Summary
Haspin phosphorylates histone H3 at Thr-3 (H3T3ph) during mitosis [1, 2], providing a chromatin binding site for the chromosomal passenger complex (CPC) at centromeres to regulate chromosome segregation [3–5]. H3T3ph becomes increasingly focused at inner centromeres during prometaphase [1, 2], but little is known about how its level or location and the consequent chromosomal localization of the CPC are regulated. In addition, CPC binding to Shugoshin proteins contributes to centromeric Aurora B localization [5, 6]. Recruitment of the Shugoshins to centromeres requires the phosphorylation of Histone H2A at T120 (H2AT120ph) by the kinetochore kinase Bub1 [7], but the molecular basis for the collaboration of this pathway with H3T3ph has been unclear. Here, we show that Aurora B phosphorylates Haspin to promote generation of H3T3ph, and that Aurora B kinase activity is required for normal chromosomal localization of the CPC, indicating an intimate linkage between Aurora B and Haspin functions in mitosis. We propose that Aurora B activity triggers a CPC-Haspin-H3T3ph feedback loop that promotes generation of H3T3ph on chromatin. We also provide evidence that the Bub1-Shugoshin-CPC pathway supplies a signal that boosts the CPC-Haspin-H3T3ph feedback loop specifically at centromeres to produce the well-known accumulation of the CPC in these regions.
Results
Aurora B kinase activity contributes to full Haspin phosphorylation in mitosis
Consistent with our previous studies of exogenous Haspin [1], we found that endogenous Haspin undergoes hyper-phosphorylation during mitosis without a clear change in intrinsic kinase activity (Figure S1A–C). To identify these phosphorylation sites, we immunoprecipitated myc-Haspin from nocodazole-arrested HeLa Tet-On transfectants in the absence of doxycycline induction, in which myc-Haspin is expressed at a low level [1]. Among a total of 29 phosphorylation sites identified by mass spectrometry, nine were potential Aurora B phosphorylation sites matching the consensus R/K-x-S/T (Tables S1 and S2). In contrast, only 4 phosphorylation sites were identified in Haspin from a predominantly interphase population (Table S2). In vitro, recombinant human Aurora B/INCENP complex was able to phosphorylate a kinase-deficient mutant of full-length Haspin, MBP-Haspin KD (Figure 1A). Mass spectrometry identified three phosphorylation sites in MBP-Haspin KD matching the Aurora consensus (Tables S1 and S2), which were all among those identified as phosphorylation sites in cells. Thus, Haspin is a direct substrate of Aurora B in vitro and is phosphorylated at Aurora B consensus sites in cells.
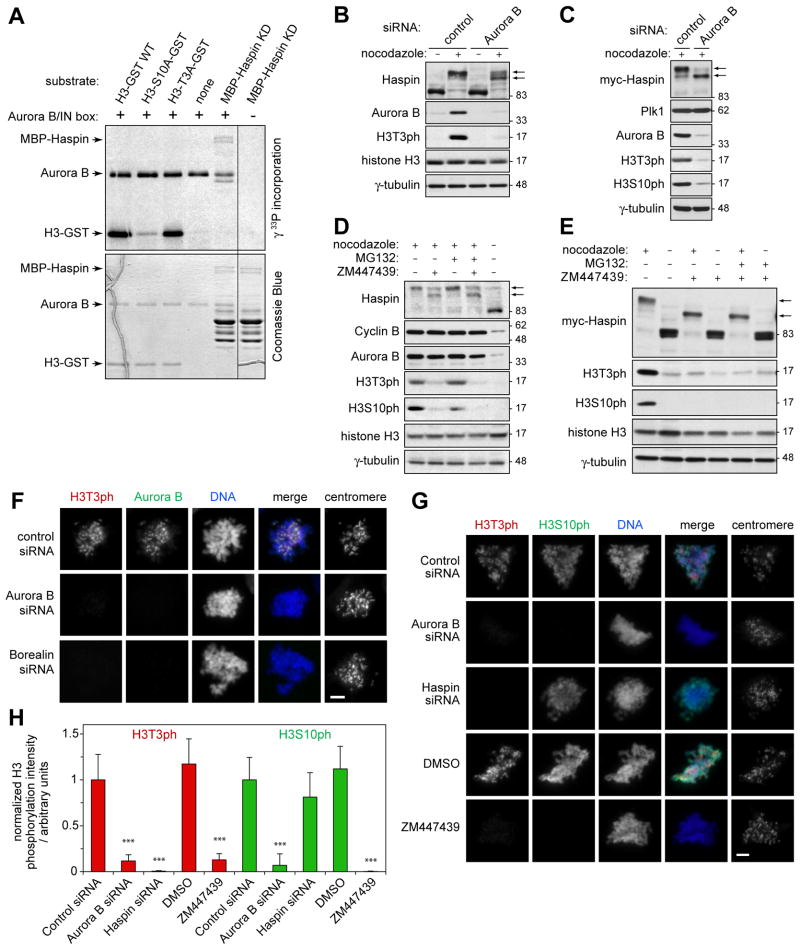
Figure 1. Phosphorylation of Haspin and H3T3 are dependent on Aurora B kinase activity in mitosis.
(A) MBP-Haspin KD or H3(1–45)-GST proteins were phosphorylated in vitro with GST-Aurora B/His-IN using [γ33P]-ATP and analyzed by SDS-PAGE and autoradiography (upper panel) and Coomassie Blue staining (lower panel). Note the presence of a significant amount of partial MBP-Haspin KD protein.
(B) HeLa cells were transfected with control or Aurora B siRNA and, after 36 h, were incubated in the absence or presence of nocodazole for 12 h to arrest cells in mitosis. Lysates were analyzed by immunoblotting.
