Abstract
STEP is a brain-specific phosphatase that opposes synaptic strengthening by the regulation of key synaptic signaling proteins. Previous studies suggest a possible role for STriatal-Enriched protein tyrosine Phosphatase (STEP) in learning and memory. To demonstrate the functional importance of STEP in learning and memory, we generated STEP knockout (KO) mice and examined the effect of deletion of STEP on behavioral performance, as well as the phosphorylation and expression of its substrates. Here we report that loss of STEP leads to significantly enhanced performance in hippocampal-dependent learning and memory tasks. In addition, STEP KO mice displayed greater dominance behavior, although they were normal in their motivation, motor coordination, visual acuity and social interactions. STEP KO mice displayed enhanced tyrosine phosphorylation of extracellular-signal regulated kinase 1/2 (ERK1/2), the NR2B subunit of the N-methyl-D-aspartate receptor (NMDAR), Proline-rich tyrosine kinase (Pyk2), as well as an increased phosphorylation of ERK1/2 substrates. Concomitant to the increased phosphorylation of NR2B, synaptosomal expression of NR1/NR2B NMDARs was increased in STEP KO mice, as was the GluR1/GluR2 containing α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPAR), providing a potential molecular mechanism for the improved cognitive performance. The data support a role for STEP in the regulation of synaptic strengthening. The absence of STEP improves cognitive performance, and may do so by the regulation of downstream effectors necessary for synaptic transmission.
Keywords: STEP, protein tyrosine phosphatase, ERK1/2, synaptic plasticity, glutamate receptors
Introduction
STriatal-Enriched protein tyrosine Phosphatase (STEP; PTPN5) is a neuronal-specific tyrosine phosphatase that has been implicated in the regulation of learning and memory. STEP is expressed in the hippocampus, cortex, striatum, and related brain regions (Lombroso et al., 1993; Boulanger et al., 1995). Alternative splicing produces several STEP isoforms (Sharma et al., 1995; Bult et al., 1997) that are differentially targeted to the post-synaptic density (Oyama et al., 1995), extrasynaptic, and cytosolic compartments (Goebel-Goody et al., 2009; Xu et al., 2009). The activity of STEP is itself regulated by both dopamine and glutamate signaling (Paul et al., 2000; Paul et al., 2003). Activation of D1 receptors by dopamine results in PKA-mediated phosphorylation of a regulatory serine residue within the Kinase Interacting Motif (KIM) of STEP (Paul et al., 2000; Paul et al., 2003). Phosphorylation at this site sterically prevents STEP from associating with and dephosphorylating its substrates (Paul et al., 2000; Xu et al., 2009). In contrast, stimulation of NMDA receptors (NMDARs) by glutamate leads to Ca2+-dependent, calcineurin/PP1-mediated dephosphorylation and activation of STEP (Paul et al., 2003; Valjent et al., 2005).
STEP associates with NMDARs, decreases NMDAR activity, and opposes the induction of LTP through dephosphorylation of Y1472 on the NR2B receptor subunit, leading to endocytosis of NR1/NR2B receptors (Pelkey et al., 2002; Snyder et al., 2005). Acute reduction in STEP expression by RNA interference increases surface expression and function of NR1/NR2B receptors (Braithwaite et al., 2006a), and STEP knockout (STEP−/−) mice have enhanced hippocampal LTP (Zhang et al., 2010). In addition, STEP inactivates ERK1/2, Fyn, and p38 kinases by dephosphorylating a regulatory tyrosine within their activation loops (Nguyen et al., 2002; Paul et al., 2003). As these kinases are required for the development of synaptic plasticity, the results suggest that STEP activity opposes the development of synaptic strengthening (Braithwaite et al., 2006b).
Earlier studies using a substrate-trapping STEP protein demonstrated that infusion of this inactive/dominant-negative protein into the lateral amygdala or ventrolateral striatum of rodents blocked consolidation of fear memories and sensitization to repeated administration of amphetamine, respectively (Paul et al., 2007; Tashev et al., 2009). However, these studies did not address the role of STEP in synaptic plasticity directly. In order to determine the role of STEP in modulating synaptic transmission, we generated mice lacking the STEP protein (STEP KO mice) (Venkitaramani et al., 2009). Mice null for STEP had increased baseline levels of phospho-ERK1/2 (pERK1/2) and enhanced activation of ERK1/2 after stimulation of hippocampal neurons (Venkitaramani et al., 2009). In the current study, we examined the effect of knocking out STEP on behavior in hippocampal-dependent learning and memory paradigms and on the Tyr phosphorylation of STEP substrates and ERK1/2 downstream signaling molecules.
Material and methods
Reagents and animals
The primary and secondary antibodies used for immunoblotting are listed in Table 1. The wildtype (WT) and STEP knockout (KO) male mice used in these experiments were 3–4 months of age, maintained on the C57Bl/6 background, and generated at Yale University from heterozygous (HET) crosses as described previously (Venkitaramani et al., 2009). STEP HET were backcrossed for at least 9 generations with C57Bl/6 WT mice from Charles River Laboratories (Wilmington, MA). Experimental mice were group-housed with a maximum of 5 mice per cage in a climate-controlled facility with 12h light-dark cycle with access to food and water ad libitum. All experiments were carried out during the light phase of the cycle. All procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Yale University.
Table 1.
Primary and secondary antibodies used in western blots
| Antibody | Format | Immunogen | Host | Dilution | Source |
|---|---|---|---|---|---|
| anti-STEP | IgG1, unconjugated | Rat synthetic peptide | Mouse | 1:1000 | Boulanger et al., 1995 |
| anti-pT202Y204 ERK1/2 | IgG1, unconjugated | Synthetic phospho-peptide | Mouse | 1:1000 | Cell Signaling Technology, Danvers, MA |
| anti-ERK2 | Whole IgG, unconjugated | C-terminus of rat sequence | Rabbit | 1:5000 | Santa Cruz Biotechnology, Santa Cruz, CA |
| anti-pY1472 NR2B | Whole IgG, unconjugated | Synthetic phospho-peptide | Rabbit | 1:1000 | Phosphosolutions, Denver, CO |
| anti-NR2B | Whole IgG, unconjugated | C-terminus of mouse NR2B | Rabbit | 1:1500 | Millipore, Billerica, MA |
| anti-NR1 | Whole IgG, unconjugated | C-terminus of rat NR1 | Mouse | 1:1000 | Millipore, Billerica, MA |
| anti-NR2A | Whole IgG, unconjugated | C-terminus of mouse NR2A | Rabbit | 1:1000 | Millipore, Billerica, MA |
| anti-GluR1 | Whole IgG, unconjugated | C-terminal peptide of rat GluR1 | Rabbit | 1:1000 | Millipore, Billerica, MA |
| anti-GluR2 | IgG2A, unconjugated | N-terminal sequence | Mouse | 1:2000 | Millipore, Billerica, MA |
| anti-GluR3 | IgG1, unconjugated | N-terminus of GluR3 | Mouse | 1:1000 | Millipore, Billerica, MA |
| anti-pS383 Elk1 | IgG1, unconjugated | Synthetic phospho-peptide | Mouse | 1:2000 | Cell Signaling Technology, Danvers, MA |
| anti-Elk1 | Whole IgG, unconjugated | Human synthetic peptide | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA |
| anti-pS133 CREB | Whole IgG, unconjugated | Synthetic phospho-peptide | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA |
| anti-CREB | Whole IgG, unconjugated | Human synthetic peptide | Rabbit | 1:1000 | Cell Signaling Technology, Danvers, MA |
| anti-pS62S67 synapsin I | Whole IgG, unconjugated | Synthetic phospho-peptide | Rabbit | 1:1000 | Thermo Fisher Scientific, Rockford, IL |
| anti-synapsin I | Whole IgG, unconjugated | Bovine synapsin I | Rabbit | 1:1000 | Millipore, Billerica, MA |
| anti-pY402 Pyk2 | Whole IgG, unconjugated | Synthetic phopho-peptide | Rabbit | 1:1000 | Invitrogen, Carlsbad, CA |
| anti-Pyk2 | IgG1, unconjugated | C-terminus of Rat Pyk2 | Mouse | 1:1000 | BD Biosciences, San Diego, CA |
| anti-rabbit | Whole IgG peroxidase-conjugated | Rabbit Fc | Donkey | 1:10,000 | Amersham Biosciences, Piscataway, NJ |
| anti-mouse | Whole IgG peroxidase-conjugated | Mouse Fc | Sheep | 1:10,000 | Amersham Biosciences |
Morris water maze
The Morris water maze was used as a measure of spatial memory and was carried out as previously described (Vorhees & Williams, 2006). Mice were handled for 4 days prior to training. The maze consisted of 1-m circular pool with a submerged 10 × 10 cm transparent platform. Spatial training consisted of 4 trials/day with the platform in the same quadrant location on all training days, an inter-trial interval of 10 min, and each trial lasting until the mouse reached the platform. Mice that failed to locate the platform within 60 s were gently guided onto the platform. All animals were allowed to remain on the platform for 30 s. The latency to reach the platform, pathlength and time spent in each quadrant was measured by a PolyTrack Video tracking System (San Diego Instruments, San Diego, CA). The animals were trained for 5 consecutive days, with a pseudo-randomized starting location (one trial was initiated from each quadrant each day). A probe trial was administered 24 h after the final training trial and the amount of time spent in the target quadrant was recorded. Following the first probe trial, animals were retrained for the same platform location for 2 additional days. The platform location was then changed to the opposite quadrant and reversal training consisted with 4 trials/day for 5 days. Twenty-four h following the last reversal trial, mice were given another probe test. Visible platform tests were also performed to control for visual acuity, motivation and swimming speed. The swim path for each trial was plotted using the Polytrack system and an investigator blind to genotype assigned a predominant search strategy as described in (Brody & Holtzman, 2006). Although the mice sometimes changed strategies during the trial, the strategy that fit the majority of the swim path was assigned.
