Abstract
In vulnerable individuals, exposure to stressors can result in chronic disorders such as generalized anxiety disorder (GAD), major depressive disorder (MDD), and post-traumatic stress disorder (PTSD). The extended amygdala is critically implicated in mediating acute and chronic stress responsivity and anxiety-like behaviors. The bed nucleus of the stria terminalis (BNST), a subregion of the extended amygdala, serves as a relay of corticolimbic information to the paraventricular nucleus of the hypothalamus (PVN) to directly influence the stress response. To investigate the influence of the corticosteroid milieu and housing conditions on BNST function, adult C57Bl/6J were either acutely or chronically administered corticosterone (CORT, 25 mg/kg in sesame oil) or vehicle (sesame oil) or were group housed or socially isolated for 1 day (acute) or 6–8 weeks (chronic). To ascertain whether these stressors could influence anxiety-like behavior, studies were performed using the novel open-field (NOF) and the elevated zero maze (EZM) tests. To investigate potential associated changes in plasticity, alterations in BNST function were assessed using ex vivo extracellular field potential recordings in the (dorsal-lateral) dlBNST and a high frequency stimulus protocol to induce long-term potentiation (LTP). Our results suggest that chronic CORT injections and chronic social isolation housing conditions lead to an increase in anxiety-like behavior on the EZM and NOF. Chronically stressed mice also displayed a parallel blunting of LTP in the dlBNST. Conversely, acute social isolation housing had no effect on anxiety-like behavior but still resulted in a blunting of LTP in the dlBNST. Collectively, our results suggest acute and chronic stressors can have a distinct profile on plasticity in the BNST that is not uniformly associated with an increase in anxiety-like behavior.
Keywords: anxiety, stress, corticosterone, housing, isolation, plasticity, bed nucleus of the stria terminalis
1. Introduction
In susceptible individuals, chronic life stressors are associated with maladaptive responses that present in clinical populations as psychopathological conditions such as generalized anxiety disorder (GAD), major depressive disorder (MDD), obsessive compulsive disorder (OCD), and post-traumatic stress disorder (PTSD). Importantly, these affective disorders may also be characterized by abnormalities in glucocorticoid secretion [1]. Elucidating the short and long-term effects of acute and chronic stress on neuroplasticity in critical brain regions implicated in hypothalamic pituitary adrenal (HPA) axis regulation is of paramount importance and may have broad implications for the treatment of affective disorders.
The bed nucleus of the stria terminalis (BNST), a subregion of the extended amygdala, has been heavily implicated in stress-induced plasticity. The BNST is considered an integral regulator of the HPA stress axis. It acts as a critical intermediary by receiving stressor inputs from the corticolimbic system and sending projections (primarily GABAergic) to the paraventricular nucleus of the hypothalamus (PVN), where corticotrophin releasing hormone (CRH) is released, inducing pituitary activation to initiate the peripheral stress response [2]. Moreover, the limbic integration and stress regulatory roles of the heterogeneous BNST nuclei are likely site-specific and quite complex [3–6].
Importantly, the BNST has been implicated in longer-duration and sustained increases in anxiety-like behavior [7]. Previous studies have demonstrated restraint stress and corticosterone (CORT) (systemic and micropellets implanted into the BNST) administered chronically can result in structural alterations in the BNST and increased anxiety-like behavior, although the effects on behavior and BNST plasticity in adult C57Bl/6J mice are unknown [8–11]. Interestingly, an additional type of stressor, chronic social isolation, may provide an understudied and uniquely relevant model for certain components of anxiety- and depression-related affective disorders that are often diagnostically characterized by, among other symptoms, social withdrawal [12–16]. Previous social isolation studies in adult C57 mice have found an increase in anxiety- and/or depressive-like behavior [17, 18]. Moreover, previous work has shown that chronic social isolation does not result in an increase in plasma corticosterone levels in C57 mice [19]. These and other studies suggest that altered anxiety-like behavior following chronic stress may be associated with distinct profiles on plasticity in the BNST, as has been reported in other limbic HPA-regulatory regions [20–22]. Therefore, we sought to utilize two unique stressors that have distinct corticosterone profiles to determine if they could similarly impact anxiety-like behavior and LTP in the dlBNST.
Given the key role the BNST plays in integrating stress and limbic inputs to regulate the stress response, it is critically important to understand the differential response acute and chronic CORT and social isolation stress may have on anxiety-like behavior and LTP in the dlBNST. Therefore, in this study we assessed the effects of acute and chronic CORT and social isolation on anxiety-like behaviors measured in the elevated zero maze (EZM) and novel open field (NOF) as well as on the early and late components of long-term potentiation in the dlBNST.
2. Methods
2.1 Subjects and Treatments
All procedures were performed according to Vanderbilt University Institutional Animal Care and Use Committee approved procedures. Male C57Bl/6J mice (7–8 weeks old on arrival; Jackson Laboratories, Bar Harbor, ME), were used for all experiments. All animals were housed in groups of two to five, unless otherwise noted. Food and water were available ad libitum. Body weights were recorded twice a week. All mice were kept on a 12-hr light-dark cycle (light on at 07:00).
