Abstract
In this study, ascorbate (Asc) and glutathione (GSH) concentrations were quantified noninvasively using double-edited 1H MRS at 4 T in the occipital cortex of healthy young [age (mean ± standard deviation) = 20.4 ± 1.4 years] and elderly (age = 76.6 ± 6.1 years) human subjects. Elderly subjects had a lower GSH concentration than younger subjects (p < 0.05). The Asc concentration was not significantly associated with age. Furthermore, the lactate (Lac) concentration was higher in elderly than young subjects. Lower GSH and higher Lac concentrations are indications of defective protection against oxidative damage and impaired mitochondrial respiration. The extent to which the observed concentration differences could be associated with physiological differences and methodological artifacts is discussed. In conclusion, GSH and Asc concentrations were compared noninvasively for the first time in young vs elderly subjects.
Keywords: ascorbate, glutathione, lactate, brain, human, aging, MEGA-PRESS, MRS
INTRODUCTION
Ascorbate (Asc, vitamin C) and glutathione (GSH) are the most concentrated chemical antioxidants in the central nervous system (1). Asc and GSH are scavengers of free radicals formed from the interaction of iron and hydrogen peroxide, and play a major role in cellular detoxification (2). Sodium-dependent maintenance of adequate Asc concentration (3,4) is important throughout life (5,6) to support additional processes, including the healthy development and function of neurons (7), neurotransmission (5), enzyme stabilization (8) and signaling in the inflammatory response (9). Numerous studies suggest that brain Asc and GSH concentrations could be affected by age-related neurological conditions. For example, although a decrease in brain Asc concentration has been consistently reported during development, conflicting evidence has been presented on whether Asc concentration declines after maturation (10). A majority of studies on animals indicate that GSH levels decline in the brain with age (11,12), but there is very little information available concerning the effect of age on human brain Asc (13) and GSH concentrations.
In MRS, resonances from Asc and GSH are difficult to resolve because of significant spectral overlap with other neurochemicals (14). They can be resolved from overlapping contributions, yet retained in the same double-edited 1H MR spectrum measured from the human brain in vivo (15). Previously reported Asc and GSH concentrations, measured in vivo via LCModel analysis of MEGA-PRESS-edited spectra (MEGA-PRESS, MEGA-point-resolved spectroscopy), were in good agreement with those measured from short-TE 1H MRS in the human brain (15–18) and from high-performance liquid chromatography (HPLC) in the rat brain ex vivo (1,19,20), which suggests that the methodology and stated assumptions on T2 (18) facilitate reliable quantification.
The goal of this study was to quantify Asc and GSH concentrations as a function of age in the human brain. To that end, double editing with (DEW) MEGA-PRESS was used for the detection of Asc and GSH resonances.
METHODS
Protocol
All double-edited spectra were measured using a 4-T, 90-cm-bore magnet (Oxford Magnet Technology, Oxford, UK) interfaced to a Varian INOVA spectrometer (Varian, Palo Alto, CA, USA) and equipped with gradients capable of switching to 40 mT/m in 400 µs (Sonata, Siemens, Erlangen, Germany), and with a surface 1H quadrature transceiver (21). Twenty-two young [age (mean ± standard deviation (SD)) = 20.4 ± 1.4 years] and 22 elderly (age = 76.6 ± 6.1 years) individuals gave informed consent for this study, which was conducted according to procedures approved by the Human Subjects’ Protection Committee at the University of Minnesota. The study was repeated three times in consecutive weeks for five subjects in each age group to evaluate measurement reliability (, the within-subject variance). Each subject was positioned supine inside the magnet with the radiofrequency (RF) transceiver (21) subjacent to their occipital lobe. To ensure consistency in positioning, the external occipital protuberance, the bridge of the nose and the midline of the chin were used to define the median plane, which was centered in the bore to ensure that the midline of the brain was parallel to the axis of the magnet. To maintain the same inclination, a level meter was placed on the chin and midpoint between the eyebrows. The protocol for each scan began with localizer multislice rapid acquisition with relaxation enhancement (RARE) images (TR = 4.0 s; TE = 60 ms; echo train length, 8; matrix, 256 × 128; two averages; slice thickness, 2 mm; five slices) to select a cubic volume of interest (VOI; 3 × 3 × 3 cm3) centered on the midsagittal plane in the occipital lobe. For the 10 subjects who repeated the study three times each, images measured from the initial scan were used to set the boundaries of the VOI for the repeat studies completed 7 and 14 days after the initial scan. The boundaries of the VOI were traced in all slices to ensure that VOIs were placed in exactly the same location and included the same structural/anatomical landmarks. The adjustment of all first- and second-order shim currents was achieved using the fast automatic shimming technique by mapping along projections (FASTMAP) with echo planar imaging readout (22,23), which resulted in water linewidths of 9 ± 2 Hz (mean ± SD). Localizer images were repeated at the end of the scan session to confirm negligible gross motion.
