Abstract
Hepatitis C Virus (HCV) infects approximately 3 percent of the world’s population, establishing a life-long infection in the majority of cases. The life cycle of HCV is closely tied to the lipid metabolism of liver cells, and lipid droplets have emerged as crucial intracellular organelles that support persistent propagation of viral infection. Here we review recent advances in our understanding of how HCV usurps intracellular lipids to propagate and highlight unique opportunities for therapeutic intervention.
The HCV life cycle
HCV is one of at least six viruses (Hepatitis A, B, C, D, and E virus, and GB virus C) that infect the liver. Long known as the agent causing non-A-non-B hepatitis, its genome was cloned in 1989 via a random-primed complementary DNA library constructed from plasma of an infected individual (for review [1]). The HCV genome is a single positive-strand ~9,600-nucleotide RNA molecule. Viral infection spreads primarily via contact with contaminated blood establishing lifelong infections; an estimated 3% of the world’s population is infected with HCV (Box 1). As a unique characteristic, about half of all individuals chronically infected with HCV develop fatty liver disease (steatosis), a condition characterized by excessive lipid accumulation in hepatocytes. Although lipid accumulation was long thought of as a mere by-product of viral infection, recent advances point to unique ties of the viral life cycle with liver lipid metabolism.
Box 1. HCV infection: the facts.
HCV is a single-stranded RNA virus of the family of flaviviridae that spreads mainly through contact with contaminated blood.
HCV infection usually causes very benign acute symptoms (loss of appetite, fatigue, abdominal pain, and flu-like symptoms).
Around 170 million people are infected; in 80% of those afflicted, the infection persists and causes variable degrees of liver damage, including steatosis, fibrosis, cirrhosis, and, hepatocellular carcinoma.
No vaccine is currently available.
Treatment options are limited; a combination of pegylated interferon-alpha and ribavirin can eradicate viral infection in ~50% of treated individuals; it is, however, a cumbersome treatment and not efficient against HCV genotype 1, the viral strain most prevalent in the US and Europe.
Our current understanding of the HCV life cycle is largely derived from a recently developed infectious cell-culture system (HCVcc) (for review [1]). Prior to this model, efforts were limited to studies of isolated steps of the viral life cycle, i.e. viral entry using pseudo-typed HIV-based lentiviruses carrying the HCV envelope proteins or viral RNA replication studies using subgenomic replicon systems. Although the discovery of a replicating infectious clone for HCV was a major breakthrough, this system remains limited to derivatives of one clinical isolate (JFH1) and specific subspecies of the Huh7 hepatoma cell line.
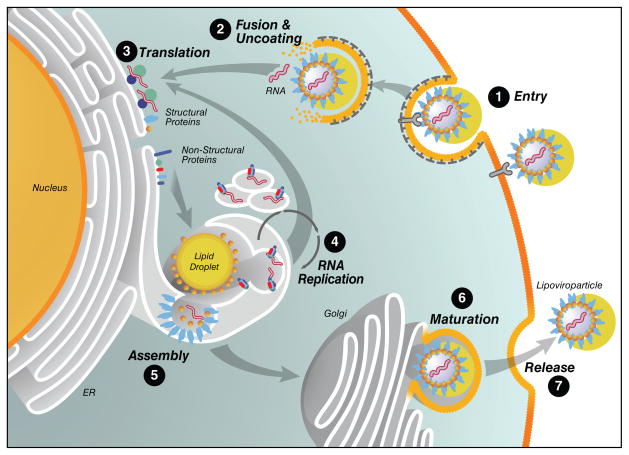
As a unique characteristic, viral particles are bound to plasma lipoproteins (also called lipoviroparticles). Viral particles enter hepatocytes via receptor-mediated endocytosis (Figure 1, step 1). After fusion/uncoating events (Figure 1, step 2), which are not yet well defined, the HCV genome becomes available for translation of viral proteins via a 5′ internal ribosomal entry site (Figure 1, step 3). This results in the production of a single 327 kDa viral precursor polyprotein at the rough endoplasmic reticulum (ER), which undergoes an ordered series of proteolytic cleavages by viral and host proteases to generate 10 viral proteins. The nucleocapsid protein core and the two envelope proteins E1 and E2 form the N terminus of the polyprotein and are the structural components of HCV virions. The precursor also gives rise to the viroporin p7 and six non-structural (NS) proteins involved in polyprotein processing, viral RNA replication, and infectious virion production: the NS2-3 autoprotease, the NS3 serine protease and RNA helicase, its cofactor NS4A, NS4B and NS5A proteins, and the NS5B RNA-dependent RNA polymerase. Nonstructural proteins form a multicomponent RNA replication complex within subdomains of the ER, called the membranous web, where positive-strand RNA is replicated via a negative strand intermediate by the virally encoded RNA-dependent RNA polymerase (Figure 1, step 4).
Figure 1. The HCV life cycle.
HCV lipoviroparticles enter target hepatocytes via receptor-mediated endocytosis (step 1). Released viral RNA is translated at the ER producing a single polyprotein precursor that is cleaved by host and viral proteases (steps 2 and 3). The viral non-structural proteins (NS2-NS5B) form RNA replication complexes in detergent-resistant lipid drafts where positive-strand RNA is replicated by the viral RNA-dependent RNA polymerase NS5B via a negative-strand intermediate (step 4). Newly synthesized positive-strand RNA is encapsidated by the viral nucleocapsid core in close proximity to lipid droplets, and envelope glycoproteins are acquired through budding into the ER lumen (step 5). Lipoviroparticles mature in the ER through interactions with lipoproteins (step 6) and exit the cell by via the cellular Golgi apparatus (step 7).