To assess the influence of acute and chronic corticosteroids on behavior and plasticity, two cohorts of mice were randomly assigned to one of four experimental groups (n=9–11 per group): acute vehicle (sesame oil; Sigma), acute corticosterone (acute CORT; 25mg/kg in sesame oil), chronic vehicle, or chronic CORT. Acute CORT and vehicle treated mice received a single subcutaneous injection while chronic vehicle and chronic CORT mice received daily subcutaneous injections over a 10 days period. We chose to use the paradigm (CORT: 25mg/kg/day for 10 days) from Pego and colleagues to further investigate their reported findings of altered BNST morphology and increased anxiety-like behavior in rodents chronically but not acutely treated with CORT [8]. The light-dark cycle utilized in our behavioral facility and the time of day of the CORT injections were in accordance with the ascending phase (13:00–16:00) of the circadian CORT cycle, as shown by previous studies [23, 24]. Prior to beginning CORT or vehicle injections, all mice were handled for 2–3 days and then administered 3 days of habituating saline injections. The first cohort (Cohort 1) underwent the EZM and NOF behavioral assays ~24 hours after the final injection. A second cohort (Cohort 2) of mice was treated in an identical manner and ~24 hours after final injection, used for electrophysiology experiments.
To assess the influence of a social stressor on behavior and plasticity, 2 additional cohorts of mice were randomly assigned to one of three treatment groups (n=10–12 per group): group housing, acute social isolation housing (1 day), or chronic social isolation housing (42–56 days). This third cohort (cohort 3) underwent the EZM and NOF behavioral assays. The fourth cohort (cohort 4) was treated in an identical manner to cohort 3 but used for electrophysiology experiments.
2.2 Behavior
Mice from Cohorts 1 and 3 were tested on the EZM and NOF 20–24 hours after their final CORT injection, final vehicle injection, acute social isolation (24 hours of isolation), chronic social isolation (42–56 days of isolation), or group housing. Mice were brought to the behavioral testing rooms to acclimate one hour prior to testing. All mice were tested between 13:00 and 17:00. All behavior tests were performed in the Vanderbilt Murine Neurobehavioral Lab shared behavior core facilities at Vanderbilt University Medical Center (https://medschool.mc.vanderbilt.edu/mnl).
2.3 Elevated zero maze (EZM)
The elevated plus and zero mazes (EPM and EZM, respectively) are behavioral assays frequently used to assess anxiety-like behavior in rodents [25]. An EZM (34 cm inner diameter, 46 cm outer diameter, placed 40 cm off the ground on 4 braced legs) was used and lux levels were assessed midway along the open and closed arms (2–6 lux for closed arms and 25–30 lux for open arms). At the start of the test, mice were placed in the center of one of the open quadrants facing the closed quadrant. The test lasted 5 minutes. In between subjects, the apparatus was cleaned with 30% ethanol. Mice were considered to be in a quadrant when 75% of their body was in the area. Time spent in the open and closed areas, as well as, number of entries into the open and closed areas were scored using ANY-Maze software (San Diego Instruments, San Diego, CA).
2.4 Novel Open Field (NOF)
Mice were tested for open field locomotor activity in a novel environment as previously described [26]. Briefly, mice were tested in a one hour session using automated experimental chambers (745 cm2 cm; MED-OFA-510; MED Associates, St Albans, VT) under constant illumination within a sound-attenuated room. The center area of the NOF measured 352cm2. Between subjects, the apparatus was cleaned with 30% ethanol. Analysis of open field activity was performed using Activity Monitor v5.10 (MED Associates).
2.5 Electrophysiology: Slice preparation
Slices were prepared as previously described [27, 28]. Mice from Cohorts 2 and 4 were brought to a holding chamber and allowed to acclimate for 1 hour. Mice were then decapitated under isoflurane. For field potential recordings, the brains were removed quickly and placed in ice-cold artificial cerebrospinal fluid (ACSF) with sucrose: (in mM) 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3 saturated with 95% O2/5% CO2 Hemisected coronal slices (300 μm) were prepared with a Vibratome (Pelco). Slices containing anterior portions of the dorsal anterolateral BNST (dlBNST; bregma, 0.26-0.02 mm) (Franklin and Paxinos, 1997) were selected using the internal capsule, anterior commissure, and stria terminalis as landmarks.
2.6 Electrophysiology: Field potential recordings
Recordings were conducted as previously described [27, 28]. Briefly, after dissection, slices were transferred to an interface recording chamber where they were perfused with heated (29°C), oxygenated (95% O2-5% CO2) ACSF at a rate of 2 ml/min. Slices were allowed to equilibrate in ACSF for at least 1 hour before experiments began. A bipolar stainless steel stimulating electrode and a borosilicate glass recording electrode filled with ACSF were placed in the dlBNST to elicit and record an extracellular field response. Baseline responses to a stimulus (50 μsec) at an intensity that produced 40% of the maximum response were recorded for no less than 20 min at a rate of 0.05 Hz. To elicit LTP, two trains of 100 Hz, 1 sec tetanus were delivered with a 20 sec intertrain interval at the same intensity as baseline test pulses. Experiments in which the N1 (an indicator of the approximate number of fibers excited and general health of the slice) changed by >20% were discarded. If the N1 could not be accurately estimated, input-output curves taken at the beginning and end of experiments were used to determine whether the N1 at higher stimulation intensities changed.
2.7 Statistical Analysis
All values are expressed as mean ± SEM. Data was analyzed using the Prism Software package. For the CORT experiments, statistical significance was assessed using a Two-Way ANOVA for all tests (P < 0.05) and when a significant effect was found, Bonferroni’s test was used for post-hoc analysis. For the Housing (isolation stress) experiments, statistical significance was assessed using a One-Way ANOVA for all tests (P < 0.05) and when a significant effect was found, Tukey’s HSD test was used for post-hoc analysis.
