Abstract
We evaluated the effects of infection of hepatocytes with the well-characterized Helicobacter species, H. pylori. Cell number doubled during each 24 h period in mock cultures or following infection with H. pylori 401C (CagA−, VacA−, BabA−, OipA−) (P < 0.05). In contrast, infection with the more virulent H. pylori NCTC11637 (CagA+, VacA+, BabA+, OipA+) resulted in cell arrest (P < 0.05). Furthermore, NCTC11637 activated caspase-3 and increased DNA fragmentation 6.1 ± 1.2 fold (P < 0.01) and the number of apoptotic bodies 9.4 ± 3.5 fold (P < 0.01) compared to controls. The effect was greater than with the less virulent strain 401C (3.8 ± 0.6 fold and 3.9 ± 1.7, respectively, P < 0.05). Strain NCTC11637 at low concentrations increased cellular DNA synthesis 139 ± 6% (P < 0.05) but decreased it to 16 ± 7% (P < 0.01) at high concentrations. In contrast, strain 401C increased DNA synthesis 155 ± 14% of controls (P < 0.05) at high concentrations. The presence of intracellular NCTC11637 within hepatocytes increased DNA fragmentation 3.0 ± 0.4 fold (P < 0.01) greater than in controls. H. pylori infection resulted in strain-species-dependent effects on hepatocytes, and virulent strain caused cell arrest and apoptosis of infected hepatocytes.
Keywords: Helicobacter, Apoptosis, Cell proliferations, Hepatocytes, Internalizations
Introduction
Helicobacter pylori and entero-hepatic Helicobacter sp. have been identified in the livers of humans and animal species [1]. The number of entero-hepatic Helicobacter sp. reported has continued to increase with H. hepaticus, H. bilis, H. cinaedi, H. canis, H. rappini, H. pullorum, and H. rodentium all having been implicated in the pathogenesis of hepatobiliary disease [1].
H. hepaticus was the first entero-hepatic Helicobacter sp. identified and is the most investigated. H. hepaticus induces liver inflammations and has been associated with developments of hepatic cancer in mice [2]. Helicobacter sp. DNA has also been identified in human hepatocellular carcinomas (HCC) suggesting a possible association between Helicobacter sp. and the development of HCC [3–7]. In the majority of cases, the DNA has been tentatively identified as H. pylori, a well-known gastric pathogen also associated with gastric carcinoma [8]. These putative Helicobacter organisms have also been identified within hepatocellular malignant tumors in the liver using immunocytochemistry and the polymerase chain reaction (PCR) [5, 9]. For example, a comparative study that examined livers and stomachs using both PCR and immunohistochemistry showed an excellent correlation between the presence of H. pylori in the stomach and in the liver [9]. However, the H. pylori in the stomach were more likely to be CagA positive than those in the liver, suggesting that this virulence factor may play different roles in the different organs [9]. Finally, there has also been a report of culture of an H. pylori-like organism from the liver [10].
H. pylori has been reported to increase apoptosis as well as cellular proliferation in gastric epithelial cells [11–13]. Details regarding possible interactions between H. pylori and hepatocytes, especially the potential role of putative virulence factors of H. pylori (e.g., CagA and VacA) in hepatocytes remains scanty. This study compared the reaction of hepatocytes to H. pylori NCTC11637, a putative, more virulent strain (positive for cag pathogenicity island [PAI], VacA, OipA, and BabA), and 401C, a less-virulent strain (negative for cag PAI, VacA, OipA, and BabA) infections in vitro. First, we evaluated the effect of H. pylori infection on cell replications by the measure of cell number during co-incubations of hepatocytes with H. pylori. Then, we investigated the effect of H. pylori infection on apoptosis and cell proliferations. To assess apoptosis or programmed cell death, we measured the expression and the activation of caspase-3, DNA fragmentation, and chromatin condensation. Caspase-3 is involved in the early stage of apoptosis [14] where it is cleaved and activates nuclear endonuclease which selectively cleaves DNA and generates mono- and oligonucleosomal DNA fragments [15]. Fragmentation of genomic DNA is a biological hallmark of apoptosis, is irreversible, and commits the cell to die. DNA fragmentation triggers chromatin condensation, producing an apoptotic body [16]. To assess the effect of H. pylori infection on hepatocyte proliferation, we measured DNA synthesis. DNA synthesis was quantified by bromodeoxyuridine (BrdU) assay which measure the incorporation of BrdU in newly synthesized cellular DNA.
In this study, we propose the disturbance of apoptosis and DNA synthesis associated by H. pylori infection of hepatocytes may have a role in the pathogenesis of liver diseases.
Materials and Methods
H. pylori Strains
H. pylori NCTC11637 (here denoted 11637), a putative more virulent strain that is positive for the cag PAI (CagA is a marker) and VacA, and H. pylori 401C, a less-virulent strain negative for the cag PAI and VacA were used in this study. Strain 11637 also possessed two other virulence factors, BabA and OipA, whereas strain 401C possessed neither of these. H. pylori 401C was isolated from a Colombian patient with gastritis. H. pylori were grown for 2 days on blood agar plates consisting of brain–heart infusion (BHI) agar (Difco, Sparks, MD) and 7% horse blood in a CO2 incubator (12% CO2) at 37°C. Bacteria were suspended in cell culture medium consisting of minimal essential medium (MEM) (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Invitrogen) and adjusted into an appropriate concentration before use. Bacterial number was standardized by optical density (OD) measurements at 600 nm.
Cell Culture
Huh 7, a human hepatoma cell line, was maintained at 37°C in a 5% CO2 atmosphere in MEM supplemented with 10% FBS. When cells were used for experiments, they were washed twice with PBS and trypsinized for harvest in the culture medium described above. Collected cells were counted by hemacytometer (Hausser Scientific, Horsham, PA) and adjusted to the desired concentration.
Effect of H. pylori on Huh7 Cells
The effect of H. pylori infection on hepatocyte cell numbers were also examined; 1.5 × 105 Huh7 cells/well were seeded on 24 well plates and incubated overnight. H. pylori were added to each well at a multiplicity of infection (MOI) of 100. After 1, 6, 24, or 48 h of incubation the cells were harvested by trypsinization and cell numbers were counted. Each experiment was performed at least in triplicate.
Effect of H. pylori Infection on Hepatocytes Apoptosis
To assess apoptosis or programmed cell death, we measured the expression and the activation of caspase-3, DNA fragmentation, and chromatin condensation.
