Abstract
Neurofibrillary tangles are a feature of Alzheimer disease and other tauopathies, and while they are generally believed to be markers of neuronal pathology, there is little evidence evaluating whether tangles directly impact neuronal function. To investigate the response of cells in hippocampal circuits to complex behavioral stimuli, we used an environmental enrichment paradigm to induce expression of an immediate-early gene, Arc, in the rTg4510 mouse model of tauopathy. These mice reversibly overexpress P301L tau and exhibit substantial neurofibrillary tangle deposition, neuronal loss, and memory deficits. Employing fluorescent in situ hybridization to detect Arc mRNA, we found that rTg4510 mice have impaired hippocampal Arc expression both without stimulation and in response to environmental enrichment; this likely reflects the combination of functional impairments of existing neurons and loss of neurons. However, tangle-bearing cells were at least as likely as non-tangle-bearing neurons to exhibit Arc expression in response to enrichment. Transgene suppression with doxycycline for 6 weeks resulted in increased percentages of Arc-positive cells in rTg4510 brains compared to untreated transgenics, restoring enrichment-induced Arc mRNA levels to that of wild-type controls despite the continued presence of neurofibrillary pathology. We interpret these data to indicate that soluble tau contributes to impairment of hippocampal function, while tangles do not preclude neurons from responding in a functional circuit.
Keywords: Alzheimer, Arc, Environmental enrichment, Neurofibrillary tangle, rTg4510, Tauopathy
INTRODUCTION
Neurofibrillary tangles (NFTs) have long been considered to be toxic aggregates that contain mislocalized and misfolded hyperphosphorylated tau and occupy the majority of the perikarya. Although most tangle-bearing neurons contain a normal complement of many mRNAs and are clearly still alive, laser capture microdissection studies have demonstrated that they have a diminished complement of synaptic mRNAs, suggesting that they may not be active (1). Nonetheless, it is not known whether a neuron containing a tangle could be a functional component of complex neural networks.
To address these questions we examined rTg4510 mice, a transgenic line that overexpresses a frontotemporal dementia-related P301L mutant form of tau via a doxycycline-responsive promoter. With age, the mice develop tangles, neuronal loss, and marked memory impairments (2–4). Surprisingly, mature mice that have already developed tau deposition and substantial neuronal loss show improved memory performance after transgene suppression, despite the continued presence of these morphological lesions (4). This implies that transgene-derived, soluble tau contributes to the behavioral phenotype and suggests 2 possibilities that are not mutually exclusive: 1) neurons containing tangles are themselves always dysfunctional but suppression of the transgene can mitigate enough soluble tau related dysfunction to allow behavioral recovery, or 2) the presence of a tangle does not preclude a neuron from participating in responses to environmentally-relevant stimuli. Because behavioral assays cannot interrogate the ability of individual neurons to function in a circuit, we used a method that combines environmental stimulation of mice with postmortem assessment of immediate-early gene activation in tangle and non-tangle-bearing neurons.
To test whether or not tangle-bearing and non-tangle-bearing neurons are able to integrate in neural systems, we took advantage of the observation that an increase in the immediate-early gene Arc occurs in specific neuronal populations in response to changes in the environment (5). Upon exposure to an enriched environment, Arc mRNA is rapidly transcribed in the nucleus and transits within ~30 minutes to the cytoplasm where it can be detected with fluorescent in situ hybridization (6, 7). We induced Arc mRNA in rTg4510 and littermate non tau expressing control mice via brief exposure to novel objects, then examined the percentage of pyramidal neurons with cytoplasmic Arc staining to determine functionality within the Cornu Ammonis fields CA1-CA3 of the hippocampus. We combined fluorescent immunostaining for neurofibrillary pathology with Arc in situ hybridization to enable the analysis of functional circuitry with the ability to determine whether individual responding neurons contain tangles, a level of analysis not possible with traditional behavioral testing.