3. Results
3.1 Chronic CORT mice display increased levels of anxiety-like behavior in the EZM and NOF
In the current study, 20–24 hours after the final chronic or acute CORT or vehicle injection, mice were tested for changes in anxiety like-behavior on the EZM and NOF. We found that mice in the chronic CORT group spent significantly less time in the open arm compared to all other groups in the EZM. There was a significant effect of treatment (F1,40=10.81; p < 0.05) and interaction of treatment x time (F1,40 = 6.409; p < 0.05) but not time, consistent with an increase in anxiety-like behavior in the chronic CORT group (Fig. 1A). For number of open arm entries on the EZM, there was no significant effect of treatment (Fig. 1B). Closed arm entries were also analyzed but no significant differences were found (data not shown; F1,40=0.3365) No differences were found in distance traveled on the EZM (data not shown). On the NOF, we found that the chronic CORT group spent significantly less time in the center zone compared to the acute vehicle treated group. There was a significant effect of treatment (Fig. 1C; F1,40=6.869; p < 0.05) and time (F1,40= 14.699; p < 0.05) but not treatment x time. No differences were found in distance travelled in the NOF in any group (Fig. 1D)
Figure 1. Anxiety-like Behavior is decreased in chronic corticosterone treated mice.
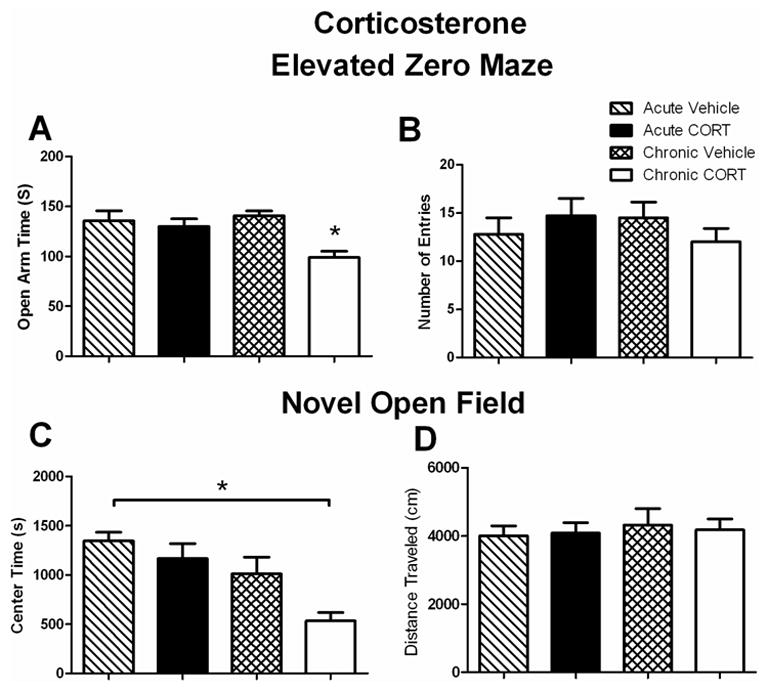
A. Time spent on the open arm of the EZM (seconds). All mice were tested 20–24 hours after the final injection of vehicle or CORT. Chronic CORT treated mice spent significantly less time in the open arm of the EZM compared to all other groups (n=9–12 per group), *p<0.05. Data are represented as mean ± SEM.
B. Number of open arm entries on the EZM. No significant differences were found on number of open arm entries. (n=9–12 per group). Data are represented as mean ± SEM.
C. Time spent in the center zone of the NOF. All mice were tested 20–24 hours after the final injection of vehicle or CORT. Chronic CORT treated mice spent significantly less time in the center zone compared to acute vehicle treated mice (n=9–12 per group), *p<0.05. Data are represented as mean ± SEM.
D. Distance Traveled on the NOF. No differences were found in the distance traveled on the NOF. (n=9–12 per group). Data are represented as mean ± SEM.
3.2 Chronic socially isolated (6–8 weeks) mice display increased levels of anxiety-like behavior in the EZM and NOF
Changes in anxiety like-behavior on the EZM and NOF were assessed in group housed or mice socially isolated for 6–8 weeks (chronic) or 24 hours (acute). We found that mice socially isolated for 6–8 weeks spent significantly less time in the open arm compared to group housed mice on the EZM (Fig. 2A; F2,28=4.485; p < 0.05) consistent with an increase in anxiety-like behavior in the chronic socially isolated group compared to group house mice. Moreover, the mice socially isolated for 24 hours showed a trend for a decrease in time spent in the open arm of the EZM (Fig. 2A; p = 0.09) compared to group housed mice. For number of open arm entries on the EZM, no significant difference was found (Fig. 2B; F2,28=0.006). In addition, no differences were found on closed arm entries on the EZM (data not shown; F2,28=1.373). No effect on distance travelled in the EZM was observed (data not shown). On the NOF, we found that the chronic socially isolated group spent significantly less time in the center zone compared to group housed mice (Fig. 2C; F2,28=3.803; p < 0.05). No differences were found in distance travelled on the NOF (Fig. 2D). Acute socially isolated mice were not significantly different than group housed mice on any behavioral measure.
Figure 2. Anxiety-like Behavior is decreased in chronically socially isolated mice compared to group housed mice.