Western Blot Analysis with Anti-caspase-3 Antibody
Huh7 cells (8 × 105 cells/well) were seeded on six well plates and incubated overnight. FBS was then removed from the culture medium and the cultures were incubated for an additional 16 h. Based on previous reports that activation of caspase-3 by H. pylori increased in a dose-dependent manner and that an MOI of 100 or more was required to reproducibly induce apoptosis, H. pylori were added to each well at an MOI of 300 [17, 18]. Cell culture medium was used as the control, mock infection. After 24 h incubation, cells were washed twice with PBS and then lysed with 200 µl/well of lyses buffer containing 1% Triton X-100, 50 mM Tris–HCl (pH 7.4), 5 mM EDTA, and 1 mM Na3VO3. The mixture was centrifuged at 10,000×g for 10 min at 4°C and the supernatant was used as to make cytosolic extracts by adding 100 µl of sample buffer containing 6 mg of SDS, 30 µl of glycerol, 19 µl of 1 M Tris (pH 6.8), 15 µl of 2-mercaptoethanol, and 3 µg of bromophenol blue. Protein samples (15 µg) were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) microporous membranes (Millipore Corporation, Billerica, Massachusetts) which were immuno-blotted overnight with rabbit anti-caspase-3 polyclonal antibody (Stressgen Biotechnologies, BC, Canada) at a 1:2,000 dilution. Anti-β-actin antibody (Santa Cruz Biotechnology, CA, USA) was used to normalize the immunoblot analyses. Horseradish peroxidase-conjugated IgG (1:5,000) (Santa Cruz Biotechnology) was used as the secondary antibody and the immuno-complexes were visualized by enhanced chemiluminescence. Densitometry analysis was performed for each band using Scion Image (Scion Corporation, Frederick, ML), and band density was compared with the β-actin band by calculation of the ratio. Western blotting bands for 36, 18 or 20, and 43 K correspond to caspase-3, activated caspase-3, and β-actin proteins, respectively.
Quantification of DNA Fragments
DNA fragmentation was quantified by an enzyme-linked immunosorbent assay (ELISA) (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. Huh7 cells (1 × 105 cells/well) were seeded on 24 well plates and incubated overnight. H. pylori was added to each well at an MOI of 30 or 300 in triplicate and incubated for up to 48 h at 37°C in a 5% CO2 atmosphere. Each experiment included control cells treated with cell culture medium instead of bacterial suspension. Cells treated with 2 µg/ml of camptothecin were the positive control. DNA fragmentation was calculated as an index (the absorbance of samples with intracellular H. pylori divided by the absorbance of the uninfected control).
Detection of Chromatin Condensation
Toluidine blue staining was used to detect chromatin condensation (apoptotic bodies) [19]. Huh7 cells (1 × 104) were seeded on eight well Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY). The cells were incubated at 37°C in 5% CO2 for 48 h after which H. pylori was added to each well at an MOI of 30 or 300. Culture medium was used as the negative control. After 48 h incubation, cells were stained by 1% of toluidine blue (w/v with PBS). The number of cells with chromatin condensation was counted per 100 hepatocytes at 400-fold magnification. The proportion of the cells with chromatin condensations or apoptotic bodies was calculated as an index (a proportion in co-incubation with H. pylori divided by the proportion in uninfected control).
Effect of H. pylori Infection on Hepatocyte Proliferation
We measured DNA synthesis to assess the effect of H. pylori infection on hepatocyte proliferation. DNA synthesis was quantified by the measure of bromodeoxyuridine (BrdU) assay (Cell Proliferation ELISA, BrdU (colorimetric), Roche Applied Science).
BrdU Assay
The incorporation of BrdU was assessed to quantify DNA synthesis and cell proliferation using a colorimetric BrdU assay performed according to the manufacturer’s instructions. Huh7 cells (1 × 104 cells/well) were cultured overnight in 96 well plates and then incubated for up to 96 h in the presence of H. pylori at an MOI of 30 or 300. Before the addition of BrdU, the cells were washed three times and then incubated for 2 h with culture medium containing 1 µg/ml amoxicillin to kill the H. pylori and prevent the detection of H. pylori DNA synthesis. The minimum inhibitory concentration (100% MIC) of the H. pylori strains to amoxicillin was assessed by agar dilution methods and was found to be 0.1 µg/ml. Culture medium without H. pylori was used as the negative control. Culture medium containing 10 pg/ml of EGF (Roche Applied Science) was used as a positive control in each experiment. Data were calculated as a percent of the absorbance value of the control.
Effect of Intracellular or Persistent H. pylori in Hepatocytes on Apoptosis and Cell Proliferation
Apoptosis ELISA and BrdU assays were used to assess the effects of intracellular H. pylori on apoptosis and cell proliferation after passage in Huh7 cells. Huh7 cells (2 × 106 cells/well) were seeded on each 10 cm dish and incubated at 37°C overnight. Culture medium containing H. pylori was applied to each well at an MOI of 300. Culture medium without H. pylori was used as a negative control. Culture medium containing 2 µg/ml of camptothecin or 0.1 ng/ml of interleukin (IL)-6 was used for a positive control for the apoptosis ELISA or BrdU assay, respectively. After 24 h incubations at 37°C in5%CO2, cells were washed three times with PBS and treated with culture medium containing 100 µg/ml of gentamicin for 2 h. Previous reports have shown that gentamicin treatment (100 µg/ml) completely killed extracellular H. pylori, whereas intracellular H. pylori remained viable [20, 21]. After washing three times with PBS, cells were harvested by trypsinization and seeded on 24 well plates for the apoptosis ELISA (5 × 104 cells/well). Cells (5 × 103 cells/well) were seeded on 96 well plates for the BrdU assay. After overnight incubation, the apoptosis ELISA and BrdU assays were performed as described above. After five passages, cells were subcultured five times with gentamicin treatment as described above before being seeded on 24 or 96 well plates. DNA fragmentation was calculated as an index (the absorbance of samples with intracellular H. pylori divided by the absorbance of the uninfected control). DNA synthesis was calculated as a percent of the control.
Statistical Analysis
All data were reported as mean ± standard error (SE) and evaluated by one-way analysis of variance (ANOVA). Statistical analysis was performed using Sigma Stat 3.01 (SPSS Inc., Chicago, IL). P ≤ 0.05 was considered statistically significant.