Radial-arm water maze
The water-escape motivated, six-arm radial task was carried out as previously described (Alamed et al., 2006; Park et al., 2006) with minor modifications. Mice were handled for 4 days before testing. The radial-arm water maze consisted of a circular 1-m pool with six arms that were 20 cm wide, 30 cm in length and that radiated from a central open area 40 cm in diameter. The submerged escape platform was located at the end of one of the arms, which was randomized for each mouse. The hidden platform location was kept constant for each mouse during the first 2 days of testing. Training consisted of 3 blocks of 5 trials/day and mice were placed at the end of a random starting arm for each trial. Mice were allowed 60 s to find the platform and each entry into an incorrect arm (all four paws in the arm without the platform) was recorded as an error. If a mouse failed to choose an arm for 20 s, it was assigned an error. The intertrial interval was 10 min and mice were allowed to rest for 30 min between each block of 5 trials. The number of errors was averaged over 5 trials for each mouse resulting in 3 error blocks per animal per day. On day 3, the location of the platform was changed to the opposite arm and mice were tested for 2 days in the reversed location. Latency to find the platform and total number of errors were recorded. Errors were further categorized as reference memory, working memory and perseverative errors. Reference memory errors were defined as errors when the mouse entered the arm that did not contain the platform. Re-entry into an arm without a platform (that had already been explored) within the same trial was scored as a working memory error. Perseverative errors, measured during reversal trials, were defined as the number of entries into the arm that contained the platform before the reversal. The strategy used to solve the maze was measured as the angle between two-arms visited successively (Lavenex & Schenk, 1996).
Open Field activity
To measure exploratory activity, mice were placed in open-field chambers (Med Associates) and activity was recorded for 30 min. Activity chambers were located in a sound-attenuated enclosure with appropriate lighting. The response to a novel environment was recorded as a function of distance traveled, ambulatory time, average velocity, ambulatory counts, and ambulatory episodes. Ambulatory counts were defined as the number of beam breaks during the period the mouse was moving, while ambulatory episodes was defined as the number of times the mouse started to move from a resting position. The distance traveled was binned into 5 min periods to monitor habituation over time.
Rotarod
To test the motor coordination of STEP KO mice, animals were trained on an accelerating rotarod (Accurotor Rotarod, Accuscan Instruments) for 2 days. The rotarod accelerated gradually from 0–40 rpm over 200 s. Maximum time allotted on the drum was 300 s. Mice were given 6 trials/day with an intertrial interval of 15 min and latency to fall off the drum onto a soft pad was recorded.
Social Discrimination task
Animals were brought to the experimental room 1 h before behavioral testing. Adult resident (test) mice were habituated to a new home cage for 20 min. Following habituation, an intruder (juvenile) was introduced for 4 min and the session was video-recorded. Test mice were returned to the animal facility 6 h following the first meeting. Twenty-four hours later, test mice were exposed to either a familiar or unfamiliar/novel juvenile intruder for 4 min in their home cage and the session was again video-recorded. The intruders were always WT, while the test mice was either STEP KO or WT. Social behaviors scored include sniffing of anogenital region, head or body, following the intruder and dominance or aggressive behaviors (Gheusi et al., 1994; Engelmann et al., 1995; Garau et al., 2000). Sniffing behaviors were considered when test mouse had its nose as close as 1 cm or less from the region of interest, i.e., anogenital, head or other body regions. Dominance behavior was scored when the resident held down the other mouse against the cage floor or cage wall, and aggressive behaviors scored if kicks and/or bites were observed. During all sessions an experienced investigator observed if any animal displayed aggressive behavior that could injure the other animal, which would lead to termination of the session, but such behaviors were not observed. A computer-assisted data acquisition system was used by an investigator blind to genotype to quantify the total time spent by test mice in each social interaction in both social meetings.
Western blotting
Immunoblot analysis was performed as described (Zhang et al., 2008). Briefly, hippocampus was dissected and homogenized in TEVP + 320 mM sucrose containing protease inhibitors. The homogenates were fractionated (Hallett et al., 2008) and the following fractions were used for immunoblotting: nuclear (P1: Elk1 and CREB), crude synaptosomal membranes (P2: Pyk2 and synapsin I) and synaptosomal membranes (LP1: ERK1/2, NMDA and AMPA receptors). Proteins (25–40 μg) were separated on 8% (LP1 fractions) or 10% (P1 and P2 fractions) SDS-PAGE and transferred to nitrocellulose membranes. Membranes were blocked and then incubated with primary antibodies overnight at 4°C followed by appropriate secondary antibodies. Signals were visualized using the SuperSignal western blot kit (Pierce Biotechnology) and captured using a Chemi-HR imaging system (Syngene). Since levels of ERK2 did not differ between WT and STEP KO mice, band intensities were analyzed using ImageJ (NIH) software and normalized to ERK2 levels from the same blot. Levels of non-phosphorylated proteins were normalized to total ERK2 levels from the same blot and WT levels were set as 100% for all comparisons with proteins from STEP KO mice. For phosphorylated proteins, we first normalized the total protein levels to ERK2 levels from the same blot and then determined the ratio of phosphorylated protein to total protein within the same blot.