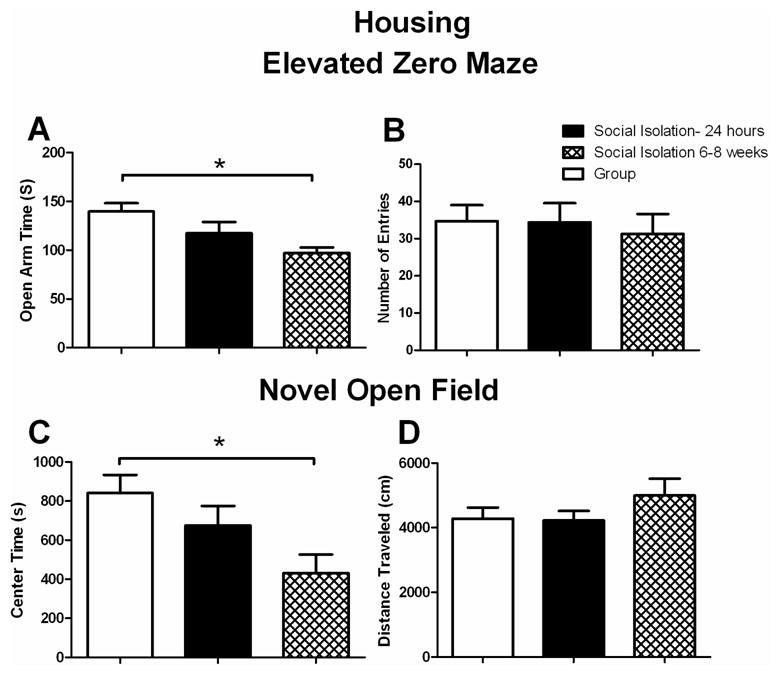
A. Time spent on the open arm of the EZM (seconds). All mice were tested after being group housed or socially isolated for 24 hours or 5–6 weeks. Chronic socially isolated mice spent significantly less time in the open arm of the EZM compared to group housed mice (n=7–13 per group), *p<0.05. Data are represented as mean ± SEM.
B. Number of open arm entries on the EZM. No significant differences were found on number of open arm entries. (n=7–13 per group). Data are represented as mean ± SEM.
C. Time spent in the center zone of the NOF. Chronic socially isolated mice spent significantly less time in the center zone of the NOF compared to group housed mice (n=7–13 per group), *p<0.05. Data are represented as mean ± SEM.
D. Distance Traveled on the NOF. No differences were found in the distance traveled on the NOF. (n=7–13 per group). Data are represented as mean ± SEM.
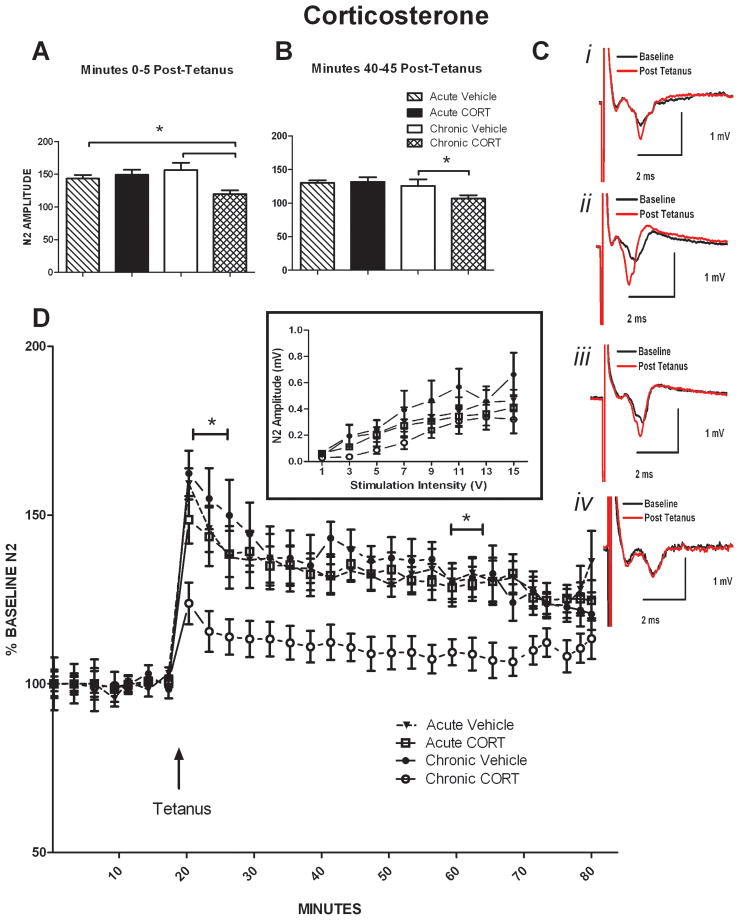
3.3 Chronic CORT mice display blunted LTP in the dlBNST compared to other groups
To investigate whether glutamatergic plasticity in the dlBNST would be altered following the chronic or acute CORT and vehicle treatments, we stimulated these afferents with a tetanus protocol (two 100 Hz, 1 sec trains with a 20 sec intertrain interval; Figure 3D) previously shown to induce NMDA receptor dependent LTP [28]. Input-output curves were generated at the start of all experiments in order for a submaximal baseline stimulation intensity to be determined (Inset, Figure 3D). An effect of stimulation intensity (V) (F7,98= 43.38; p < 0.05) but not stress conditions nor stress-conditions × stimulation intensity (V) interactions was observed across these input-output plots. Based on previous research from our lab, this NMDA receptor-dependent LTP can be separated into an early (0–5 minutes post-tetanus) and late (40–45 minutes) component. Thus, ethanol has been shown to impair, whereas cocaine has been shown to selectively enhance, the early but not late component of LTP in the dlBNST [27, 28]. Here, we found this protocol elicited significantly impaired LTP of the N2 [6-cyano-7-nitroquinoxaline-2,3-dione (CNQX)-sensitive synaptic response] 0–5 minutes after tetanus in the chronic CORT chronic group compared to the chronic vehicle and acute CORT groups (Fig. 3A; acute vehicle: 143.4 ± 4.52%; acute CORT: 156.4 ± 7.05%; chronic vehicle: 149.3 ± 8.57%; chronic CORT: 105.584 ± 3.83%; effect of treatment: F1,26= 4.746; interaction of treatment x time F1,26= 9.046; p < 0.05) but no effect of time was observed. This protocol also elicited significantly blunted LTP of the N2 40–45 minutes after tetanus in the chronic CORT group compared to acute vehicle and acute CORT treatment groups (Fig. 3B; acute vehicle: 131.12 ± 3.07%; acute CORT: 131.79 ± 5.40%; chronic vehicle: 125.82 ± 9.36%; chronic CORT: 105.48 ± 4.75%; effect of time: F1,26= 6.941; p < 0.05) but no effect of treatment or an interaction of treatment × time was observed. Representative traces of field potential recordings post-tetanus (Fig.3C) i) acute vehicle, ii) acute CORT, iii) chronic vehicle, and iv) chronic CORT.