Results
Effect of H. pylori on Huh7 Cells
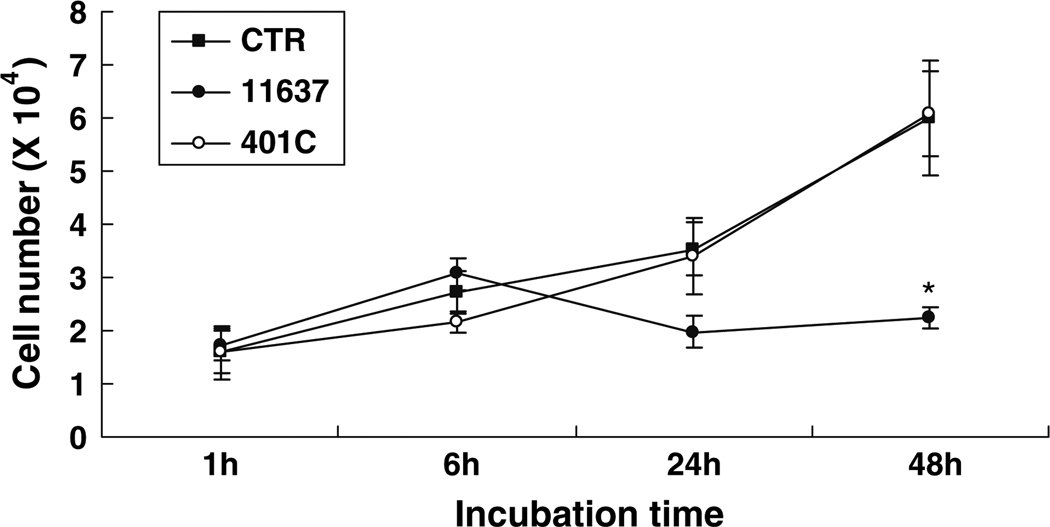
The cell number of uninfected control hepatocytes doubled during each 24 h period for up to 48 h (e.g., cell numbers were 1.6 ± 0.4 × 104/well for 1 h increasing to 3.5 ± 0.5 × 104/well for 24 h; P < 0.05; Fig. 1). The cell number of control cells and those infected with strain 401C were similar (P = 0.13). In contrast, no significant increases were observed following infection with strain 11637 during the observation period of 48 h (e.g., 1.7 ± 0.3 × 104/well after 1 h incubation and 2.2 ± 0.2 × 104/well after 48 h incubation, respectively; P = 0.26; Fig. 1). The number of hepatocytes infected with strain 11637 at 48 h was significantly reduced compared to cultures with strain 401C (6.0 ± 0.8 × 104/well) or uninfected control cells (6.0 ± 1.1 × 104/well; P < 0.05).
Fig. 1.
The effect of H. pylori 11637 or 401C infection on the number of Huh7 cells. 1.5 × 105 of Huh7 cells infected without (■) or with H. pylori 11637 (●) or 401C (○) at MOI of 100 for up to 48 h. Data are presented as mean ± SEM. *P < 0.05 compared with an infected control
Effect of H. pylori Infection on Hepatocytes Apoptosis
Caspase-3 Activations
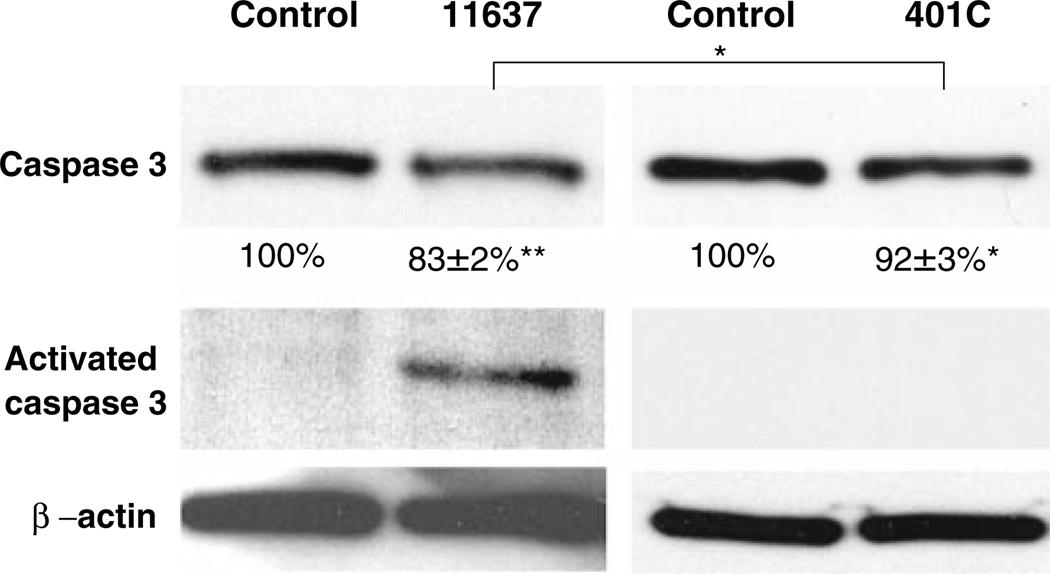
Semi-quantitative western blot analyses showed that caspase-3 protein expression in Huh7 cells decreased following infection with both H. pylori strains (Fig. 2). Densitometry analysis showed that the reduction was 83 ± 2% (P < 0.01) and 92 ± 3% (P < 0.05) of uninfected controls with strain 11637 and 401C infections, respectively. The amount of reduction was the greatest with the more virulent strain 11637 (P < 0.05). Importantly, activated caspase-3 band 18 or 20 K was only detected in cells infected with the more virulent strain 11637.
Fig. 2.