Statistical Analysis
All analyses were performed using statistical software SPSS (Chicago, IL, USA). Statistical analyses were performed using one-way (genotype) repeated-measures ANOVA on latency (to find platform), number of errors per block, bins or trials and groups with significant differences were compared using one-way ANOVA with genotype as factor. The search strategy used during Morris water maze trials and turn angle during radial-arm water maze were analyzed using the non-parametric Mann-Whitney U test. The time spent by the test mice (resident) performing social behaviors was analyzed by a two-way ANOVA with duration of the interval (30 min vs. 24 hours) and familiarity (familiar vs. unfamiliar) as factors. Analysis of experiments involving two groups including western blots was performed using unpaired two-tailed (non-directional) t-tests. All data are presented as means ± SEM and P < 0.05 was considered to be significant.
Results
STEP KO mice showed increased behavioral flexibility and accuracy in tests of spatial learning and memory
We used two hippocampus-dependent spatial tasks to assess learning and memory in STEP KO mice. First, we tested STEP WT and KO mice in the Morris water maze. The latency to find the hidden platform as well as the pathlength was similar between the WT and KO mice on all training days (Fig. 1A and B). Both STEP WT and KO mice showed improvement in performance over the training blocks, and the latency and pathlength decreased significantly over training blocks (F6,180 = 29.49, P < 0.001 for latency; F6,156 = 24.81, P < 0.001 for pathlength). The performance on the probe trial on day 6 did not differ significantly between WT and KO mice (Fig. 1C). Both groups spent significantly more time in the target quadrant as opposed to other quadrants during this probe trial (t31 = 4.18, P < 0.001 as compared to chance). The latency to find the platform and pathlength decreased significantly during reversal trials for both genotypes (F4,120 = 18.81, P < 0.001 for latency; F4,112 = 17.55, P < 0.001 for pathlength).
FIG. 1. Altered performance of STEP KO mice on the Morris water maze.
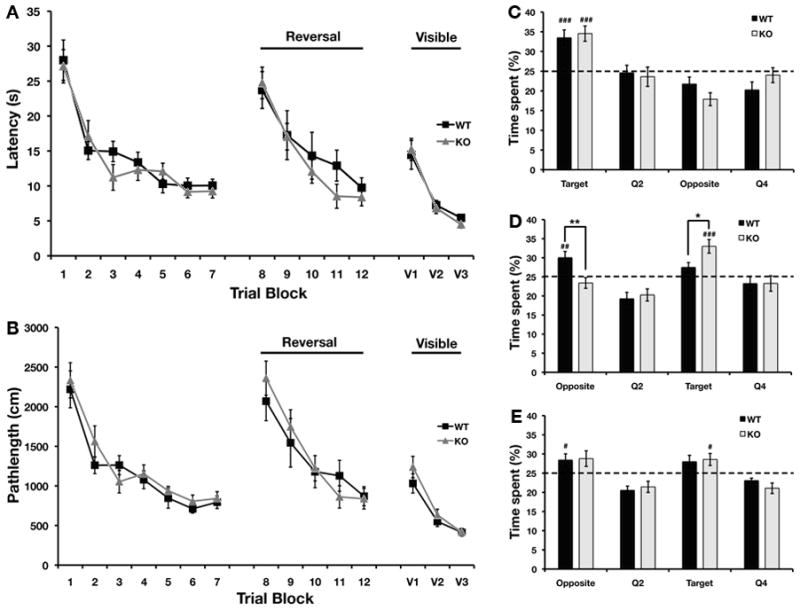
The latency to find the platform (A) as well as the pathlength (B) were not different between WT and KO mice during all training blocks. The time spent in the four quadrants during probe trial I (Day 6) were similar between WT and STEP KO mice (C). Both groups spent significantly more time than chance in the target quadrant (p < 0.001). During probe trial II (Day 13) after reversal (D), the STEP KO mice spent significantly more time in the new target quadrant than WT mice (p < 0.05 as compared to WT). In probe trial III (Day 20), there were no significant differences between the WT and STEP KO mice (E) (n=14–16 per genotype).
However, during the second probe trial after reversal training on day 13, the STEP KO mice spent significantly more time in the target quadrant as compared to WT mice (Fig. 1D, t31 = 4.46, P < 0.001 for STEP KO mice in the new target quadrant as compared to chance). Although there was no difference in the latencies to find the platform on training trials during reversal learning, WT mice spent significantly more time in the quadrant that housed the hidden platform before reversal (for STEP WT mice, t31 = 3.09, P < 0.01 in the opposite quadrant as compared to chance). These results indicate that STEP KO mice showed enhanced performance during the reversal probe test, which is a measure of cognitive flexibility. This difference between STEP WT and KO mice was not detected during a probe trial on day 20 (Fig. 1E), suggesting that the STEP KO mice were able to learn and consolidate the new location of the platform better than WT controls, but were also able to extinguish the memory. We also tested the ability of WT and KO mice to locate the visible platform. There were no significant differences in either the latency to find the platform or the pathlength between the two genotypes, suggesting that the differences observed in the hidden platform trials cannot be attributed to changes in motivation or ability to perform the task.
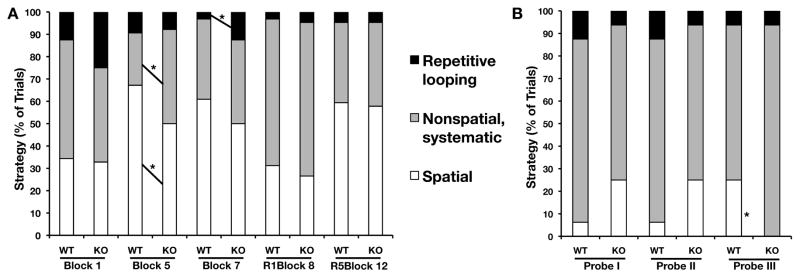
We analyzed the strategy used by the WT and STEP KO mice to complete this spatial task. The search strategy was scored during training blocks 1, 5, 7, 8 and 12 as well as during probe trials I, II and III. Search strategies were grouped into 3 main categories, spatial, non-spatial/systematic and repetitive looping. The search strategies used by STEP KO mice did not differ from those used by WT controls during first training block (Trial block 1) or training blocks after reversal (Trial blocks 8 and 12 (Fig 2B)). During trial block 5, WT mice predominantly used a spatial search strategy, whereas STEP KO mice used both spatial (Z = −1.97, P < 0.05) and non-spatial/systematic strategies (Z = −2.25, P < 0.024) equally. STEP KO mice exhibited significantly more repetitive looping than WT mice during trial block 7 (Z = −1.97, P < 0.05). In the first two probe trials there was no difference in the strategy used between the WT controls and STEP KO mice. However, during probe trial III, STEP KO mice did not use a spatial strategy as often as WT mice (Z = −2.10, P < 0.04). We sub-categorized each group of search strategies (Table 2.) and found that STEP KO mice preferred the non-spatial/systematic strategy more than the spatial strategy during trial block 5 and probe trial III. Interestingly, the latency and pathlength did not differ between the two genotypes during trial block 5.
FIG. 2. Differences in search strategy used by STEP KO during first and last blocks of training as well as probe trials.
The search strategy (A) used by STEP KO mice did not differ from WT mice during the first training block or training blocks after reversal. The STEP KO mice used different search strategy during training blocks 5 and 7. The search strategy (B) of STEP KO mice was similar to that of WT controls during probe trials I and II. The STEP KO mice did not utilize spatial strategy during probe trial III.
Table 2a.