Figure 3. Chronic Corticosterone group displays blunted LTP in the dlBNST.
A. 0–5 minutes after administering tetanus, N2 amplitude was significantly blunted in the chronic CORT group compared to chronic vehicle and acute CORT.
B. 55–60 minutes after administering tetanus, N2 amplitude was lower in the chronic CORT group compared to acute vehicle and acute CORT groups.
C. Representative traces from: i) acute vehicle, ii) acute CORT, iii) chronic vehicle, and iv) chronic CORT.
D. ▴ Time of tetanus [two trains (20 sec interstimulus interval); 100 Hz, 1 sec]. Inset) Input [stimulation intensity(v)] – Output [Amplitude of N2 (mV)] curves taken at start of each experiment.
3.4 Socially isolated mice display blunted LTP in the dlBNST compared to group housed mice
To investigate whether glutamatergic plasticity in the dlBNST would be altered following acute or chronic social isolation versus group housing, we stimulated the stria terminalis afferents with the same tetanus protocol described above (two 100 Hz, 1 sec trains with a 20 sec intertrain interval; Figure 4D). Again, input-output curves were generated at the start of each experiment (Inset, Figure 4D). An effect of stimulation intensity (V) (F7,98= 22.51; p < 0.05) but not stress conditions nor stress-conditions × stimulation intensity (V) interactions was observed in these plots. This protocol had no effect on the early (0–5 minutes post-tetanus) component of LTP of the N2 (Fig. 4A; F2,23= 3.073; p =0.0675), although a trend for a decrease was observed in the 6–8 week socially isolated compared to the group housed mice. This protocol did elicit significantly blunted LTP of the N2 40–45 minutes after tetanus chronic (6–8 weeks) and acute (24 hours) socially isolated mice compared to group housed mice (Fig. 4B; chronic socially isolated (6–8 weeks): 105.42 ± 5.21%; acute socially isolated (1 day): 110.78 ± 6.05%; group housed: 142.01 ± 7.24%; F2,23= 8.807; p < 0.05). Representative traces of field potential recordings post-tetanus (Fig. 4C) i) acute socially isolated, ii) chronic socially isolated, and iii) group housed.
Figure 4. Socially isolated mice display blunted LTP in the dlBNST compared to group housed mice.
A. 0–5 minutes after administering tetanus, there was no significant difference in N2 amplitude in any group.
B. 55–60 minutes after administering tetanus, N2 amplitude was lower in the isolated compared to group housed mice.
C. Representative traces from: i) acute (1 Day) socially isolated, ii) chronic (6–8 weeks) socially isolated, and iii) group housed.
D. ▴ Time of tetanus [two trains (20 sec interstimulus interval); 100 Hz, 1 sec]. Inset) Input [stimulation intensity(v)] – Output [Amplitude of N2 (mV)] curves taken at start of each experiment.
4. Discussion
In this study, we found that chronic CORT treatment and long-term social isolation (6–8 weeks) results in an increase in anxiety-like behavior in adult male C57Bl/6J mice. The increase in anxiety like behavior was accompanied by an impairment of LTP in the dlBNST in the chronically stressed mice. In contrast to the altered behavior in chronically stressed mice, we did not observe an increase in anxiety-like behavior in the short-term socially isolated mice but did observe a blunting of LTP in the dlBNST.
Our data indicate that chronic CORT administration can lead to an increase in anxiety-like behavior (measured as time spent on the open arm of the EZM and in the center of the NOF) in adult C57Bl/6J mice. Our results are consistent with previous data suggesting that stressors which lead to increased levels of plasma CORT are associated with enhanced anxiety-like behavior [8]. It is important to note, at the time of behavioral testing, exogenously administered CORT is no longer actively on-board, as plasma CORT levels return to baseline levels within 2 hours following an intraperitoneal injection of CORT in adrenalectomized (ADX) rats similar to the CORT time-course profile of many acute stressors [29–31]. However, repeated chronic CORT treatment results in dysregulation of the HPA axis and plasma CORT levels are reportedly still elevated at 12 hours but return to baseline 2 days after termination of a chronic CORT paradigm in adrenal intact rats [8, 32–35]. Similar to what has been observed in other chronic stress models, the repeated chronic CORT administration paradigm was chosen based on several studies that have demonstrated an impairment in HPA axis negative feedback regulation, increases in anxiety- and/or depressive-like behaviors, and a downregulation in hippocampual glucocorticoid receptors (GR) in rodents [8] [36–40] but see [32]. However, there are numerous studies that have looked at chronic CORT administration at different times of day, doses, and for varying lengths of time. These variables likely account for at least some reported differential anxiety-like behavioral effects following chronic CORT. Although we found no effects of acute CORT treatment on anxiety-like behavior, it is possible that the time point used in our study, 24 hours following the single CORT injection, failed to capture differences in anxiety-like behavior. Indeed, Mitra and Saplosky found an increase in anxiety-like behavior on the EPM in rats following acute CORT treatment, however, this increase was apparent 12 days but not 24 hours after a single CORT injection, suggesting a delayed anxiogenic effect of acute CORT [32]. Finally, species, strain, and methodological differences may have a tremendous influence on CORT-induced anxiety-like behavior, thus, it would also be prudent to assess this regimen in other strains of mice, since the literature on acute and chronic CORT-induced behavioral changes in mice is relatively limited.