Assessment of caspase-3 expression and activation. Huh7 cells were incubated with or without H. pylori 11637 or 401C at MOI of 300 for 24 h. Expression of β-actin was measured as internal control. Western blot analysis was made for the expression of caspase-3 and activated caspase-3 using anti-caspase-3 antibody. Densitometry analysis of caspase-3 protein expression was compared to the respective protein for β-actin (caspase-3/β-actin ratio) and shown by calculations of percentages of the ratio in control under each western band (shown below each band). Data are presented as mean ± SEM. **P < 0.01, *P < 0.05
DNA Fragmentation
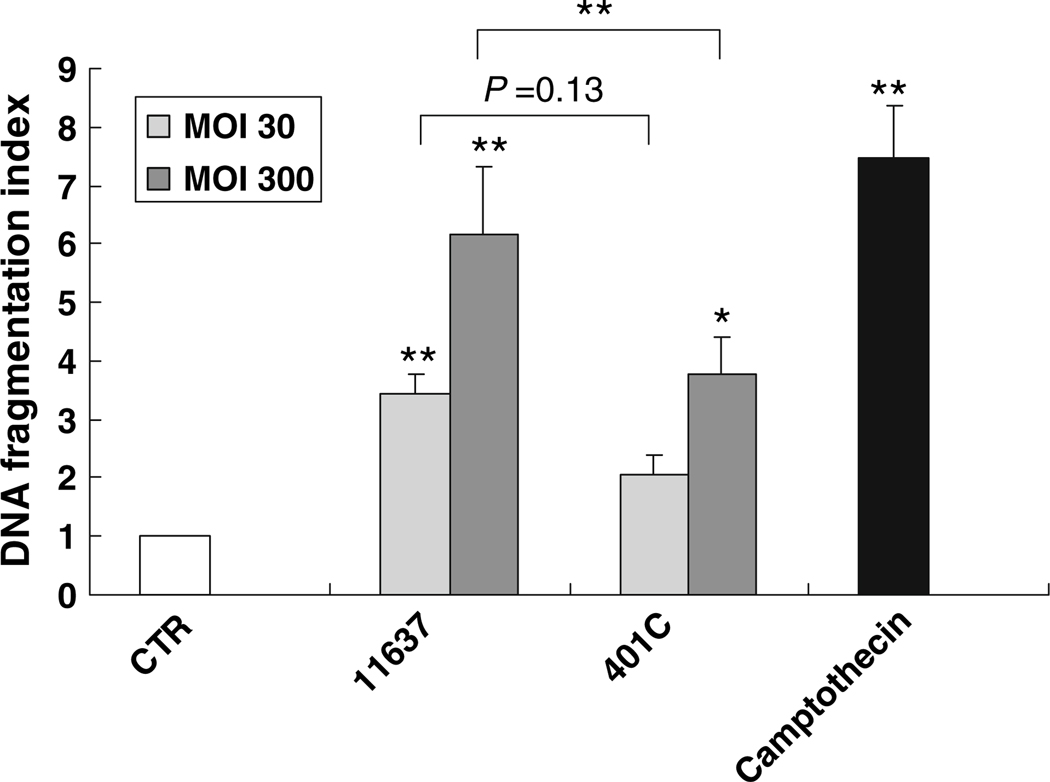
An ELISA to detect histone-associated DNA fragments in the cytoplasm was performed to quantify apoptosis of hepatocytes. Both strains were associated with an increase in DNA fragmentation after 48 h infection (P < 0.05; Fig. 3). At an MOI of 30, DNA fragmentation increased 3.4 ± 3 fold (P < 0.01) and 2.9 ± 0.3 fold (P = 0.15) following infection with strain 11637 and 401C, respectively. At an MOI of 300, DNA fragmentation further increased 6.1 ± 1 fold (P < 0.01) and 3.8 ± 0.6 fold (P < 0.05) following infection with stain 11637 and 401C, respectively. The augmentation of DNA fragmentation was significantly greater with the more virulent stain (P = 0.01) at an MOI of 300, but not at an MOI of 30 (P = 0.13).
Fig. 3.
Comparison of DNA fragmentation in Huh7 cells co-incubated with or without H. pylori 11637 or 401C by quantitative apoptosis ELISA. Quantitative apoptosis ELISA methods quantify histon-complexed DNA fragments, which are biological hallmarks of apoptosis. DNA fragmentation index (405 nm absorbance of samples per 405 nm absorbance of control) was used for comparison of DNA fragmentation quantity. Huh7 cells were co-cultured with H. pylori 11637 or 401C at MOI of 30 (light gray bars) or 300 (dark gray bars) for 48 h. Control cells were incubated with cell culture medium absent with H. pylori (white bar). 2 µg/ml of camptothecin was used for positive control (black bar). Data are presented as mean ± SEM. **P ≤ 0.01, *P < 0.05
Comparative Analysis of Apoptotic Bodies
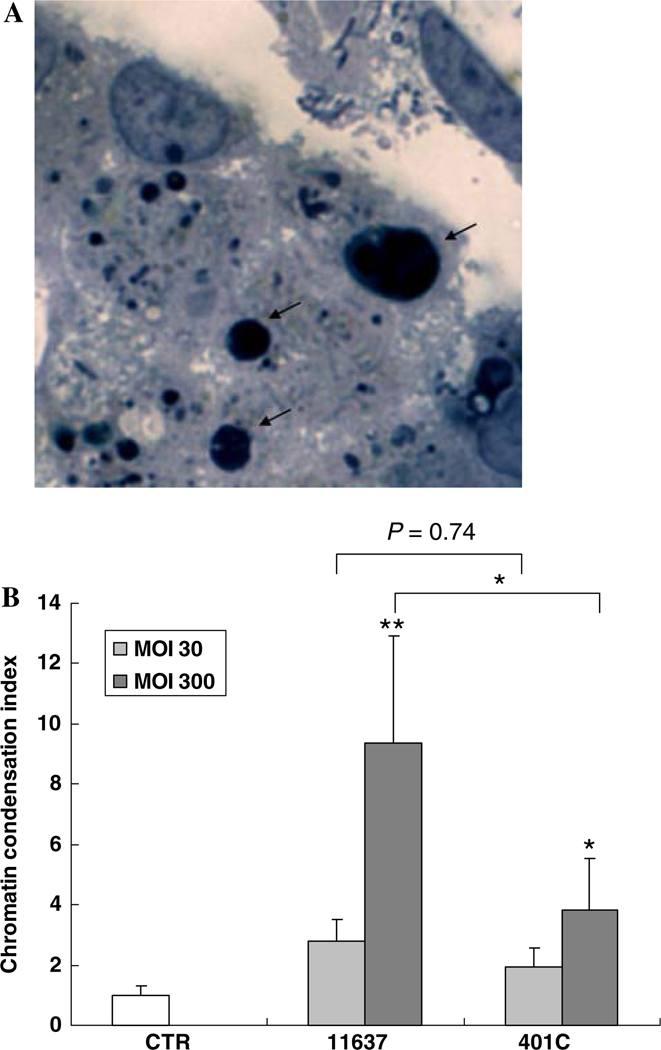
Toluidine blue staining was used to identify chromatin condensations (apoptotic bodies) that occur in the late phase of the apoptotic process (Fig. 4a). H. pylori infection augmented the proportion of cells with chromatin condensation with the actual results depending on the MOI and the virulence of the strain (P < 0.05; Fig. 4b). At an MOI of 300, infection of both strains 11637 and 401C increased chromatin condensation 9.4 ± 3 fold and 3.9 ± 1 fold, respectively (P < 0.01 and P < 0.05 compared with uninfected control, respectively). The increase of apoptotic bodies with strain 11637 was greater than with strain 401C (P < 0.05). At an MOI of 30, strain 11637 infections increased chromatin condensation 2.8 ± 0.7 fold which was not significantly different from the 1.9 ± 0.6 fold increase with 401C infection (P = 0.74).