(C) As for (B), but in HeLa Tet-On/myc-Haspin cells.
(D) HeLa cells were incubated with or without the Aurora B inhibitor ZM447439, nocodazole and MG132 for 3 h and lysates were analyzed by immunoblotting.
(E) HeLa Tet-On/myc-Haspin cells were treated with or without nocodazole for 2 h followed by 1 h treatment with ZM447439 with or without MG132. Lysates were analyzed by immunoblotting.
(F) At 48 h after transfection with the siRNAs indicated, U2OS cells were treated with nocodazole for 1 h and then stained for immunofluorescence microscopy.
(G) At 48 h after transfection with siRNAs, U2OS cells were treated with nocodazole and MG132 for 1.5 h. Alternatively, untransfected U2OS cells were treated with nocodazole and MG132 with or without ZM447439 for 1.5 h, and stained with the antibodies indicated. Scale bars = 5 μm.
(H) Quantification of H3T3ph and H3S10ph levels in mitotic U2OS cells treated as described in (G). Means +/− SD are shown for 7–8 cells in each condition. *** p<0.001 compared to the respective control siRNA transfection. See also Figure S1.
RNAi-mediated knockdown of Aurora B (or Survivin, see below) reduced phosphorylation of both endogenous (Figure 1B) and myc-tagged Haspin (Figure 1C) in nocodazole-arrested mitotic HeLa cells. The reduction in mitotic Haspin phosphorylation was partial, consistent with the fact that only 9 of the 29 identified mitotic phosphorylation sites in Haspin match the Aurora B consensus. Treatment of cells with the Aurora B inhibitor ZM447439 caused a similar reduction in phosphorylation of endogenous Haspin (Figure 1D) and myc-Haspin (Figure 1E) in nocodazole-arrested cells in the presence or absence of the proteasome inhibitor MG132 (added to further prevent mitotic exit). Over-expression of a dominant negative Aurora B mutant deficient in kinase activity (myc-Aurora B K106R) [8] also reduced Haspin phosphorylation in nocodazole-arrested cells (Figure S1D). We conclude that Aurora B kinase activity is required for full phosphorylation of Haspin during mitosis.
Aurora B activity is required for generation of H3T3ph in mitosis
The Aurora B-dependent phosphorylation of Haspin prompted us to examine whether Aurora B activity influences H3T3ph in mitosis. Immunoblotting of mitotic HeLa cell lysates revealed a clear reduction in total H3T3ph upon depletion of Aurora B (Figure 1B, C), treatment with ZM447439 (Figure 1D, E) or over-expression of myc-Aurora B K106R (Figure S1D). To exclude direct inhibition of Haspin by ZM447439, we determined that ZM447439 did not significantly inhibit the activity of purified MBP-Haspin in vitro (Figure S1E). Immunofluorescence microscopy confirmed that RNAi of Aurora B, Borealin or INCENP, or treatment with ZM447439, all reduced H3T3ph in mitotic U2OS cells treated with nocodazole (Figure 1F; Figure S1F) or nocodazole and MG132 (Figure 1G), albeit less efficiently than Haspin RNAi (Figure 1H). Similar results were obtained in ZM447439-treated DLD-1 (Figure S1G) and HeLa cells, and upon depletion of Survivin or over-expression of myc-Aurora B K106R, or when using a chemically distinct Aurora B inhibitor, Hesperadin (see below). These results revealed that generation of H3T3ph in mitosis is partly dependent on Aurora B kinase activity.
Aurora B does not directly phosphorylate H3 at Thr-3
Two lines of evidence suggested that H3T3 is unlikely to be directly phosphorylated by Aurora B. First, whereas mutation of the known target site Ser-10 dramatically reduced phosphorylation of H3(1–45)-GST (the N-terminal 45 residues of H3 fused to GST) by recombinant Aurora B/INCENP in vitro, mutation of Thr-3 did not detectably affect H3(1–45)-GST phosphorylation (Figure 1A), and similar results were obtained using immunoprecipitated Aurora B complex [1]. Second, artificial re-targeting of Aurora B to centromeres by expression of CENP-B-INCENP restored centromeric MCAK localization but not H3T3ph in Haspin-depleted cells [3], suggesting that Aurora B/INCENP does not phosphorylate H3T3 in the absence of Haspin.
To more rigorously exclude direct phosphorylation of H3T3 by Aurora B, we carried out in vitro assays with synthetic peptide substrates. We confirmed that H3(1–21) and H3(1–8) peptides were phosphorylated by MBP-Haspin, and that pre-phosphorylation at Thr-3 abolished this activity (Figure S1H). In contrast, H3(1–8) peptide lacking Ser-10 was not phosphorylated by Aurora B/INCENP, whereas H3(1–21) peptide containing Ser-10 was a good substrate (Figure S1I). Thus, Aurora B did not directly phosphorylate H3T3 in vitro.
To further confirm this conclusion, H3(1–45)-GST was subjected to in vitro phosphorylation using mitotic HeLa cell extract as a source of kinases. Without ZM447439, phosphorylation of H3(1–45)-GST at Thr-3 and Ser-10 was detected with phosphospecific antibodies (Figure S1J). As expected, H3S10ph was reduced by ZM447439, presumably due to inhibition of Aurora B activity. In contrast, ZM447439 treatment had little effect on H3T3ph, suggesting that Aurora B is not responsible for H3T3ph in this system. We conclude that Aurora B does not phosphorylate H3T3 directly.
