Abstract
Background
Chemotherapy-induced nausea and vomiting (CINV) is a debilitating side effect. Previous studies have primarily focused on prophylactic therapy, but no published reports have evaluated the treatment of breakthrough CINV.
Methods
A prospective, pilot study was performed to provide preliminary prospective evidence of the efficacy of individual agents prescribed for the treatment of breakthrough CINV. Enrolled patients were receiving moderately or highly emetogenic chemotherapy and prophylactic treatment of CINV based on antiemetic guidelines. Patients were prescribed an antiemetic for breakthrough CINV at the discretion of their treating oncologist. If patients had breakthrough CINV that required a breakthrough antiemetic medication, they were instructed to complete a questionnaire every 30 minutes for 4 hours after taking the antiemetic. Levels of nausea (0–10), vomiting, and side effects were recorded.
Results
Of the 96 patients enrolled, 27 (28%) reported breakthrough nausea and/or vomiting requiring medication and completed the questionniare. Eighty-eight percent (n = 24) reported the use of prochlorperazine; they experienced a 75% median nausea reduction after 4 hours, with minimal side effects. Three patients (12%) reported the use of a 5-hydroxytryptophan (5-HT) receptor antagonist for treatment of breakthrough nausea. These patients reported a median nausea reduction of 75% after 4 hours and no perceived toxicities.
Conclusions
Prochlorperazine and 5-HT receptor antagonists appear to be effective breakthrough antiemetic therapies. The described study methodology can be used to conduct randomized clinical trials to find more effective drugs for treating established nausea.
Introduction
Chemotherapy-induced nausea and vomiting (CINV) has been commonly cited by patients as being among the “most unpleasant and distressing” side effects associated with chemotherapy.1 It can lead to dehydration, anorexia, weight loss, and fluid/electrolyte disturbances, and it can result in dose reductions, treatment delays, or discontinuation of treatment, threatening the potential for a good outcome. It has also been associated with higher overall costs of cancer care and significant deterioration in quality of life.2–4
With the use of prophylactic regimens, including such things as 5-HT receptor antagonists, dexamethasone, and aprepitant, the incidence of acute vomiting with highly emetogenic chemotherapy has decreased, from nearly 90% down to 30% to 40%.5,6 In moderately emetogenic chemotherapy, the incidence of acute vomiting has correspondingly decreased, from over 70% down to 15% to 30%. So, although the incidence of breakthrough CINV has decreased, a relatively large proportion of patients still experience breakthrough CINV, and there is a paucity of trials assessing how to best treat it.3,4,7,8
The American Society of Clinical Oncology (ASCO) and National Cancer Care Network (NCCN) guidelines recommend that, for breakthrough CINV, physicians should consider adding an additional agent such as lorazepam or prochlorperazine, or substituting high-dose metoclopramide for the utilized 5-HT antagonist.6,9 However, these guidelines do not provide any specifics regarding whether these agents should be used sequentially or in combination, or regarding dosing, timing, and duration of these therapies. Most of these recommendations actually appear to be for the prophylactic use of antiemetics for subsequent cycles of chemotherapy, as opposed to treating the breakthrough nausea and vomiting that occurs after chemotherapy has been given.
A relatively recent editorial10 reports that agents used for prevention of nausea and vomiting are not helpful for the treatment of nausea and vomiting. Echoing this sentiment, the Physician Data Query (PDQ) for supportive care section on chemotherapy-induced nausea and vomiting states that there is no known effective therapy for treatment of nausea and vomiting that occurs after chemotherapy.11 An antiemetic chapter in Cancer Medicine, 8th edition also notes that there is no established treatment for breakthrough nausea and vomiting that occurs after chemotherapy.12 Thus, this is an important unexplored area of clinical care.
The current exploratory observation study was designed to record the response to antiemetic therapy that had been taken for breakthrough chemotherapy-induced nausea and vomiting. This study was also designed to test a potential methodology that might be used to evaluate the treatment of breakthrough nausea and vomiting in future trials.
Methods
Eligibility requirements included patients older than 18, scheduled to receive highly or moderately emetogenic chemotherapy, who received prophylactic treatment for CINV. The primary oncologist prescribed breakthrough antiemetic medication according to his or her usual practice. Participants were approached for the current trial when they were in the chemotherapy unit, after their chemotherapy and antiemetic drugs had been prescribed. They were asked to complete a nausea and vomiting questionnaire if they developed nausea and/or vomiting requiring antiemetic rescue medication at any time during the first 3 days after getting their chemotherapy. The questionnaire required participants to record information at baseline (when the rescue antiemetic medication was taken) and every half hour for 4 hours. The data gathered included a nausea score (scale, 0–10), the number of episodes of vomiting, and side effects, such as agitation, drowsiness (scale, 0–10), and headache.