Morris Water Maze search strategies during trial blocks.
| Block N o. | Genotype | Spatial Direct | Spatial Indirect | Focal Correct | Scanning | Random | Focal Incorrect | Chaining | Peripheral Looping | Circling |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WT | 1.56 | 23.44 | 9.38 | 6.25 | 34.38 | 12.50 | 0.00 | 4.69 | 7.81 |
| KO | 4.69 | 26.56 | 1.56 | 6.25 | 34.38 | 1.56* | 0.00 | 12.50 | 12.50 | |
| 5 | WT | 26.56 | 40.63 | 0.00 | 17.19 | 6.25 | 0.00 | 0.00 | 1.56 | 7.81 |
| KO | 17.19 | 32.81 | 0.00 | 23.44 | 18.75* | 0.00 | 1.56 | 0.00 | 6.25 | |
| 7 | WT | 20.31 | 32.81 | 7.81 | 21.88 | 14.06 | 0.00 | 0.00 | 0.00 | 3.13 |
| KO | 18.75 | 25.00 | 6.25 | 32.81 | 4.69 | 0.00 | 1.56 | 1.56 | 9.38 | |
| 8 | WT | 18.75 | 10.94 | 1.56 | 25.00 | 25.00 | 15.63 | 1.56 | 0.00 | 1.56 |
| KO | 7.81 | 18.75 | 0.00 | 56.25*** | 7.81** | 4.69* | 4.69 | 0.00 | 0.00 | |
| 12 | WT | 28.13 | 26.56 | 4.69 | 25.00 | 10.94 | 0.00 | 1.56 | 1.56 | 1.56 |
| KO | 12.50* | 39.06 | 6.25 | 29.69 | 7.81 | 0.00 | 0.00 | 0.00 | 4.69 |
Mann-Whitney U test,
P < 0.05,
P < 0.01,
P < 0.001
Next, we evaluated performance in the radial-arm water maze, which tests spatial working memory in addition to spatial reference memory. Unlike the Morris water maze, there was a significant main effect of genotype (F1,28 = 4.32, P < 0.05 for reference memory error; F1,28 = 4.65, P < 0.04 for latency; F1,28 = 4.40, P < 0.05 for working memory error) and an interaction between genotype and blocks (F5,140 = 2.74, P < 0.03 for reference memory error; F5,140 = 2.29, P < 0.05 for latency; F5,140 = 2.72, P < 0.03 for working memory error) during the first two days of testing. STEP KO mice committed fewer reference errors (Fig. 3A, F1,28 = 5.18, P < 0.02 for Block 1; F1,28 = 10.60, P < 0.003 for Block 3) and working memory errors (Fig. 3C, F1,28 = 4.32, P < 0.05 for Block 1; F1,28 = 7.89, P < 0.009 for Block 3) on the first day of testing. The latency to find the hidden platform was also significantly faster in the STEP KO as compared to their WT controls (Fig. 3B, F1,28 = 10.81, P < 0.003 for Block 3).
FIG. 3. Enhanced performance of STEP KO mice on radial-arm water maze.
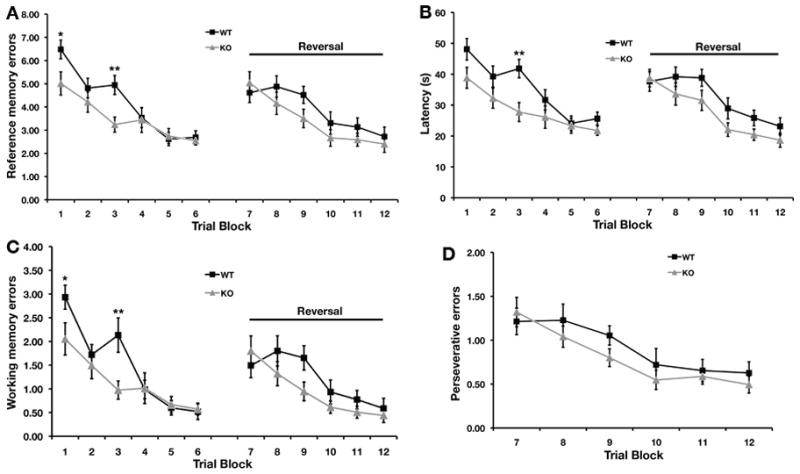
The number of reference memory errors (A), latency to find the platform (B) as well as the working memory errors (C) were significantly lower in STEP KO mice as compared to WT controls during the first two days of testing. The STEP KO mice and WT littermates did not differ significantly in error rate or latency during reversal trials. However, the STEP KO mice had consistently exhibited lower error rates and latency. Perseverative errors (D) during reversal trials were similar between WT and STEP KO mice (n=15 per genotype).
There were no significant effects of genotype or interactions between genotype and block during reversal trials. Although there was no significant difference in the latency to find the platform or in errors on the second testing day or during reversal training and testing, STEP KO mice committed fewer errors and, on average, located the platform more quickly than their WT controls (Fig. 3A, 3B and 3C). The two groups did not differ in the perseverative error rate during reversal learning (Fig. 3D). This suggests that in a more complex learning spatial task, STEP KO mice show enhanced acquisition of the behavior.
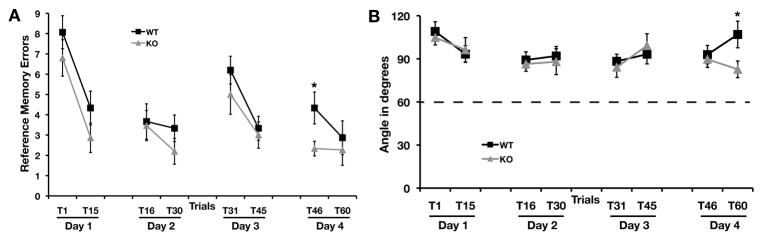
We assessed the strategy used to solve the radial-arm water maze by measuring the angle of turn between two successive arm choices. We also evaluated the reference memory errors during the first and last trials on each training day (Fig. 4A). The number of reference memory errors decreased across trials in both genotypes. However, STEP KO mice committed significantly fewer reference memory errors than WT controls during trial 46 (F1,28 = 5.36, P < 0.03). The turn angle was not different between WT and STEP KO mice (Fig. 4B) across most trials except the last trial (trial 60) after reversal (Z = −2.48, P < 0.014). An average turn angle value close to 60° would suggest a serial/sequential search strategy was used while a larger turn angle values is indicative of spatial search strategy. During trial 60, WT mice made more 180° arm choices than STEP KO mice (Table 3.). However, both the WT and KO mice used a spatial strategy as indicated by average turn angles greater than 60°.
FIG. 4. Comparison of Reference memory errors and turn angles between successive arm visits.
The number of reference memory errors (A) during the first and last trial of each training day was similar between WT and STEP KO mice except for the first trial of the last experimental day (Day 4). The number of reference memory errors decreased over trials in both genotypes. WT mice committed significantly more reference memory errors during the first trial on day 4. The average turn angle (B) during each arm visit was comparable between WT and STEP KO mice except during the last trial of the experiment. During the last trial on day 4, STEP KO mice used a strategy that significantly reduced the turn angle.
Table 3.
