Abstract
Objective
Certain cervicovaginal lavage (CVL) fluid samples obtained from HIV-1–infected and uninfected women stimulate in vitro HIV-1 replication. This activity, HIV-inducing factor (HIF), changes when CVL fluid is heated. We sought to confirm a previous observation that HIF was associated with bacterial vaginosis (BV).
Methods
HIF was measured in unheated and heated CVL fluid obtained from HIV-1–infected women and compared with the presence of BV by Nugent scores, other genital tract conditions, and cervicovaginal HIV-1 shedding.
Results
Among the 295 women studied, 54% of CVL samples had HIF activity and 21% showed heat-stable HIF activity. In adjusted logistic regression, heat-stable HIF was associated with BV (odds ratio [OR] = 51.7, 95% confidence interval [CI]: 5.0, 530.7) and with intermediate flora (OR = 43.3, 95% CI: 3.6, 521.1); heat-labile HIF was not associated with BV. Neither heat-stable nor heat-labile HIF was associated with other cervicovaginal conditions nor, after controlling for plasma viral load, with genital tract HIV-1 shedding.
Conclusion
We confirmed the association of HIF with BV and attribute it to the heat-stable component. Heat-stable activity is also associated, although less strongly, with intermediate vaginal flora. We propose that heat-stable HIF is a result of products of BV-associated bacteria.
Keywords: HIV-1, bacterial vaginosis, vaginitis, cervicovaginal lavage, sexually transmitted diseases, MRP-8 protein
Many lines of evidence indicate that sexually transmitted infections may enhance sexual HIV-1 transmission.1 Nine epidemiologic studies have been interpreted to suggest that bacterial vaginosis (BV) increases women’s susceptibility to vaginally transmitted HIV-1 infection,2–10 although another study has not found such an effect.11 Unlike other sexually transmitted infections associated with HIV-1 transmission,12,13 BV is neither an inflammatory nor an ulcerative condition. The biologic mechanism through which BV might affect transmission is therefore uncertain.
In 1997, Spear and colleagues14 reported that cervicovaginal lavage (CVL) fluid from some HIV-1–infected and uninfected women increased HIV-1 replication in vitro. This in vitro activity was attributed to an HIV-inducing factor (HIF) in CVL fluid. The effect was increased when the original CVL fluid samples with activity were heated. As reported in the initial study, HIF was more often present in CVL fluid obtained from women with vaginitis or vaginosis than from women without vaginal abnormalities. A subsequent study of 26 CVL fluid specimens obtained from 17 women found a statistically significant association of HIF with a vaginal fluid pH >4.5 and Nugent criteria for BV.15 Therefore, laboratory studies suggest that BV could enhance in vivo HIV-1 replication, providing a possible mechanism whereby BV could increase sexual transmission of HIV-1.
We analyzed data and specimens collected in a large cross-sectional study of HIV-1 in the female reproductive tract16 to confirm the association between HIF and BV in a much larger group of women, to investigate the effect of heating CVL fluid on HIF, and to assess the relation between HIF and genital tract HIV-1 shedding.
METHODS
Study Design
This was a substudy of the Division of AIDS Treatment Research Initiative (DATRI) 009, a previously published cross-sectional study of genital tract HIV-1 shedding.16 Participants were HIV-1–infected nonpregnant women 18 to 45 years of age, with an intact uterus and cervix, who had been on no or stable antiretroviral therapy for 1 month preceding the study visit and who had abstained from vaginal intercourse and use of topical vaginal products in the 48 hours before the study visit. Details regarding specimen collection have been published.16 After study approval was granted by the institutional review boards of each participating institution, each subject signed a consent form agreeing to join DATRI 009 and to permit subsequent laboratory and data analyses such as this substudy.
Laboratory Procedures
The methods used for assessing genital tract conditions, for HIV-1 detection and quantitation, and for lymphocyte subset measurements have been described.16–18 HIV-1 RNA in plasma, CVL fluid, and extraction media containing endocervical swabs were quantified by reverse transcriptase polymerase chain reaction (RT-PCR; Monitor Assay, Roche Molecular Systems, Branchburg, NJ; lower limit of detection of 400 copies/mL).
Women with at least 1 genital tract specimen (CVL fluid, swab, or cytobrush) positive for HIV-1 (by RNA detection or qualitative culture) were considered to have genital tract HIV-1 shedding; women with all CVL fluid and cervical swabs and/or cytobrushes negative for HIV-1 by PCR and culture were considered to have no genital shedding. Forty-six women missing 1 or more genital specimens and whose other specimens were negative were considered to be indeterminate for shedding, as in the original report, and were excluded from the analysis of HIV-1 shedding. Gram stains of vaginal fluids were assessed at a central laboratory and categorized as normal vaginal flora (Nugent score of 0–3), intermediate vaginal flora (Nugent score of 4–6), or BV (Nugent score of 7–10).19
CVL fluid samples were prepared for detection of HIF as follows. Uncentrifuged CVL fluid samples were frozen in 1-mL aliquots at −70°C. Samples were thawed, vortexed, and centrifuged in an Eppendorf microfuge (Model 5415C; Brinkman Instruments, Westbury, NY) set on high speed for 2 minutes to pellet cellular debris. A portion of the supernatant fluid was transferred to a sterile tube, and aliquots were directly added from this tube to assay wells to measure activity of “unheated” HIF. The remaining sample was then heated in a boiling water bath for 5 minutes, and aliquots were added to assay wells to measure activity of “heated” HIF.
