis function must also be considered in our findings on central nervous system activational responses to stress. It has been shown previously that circulating glucocorticoid levels are higher in females than males under resting conditions [29]. Furthermore, average daily corticosterone levels fluctuate according to estrus cycle stage in females, with females showing equivalent levels to males during estrus but higher values during proestrus, metestrus and diestrus [30]. It is therefore likely that our female subjects had higher concentrations of glucocorticoid hormones on board throughout the majority of our experimental paradigm, which could play a role in the central nervous system activational responses shown in our data. For example, heightened glucocorticoid negative feedback in females could contribute to the lower numbers of Fos-positive neurons seen after acute stress exposure, or the relatively higher numbers of activated AVP- or GR-expressing neurons in females at baseline.
It is also clear from the aforementioned references that estrogen has a significant impact on the HPA axis, and this has also been found to be true under conditions of stress. Estrogen has been shown to regulate both HPA axis function and the sensitivity of the axis to glucocorticoid negative feedback [3, 27]. In addition, Weiser and Handa [28] have demonstrated a mechanism through which estrogen can impair glucocorticoid negative feedback on the HPA axis by acting through the estrogen receptor-α in the PVH. Additional experiments are needed to more fully determine the basis for the sex differences seen in our results. However, we have identified critical differences in the stress-induced activation of neurons which may underlie sex-specific mechanisms involved in the neural processing of stress.
Future studies will need to include assessments of ACTH and/or corticosterone levels to more accurately outline the complex and interactive mechanisms responsible for our observed results. The existing literature shows larger responses to a multitude of stressors in females compared to males, but a majority of these studies show that the heightened responses in females originate from increased adrenal sensitivity to ACTH and higher adrenal hormone secretion. Less is known about sex differences in stress responses at the level of the central nervous system and, specifically, the PVH level of the HPA axis. Our data show that females display fewer Fos-expressing cells than males after acute restraint (although the percent increase from control is similar), but habituate similarly to males after repeated restraint exposure. In addition, females have a higher level of activation of GR-expressing cells than males after both acute and repeated stress exposure, suggesting that they may be able to respond more rapidly to hormonal signals designed to terminate the response to stress. Whether or not PVH responses are actually terminated faster, or subsequent responses are dampened, cannot be determined by the present data. It is possible, however, that the longer-lasting circulatory hormone profiles previously seen in females could be due to changes in peripheral secretory or clearance mechanisms which are regulated separately from the activities of the central nervous system. Future work will be needed to appropriately address these questions.
In conclusion, we have observed significant differences in the activation of PVH neurons that co-express neuropeptides known to function in the regulation of the HPA axis. Expression profiles that differ by sex suggest that there are substantial variations in the mechanisms females use to generate responses to stress, as compared to males. Females appear to be less sensitive than males to acute stress, in that they have fewer Fos-positive cells in the PVH under this condition. However, they may, in fact, produce equal or even greater responses to acute stressors through the specific recruitment of AVP and, possibly, increased adrenal sensitivity to ACTH. The enhanced activation of GR-expressing neurons seen in the PVH of female rats may indicate a heightened sensitivity to negative feedback that is not seen in males. This could lead to constrained stress responses at the level of the PVH, decreased sensitivity to subsequent stressors, and/or more rapid termination once a stress response is initiated. Taken together, these data suggest that female rats may respond to acute stress in a manner that is equal to or even greater than that seen in males, but might also have an increased efficiency in their ability to process that stress. Our findings that females appear to respond to increasing stress exposure by selectively activating more GR-expressing and fewer AVP-expressing neurons warrant further study, and may provide insight into the mechanisms through which men and women differentially manifest stress-related illnesses.