The late steps of the HCV life cycle are less well understood. The prevailing model suggests that newly synthesized positive-strand viral RNA genomes are recruited to ER membranes surrounding lipid droplets where genomic RNA is encapsidated into progeny virions (Figure 1, step 5). The viral nucleocapsid protein core itself associates with the surface of lipid droplets and orchestrates this assembly process. Progeny virions are thought to bud into the ER lumen thereby acquiring envelope proteins bound to the ER membrane before release via the Golgi apparatus through exocytosis (Figure 1, step 7). Somewhere along this route, the virus matures from a high-density viroparticle to an infectious low-density lipoviroparticle ready to infect naïve target cells (Figure 1, step 6). However, the molecular details of viral assembly, maturation, and release remain to be determined.
HCV: a lipoviroparticle
In 1992, Thomssen and colleagues first demonstrated an association of HCV particles with beta-lipoproteins, an observation that partially explained the density heterogeneity of circulating HCV particles in the blood of infected patients [2]. In 2002, the term lipoviroparticles (LVP) was coined based on large spherical particles isolated from low-density fractions of plasma from infected patients that were rich in triglycerides and contained at least apolipoprotein B (ApoB), HCV RNA and the viral core protein [3]. Further studies of virus preparations isolated from the blood or from the liver of infected patients showed that the majority of HCV RNA is found in particles at densities below 1.08 g/ml and can be precipitated with antibodies against ApoB or Apolipoprotein E (ApoE) proteins pointing to a tight association of HCV particles with very low-density lipoproteins (VLDL) [4, 5].
Interestingly, laboratory-grown HCVcc particles have a slightly higher density than plasma-derived particles (~1.15 g/ml) [6, 7] and are found associated with ApoE and ApoCI, but mostly lack ApoB [8–10]. This could be explained by the fact that the VLDL assembly pathway is impaired in the Huh7-based producer cell lines used to generate HCVcc in vitro. In Huh7 cells, ApoB-containing VLDL precursors are not fully lipidated and do not fuse to ApoE-containing precursors [11]. It is therefore likely that laboratory-grown HCVcc particles have different biochemical characteristics regarding density and association with lipoproteins than particles isolated from infected patients (Box 2).
Box 2. Some outstanding questions.
Virion
What is the composition of HCV virions in vivo?
What are the host proteins associated with HCV virions, ApoE, ApoB or both?
Entry
Does the virus enter through tight junctions or does it induce relocalization of the two tight junction proteins involved in entry?
Are there liver-specific entry factors yet to be discovered?
RNA Replication
What is the precise nature of the membranous web?
How is viral RNA replication and virion assembly mechanistically linked?
Assembly, Maturation, and Release
Why does HCV assembly require lipid droplets and how do cytoplasmic lipid droplets communicate with luminal VLDLs?
When do newly generated virions assembly with VLDLs and where in the VLDL secretion pathway do virions get lipidated?
Accordingly, when chimpanzees were infected with laboratory-grown HCVcc particles, viral particles isolated from the bloodstream of infected animals showed higher specific infectivity and lower densities as compared to the HCVcc inoculum, suggesting that full lipidation is required for optimal infectivity of HCV lipoviroparticles in vivo [7]. However, partial lipidation is nevertheless key to the infectivity of laboratory-grown HCVcc particles, because addition of lipoprotein lipase to HCVcc-containing media decreases infectivity and HCVcc’s association with ApoE while increasing the densities of the lipoviroparticles [12].
The “lipidome” of laboratory-grown HCVcc particles – recently determined by mass spectrometry [8] – reveals a unique lipid composition of HCV particles, distinct from human immunodeficiency virus and from HCV-producing cells. As a characteristic of HCVcc, cholesterol esters account for nearly half of the lipids, a finding that underlies the tight association of the lipoviroparticles with low-density lipoproteins (LDLs) or VLDLs. However, lipidation is not the only determinant of HCV particle infectivity. Recent electron microscopic analysis of HCVcc preparations revealed two main particle sizes: highly infectious particles (<60 nm in diameter) that contain a membrane bilayer (presumably the viral envelope) and less infectious particles (<45 nm in diameter) that lack the membrane bilayer indicating that defective non-enveloped particles are secreted from hepatoma cells during viral propagation [9]. The significance of the defective particles is not yet known.