Fig. 4.
Comparison of chromatin condensations in Huh7 cells co-incubated with or without H. pylori 11637 or 401C. a Chromatin condensations (apoptotic bodies) were observed by toluidine blue stain (arrow). b Huh7 cells co-incubated with H. pylori 11637 or 401C at MOI of 30 (light gray bars) and 300 (dark gray bars). Uninfected cells were used as control (white bar) for 48 h. The number of Huh7 cells containing chromatin condensation was calculated as index (number in Huh7 cells co-incubated with H. pylori/number in control). Data are presented as mean ± SEM. **P < 0.01, *P < 0.05
Effect of H. pylori Infection on Hepatocytes Proliferation
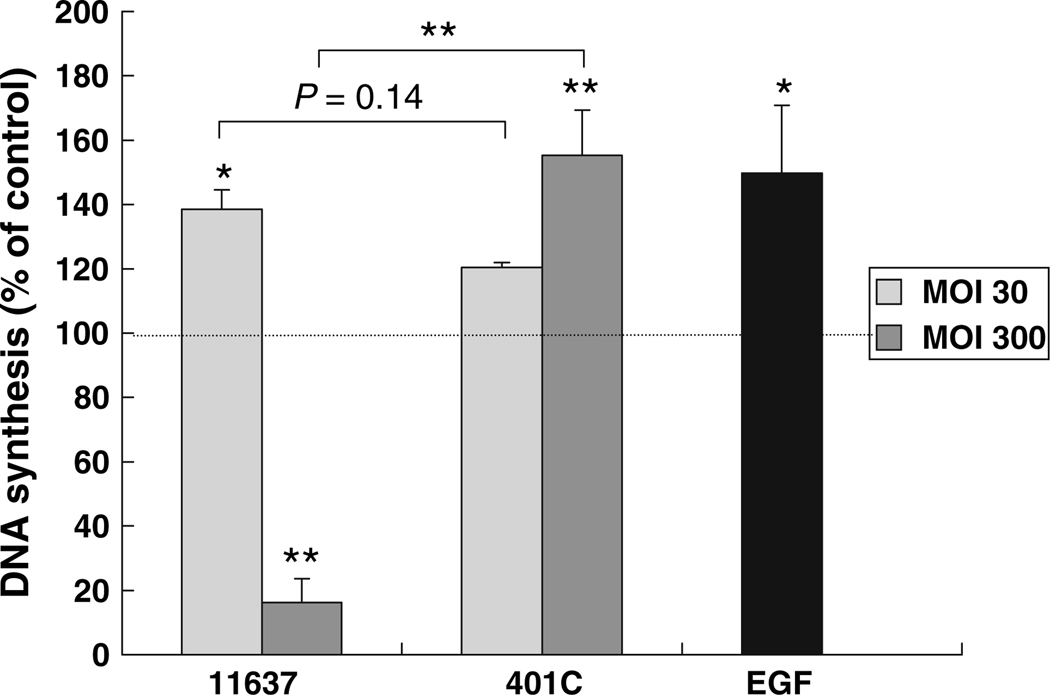
DNA synthesis of infected or mock infected Huh7 cells was analyzed by the BrdU assay. EGF was used as a positive control. H. pylori 11637 infection at an MOI of 30 caused 139 ± 6% augmentation of DNA synthesis as compared with mock cultures (P < 0.05) after 96 h infection (Fig. 5). Interestingly, a high bacterial concentration (MOI of 300) was associated with a dramatic inhibition of DNA synthesis with strain 11637 (to 16 ± 7% of control; P < 0.01). In contrast, the less virulent strain 401C infection resulted in augmented DNA synthesis at both MOIs; 120 ± 1% and 155 ± 14% of control at an MOI of 30 and 300, respectively (P = 0.10 and P < 0.01 compared with control, respectively). There was no significant difference between DNA synthesis following infections with strains 11637 and 401C at an MOI of 30 (P = 0.14).
Fig. 5.
Comparison of DNA synthesis of Huh7 cells co-incubated with or without H. pylori 11637 or 401C by BrdU assay. Huh7 cells incubated with H. pylori 11637 or 401C at MOI of 30 (light gray bars) or 300 (dark gray bars) for up to 96 h. Uninfected cells were used as control. 10 pg/ml of EGF was used for positive control (black bar). BrdU incorporation rates reflecting DNA synthesis were analyzed by immunocytochemistry and were expressed as percentage of control absorbance. Data are presented as mean ± SEM. **P < 0.01, *P < 0.05
Effect of Intracellular or Persistent H. pylori on Apoptosis and Cell Proliferation after Passages of Infected Hepatocytes Cell Cultures
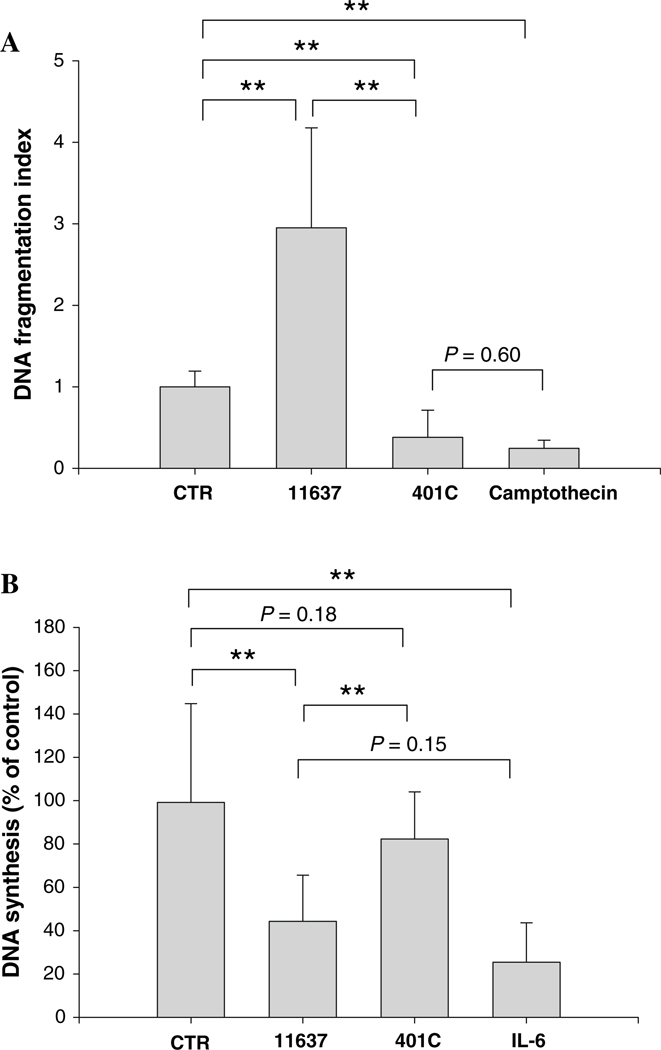
H. pylori is known to invade human cells [21–23]. Preliminary studies found that approximately 1% of adherent H. pylori were internalized into hepatocytes when 107 CFU of bacteria were inoculated to 105 hepatocytes and persisted during several passages of cell culture (data not shown). Further, the adherence and the internalization of H. pylori into hepatocytes were independent of the presence of CagA and VacA [24]. We therefore assessed the effect of