Phosphorylation by Aurora B is required for full Haspin activity toward H3T3 in mitosis
To determine if direct phosphorylation of Haspin by Aurora B might regulate Haspin function, serine and threonine residues within Aurora B consensus sites were mutated to alanine in an siRNA-resistant myc-Haspin construct (myc-Haspin 11A). The mutations included eight sites identified in cells (a ninth was excluded because it also matches the Cdk consensus), and three that are conserved in Haspin from different species (see Table S1). Immunoblotting of lysates of transfected mitotic HeLa cells showed that the highest molecular weight band corresponding to myc-Haspin WT was not present in myc-Haspin 11A expressing cells (Figure 2A), consistent with the expected loss of phosphorylation. In fact, myc-Haspin 11A migrated essentially identically to myc-Haspin WT from ZM447439-treated cells and, while ZM447439 treatment reduced phosphorylation of myc-Haspin WT, it had no detectable effect on phosphorylation of myc-Haspin 11A. Moreover, myc-Haspin 11A immunoprecipitated from asynchronous cells was a poorer substrate for recombinant Aurora B/INCENP than myc-Haspin WT (Figure 2B). Thus, the majority of Aurora B-dependent phosphorylation sites were mutated in myc-Haspin 11A.
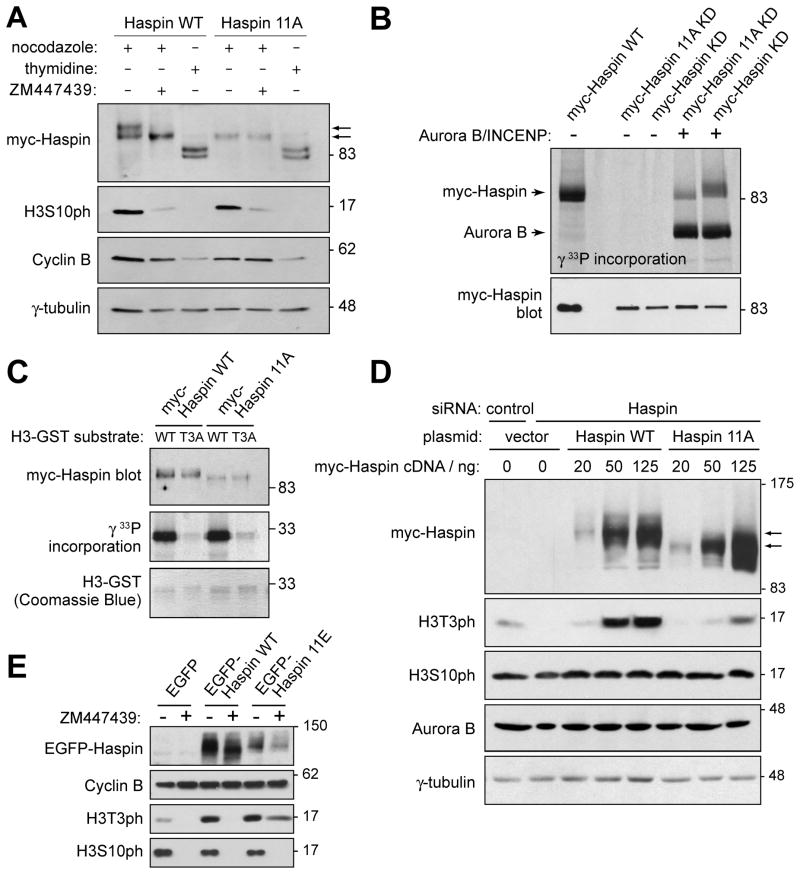
Figure 2. Aurora B indirectly regulates H3T3ph by phosphorylating Haspin.
(A) Myc-Haspin 11A is resistant to Aurora B-mediated phosphorylation in cells. At 19 hr after transfection with myc-Haspin constructs, HeLa cells were blocked at G1/S with thymidine for 18 h, or blocked in mitosis with nocodazole for 3 h followed by a further 3 h in nocodazole with or without ZM447439. Lysates were analyzed by immunoblotting.
(B) Myc-Haspin 11A is resistant to Aurora B-mediated phosphorylation in vitro. Myc-Haspin immunoprecipitates from transiently transfected asynchronous HeLa cells were divided in two and subjected to either phosphorylation in vitro by GST-Aurora B/His-IN with [γ33P]-ATP and analyzed by SDS-PAGE and autoradiography (upper panel) or immunoblotting (lower panel). Kinase deficient (KD) forms of Haspin were used to eliminate autophosphorylation.
(C) The kinase activity of myc-Haspin 11A in vitro is intact. Myc-Haspin WT or myc-Haspin 11A immunoprecipitated from transiently transfected nocodazole-arrested mitotic HeLa cells were exposed to H3(1–45)-GST or H3(1–45)T3A-GST substrates in vitro and analyzed by SDS-PAGE and immunoblotting (top panel) and autoradiography (middle panel). H3(1–45)-GST was visualized by Coomassie Blue staining (lower panel).
(D) Myc-Haspin 11A is defective in restoring H3T3ph following endogenous Haspin RNAi. HeLa cells were transfected with indicated siRNAs, followed by transfection with various doses of myc-Haspin WT or 11A mutant plasmids. At 24 hr after DNA transfection, cells were blocked in mitosis by nocodazole treatment for 14 hr, and lysates analyzed by immunoblotting.
(E) EGFP-Haspin 11E prevents loss of H3T3ph upon Aurora B inhibition. HeLa cells were transfected with EGFP, EGFP-Haspin WT or 11E plasmids. Cells were released from an 18 h thymidine treatment into fresh medium for 5.5 h and then nocodazole with or without ZM447439 was added for 5.5 h. Finally, MG132 was added for an additional 2.5 h and lysates were analyzed by immunoblotting. See also Figure S2.