The study was reviewed and approved by the Mayo Clinic Internal Review Board. Signed consent was obtained from all patients who enrolled into the study.
Descriptive statistics including mean, median, and frequency (percentage) were used to summarize and evaluate the data collected. Due to the sample size and exploratory nature, the results are presented in a hypothesis-generating fashion.
Results
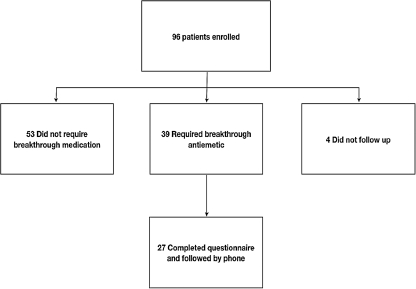
Ninety-six patients were enrolled in this trial from April to October of 2008. Baseline characteristics are shown in Table 1. Thirty-nine patients reported nausea and/or vomiting requiring breakthrough medication (41%). Twenty-seven of these patients completed the 4-hour questionnaire and are included in the final analytical cohort (Fig. 1).
Table 1.
Patient Characteristics
| Total (n = 96) | Completed questionnaire (n = 27) | |
|---|---|---|
| Sex | ||
| Female | 53 (55%) | 20 (74%) |
| Male | 43 (45%) | 7 (26%) |
| Race/Ethnicity | ||
| White, non-Hispanic | 91 (95%) | 26 (96%) |
| Hispanic | 2 (2%) | 1 (4%) |
| Black | 1 (1%) | 0 (0%) |
| Unknown | 2 (2%) | 0 (0%) |
| Age | ||
| Median | 58 | 57 |
| Range | (30–86) | (30–72) |
| Type of cancer | ||
| Breast | 7 (7%) | 3 (11%) |
| Gastrointestinal | 25 (26%) | 7 (26%) |
| Genitourinary | 4 (4%) | 1 (4%) |
| Skin | 4 (4%) | 1 (4%) |
| Lung | 12 (13%) | 2 (7%) |
| Gynecologic | 22 (23%) | 7 (26%) |
| Head and Neck | 4 (4%) | 0 (0%) |
| Neuroendocrine | 1 (1%) | 0 (0%) |
| Sarcoma | 3 (3%) | 0 (0%) |
| Hematologic | 14 (15%) | 2 (7%) |
| Degree of emetogenicity | ||
| High | 25 (26%) | 5 (19%) |
| Moderate | 71 (74%) | 22 (81%) |
| Prophylactic regimen | ||
| Dexamethasone | 88 (92%) | 25 (93%) |
| Granisetron | 75 (78%) | 20 (74%) |
| Palonosetron | 21 (22%) | 7 (26%) |
| Aprepitant | 5 (5%) | 1 (4%) |
FIG. 1.
Consort diagram.
Twenty-four of the 27 patients (88%) took oral prochlorperazine (10 mg), whereas 3 (12%) took a 5-HT receptor antagonist (granisetron 1 mg orally, odansetron 8 mg intravenously, and odansetron 8 mg sublingually) for breakthrough CINV.
Efficacy
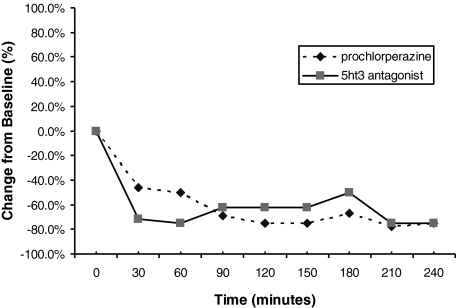
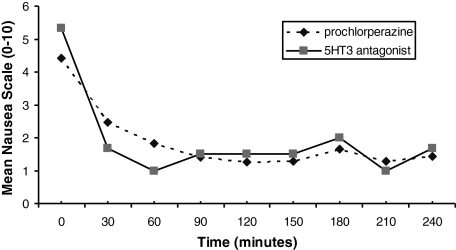
In patients receiving prochlorperazine, there was a median reduction of 75% (interquartile range 45%) in nausea at the end of 240 minutes from baseline (Fig. 2). Twenty-one percent reported vomiting at baseline but only 4% during the 4-hour evaluation period of the study. Fig. 3 illustrates the data, in terms of changes in mean nausea scores over the 4-hour study time period, whereas Table 2 provides individual patient data. The majority (96%) of these patients noted that they would recommend this medication to other patients. At study completion, patients rated their median satisfaction as 8, on a scale of 0 to 10, with 10 being extremely satisfied.