To control for dietary influence on brain Asc concentration, candidates who consumed fewer than five fruits and vegetables per day (i.e. typical consumption according to the National Center for Chronic Disease Prevention and Health Promotion), and who were willing to refrain from taking vitamin C supplements, were recruited. Candidates with neurological disorders or chronic diseases were excluded. Participants started food records 6 days prior to spectroscopy for the calculation of vitamin C consumption. Three days prior to spectroscopy, subjects began a diet designed to contain the recommended dietary allowance (30 mg of vitamin C/1000 kcal/day). Food was provided for the 3 days prior to spectroscopy, and participants documented their compliance, which was computed as the percentage of calories and vitamin C eaten. To measure blood serum Asc concentration ([Asc]blood) on the same morning as each MR scan (which always began between 09.00 and 12.00 h), 8 mL of whole blood was collected and placed immediately on wet ice. Serum was then separated at 21°C and aliquoted into preservative. Ampoules were submitted frozen (− 70°C) to ARUP Laboratories, Inc. (Salt Lake City, UT, USA) for analysis by spectrophotometry. Three tests for cognitive status were administered to each participant. The Montreal Cognitive Assessment (MoCA) was administered to detect mild cognitive impairment with high sensitivity (24). To detect more subtle changes, timed performances on the symbol digit modalities test (Psychological Assessment Resources, Inc., FL, USA) and on the Trail Making Test (parts A and B) were also evaluated. Each subject completed the Geriatric Depression Scale (25). All measures of vitamin C consumption, compliance to the study diet, [Asc]blood, cognitive status and depression were analyzed for confounding with brain Asc and GSH concentration using linear regression. The significance level for any single association, without correction for multiple comparisons, was set at p < 0.025.
Homonuclear editing
Double editing for Asc and the cysteine residue of GSH (2.95 ppm) was achieved via DEW MEGA-PRESS, as described previously (26). Water suppression using variable-power RF pulses with optimized relaxation delays (VAPOR) (27) and outer volume saturation were applied before the DEW MEGA-PRESS pulse train. Slice-selective excitation was achieved using a 2-ms sinc RF pulse with a bandwidth of 2.0 kHz (γB1 = 706 Hz). Slice-selective refocusing used a 3-ms sinc RF pulse with a bandwidth of 1.5 kHz (γB1 = 942 Hz). Edited spectra were measured as described above (TE = 122 ms; number of excitations = 512; TR = 4.5 s) from the 27-mL VOI. This TE was set to minimize contributions from coedited resonances, such as phosphorylcholine (PC), glycerophosphorylcholine (GPC), myo-inositol (Ins) and N-acetylaspartate (NAA). At this TE, optimal editing efficiency was achieved using a 45-ms Gaussian pulse set at 4.10 ppm for Asc and 4.56 ppm for the cysteine β-CH2 of GSH. The chemical shift for localization was set to 3.26 ppm to minimize the chemical shift displacement artifact for the detection of GSH and Asc. Field drift was monitored to ensure that it did not exceed ± 10 Hz. The entire scan took approximately 1.5 h per session. The spectral width was 6000 Hz and 4035 complex points were acquired for each spectrum. Each single free induction decay (FID) was stored separately in memory, and the frequency and phase were corrected on the basis of the NAA methyl signal prior to summation of all FIDs (28). To ensure a flat baseline, residual unsuppressed water was removed from the spectrum using the Hankel–Lanczos singular value decomposition (HLSVD) (29). Resonances between 4.5 and 4.9 ppm (water = 4.7 ppm), as determined by the HLSVD algorithm, were subtracted from the data.
Quantification
For each subject, the metabolite concentrations were quantified from the DEW MEGA-PRESS-edited spectra using LCModel version 6.2 with optimized parameters for a flat baseline (30). The node spacing was set high using the DKNTMN parameter and the baseline was held flat using the ALPBMN parameter. The following were included in the basis set: Asc, GSH, Ins, lactate (Lac), NAA, PC, GPC, glycerophosphorylethanolamine (GPE) and phosphorylethanolamine (PE). These spectra were simulated using the quantum mechanics density matrix formalism with published values of J-coupling constants and chemical shifts (14,26,31). The accuracy of simulation was verified by ensuring that concentrations quantified using experimentally measured and simulated basis sets were not different (p > 0.05). Concentrations were calculated using tissue water as an internal reference, i.e.
| [1] |
where SImet corresponds to the signal intensity of metabolites measured by LCModel and SIH2O is the integral of the water resonance acquired using stimulated echo acquisition mode (STEAM) spectroscopy (TR = 4.5 s, TM = 42 ms, TE = 4 ms). [H2O] denotes the concentration of pure water. WC refers to the water content of the gray matter, and is assumed to be 0.8 (32). The constant k was determined using a phantom experiment in which concentrations of NAA, Asc, GSH and Lac (10 mm) were known. The constant k accounted for the editing efficiency. As the effects of T2 are not accounted for in eqn (1), concentrations are reported in institutional units (i.u.), which are intended to approximate micromoles per gram of brain tissue. Next, the cerebrospinal fluid (CSF) fraction [obtained by fitting integrals of unsuppressed water spectra measured at several TE with a biexponential decay function (33)] in each voxel (fCSF) was used to correct for the partial volume effect, assuming a negligible concentration of metabolites in CSF (34). To account for the high concentration of Lac in CSF, tissue concentrations of Lac were corrected by subtracting the same CSF contribution for young and elderly subjects (35).
The criteria for the reliable quantification of metabolite concentrations were based on negligible fitted residuals and the Cramer–Rao lower bounds (CRLBs), which are estimated SDs, expressed as a percentage of the estimated concentrations (36). Concentrations with CRLB > 50% were classified as not detected. Only metabolites for which CRLBs were below 50% in at least half of the spectra were reported.
Statistical methods
The concentrations of metabolites in young and elderly subjects were compared using a two-tailed, unpaired, Student’s t-test without correction for multiple comparisons. For the five subjects in each age group who underwent three repeat studies, the average of the three studies was used as a single measurement for the statistical analysis. To examine measurement reliability, a mathematical model was built to describe the contributions of between-subject (interindividual) and within-subject (intraindividual) variation. The overall measurement variance (SD squared) in one measurement can be expressed as . The information about , the within-subject variance, came from the 10 repeat studies, whereas all 44 subjects provided information about the within-subject variance. Variance components were estimated by maximizing the restricted likelihood.