We also found that myc-Haspin 11A immunoprecipitated from nocodazole-arrested HeLa cells phosphorylated H3(1–45)-GST in vitro as efficiently as myc-Haspin WT (Figure 2C), indicating that the mutant was not grossly misfolded and that phosphorylation by Aurora B does not dramatically alter the intrinsic kinase activity of Haspin. In addition, biochemical fractionation showed that both myc-Haspin 11A and WT were present in the chromatin-enriched pellet (Figure S2A), and immunofluorescence microscopy showed that, at least when over-expressed, myc and EGFP-tagged forms of both Haspin WT and 11A were localized to mitotic chromosomes, even when endogenous Haspin was depleted (Figure S2B, C).
We then carried out Haspin RNAi rescue experiments to examine the cellular activity of Haspin 11A. HeLa cells were depleted of endogenous Haspin by RNAi, followed by transfection with increasing doses of siRNA-resistant Haspin WT or 11A mutant plasmids. Mitotic cells were harvested after nocodazole treatment and analyzed by immunoblotting. Myc- or EGFP-tagged Haspin 11A was less effective than Haspin WT in restoring H3T3ph in mitotic HeLa cells depleted of endogenous Haspin (Figure 2D, S2D). Furthermore, transfection of cells with EGFP-Haspin 11E (containing glutamate substitutions at the same eleven sites to mimic the charge introduced by phosphorylation), but not EGFP-Haspin WT, allowed maintenance of substantial levels of H3T3ph in mitosis even when Aurora B was inhibited (Figure 2E, Figure S2E, F). EGFP-Haspin 11E also localized to mitotic chromosomes (Figure S2C) and restored H3T3ph in mitotic HeLa cells depleted of endogenous Haspin (Figure S2D). These results suggest that direct phosphorylation by Aurora B is required for full Haspin-mediated H3T3 phosphorylation in mitosis.
Aurora B kinase activity contributes to normal CPC localization on chromosomes
We recently showed that Haspin-mediated H3T3ph helps position the CPC at inner centromeres in mitosis [3]. Combined with our finding here that Aurora B activity promotes H3T3ph in mitosis, a model can be proposed in which Aurora B acts through Haspin to regulate its own chromosomal localization (Figure 3A). We sought to test this possibility in a number of ways.
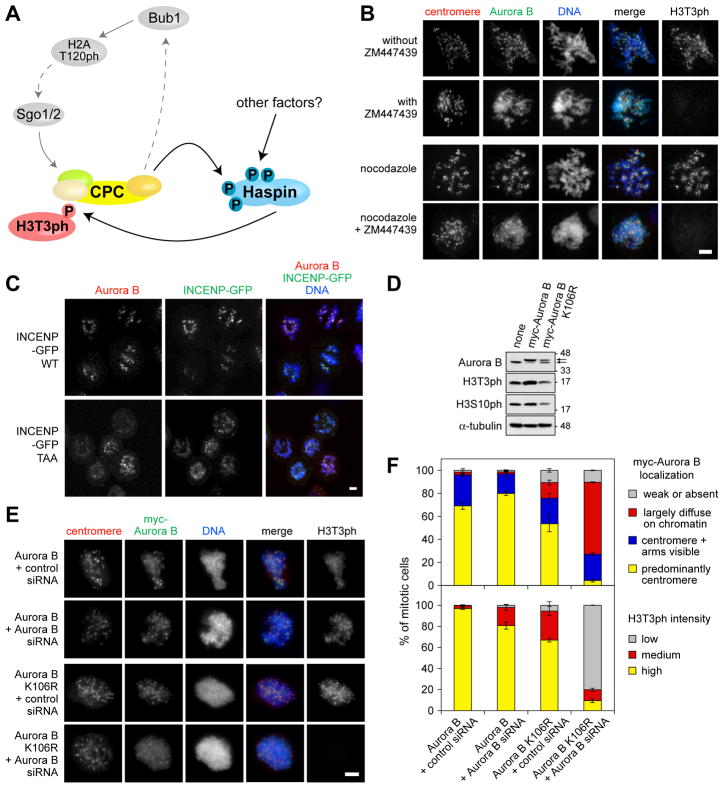
Figure 3. Aurora B kinase activity contributes to CPC localization in cells.
(A) Model for the functional interplay of Aurora B, Haspin and the Bub1-Shugoshin pathway in mitosis.
(B) Chemical inhibition of Aurora B alters its distribution on mitotic chromosomes. U2OS cells were treated with ZM447439 plus nocodazole or DMSO (vehicle control) for various times, and stained with the antibodies indicated. For quantification see Figure S3C.
(C) The INCENP-TAA mutant compromises the centromeric localization of the CPC. Confocal immunofluorescence microscopy of U2OS cells expressing INCENP-GFP WT or INCENP-GFP TAA mutant under doxycycline-inducible promoters. Cells were treated with thymidine for 24 h, then released into low dose nocodazole (25 ng/ml) plus doxycycline (to induce GFP-INCENP expression) for 14 h.
(D) Parental HeLa cells or a HeLa-derived cell lines stably expressing myc-Aurora B WT or K106R were treated with nocodazole for 14 h. Mitotic cells were collected and analyzed by immunoblotting.
(E) A kinase-deficient Aurora B mutant has compromised centromeric localization. Immunofluorescence microscopy of nocodazole-treated mitotic HeLa cells stably expressing myc-Aurora B WT or K106R 36 h after depletion of endogenous Aurora B by siRNA transfection. Scale bars = 5 μm.
(F) Quantification of Aurora B localization and H3T3ph intensity in cells treated as in (E). Approximately 100 cells were counted in each condition. Means +/− SD are shown (n = 3). See also Figure S3.
