Abstract
Carotid body glomus (CB) cells express different types of K+ channels such as TASK, BK, and Kv channels, and hypoxia has been shown to inhibit these channels. Here we report the presence of a ~72-pS channel that has not been described previously in CB cells. In cell-attached patches with 150 mM K+ in the pipette and bath solutions, TASK-like channels were present (~15 and ~36-pS). After formation of inside-out patches, a 72-pS channel became transiently active in ~18% of patches. The 72-pS channel was K+-selective, inhibited by 2–4 mM ATP and 10–100 μM glybenclamide. The 72-pS channel was observed in CB cells isolated from newborn, 2–3 week and 10–12 week-old rats. Reverse transcriptase-PCR and immunocytochemistry showed that Kir6.1, Kir6.2, SUR1 and SUR2 were expressed in CB glomus cells as well as in non-glomus cells. Acute hypoxia (~15 mmHg O2) inhibited TASK-like channels but failed to activate the 72-pS channel in cell-attached CB cells. K+ channel openers (diazoxide, pinacidil, levcromakalim), sodium cyanide and removal of extracellular glucose also did not activate the 72-pS channel in the cell-attached state. The hypoxia-induced elevation of intracellular [Ca2+] was unchanged by glybenclamide or diazoxide. NaCN-induced increase in [Ca2+] was not affected by 10 μM glybenclamide but inhibited by 100 μM glybenclamide. Acute glucose deprivation did not elevate [Ca2+] in the presence or absence of glybenclamide. These results show that an ATP-sensitive K+ channel is expressed in the plasma membrane of CB cells, but is not activated by short-term metabolic inhibition. The functional relevance of the 72-pS channel remains to be determined.
1. Introduction
Carotid body glomus (CB) cells sense low pO2 and initiate a cascade of events that ultimately leads to an increase in breathing and other cardiorespiratory responses. Initiation of excitation of CB cells by hypoxia is caused in large part by inhibition of background K+ channels that allow cell depolarization to occur as a result of a resting a Na+ conductance (Buckler, 2007; Carpenter and Peers, 2001). K+ channels that give rise to the O2-sensitive background K+ conductance have generally been described to be TASK, BK and/or KV channels (Buckler et al., 2000; Lopez-Barneo et al., 2004; Lopez-Lopez and Perez-Garcia, 2007; Peers and Wyatt, 2007). In isolated rat CB cells, TASK is the most active K+ channel near the resting membrane potential, and is inhibited by hypoxia (Buckler et al., 2000; Kim et al., 2009). This is consistent with recent findings that the basal catecholamine secretion was increased in TASK-1/3 null mice and the carotid nerve response to hypoxia was reduced in these mice (Ortega-Saenz et al., 2010; Trapp et al., 2008). However, mice lacking TASK still show a hypoxia-induced increase in catecholamine secretion from the CB and increase in ventilation, indicating that other ion channels are involved in hypoxia sensing.
In many cell types including neurons, a decrease in [ATP] has been shown to activate an ATP-sensitive K+ (KATP) channel. If present and active in the glomus cell, such activation would oppose the depolarization produced by leak channel inhibition, but these channels have not yet been identified in glomus cells. In the course of studying TASK function in excised patches of CB cells, we found the presence of an ion channel that was not open in cell-attached patches, but appeared after formation of inside-out and outside-out patches. In the present study, we characterized the biophysical properties of this channel. Single channel analysis and sensitivity of the channel by ATP and glybenclamide indicated that it is similar to those of ATP-sensitive K+ channels previously described in cardiac and neuronal cells. However, K+ channel openers failed to open the channel in CB cells. Acute hypoxia and glucose deprivation also did not cause activation of this K+ channel. Therefore, it is unlikely that the 72-pS channel is involved in acute hypoxia-induced excitation of CB. The potential role of this K+ channel in chronic hypoxia and glucose deprivation remains to be determined.
2. Material and methods
2.1. Carotid body cell isolation
The protocols for animal use in this study were approved by the Animal Care and Use Committees of Rosalind Franklin University and University of Arkansas for Medical Sciences. Rats (postnatal 0–18: 484 rats; 10–12 weeks: 15 rats) were anesthetized with isoflurane, decapitated, and the heads placed in ice-cold buffered saline solution (118 mM NaCl, 23 mM NaHCO3, 3 mM KCl, 2 mM KH2PO4, 1.2 mM CaCl2, 1 mM MgCl2, 10 mM Glucose, pH 7.2). CBs from both sides were dissected and placed in ice-cold low-Ca2+, low-Mg2+ phosphate buffered saline solution (low Ca2+/Mg2+-PBS: 137 mM NaCl, 2.8 mM KCl, 2 mM KH2PO4, 0.07 mM CaCl2, 0.05 mM MgCl2, pH 7.4). Each CB was cut into 3–4 pieces and placed in a solution containing trypsin (400 μg/ml) and collagenase (400 μg/ml) in low Ca2+/Mg2+-PBS and incubated at 37°C for 20–25 minutes. CBs were gently triturated using a fire polished Pasteur pipette to mechanically dissociate the cells. Enzymatic digestion was continued for additional few minutes when necessary. CB growth medium (Ham’s F-12, 10% fetal bovine serum, 23 mM glucose, 4 mM L-alanyl-glutamine (Glutamax-1), 10K units penicillin/streptomycin, and 300 μg/ml insulin) was added to stop enzyme activity. After brief trituration, the solution containing the digested CBs was centrifuged for 4 minutes at ~6000 rpm (~2000 × g) using a microcentrifuge. The supernatant was removed and warm CB growth media added to gently resuspend the pellet. This step was repeated to remove traces of enzymes. Suspended CB cells were placed on glass coverslips coated with polylysine, and incubated at 37°C for 50 minutes to allow settling and attachment of the cells. CB growth medium was further added to the plate containing the coverslips and then incubated at 37°C for additional 2 hours. Coverslips were then transferred to the recording chamber for electrophysiological experiments.