RESULTS
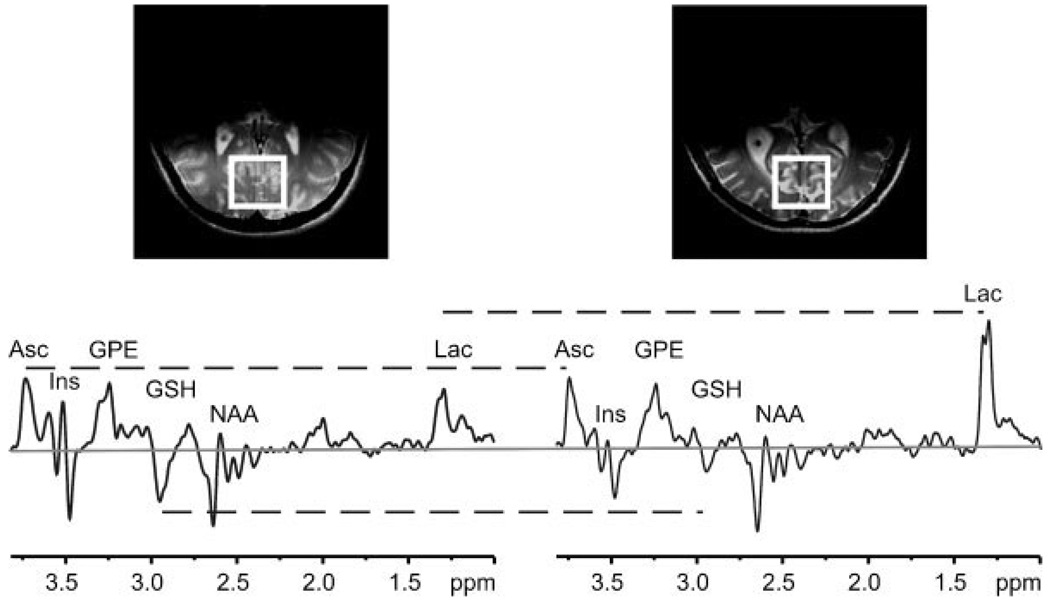
Figure 1 compares the representative double-edited spectra measured from young and elderly subjects. The double-edited spectra are characterized by several major peaks, including Asc at 3.73 ppm, GSH at 2.95 ppm and Lac at 1.33 ppm. Less intense GSH and more intense Lac resonances are discernible in the spectrum measured from elderly subjects.
Figure 1.
Representative double editing with MEGA-point-resolved spectroscopy (DEW MEGA-PRESS) spectra (TE = 122 ms; TR = 4.5 s; number of excitations = 512) from one young (left) and one elderly (right) subject. Constancy or differences in ascorbate (Asc) (3.73 ppm), glutathione (GSH) (2.95 ppm) and lactate (Lac) (1.33 ppm) resonances are illustrated with horizontal broken lines. Gaussian filtering with σ = 0.15 s was applied. Spectra are scaled such that the heights of the Asc peaks are equal. The zero level is indicated by a gray horizontal line. Top: transverse MRI illustrating (box) the location of the volume of interest in the occipital lobe. GPE, glycerophosphorylethanolamine; Ins, myo-inositol; NAA, N-acetylaspartate.
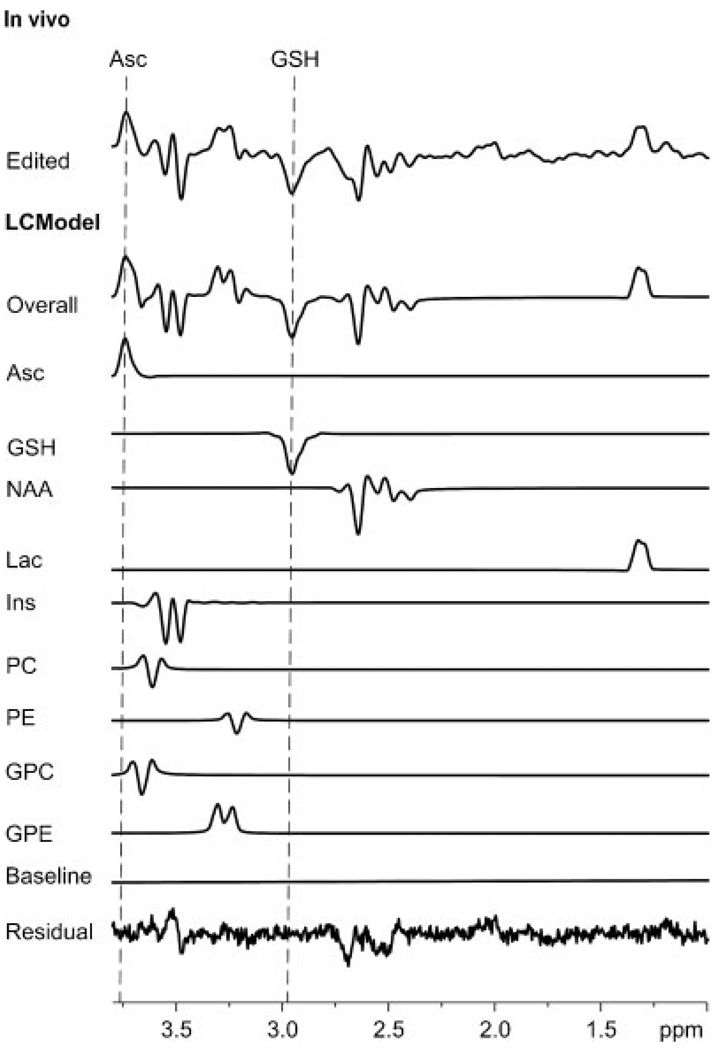
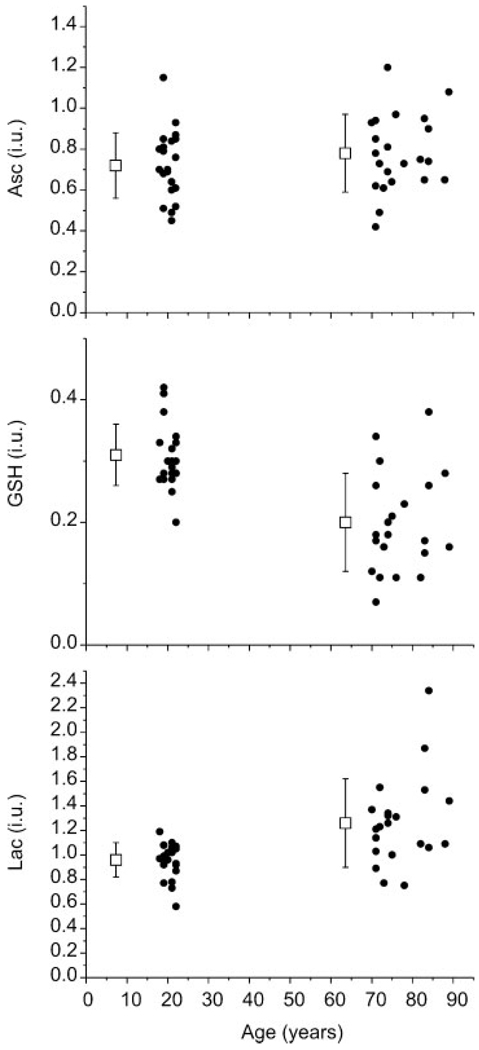
Figure 2 illustrates a representative Asc and GSH double-edited spectrum from one of the young subjects and the output of LCModel analysis that was used to quantify concentrations. Although CRLBs for Ins and NAA were less than 20%, they were classified as not detected because of the large fitted residuals (Fig. 2). Mean metabolite concentrations for each study group are reported in Table 1. The GSH concentration was lower (p = 0.000002) and the Lac concentration was higher (p = 0.0006) in elderly subjects. Asc was not associated with age. Figure 3 displays the concentrations of Asc, GSH and Lac from each subject as a function of age, together with means and SDs. Table 2 details the contributions of the between-subject and within-subject variation to the overall measurement variance in Asc and GSH concentrations.
Figure 2.
A representative result of quantification via LCModel. In vivo: edited spectrum. LCModel: overall fit of the edited spectrum; fitted components for ascorbate (Asc), glutathione (GSH), N-acetylaspartate (NAA), lactate (Lac), myo-inositol (Ins), phosphorylcholine (PC), phosphorylethanolamine (PE), glycerophosphorylcholine (GPC) and glycerophosphorylethanolamine (GPE); baseline component of LCModel fitting and fitted residual.
Table 1.
Metabolite concentrations (mean ± standard deviation) in young and elderly subjects. Institutional units (i.u.) estimate the number of micromoles per gram wet weight without correction for T2
| Ascorbate (i.u.) | Glutathione (i.u.)a | Lactate (i.u.)a | |
|---|---|---|---|
| Young | 0.72 ± 0.16 | 0.31 ± 0.05 | 0.96 ± 0.14 |
| Elderly | 0.78 ± 0.19 | 0.20 ± 0.08 | 1.26 ± 0.36 |
p < 0.05.
Figure 3.