Our results indicate that chronic social isolation (6–8 weeks), can lead to an increase in anxiety-like behavior in C57Bl/6J mice. Similar to the chronic CORT mice, we also found an increase in anxiety-like behavior in the chronic (6–8 weeks) socially isolated mice on the EZM and NOF but no change in locomotor activity. Moreover, although it did not reach significance, a trend for an increase in anxiety-like behavior in the 24 hours socially isolated mice was observed. It is possible that under more anxiogenic testing conditions, this trend for an increase in anxiety-like behavior may be more apparent. We chose prolonged social isolation because of studies demonstrating prolonged environmental social deprivation in C57Bl/6J mice and a sub-strain of C57 mice, C57BL/6ByJ, results in altered affective behaviors, and emotional reactivity [15, 42–45]. It is also important to note that previous studies have found inconsistent social isolation induced effects in C57 mice compared to DBA/2 and AJ strains [43–45]. In comparing this data to the CORT behavioral results, previous work has shown there is not a corresponding increase in CORT in prolonged socially isolated C57 mice [ 19]. Thus, we sought to take advantage of stressors that had distinct CORT profiles. Social isolation stress may serve as a valuable model for anxiety- and depressive like behaviors and it would be interesting in the future to examine whether brief daily exposure to another mouse may counter the effects of isolation on anxiety-like behaviors and plasticity.
Our findings also provide new data in support of the involvement of the dlBNST following chronic but not acute CORT treatment. Thus, chronic CORT treatment results in an impairment of the early component of LTP (0–5 minutes post-tetanus) compared to acute CORT and chronic vehicle treated mice. In addition, the late component (40–45 minutes post-tetanus) of LTP was lower in the chronic CORT compared to acute CORT and chronic vehicle treated mice. It is also possible the stress of injections may have influenced the expression of LTP in the dlBNST, however, we believe this possibility unlikely given that the group housed mice in the Housing experiments displayed a similar level of LTP as the vehicle injected mice in this study (~130 for vehicle treated compared to ~140 for group housed; p=0.283). Previous work has shown glucocorticoids can have direct effects on neuronal excitability [46, 47] and alter LTP in the hippocampus but not the basolateral amygdala [35, 48]. For example, previous studies have shown a CORT-mediated enhancement of LTP in the CA1 region of the hippocampus when CORT was applied immediately prior to tetanus induction, however, if CORT was pre-applied for several hours or was administered chronically and systemically, it suppressed LTP [35, 49, 50]. Our results appear consistent with most hippocampal studies that have demonstrated hypercorticolism results in facilitation of long-term depression (LTD) or impairment of LTP. The mechanisms underlying the changes in LTP are still unknown but many studies suggest the degree of GR activation may be an underlying factor [22, 51, 52]. Importantly, CORT enters the brain and rapidly binds to the nuclear mineralocorticoid and glucocorticoid receptors (MRs and GRs, respectively) and these receptors can then act as transcriptional regulators. Thus, it is interesting to speculate that hypercorticolism resulting from chronic CORT treatment may be necessary to alter transcription and lead to an upregulation of target proteins required to alter LTP induction and expression. Notably, the BNST contains a high density of GRs [53], in addition to norepinephrine, dopamine (DA), glutamate, and CRH receptors that are also heavily implicated in stress- and drugs of abuse-dependent effects on LTP [27, 47, 54].
This study provides the first demonstration, to our knowledge, of the ability of an acute and chronic social isolation stressor to result in blunted LTP in the dlBNST. Thus, we found no significant differences of housing conditions on the early (0–5 minutes post-tetanus) component of LTP, although there was a trend for a decrease in the 6–8 week socially isolated compared to group housed mice. However, we found that the late (40–45 minutes post-tetanus) component of LTP was significantly blunted in the short- and long-term socially isolated mice compared to the group housed mice. There are no studies, to our knowledge, that have examined LTP in mice first socially isolated in adulthood. Studies in rodents socially isolated at weaning (~postnatal day 21) for a minimum of 1 month, suggest a blunting of LTP in the hippocampus [55, 56] but see [57]. Surprisingly, we also observed impaired LTP in the acute socially isolated mice but no increase in anxiety-like behavior, whereas we observed both a blunting of LTP and an increase in anxiety-like behavior in both the chronic CORT and chronic social isolation groups. It is possible that an enhanced anxiety-like state, driven in part by changes in plasticity in the dlBNST and downstream targets, was only beginning to develop at 24 hours of social isolation. As a model for anxiety- and depressive-like behavior, acute and chronic social isolation could prove a useful and unique assay that may have significant construct validity for the anxiety and depressive symptoms present in a clinical population.