2.2. Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA). Channel current was filtered at 3 kHz using 8-pole Bessel filter (−3 dB; Frequency Devices, Haverhill, MA) and transferred to a computer using the Digidata 1320 interface at a sampling rate of 20 kHz. Single-channel currents were analyzed with the pCLAMP program (Version 10). Channel openings were analyzed to obtain channel activity (NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open). NPo was determined from ~20 s of current recording. The single channel current tracings shown in the figures were filtered at 1 kHz. In experiments using cell-attached and inside-out patches, pipette and bath solutions contained (mM): 150 KCl, 1 MgCl2, 5 EGTA, 11 glucose and 10 HEPES (pH 7.3). In other cell-attached recordings for testing the effect of NaCN and K+ channel openers, the bath solution contained (mM): 117 NaCl, 23 NaHCO3, 5 KCl, 1 CaCl2, 1 MgCl2, 11 glucose (pH 7.3). The bath solution was quickly switched to that without CaCl2 before forming inside-out patches to help detect the opening of the 72-pS channel. Outside-out patch was formed from the whole-cell configuration by gently lifting up the pipette. MgATP (0.1 mM) was added to the pipette solution when using outside-out patches. For statistics, Student’s t-test (for comparison of two sets of data) was used with p<0.05 as the criterion for significance. Data were analyzed using the Origin program and presented as mean±S.D.
2.3. Intracellular [Ca2+] measurement
Intracellular [Ca2+]i was measured by quantitative epifluorescence imaging using the calcium-sensitive dye fura-2. Cells plated on the coverslip were incubated with 4 μM fura-2 acetoxymethyl ester (fura-2 AM; Molecular Probes) for 30 minutes at 37°C. Fura-2 fluorescence emission was measured at 510 nm in response to alternating excitation at 340 and 380 nm. Images were acquired and stored using a NIKON TE2000 microscope and CCD (CoolSNAP HQ2) camera under computer control (MetaFluor: Molecular Devices). For each coverslip, the background light levels were determined and subtracted from each image before measurement of the fluorescence intensity ratio. [Ca2+]i was determined using the 340/380 fluorescence ratio as described previously (Wasicko et al., 1999). Calibration was performed using cell-free solutions (Grynkiewicz et al., 1985).
2.4. Hypoxia studies
Cell-attached patches were formed on CB cells and perfused with a bicarbonate-buffered solution containing 117 mM KCl, 23 mM NaHCO3, 1 mM MgCl2, 11 mM glucose and 10 mM HEPES and gassed with 5% CO2/95% air mixture (normoxia) for at least 60 min. After steady state channel activity was obtained, the perfusion solution was switched to solution gassed (for ~60 min) with 5% CO2/95% N2 mixture (hypoxia) for ~5 minutes (34°C). Glucose oxidase (24 units/ml) and catalase (135 units/ml) were added to the hypoxic solution to further reduce O2 pressure to <1% in one set of single channel recording experiments as indicated. The pipette solution contained (mM) 150 KCl, 1 MgCl2, 5 EGTA, 10 glucose and 10 HEPES (pH 7.3). The temperature of the perfusion solutions was kept at ~34°C, and the rate of perfusion was ~2.2 ml/min. Oxygen pressure of the solutions was checked using an oxygen meter (ISO2, WPI, Sarasota, USA) that was calibrated to 0% with solution gassed with pure nitrogen for 60 min and to 21% with solution gassed with air for 60 min at 34°C. The O2 partial pressure as judged by the reading on the meter for the hypoxic solution inside the recording chamber used in this study was ~2–3% (15–22 mmHg O2).
2.5. Real Time Reverse-transcriptase (RT)-PCR (qPCR)
Total RNA was prepared from whole CB isolated from rats age P14–16. cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). Using specific primers designed to yield a product of 100–150 bases, real time RT-PCR (qPCR) reaction was carried out for Kir6.1, Kir6.2B, SUR1, SUR2 and two reference genes, PPIA (peptidyl-proyl-isomerase A) and TBP (TATA-box binding protein). qPCR using SYBR green technology was performed on an iCycler iQ real-time detection system (Bio-Rad) in 96-well plates. The following amplification program was used: after 5 min of denaturation at 95°C, 50 cycles of real time PCR with 2-step amplification were performed consisting of 15 s at 95°C for denaturation, 45 s at 60°C for annealing and 1 min at 95°C for polymerase elongation. In each qPCR run, two reference genes and four KATP subtypes were run with cDNA in one 96 well PCR plate simultaneously. All samples were amplified in triplicate. The qPCR products after cleaning by MiniElute PCR purification kit (Qiagen) were run by 2% agarose gel electrophoresis to verify the expected qPCR products.
2.6. Immunocytochemistry
Dissociated CB cells of rats age P14–18 were plated on poly-D-lysine coated glass coverslip and cultured for 3–4 hrs in incubator. The cells were fixed with 4% paraformaldehyde and treated with PBS containing 0.4% Trion X-100 for 10 min at 24°C. After wash with PBS, cells were incubated with rabbit polyclonal antibodies against Kir6.1 (1:400 dilution, Alomone), Kir6.2 (1:400 dilution, Alomone), SUR1 (1:150 dilution, Santa Cruz), SUR2 (1:200 dilution, Santa Cruz) for overnight at 4 °C. Anti-TH (tyrosine hydroxylase) (1:500 dilution, Santa Cruz) monoclonal antibody was used for double labeling as a marker of the type I cells. The non-specific binding sites were blocked overnight by 5% donkey serum and 3% BSA in PBS containing 0.1% Trion X-100. Donkey anti-rabbit (DR) IgG tagged with Alexa Fluor 488 and donkey anti-mouse (DM) IgG tagged with Alexa Fluor 546 (Invitrogen) were used as secondary antibodies and incubated for 2 hrs at 24°C. After final wash, the cells were mounted on a glass slide with Prolong Gold anti-fade reagent containing DAPI (Invitrogen). Cells were observed under Nikon TE-2000 microscope at 400X and the images were taken, merged and analyzed by NIS Element software (Nikon). For control, Kir6.1 antibody (diluted 1:200) was incubated with Kir6.1 antigen (antibody and antigen ratio at 1:1) for 1 hour at 24°C. The mixture was spun at 10,000×g for 5 min and the supernatant was used for staining. For other controls, the procedure was the same except that the primary antibody was omitted.