Our results demonstrate that overexpression of P301L tau causes marked impairments in Arc upregulation in response to environmental enrichment in both tangle-bearing and morphologically normal neurons. Surprisingly, tangle-bearing neurons were somewhat more preserved in their responsiveness. Suppression of the transgene reversed this effect, suggesting that tangle-bearing neurons are able to participate in functional circuits as well as non-tangle-bearing neighboring neurons. These data reinforce the hypothesis that soluble tau species, rather than fibrillar aggregates, are most relevant for neural system dysfunction in the rTg4510 model of tauopathy.
MATERIALS AND METHODS
Animals and Enrichment
rTg(tauP301L)4510 mice and littermates expressing the activator CK-tTA transgene but not the tau responder transgene (non tau-expressing controls) were group housed on a 12-hour light/dark cycle with access to food and water ad libitum. Those treated to suppress the tau transgene (n = 10 wild-type, 11 transgenic) received chow containing 200 mg/kg doxycycline (Harlan Teklad, South Easton, MA) for 6 weeks prior to enrichment experiments and the remainder received standard chow. Treatment groups are outlined in the Table.
Table.
Subjects and Treatment Groups
| Genotype | Enrichment | Doxycycline treatment | Number of mice |
|---|---|---|---|
| wild-type | enriched | 6 weeks dox | 4 |
| wild-type | enriched | none | 4 |
| wild-type | non-enriched | 6 weeks dox | 7 |
| wild-type | non-enriched | none | 4 |
| rTg4510 | enriched | 6 weeks dox | 6 |
| rTg4510 | enriched | none | 5 |
| rTg4510 | non-enriched | 6 weeks dox | 4 |
| rTg4510 | non-enriched | none | 4 |
dox = doxycline
For the enriched groups, 3 novel objects (colored dome, cardboard tube, and chewable cardboard box) were placed in the animals’ home cages for 25 minutes, as previously described (8, 9). Enriching components were then removed and after a 25-minute rest period, animals were sacrificed by CO2. Non-enriched animals remained in the home cage for 25 minutes and were killed. Brains were rapidly removed and the left hemisphere was dropped into a disposable cryomold containing M1 embedding matrix (Thermo Electron Corporation, Pittsburgh, PA), frozen over an ethanol/dry ice bath, and stored at −80°C. Animal work was approved by institutional committees and conformed to NIH guidelines.
In Situ Hybridization
Ten-μm-thick sagittal sections were prepared using a Thermo Shandon SME Cryotome (Pittsburgh, PA). A series of every 50th section from each animal was collected throughout the entire hemisphere to sample every 500 μm. Slides were stored at -80°C until further processing. Fluorescent in situ hybridization with anti-phosphorylated tau antibody (PHF-1, courtesy of Peter Davies) co-immunohistochemistry was performed, as described in (10). Arc anti-sense probe template was derived by RT-PCR from mouse brain mRNA (Genbank ID: NM018790, nt 262..1338). Briefly, sections were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 minutes, treated with 3% H2O2 in PBS for 30 minutes, and acetylated for 10 minutes (5.3 mL triethanolamine, 0.7 mL glacial HCl, 1 mL acetic anhydride in 400 mL final volume), with 3 PBS washes between each step. Slides were then covered in hybridization solution (50% formamide, 5× SSC, 1× Denhardt’s solution, 100 μg/mL yeast RNA, 100 μg/mL salmon sperm DNA) for 1 hour at room temperature and incubated in hybridization solution with a 1:1000 dilution of digoxigenin-labeled Arc riboprobe overnight at 68°C. Slides were washed twice in 0.2× SSC at 68°C (45 minutes/wash), covered for 1 hour with 10% goat serum in Tris-buffered saline (TBS) at room temperature, and incubated overnight at 4°C with anti-digoxigenin, peroxidase-conjugated, Fab fragments (Roche Applied Science, Indianapolis, IN) and PHF-1 (1:200). Slides were then washed in TBS, goat anti-mouse Cy3 IgG (Jackson Immuno, West Grove, PA) was applied for 1 h followed by TBS washes. Sections were incubated for 5 to 10 minutes using a kit containing tyramide-alexa-488 with HRP-streptavidin (Invitrogen, Carlsbad, CA), washed in TBS, and coverslipped with Vectashield mounting medium with DAPI (Vector Labs, Burlingame, CA).