In addition to the role of CRH in activating the HPA axis, extrahypothalamic CRH has been implicated in stress-induced drug-seeking and the actions of drugs of abuse on LTP in the dlBNST [58, 59]. Thus, an acute cocaine injection can lead to a DA receptor- and CRH receptor 1 (CRHR1)-mediated enhancement of an NMDA-receptor-dependent early component of LTP, short-term potentiation (STP), in the dlBNST [27]. In contrast to the cocaine-induced enhancement of the early component of LTP, Francesconi and colleagues demonstrated [54] a CRHR1-dependent impairment of LTP of the intrinsic excitability of juxtacapsular (dorsal) BNST neurons during protracted abstinence from alcohol self-administration, a known stressor that results in increased anxiety-like behavior. These studies suggest CRHR1 may indeed be a good candidate for mediating the impairment of LTP in chronic-CORT treated and socially isolated mice. Given the critical role of the BNST in integrating limbic and stress information, it will be important for future studies to investigate the underlying mechanisms of stress- dependent blunting of LTP in the dlBNST.
Taken together, our results add to our understanding of the effects acute versus chronic stressors have on anxiety-like behavior and LTP in the dlBNST, Our findings also highlights the caution needed in determining housing conditions for experimental subjects. Garnering a better understanding of the behavioral and functional consequences of housing conditions is critical for an accurate interpretation of experimental findings and further research in this area is clearly warranted. Moreover, given that numerous stressors of varying magnitude, intensity, and duration can increase CORT levels, it is imperative that we understand the impact of elevated CORT on plasticity in the BNST, a region critical for integrating and processing stress information and shaping the stress response. Future studies should address the effects of bath applied CORT on LTP in the dlBNST, the number of daily CORT injections needed to observe alterations in LTP and the role time of day (rising or falling pulsatile CORT) plays in observing stress effects in chronically CORT treated mice. Moreover, our understanding of the effects of acute and chronic CORT and altered housing conditions in different inbred mouse strains is very limited and further studies are needed to fill this gap in our understanding of strain-dependent stress effects. Finally, since chronic social isolation reportedly does not lead to an increase in CORT levels, and even short-term isolation can blunt LTP in the dlBNST, additional studies should also assess the underlying similarities and differences in the mechanisms responsible for stressor mediated-impairment of LTP in the dlBNST.
Acknowledgments
The behavioral data presented in this manuscript were generated at the Vanderbilt Murine Neurobehavioral Laboratory: http://kc.vanderbilt.edu/mnlcore/.
This work was supported by National Institutes of Health Grants NS07491 and MH065215-08 (KLC), MH064913 (KML) and AA019455 AND DA019112 (DGW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marques AH, Silverman MN, Sternberg EM. Glucocorticoid dysregulations and their clinical correlates. From receptors to therapeutics. Ann N Y Acad Sci. 2009;1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24(3):151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Dunn JD. Plasma corticosterone responses to electrical stimulation of the bed nucleus of the stria terminalis. Brain Res. 1987;407(2):327–31. doi: 10.1016/0006-8993(87)91111-5. [DOI] [PubMed] [Google Scholar]
- 4.Casada JH, Dafny N. Restraint and stimulation of bed nucleus of the stria terminalis produce similar stress-like behaviors. Brain Res Bull. 1991;27(2):207–12. doi: 10.1016/0361-9230(91)90069-v. [DOI] [PubMed] [Google Scholar]
- 5.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27(8):2025–34. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis M, Walker DL, Miles L, Grillon C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OF, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. Eur J Neurosci. 2008;27(6):1503–16. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepard JD, Chambers CO, Busch C, Mount A, Schulkin J. Chronically elevated corticosterone in the dorsolateral bed nuclei of stria terminalis increases anxiety-like behavior. Behav Brain Res. 2009;203(1):146–9. doi: 10.1016/j.bbr.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res. 2006;174(1):193–6. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 11.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965(1–2):290–4. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- 12.Heinrich LM, Gullone E. The clinical significance of loneliness: a literature review. Clin Psychol Rev. 2006;26(6):695–718. doi: 10.1016/j.cpr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Barrot M, Wallace DL, Bolanos CA, Graham DL, Perrotti LI, Neve RL, et al. Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc Natl Acad Sci U S A. 2005;102(23):8357–62. doi: 10.1073/pnas.0500587102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costello J, Kendrick K. Grief and older people: the making or breaking of emotional bonds following partner loss in later life. J Adv Nurs. 2000;32(6):1374–82. doi: 10.1046/j.1365-2648.2000.01625.x. [DOI] [PubMed] [Google Scholar]
- 15.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12(2):200–9. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29(24):7820–32. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chourbaji S, Zacher C, Sanchis-Segura C, Spanagel R, Gass P. Social and structural housing conditions influence the development of a depressive-like phenotype in the learned helplessness paradigm in male mice. Behav Brain Res. 2005;164(1):100–6. doi: 10.1016/j.bbr.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Kwak C, Lee SH, Kaang BK. Social Isolation Selectively Increases Anxiety in Mice without Affecting Depression-like Behavior. Korean J Physiol Pharmacol. 2009;13(5):357–60. doi: 10.4196/kjpp.2009.13.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106(14):5895–900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5(2):205–16. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 22.Sousa N, Cerqueira JJ, Almeida OF. Corticosteroid receptors and neuroplasticity. Brain Res Rev. 2008;57(2):561–70. doi: 10.1016/j.brainresrev.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Girotti M, Weinberg MS, Spencer RL. Differential responses of hypothalamus-pituitary-adrenal axis immediate early genes to corticosterone and circadian drive. Endocrinology. 2007;148(5):2542–52. doi: 10.1210/en.2006-1304. [DOI] [PubMed] [Google Scholar]