3. Results
3.1. Activation of a 72-pS channel in inside-out patches
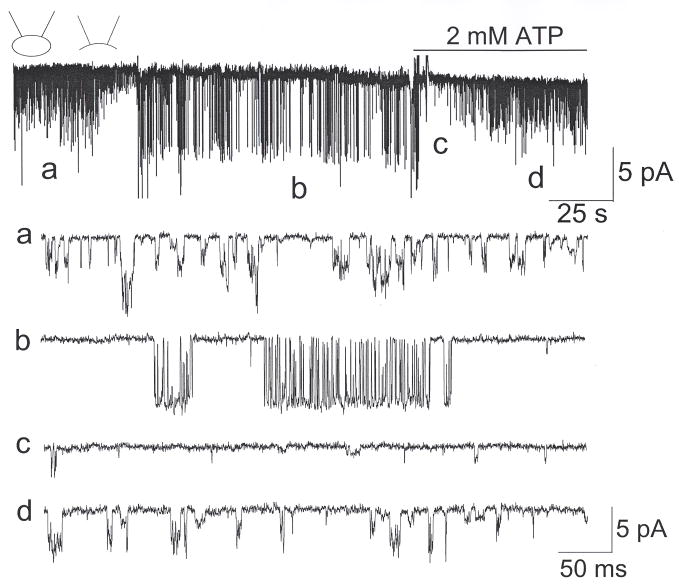
Cell-attached patches were formed on CB cells with pipette and bath solution containing 150 mM KCl. At pipette potential of +60 mV (membrane potential of ~−60 mV), single channel openings with kinetic properties similar to those of TASK-1 (~16-pS) and TASK-3 (~36-pS) were observed in >95% of cells chosen from clusters of 2–6 cells (Fig. 1). These channels have previously been identified to be TASK-like, based their sensitivity to acid, anandamide and divalent cations (Kim et al., 2009; Williams and Buckler, 2004). When inside-out patches were formed, activation of a 72-pS channel was observed in 18% of patches, as illustrated by two current tracings (#1 and #2). In some patches, the 72-pS channel opened and then closed immediately after one or two openings. In other patches, the 72-pS channel opened and remained open for several minutes and then closed. Thus, the ~72-pS channel was activated by washout of the intracellular compartment, but activation was usually not persistent. The 72-pS channel was present in CB cells isolated from 2–3 week-old (19/106 patches) as well as from 10–12 week-old rats (4/23 patches). For the rest of the study, we used CB cells isolated from 2–3 week old rats.
Figure 1. Opening of the 72-pS channel in inside-out patches.
Cell-attached patch was formed with pipette potential set at +60 mV. TASK-like channel openings are present in both patches. Upon forming the inside-out patch, 72-pS channels are activated. Expanded current tracings from the tracing #2 are shown.
The single channel openings of TASK-like and the 72-pS channels from tracing #2 are shown in expanded time scale at the bottom of Fig. 1. These current tracings show that the 72-pS channel opens in bursts of variable durations. Single channel openings were recorded at various membrane potentials ranging from −80 mV and +80 mV. Tracings obtained at −60 mV, 0 mV and +60 mV (membrane potentials) are shown in Fig. 2A. Outward currents showed less frequent transitions between open and closed states within each burst. Amplitude histograms were obtained from these tracings (Fig. 2B), and the mean amplitude levels were used to plot the current-voltage relationship that showed a weak inward rectification (Fig. 2C). At +60 mV, the single channel conductance was 38-pS. The mean open time durations determined by fitting the duration histograms were 1.1±0.1 ms at −60 mV and 3.6±0.9 ms at +60 mV (n=3). When bath [K+] was switched from 150 mM to 70 mM and 35 mM in inside-out patches, the reversal potential shifted from 0 mV to +16±3 mV and +32±3 mV (n=3), respectively. When K+ in the bath solution was replaced with Na+, the outward current of the 72-pS channel was no longer observed in inside-out patches (n=4). Thus, the 72-pS channel is a K+-selective ion channel.
Figure 2. Kinetic analysis of the 72-pS channel.
(A) Current tracings show openings of the 72-pS channel at membrane potentials of +60 mV and −60 mV.
(B) Graphs show amplitude and duration histograms of openings at both voltages.
(C) Current-voltage relationship is shown. Each point is the mean of 3 determinations.
3.2. Modulation of the 72-pS channel by ATP and glybenclamide
The biophysical properties of the 72-pS channel suggest that it is similar to the cardiac and neuronal KATP channels (Hibino et al., 2010). This would indicate that the activation of the 72-pS channel in inside-out patches was presumably due to loss of ATP. To test this further, 2 mM ATP was applied to the inside-out patch showing opening of the 72-pS channel. ATP caused an immediate closing of the 72-pS channel followed by slower activation of the TASK-like channels (Fig. 3). This is more clearly illustrated in the expanded current tracings. In two patches, washout of ATP quickly reactivated the 72-pS channel, and reapplication of ATP closed it, as expected of a KATP channel. As reported previously (Varas et al., 2007), activity of the TASK-like channels decreased following patch excision, and recovered after addition of ATP in the inside-out patch.
Figure 3. Inhibition of the 72-pS channel by ATP.
A cell-attached patch shows TASK-like activity (a). In this patch, formation of inside-out state caused rundown of TASK activity and also opened the 72-pS channel (b). Application of 2 mM MgATP to the cytosolic side of the membrane quickly closed the channel (c) and slowly activated TASK-like channels (d).