Viral entry via lipoprotein receptors LDL-R and SR-B1
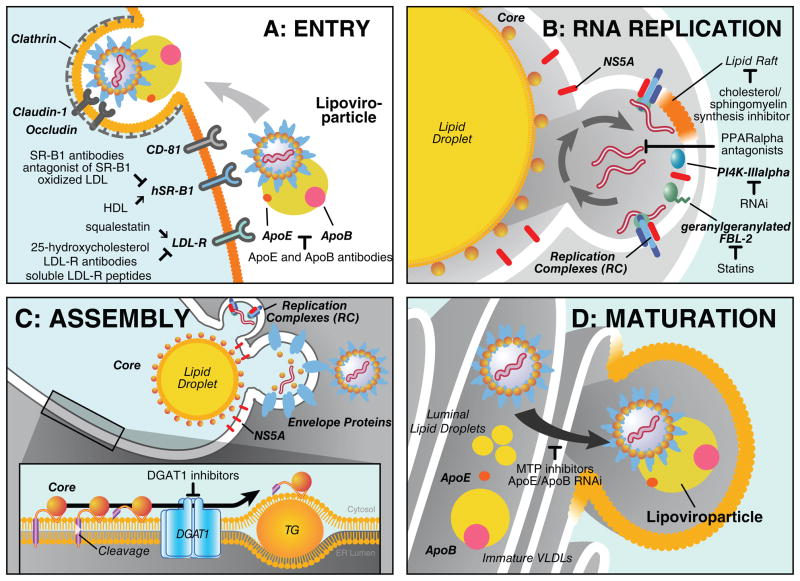
A role of the LDL receptor (LDL-R) in HCV entry — either directly or indirectly — was long suspected due to the unique nature of the lipoviroparticles, which are in densities and association with lipoproteins reminiscent of VLDLs (Figure 1, step 1). Indeed, the LDL-R together with the tetraspanin protein CD81, scavenger receptor B1 (SR-B1), and the tight-junction proteins claudin 1 and occludin is regarded as one of the HCV co-receptors (reviewed in [13]). The current multistep model predicts that early attachment steps of the lipoviroparticle are mediated by lipoprotein-binding receptors LDL-R and SR-B1, the latter a receptor for high-density lipoproteins (HDL) (Figure 2, Entry). After primary attachment, the virus is thought to engage with CD81 and subsequently claudin 1 and occludin, which interact with HCV envelope proteins before endocytosis in a clathrin-dependent manner [14].
Figure 2. Role of host cell lipid metabolism in different steps of the viral life cycle.
(a) Entry: After attachment of the lipoviroparticles to LDL-R and SR-B1 the virus binds to CD81 and subsequently claudin-1 and occludin, which mediate the late steps of viral entry. The virus is internalized by clathrin-dependent endocytosis. Antibodies against ApoB, ApoE, LDL-R, and SR-B1 inhibit viral entry. Modulation of cellular LDL-R expression through treatment with squalestatin or 25-hydroxycholesterol enhances or decreases HCV entry efficiency. Natural ligands of SR-B1 as HDL and oxidized LDL also affect entry. (b) RNA replication: Viral RNA replication complexes localize to lipid raft-containing, detergent-resistant membranes created by the viral protein NS4B. For full viral replication, these replication complexes need to be in close proximity to lipid droplets, a process that requires NS5A. Host factors required for the formation of replication complexes include geranylgeranylated FBL-2, which is a target for statins, and PI4K-IIIalpha. Cholesterol and sphingomyelin synthesis inhibitors impair viral RNA replication. PPARalpha antagonists disperse viral replication complexes. (c) Assembly at lipid droplets: Core is translated at the ER and released from the polyprotein through cleavage by SPP. Core interacts in the ER with DGAT1 and localizes to lipid droplets in a DGAT1-dependent manner. Once bound to the lipid droplet surface core recruits the viral RNA for encapsidation forming high-density immature virions. (d) Maturation: High-density virions mature by fusion with ApoB/ApoE-containing lipoproteins and/or lipidation to form mature low-density lipoviroparticle. This process can be impaired by treatment with MTP inhibitors and ApoB/ApoE siRNAs.
Interestingly, none of the receptors is exclusively expressed on the surface of liver cells, which would explain the liver tropism of HCV. Of note, much of our current understanding of HCV entry is based on studies of lentiviral particles pseudotyped with HCV envelope proteins and HCVcc particles. Since both types of particles are not or not fully lipidated, particles derived from patients or primary hepatocyte cultures should be probed to identify a potential liver-specific HCV receptor.
The two lipoprotein receptors, LDL-R and SR-B1, implicated in HCV attachment differ in their mode of action. The LDL-R makes contact with the lipoviroparticle via its ApoB and ApoE content. Indeed, antibodies directed against ApoE and ApoB are effective in blocking HCVcc entry into hepatoma cells [15, 16]. The LDL-R was further defined as a HCV co-receptor that interacts with ApoE present in HCVcc particles [17]. This function is not restricted to the HCVcc system, since antibodies directed against LDL-R or the use of recombinant soluble LDL-R peptides effectively blocked entry of virus isolated from infected patients into primary human hepatocytes [18]. Moreover, treatment with squalestatin, which elevates, or 25-hydroxycholesterol, which decreases LDL-R levels correlated with HCV entry efficiencies in primary human hepatocytes [18] and in the HCVcc system [17] (Figure 2, Entry).
In contrast to LDL-R, SR-B1 directly interacts with the HCV envelope protein E2 [19]. SR-B1 is essential for viral entry as shown in the infectious cell culture system and in cells infected with pseudotyped particles [20, 21]. In fact, treatment with a small-molecule antagonist of SR-B1 potently inhibits HCVcc entry into hepatoma cells [22]. Similarly, treatment with natural ligands of SR-B1, HDL or oxidized LDL, modulates HCV entry efficiencies: HDL enhances while oxidized LDL decreases viral entry in this system [23, 24]. Interestingly, SR-B1, but not CD81, is essential for direct cell-to-cell spread of viral infection pointing to a unique role of the SR-B1 receptor in the viral entry process [25–27]. Overall, the individual molecular functions and the precise mechanisms of collaboration between the different HCV receptor molecules are still under active investigation.
RNA-dependent RNA replication in intracellular lipid rafts
Once viral particles have successfully entered hepatocytes, the expression of the viral NS4B protein induces extensive membrane alterations and formation of vesicular structures thought to be consistent with a membranous web identified independently by electron microscopy (for review [1]). Within these membranous structures, nonstructural proteins NS3, NS4A, NS4B, NS5A, and NS5B cluster to form multi-protein complexes devoted to the replication of positive-strand viral RNA via negative-strand RNA intermediates (Figure 1, step 4). These replication “niches” are derived from the ER and show many characteristics of lipid rafts, i.e. they colocalize with calveolin-2 and are rich in sphingomyelin and cholesterol (Figure 2, Replication)[28, 29]. Indeed, treatment with cellular cholesterol and sphingomyelin synthesis inhibitors suppresses HCV RNA replication, underscoring the importance of lipid rafts for HCV RNA replication [29–31].