First, the model predicts that the chromosomal localization of Aurora B will be altered when Haspin is mutated to prevent phosphorylation by Aurora B. We were unable to directly test this possibility at centromeres in RNAi-rescue experiments because we could not control expression levels sufficiently to prevent increased H3T3ph and CPC localization to chromosome arms caused by Haspin overexpression [3]. However, overexpression of EGFP-Haspin 11A was less effective than EGFP-Haspin WT in increasing H3T3ph and CPC localization on chromosome arms (Figure S3A, B), confirming that mutation of Aurora B phosphorylation sites on Haspin compromises mechanisms of CPC localization.
Second, the model suggests that indirectly diminishing H3T3ph by inhibiting Aurora B will affect chromosomal localization of the CPC. Indeed, ZM447439 treatment decreased the percentage of U2OS cells in which Aurora B was enriched at centromeres, an effect that was particularly apparent when cells were also treated with nocodazole, which enhances Aurora B localization to centromeres in control cells (Figure 3B, Figure S3C). The Aurora B inhibitor Hesperadin also caused delocalization of the CPC from centromeres, even in the presence of MG132 to prevent mitotic exit (Figure S3D-F). Similar results were observed in HeLa cells (Figure S3C), where the effect of ZM447439 treatment on Aurora B localization was particularly clear on spread mitotic chromosomes (Figure S3G). In contrast, Aurora B inhibition did not significantly alter formation of CPC, as judged by the co-immunoprecipitation of Survivin with Aurora B (Figure S3H). Thus, Aurora B inhibition compromises centromeric localization of the CPC.
Third, we examined the localization of overexpressed CPC components mutated to reduce Aurora B activity. As expected, GFP-INCENP WT concentrated at centromeres when expression was induced in U2OS cells (Figure 3C). In contrast, a GFP-INCENP TAA mutant that is compromised in its ability to activate Aurora B [9, 10] was more diffusely localized on chromatin and caused delocalization of endogenous centromeric Aurora B (Figure 3C). Similar results were obtained using kinase-deficient Aurora B. Except when expressed at very high levels, myc-Aurora B WT localized predominantly to centromeres in mitotic HeLa cells and H3T3ph levels were normal or slightly increased (Figure S3I). In contrast, expression of myc-Aurora B K106R resulted in strongly decreased H3T3ph, presumably due to its dominant-negative effect, as previously noted in immunoblotting experiments (Figure S1D). In cells with reduced H3T3ph, myc-Aurora B K106R showed decreased localization to centromeres when compared to cells expressing comparable levels of myc-Aurora B WT (Figure S3I). Thus, two Aurora B inhibitors and overexpression of two different forms of the CPC deficient in kinase activity all caused similar defects in Aurora B localization.
Fourth, we wished to examine the localization of kinase-deficient Aurora B when expressed at near-normal levels in the absence of endogenous Aurora B. We established HeLa-derived cell lines stably expressing myc-Aurora B WT or K106R at levels comparable to endogenous Aurora B in parental HeLa cells (Figure 3D). Although the Aurora B K106R-expressing cell line showed a slight reduction in H3S10ph and evidence of altered DNA content, both cell lines could be propagated for multiple passages in culture. Therefore, myc-Aurora B K106R did not show lethal dominant-negative effects when expressed at this level. In control siRNA-transfected cells, myc-Aurora B K106R localized predominantly to centromeres in mitosis and H3T3ph was maintained at near normal levels (Figure 3E, F). However, upon RNAi-mediated depletion of endogenous Aurora B, H3T3ph was strongly reduced and myc-Aurora B K106R localized diffusely to chromosome arms and only weak centromeric accumulation was observable, while myc-Aurora B WT was little affected (Figure 3E, F). Taken together, these data indicate that Aurora B kinase activity is required for its normal chromosomal localization during mitosis.
A positive feedback loop between Aurora B and Haspin
Because H3T3ph generated by Haspin produces a binding site for the CPC on chromosomes [3–5], chromatin-bound Aurora B could act on Haspin to further stimulate local H3T3 phosphorylation, generating a positive feedback loop between Haspin and Aurora B to help drive CPC accumulation on chromatin, particularly at inner centromeres (Figure 3A). To determine if the H3T3ph-bound population of Aurora B plays a role in the generation of H3T3ph, we made use of a HeLa cell line stably expressing a mutant form of Survivin (D70A/D71A) that prevents the binding of the CPC to H3T3ph [3]. As expected, depletion of endogenous Survivin from control cells decreased the level of H3T3ph (and H3S10ph). Exogenously expressed Survivin-myc WT restored total H3T3ph (and H3S10ph) in Survivin-depleted cells (Figure 4A), and also restored the normal concentration of both H3T3ph and the CPC at mitotic centromeres (Figure 4B; Figure S4A). In contrast, expression of Survivin-myc D70A/D71A (which rendered the distribution of Aurora B diffuse on chromatin) was less able to restore H3T3ph and its accumulation at centromeres (Figure 4A, B; Figure S4A), though it did restore H3S10ph as previously reported [3]. Consistent with this effect, Survivin-myc D70A/D71A was unable to support full phosphorylation of Haspin in mitosis (Figure 4A). These results indicate that binding of the CPC to H3T3ph enhances the further generation of H3T3ph and accumulation of this modification at centromeres.
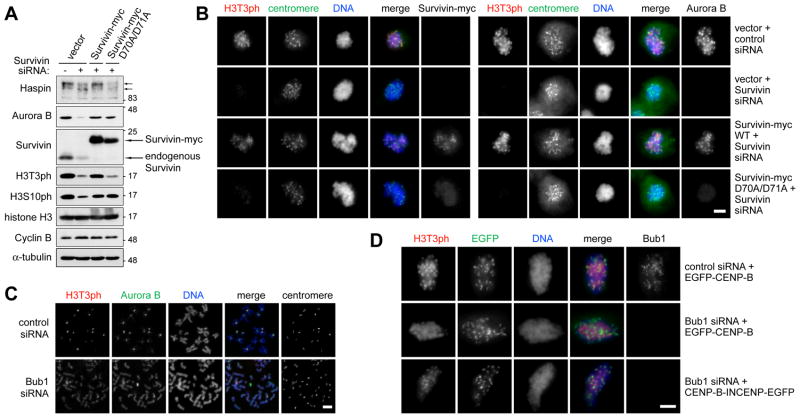
Figure 4. Survivin binding to H3T3ph is required for normal H3T3ph generation in cells, and the Bub1-Shugoshin pathway focuses H3T3ph and the CPC at centromeres.