intracellular H. pylori on hepatocytes using quantitative assays for apoptosis (ELISA) and cell proliferations (BrdU). To assess the effect of intracellular H. pylori, the assays were performed after one passage with removal of the adherent H. pylori (Fig. 6). Intracellular H. pylori 11637 was associated with a 3.0 ± 0.4 fold augmentation of DNA fragmentation as compared to control cultures (P < 0.01; Fig. 6a). In contrast, intracellular H. pylori 401C was associated with a 38 ± 8% inhibition of DNA fragmentation as compared to uninfected control (P < 0.01). Our preliminary data showed that camptothecin and H. pylori treatments increased DNA fragmentation after 24 h of co-incubation with Huh7 cells (data not shown). However, since treatment with camptothecin also inhibited DNA fragmentation (25 ± 4% in uninfected control; P < 0.01), there was no significant difference between the results of camptothecin treatments and strain 401C infections (P = 0.60), suggesting that intracellular bacteria alone have little effect on DNA fragmentations of hepatocytes and the observed effects were results of adherent H. pylori 401C.
Fig. 6.
Effect of intracellular H. pylori on hepatocyte DNA fragmentation or DNA synthesis after one passage with a removal of adherent bacteria. DNA fragmentation (a) and DNA synthesis (b) was assessed after one passage of Huh7 cells sub-cultured with or without intracellular H. pylori 11637 or 401C after removal of adherent H. pylori. Camptothecin or IL-6 was used instead of H. pylori for a positive control in the experiments of DNA fragmentation or DNA synthesis, respectively. Data are presented as mean ± SEM. **P < 0.01
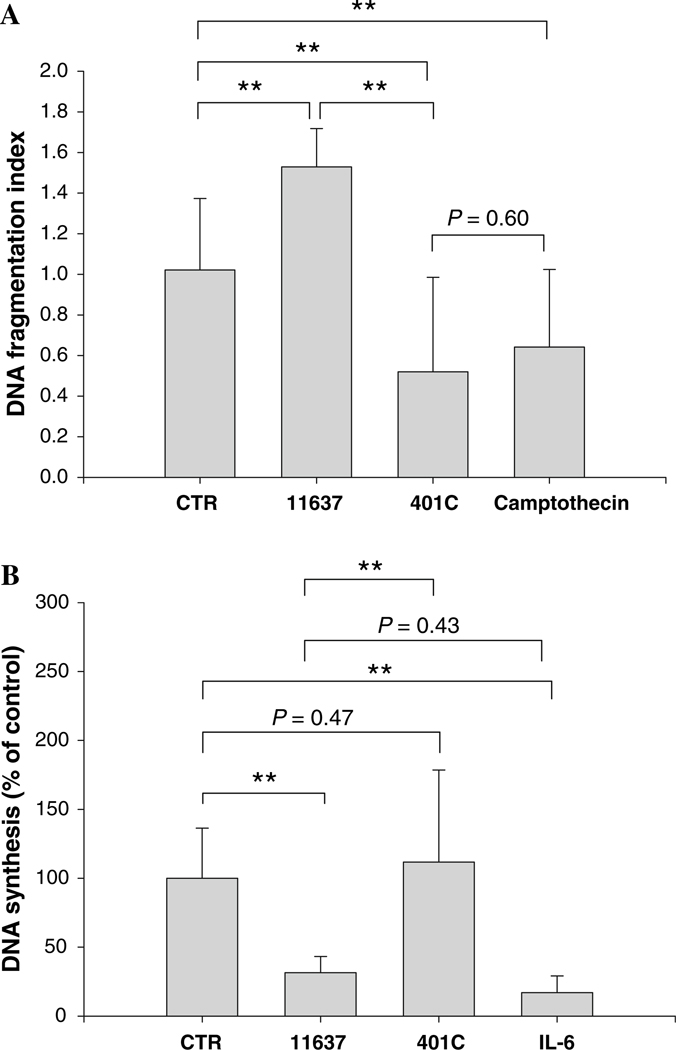
As for DNA synthesis (Fig. 6b), intracellular H. pylori 11637 resulted in a 44 ± 6% inhibition of DNA synthesis compared with the absence of H. pylori after one passage with removal of adherent bacteria (P < 0.01) and was comparable to the effect seen in cultures initially treated with IL-6 (25 ± 6% of uninfected control; P = 0.152 versus strain 11637 infections). Inhibition of DNA synthesis with strain 11637 infections was related to the removal of adherent bacteria rather than the presence of intracellular bacteria, again consistent with the notion that intracellular H. pylori had minimal effects on hepatocyte DNA synthesis. In contrast, no significant changes in DNA synthesis were seen following invasive infection with H. pylori 401C (P = 0.18; Fig. 6b). Thus, the changes seen after the removal of adherent H. pylori appear to be related to the presence of the virulence factors CagA and VacA. No changes were observed even after five passages (Fig. 7).
Fig. 7.