The assay for HIF activity was performed as follows. Aliquots of CVL fluid (10 µL) were added to wells of 96-well flat-bottom microtiter plates (Costar, Cambridge, MA) that contained 5 × 104 U1 cells (obtained from the AIDS Research and Reference Reagent Program, contributed by T. Folks) in 190 µL of culture medium consisting of RPMI-1640 medium (Whittaker Bioproducts, Walkersville, MD) supplemented with 10% heat-inactivated fetal bovine serum (Whittaker Bioproducts) and 50 µg/mL of gentamicin (Sigma Chemical Co., St. Louis, MO). Cells were cultured for 3 days, and the amount of p24 in culture supernatants was measured by enzyme-linked immunosorbent assay (ELISA; National Institutes of Health, AIDS Vaccine Program, Fredrick, MD). Duplicate cultures of each CVL fluid sample were set up, and the p24 values were averaged. Negative and positive controls for expression of p24 by U1 cells consisted of saline and phorbol myristate acetate (100 ng/mL; Sigma Chemical Co.). A CVL fluid sample was considered positive for HIF if p24 values were greater than the mean plus 3 standard deviations (SDs) of the negative unheated controls.
Statistical Analysis
The Fisher exact test was used to assess the statistical significance of categoric variables, and an exact form of the McNemar test was used for paired categorical variables. The Cochran-Armitage trend test was used for ordered categorical data. Odds ratios (ORs) and their 95% confidence intervals (95% CIs) were obtained from contingency tables or multivariate logistic regression.
Correlation among nonnormally distributed variables was measured with the Spearman coefficient. Measures of quantitative p24 in culture supernatants (quantitative HIF) were approximately exponentially distributed; therefore, the Savage test was used to assess differences between 2 or more groups, and multivariate analyses were performed with generalized linear models using the gamma distribution.20
Quantitative measures of HIV-1 RNA in CVL fluid and endocervical swab extraction media had a Weibull distribution; therefore, the associations of genital tract HIV-1 RNA with genital and blood compartment factors were analyzed using Weibull survival regression models adjusting for log10 plasma HIV-1 RNA levels.20 HIV-1 RNA levels below the limit of quantification (400 copies/mL) in CVL fluid (75% of values) or endocervical swab extraction media (54% of values) were left-censored. Twenty-three percent of plasma RNA levels were below the limit of quantitation (400 copies/mL). Regression models in which undetectable plasma HIV-1 RNA levels were replaced with values at the limit (400 copies/mL) or replaced with randomly imputed values between 0 and 400 copies/mL gave similar results. Finally, the associations of HIF with genital tract HIV-1 shedding were analyzed using multivariate logistic regression, with and without adjustment for log10 plasma HIV-1 RNA.
Statistical significance was defined as a P value ≤0.05. Analyses were performed with SAS Release 8.1 (SAS Institute, Cary, NC), S-PLUS 2000 (Data Analysis Products Division, Insightful, Seattle, WA), and StatExact 4 (Cytel Corporation, Cambridge MA).
RESULTS
Among the 311 women in DATRI 009, 297 had sufficient remaining CVL fluid to perform HIF assays and are included in this substudy. Participants were predominantly African American, and all were of reproductive age, as described in Table 1. One hundred fifty-one (60%) of 251 women had genital tract HIV-1 shedding. Genital tract infections and disorders were frequent among our participants (Table 2). Approximately two thirds of women had vaginal discharge or an elevated pH. Human papillomavirus (in CVL fluid by PCR) and BV (by Nugent criteria) were the most common infections, with approximately one third of women having each condition.
TABLE 1.