Radial-arm Water Maze turning angle percentages.
| Trial No. | Genotype | 0° turn | 60° turn | 120° turn | 180° turn |
|---|---|---|---|---|---|
| 1 | WT | 1.16 ± 0.79 | 37.61 ± 8.03 | 36.25 ± 6.22 | 24.37 ± 5.24 |
| KO | 2.41 ± 1.77 | 38.61 ± 8.03 | 40.53 ± 4.91 | 18.29 ± 3.19 | |
| 15 | WT | 3.85 ± 1.72 | 44.60 ± 9.52 | 43.78 ± 9.62 | 7.76 ± 2.56 |
| KO | 1.69 ± 1.16 | 48.27 ± 9.90 | 37.98 ± 8.52 | 12.06 ± 7.31 | |
| 16 | WT | 3.54 ± 1.94 | 53.06 ± 7.95 | 34.56 ± 8.06 | 8.84 ± 3.70 |
| KO | 0.67 ± 0.67 | 63.02 ± 7.34 | 28.03 ± 6.97 | 8.29 ± 3.49 | |
| 30 | WT | 0.00 ± 0.00 | 60.11 ± 7.45 | 26.50 ± 6.10 | 13.39 ± 4.93 |
| KO | 0.83 ± 0.83 | 60.28 ± 10.63 | 23.61 ± 7.56 | 13.06 ± 7.31 | |
| 31 | WT | 4.15 ± 2.22 | 54.81 ± 6.99 | 30.86 ± 5.17 | 10.19 ± 2.48 |
| KO | 10.72 ± 4.51 | 44.67 ± 8.66 | 38.32 ± 7.80 | 6.29 ± 2.66 | |
| 45 | WT | 0.00 ± 0.00 | 58.37 ± 7.32 | 26.75 ± 6.02 | 14.89 ± 5.07 |
| KO | 0.00 ± 0.00 | 48.83 ± 8.94 | 37.06 ± 8.12 | 14.11 ± 6.89 | |
| 46 | WT | 1.33 ± 1.33 | 53.15 ± 7.77 | 34.80 ± 7.51 | 10.72 ± 3.82 |
| KO | 1.67 ± 1.67 | 53.89 ± 7.49 | 37.78 ± 6.99 | 6.67 ± 3.92 | |
| 60 | WT | 1.78 ± 1.25 | 40.73 ± 8.58 | 34.98 ± 9.27 | 22.51 ± 9.00 |
| KO | 0.00 ± 0.00 | 63.83 ± 9.33 | 31.17 ± 8.12 | 1.67 ± 1.67* |
Mann-Whitney U test,
P < 0.05
Activity levels and motor coordination were similar in STEP KO and WT mice
We examined the effect of the STEP knockout on baseline locomotor activity and motor coordination, since locomotor activity is essential for performing the behavioral tasks used. STEP WT and KO mice were allowed to explore an open field for 30 min on 3 consecutive days. Baseline activity was not significantly different between the STEP WT and KO mice, suggesting similar locomotor responses to a novel environment. STEP WT and KO mice did not differ in measures of total distance travelled (F1,26 = 0.003, P > 0.05; Fig. 5A), showed a similar significant decrease in activity over time (F5,22 = 7.517, P < 0.001; Fig. 5B), time of ambulation (F1,26 = 0.003, P > 0.05; Fig. 5C), average velocity (F1,26 = 0.797, P > 0.05; Fig. 5D), ambulatory counts (F1,26 = 0.013, P > 0.05; Fig. 5E) or ambulatory episodes (F1,26 = 0.214, P > 0.05; Fig. 5F).
FIG. 5. Comparison of open field activity, motor coordination and social interaction between WT controls and STEP KO mice.
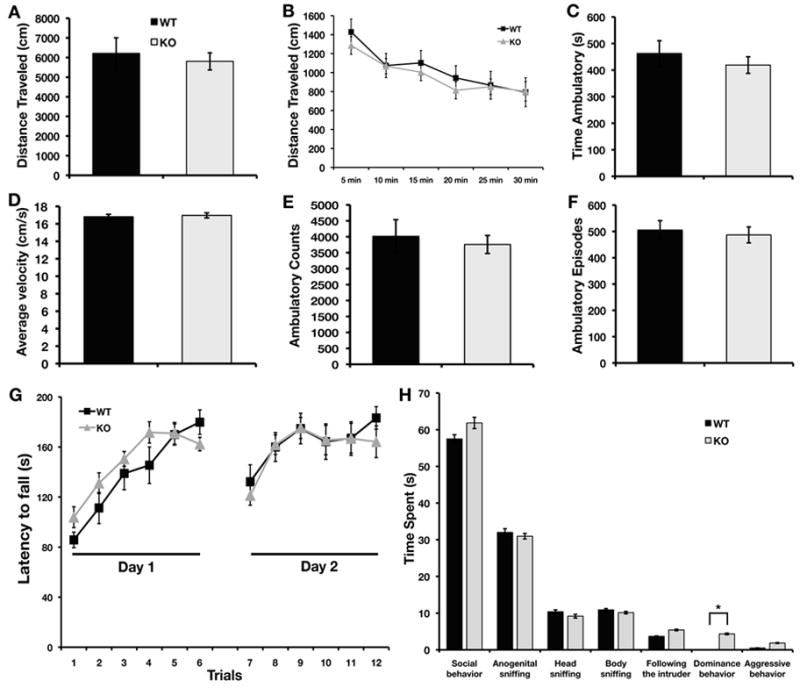
The total distance travelled (A), time of ambulation (C), average velocity (D), ambulatory counts (E) and ambulatory episodes (F) averaged over 3 consecutive days were not different between STEP WT and KO mice. Total distance moved per 5 min blocks (B) over 30 min showed that both WT and KO mice habituated to the environment. The latency to fall (G) from a rotarod increased significantly over 6 trials on both testing days. Total time spent in social behaviors as well as specific categories of behaviors are shown in (H). STEP KO mice showed significantly more dominance behaviors than WT controls (p < 0.03) (n=10–14 for open field and rotarod, n=5–7 for social interaction for each genotype).
Motor coordination and learning were assessed using an accelerating rotarod test. The latency to fall from the rotarod within each day and over the 2 days of testing did not differ significantly between the STEP WT and KO mice (Fig. 5G). The latency to fall off the drum increased significantly over trials for both genotypes, suggesting improved performance with training (F5,95 = 25.703, P < 0.001 for day 1; F5,95 = 9.273, P < 0.001 for day 2). There was no interaction between trials and genotype. These results suggest that STEP KO mice do not exhibit deficits in motor coordination or motor learning.
STEP KO mice differ from WT mice in specific social behaviors
Social recognition memory is a measure of the ability of mice to discriminate between familiar and unfamiliar (novel) conspecifics. The analysis of the baseline social interaction of STEP KO and WT observed during the first meeting shows that STEP KO mice spent significantly more time showing dominance behavior as compared to WT mice (Fig. 5H, F1,20 = 6.07, P < 0.03). STEP WT and KO mice did not differ significantly in the total amount of time spent in social behaviors during either a first or second meeting. Both groups showed a decrease in social behaviors towards the familiar intruder after 30 min or 24 h, and spent more time with the unfamiliar intruder (data not shown). These data suggest that STEP KO mice showed intact short- and long-term social memory, but presented high levels of dominance behavior at baseline.
Increased tyrosine phosphorylation and expression of ionotropic glutamate receptors
It is possible that the decreased ability of STEP to dephosphorylate its molecular targets in the hippocampus underlies the improved performance of these mice in these learning tasks. We previously found that the Tyr phosphorylation of ERK1/2 was elevated in the hippocampus of STEP KO mice (Venkitaramani et al., 2009). Here, we examined the phosphorylation state of glutamate receptors and an upstream kinase known to regulate their Tyr phosphorylation in synaptosomal membrane fractions from the hippocampus of STEP WT and KO mice. We confirmed the absence of STEP in KO mice, and detected STEP61, the only isoform expressed in the hippocampus, in blots from WT samples. The level of activated ERK1/2 served as a positive control and was significantly increased in the STEP KO mice as compared to WT controls (Fig. 6A and 6B, t4 = 2.78, P < 0.04) (Venkitaramani et al., 2009).
FIG. 6. STEP KO mice have elevated Tyr phosphorylation of STEP substrates and increased surface expression of ionotropic glutamate receptors.