Concentrations of ascorbate (Asc) (top), glutathione (GSH) (middle) and lactate (Lac) (bottom) by age group, together with means (boxes) and standard deviations (error bars). Concentrations were quantified in institutional units (i.u.) which were designed to estimate the concentration (µmol/g wet weight) without correction for T2. Means and standard deviations of the concentrations were intentionally shifted to the left of the mean age of each group for clarity.
Table 2.
Between-subject (σs–s) and within-subject (σwithin) variance of ascorbate (Asc) and glutathione (GSH) expressed in terms of standard deviation (square root of variance)
| Ascorbate | Glutathione | |
|---|---|---|
| σs–s | 0.14 | 0.05 |
| σwithin | 0.10 | 0.05 |
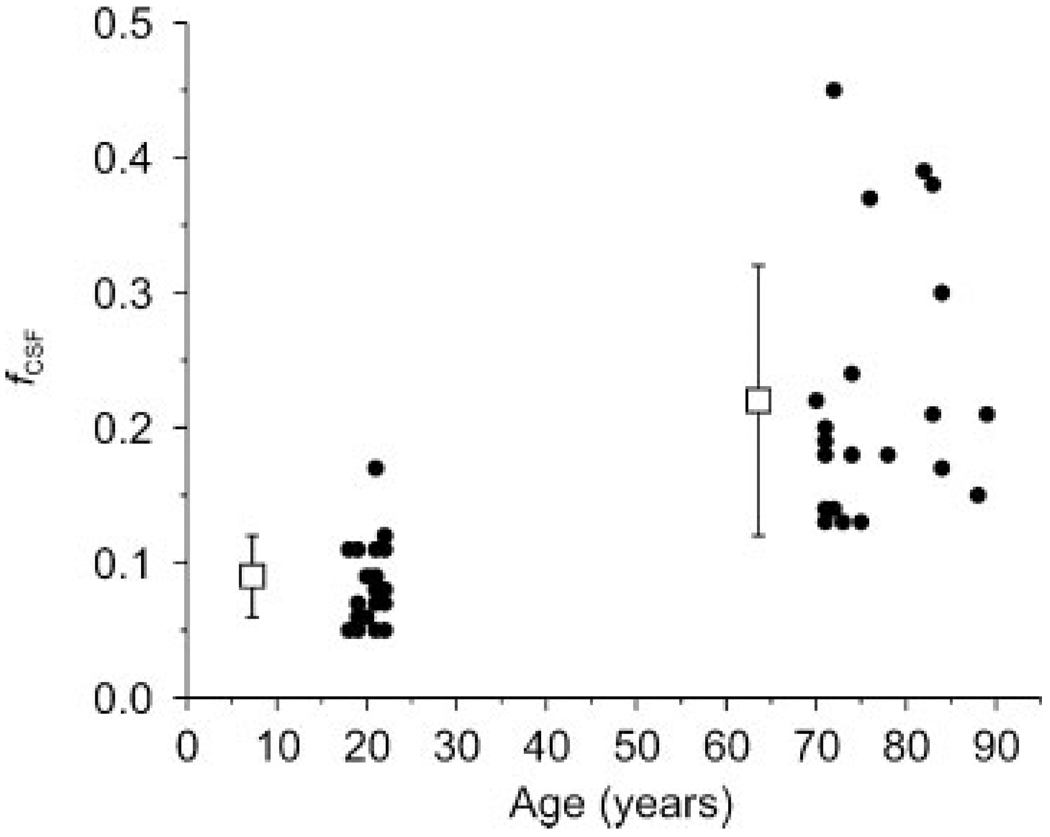
Figure 4 shows the difference in CSF fraction (fcsf) between young and elderly subjects in the occipital cortex. The CSF fraction in elderly subjects (0.22 ± 0.1, mean ± SD) was higher (p = 0.0000001) than that of young subjects (0.09 ± 0.03), which was expected on the basis of age-related atrophy. The variance in CSF fraction among repeat measurements was less than 10% of the mean CSF fraction for both young and elderly subjects, attesting to the good reproducibility of the VOI positioning.
Figure 4.
The voxel fraction of cerebrospinal fluid (fCSF) by age group, together with means (boxes) and standard deviations (error bars). fCSF was significantly higher in elderly subjects (p < 0.05). Means and standard deviations of fCSF were intentionally shifted to the left of the mean age of each group for clarity.
Brain Asc and GSH concentrations were not associated with any measure of vitamin C consumption, compliance with the study diet, [Asc]blood, cognitive status or depression.
DISCUSSION
The main findings of this study were that the GSH concentration was significantly lower in elderly subjects, whereas the concentration of Asc did not differ significantly. Higher Lac concentration was also found in elderly subjects. Furthermore, the CSF fraction was higher in elderly subjects.
A reduction in GSH concentration with increasing age has been reported in several species, including mosquitoes, adult housefly, fruit fly and mouse and rat brain (12,37,38). The GSH concentration in the CSF of humans decreases with aging (39). The finding of a lower GSH concentration in elderly human subjects in this study is consistent with findings from animal studies (40,41), which show deficits in the detoxification of reactive oxygen species in the aging brain (11). GSH is slightly more predominant in glia than in neurons (1), with the extracellular concentration of GSH normally remaining at relatively low levels (11). GSH concentration in the CSF is very low (11). Therefore, signal contributions to the GSH resonance detected in the VOI were predominantly intracellular. A reduction in intracellular GSH concentration with age may signify deficits in the prevention of oxidative damage.