Although the KATP channel is inhibited by high [ATP], ATP (with Mg) is required to maintain channel function, presumably by keeping the channels phosphorylated and maintaining the membrane PIP2 concentration (Baukrowitz et al., 1998; Shyng and Nichols, 1998). Thus, at low concentrations of MgATP (~100–300 μM), opening of KATP channel is observed in inside-out patches (Terzic et al., 1995). Normally, when inside-out patches are formed from CB cells, the 72-pS channel opens, but closes after various time periods ranging from seconds to minutes. When 0.1 mM MgATP was present in the bath solution, the 72-pS channel opened persistently, and closed only when a high [ATP] was applied (Fig. 4A). This represents another piece of evidence indicating that the 72-pS channel is a KATP channel.
Figure 4. Modulation of the 72-pS channel by low [ATP] and glybenclamide.
(A) An inside-out patch initially shows only TASK-like channels. Pipette potential was +60 mV. When 0.1 mM ATP was applied to the bath solution, a 72-pS channel became active together with the TASK-like channel. Addition of 4 mM MgATP quickly closed the 72-pS channel, but TASK-like channels remained active. (B) An outside-out patch shows opening of the 72-pS channel. Application of 50 μM glybenclamide quickly closed the channel. Expanded current tracings are also shown. (C) Graph shows the strong inhibitory effect of glybenclamide on KATP activity (mean±SD of 4 determinations).
Sulphonylurea compounds are known to bind to the SUR subunit and inhibit the KATP channel activity (Ashcroft, 2005; Ashcroft, 2006; Inagaki et al., 1996). To further show that the 72-pS channel in CB cells represents the KATP channel, outside-out patches were formed and glybenclamide (50 μM) applied to the extracellular perfusion solution. As shown in Fig. 4B–C, glybenclamide produced a rapid inhibition of the 72-pS channel in 4 patches tested. In two outside-out patches showing opening of the KATP channel, 10 μM glybenclamide was also effective in closing the channel. These results show that the 72-pS channel in CB cells is a KATP channel whose complex contains the SUR subunit.
3.3. Lack of activation of the 72-pS channel by hypoxia, cyanide, low glucose and K+ channel openers
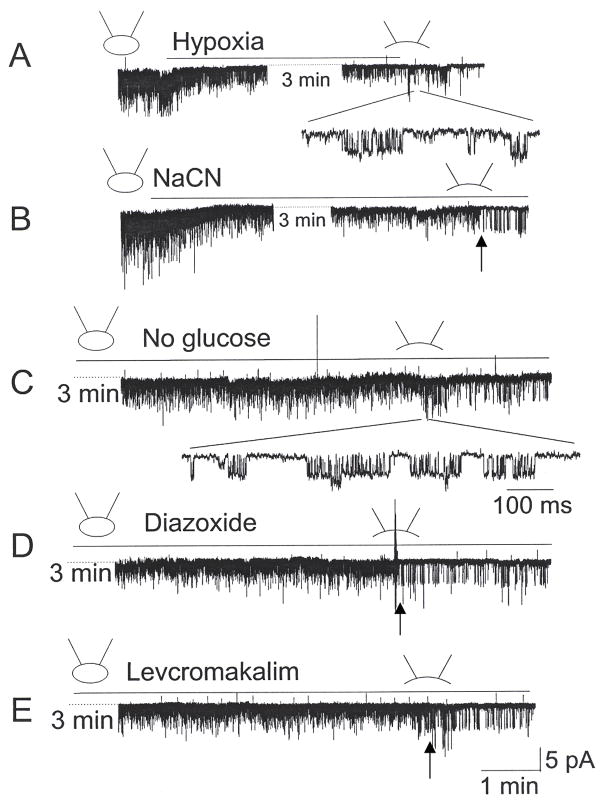
As hypoxia has been reported to activate KATP channels in certain neurons (Yamada and Inagaki, 2002; Yamada et al., 2001), we tested the possibility that hypoxia also activates the 72-pS channel in CB cells. Cell-attached patches were formed and perfused first with a bicarbonate-buffered normoxic solution (bubbled with 21% O2) for ~2 min and then switched to the hypoxic solution (bubbled with 0% O2) for ~5 min. In all 92 patches tested, hypoxia did not activate the 72-pS channel, although TASK was strongly inhibited. Whenever possible, at the end of each experiment, inside-out patch was formed to record opening of the 72-pS channel. The presence of the 72-pS channel in the patch was confirmed in four patches, and one such patch is shown in Fig. 5A. Brief openings of the ~72-pS channel indicated that the KATP channel was indeed present in the cell-attached patch (see expanded tracing). As the KATP channel does not always open after inside-out patch formation, we are uncertain as to how many patches actually contained the channel. In another set of experiments, cells were perfused with hypoxia solution containing glucose oxidase and catalase to further reduce O2 pressure. None of the cell-attached patches showed opening of the KATP channel (n=16). These results show that acute hypoxia does not lead to activation of the KATP channel in rat CB cells.
Figure 5. Hypoxia, NaCN, no glucose and K+ channel openers do not activate the KATP channel in cell-attached patches.
(A–B) Hypoxia (~1% O2) and NaCN (1 mM) inhibits TASK-like channel activity in cell-attached patches. A 72-pS channel is activated upon formation of inside-out patch, as shown by expanded current tracing or arrow.
(C–E) Removal of glucose, application of diazoxide (300 μM) or levocromakalim (200 μM) show no effect on TASK activity. Opening of the 72-pS channel following formation of inside-out patch is shown by arrows (D–E) or expanded tracing (C).
Another potential activator of the KATP channel is cyanide (CN) that inhibits mitochondrial cytochrome oxidase and prevents ATP generation. To test the effect of CN, cell-attached patches were formed on CB cells and perfused with solution containing 1 mM NaCN for ~6 min. NaCN decreased TASK activity to 26±5% of control (n=4), qualitatively similar to that observed with hypoxia (Fig. 5B). However, no opening of KATP channel was observed in the cell-attached state (n=26). In 4 of 29 patches, opening of the 72-pS channel could be confirmed following formation of inside-out patches, indicating the presence of the KATP channel in the cell-attached patch membrane (Fig. 5B). In another set of experiments, reduction of [glucose] from 11 mM to 0 mM in the perfusion solution produced no activation of the KATP channel in all 21 cell-attached patches. The presence of the 72-pS channel was confirmed in three inside-out patches (see expanded single channel current in Fig. 5C), showing that removal of glucose did not result in opening of the KATP channel in CB cells. Removal of glucose also produced no significant effect on TASK activity (0.21±0.03 to 0.20±0.03; p>0.05; n=6).