All viral proteins involved in intracellular viral RNA replication and newly synthesized viral RNA are found in lipid raft fractions of infected cells [28]. This localization is dependent on the presence of the viral NS4B protein [32], which itself is palmitoylated at two cysteine residues in its C-terminal region [33]. Mutation of these residues block HCV RNA replication, presumably by disturbing protein-protein interactions and viral protein recruitment to replicating RNA complexes [33].
While the association with lipid raft-like microdomains is thought to protect viral RNA replication complexes from the influence of cellular proteases and nucleases, infection with HCV or continuous HCV RNA replication also causes extensive alterations of the lipid raft proteome [34]. Among 150 proteins found to be up- or downregulated in lipid rafts of HCVcc-infected cells, proteins involved in vesicular trafficking and in cell signaling were enriched, many of which relocalized from or to lipid rafts as a consequence of viral infection.
The formation of a membranous web and the association of viral RNA replication complexes with cholesterol-rich intracellular lipid rafts represent unique opportunities for antiviral intervention. Indeed, treatment with cholesterol-reducing 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors or statins potently suppresses HCV RNA replication in vitro [35, 36]. Mechanistically this inhibition was linked to the F-Box/Leucine-rich repeat protein 2 (FBL-2), a host protein that is geranylgeranylated in an HMG-CoA reductase-dependent manner [37] (Figure 2, Replication). The geranylgeranylation of FBL-2 is thought to regulate the membrane attachment of FBL-2 and the interaction with the HCV NS5A protein, two critical events in HCV RNA replication [37]. However, clinical trials with statins showed controversial results: single treatment atorvastatin or fluvastatin did not reduce viral load in HCV-infected or HCV/HIV-co-infected patients, respectively [38, 39], whereas in a different study fluvastatin showed modest and variable suppressive effects in HCV-infected patients [40]. Interestingly, high LDL levels, low HDL levels, and fluvastatin use are independently associated with a sustained virological response in patients treated with the standard therapy of pegylated interferon-alpha and ribavirin [41]. These results indicate a positive effect of statins when used in combination with the standard therapy; however, prospective studies need to be undertaken to evaluate the effect in greater detail.
Several independent genomic screens using siRNA or shRNA libraries recently identified a second key cofactor required for the formation of RNA replication niches: phosphatidylinositol 4-kinase (PI4K)-IIIalpha, a kinase that produces the membrane lipid phosphatidylinositol-4-phosphate (PI4P), a mediator of protein attachment [42–45] (Figure 2, Replication). Interestingly, PI4K-IIIalpha was previously found as a binding partner for the HCV NS5A protein in a yeast-2-hybrid screen, where the interaction domain with PI4K-IIalpha was mapped to domains II–III of NS5A (aa 2277–2424, Figure 3, NS5A) [46]. PI4K-IIIalpha, NS5A, and double-stranded RNA co-localized in detergent-resistant membranes and RNAi-mediated knockdown of PI4K-IIIalpha reduced HCV-induced membrane alterations as shown by electron microscopy [43]. HCV NS5A directly interacts and stimulates PI4K-IIIalpha activity, leading to elevated PI4P levels in HCV-infected cells [45]. Lowering PI4K-IIIalpha activity could lead to an altered lipid composition in the membranous web or could disturb recruitment of host factors required for membranous web formation and efficient RNA replication.
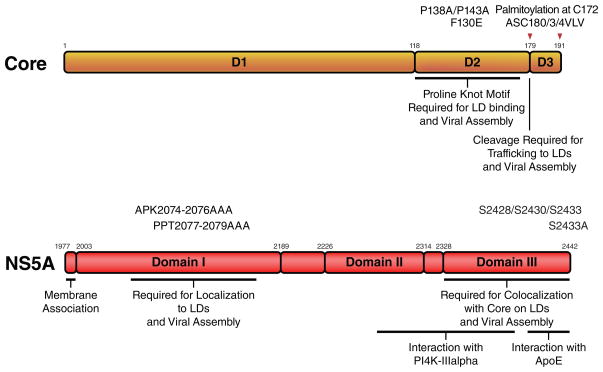
Figure 3. Domain structure of the viral core and NS5A proteins.
Core and NS5A are viral proteins with intrinsic lipid droplet-localizing properties. Depicted are domains and mutations connected with lipid droplet association and host factor interactions. Red arrows indicate the cleavage sites in core by host peptidases.
Another approach to disrupt formation of HCV RNA replication complexes is the use of antagonists of the peroxisome proliferator-activated receptor alpha (PPARalpha) [47, 48]. This treatment inhibited HCV RNA replication and dispersed HCV RNA from replication sites as visualized by two-photon fluorescence and coherent anti-Stokes Raman scattering (CARS) microscopy [48]. Dispersion from replication sites induced by PPAR alpha antagonist occurred at a faster rate than dispersion observed in response to lovastatin treatment in living cells and simultaneously increased the size of intracellular lipid storage droplets underscoring potential connections between both subcellular compartments (Figure 2, Replication).