- 24.Sage D, Maurel D, Bosler O. Corticosterone-dependent driving influence of the suprachiasmatic nucleus on adrenal sensitivity to ACTH. Am J Physiol Endocrinol Metab. 2002;282(2):E458–65. doi: 10.1152/ajpendo.00287.2001. [DOI] [PubMed] [Google Scholar]
- 25.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 26.Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacology. 2009;34(7):1685–94. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28(51):13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24(25):5741–7. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway-Campbell BL, McKenna MA, Wiles CC, Atkinson HC, de Kloet ER, Lightman SL. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology. 2007;148(11):5470–7. doi: 10.1210/en.2007-0585. [DOI] [PubMed] [Google Scholar]
- 30.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–75. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 31.Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19(7):1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- 32.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci U S A. 2008;105(14):5573–8. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998;2(4):237–49. doi: 10.3109/10253899809167288. [DOI] [PubMed] [Google Scholar]
- 34.Sousa N, Madeira MD, Paula-Barbosa MM. Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 1998;794(2):199–210. doi: 10.1016/s0006-8993(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 35.Pavlides C, Watanabe Y, McEwen BS. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3(2):183–92. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 36.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52(3):597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 37.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–28. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 38.Gregus A, Wintink AJ, Davis AC, Kalynchuk LE. Effect of repeated corticosterone injections and restraint stress on anxiety and depression-like behavior in male rats. Behav Brain Res. 2005;156(1):105–14. doi: 10.1016/j.bbr.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 40.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73(5):705–17. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 42.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4(4):240–52. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 43.Gendreau PL, Gariepy JL, Petitto JM, Lewis MH. D1 dopamine receptor mediation of social and nonsocial emotional reactivity in mice: effects of housing and strain difference in motor activity. Behav Neurosci. 1997;111(2):424–34. doi: 10.1037//0735-7044.111.2.424. [DOI] [PubMed] [Google Scholar]
- 44.Gendreau PL, Petitto JM, Gariepy JL, Lewis MH. D2-like dopamine receptor mediation of social-emotional reactivity in a mouse model of anxiety: strain and experience effects. Neuropsychopharmacology. 1998;18(3):210–21. doi: 10.1016/S0893-133X(97)00131-0. [DOI] [PubMed] [Google Scholar]
- 45.Misslin R, Belzung C, Vogel E. Behavioural Validation of a Light/Dark Choice Procedure For Testing Anti-anxiety Agents. Behavioral Processes. 1989;18:119–132. doi: 10.1016/S0376-6357(89)80010-5. [DOI] [PubMed] [Google Scholar]
- 46.Joels M, de Kloet ER. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15(1):25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- 47.Joels M, Krugers HJ. LTP after stress: up or down? Neural Plast. 2007;2007:93202. doi: 10.1155/2007/93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kavushansky A, Richter-Levin G. Effects of stress and corticosterone on activity and plasticity in the amygdala. J Neurosci Res. 2006;84(7):1580–7. doi: 10.1002/jnr.21058. [DOI] [PubMed] [Google Scholar]
- 49.Wiegert O, Joels M, Krugers H. Timing is essential for rapid effects of corticosterone on synaptic potentiation in the mouse hippocampus. Learn Mem. 2006;13(2):110–3. doi: 10.1101/lm.87706. [DOI] [PubMed] [Google Scholar]
- 50.Zhou J, Zhang F, Zhang Y. Corticosterone inhibits generation of long-term potentiation in rat hippocampal slice: involvement of brain-derived neurotrophic factor. Brain Res. 2000;885(2):182–91. doi: 10.1016/s0006-8993(00)02934-6. [DOI] [PubMed] [Google Scholar]
- 51.Krugers HJ, Alfarez DN, Karst H, Parashkouhi K, van Gemert N, Joels M. Corticosterone shifts different forms of synaptic potentiation in opposite directions. Hippocampus. 2005;15(6):697–703. doi: 10.1002/hipo.20092. [DOI] [PubMed] [Google Scholar]
- 52.Pavlides C, McEwen BS. Effects of mineralocorticoid and glucocorticoid receptors on long-term potentiation in the CA3 hippocampal field. Brain Res. 1999;851(1–2):204–14. doi: 10.1016/s0006-8993(99)02188-5. [DOI] [PubMed] [Google Scholar]
- 53.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: an immunohistochemical and in situ hybridization study. Neurosci Res. 1996;26(3):235–69. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 54.Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29(17):5389–401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu L, Bao G, Chen H, Xia P, Fan X, Zhang J, et al. Modification of hippocampal neurogenesis and neuroplasticity by social environments. Exp Neurol. 2003;183(2):600–9. doi: 10.1016/s0014-4886(03)00248-6. [DOI] [PubMed] [Google Scholar]
- 56.Roberts L, Greene JR. Post-weaning social isolation of rats leads to a diminution of LTP in the CA1 to subiculum pathway. Brain Res. 2003;991(1–2):271–3. doi: 10.1016/j.brainres.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 57.Ashby DM, Habib D, Dringenberg HC, Reynolds JN, Beninger RJ. Subchronic MK-801 treatment and post-weaning social isolation in rats: differential effects on locomotor activity and hippocampal long-term potentiation. Behav Brain Res. 212(1):64–70. doi: 10.1016/j.bbr.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 58.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarnyai Z. Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans. Ann N Y Acad Sci. 1998;851:371–87. doi: 10.1111/j.1749-6632.1998.tb09011.x. [DOI] [PubMed] [Google Scholar]