Next, we tested the effect of K+ channel openers by perfusing cell-attached patches with solution containing 300 μM diazoxide, 200 μM pinacidil or 200 μM levcromakalim. None of the cell-attached patches showed opening of the KATP channel during the ~6 min perfusion period. Again, the presence of the KATP channel was confirmed by forming inside-out patches at the end of the experiment. Although most inside-out patches did not show opening of the 72-pS channel, we repeated the experiments until we were able to clearly record the KATP channel in 3 inside-out patches for each of the K+ channel openers. Tracings shown in Fig. 5D–5E are examples of the current recording in CB cells perfused with diazoxide and levcromakalim. Together, these results show that the 72-pS channel in CB cells is not easily activated by agents that are known to activate the KATP channels.
3.4. Studies on intracellular [Ca2+]
The potential role of KATP channel in the hypoxic elevation of [Ca2+] was examined by studying the effect of glybenclamide. We hypothesized that if hypoxia were to activate the KATP channel, the ensuing hyperpolarization would reduce the hypoxia-induced depolarization and subsequent elevation of [Ca2+]. Fig. 6A shows the effect of 20 mM KCl and hypoxia on intracellular [Ca2+] in CB cells. As expected, 20 mM KCl produced a rapid and reversible elevation of intracellular [Ca2+], due to depolarization and opening of the voltage-gated Ca2+ channels. Glybenclamide (50 μM) itself had no effect on the basal [Ca2+] and also did not significantly affect the hypoxia-induced elevation of [Ca2+]. This is in keeping with the above finding (Fig. 5) that the KATP channel is not open at rest and not activated by hypoxia. The results are also in agreement with the recent observation that glybenclamide failed to prevent hypoxia-induced increase in carotid body sensory discharge (Peng et al., 2010).
Figure 6. Glybenclamide and diazoxide do not affect hypoxia-induced elevation of intracellular [Ca2+].
(A) Change in intracellular [Ca2+] in response to 20 mM KCl and hypoxic solution is shown in the presence and absence of 50 μM glybenclamide. The effect of glybenclamide is summarized in the bar graph below.
(B) Change in intracellular [Ca2+] in response to 20 mM KCl and hypoxic solution is shown in the presence and absence of 300 μM diazoxide. The effect of diazoxide is summarized in the bar graph below. NS=not significant (p>0.05). Each bar represents the mean±SD from 16–21 cells.
We also tested the effect of diazoxide, a KATP channel activator, on the hypoxia-induced elevation of [Ca2+]. CB cells were perfused with diazoxide (300 μM) for 5 min in normoxic solution, and then perfused with hypoxic solution containing diazoxide for 2 min. Averaged increase (n=21 cells) in [Ca2+] by hypoxia was not significantly affected by diazoxide (Fig. 6B). Diazoxide at a higher concentration (500 μM) also failed to significantly alter the hypoxia-induced elevation of [Ca2+] in 4 experiments. These results are also consistent with the single channel recording showing that diazoxide did not activate the KATP channel.
Cyanide has been shown to elevate intracellular [Ca2+] in rat CB cells (Buckler and Vaughan-Jones, 1998). In our experiments, NaCN (1 mM) produced a strong increase in [Ca2+] above that produced by 20 mM KCl (Fig. 7A). In the presence of 100 μM glybenclamide, NaCN-induced increased in [Ca2+] was smaller than that observed in the absence glybenclamide. This is probably due to non-specific effects of high concentration of glybenclamide that also affect other ion transporters such as Cl− channels, L-type Ca channels and Na-pump (Lee and Lee, 2005; Tominaga et al., 1995). At 10 μM glybenclamide, a concentration that is effective in blocking the KATP channel, the NaCN-induced increase in [Ca2+] was not affected (Fig. 7B–C). Removal of glucose had no effect on basal [Ca2+] either in the presence or absence of 100 μM glybenclamide (Fig. 7D). These results show that the KATP channel in CB cells does not play a significant role in hypoxia- or CN-induced excitation of rat CB cells.
Figure 7. Effect of glybenclamide on NaCN-induced increase in intracellular [Ca2+].
(A–B) NaCN (1 mM) produced a large increase in [Ca2+] and this was reduced by 100 μM glybenclamide (A) but not by 10 μM glybenclamide (B).
(C) Bar graph shows the summary of the effect of glybenclamide on CN-induced increase in [Ca2+]. Each bar is mean±SE of 18–32 cells.
(D) Removal of glucose shows no effect on basal [Ca2+] and no further effect by glybenclamide.
3.5. Expression of KATP channel subunits in CB cells
KATP channels are heterooctamers composed of four pore-forming Kir6.x subunits and four regulatory sulfonylurea receptor (SUR) subunits. To provide further evidence that the KATP channel is expressed in CB cells, RT-PCR and immunocytochemistry were performed in whole CB and isolated CB cells, respectively, using primers and antibodies designed to detect SUR1, SUR2, Kir6.1 and Kir6.2. RT-PCR analysis showed that all four subunits were expressed in the whole CB (Fig. 8A). To show that the KATP channel subunits are expressed in CB cells, immunocytochemistry was performed using antibodies directed against Kir6.1, Kir6.2, SUR1, and SUR2. Antibody against tyrosine hydroxylase (TH), a marker for CB glomus cells, was used to identify the glomus cells. CB glomus cells stained positive for both Kir6.1 and Kir6.2 subunits (Fig. 8Ba–b), and also positive for both SUR1 and SUR2 subunits (Fig. 8Bc–d). The majority of non-glomus cells (staining negative for TH) were also labeled with Kir6.1, Kir6.2, SUR1 and SUR2 antibodies. For Kir6.1 and Kir6.2, antibodies raised against a different epitope produced qualititatively similar results, i.e., both glomus and non-glomus cells were labeled with both Kir6.1 and Kir6.2, antibodies. No cells were labeled when the primary antibody was absent (see an example in Fig. 8E). For Kir6.1 for which the antigen is available, addition of antigen blocked the labeling of all cells (Fig. 8F). These data indicate that CB glomus cells express all four subunits of the KATP channel.