Cytosolic lipid droplets as virion assembly platforms
Studies using the infectious HCVcc system have confirmed the critical role for lipid droplets in HCV assembly (Figure 1, step 5). Lipid droplets originate from the ER where triglycerides and cholesterol esters are synthesized and eventually form the core of lipid droplets surrounded by a phospholipid monolayer and a host of proteins at their surface. Two viral proteins expressed by HCV, core and the nonstructural protein NS5A, localize to lipid droplets. Both play key roles in the viral assembly process: core forms the nucleocapsid surrounding the viral RNA genome while NS5A is thought to mediate the switch from intracellular viral RNA replication to RNA packaging into progeny virions. ER membranes in close proximity to lipid droplets are thought to serve as viral assembly sites as they host budding virion-like structures in HCVcc-infected hepatoma cells [49]. Similar structures were observed in non-liver cells where assembly and release of HCV are impaired and these budding-like structures accumulate in ER membranes close to lipid droplets [50].
Core is the first protein of the viral precursor polyprotein, and is released by two proteolytic cleavages: first, cleavage at position 191 by cellular signal peptidase occurs in the ER lumen and separates core from the subsequent E1 protein (Figure 3, Core). A second intramembranous cleavage at position 179 by cellular signal peptide peptidase (SPP) generates the 179-amino acid mature core protein. The majority of the core protein is localized at the cytosolic side of the ER membrane with its C terminus inserted into one layer of the ER membrane where it is believed to migrate via lateral diffusion into ER subcompartments and subsequently onto the surface of lipid droplets (for review [51], Figure 2, Assembly). Accordingly, mutation of the SPP cleavage site (i.e. the second cleavage) renders core incapable of trafficking to the lipid droplet surface. Core binds to lipid droplets via an amphipathic helix-turn-helix motif located in its so-called D2 domain, termed a proline knot motif [52] (Figure 3, Core).
The localization of core to lipid droplets is critical for successful viral assembly [49]. Viruses expressing a core protein mutated in the lipid droplet-binding domain (P138A/P143A or F130E) are dysfunctional in viral assembly [49] [53] (Figure 3, Core). In addition, mutations in the SPP cleavage site (ASC180/3/4VLV) render the virus non-infectious [54] (Figure 3, Core). However, although core binding to lipid droplets is essential for the production of infectious virions, the affinity with which core binds to lipid droplets appears precisely fine-tuned, as viruses with a very hydrophobic lipid droplet-binding domain produce less infectious particles than viruses harboring a less hydrophobic domain [55]. Core itself is palmitoylated at cysteine 172 [56], and mutation of this residue, does not change core’s affinity to lipid droplets but still results in severely impaired viral output via a mechanism not yet understood.
The localization of core to lipid droplets is tightly controlled by the triglyceride-synthesizing enzyme diacylglycerol acyltransferase 1 (DGAT1). Core specifically interacts with DGAT1, but not DGAT2, and co-localizes with DGAT1 at the ER. This interaction is thought to recruit core to specific areas of the ER where DGAT1-generated lipid droplets form (Figure 2, Assembly). Although the catalytic activity of DGAT1 is not required for the interaction with core, it is required for the formation of lipid droplets that core can access. Indeed, treatment with a small-molecule inhibitor of DGAT1 effectively blocks access of core to lipid droplets and suppresses infectious viral particle formation in hepatoma cells and primary hepatocytes infected with HCVcc [57].
Interestingly, the second cellular DGAT enzyme, DGAT2, cannot compensate for the core-localizing function of DGAT1 despite its ability to maintain normal lipid droplet content in infected hepatoma cells in the absence of DGAT1 [57]. Interestingly, core also causes intracellular accumulation of lipids (steatosis), which is frequently observed in infected patients (for review see [58]). Core’s steatogenic properties are more pronounced in HCV genotype 3 strains, where the level of lipid accumulation corresponds with HCV RNA levels in the plasma. It has been suggested that HCV core protein inhibits microsomal triglyceride transfer protein (MTP) activity, a key protein in VLDL assembly. Core expression also alters the expression of genes involved in fatty acid biosynthesis and the development of steatosis, as the severity of clinical steatosis is linked to polymorphisms in the lipid droplet-binding domain of core in patients infected with HCV [59].
In cultured hepatoma cells, core induces lipid droplet redistribution causing clustering of lipid droplets in the perinuclear region [60, 61]. This process is microtubule and dynein-dependent, and disruption of the microtubule network decreases viral assembly and release [60]. One possibility is that this clustering generates “hotspots” of viral assembly in the cytoplasm of infected cells.
A key event in viral assembly is the successful encapsidation of viral RNA into progeny virions. Therefore, close contact exists between cellular compartments where viral RNA replication and virion assembly take place. In cells infected with HCVcc, components of viral RNA replication complexes as well as viral RNA can be found in close proximity to lipid droplets [49]. In addition, viral RNA synthesis is detected in isolated lipid droplet fractions [49]. The viral NS5A protein is thought to play a critical role in bridging viral RNA replication and virion assembly processes, a function regulated by different domains and the phosphorylation status of NS5A [62]. Domains I and III are necessary for successful viral assembly, whereas domains I and II are required for NS5A’s function in viral RNA replication (Figure 3, NS5A). Mutations in domain I of NS5A that abolish lipid droplet localization (APK2074–2076AAA and PPT2077–2079AAA) block viral assembly and infectious virion production, as viral RNA replication complexes are not recruited to the sites of assembly [49].