Acknowledgements
The authors would like to acknowledge the technical support of the NIH/RCMI-funded core facilities of the Border Biomedical Research Center at UTEP, especially the Analytical Cytology Core. We would also like to express our appreciation for UTEP’s Veterinary Services Department which provided excellent support for our vivarium work. This project was supported by NIH Grant RR008124 (RCMI, to UTEP), NSF Grant No. 0245071 (ADVANCE GRA, to JKZ), and HHMI Grant No. 52005908 (to UTEP, supporting JKZ and AAF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosomatic Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Grippo AJ, Johnson AK. Stress, depression, and cardiovascular dysregulation: A review of neurobiological mechanisms and the integration of research from preclinical disease models. Stress. 2009;12(1):1–21. doi: 10.1080/10253890802046281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psych. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinol. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Pirnik Z, Mravec B, Kiss A. Fos protein expression in mouse hypothalamic paraventricular and supraoptic nuclei upon osmotic stimulus: colocalization with vasopressin, oxytocin and tyrosine hydroxylase. Neurochem. Int. 2004;45:597–607. doi: 10.1016/j.neuint.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neurosci. 2006;138(4):1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of CRF mRNA in rat brain regions by glucocorticoids and stress. J. Neurosci. 1991;11(3):585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Kloet ER, Vreugdenhi l E., Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endo. Rev. 1998;19(3):269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 9.Fenoglio KA, Chen Y, Baram TZ. Neuroplasticity of the hypothalamic-pituitary-adrenal axis early in life requires recurrent recruitment of stress-regulating brain regions. J. Neurosci. 2006;26(9):2434–2442. doi: 10.1523/JNEUROSCI.4080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viau V, Sawchenko PE. Hypophysiotropic neurons of the paraventricular nucleus respond in spatially, temporally, and phenotypically differentiated manners to acute vs. repeated restraint stress. J. Comp. Neurol. 2002;445:229–307. doi: 10.1002/cne.10178. [DOI] [PubMed] [Google Scholar]
- 11.Keck ME. Corticotropin-releasing factor, vasopressin and receptor systems in depression and anxiety. Amino Acids. 2006;31(3):241–250. doi: 10.1007/s00726-006-0333-y. [DOI] [PubMed] [Google Scholar]
- 12.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinol. 1999;140(8):3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 13.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 14.Spencer RL, Kim P, Kalman B, Cole M. Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinol. 1998;139(6):2718–2726. doi: 10.1210/endo.139.6.6029. [DOI] [PubMed] [Google Scholar]
- 15.Yudt M, Cidlowski J. The glucocorticoid receptor: coding a diversity of proteins and responses through a single gene. Mol. Endocrinol. 2002;16(8):1719–1726. doi: 10.1210/me.2002-0106. [DOI] [PubMed] [Google Scholar]
- 16.Charmandari E, Kino T, Chrousos GP. Glucocorticoids and their actions: An introduction. Ann. N.Y. Acad. Sci. 2004;1024:1–8. doi: 10.1196/annals.1321.001. [DOI] [PubMed] [Google Scholar]
- 17.Shu S, Ju G, Fan L. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci. Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 18.Paxinos G, Watson C. The Rat Brain: In Stereotaxic Coordinates. 4th Ed Elsevier (Academic Press); San Diego: 1998. [Google Scholar]
- 19.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer D. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Cahill L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 21.Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinol. 1961;68(5):818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 22.Surget A, Belzung C. Involvement of vasopressin in affective disorders. Eur. J. Pharmacol. 2008;583(2-3):340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 23.Ma XM, Levy A, Lightman S. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: A study of both AVP and CRH messenger ribonucleic acid and heteronuclear RNA. Endocrinol. 1997;138(10):4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 24.Makara GB, Mergl Z, Zelena D. The role of vasopressin in HPA axis activation during stress: An assessment of the evidence. Ann. NY Acad. Sci. 2004;1018:151–161. doi: 10.1196/annals.1296.018. [DOI] [PubMed] [Google Scholar]
- 25.Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: Implications for the pathophysiology of depression. Life Sci. 1998;62(22):1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- 26.Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the HPA axis: implications for stress adaptation. Reg. Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- 27.Louvart H, Maccari S, Lesage J, Léonhardt M, Dickes-Coopman A, Darnaudéry M. Effects of a single footshock followed by situational reminders on HPA axis and behaviour in the aversive context in male and female rats. Psychoneuroendocrinol. 2006;31:92–99. doi: 10.1016/j.psyneuen.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neurosci. 2009;159(2):883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinol. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- 30.Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinol. 1997;138(9):3842–3848. doi: 10.1210/endo.138.9.5395. [DOI] [PubMed] [Google Scholar]