Figure 8. Expression of KATP channel subunits in CB cells.
(A) RT-PCR analysis for expression of Kir6.1, Kir6.2, SUR1 and SUR2. Expression of PP1A and TBP were used as controls. A single band on agarose gel was obtained for each channel subunit.
(B) Dissociated CB cells were labeled with polyclonal antibodies against Kir6.1, Kir6.2, SUR1 and SUR2, and identified using Alexa Fluor 488 (green). The same cells were also immunostained for tyrosine hydroxylase (TH), and identified using Alexa Fluor 546 (red). Cells expressing both KATP channel subunit and TH are identified as yellow-orange. Cells were also stained with DAPI (blue). Cells stained with DAPI and expressing TH are shown in pink. Scale bar is 50 μm.
4. Discussion
CB cells express a number of different K+ channels such as TASK, BK, HERG and Kv that help to modulate cell excitability at rest and during hypoxia. In this study, we show that a KATP channel is also functionally expressed in rat CB cells. Although electrophysiological analyses of CB cells have been performed in several laboratories, a KATP channel has not been previously identified. Based on our single-channel recording, the kinetic properties of the KATP channel in rat CB cells are similar to those of cardiac and pancreatic β cells, i.e., a high single channel conductance (72-pS) and inhibition by intracellular ATP and glybenclamide. This is further confirmed by immunocytochemical studies showing that both the Kir6.1, Kir6.2 and the sulphonylurea binding subunits (SUR1 and SUR2) that combine to form the KATP channel are present in CB cells (Aguilar-Bryan et al., 1998).
4.1. General properties of the KATP channel in CB cells
The single channel conductance (72-pS), the mild inward rectification, and the general kinetics of opening of the KATP channel in CB cells are similar to those of cardiac and pancreatic β cells (Ashcroft, 1988; Sakmann and Trube, 1984). Many neurons also express KATP channels with similar biophysical properties. With the exception of pancreatic β cells, the KATP channel is normally inactive in intact cells, due to closure by high intracellular [ATP]. The KATP channel opens when an inside-out patch is formed or intracellular [ATP] is reduced. In our studies, about 18% of patches showed opening of mostly one KATP channel upon patch excision, indicating a low cell surface density compared with two-three TASK-like channels that are present in almost every patch. Therefore, the number of KATP channels is ~3% of that of TASK channels in CB cells, if we assume that TASK open probability at rest is ~0.5. Assuming a CB cell capacitance of ~6 pF, 1 μF=1 cm2, and a pipette tip diameter of ~1–3 μm2 for 3–5 MΩ tip resistance (Sakmann and Neher, 1995), this would translate to ~18–50 KATP channels and ~600–1800 TASK channels in each CB cell.
Modulation of the KATP channel in CB cells by ATP and glybenclamide are similar to those of KATP channels in other cell types. Thus, 2–4 mM ATP applied to the cytosolic side of the membrane and glybenclamide (a sulphonylurea) applied to the extracellular side of the membrane inhibit the KATP channel. Activation of the KATP channel by 0.1 mM ATP is also consistent with the generally known property of the KATP channel that requires a low level of ATP for phosphorylation. Therefore, we were surprised to find that KATP channel openers such as diazoxide, pinacidil and levcromakalim did not activate the 72-pS channel. One possible reason that the drugs do not open the KATP channel in CB cells could be that the intracellular ATP strongly blocks the KATP channel, sufficient to counter the effect of K+ channel openers. In rat hypothalamic neurons, pinacidil, diazoxide and levcromakalim were not effective in activating the KATP channel when intracellular [ATP] was 3 mM, despite the presence of the KATP channel in the membrane (Sellers et al., 1992). In mouse skeletal muscle fibers, levcromakalim activated the KATP channel when intracellular [ATP] was 0.1 mM, but not when it was 1.0 mM (Hussain et al., 1994). Studies in general show that KATP channel openers are very weak activators in the presence of high [ATP] in the cell. Thus, many studies have used low intracellular [ATP] to achieve activation of the KATP channel by KATP channel openers (Hibino et al., 2010; Teramoto et al., 1997; Terzic et al., 1995). When only one KATP channel is present in the patch, the probability of its openings would be very low, and this may also explain the lack of KATP channel opening in CB cells in response to KATP channel openers. The failure of NaCN to open the KATP channel could be that CN-induced decrease in [ATP] is not sufficient to open the KATP channel. It is also possible that the large increase in [Ca2+] produced by CN blocks opening of the KATP channel in CB cells in the cell-attached condition, as high [Ca2+] is known to inhibit the KATP channel by depleting membrane PIP2 (Kim and Bang, 1999).
The immunocytochemistry data that Kir6.1, Kir6.2, SUR1 and SUR2 are present in CB cells suggest that multiple combinations of subunits may exist. Studies using cloned subunits have shown that Kir6.1/SUR and Kir6.2/SUR form channels with single channel conductance levels of ~30–40-pS and ~70–80 pS, respectively (Repunte et al., 1999). Therefore, the ~72-pS channel in the CB cells is most likely made up of Kir6.2/SUR. Kir6.2/SUR2B is an isoform present in the nonvascular smooth muscle cells (Hibino et al., 2010). Thus, the CB KATP channel could be made up of Kir6.2/SUR1 and/or Kir6.2/SUR2A. The subunit composition of the KATP channel is highly variable in neurons. For example, rat dorsal vagal neurons express Kir6.2/SUR1 whereas rat dorsal root ganglion neurons express both Kir6.2/SUR1 and Kir6.2/SUR2 (Karschin et al., 1998). In glucose sensing neurons in rat ventromedial hypothalamus and the cholinergic inter-neurons in the striatum, Kir6.1/SUR1 is the main KATP channel (Lee et al., 1998; Lee et al., 1999). Thus, neuronal KATP channels are more diverse in subunit composition than cardiac or pancreatic KATP channels. The kinetics properties of the KATP channel in rat CB cells resemble those of the pancreatic β cell (Kir6.2/SUR1) and cardiac myocytes (Kir6.2/SUR2A), based on their single channel conductance. However, the pharmacology seems different from those of pancreatic β cell and cardiac myocytes, as K+ channel openers failed to activate the KATP channel in rat CB cells. The reason for this difference is not clear at present. Expression of Kir6.1 has recently been shown in glomus cells from cat carotid body, indicating that the KATP channel is likely to be present in other species (Fitzgerald et al., 2011).