(A) HeLa cells stably expressing vector alone, Survivin-myc or Survivin-myc D70A/D71A were transfected with siRNA for 40 h and arrested in mitosis with nocodazole for 14 h. Lysates were analyzed by immunoblotting.
(B) Stable HeLa cells were transfected as in (A). After 2 days, immunofluorescence microscopy of mitotic cells treated with nocodazole for 2 h was carried out. For quantification see Figure S4A.
(C) HeLa cells were transfected with the siRNAs indicated and, after 2 days, treated with nocodazole and MG132 for 3 h. Chromosome spreads were then prepared for immunofluorescence. See also Figure S4B.
(D) U2OS cells were transfected with the siRNAs indicated, followed by transfection with EGFP-CENP-B or CENP-B-INCENP-EGFP plasmids. After 2.5 to 3 h treatment with nocodazole and MG132, the distribution of H3T3ph was determined by immunofluorescence microscopy. Scale bars = 5 μm. For quantification, see Figure S4C.
Interplay with the Bub1-Shugoshin pathway
To determine the influence of the Bub1-Shugoshin pathway on Haspin-H3T3ph-mediated regulation of CPC localization, we depleted Bub1 by siRNA transfection and analyzed HeLa cells treated with nocodazole and MG132 to prevent mitotic exit. As previously reported [6], Bub1 depletion diminished the centromeric accumulation of Aurora B in HeLa cells and increased its visibility on chromosome arms (Figure 4C). Bub1 loss also caused H3T3ph to become diffusely distributed on chromosomes (Figure 4C), though the total levels of H3T3ph were not greatly reduced (Figure S4B), resembling the effects of Sgo1 depletion [11]. Similar findings were obtained in U2OS cells. Interestingly, artificial retargeting of Aurora B to centromeres with CENP-B-INCENP [12] partially restored the centromeric accumulation of H3T3ph in Bub1-depleted cells (Figure 4D, Figure S4C). Therefore, the Bub1-Shugoshin pathway appears to contribute to the normal centromeric concentration of H3T3ph in part by localizing the CPC to centromeres.
Discussion
Haspin is a substrate of Aurora B
Our results reveal that Haspin is a direct substrate of Aurora B, and that Aurora B facilitates generation of H3T3ph during mitosis. Although we do not rule out a contribution of other mechanisms (such as regulation of an H3T3ph phosphatase), our results suggest that direct phosphorylation of Haspin is a key means by which Aurora B regulates H3T3ph. The crystal structure of the Haspin kinase domain suggests that it has constitutive activity that does not require activation loop phosphorylation [13, 14]. Consistent with this, we found no evidence that phosphorylation affects the intrinsic kinase activity of Haspin. Also, mutation of Aurora B phosphorylation sites did not noticeably alter the localization of over-expressed Haspin to mitotic chromosomes. Additionally, we found that in cell extracts in which many normal intermolecular associations are disrupted, H3T3ph was much less sensitive to Aurora B inhibition than in intact cells. Together these findings suggest that phosphorylation by Aurora B on the Haspin N-terminal domain might modulate the binding of a regulatory protein. An appealing hypothesis is that phosphorylation in mitosis displaces an inhibitory protein that binds to Haspin in interphase. Alternatively, phosphorylation by Aurora B might regulate access of Haspin to nucleosomal H3 in cells. Strictly speaking, because we do not yet know the distribution of Haspin expressed at endogenous levels, it remains possible that Haspin localization is influenced by Aurora B. Further studies are required to fully understand the mechanism by which Aurora B regulates Haspin.
Aurora B is a master regulator of histone phosphorylation in mitosis
Aurora B directly phosphorylates H3S10, H3S28, and the centromeric histone CENP-A at Ser-7 [15]. We find here that Aurora B stimulates phosphorylation of H3T3 by Haspin. Another report indicates that Aurora B is required for phosphorylation of centromeric H2A at the residue equivalent to T120 in Drosophila cells [16]. A recent study revealing that Bub1 is responsible for phosphorylation of this residue in human and budding yeast [7], coupled with work indicating that Aurora B influences Bub1 localization [17–19], suggests that Bub1 might be the intermediary kinase in this case (see Figure 3A). Thus, Aurora B can be considered a “master regulator” of mitotic histone phosphorylation, serving both as a direct histone kinase and to coordinate the activity of other histone kinases.
Functional interplay between Aurora B and Haspin in mitosis
H3T3ph created by Haspin is required to position the CPC at inner centromeres [3, 5], and H3T3ph also facilitates activation of Aurora B on chromatin [4, 20]. Taken together with the data reported here, a model can be envisioned in which Aurora B phosphorylates Haspin to promote H3T3ph in mitosis, enhancing localization of the CPC to chromatin. Aurora B then acts in a local positive feedback loop to sustain Haspin activity and therefore contributes to its own accumulation at the inner centromere (Figure 3A). Indeed, a Survivin mutant (D70/D71A) that is unable to bind to H3T3ph [3] is compromised in its ability to restore H3T3ph, suggesting that binding of the CPC to H3T3ph enhances generation of H3T3ph.