Imaging and Image Analysis
Images were collected and analyzed by observers blind to treatment and genotype. Sections were imaged on a Zeiss LSM 510 META confocal system. Collection parameters (laser power and detector gain) remained the same for all imaging sessions. Three images were taken at equal intervals through the pyramidal cell layers of CA1-3 of hippocampus starting at a random location in CA1 on each sagittal section (cut at 500 μm intervals), resulting in 20 to 25 images per animal sampled in a systematic random fashion throughout the area of interest. Cell counts were performed in Adobe Photoshop CS3. Images were separated into red, green, and blue color channels to count cells PHF-1-positive cells (tangles), Arc mRNA, and DAPI (nuclei) respectively. Cells were marked as Arc-positive, PHF-1-positive, or both. Analysis by 2 blinded observers found less than 5% inter-observer variability.
Stereology
CA1-3 neuron numbers were estimated as described previously (2), using the optical dissector method on an Olympus upright BX51 microscope with a motorized stage (Prior Scientific, Rockland, MA) and the CAST stereology program (CAST, Olympus, Denmark). The region was outlined on every 50th section of the 10-μm cryostat sections through the entire hippocampus and neurons were counted in 21.8×21.8×10 μm dissectors in a grid with a step length of 150 μm. Neuron density was calculated and total neuron number estimated by multiplying the neuron density by total volume of the CA1-3 obtained by the Cavalieri method.
Statistical Analysis
Normality of data was assessed using a Shapiro-Wilk test. The percentage of neurons with Arc mRNA in the cytoplasm was calculated by dividing the number of Arc mRNA-positive cells by the number of DAPI-positive neurons in the same field. Means of groups were compared by post-hoc ANOVA with the percentage Arc-positive cells as dependent variable and depending on the comparison either enrichment status, genotype, or doxycycline treatment as independent variable split by the other 2 factors. To assess the effect of tau inclusions on the ability of neurons to upregulate Arc mRNA, we calculated the percentage of non-tangle-bearing neurons (PHF1-negative) and tangle-bearing neurons (PHF1-positive) with Arc mRNA activation and compared means using post-hoc ANOVAs with either enrichment or doxycycline treatment as independent variables split by the other factor. To compare the percentage of tangle-bearing vs. non-tangle-bearing neurons expressing Arc mRNA, within animal ratios were also calculated of the % non-tangle-bearing neurons with cytoplasmic Arc : % tangle-bearing neurons expressing Arc. For each group of rTg4510 mice, the mean of these ratios for all animals in the group was compared to the null hypothesis of mean = 1 (meaning tangle and non-tangle-bearing neurons are equally likely to express Arc), using a z-test and the actual standard deviations. Neuron numbers in the CA1-3 were compared with ANOVA using genotype and doxycycline treatment as independent variables.
RESULTS
Tg4510 Show Impaired Arc Response to Environmental Enrichment
Previous studies have demonstrated that exposure to a novel environment results in a brief period of Arc transcription in hippocampal neurons that reflects electrophysiological responsiveness (11, 12), and so marks neurons that are integrated into neural systems receiving complex environmental stimuli (13). The rTg4510 mouse develops tau deposition and substantial neuronal loss throughout forebrain structures, notably the hippocampus including the large pyramidal neurons in areas CA1-CA3 (2). To determine whether hippocampal Arc transcription is impaired in this model, we exposed 11.5-month-old transgenic animals (which have tangle pathology and neuronal loss [2]) and non-tau expressing controls to an enriched environment. The Arc response was recorded as the percentage of DAPI-stained nuclei surrounded by cytoplasmic Arc staining, as detected using fluorescent in situ hybridization techniques (Fig. 1).
Figure 1.
rTg4510 mice have an impaired Arc response to environmental enrichment that is rescued by transgene suppression. Micrographs of fluorescent in situ hybridization show Arc mRNA (green), PHF1 immunostaining (red) and DAPI nuclear stain (blue). There is a robust response to enrichment in non-transgenic (wild-type) mice (top), and less response both at baseline and in response to enrichment in rTg4510 mice. This can be rescued with transgene suppression (6 weeks doxycycline). Scale bar = 50 μm.