4.2. Hypoxia and the KATP channel in CB cells
KATP channels in cardiac and neuronal cells are not active under normoxic conditions; thus, no significant biological role is anticipated under normal physiological conditions. However, under stressed conditions, altered phenotypes are observed when the KATP channel is made non-functional. For example, wild type mice show elevated S-T segment on the electrocardiogram (EKG) in response to ischemia. In Kir6.2 knockout mice, the elevation of the S-T segment was reduced, indicating that the KATP channel was the cause of the EKG abnormality (Li et al., 2000; Suzuki et al., 2002). In Kir6.2 knockout mice, ischemic preconditioning was absent, indicating that the KATP channel provided a protective role against ischemia (Gumina et al., 2003). These mutant mice also showed increased sensitivity to sympathetic stress, resulting in arrhythmia in response to high sympathetic challenge (Zingman et al., 2002). Mutant mice lacking Kir6.2 were susceptible to generalized seizures resulting in severe tonic-chronic convulsion and death within 22 s in response to hypoxia (~5% O2), due to the missing KATP channels at the postsynaptic site of the substantia nigra reticulata (Yamada et al., 2001). These studies show that the KATP channels are activated under stressed conditions to provide a neuroprotective role.
In CB cells, we observed no activation of the KATP channels in response to short-lasting hypoxia. This result is generally consistent with previous measurements of ATP content. Moderate hypoxia causes only a slight decrease in ATP content (Obeso et al., 1985) and the metabolic poison, cyanide, causes a 45% decrease in ATP content over 5 min (Obeso et al., 1989). Furthermore, uncouplers DNP and CCCP failed to alter ATP content (Obeso et al., 1989). This suggests that ATP levels are maintained at millimolar levels during even prolonged hypoxic stress, and millimolar levels of ATP prevent KATP activation. This likely explains why previous investigators failed to detect the KATP channel in earlier studies (Peers and O’Donnell, 1990). If so, then a modulatory role for the KATP channel might only be manifest under conditions where ATP production is significantly impaired such as during prolonged glucose deprivation, but this may take considerable time depending on the metabolic reserves of the CB cells. Although ATP is considered to be the primary determinant of KATP channel activity, other factors such as ADP, pH, divalent cations and membrane PIP2 are also significant modulators (Ashcroft, 1988; Nichols, 2006). For example, divalent cations inhibit KATP channel activity (Findlay, 1988). The lack of activation of KATP channel by hypoxia (and by CN) in CB cells could therefore partly be due to the elevated [Ca2+]. Alternatively, activation of the KATP channel may be dependent on cell-to-cell interactions within the intact organ, and this would be disrupted following dissociation. Fitzgerald et al. demonstrated an inhibition of acetylcholine and ATP secretion by exogenous H2S, which acted through activation of KATP channel (Fitzgerald et al., 2011). Since H2S is endogenously produced in the CB (Peng et al., 2010), it is a potential pathway for modulating organ function through the KATP channels present in the glomus cells.
In keeping with the single channel recording data, our studies using glybenclamide show that KATP channels are not involved in hypoxia- and CN- induced elevation of intracellular [Ca2+]. The lack of effect of K+ channel openers on the KATP channel also agrees with the finding that diazoxide has no effect on the hypoxia-induced elevation of intracellular [Ca2+]. These results are consistent with the observation that pinacidil and levcromakalim (activators of KATP channel) showed no effect on the CB afferent nerve response to acute hypoxia (Takahashi et al., 2005). In our studies, removal of glucose did not activate the KATP channel and showed no effect on basal [Ca2+] in the presence or absence of glybenclamide, indicating that the KATP channel is not involved in acute metabolic stress-induced CB excitation. Our result is in agreement with an earlier study showing no effect of removal of glucose on CB nerve activity (Conde et al., 2007), but contradicts another study in which removal of glucose enhanced intracellular [Ca2+] (Garcia-Fernandez et al., 2007).
In conclusion, the present results demonstrate that a K+ channel is expressed in carotid body glomus cells with characteristics consistent with a KATP channel, including channel conductance, sensitivity to ATP and sensitivity to KATP blockers. The channel was inactive under normal physiological conditions or during acute hypoxia and during exposure to metabolic inhibitors (CN, no glucose) and K+ channel openers. At present, it is not clear under which conditions this channel may be activated, but one may speculate that it may be activated during long-term metabolic stresses such as long-term glucose deprivation or chronic hypoxia.