Also consistent with this model, we find that inactivation of Aurora B in a number of different ways compromises the centromeric accumulation of the CPC, increasing its localization on chromosome arms. As previously reported, we find that when an Aurora B kinase-deficient mutant is expressed at low levels, it localizes normally to centromeres [8, 10, 21], but that it does not localize efficiently when expressed at higher levels [10, 21]. From this apparent dominant-negative effect of kinase-deficient Aurora B, Honda et al. (2003) concluded “phosphorylation of an as yet unidentified Aurora B substrate seems to be essential for efficient association of the Aurora B/INCENP/Survivin complex with the centromere.” Indeed, our results suggest that Haspin is such a substrate, and that loss of H3T3ph at least partly explains the dominant-negative effect of Aurora B kinase-deficient mutants on CPC localization. Supporting this view, we find that when endogenous Aurora B is depleted (causing a decline in H3T3ph), a kinase-deficient mutant of Aurora B (when expressed at a low level) changes from a predominantly centromeric localization to a diffuse distribution on chromosomes. Indeed, similar results have been obtained by others [22], although there is also a conflicting report [23].
Again consistent with a positive feedback model, we find that inhibition of Aurora B with ZM447439 or Hesperadin compromises the centromeric concentration of the CPC. In other studies, Aurora inhibitors have been reported either to cause loss of centromeric Survivin [24], Aurora B [25] and INCENP [26] or to allow centromeric CPC localization [21, 22, 27]. The reasons for these apparent discrepancies are not clear but, in light of our results with the Aurora B kinase-deficient mutant, differences in the extent of kinase inhibition in the various studies are a plausible explanation. It is also possible that the delocalization of Aurora B from centromeres to arms was overlooked or considered unimportant in some prior studies. Nevertheless, multiple lines of evidence support the idea that Aurora B kinase activity contributes to focusing of the CPC at centromeres.
How is H3T3ph concentrated at inner centromeres?
Notably, the proposed positive feedback loop between Haspin and Aurora B does not in itself provide an explanation for the centromeric focusing of H3T3ph and the CPC. We and others find that Bub1 or Sgo1 depletion causes delocalization of H3T3ph from centromeres [5, 11, 28], and that artificially retargeting Aurora B to centromeres in the absence of Bub1 partially restores the centromeric concentration of H3T3ph (this study). Therefore, centromeric localization of the CPC via the Bub1-H2AT120ph-Shugoshin-CPC pathway [5] appears to provide an input signal that boosts the Haspin-H3T3ph-CPC feedback loop specifically at centromeres to drive H3T3ph and CPC concentration at this location. Additionally, because Aurora B influences Bub1 [17–19] and Sgo1 localization [11, 29], and centromeric H2A phosphorylation [16] (see above), it is possible that the Bub1-H2AT120ph-Shugoshin-CPC pathway constitutes a second feedback loop regulating centromeric CPC (Figure 3A) [30]. The combination of these feedback loops would ensure robust CPC localization and function at centromeres.
We note that inhibition of Aurora B does not eliminate H3T3ph or completely delocalize centromeric CPC. There are likely to be additional factors that regulate Haspin to control its activity at centromeres (see Figure 3A). For example, Haspin localization may depend on cohesin complexes, and the Bub1-Shugoshin pathway might have an additional influence on H3T3ph distribution through its effects on cohesion [5]. Also, residual phosphorylation of Haspin remains in Aurora B-inhibited mitotic cells. It will be interesting to test whether these Aurora B-independent phosphorylation sites regulate Haspin activity at centromeres, and whether they modulate interactions of Haspin with cohesion proteins at centromeres [5].
Conclusion
We show that Aurora B phosphorylates Haspin to enhance H3T3ph and to regulate its own localization on chromatin. This mechanism resembles the ability of Cyclin B1-Cdk1 to prime its own import into the nucleus during mitotic entry [31] and the self-regulated recruitment of Plk1 to its phosphorylated substrates [32]. In addition, “reading” of H3T3ph by Survivin, leading to Aurora B recruitment, phosphorylation of Haspin, and increased “writing” of H3T3ph may share similarities with the spreading of the heterochromatin protein HP1 driven by recognition of H3K9me3, and the subsequent recruitment of histone H3K9 methyltransferases that associate with H3K9me3 and/or HP1 [33]. Indeed, it is possible that CPC deposition at centromeres (perhaps by the centromere-specific Bub1-Shugoshin pathway) triggers the Haspin-H3T3ph-CPC feedback loop, which then spreads H3T3ph across the inner centromere, yielding the well-known CPC localization pattern in this region. The recruitment of enzyme complexes to chromatin by the modification that they generate (either directly or indirectly) appears to be a common theme in chromatin biology. Such “self-priming” loops may be a widespread mechanism regulating the localization of key kinases and chromatin-modifying enzymes during mitosis.