At baseline, as expected, only a small percentage of neurons express detectable Arc mRNA. Environmental enrichment in wild-type animals caused an increase in the percentage of cells expressing Arc mRNA, from 3.3% at baseline to 17.6% after enrichment (Fig. 2, F[1,6]=40.95, p = 0.0007). The 11.5-month-old rTg4510 mice had markedly diminished Arc expression both at baseline (0.5% of cells) and after enrichment (3.9%). As this nearly 8-fold increase in expression levels in enriched transgenics reaches statistical significance (F[1,7]=7.41, p = 0.03), some response to environmental enrichment is possible, but the tau overexpression is associated with significantly and dramatically decreased Arc responses in comparison to those in wild-type animals, both at baseline (F[1,6]=7.78, p=0.032) and in response to a novel experience (F[1,7]=40.65, p = 0.0004). Stereological estimates of neuron numbers in the CA region, in which we observed Arc activation, revealed that rTg4510 mice had significant neuronal loss of 56% compared to wild-type controls (Fig. 2, p < 0.0001). This loss of neurons in the circuit must contribute to the loss of functional response to environmental stimulation. The presence of pathologically mislocalized, hyperphosphorylated, and/or aggregated tau could also contribute to dysfunction.
Figure 2.

Quantification of impaired Arc response and neuronal loss in rTg4510 mice. (A) Enriched wild-type mice exhibit a significant increase in the percentage of cells expressing Arc compared to non-enriched wild-type mice. At baseline, Tg4510 mice show significantly lower Arc levels than wild-type mice. Enriched transgenic animals have more Arc expression with environmental enrichment, but this response is impaired in comparison to that in wild-type animals. Right, transgene suppression with doxycycline results in increased percentages of Arc-positive cells in rTg4510 brain compared to untreated transgenics, restoring enrichment-induced Arc mRNA levels to that of wild-type controls. Doxycycline does not have a significant effect on baseline or enriched Arc levels in wild-types. (B) rTg4510 mice have significant neuronal loss in the CA region of the hippocampus at 11.5 months of age, which is ameliorated by 6 weeks of transgene suppression with doxycycline. In contrast, doxycycline treatment of wild-type animals caused a small amount of neuronal loss (16% compared to wild-type untreated mice). * p < 0.05 post-hoc ANOVA comparisons; N.S. = not significant.
Transgene Suppression with Doxycycline Rescues Arc Response in Tg4510
Expression of P301L human tau in rTg4510 mice is driven by a responder transgene consisting of a tetracycline-operon responsive element that is suppressible with doxycycline. Even at very late stages (when many tau-expression neurons have died), 6 weeks of transgene suppression reduced both tau mRNA and sarkosyl-soluble tau levels by 3-fold (4). Brief treatment, begun even after considerable tau deposition in this model, is also sufficient to improve performance in a spatial memory task (4). To determine whether suppression of the tau transgene could improve the Arc response to enrichment, we administered doxycycline feed for 6 weeks to transgenic animals and performed enrichment experiments.
Enriched, doxycycline-treated rTg4510 mice showed a significant Arc response compared to non-enriched doxycycline-treated rTg4510 mice (from 2.0% to 13.1% of neurons responding, F[1,8]=17.27, p=0.003, Fig. 2). This is higher than that achieved with enrichment in non-doxycycline-treated animals (F[1,9]=14.49, p = 0.004 comparing enriched doxycycline-treated rTg4510 to enriched non-doxycycline-treated rTg4510). Baseline Arc levels (non-enriched) were not significantly higher in doxycycline-treated rTg4510 animals than without transgene suppression. (F[1,6]=2.11, p = 0.20). Transgene suppression restored both baseline and enrichment-induced Arc expression levels in Tg4510 to levels not statistically significantly different from that of wild-type controls. The percentage of neurons containing tangles was unchanged after 6 weeks of doxycycline treatment (p > 0.05 t-test).
Doxycycline had no effect on non-tau expressing mice (Fig. 2). With doxycycline treatment, environmental enrichment still caused a significant several-fold increase in Arc expression compared to baseline (F[1,9]=80.68, p < 0.0001). In both doxycycline-treated and untreated non-tau expressing mice, Arc expression increased to a similar extent in enriched vs. non-enriched groups.