Effect of persistent intracellular H. pylori on hepatocyte DNA fragmentation or DNA synthesis after five passages with removals of adherent bacteria. DNA fragmentation (a) and DNA synthesis (b) was assessed after five passages of Huh7 cells sub-cultured with or without intracellular H. pylori 11637 or 401C after removal of adherent H. pylori. Camptothecin or IL-6 was used instead of H. pylori as a positive control in the experiments of DNA fragmentation and DNA synthesis, respectively. Data are presented as mean ± SEM. **P < 0.01
Discussion
H. pylori infection–hepatocyte interactions differed in relation to differences in H. pylori virulence (putative more virulent strains versus less-virulent strains), inoculation dose (i.e., MOI), and incubation time. Overall, the most striking effects were seen with the more virulent strains at a high MOI and following longer incubation time. These results were similar to those reported with gastric cancer cell lines [11] where low concentrations of H. pylori (e.g., an MOI < 100) resulted in both DNA fragmentation and increased DNA synthesis. Increased DNA synthesis likely represents a compensatory mechanism to counteract the increased cell loss caused by the increased rate of cell death. High MOIs resulted in an increased DNA fragmentation and diminished DNA synthesis suggesting failure to compensate at higher MOIs [11]. Therefore, it is conceivable that the discrepancies among the previous reports regarding the effects of H. pylori on cell proliferation and apoptosis [11, 25–29] are actually due to differences in study parameters such as the density and virulence of H. pylori used for the infections.
Based on our observations and the reported evidence that the number of Helicobacter in the liver is likely to be very small [2, 30, 31], we postulate that overall effect of Helicobacter infection in the liver would possibly include and increase in DNA fragmentation and DNA synthesis. However, invasive infection with H. pylori may have only minimal overall effects on cell replication because of the low proportion of infected cells. It is possible that persistent infection with H. pylori could lead to subtle modulations in hepatocytes replication and play a role in the pathogenesis of liver disease. Of interest, intracellular H. pylori 11637 induced apoptosis, whereas strain 401C did not, suggesting that virulent factors released inside the hepatocytes may be important in the induction of apoptosis. At low density, the effects of H. pylori virulence factors were less dramatic and the results with the virulent and less virulent strains appeared similar (e.g., changes in DNA fragmentation and DNA synthesis were not significantly different between infection with strain 11637 or 401C at low bacterial concentrations), suggesting that adherence or the process of an invasion may be important in hepatocyte injury. One possible factor may be β-1 integrin which is a major cell surface adhesion receptor. Su et al. showed that H. pylori invasion into gastric epithelial cells was associated with β-1 integrin-mediated cytoskeletal rearrangements [23]. The attachment of H. pylori to β-1 integrin receptors promoted phosphotyrosine signaling resulting in a tyrosine phosphorylation cascade and internalization of the bacteria [23, 32, 33]. Once the bacteria initiate the process of internalization, contact between the extracellular matrix (ECM) and integrin are broken, allowing endocytotic factors to concentrate receptors at the site of bacterial binding [34]. Loss of adherence of integrin to ECM then induces apoptosis [35, 36].
In conclusion, we found that H. pylori affected hepatocyte replication mainly by inducing apoptosis with a compensatory increase in DNA synthesis to balance the increased cell loss. Persistent infections of H. pylori in the liver may allow both apoptosis and DNA synthesis to increase continuously. Therefore, we postulate that the overall effect is to promote an increase in the cell cycle. Furthermore, the presence of intracellular virulent strain H. pylori increased apoptosis during several passages, suggesting that persistence of H. pylori inside hepatocytes may play an important role in the pathogenesis of liver disease by disturbing the critical balance between cell proliferations and apoptosis. The failure of cells to undergo apoptosis is thought to be involved in the pathogenesis of a variety of human diseases including cancer [37, 38]. Our findings of an increase in compensatory proliferation of hepatocytes suggest a potential contributory role of Helicobacter in the development of HCC.
Acknowledgements
We are grateful to Dr. Andre Dubois and Dr. Cristina Semino-Mora for insightful discussions as assistance. We also thank Mr. Giovanni Suarez for performing the quantitative apoptosis ELISA assays and Dr. Reyes for his excellent advice. This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338 which funds the Texas Gulf Coast Digestive Diseases Center. Dr. Yoshio Yamaoka is supported in part by NIH grant DK 62813. Dr. Kyoko Ito was supported in part by an International GI Training Grant from the American College of Gastroenterology.
Contributor Information
Kyoko Ito, Department of Medicine, GI Section, Michael E DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA; Department of Medicine, Division of Gastroenterology and Hepatology, The Jikei University School of Medicine, Tokyo, Japan.
Yoshio Yamaoka, Department of Medicine, GI Section, Michael E DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA.
Boris Yoffe, Department of Medicine, GI Section, Michael E DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA.
David Y. Graham, Email: dgraham@bcm.edu, Department of Medicine, GI Section, Michael E DeBakey Veterans Affairs Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA.