Published data suggest that the Asc concentration declines with age (42,43). However, it is unclear whether this phenomenon occurs during the process of development or aging, as most previous studies have focused on development. Guinea pig Asc concentration decreased in young animals from the age of 3 to 6 months, but, during the next 3 months, the Asc concentration in these animals remained unchanged (10). Furthermore, postmortem analyses of animals aged between 9 and 42 months showed no difference in Asc concentration in many tissues, including the brain (10). These findings are supported by several other studies of animal brains (44,45). In the present study, human brain Asc concentration did not differ significantly between young and elderly subjects. Asc is localized primarily in neurons (1) and Asc concentrations in extracellular tissue and CSF are very low (8,19). Therefore, the signal contributions to the Asc resonance detected in the VOI were predominantly neuronal. As such, the findings for Asc concentration reflect neuronal status. The existing literature and the constant Asc concentration found in this study suggest that this study’s young cohort had already completed their maturation phase, and thus no reduction in Asc concentration could be observed in the elderly cohort.
The present study also found a higher Lac concentration in elderly subjects. Mitochondrial diseases have been shown to elevate MRS-measured cerebral and CSF Lac levels (46,47). Such elevation could be related to local oxidative stress associated with reduced oxygen supply, increased oxygen demand or impairment in oxidative metabolism. Thus, a higher Lac concentration could reflect mitochondrial respiratory chain problems in the elderly brain. Impaired mitochondrial respiration is consistent with deficits in protection against oxidative damage, and therefore with the lower GSH concentration measured in the elderly cohort.
Because methodological limitations could confound age-related differences in measured metabolite concentrations, such limitations were addressed as follows. In this study, cerebral water was used as an internal reference to quantify metabolite concentrations. Water is a better internal reference than NAA because it can be quantified with higher precision (via sensitivity) and greater accuracy, as the extent to which brain NAA concentration remains constant throughout aging is controversial. However, partial volume effects from CSF can diminish this advantage. Cerebral atrophy associated with aging can potentially lead to an overestimation of brain water concentration within the voxel because of the inclusion of CSF, if appropriate correction is not made. In this study, correction was made for cerebral atrophy via measurement of the CSF content in the VOI. The potential decrease in water concentration in gray matter with age could be another limitation of the use of water as an internal reference (48). However, neither biochemical studies ex vivo nor the study of T1 relaxation times in vivo support a significant reduction in gray matter water content with age (48). We have assumed constant water content in gray matter with respect to age (49). Any reduction in gray matter water concentration as a result of aging would lead to an overestimation of the metabolite concentrations in the elderly group.