NS5A colocalizes with core on lipid droplets, and deletions in domain III of NS5A that disrupt this colocalization lead to impaired virion assembly [63]. Interestingly, these deletion mutants of NS5A still localize to lipid droplets but do not accumulate on the same droplets as core. However, a direct interaction of core with NS5A in infected cells was not observed [63]. In contrast, mutations in the C-terminal serine cluster in domain III of NS5A (S2428/S2430/S2433) reduced the basal phosphorylation of NS5A, causing a disruption of the NS5A core interaction, mislocalization of NS5A, and decreased virion production [64]. Replacing the same serines with glutamic acid, which mimics phosphoserines, partially restored the NS5A core interaction and virion production, suggesting that phosphorylation of these serine residues are important for virion production [64]. Indeed a single point mutation in NS5A (S2433A) abrogates infectious virion assembly [62], as does inhibition of the phosphorylation by chemical inhibition of the casein kinase II [62]. Therefore phosphorylation of the C-terminal serine cluster in domain III of NS5A by casein kinase II and putative other kinases might determine when the virus switches from RNA replication to packaging. Interestingly, the C-terminal region of NS5A also interacts with ApoE, a process that could facilitate the recruitment of viral RNA to sites of viral assembly [65, 66], Other nonstructural viral proteins implicated as key players in the infectious particle production process are NS2 and NS3 [67–71]. A mutant NS2 protein deficient in supporting viral assembly failed to colocalize with E2 and NS3 around lipid droplets, underscoring that proper localization of the non-structural protein complex at lipid droplets is a prerequisite for infectious virion production [67]
ApoB and ApoE in HCV Assembly, Maturation, and Release
The lipoprotein secretion pathway has evolved as a critical pathway in the assembly and release of infectious HCV lipoviroparticles [16, 72, 73]. Evidence comes from experiments using inhibitors of the microsomal transfer protein (MTP) that abolished infectious HCVcc secretion in infected hepatoma cells [72, 73]. Specifically, ApoB downregulation by RNAi inhibited secretion [72] and intracellular maturation of HCVcc [73]. Immature, high-density intracellular viral particles are degraded in a post-ER compartment in a proteasome-independent manner similar to the posttranslational degradation of unlipidated or misfolded ApoB [73]. However, pharmacological inhibition of MTP at concentrations that block ApoB, but not ApoE, secretion had no effect on HCV release supporting a specific role of ApoE in viral release [74]. Accordingly, knockdown of ApoE expression in infected hepatoma cells reduced levels of intracellular as well as secreted HCVcc [16]. Moreover, the reported interaction of ApoE with the viral NS5A protein underscores a specific role of ApoE in the viral life cycle [65, 66].
Whether different ApoE isoforms are involved remains to be conclusively determined. One study reported that different ApoE isoforms have similar interaction kinetics with NS5A and do not differentially influence production of HCVcc [65]. However, re-introduction of ApoE3 and ApoE4 into hepatoma cells after ApoE knockdown nearly completely rescued infectious virion production while ApoE2 poorly restored HCVcc infectivity [75], which could point to genetic polymorphisms relevant for HCV infection.
It is important to note that the precise mechanisms governing viral assembly, maturation and release may not be resolved in the HCVcc system, which is currently defective in VLDL secretion. Recent findings that low-density infectious HCV particles can be recovered from infected primary human hepatocyte cultures [76] are encouraging and will allow molecular studies of late steps of the HCV life cycle in cells with intact VLDL secretion.
Outlook
Although it is apparent that HCV infection and liver lipid metabolism are closely intertwined, the precise details of this interaction are only starting to be unraveled. Important insight into these mechanisms will come from more physiological models of viral infection and pathogenesis. The development of infectious cell culture systems using primary human hepatocytes [76, 77] is an important step forward as are new elegant advances to monitor infectivity of primary viral isolates in cell culture [78]. Three-dimensional cell culture systems that mimic the environment of hepatocytes in vivo [79] may also help further decipher the complex HCV entry process, in which tight junction proteins play an important role. Humanized mouse models such as chimeric uPA-SCID [80] or Fah−/−, Rag2−/− or IL2rg−/− [81] mice represent important new in vivo models but remain restricted to expert laboratories. One important step towards a readily available small animal model for HCV was made in recent studies where human versions of the viral receptors were introduced into mouse cell lines then rendered permissive for HCV entry [82].
Despite the current limitations to study HCV infection in its physiological context, new and exciting opportunities to therapeutically interfere with HCV infection have arisen from its complex interplay with the host cell lipid metabolism (outlined in Figure 2). New therapeutic strategies are clearly needed to combat acute and chronic HCV infection, and antiviral drugs targeting the viral NS3 and NS5B proteins are currently being developed. However, the high error rate of the viral RNA-dependent RNA polymerase leads to frequent and rapid emergence of drug-resistant viral strains. Understanding the virus-lipid interactions and developing highly targeted strategies to block these interactions may provide necessary alternatives to supplement antiviral therapy.