Stereological estimates of neuron numbers in the CA region showed that transgene suppression for 6 weeks partially ameliorated the 56% neuronal loss observed without suppression resulting in only 30% decrease from wild-type untreated levels, which did not reach statistical significance (p = 0.098). These data show that suppression of the tau transgene from 10 to 11.5 months of age rescues the ability of hippocampal neural systems in rTg4510 mice to respond to environmental stimuli and prevents further neuronal loss without removing neurofibrillary pathology (2, 4). Thus, removal of soluble tau and prevention of further neuronal loss were sufficient to restore function.
Tangle-Bearing Neurons Respond to Environmental Enrichment
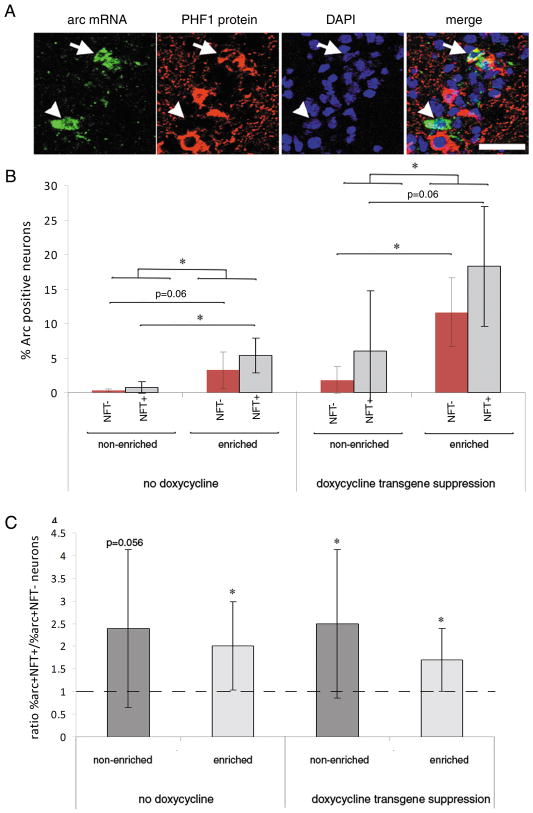
To address whether the presence or absence of a tangle affects the likelihood that a CA neuron will have an Arc response both at baseline and in response to enrichment, we examined the percentage of Arc-positive cells in tangle-bearing vs. non-tangle-bearing neurons. Tangle-bearing neurons were defined by staining with PHF1 antibody, which recognizes tau phosphoepitope Ser396,404 (14, 15). In human brains, PHF1 detects a wide range of neurofibrillary pathology including pre-tangles (16); however, in the rTg4510 model at these ages, PHF1 staining is always present in Thioflavin-S-positive and silver positive tangles (2). We also confirmed in these animals that all PHF1-positive neurons are Thioflavin-S-positive, β-sheet containing tangles (Figure, Supplemental Digital Content 1, http://links.lww.com/NEN/A245) (2). Thus, PHF1-positive neurons are referred to as tangle-bearing neurons. We were also interested in the effect of transgene suppression on the likelihood of a tangle-positive vs. tangle-negative neuron to be Arc-positive after enrichment. During image analysis, the number of Arc-positive neurons and the number of tangles in the area of interest were recorded, and cells expressing Arc were also recorded as either tangle-positive or tangle negative (Fig. 3A). Both tangle-bearing and non-tangle-bearing neurons were Arc-positive Group means show that without transgene suppression, enrichment increased the percentage of NFT-bearing neurons that express Arc mRNA (Fig. 3B, F[1,7]=12.30, p = 0.010) and tended to increase the percentage of non tangle-bearing cells with Arc mRNA (F[1,7]=4.93, p = 0.062). Suppression of the transgene resulted in a dramatic increase in the likelihood that tangle-negative neurons responded to environmental enrichment by expressing Arc (F[1,8]=13.51, p = 0.0063), and a trend toward an increase in tangle-positive neurons expressing Arc mRNA (F[1,8]=4.75, p = 0.06). Interestingly, when examined within animals, tangle-bearing neurons were significantly more likely to be Arc-positive than non-tangle-bearing neurons, with an average of 2-fold higher percentage of tangle-bearing neurons expressing Arc than non-tangle-bearing neurons (Fig. 3C, p < 0.05 z-test mean significantly greater than 1 for all groups except non-enriched, non-doxycycline-treated animals, which had a trend p = 0.060). Neither enrichment nor transgene suppression with doxycycline significantly altered the likelihood of NFT vs. non-NFT cells to express Arc. We interpret these data to indicate that tangle-bearing neurons can remain important in functional circuits.