Another advantage of using water as an internal reference was the measurement at short TE, which minimized the dependence of the reference signal on T2. The concentrations approximated as µmol/g in young subjects in this study were lower ([Asc] = 0.72 ± 0.16 i.u., [GSH] = 0.31 ± 0.05 i.u., mean ± SD) than those quantified previously (26) at TE = 112 ms ([Asc] = 0.84 ± 0.11 µmol/g, [GSH] = 1.02 ± 0.09 µmol/g) when NAA was used as internal reference. Quantification using NAA as internal reference was based on the assumption that differences between the T2 relaxation of NAA and the T2 relaxation of the antioxidant had the same impact on the resonance intensity in vitro and in vivo, i.e. the ratio exp[–TE/T2(NAA)]/exp[–TE/T2(antioxidant)] was approximately the same under in vitro and in vivo conditions. The lower concentrations approximated in the current study probably reflect the actual influence of T2 on the quantification of metabolite concentrations. In particular, the T2 value of GSH in vivo is very short (30).
In addition, differences in T2 with age have the potential to confound the quantification of metabolite concentrations. As atrophy in normal aging involves neuronal shrinkage, as well as axonal and myelin degeneration (50,51), the resulting increase in the fraction of small neurons could lead to shorter metabolite T2 values (52). Furthermore, age-dependent iron deposition has shown a strong correlation with T2 shortening (53). In other words, not accounting for T2 [eqn (1)] could lead to the underestimation of concentrations in the elderly group.
Additional mechanisms that could lead to resonance intensity differences between elderly and young subjects include B1 inhomogeneity and the four-compartment artifact that is associated with J modulation (54–56). If water and metabolite signals are evenly distributed throughout the VOI, the constant k in eqn (1) will account for the sensitivity profile and four-compartment artifacts. However, if water and/or metabolite signal contributions are asymmetric, these factors can introduce error to the measured concentration. In this study, the percentage CSF was higher in the elderly, in part as a result of brain shrinkage and widening of the sagittal sinus. This asymmetry overlain with the sensitivity profile and four-compartment artifact regions led to an artifactual 6% lower [GSH]brain and 4% lower [Lac]brain in elderly than in young subjects (Supporting information Fig. S1). Because displacements were small for Asc, [Asc]brain was affected minimally. Therefore, the influence of asymmetric tissue composition on age-associated differences in [Asc]brain, [GSH]brain and [Lac]brain was negligible relative to the scatter in the data (SD, Table 1). Alternatively, the four-compartment artifact could be suppressed with inner volume saturation (55).
The large fitted residuals near the chemical shifts of Ins (1,3CH at 3.52 ppm) and the aspartate moiety of NAA (3CH2 at 2.49 and 2.67 ppm) suggest either inaccurate basis spectra or the presence of compounds that are not accounted for. For example, the aspartyl moiety of N-acetylaspartylglutamate coedits with Asc more efficiently than does the aspartate moiety of NAA. Fortunately, these large residuals are fully resolved from Asc and GSH, and thus have a trivial influence on the quantification of antioxidant concentrations.
CONCLUSIONS
Differences in the concentrations of two important antioxidants in the elderly human brain have been measured for the first time using noninvasive methods. The GSH concentration was found to be lower in elderly subjects, although a shorter T2 of GSH in the elderly would also lead to this finding. Moreover, the Asc concentration did not differ between young and elderly subjects, provided that the assumptions on Asc T2 are valid. These results provide an insight into the association between age-related neurodegeneration and oxidative stress.
Supplementary Material
Acknowledgements
We thank Pierre-Gilles Henry and Dinesh Deelchand for developing the software for LCModel analysis of the edited spectra and simulation. We thank Ivan Tkac for MRS-related advice throughout the project. We appreciate the professionalism with which nutrition and nursing staff at the Clinical and Translational Science Unit, University of Minnesota, executed this protocol. We thank Dianne Hutter, RN for assistance with administering the protocol and managing blood samples, the University of Minnesota Alumni Association and the Metropolitan Area Agency on Aging (MAAA) for assistance in recruiting elderly subjects, and colleagues at the Center for Magnetic Resonance Research (CMRR) for maintaining spectrometer performance.
This work was supported by the National Institutes of Health through grant numbers R21-AG029582, P41-RR008079, P30-NS057091, M01-RR00400 and S10 RR023730.
Abbreviations used
- Asc
ascorbate
- [Asc]blood
blood serum ascorbate concentration
- Cho
choline
- Cr
creatine
- CRLB
Cramer–Rao lower bound
- CSF
cerebrospinal fluid
- DEW MEGA-PRESS
double editing with MEGA-point-resolved spectroscopy
- FASTMAP
fast automatic shimming technique by mapping along projections
- fCSF
cerebrospinal fluid fraction
- FID
free induction decay
- GPC
glycerophosphorylcholine
- GPE
glycerophosphoryl-ethanolamine
- GSH
glutathione
- HLSVD
Hankel–Lanczos singular value decomposition
- Ins
myo-inositol
- Lac
lactate
- MoCA
the Montreal Cognitive Assessment
- NAA
N-acetylaspartate
- OVS
outer volume suppression
- PC
phosphorylcholine
- PE
phosphorylethanolamine
- RARE
rapid acquisition with relaxation enhancement
- RF
radiofrequency
- SD
standard deviation
- SNR
signal-to-noise ratio
- STEAM
stimulated echo acquisition mode
- VAPOR
variable-power radiofrequency pulses with optimized relaxation delays
- VOI
volume of interest
Footnotes
Supporting information may be found in the online version of this article.