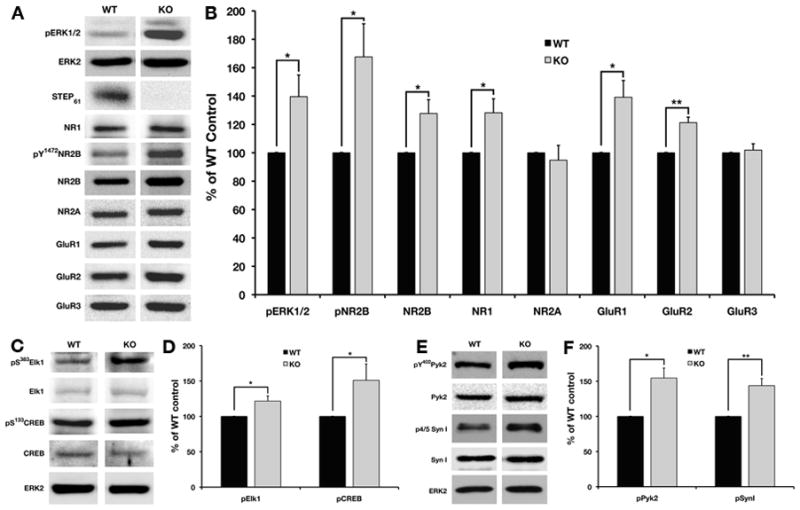
Synaptosomal membrane fractions from WT and STEP KO hippocampi were probed with p-ERK1/2, ERK2, STEP, pY1472-NR2B, NR2B, NR2A, GluR1, GluR2, and GluR3. Representative immunoblots are shown in (A) and histograms in (B). The STEP KO mice show no detectable STEP expression and increased Tyr phosphorylation of ERK1/2 and NR2B. The expression levels of NR1, NR2B, GluR1, and GluR2 but not NR2A and GluR3 are elevated in the STEP KO mice. The levels of phosphorylation of ERK substrates in nuclear fraction were detected by immunoblotting (C, E) and quantified (D, F). There were significant increases in activated CREB, Elk1 and synapsin I in STEP KO mice. The Tyr phosphorylation of Pyk2 was significantly enhanced in crude synaptosomal fractions from STEP KO mice (E, F) (n=5 per genotype).
We next measured the Tyr phosphorylation of NR2B at Y1472. Phosphorylation of NR2B at Y1472 by Fyn is associated with increased NMDAR surface expression, increased NMDAR channel activity, and enhanced synaptic transmission (Xu et al., 2006). There was a significant elevation in the phosphorylation of Y1472 in hippocampal synaptosomal fractions from STEP KO mice (Fig. 6A and 6B, t4 = 2.90, P < 0.05). We also examined the total levels of the NR1, NR2B and NR2A subunits of the NMDAR complex. There were significant increases in total levels of the NR1 subunit (t4 = 2.88, P < 0.05), which is essential for NMDAR assembly and function, as well as total levels of the NR2B subunit (t4 = 2.86, P < 0.05) in the hippocampal synaptosomal membrane compartment of STEP KO mice compared to WT controls (Fig. 6A and 6B); however, we did not observe any differences in the total levels of the NR2A subunit between STEP WT and KO mice.
We next examined the level of AMPAR subunits in synaptosomal membrane fractions. We found a significant increase in the expression levels of both GluR1 (t4 = 3.33, P < 0.03) and GluR2 (t4 = 5.60, P < 0.005) subunits in STEP KO mice, while GluR3 subunit levels were similar between the two genotypes (Fig. 6A and 6B). These results suggest that STEP activity is important for regulating the trafficking of two classes of glutamate receptors, and are consistent with earlier findings that mice null for STEP have significant increases in surface expression of GluR1/GluR2 subunits of AMPAR (Zhang et al., 2008).
Calcium entry through NMDARs results in the tyrosine phosphorylation of Pyk2, leading to activation of ERK1/2 (Lev et al., 1995). Pyk2 also induces the phosphorylation of glutamate receptors on residues important for membrane insertion via activation of Src kinase, and is necessary for the induction of hippocampal LTP (Huang et al., 2001). Recent studies in our lab have shown that Pyk2/CAKβ, a member of FAK family of kinases, is a substrate of STEP (Xu and Lombroso, unpublished results). We therefore examined the Tyr phosphorylation of Pyk2 (Y402) in the crude synaptosomal fractions of STEP WT and KO mice. There were significant increases in pY402 Pyk2 phosphorylation in STEP KO mice compared to their WT controls (Fig. 6E and 6F, t4 = 3.75, P < 0.02).
We examined the phosphorylation levels of downstream targets of pERK1/2 known to contribute to synaptic plasticity in the hippocampus. We observed a significant increase in the phosphorylation of two transcription factors with roles in synaptic plasticity and performance in tests of learning and memory (Thomas & Huganir, 2004), S383-Elk1 (t4 = 3.02, P < 0.04) and S133-CREB (t4 = 2.82, P < 0.05) in the nuclear fraction of STEP KO mice as compared to WT controls (Fig. 6C and 6D). In addition, we detected a significant increase in synapsin I phosphorylation at ERK1/2 specific sites in the crude synaptosomal fraction of STEP KO mice (Fig. 6E and 6F, t4 = 5.95, P < 0.004). Phosphorylation of synapsin I at these sites is associated with an increased probability of vesicle release (Jovanovic et al., 2001).
Discussion
We report for the first time that STEP KO mice show improved hippocampal learning and memory and that the loss of STEP is associated with increased tyrosine phosphorylation of STEP substrates that are essential for synaptic plasticity in the hippocampus. The altered performance of STEP KO mice in these behavioral paradigms is not due to lack of motivation, impaired motor coordination, or loss of visual acuity.
Previous studies suggested a role for STEP in the regulation of synaptic plasticity in the amygdala and striatum (Paul et al., 2007; Tashev et al., 2009). Here, we directly examined whether STEP modulates hippocampal learning and memory. Mice null for STEP did not show altered acquisition of spatial memory in the Morris water maze. There were also no differences during the first probe trial, which is a measure of memory consolidation. However, loss of STEP enhanced performance and resulted in more selective search of the target quadrant during the probe trial after reversal learning. The improved performance during reversal in the STEP KO mice could be due to increased cognitive flexibility or due to enhanced extinction of initial learning. The lack of detectable differences during the initial acquisition phase and first probe trial may indicate a ceiling effect. It is possible that we might have detected differences in the rate of acquisition of spatial memory between WT and STEP KO mice using a pool of larger diameter.
Search strategy analysis suggested that STEP KO mice employed both spatial and non-spatial/systematic approaches equally during most trial blocks as compared to WT controls. During probe trial III, STEP KO failed to use a spatial strategy. Typically, use of a spatial strategy is associated with decreased latency to find the platform, but STEP KO mice were not outperformed by WT controls during any of the trial blocks.
To examine whether STEP KO mice have increased cognitive flexibility, we subjected them to a more complex spatial task, the radial-arm water maze. In this task, we detected significant differences in latency and error rates between STEP KO and control mice. STEP KO mice committed significantly less reference and working memory errors. Thus, the enhanced acquisition of spatial memory by STEP KO mice was not discernible in the traditional water maze task, but was demonstrable in the more complex radial-arm water maze.
The search strategy used to perform the radial-arm water maze task did not differ significantly between WT and STEP KO mice until the last trial after reversal. Although the turn angle was smaller for the STEP KO mice, the latency to find the platform was similar to that of WT controls. Both groups of mice used a spatial strategy as indicated by average turn angles greater than 60°.
To investigate baseline activity and motor coordination, we tested open field activity and rotarod performance of STEP KO mice. Knocking out STEP did not affect exploratory behavior or motor learning. In contrast, the knock-out of a highly related phosphatase (PTP-STEP-like; PTPRR) that regulates MAPK activity in the cerebellum (Hendriks & Stoker, 2008) resulted in deficits in motor coordination and balance (Chirivi et al., 2007). Interestingly, STEP is not expressed in the cerebellum and deletion of STEP does not alter phospho-ERK1/2 levels in this brain region (Venkitaramani et al., 2009). This suggests that improved performance of STEP KO mice in hippocampal-dependent tasks is not associated with altered activity, motivation or motor coordination.