Figure 3.
(A) In situ hybridization for Arc mRNA (green) with PHF1 co-immunostain for tau tangles (red) and DAPI nuclear counterstain (blue) show that Arc mRNA can be readily detected in both tangle (arrow) and non-tangle-bearing (arrowhead) neurons. Scale bar = 50 μm. (B) Quantification of group means shows that both tangle-bearing and non-tangle-bearing neurons can express Arc mRNA and that enrichment increases expression in both types of neurons. *p < 0.05 post-hoc ANOVA comparisons. (C) Within animal ratios of the percentage tangle-bearing to non-tangle-bearing neurons shows that independent of doxycycline treatment or enrichment, tangle-bearing neurons are more likely to express Arc than non tangle-bearing neurons. *p < 0.05 z-test mean is greater than 1 as would be expected with no effect of tangles on Arc response.
DISCUSSION
Arc is a versatile tool in the study of memory-related pathological changes because its expression can be induced by a range of behavioral, pharmacological, and pathological stimuli. We used environmental enrichment to induce Arc expression in the hippocampus of Tg4510 and wild-type mice because it leads to a robust increase in expression and provides a read-out of neural system integrity in neurons on a cell-by-cell basis. To our knowledge this is the first time individual neurons in an intact neural system have been interrogated for function. No other approach other than slice electrophysiology has been attempted to address the fundamental question of whether tangle-bearing neurons are functionally active, integrated into a neural circuits, or indeed essentially dead, as many have assumed. Interpretation of electrophysiology in slice preps is limited because responses to a physiologically relevant stimulus such as a novel environment cannot be done, and in vivo electrophysiology (which has not yet been published for these animals) can only interrogate a very limited number of neurons in an anesthetized animal, again limiting its use for investigating physiologically relevant stimuli. We used a method that combines environmental stimulation of mice with postmortem assessment of immediate-early gene activation in tangle and non-tangle-bearing neurons that allowed us to address explicitly the question of the responses of many individual cells to environmental stimuli.
The enhanced Arc response after enrichment that we observed in wild-type mice is consistent with previous studies showing hippocampal Arc upregulation following environmental enrichment (13, 17). Arc transcription upon exposure to a novel environment is detectable within 5 to 10 minutes as distinct nuclear foci and within ~30 minutes spreads to the cytoplasm. This staining profile has been exploited using the catFISH technique to determine that distinct populations of neurons are activated by exposure to different environments (17), and that integration of CA1 and CA3 ensembles may help to refine the neural representation of a behavioral context (18).
Observations that Arc dysregulation is directly linked to memory impairments are also relevant. Intra-hippocampal infusion of Arc antisense RNA results in impaired consolidation of long-term potentiation and long-term spatial memory (19), and inhibitory avoidance memory (20); Arc knockout mice fail to retain memory in a number of tasks, including spatial learning, fear conditioning, object recognition, and taste aversion (21).