FIG. 2.
Median nausea, presented as the percent of baseline.
FIG. 3.
Mean nausea over time, on scale of 0 to 10.
Table 2.
Nausea Scales per Patient Taking Prochlorperazine or a 5-HT Receptor Antagonist, Over Time
| |
Time intervals |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | 0 hours | 0.5 hours | 1 hour | 1.5 hours | 2 hours | 2.5 hours | 3 hours | 3.5 hours | 4 hours |
| Prochloperazine | |||||||||
| 1 | 3 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 |
| 2 | 3 | 3 | 3 | 2 | 1 | 0 | 0 | 0 | 0 |
| 3 | 4 | 4 | 3 | 3 | 3 | 2 | 2 | 2 | 2 |
| 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 2 |
| 6 | 8 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7 | 4 | 2 | 2 | 2 | 1 | 2 | 1 | 1 | |
| 8 | 5 | 6 | 4 | 3 | 3 | 2 | 3 | 2 | 3 |
| 9 | 6 | 5 | 4 | 5 | 5 | 5 | 4 | 4 | 4 |
| 10 | 5 | 4 | 2 | 1 | 1 | 0 | 0 | 1 | 0 |
| 11 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 12 | 7 | 4 | 2 | 2 | 2 | 7 | 4 | ||
| 13 | 10 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 14 | 5 | 2 | 1 | 0 | 0 | 0 | 9 | 5 | 2 |
| 15 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 17 | 3 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| 18 | 5 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | |
| 19 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 4 | 3 | 3 | 3 | 3 | 3 | 2 | 1 | 1 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 10 |
| 22 | 4 | 3 | 3 | 1 | 1 | 0 | 1 | 1 | 0 |
| 23 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 4 | 3 | 1 | 1 | 1 | 1 | 2 | 3 | 3 |
| 5-HT3 receptor antagonist | |||||||||
| 25 | 4 | 3 | 2 | 3 | 3 | 3 | 4 | 2 | 1 |
| 26 | 7 | 2 | 3 | 4 | |||||
| 27 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Blank sites represent times that patients did not report data.
Three patients completed the questionnaire after taking a 5-HT antagonist, revealing a 75% median reduction in nausea from baseline (Fig. 2). Fig. 3 illustrates the data, in terms of changes in mean nausea scores over the 4-hour study time period, whereas Table 2 provides individual patient data. One patient had vomiting at baseline, but none reported vomiting during the 4 hours after taking the 5-HT antagonist. At study completion, these 3 patients rated their satisfactions as 0, 4, and 8 on a scale of 0 to 10 (with 0 being “not at all satisfied” and 10 being “very satisfied”). However, the patient who rated satisfaction as 0 had complete resolution of nausea by 30 minutes, and the patient also stated that she would recommend the medications to others. Therefore, it is likely that the patient mistakenly marked this as 0 and probably actually meant 10.
Toxicity
Patients who received prochlorperazine reported a median drowsiness, at baseline, of 3, which was reduced to 2 after 4 hours. Twenty percent of patients taking prochlorperazine had a headache at baseline, which was reduced to 0% after 4 hours. Twenty percent also reported agitation at baseline, which was reduced to 4% at the conclusion. Complaints of abdominal cramping (2 patients), dry mouth (1), tachycardia (1), and blurry vision (1) were also recorded.
One patient receiving a 5-HT receptor antagonist reported a baseline drowsiness of 5; this remained unchanged after 4 hours. One patient, without drowsiness at baseline, reported drowsiness of 1 after taking the medication. No patients receiving a 5-HT receptor antagonist reported headache, agitation, or other toxicities.
Discussion
The majority of the patients enrolled in this pilot study used prochlorperazine for breakthrough nausea and vomiting, because prochlorperazine was the most commonly prescribed drug for breakthrough antiemesis in this clinic. This current pilot trial supports the use of prochlorperazine for breakthrough CINV. Although one might claim that this is not news, as prochlorperazine has been used for a long time as a rescue medication, we are unaware of any previous studies that have studied this issue.
Results were achieved with minimal perceived side effects. In fact, contrary to what was expected, drowsiness improved in patients taking prochlorperazine. Ideally, the efficacy and toxicity of prochlorperazine as a breakthrough antiemetic therapy should be more clearly delineated in a placebo-controlled, double-blinded clinical trial.