References
- 1.Fox JG. The non-H. pylori helicobacter: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50:273–283. doi: 10.1136/gut.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward JM, Fox JG, Anver MR, Haines DC, George CV, Collins MJ, Jr, Gorelick PL, Nagashima K, Gonda MA, Gilden RV, Tully JG, Russel RJ, Benvensite RE, Paster BJ, Dewhirst FE, Donovan JC, Anderson LM, Rice JM. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst. 1994;86(16):1222–1227. doi: 10.1093/jnci/86.16.1222. [DOI] [PubMed] [Google Scholar]
- 3.Avenaud P, Marais A, Monterio L, Le Bail B, Bioulac Sage P, Balabaud C, Mégraud F. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431–1439. [PubMed] [Google Scholar]
- 4.Verhoef C, Pot RGJ, de Man RA, Zondervan PE, Kuipers EJ, IJzermans JN, Kusters JG. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15(11):1171–1174. doi: 10.1097/00042737-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Nakamura M, Toda Negishi M, Torii A, Ohno T. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221–227. [PubMed] [Google Scholar]
- 6.Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47(7):1638–1643. doi: 10.1023/a:1015848009444. [DOI] [PubMed] [Google Scholar]
- 7.Rocha M, Avenaud P, Menard A, Le Bail B, Balabaud C, Bioulac-Sage P, de Magalhães Queiroz DM, Mégraud F. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396–401. doi: 10.1136/gut.2004.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC. IARC working group on the evaluation of carcinogenic risk of humans. vol 61. Lyon: IARC; 1994. [Google Scholar]
- 9.Stalke P, Al-Soud WA, Bielawski KP, Bakowska A, Trocha H, Stepinski J, Wadström T. Detection of Helicobacter species in liver and stomach tissues of patients with chronic liver diseases using polymerase chain reaction-denaturing gradient gel electrophoresis and immunohistochemistry. Scand J Gastroenterol. 2005;40:1032–1041. doi: 10.1080/00365520510023251. [DOI] [PubMed] [Google Scholar]
- 10.de Magalhaes Queiroz DM, Santos A. Isolation of a Helicobacter strain from the human liver. Gastroenterology. 2001;121(4):1023–1024. doi: 10.1053/gast.2001.28574. [DOI] [PubMed] [Google Scholar]
- 11.Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of growth epithelial cell growth by Helicobacter pylori: evidence of major role of apoptosis. Gastroenterology. 1997;113(6):1836–1847. doi: 10.1016/s0016-5085(97)70003-9. [DOI] [PubMed] [Google Scholar]
- 12.Peek RM, Blaser MJ, Mays DJ, Forsyth MH, Cover TL, Song SY, Krishna U, Pietenpol JA. Helicobacter pylori strain-specific genotypes and modulation of the gastric epithelial cell cycle. Cancer Res. 1999;59:6124–6131. [PubMed] [Google Scholar]
- 13.Anti M, Armuzzi A, Gasbarrini A, Gasbarrini G. Importance of change in epithelial cell turnover during Helicobacter pylori infection in gastric carcinogenesis. Gut. 1998;43:S27–S32. doi: 10.1136/gut.43.2008.s27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Huhman ICE/CED-3 protease nomenclature. Cell. 1996;87(2):171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 15.Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136:593–601. [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashktorab H, Frank S, Khaled AR, Durum SK, Kifle B, Smoot DT. Bax translocation and mitochondrial fragmentation induced by Helicobacter pylori. Gut. 2004;53:805–813. doi: 10.1136/gut.2003.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potthoff A, Ledig S, Martin J, Jandl O, Cornberg M, Obst B, Beil W, Manns MP, Wagner S. Significance of the caspase family in Helicobacter pylori induced gastric epithelial apoptosis. Helicobacter. 2002;7(6):367–377. doi: 10.1046/j.1523-5378.2002.00112.x. [DOI] [PubMed] [Google Scholar]
- 19.Erenpreisa J, Erenpreiss J, Freivalds T, Slaidina M, Krampe R, Butikova J, Ivanov A, Pjanova D. Toluidine blue test for sperm DNA integrity and elaboration of image cytometry algorithm. Cytometry. 2003;2:19–27. doi: 10.1002/cyto.a.10015. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimatric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Evans DG, Evans DJ, Graham DY. Adherence and internalization of Helicobacter pylori by Hep-2 cells. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 22.Petersen AM, Sorensen K, Blom J, Krogfelt KA. Reduced intracellular survival of Helicobacter pylori vac A mutants in comparison with their wild-types indicated the role of VacA in pathogenesis. FEMS. 2001;30:103–108. doi: 10.1111/j.1574-695X.2001.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 23.Su B, Johansson S, Fällman M, Patarroyo M, Granström M, Normark S. Signal transduction-mediated adherence and entry of Helicobacter pylori cultured cells. Gastroenterology. 1999;117:595–604. doi: 10.1016/s0016-5085(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Yamaoka Y, Ota H, El-Zimaity H, Graham DY. Adherence, internalization, and persistence of Helicobacter pylori in hepatocytes. Dig Dis Sci. 2007 doi: 10.1007/s10620-007-0164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang K, Fujiwara Y, Wyle F, Tarnawski A. Helicobacter pylori toxin inhibits growth and proliferation of cultured gastric cells-KATO III. J Physiol Pharmacol. 1993;44:17–22. [PubMed] [Google Scholar]
- 26.Knipp U, Birkholz S, Kaup W, Opferkuch W. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect Immun. 1996;64:3491–3496. doi: 10.1128/iai.64.9.3491-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricci V, Ciacci C, Zarrilli R, Sommi P, Tummuru MK, Del Vecchio Blanco C, Bruni CB, Cover TL, Blaser MJ, Romano M. Helicobacter pylori on gastric epithelial cell migration and proliferation in vitro: role of VacA and CagA. Infect Immun. 1996;64:2829–2833. doi: 10.1128/iai.64.7.2829-2833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smoot DT, Wynn Z, Elliott TB, Allen CR, Mekasha G, Naab T, Ashktorab H. Effect of Helicobacter pylori on proliferation of gastric epithelial cells in vitro. Am J Gastroenterol. 1999;94(6):1508–1511. doi: 10.1111/j.1572-0241.1999.01134.x. [DOI] [PubMed] [Google Scholar]
- 29.Fan XG, Kelleher D, Fan XJ, Xia HX, Keeling PW. Helicobacter pylori increases proliferation of gastric epithelial cells. Gut. 1996;38:19–22. doi: 10.1136/gut.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox JG, Yan LL, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33(2):445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox JG, Drolet R, Higgins R, Messier S, Yan L, Coleman BE, Paster BJ, Dewhirst FE. Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. J Clin Microbiol. 1996;34(10):2479–2482. doi: 10.1128/jcm.34.10.2479-2482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187(8):1165–1177. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyle EC, Finlay BB. Bacterial pathogenesis: exploiting cellular adherence. Curr Opin Cell Biol. 2003;15:633–639. doi: 10.1016/s0955-0674(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 34.Isberg RR, van Nhieu GT. The mechanism of phagocytic uptake promoted by invasion-integrin interaction. Trends Cell Biol. 1995;5:120–124. doi: 10.1016/s0962-8924(00)88962-x. [DOI] [PubMed] [Google Scholar]
- 35.Newsome PN, Tsiaoussis J, Masson S, Buttery R, Livingston C, Ansell I, Ross JA, Sethi T, Hayes PC, Plevris JN. Serum from patients with fulminant hepatic failure causes hepatocytes detachment and apoptosis by beta(1)-integrin pathway. Hepatology. 2004;40(3):636–645. doi: 10.1002/hep.20359. [DOI] [PubMed] [Google Scholar]
- 36.Pinkse GG, Voorhoeve MP, Noteborn M, Terpstra OT, Bruijn JA, De Heer E. Hepatocytes survival depends on beta1-integrin-mediated attachment of hepatocytes to hepatic extracellular matrix. Liver Int. 2004;24(3):218–226. doi: 10.1111/j.1478-3231.2004.0914.x. [DOI] [PubMed] [Google Scholar]
- 37.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1447. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 38.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]