Here, we first tested the hypothesis that rTg4510 mice have impairments in hippocampal circuitry that result in a diminished immediate-early gene response. rTg4510 mice have lower Arc levels at baseline and in response to novel environmental stimuli compared to wild-type mice: only 4% of neurons respond to enrichment in rTg4510 hippocampus whereas 18% of non-tau expressing control neurons respond. This could be due to the pathological neuronal loss observed in this model, to loss of synapses, to impairment of cellular processes such as axon transport due to soluble or fibrillar tau in the cells, or a combination of these factors. The observation that P301L tau overexpression leads to diminished Arc expression is consistent with our previous observations that rTg4510 animals have impaired memory, and with an established role for Arc as essential for memory consolidation.
rTg4510 mice have a regulatable promoter system allowing the P301L tau transgene to be suppressed with doxycycline. Here, we demonstrate that despite heavy tau deposition, brief suppression of the transgene caused the level of Arc activation in response to enrichment to return to that of wild-type animals. Doxycycline treatment in Tg4510 mice previously revealed that tau pathology progresses independent of transgenic tau expression beginning at 4 months of age; 6 weeks of transgene suppression at an advanced age is sufficient to improve or stabilize performance in the Morris water maze task. Although sarkosyl-insoluble (64 kDa) tau continues to accumulate, transgene suppression reduces levels of a soluble 55-kDa species (4). Doxycycline treatment causes recovery of Arc expression despite the persistence of neurofibrillary pathology both at baseline and in response to environmental stimuli, suggesting that the increase in Arc activation may be attributable to decreased levels of soluble tau.
While limited direct evidence exists that soluble tau has specific damaging effects on neurons, the detrimental effects of oligomeric β-amyloid (Aβ) have been widely studied (22). There is evidence that soluble Aβ can cause dysregulation of Arc; in cultured neurons, brain-derived neurotrophic factor-induced Arc expression is impaired by application of low levels of Aβ peptide (23); and sustained Arc upregulation has been observed in primary neurons in response to Aβ oligomers, leading to spine morphology changes (24).
We tested the hypothesis that tangle-bearing neurons have an impaired ability to respond to environmental stimuli and found that this was not the case. Although there is evidence that increasing numbers of NFTs correlate with neuronal death and cognitive decline, and laser capture studies have shown differences in gene expression in NFT-bearing neurons (1, 25), there is debate as to whether NFTs are protective or destructive pathological features (26, 27). There is, in fact, limited direct evidence that NFT themselves are detrimental to individual neurons. A study of cortical sections prepared from Tg4510 mice demonstrated that neurons in transgenic animals showed reductions in morphological complexity and altered electrophysiological properties compared with neurons from wild-type mice, but found no significant differences between NFT-bearing and non-NFT-bearing neurons within transgenic brains (28). With regards to Arc upregulation, NFT-bearing neurons respond to environmental enrichment to a greater extent than non-NFT- bearing neurons. Several possibilities could account for this unexpected result: we postulate that tangles may sequester pathogenic soluble tau, allowing better functionality than in non-NFT-bearing neurons. Alternatively, neurons that are more functionally active and well-connected in neural circuits may be more likely to form a tangle, and these remain the important cells in functional circuits after NFTs form. It is also possible that tangle-bearing cells could be expressing more Arc as a compensatory reaction to insure that sufficient downstream components are produced, or that tangles disrupt the excitatory/inhibitory balance in neurons, making them more likely to respond. Finally, increased responsiveness could be a lack of tuning or selectivity deficit induced by NFTs. In any case, our data indicate that NFTs do not preclude a neuron from responding in a functional circuit. These results suggest that it might be possible to restore function in neurons containing neurofibrillary lesions in Alzheimer disease despite the presence of those lesions. It is worth noting that tangles in this mouse model contain only 4R tau, which has different amyloidogenic properties from the mixed 3R/4R tau tangles in Alzheimer disease (29, 30). However, we have confirmed by electron microscopy that the tangles in the transgenic mice are filamentous; thus, their contributions to function are probably similar.
In summary, we confirm that the rTg4510 mouse model of tauopathy has impaired function of hippocampal circuitry using a technique that also allows us to interrogate individual neuronal responses to complex behavioral stimuli. We find that impaired hippocampal function can be rescued with suppression of soluble tau expression without removing neurofibrillary pathology. Finally, we demonstrate that neurons with NFTs are able to respond as well or more strongly than neighboring neurons to environmental stimulation.
Supplementary Material
Acknowledgments
This work was supported by an Alzheimer’s Association Zenith Award, NIH grants AG08487, K99AG33670, K08 NS069811, and AG026249.