Acknowledgments
We apologize to colleagues whose work could not be cited due to limits in reference number. We thank John Carroll and Giovanni Maki for help preparing the figures. This work was supported by funds from the Gladstone Institutes, the Hellman Family Foundation and the NIH (R56 AI085056 and P30 DK026743 (UCSF Liver Center)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moradpour D, et al. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 2.Thomssen R, et al. Association of hepatitis C virus in human sera with beta-lipoprotein. Med Microbiol Immunol (Berl) 1992;181:293–300. doi: 10.1007/BF00198849. [DOI] [PubMed] [Google Scholar]
- 3.Andre P, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen SU, et al. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418–2428. doi: 10.1128/JVI.80.5.2418-2428.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen SU, et al. Characterization of hepatitis C RNA-containing particles from human liver by density and size. J Gen Virol. 2008;89:2507–2517. doi: 10.1099/vir.0.2008/000083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gastaminza P, et al. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol. 2006;80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindenbach BD, et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103:3805–3809. doi: 10.1073/pnas.0511218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merz A, et al. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem. 2011;286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gastaminza P, et al. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84:10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meunier JC, et al. Apolipoprotein c1 association with hepatitis C virus. J Virol. 2008;82:9647–9656. doi: 10.1128/JVI.00914-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Icard V, et al. Secretion of hepatitis C virus envelope glycoproteins depends on assembly of apolipoprotein B positive lipoproteins. PLoS ONE. 2009;4:e4233. doi: 10.1371/journal.pone.0004233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimizu Y, et al. Lipoprotein lipase and hepatic triglyceride lipase reduce the infectivity of hepatitis C virus (HCV) through their catalytic activities on HCV-associated lipoproteins. Virology. 2010;407:152–159. doi: 10.1016/j.virol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Sabahi A. Hepatitis C Virus entry: the early steps in the viral replication cycle. Virol J. 2009;6:117. doi: 10.1186/1743-422X-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanchard E, et al. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andreo U, et al. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell Microbiol. 2007;9:2445–2456. doi: 10.1111/j.1462-5822.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang KS, et al. Human apolipoprotein e is required for infectivity and production of hepatitis C virus in cell culture. J Virol. 2007;81:13783–13793. doi: 10.1128/JVI.01091-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen DM, et al. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology. 2009;394:99–108. doi: 10.1016/j.virol.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina S, et al. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411–419. doi: 10.1016/j.jhep.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Scarselli E, et al. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017–5025. doi: 10.1093/emboj/cdf529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Catanese MT, et al. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol. 2007;81:8063–8071. doi: 10.1128/JVI.00193-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapadia SB, et al. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374–383. doi: 10.1128/JVI.01134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Syder AJ, et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48–55. doi: 10.1016/j.jhep.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 23.Dreux M, Cosset FL. The scavenger receptor BI and its ligand, HDL: partners in crime against HCV neutralizing antibodies. J Viral Hepat. 2007;14(Suppl 1):68–76. doi: 10.1111/j.1365-2893.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- 24.von Hahn T, et al. Oxidized low-density lipoprotein inhibits hepatitis C virus cell entry in human hepatoma cells. Hepatology. 2006;43:932–942. doi: 10.1002/hep.21139. [DOI] [PubMed] [Google Scholar]
- 25.Timpe JM, et al. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17–24. doi: 10.1002/hep.21959. [DOI] [PubMed] [Google Scholar]
- 26.Witteveldt J, et al. CD81 is dispensable for hepatitis C virus cell-to-cell transmission in hepatoma cells. J Gen Virol. 2009;90:48–58. doi: 10.1099/vir.0.006700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brimacombe CL, et al. Neutralizing Antibody-Resistant Hepatitis C Virus Cell-to-Cell Transmission. J Virol. 2011;85:596–605. doi: 10.1128/JVI.01592-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi ST, et al. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J Virol. 2003;77:4160–4168. doi: 10.1128/JVI.77.7.4160-4168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aizaki H, et al. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450–461. doi: 10.1016/j.virol.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Su AI, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amemiya F, et al. Targeting lipid metabolism in the treatment of hepatitis C virus infection. J Infect Dis. 2008;197:361–370. doi: 10.1086/525287. [DOI] [PubMed] [Google Scholar]
- 32.Gao L, et al. Interactions between viral nonstructural proteins and host protein hVAP-33 mediate the formation of hepatitis C virus RNA replication complex on lipid raft. J Virol. 2004;78:3480–3488. doi: 10.1128/JVI.78.7.3480-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu GY, et al. Palmitoylation and polymerization of hepatitis C virus NS4B protein. J Virol. 2006;80:6013–6023. doi: 10.1128/JVI.00053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannova P, et al. Modification of host lipid raft proteome upon hepatitis C virus replication. Mol Cell Proteomics. 2006;5:2319–2325. doi: 10.1074/mcp.M600121-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Ye J, et al. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci U S A. 2003;100:15865–15870. doi: 10.1073/pnas.2237238100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C, et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Mol Cell. 2005;18:425–434. doi: 10.1016/j.molcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.O’Leary JG, et al. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895–898. doi: 10.1002/hep.21554. [DOI] [PubMed] [Google Scholar]
- 39.Milazzo L, et al. Does fluvastatin favour HCV replication in vivo? A pilot study on HIV-HCV coinfected patients. J Viral Hepat. 2009;16:479–484. doi: 10.1111/j.1365-2893.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 40.Bader T, et al. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383–1389. doi: 10.1111/j.1572-0241.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 41.Harrison SA, et al. Serum cholesterol and statin use predict virological response to peginterferon and ribavirin therapy. Hepatology. 2010;52:864–874. doi: 10.1002/hep.23787. [DOI] [PubMed] [Google Scholar]