It is logical that prochlorperazine might be a good agent to use, when patients develop nausea/vomiting after receiving antiemetic regimens that focus on 5-HT receptor antagonism and blocking of the NK-1 receptor, as prochlorperazine acts on a different pathway, by blocking postsynaptic mesolimbic dopaminergic D1 and D2 receptors. If a patient fails prophylaxis, it makes intuitive sense to try to influence alternate pathways, such as antidopaminergic, to achieve treatment goals, rather than utilizing more of the mechanism that has already failed.
Having stated this, the three participants who took a 5-HT antagonist for breakthrough also showed a 75% reduction in nausea from baseline after 4 hours.
Another interesting observation is the rapidity of activity of treatment. Patients receiving both types of medications experienced marked symptom control in as little as 30 minutes. This may be due to medication effect. However, it may also represent the natural course of nausea and vomiting. The natural course of breakthrough nausea and vomiting has not been well described. This could be more clearly defined with a placebo arm in future trials.
Limitations of this study include that it is an observational pilot study. The treatment arms were not compared with a placebo arm, and relief may have occurred if no medication was given. Randomized trials evaluating efficacy of frequently prescribed antiemetics would provide needed information on the best agent for this troubling symptom.
Noting that prochlorperazine is an older established drug for treating nausea and vomiting and that the findings from this study are not a total surprise, the other major impact of this report is that it describes a method that could be used to compare and contrast various drugs, against each other or placebos. This could lead to some real science that could change the claims that there is not an established treatment for established nausea/vomiting.10–12
Acknowledgments
This work was supported in part by the United States National Institutes of Health Grant-CA 124477 (PI Charles Loprinzi, M.D.).
Author Disclaimer Statement
No competing financial interests exist.
References
- 1.Morrow GR. Hickok JT. Burish TG. Rosenthal SN. Frequency and clinical implications of delayed nausea and delayed emesis. Am J Clin Oncol. 1996;19:199–203. doi: 10.1097/00000421-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 2.Ballatori E. Roila F. Ruggeri B. Betti M. Sarti S. Soru G. Cruciani G. Di Maio M. Andrea B. Deuson RR. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15:179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 3.Cohen L. de Moor CA. Eisenberg P. Ming EE. Hu H. Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15:497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 4.Tina Shih YC. Xu Y. Elting LS. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110:678–685. doi: 10.1002/cncr.22823. [DOI] [PubMed] [Google Scholar]
- 5.NCCN. Antiemesis: Clinical practice guidelines in oncology v.2. www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.%20Accessed%20October%2015th,%202006. [Oct 15;2006 ]. www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.%20Accessed%20October%2015th,%202006
- 6.Roila F. Herrstedt J. Aapro M. Gralla RJ. Einhorn LH. Ballatori E. Bria E. Clark-Snow RA. Espersen BT. Feyer P. Grunberg SM. Hesketh PJ. Jordan K. Kris MG. Maranzano E. Molassiotis A. Morrow G. Olver I. Rapoport BL. Rittenberg C. Saito M. Tonato M. Warr D. ESMO/MASCC Guidelines Working Group: Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: Results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 7.Grunberg SM. Deuson RR. Mavros P. Geling O. Hansen M. Cruciani G. Daniele B. De Pouvourville G. Rubenstein EB. Daugaard G. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–2268. doi: 10.1002/cncr.20230. [DOI] [PubMed] [Google Scholar]
- 8.Hickok JT. Roscoe JA. Morrow GR. King DK. Atkins JN. Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: A University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 9.American Society of Clinical Oncology. Kris MG. Hesketh PJ. Somerfield MR. Feyer P. Clark-Snow R. Koeller JM. Morrow GR. Chinnery LW. Chesney MJ. Gralla RJ. Grunberg SM. American Society of Clinical Oncology guideline for antiemetics in oncology: Update 2006.[see comment][erratum appears in J Clin Oncol 2006;24:5341–5342] J Clin Oncol. 2006;24:2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 10.Kris MG. Why do we need another antiemetic? Just ask.[comment] J Clin Oncol. 2003;21:4077–4080. doi: 10.1200/JCO.2003.07.968. [DOI] [PubMed] [Google Scholar]
- 11.NCI. Nausea and vomiting. www.cancer.gov/cancertopics/pdq/supportivecare/nausea/healthprofessional. [Feb 12;2008 ]. www.cancer.gov/cancertopics/pdq/supportivecare/nausea/healthprofessional
- 12.Bardia >A. Ettinger D. Antiemetic therapy. In: Waun Ki., editor; Hong RCBJ, editor; Hait W, editor; Kufe DW, editor; Pollock RE, editor; Weichselbaum RR, editor; Holland JF, editor; Emil Frei III, editor. 8th. Shelton, CT: People's Medical Publishing House; 2010. pp. 1757–1762. Cancer Medicine. [Google Scholar]