References
- 1.Callahan LM, Vaules WA, Coleman PD. Quantitative decrease in synaptophysin message expression and increase in cathepsin D message expression in Alzheimer disease neurons containing neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:275–87. doi: 10.1097/00005072-199903000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Spires TL, Orne JD, SantaCruz K, et al. Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. Am J Pathol. 2006;168:1598–607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsden M, Kotilinek L, Forster C, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–47. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–81. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramham CR, Alme MN, Bittins M, et al. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–40. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramham CR, Worley PF, Moore MJ, et al. The immediate early gene arc/arg3.1: regulation, mechanisms, and function. J Neurosci. 2008;28:11760–7. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grinevich V, Kolleker A, Eliava M, et al. Fluorescent Arc/Arg3.1 indicator mice: a versatile tool to study brain activity changes in vitro and in vivo. J Neurosci Methods. 2009;184:25–36. doi: 10.1016/j.jneumeth.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer-Luehmann M, Mielke M, Spires-Jones TL, et al. A reporter of local dendritic translocation shows plaque- related loss of neural system function in APP-transgenic mice. J Neurosci. 2009;29:12636–40. doi: 10.1523/JNEUROSCI.1948-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spires TL, Grote HE, Varshney NK, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–6. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price SR, De Marco Garcia NV, et al. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–16. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 11.Miyashita T, Kubik S, Haghighi N, et al. Rapid activation of plasticity-associated gene transcription in hippocampal neurons provides a mechanism for encoding of one-trial experience. J Neurosci. 2009;29:898–906. doi: 10.1523/JNEUROSCI.4588-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzowski JF, Miyashita T, Chawla MK, et al. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–82. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazdarjanova A, McNaughton BL, Barnes CA, et al. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–71. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otvos L, Jr, Feiner L, Lang E, et al. Monoclonal antibody PHF-1 recognizes tau protein phosphorylated at serine residues 396 and 404. J Neurosci Res. 1994;39:669–73. doi: 10.1002/jnr.490390607. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg SG, Davies P, Schein JD, et al. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992;267:564–9. [PubMed] [Google Scholar]
- 16.Augustinack JC, Schneider A, Mandelkow EM, et al. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol. 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 17.Guzowski JF, McNaughton BL, Barnes CA, et al. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 18.Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–96. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzowski JF, Lyford GL, Stevenson GD, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntyre CK, Miyashita T, Setlow B, et al. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–23. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plath N, Ohana O, Dammermann B, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–44. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–84. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang DC, Chen SS, Lee YC, et al. Amyloid-beta at sublethal level impairs BDNF-induced arc expression in cortical neurons. Neurosci Lett. 2006;398:78–82. doi: 10.1016/j.neulet.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 24.Lacor PN, Buniel MC, Chang L, et al. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callahan LM, Vaules WA, Coleman PD. Progressive reduction of synaptophysin message in single neurons in Alzheimer disease. J Neuropathol Exp Neurol. 2002;61:384–95. doi: 10.1093/jnen/61.5.384. [DOI] [PubMed] [Google Scholar]
- 26.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–4. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Congdon EE, Duff KE. Is tau aggregation toxic or protective? J Alzheimers Dis. 2008;14:453–7. doi: 10.3233/jad-2008-14415. [DOI] [PubMed] [Google Scholar]
- 28.Rocher AB, Crimins JL, Amatrudo JM, et al. Structural and functional changes in tau mutant mice neurons are not linked to the presence of NFTs. Exp Neurol. 2010;223:385–93. doi: 10.1016/j.expneurol.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchihara T, Nakamura A, Yamazaki M, et al. Tau-positive neurons in corticobasal degeneration and Alzheimer’s disease--distinction by thiazin red and silver impregnations. Acta Neuropathol. 2000;100:385–9. doi: 10.1007/s004010000186. [DOI] [PubMed] [Google Scholar]
- 30.Uchihara T, Shibuya K, Nakamura A, et al. Silver stains distinguish tau-positive structures in corticobasal degeneration/progressive supranuclear palsy and in Alzheimer’s disease--comparison between Gallyas and Campbell-Switzer methods. Acta Neuropathol. 2005;109:299–305. doi: 10.1007/s00401-004-0947-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.