- 42.Tai AW, et al. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe. 2009;5:298–307. doi: 10.1016/j.chom.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger KL, et al. Roles for endocytic trafficking and phosphatidylinositol 4-kinase III alpha in hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009;106:7577–7582. doi: 10.1073/pnas.0902693106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaillancourt FH, et al. Identification of a lipid kinase as a host factor involved in hepatitis C virus RNA replication. Virology. 2009;387:5–10. doi: 10.1016/j.virol.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 45.Reiss S, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9:32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn J, et al. Systematic identification of hepatocellular proteins interacting with NS5A of the hepatitis C virus. J Biochem Mol Biol. 2004;37:741–748. doi: 10.5483/bmbrep.2004.37.6.741. [DOI] [PubMed] [Google Scholar]
- 47.Rakic B, et al. Peroxisome proliferator-activated receptor alpha antagonism inhibits hepatitis C virus replication. Chem Biol. 2006;13:23–30. doi: 10.1016/j.chembiol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Lyn RK, et al. Direct imaging of the disruption of hepatitis C virus replication complexes by inhibitors of lipid metabolism. Virology. 2009;394:130–142. doi: 10.1016/j.virol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Miyanari Y, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 50.Roingeard P, et al. Hepatitis C virus budding at lipid droplet-associated ER membrane visualized by 3D electron microscopy. Histochem Cell Biol. 2008;130:561–566. doi: 10.1007/s00418-008-0447-2. [DOI] [PubMed] [Google Scholar]
- 51.Roingeard P, Hourioux C. Hepatitis C virus core protein, lipid droplets and steatosis. J Viral Hepat. 2008;15:157–164. doi: 10.1111/j.1365-2893.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 52.Boulant S, et al. Structural determinants that target the hepatitis C virus core protein to lipid droplets. J Biol Chem. 2006;281:22236–22247. doi: 10.1074/jbc.M601031200. [DOI] [PubMed] [Google Scholar]
- 53.Boulant S, et al. Disrupting the association of hepatitis C virus core protein with lipid droplets correlates with a loss in production of infectious virus. J Gen Virol. 2007;88:2204–2213. doi: 10.1099/vir.0.82898-0. [DOI] [PubMed] [Google Scholar]
- 54.Targett-Adams P, et al. Maturation of hepatitis C virus core protein by signal peptide peptidase is required for virus production. J Biol Chem. 2008;283:16850–16859. doi: 10.1074/jbc.M802273200. [DOI] [PubMed] [Google Scholar]
- 55.Shavinskaya A, et al. The lipid droplet binding domain of hepatitis C virus core protein is a major determinant for efficient virus assembly. J Biol Chem. 2007;282:37158–37169. doi: 10.1074/jbc.M707329200. [DOI] [PubMed] [Google Scholar]
- 56.Majeau N, et al. Palmitoylation of hepatitis C virus core protein is important for virion production. J Biol Chem. 2009;284:33915–33925. doi: 10.1074/jbc.M109.018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herker E, et al. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat Med. 2010;16:1295–1298. doi: 10.1038/nm.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Syed GH, et al. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21:33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jhaveri R, et al. Specific polymorphisms in hepatitis C virus genotype 3 core protein associated with intracellular lipid accumulation. J Infect Dis. 2008;197:283–291. doi: 10.1086/524846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulant S, et al. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule- and Dynein-dependent manner. Traffic. 2008;9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- 61.Depla M, et al. Ultrastructural and quantitative analysis of the lipid droplet clustering induced by hepatitis C virus core protein. Cell Mol Life Sci. 2010;67:3151–3161. doi: 10.1007/s00018-010-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tellinghuisen TL, et al. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032. doi: 10.1371/journal.ppat.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Appel N, et al. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 2008;4:e1000035. doi: 10.1371/journal.ppat.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Masaki T, et al. Interaction of hepatitis C virus nonstructural protein 5A with core protein is critical for the production of infectious virus particles. J Virol. 2008;82:7964–7976. doi: 10.1128/JVI.00826-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cun W, et al. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 2010;84:11532–11541. doi: 10.1128/JVI.01021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Benga WJ, et al. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43–53. doi: 10.1002/hep.23278. [DOI] [PubMed] [Google Scholar]
- 67.Jirasko V, et al. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog. 2010;6:e1001233. doi: 10.1371/journal.ppat.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stapleford KA, Lindenbach BD. Hepatitis C virus NS2 coordinates virus particle assembly through physical interactions with the E1-E2 glycoprotein and NS3-NS4A enzyme complexes. J Virol. 2011;85:1706–1717. doi: 10.1128/JVI.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Y, et al. Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J Virol. 2011;85:86–97. doi: 10.1128/JVI.01070-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y, et al. NS3 helicase domains involved in infectious intracellular hepatitis C virus particle assembly. J Virol. 2008;82:7624–7639. doi: 10.1128/JVI.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mousseau G, et al. Dimerization-driven interaction of hepatitis C virus core protein with NS3 helicase. J Gen Virol. 2011;92:101–111. doi: 10.1099/vir.0.023325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang H, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gastaminza P, et al. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang J, Luo G. Apolipoprotein E but not B is required for the formation of infectious hepatitis C virus particles. J Virol. 2009;83:12680–12691. doi: 10.1128/JVI.01476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hishiki T, et al. Infectivity of hepatitis C virus is influenced by association with apolipoprotein E isoforms. J Virol. 2010;84:12048–12057. doi: 10.1128/JVI.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Podevin P, et al. Production of infectious hepatitis C virus in primary cultures of human adult hepatocytes. Gastroenterology. 2010;139:1355–1364. doi: 10.1053/j.gastro.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 77.Ploss A, et al. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc Natl Acad Sci U S A. 2010;107:3141–3145. doi: 10.1073/pnas.0915130107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones CT, et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol. 2010;28:167–171. doi: 10.1038/nbt.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sainz B, Jr, et al. Three-dimensional Huh7 cell culture system for the study of Hepatitis C virus infection. Virol J. 2009;6:103. doi: 10.1186/1743-422X-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mercer DF, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 81.Bissig KD, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ploss A, et al. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]