We also examined the performance of STEP KO mice in social behaviors, and found that STEP KO and WT mice showed similar short- and long-term social memory as WT controls. However, the analysis of specific social behaviors during an initial exposure to an intruder revealed that STEP KO mice exhibit more dominance behavior. Dominance behavior is important for establishing hierarchy amongst conspecifics and has evolutionary advantages. Changes in dominance behavior between conspecific males have been noted in a variety of genetic mutants, including mice null for neuronal nitric oxide synthase and the dopamine transporter, and p25 over-expressing mice (Nelson & Chiavegatto, 2001; Angelo et al., 2003; Nelson & Trainor, 2007). Reduction in aggression has been shown in mice null for GluR1 and aromatase (Toda et al., 2001; Vekovischeva et al., 2004).
Previous studies have shown that mice exhibiting dominance behavior during social interaction commit fewer errors than subordinates when the radial maze test is administered immediately after the social interaction (Barnard & Luo, 2002). This improvement has been attributed to state-dependent change in learning and memory (Barnard & Luo, 2002). In the current work, different mice were used in each behavioral test, which rules out the possibility of a state-dependent change in learning. However, STEP KO mice showed increased levels of dominance behavior as well as improved hippocampal learning and memory suggesting a possible correlation between the two behaviors. Thus, one may speculate that STEP could be the common link leading to changes in dominance behavior and also learning and memory. The effects of knocking down STEP and the concomitant effects on dominance and learning and memory should be addressed in future studies.
Improved performance in learning and memory tasks is generally associated with altered expression, post-translational modification, or targeting of signaling molecules that modulate synaptic plasticity. Post-translational modification by phosphorylation is an important mechanism underlying synaptic plasticity (Lee, 2006). STEP regulates the activity of ERK1/2 (Paul et al., 2003), p38 kinase (Xu et al., 2009), and Fyn (Nguyen et al., 2002), as well as the trafficking of NMDARs and AMPARs (Pelkey et al., 2002; Snyder et al., 2005; Kurup et al., 2010b; Zhang et al., 2010), all of which are critical for synaptic plasticity. Thus, the activity of STEP is likely to be critical for the regulation of synaptic strengthening. The NR2B subunit can interact with αCaMKII at synaptic membrane via its C-terminal domain resulting in phosphorylation of GluR1 subunit of AMPAR, which is essential for spatial learning and synaptic plasticity (Zhou et al., 2007). In addition, the baseline tyrosine phosphorylation of ERK1/2 and NR2B subunit of the NMDAR is increased in the STEP KO mice. Interestingly, both p25 over-expressing mice (Angelo et al., 2003) and cdk5 conditional knockout mice (Hawasli et al., 2007), in which baseline pY1472-NR2B levels is enhanced, show increased aggression, reversal learning in spatial tasks and enhanced spatial learning respectively, suggesting that increased baseline pY1472 of NR2B may be an important molecular event underlying the behavioral changes reported here.
In addition, expression of NR1, NR2B, GluR1 and GluR2 subunits, but not NR2A and GluR3, were enhanced in synaptosomal membrane fraction of STEP deficient mice. The ability to learn hippocampal-dependent tasks has been shown to correlate with NR1 levels in the CA3 region (Adams et al., 2001). Region specific knockdown of GluR2 subunit of AMPAR results in impaired hippocampal-dependent spatial learning (Wiltgen et al., 2010). The results presented here confirm earlier findings that STEP regulates the surface expression of specific NMDAR and AMPAR subunits (Zhang et al., 2008; Zhang et al., 2010). We have also previously shown that endocytosis of NMDARs by amyloid-β (Kurup et al., 2010a; Kurup et al., 2010b) and internalization of AMPARs in response to DHPG (Zhang et al., 2008) are both reduced in STEP KO mice and this is reversed by the restoration of active STEP protein to neuronal cultures. Thus, STEP is likely to have a regulatory role in activity-dependent trafficking of glutamate receptors.
Recent data from our lab have suggested that Pyk2 is a substrate of STEP (Xu and Lombroso, unpublished results). Pyk2/CAKβ is a FAK family kinase that is required for induction of hippocampal LTP and is necessary for src kinase-mediated phosphorylation and upregulation of NMDAR surface expression (Huang et al., 2001). Stimulation of ionotropic and metabotropic glutamate receptors leads to the activation of Pyk2 and subsequent increase in the phosphorylation of ERK1/2 (Nicodemo et al., 2010). Tyrosine phosphorylation of Pyk2 at Y402 is elevated in the STEP KO mice as compared to WT controls, suggesting that STEP normally decreases the activity of Pyk2.
Similarly, ERK/MAPK regulates synaptic strengthening in part by targeting the CREB and Elk1 transcription factors, leading to activity-dependent gene transcription (Davis et al., 2000). In STEP KO mice, the baseline phosphorylation of ERK1/2-dependent phosphorylation sites on both CREB and Elk1 was increased. Blockade of ERK1/2-mediated activation of CREB and Elk1 have been shown to prevent maintenance of LTP (Davis et al., 2000). Thus, the increased basal activity of CREB and Elk1 in the STEP KO mice may contribute to the enhanced learning observed here. ERK1/2 also phosphorylates synapsin I after depolarization, which leads to an increased probability of vesicle release (Jovanovic et al., 2001; Kushner et al., 2005). The ERK1/2 sites 4 and 5 on synapsin I are hyperphosphorylated in STEP KO mice, suggesting that there may also be presynaptic changes associated with the knockdown of STEP.
Since the current studies used mice with constitutive loss of the STEP protein, it is possible that some of the molecular changes observed here might be attributed to the absence of STEP during development. The levels of synaptic proteins (NR1, NR2B, GluR1 and GluR2) are elevated in primary hippocampal or cortical neurons prepared from embryonic tissue. However, restoration of wildtype, but not inactive, STEP protein normalized these receptor levels to that of WT controls (Zhang et al., 2008; Kurup et al., 2010a). Although, we cannot rule out the possibility that STEP’s function during development increases expression and localization of synaptic proteins, our data suggest that constitutive knockdown of STEP does not irreversibly alter markers of synaptic plasticity. Further studies are needed to evaluate whether selective ablation of STEP in adult mice can improve memory.
It is interesting to speculate about how a protein that impairs memory might have evolved. One of the functions of STEP is to dephosphorylate and internalize both AMPA and NMDA receptor complexes. In the absence of STEP, the internalization of these receptors would be hampered, and there would soon be an over-expression of glutamate receptors on synaptic membrane surfaces. Thus, STEP may have evolved to return glutamate receptors to baseline levels so that further learning can occur.
In summary, we examined the hypothesis that reducing STEP levels would facilitate learning and memory. Genetic ablation of STEP resulted in enhanced performance in hippocampal-dependent spatial tasks without affecting baseline activity or motor coordination. We detected increased tyrosine phosphorylation of substrates of STEP as well as augmented expression of selected NMDAR and AMPAR subunits in the synaptosomal membrane fraction. In addition, activation of downstream substrates of phospho-ERK1/2 was enhanced in the STEP KO mice. Given that phosphorylation of signaling molecules as well as the synaptic distribution of ionotropic receptors significantly affects synaptic transmission, these changes may account for the improved performance of STEP KO in spatial tasks. Thus, reduction of STEP activity may be valuable in reversing synaptic deficits associated with various neurological disorders.
Table 2b.