Supplementary Material
Acknowledgments
We thank Claire Crafter of AstraZeneca for the gift of ZM447439, Ross Tomaino for mass spectrometry, and Hiroshi Kimura, Stephen Taylor and Linda Wordeman for kindly providing plasmids and/or antibodies. This work was funded by grants to J.M.G.H. from the American Cancer Society (RSG 05–13401-GMC) and the National Institutes of Health (R01 GM074210). M.S.W and S.M.A.L. are supported by the Netherlands Organization of Scientific Research (NWO-Vidi 917.66.332)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dai J, Sultan S, Taylor SS, Higgins JMG. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev. 2005;19:472–488. doi: 10.1101/gad.1267105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markaki Y, Christogianni A, Politou AS, Georgatos SD. Phosphorylation of histone H3 at Thr3 is part of a combinatorial pattern that marks and configures mitotic chromatin. J Cell Sci. 2009;122:2809–2819. doi: 10.1242/jcs.043810. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JMG. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science. 2010;330:231–235. doi: 10.1126/science.1189435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science. 2010;330:235–239. doi: 10.1126/science.1189505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science. 2010;330:239–243. doi: 10.1126/science.1194498. [DOI] [PubMed] [Google Scholar]
- 6.Tsukahara T, Tanno Y, Watanabe Y. Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature. 2010;467:719–723. doi: 10.1038/nature09390. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science. 2010;327:172–177. doi: 10.1126/science.1180189. [DOI] [PubMed] [Google Scholar]
- 8.Girdler F, Gascoigne KE, Eyers PA, Hartmuth S, Crafter C, Foote KM, Keen NJ, Taylor SS. Validating Aurora B as an anti-cancer drug target. J Cell Sci. 2006;119:3664–3675. doi: 10.1242/jcs.03145. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JD, Schumacher JM. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B Kinase stimulates Aurora B kinase activity. J Biol Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honda R, Korner R, Nigg EA. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol Biol Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai J, Sullivan BA, Higgins JMG. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell. 2006;11:741–750. doi: 10.1016/j.devcel.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SMA. Sensing Chromosome Bi-Orientation by Spatial Separation of Aurora B Kinase from Kinetochore Substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eswaran J, Patnaik D, Filippakopoulos P, Wang F, Stein RL, Murray JW, Higgins JMG, Knapp S. Structure and functional characterization of the atypical human kinase haspin. Proc Natl Acad Sci U S A. 2009;106:20198–20203. doi: 10.1073/pnas.0901989106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa F, Capasso P, Tortorici M, Forneris F, de Marco A, Mattevi A, Musacchio A. Crystal structure of the catalytic domain of Haspin, an atypical kinase implicated in chromatin organization. Proc Natl Acad Sci U S A. 2009;106:20204–20209. doi: 10.1073/pnas.0908485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116:3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 16.Brittle AL, Nanba Y, Ito T, Ohkura H. Concerted action of Aurora B, Polo and NHK-1 kinases in centromere-specific histone 2A phosphorylation. Exp Cell Res. 2007;313:2780–2785. doi: 10.1016/j.yexcr.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson VL, Scott MI, Holt SV, Hussein D, Taylor SS. Bub1 is required for kinetochore localization of BubR1, Cenp-E, Cenp-F and Mad2, and chromosome congression. J Cell Sci. 2004;117:1577–1589. doi: 10.1242/jcs.01006. [DOI] [PubMed] [Google Scholar]
- 19.Boyarchuk Y, Salic A, Dasso M, Arnaoutov A. Bub1 is essential for assembly of the functional inner centromere. J Cell Biol. 2007;176:919–928. doi: 10.1083/jcb.200609044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosasco-Nitcher SE, Lan W, Khorasanizadeh S, Stukenberg PT. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 21.Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267–280. doi: 10.1083/jcb.200208091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Miranda G, de Castro IP, Carmena M, Aguirre-Portoles C, Ruchaud S, Fant X, Montoya G, Earnshaw WC, Malumbres M. SUMOylation modulates the function of Aurora-B kinase. J Cell Sci. 2010;123:2823–2833. doi: 10.1242/jcs.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scrittori L, Skoufias DA, Hans F, Gerson V, Sassone-Corsi P, Dimitrov S, Margolis RL. A small C-terminal sequence of Aurora B is responsible for localization and function. Mol Biol Cell. 2005;16:292–305. doi: 10.1091/mbc.E04-06-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beardmore VA, Ahonen LJ, Gorbsky GJ, Kallio MJ. Survivin dynamics increases at centromeres during G2/M phase transition and is regulated by microtubule-attachment and Aurora B kinase activity. J Cell Sci. 2004;117:4033–4042. doi: 10.1242/jcs.01242. [DOI] [PubMed] [Google Scholar]
- 25.Emanuele MJ, Lan W, Jwa M, Miller SA, Chan CS, Stukenberg PT. Aurora B kinase and protein phosphatase 1 have opposing roles in modulating kinetochore assembly. J Cell Biol. 2008;181:241–254. doi: 10.1083/jcb.200710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nozawa RS, Nagao K, Masuda HT, Iwasaki O, Hirota T, Nozaki N, Kimura H, Obuse C. Human POGZ modulates dissociation of HP1alpha from mitotic chromosome arms through Aurora B activation. Nat Cell Biol. 2010;12:719–727. doi: 10.1038/ncb2075. [DOI] [PubMed] [Google Scholar]
- 27.Klein UR, Nigg EA, Gruneberg U. Centromere targeting of the chromosomal passenger complex requires a ternary subcomplex of Borealin, Survivin, and the N-terminal domain of INCENP. Mol Biol Cell. 2006;17:2547–2558. doi: 10.1091/mbc.E05-12-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eot-Houllier G, Fulcrand G, Watanabe Y, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase 3 is required for centromeric H3K4 deacetylation and sister chromatid cohesion. Genes Dev. 2008;22:2639–2644. doi: 10.1101/gad.484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resnick TD, Satinover DL, MacIsaac F, Stukenberg PT, Earnshaw WC, Orr-Weaver TL, Carmena M. INCENP and Aurora B promote meiotic sister chromatid cohesion through localization of the Shugoshin MEI-S332 in Drosophila. Dev Cell. 2006;11:57–68. doi: 10.1016/j.devcel.2006.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vader G, Lens SMA. Chromosome segregation: taking the passenger seat. Curr Biol. 2010;20:R879–881. doi: 10.1016/j.cub.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 31.Gavet O, Pines J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J Cell Biol. 2010;189:247–259. doi: 10.1083/jcb.200909144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petronczki M, Lenart P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–659. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Grewal SI. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev. 2010;20:134–141. doi: 10.1016/j.gde.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.