Acknowledgments
This work was funded by NIH grant HL054621.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar-Bryan L, et al. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–45. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. Adenosine 5′-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–58. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM. From molecule to malady. Nature. 2006;440:440–7. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Buckler KJ. TASK-like potassium channels and oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2007;157:55–64. doi: 10.1016/j.resp.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513(Pt 3):819–33. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, et al. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525(Pt 1):135–42. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter E, Peers C. A standing Na+ conductance in rat carotid body type I cells. Neuroreport. 2001;12:1421–5. doi: 10.1097/00001756-200105250-00025. [DOI] [PubMed] [Google Scholar]
- Conde SV, et al. Low glucose effects on rat carotid body chemoreceptor cells’ secretory responses and action potential frequency in the carotid sinus nerve. J Physiol. 2007;585:721–30. doi: 10.1113/jphysiol.2007.144261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay I. Calcium-dependent inactivation of the ATP-sensitive K+ channel of rat ventricular myocytes. Biochim Biophys Acta. 1988;943:297–304. doi: 10.1016/0005-2736(88)90561-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RS, et al. The impact of hydrogen sulfide (H(2)S) on neurotransmitter release from the cat carotid body. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fernandez M, et al. Mechanisms of low-glucose sensitivity in carotid body glomus cells. Diabetes. 2007;56:2893–900. doi: 10.2337/db07-0122. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, et al. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–50. [PubMed] [Google Scholar]
- Gumina RJ, et al. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–13. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Hibino H, et al. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Hussain M, et al. Mechanism of action of a K+ channel activator BRL 38227 on ATP-sensitive K+ channels in mouse skeletal muscle fibres. J Physiol. 1994;478(Pt 3):523–32. doi: 10.1113/jphysiol.1994.sp020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Karschin A, et al. KATP channel formation by the sulphonylurea receptors SUR1 with Kir6.2 subunits in rat dorsal vagal neurons in situ. J Physiol. 1998;509(Pt 2):339–46. doi: 10.1111/j.1469-7793.1998.339bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Bang H. Modulation of rat atrial G protein-coupled K+ channel function by phospholipids. J Physiol. 1999;517(Pt 1):59–74. doi: 10.1111/j.1469-7793.1999.0059z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, et al. Heteromeric TASK-1/TASK-3 is the major oxygen-sensitive background K+ channel in rat carotid body glomus cells. J Physiol. 2009;587:2963–75. doi: 10.1113/jphysiol.2009.171181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, et al. Identification of an ATP-sensitive potassium channel current in rat striatal cholinergic interneurones. J Physiol. 1998;510(Pt 2):441–53. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, et al. Glucose-receptive neurones in the rat ventromedial hypothalamus express KATP channels composed of Kir6.1 and SUR1 subunits. J Physiol. 1999;515(Pt 2):439–52. doi: 10.1111/j.1469-7793.1999.439ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Lee CO. Inhibition of Na+-K+ pump and L-type Ca2+ channel by glibenclamide in Guinea pig ventricular myocytes. J Pharmacol Exp Ther. 2005;312:61–8. doi: 10.1124/jpet.104.074369. [DOI] [PubMed] [Google Scholar]
- Li RA, et al. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837–9. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, et al. Regulation of oxygen sensing by ion channels. J Appl Physiol. 2004;96:1187–95. doi: 10.1152/japplphysiol.00929.2003. discussion 1170–2. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Perez-Garcia MT. Oxygen sensitive Kv channels in the carotid body. Respir Physiol Neurobiol. 2007;157:65–74. doi: 10.1016/j.resp.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Obeso A, et al. Correlation between adenosine triphosphate levels, dopamine release and electrical activity in the carotid body: support for the metabolic hypothesis of chemoreception. Brain Res. 1985;348:64–8. doi: 10.1016/0006-8993(85)90360-9. [DOI] [PubMed] [Google Scholar]
- Obeso A, et al. Effects of cyanide and uncouplers on chemoreceptor activity and ATP content of the cat carotid body. Brain Res. 1989;481:250–7. doi: 10.1016/0006-8993(89)90801-9. [DOI] [PubMed] [Google Scholar]
- Ortega-Saenz P, et al. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010;135:379–92. doi: 10.1085/jgp.200910302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C, O’Donnell J. Potassium currents recorded in type I carotid body cells from the neonatal rat and their modulation by chemoexcitatory agents. Brain Res. 1990;522:259–66. doi: 10.1016/0006-8993(90)91470-2. [DOI] [PubMed] [Google Scholar]
- Peers C, Wyatt CN. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007;157:75–82. doi: 10.1016/j.resp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Peng YJ, et al. H(2)S mediates O(2) sensing in the carotid body. Proc Natl Acad Sci U S A. 2010;107:10719–24. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repunte VP, et al. Extracellular links in Kir subunits control the unitary conductance of SUR/Kir6.0 ion channels. EMBO J. 1999;18:3317–24. doi: 10.1093/emboj/18.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Neher E. Chapter 21: Geometric parameters of pipettes and membrane patches. plenum press; 1995. Single channel recording. [Google Scholar]
- Sakmann B, Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984;347:641–57. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–41. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Suzuki M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, et al. Doxapram stimulates the carotid body via a different mechanism than hypoxic chemotransduction. Respir Physiol Neurobiol. 2005;147:1–9. doi: 10.1016/j.resp.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Teramoto N, et al. Effects of levcromakalim and nucleoside diphosphates on glibenclamide-sensitive K+ channels in pig urethral myocytes. Br J Pharmacol. 1997;120:1229–40. doi: 10.1038/sj.bjp.0701033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A, et al. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–45. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Tominaga M, et al. Glibenclamide, an ATP-sensitive K+ channel blocker, inhibits cardiac cAMP-activated Cl− conductance. Circ Res. 1995;77:417–23. doi: 10.1161/01.res.77.2.417. [DOI] [PubMed] [Google Scholar]
- Trapp S, et al. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008;28:8844–50. doi: 10.1523/JNEUROSCI.1810-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varas R, et al. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol. 2007;583:521–36. doi: 10.1113/jphysiol.2007.135657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasicko MJ, et al. Resetting and postnatal maturation of oxygen chemosensitivity in rat carotid chemoreceptor cells. J Physiol. 1999;514 (Pt 2):493–503. doi: 10.1111/j.1469-7793.1999.493ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA, Buckler KJ. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L221–30. doi: 10.1152/ajplung.00010.2003. [DOI] [PubMed] [Google Scholar]
- Yamada K, Inagaki N. ATP-sensitive K(+) channels in the brain: sensors of hypoxic conditions. News Physiol Sci. 2002;17:127–30. doi: 10.1152/nips.01384.2001. [DOI] [PubMed] [Google Scholar]
- Yamada K, et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- Zingman LV, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–83. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]