REFERENCES
- 1.Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82(4):1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- 2.Rice ME, Forman RE, Chen BT, Avshalumov MV, Cragg SJ, Drew KL. Brain antioxidant regulation in mammals and anoxia-tolerant reptiles: balanced for neuroprotection and neuromodulation. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 2002;133(4):515–525. doi: 10.1016/s1532-0456(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 3.Hediger MA. New view at C. Nat. Med. 2002;8(5):445–446. doi: 10.1038/nm0502-445. [DOI] [PubMed] [Google Scholar]
- 4.Spector R. Nutrient transport systems in brain: 40 years of progress. J. Neurochem. 2009;111(2):315–320. doi: 10.1111/j.1471-4159.2009.06326.x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison FE, Hosseini AH, McDonald MP, May JM. Vitamin C reduces spatial learning deficits in middle-aged and very old APP/PSEN1 transgenic and wild-type mice. Pharmacol. Biochem. Behav. 2009;93(4):443–450. doi: 10.1016/j.pbb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tveden-Nyborg P, Johansen LK, Raida Z, Villumsen CK, Larsen JO, Lykkesfeldt J. Vitamin C deficiency in early postnatal life impairs spatial memory and reduces the number of hippocampal neurons in guinea pigs. Am. J. Clin. Nutr. 2009;90(3):540–546. doi: 10.3945/ajcn.2009.27954. [DOI] [PubMed] [Google Scholar]
- 7.Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 2007;85(5):1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- 8.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009;46(6):719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casetta I, Govoni V, Granieri E. Oxidative stress, antioxidants and neurodegenerative diseases. Curr. Pharm. Des. 2005;11(16):2033–2052. doi: 10.2174/1381612054065729. [DOI] [PubMed] [Google Scholar]
- 10.Lykkesfeldt J, Moos T. Age-dependent change in vitamin C status: a phenomenon of maturation rather than of ageing. Mech. Ageing Dev. 2005;126(8):892–898. doi: 10.1016/j.mad.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Janáky R, Cruz-Aguado R, Oja S, Shaw C. 15 Glutathione in the nervous system: roles in neural function and health and implications for neurological disease. In: Lajtha A, editor. Handbook of Neurochemistry and Molecular Neurobiology. 3rd ed. Springer: New York; 2007. pp. 347–399. [Google Scholar]
- 12.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res. Rev. 2005;4(2):288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Schaus R. The ascorbic acid content of human pituitary, cerebral cortex, heart, and skeletal muscle and its relation to age. Am. J. Clin. Nutr. 1957;5(1):39–41. doi: 10.1093/ajcn/5.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Terpstra M, Marjanska M, Henry PG, Tkac I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn. Reson. Med. 2006;56(6):1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 16.Terpstra M, Henry PG, Gruetter R. Measurement of reduced glutathione (GSH) in human brain using LCModel analysis of difference-edited spectra. Magn. Reson. Med. 2003;50(1):19–23. doi: 10.1002/mrm.10499. [DOI] [PubMed] [Google Scholar]
- 17.Tkáč I, Öz G, Adriany G, Uğurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs.7T. Magn. Reson. Med. 2009;62(4):868–879. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terpstra M, Uğurbil K, Tkáč I. Noninvasive quantification of human brain ascorbate concentration using 1H NMR spectroscopy at 7 T. NMR Biomed. 2010;23(3):227–232. doi: 10.1002/nbm.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23(5):209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 20.Terpstra M, Tkac I, Rao R, Gruetter R. Quantification of vitamin C in the rat brain in vivo using short echo-time 1H MRS. Magn. Reson. Med. 2006;55(5):979–983. doi: 10.1002/mrm.20854. [DOI] [PubMed] [Google Scholar]
- 21.Adriany G, Gruetter R. A half-volume coil for efficient proton decoupling in humans at 4 tesla. J. Magn. Reson. 1997;125(1):178–184. doi: 10.1006/jmre.1997.1113. [DOI] [PubMed] [Google Scholar]
- 22.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn. Reson. Med. 2000;43(2):319–323. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn. Reson. Med. 1993;29(6):804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Onishi J, Suzuki Y, Umegaki H, Endo H, Kawamura T, Iguchi A. A comparison of depressive mood of older adults in a community, nursing homes, and a geriatric hospital: factor analysis of Geriatric Depression Scale. J. Geriatr. Psychiatry Neurol. 2006;19(1):26–31. doi: 10.1177/0891988705284725. [DOI] [PubMed] [Google Scholar]
- 26.Terpstra M, Marjanska M, Henry PG, Tkáč I, Gruetter R. Detection of an antioxidant profile in the human brain in vivo via double editing with MEGA-PRESS. Magn. Reson. Med. 2006;56(6):1192–1199. doi: 10.1002/mrm.21086. [DOI] [PubMed] [Google Scholar]
- 27.Tkac I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1ms echo time. Magn. Reson. Med. 1999;41(4):649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Terpstra M, Uğurbil K, Gruetter R. Direct in vivo measurement of human cerebral GABA concentration using MEGA-editing at 7 Tesla. Magn. Reson. Med. 2002;47(5):1009–1012. doi: 10.1002/mrm.10146. [DOI] [PubMed] [Google Scholar]
- 29.Cabanes E, Confort-Gouny S, Le Fur Y, Simond G, Cozzone PJ. Optimization of residual water signal removal by HLSVD on simulated short echo time proton MR spectra of the human brain. J. Magn. Reson. 2001;150(2):116–125. doi: 10.1006/jmre.2001.2318. [DOI] [PubMed] [Google Scholar]