Morris Water Maze search strategies during probe trials.
| Probe No. | Genotype | Spatial Direct | Spatial Indirect | Focal Correct | Scanning | Random | Focal Incorrect | Chaining | Peripheral Looping | Circling |
|---|---|---|---|---|---|---|---|---|---|---|
| I | WT | 0.00 | 0.00 | 6.25 | 31.25 | 50.00 | 0.00 | 0.00 | 6.25 | 6.25 |
| KO | 0.00 | 6.25 | 18.75 | 31.25 | 37.50 | 0.00 | 6.25 | 0.00 | 0.00 | |
| II | WT | 0.00 | 0.00 | 6.25 | 31.25 | 50.00 | 0.00 | 0.00 | 6.25 | 6.25 |
| KO | 0.00 | 6.25 | 18.75 | 31.25 | 37.50 | 0.00 | 6.25 | 0.00 | 0.00 | |
| III | WT | 0.00 | 6.25 | 18.75 | 25.00 | 43.75 | 0.00 | 6.25 | 0.00 | 0.00 |
| KO | 0.00 | 0.00 | 0.00 | 56.25 | 37.50 | 0.00 | 6.25 | 0.00 | 0.00 |
Acknowledgments
This work was funded by NIH grants MH01527 and MH52711, a National Association of Research on Schizophrenia and Depression (NARSAD) grant to PJL, and a Brown-Coxe postdoctoral fellowship to DVV. MRP was supported by DA14241, and PJM was supported by National Council for the Improvement of Higher Education (CAPES-Brazil) scholarship. We would like to thank laboratory members for helpful discussions and critical reading of the manuscript. The authors of this research article report no biomedical, financial interests or potential conflict of interests.
Abbreviations
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
- CREB
cyclic AMP response element binding protein
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- KIM
kinase interacting motif
- LTP
long-term potentiation
- NMDAR
N-methyl D-aspartate receptor
- PAGE
polyacrylamide gel electrophoresis
- Pyk2
proline-rich tyrosine kinase 2
- PKA
protein kinase A
- STEP
STriatal-Enriched protein tyrosine Phosphatase
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Angelo M, Plattner F, Irvine EE, Giese KP. Improved reversal learning and altered fear conditioning in transgenic mice with regionally restricted p25 expression. Eur J Neurosci. 2003;18:423–431. doi: 10.1046/j.1460-9568.2003.02746.x. [DOI] [PubMed] [Google Scholar]
- Barnard CJ, Luo N. Acquisition of dominance status affects maze learning in mice. Behav Processes. 2002;60:53–59. doi: 10.1016/s0376-6357(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, Oksenberg D, Nikolich K. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006a;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- Braithwaite SP, Paul S, Nairn AC, Lombroso PJ. Synaptic plasticity: one STEP at a time. Trends Neurosci. 2006b;29:452–458. doi: 10.1016/j.tins.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Holtzman DM. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006;197:330–340. doi: 10.1016/j.expneurol.2005.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Zhao F, Dirkx R, Jr, Raghunathan A, Solimena M, Lombroso PJ. STEP: a family of brain-enriched PTPs. Alternative splicing produces transmembrane, cytosolic and truncated isoforms. Eur J Cell Biol. 1997;72:337–344. [PubMed] [Google Scholar]
- Chirivi RG, Noordman YE, Van der Zee CE, Hendriks WJ. Altered MAP kinase phosphorylation and impaired motor coordination in PTPRR deficient mice. J Neurochem. 2007;101:829–840. doi: 10.1111/j.1471-4159.2006.04398.x. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Garau A, Marti MA, Sala J, Balada F. Age effects on the social interaction test in early adulthood male rats. Depress Anxiety. 2000;12:226–231. doi: 10.1002/1520-6394(2000)12:4<226::AID-DA6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthe RM, Goodall G, Dantzer R. Ethological study of the effects of tetrahydroaminoacridine (THA) on social recognition in rats. Psychopharmacology (Berl) 1994;114:644–650. doi: 10.1007/BF02244996. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1446–1459. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Collins TL, Standaert DG, Dunah AW. Curr Protoc Neurosci. Unit 1.16. Chapter 1. 2008. Biochemical fractionation of brain tissue for studies of receptor distribution and trafficking. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks WJ, Stoker AW. Protein tyrosine phosphatases: sequences and beyond. FEBS J. 2008;275:815. doi: 10.1111/j.1742-4658.2008.06248.x. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Venkitaramani DV, Xu J, Lombroso PJ. The role of STEP in Alzheimer disease. Channels (Austin) 2010a;4:347–350. doi: 10.4161/chan.4.5.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010b;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR, Cui Y, LeBoutillier JC, Marrone DF, Choi ES, De Zeeuw CI, Petit TL, Pozzo-Miller L, Silva AJ. Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci. 2005;25:9721–9734. doi: 10.1523/JNEUROSCI.2836-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Schenk F. Integration of olfactory information in a spatial representation enabling accurate arm choice in the radial arm maze. Learn Mem. 1996;2:299–319. doi: 10.1101/lm.2.6.299. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacol Ther. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Chiavegatto S. Molecular basis of aggression. Trends Neurosci. 2001;24:713–719. doi: 10.1016/s0166-2236(00)01996-2. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- Nicodemo AA, Pampillo M, Ferreira LT, Dale LB, Cregan T, Ribeiro FM, Ferguson SS. Pyk2 uncouples metabotropic glutamate receptor G protein signaling but facilitates ERK1/2 activation. Mol Brain. 2010;3:4. doi: 10.1186/1756-6606-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience. 1995;69:869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- Park JH, Widi GA, Gimbel DA, Harel NY, Lee DH, Strittmatter SM. Subcutaneous Nogo receptor removes brain amyloid-beta and improves spatial memory in Alzheimer’s transgenic mice. J Neurosci. 2006;26:13279–13286. doi: 10.1523/JNEUROSCI.4504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, Mills E, Hakim S, Salter MW, Taylor JR, Lombroso PJ. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The Dopamine/D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Sharma E, Zhao F, Bult A, Lombroso PJ. Identification of two alternatively spliced transcripts of STEP: a subfamily of brain-enriched protein tyrosine phosphatases. Brain Res Mol Brain Res. 1995;32:87–93. doi: 10.1016/0169-328x(95)00066-2. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Tashev R, Moura PJ, Venkitaramani DV, Prosperetti C, Centonze D, Paul S, Lombroso PJ. A substrate trapping mutant form of striatal-enriched protein tyrosine phosphatase prevents amphetamine-induced stereotypies and long-term potentiation in the striatum. Biol Psychiatry. 2009;65:637–645. doi: 10.1016/j.biopsych.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Toda K, Saibara T, Okada T, Onishi S, Shizuta Y. A loss of aggressive behaviour and its reinstatement by oestrogen in mice lacking the aromatase gene (Cyp19) J Endocrinol. 2001;168:217–220. doi: 10.1677/joe.0.1680217. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekovischeva OY, Aitta-Aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A, Sprengel R, Korpi ER. Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav. 2004;3:253–265. doi: 10.1111/j.1601-1848.2004.00075.x. [DOI] [PubMed] [Google Scholar]
- Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of Striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Royle GA, Gray EE, Abdipranoto A, Thangthaeng N, Jacobs N, Saab F, Tonegawa S, Heinemann SF, O’Dell TJ, Fanselow MS, Vissel B. A role for calcium-permeable AMPA receptors in synaptic plasticity and learning. PLoS ONE. 2010;5:e12818. doi: 10.1371/journal.pone.0012818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Takahashi E, Li W, Halt A, Wiltgen B, Ehninger D, Li GD, Hell JW, Kennedy MB, Silva AJ. Interactions between the NR2B receptor and CaMKII modulate synaptic plasticity and spatial learning. J Neurosci. 2007;27:13843–13853. doi: 10.1523/JNEUROSCI.4486-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]