- 30.Emir UE, Deelchand D, Henry PG, Terpstra M. Noninvasive quantification of T(2) and concentrations of ascorbate and glutathione in the human brain from the same double-edited spectra. NMR Biomed. 2010 doi: 10.1002/nbm.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry PG, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn. Reson. Med. 2006;55(2):250–257. doi: 10.1002/mrm.20764. [DOI] [PubMed] [Google Scholar]
- 32.Siegel GJ, editor. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. Philadelphia: Lippincott-Raven Publishers; 1999. [Google Scholar]
- 33.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J. Magn. Reson., Ser. B. 1993;102(1):1–8. [Google Scholar]
- 34.Kruse T, Reiber H, Neuhoff V. Amino acid transport across the human blood-CSF barrier. An evaluation graph for amino acid concentrations in cerebrospinal fluid. J. Neurol. Sci. 1985;70(2):129–138. doi: 10.1016/0022-510x(85)90082-6. [DOI] [PubMed] [Google Scholar]
- 35.Regenold WT, Phatak P, Marano CM, Sassan A, Conley RR, Kling MA. Elevated cerebrospinal fluid lactate concentrations in patients with bipolar disorder and schizophrenia: implications for the mitochondrial dysfunction hypothesis. Biol. Psychiatry. 2009;65(6):489–494. doi: 10.1016/j.biopsych.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 37.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273(5271):59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sohal RS, Sohal BH, Brunk UT. Relationship between antioxidant defenses and longevity in different mammalian species. Mech. Ageing Dev. 1990;53(3):217–227. doi: 10.1016/0047-6374(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 39.Cudkowicz ME, Sexton PM, Ellis T, Hayden DL, Gwilt PR, Whalen J, Brown RH., Jr The pharmacokinetics and pharmaco-dynamics of procysteine in amyotrophic lateral sclerosis. Neurology. 1999;52(7):1492–1494. doi: 10.1212/wnl.52.7.1492. [DOI] [PubMed] [Google Scholar]
- 40.Hazelton GA, Lang CA. Glutathione biosynthesis in the aging adult yellow-fever mosquito [Aedes aegypti (Louisville)] Biochem. J. 1983;210(2):289–295. doi: 10.1042/bj2100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hazelton GA, Lang CA. Glutathione contents of tissues in the aging mouse. Biochem. J. 1980;188(1):25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michels AJ, Joisher N, Hagen TM. Age-related decline of sodium-dependent ascorbic acid transport in isolated rat hepatocytes. Arch. Biochem. Biophys. 2003;410(1):112–120. doi: 10.1016/s0003-9861(02)00678-1. [DOI] [PubMed] [Google Scholar]
- 43.Svensson L, Wu C, Hulthe P, Johannessen K, Engel JA. Effect of ageing on extracellular ascorbate concentration in rat brain. Brain Res. 1993;609(1–2):36–40. doi: 10.1016/0006-8993(93)90851-d. [DOI] [PubMed] [Google Scholar]
- 44.Sahoo A, Chainy GB. Alterations in the activities of cerebral antioxidant enzymes of rat are related to aging. Int. J. Dev. Neurosci. 1997;15(8):939–948. doi: 10.1016/s0736-5748(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 45.Rikans LE, Moore DR. Effect of aging on aqueous-phase antioxidants in tissues of male Fischer rats. Biochim. Biophys. Acta. 1988;966(3):269–275. doi: 10.1016/0304-4165(88)90076-1. [DOI] [PubMed] [Google Scholar]
- 46.Jose da Rocha A, Tulio Braga F, Carlos Martins Maia A, Jr, Jorge da Silva C, Toyama C, Pereira Pinto Gama H, Kok F, Rodrigues Gomes H. Lactate detection by MRS in mitochondrial encephalopathy: optimization of technical parameters. J. Neuroimaging. 2008;18(1):1–8. doi: 10.1111/j.1552-6569.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 47.Lin DD, Crawford TO, Barker PB. Proton MR spectroscopy in the diagnostic evaluation of suspected mitochondrial disease. Am. J. Neuroradiol. 2003;24(1):33–41. [PMC free article] [PubMed] [Google Scholar]
- 48.Chang L, Ernst T, Poland RE, Jenden DJ. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sci. 1996;58(22):2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- 49.Brooks JC, Roberts N, Kemp GJ, Gosney MA, Lye M, Whitehouse GH. A proton magnetic resonance spectroscopy study of age-related changes in frontal lobe metabolite concentrations. Cereb. Cortex. 2001;11(7):598–605. doi: 10.1093/cercor/11.7.598. [DOI] [PubMed] [Google Scholar]
- 50.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J. Neurocytol. 2002;31(8–9):581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- 51.Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6(3):275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirov II, Fleysher L, Fleysher R, Patil V, Liu S, Gonen O. Age dependence of regional proton metabolites T2 relaxation times in the human brain at 3 T. Magn. Reson. Med. 2008;60(4):790–795. doi: 10.1002/mrm.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schenker C, Meier D, Wichmann W, Boesiger P, Valavanis A. Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology. 1993;35(2):119–124. doi: 10.1007/BF00593967. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21(1):22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 55.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: improved sensitivity at high fields using inner volume saturation. Magn. Reson. Med. 2007;58(6):1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 56.Yablonskiy DA, Neil JJ, Raichle ME, Ackerman JJ. Homonuclear J coupling effects in volume localized NMR spectroscopy: pitfalls and solutions. Magn. Reson. Med. 1998;39(2):169–178. doi: 10.1002/mrm.1910390202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.