Demographics, Clinical Characteristics, and Plasma and Genital Tract HIV-1 Detection Among Participating HIV-Infected Women (n = 297)
| Characteristic | No. (%) With Available Data |
Mean (SD) | Median (range) |
|---|---|---|---|
| Race | 297 | ||
| White | 61 (20.5%) | ||
| African American | 178 (59.9%) | ||
| Hispanic/Latina | 51 (17.2%) | ||
| Other | 7 (2.4%) | ||
| Age (y) | 297 | 35.2 (6.0) | 36.0 (19–45) |
| Risk behaviors | 297 | ||
| Sex with intravenous drug user | 95 (32.0%) | ||
| Sex with bisexual man | 46 (15.5%) | ||
| Sex with HIV+ partner | 20 (6.7%) | ||
| Sex with partner of unknown risk | 101 (34.0%) | ||
| Intravenous drug use | 69 (23.3%) | ||
| Blood transfusion | 24 (8.1%) | ||
| Other or unknown | 15 (5.1%) | ||
| Reporting more than 1 risk | 54 (18.0%) | ||
| CD4+ lymphocytes/mm3 | 296 | 423 (248) | 406 (0–1285) |
| ≤200 | 51 (17.2%) | ||
| 201–499 | 149 (50.3%) | ||
| ≥500 | 96 (32.4%) | ||
| ART summary | 297 | ||
| None | 115 (38.7%) | ||
| NRTI only* | 73 (24.6%) | ||
| NRTI combination with PI and/or NNRTI | 109 (36.7%) | ||
| Plasma HIV-1 RNA copies/mL | 290 | 30,843 (100,418)† | 4729 (402–1,127,500)† |
| <400 | 67 (23.1%) | ||
| 400–4999 | 115 (39.6%) | ||
| 5000–49,999 | 80 (27.6%) | ||
| ≥50,000 | 28 (9.7%) | ||
| CVL fluid HIV-1 RNA copies/mL | 286 | 9847 (25,924)† | 1629 (411–184,148)† |
| <400 | 214 (74.8%) | ||
| 400–4999 | 50 (17.5%) | ||
| 5000–49,999 | 19 (6.6%) | ||
| ≥50,000 | 3 (1.1%) | ||
| Endocervical swab HIV-1 RNA copies/mL | 267 | 20,299 (56,720)† | 3342 (404–420,221)† |
| <400 | 143 (53.6%) | ||
| 400–4999 | 71 (26.6%) | ||
| 5000–49,999 | 42 (15.7%) | ||
| ≥50,000 | 11 (4.1%) | ||
| CVL fluid HIV-1 culture | 264 | ||
| Positive | 7 (2.7%) | ||
| Negative | 257 (97.3) | ||
| Endocervical swab HIV-1 culture | 277 | ||
| Positive | 8 (2.9%) | ||
| Negative | 269 (97.1%) | ||
| Any genital HIV-1 shedding‡ | 251 | ||
| Positive | 151 (60.2%) | ||
| Negative | 100 (39.8%) |
Includes 8 women on NRTI monotherapy.
Among women with quantifiable levels of HIV-1 RNA in plasma, CVL fluid, or endocervical swab extraction media.
Women with at least 1 genital tract specimen (CVL fluid, swab, or cytobrush) positive for HIV-1 (by RNA detection or culture) were considered to have genital tract HIV-1 shedding. Women with all genital tract specimens (CVL, swab, or cytobrush) negative for HIV-1 (by RNA detection or culture) were considered not to have genital tract HIV-1 shedding.
ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
TABLE 2.
Genital Tract Disorders and Associations with Heat-Stable HIF
| Variable | Present/Number Women Assessed (%) |
Unadjusted Association With Heat-Stable HIF OR (95% CI) |
Full Model: Association With Heat-Stable HIF OR (95% CI) |
Reduced Model: Association With Heat-Stable HIF OR (95% CI) |
|---|---|---|---|---|
| Bacterial vaginosis by Nugent criteria | ||||
| Normal flora (0–3) | 112/236 (47.5%) | 1.0 | 1.0 | 1.0 |
| Intermediate flora (4–6) | 34/236 (14.4%) | 19.64 (2.21, 174.94)† | 43.32 (3.60, 521.07)# | 26.00 (2.63, 256.80)†† |
| BV(7–10) | 90/236 (38.1%) | 100.64 (13.46, 752.47)‡ | 51.74 (5.04, 530.72)** | 78.00 (10.09, 602.91)‡‡ |
| Vaginal discharge | 186/297 (62.6%) | 2.74 (1.41, 5.32)§ | 4.94 (0.66, 36.91) | |
| Trichomonas by microscopy | 10/294 (3.4%) | 6.33 (1.73, 23.23)¶ | 0.20 (0.02, 2.53) | |
| Vaginal fluid pH >4.5 | 183/297 (61.6%) | 9.22 (4.46, 19.10)‖ | 3.41 (0.80, 14.63) | |
| Vaginal candidiasis* | 55/269 (20.5%) | 1.52 (0.77, 3.00) | 1.96 (0.49, 7.88) | |
| Herpes simplex PCR on genital swabs | 28/275 (10.2%) | 0.83 (0.30, 2.28) | 1.24 (0.27, 5.75) | |
| Human papilloma virus PCR, any type, in CVL fluid | 109/297 (36.7%) | 1.33 (0.75, 2.36) | 0.52 (0.14, 2.01) | |
| Warts on examination | 38/206 (18.5%) | 1.75 (0.82, 3.75) | 1.83 (0.41, 8.21) | |
| Pap smear: ASCUS | 54/297 (18.2%) | 0.95 (0.46, 1.98) | 1.98 (0.41, 9.61) | |
| Pap smear: SIL | 47/297 (15.8%) | 1.77 (0.88, 3.56) | 1.14 (0.21, 6.35) | |
| RBCs in CVL fluid | 210/289 (72.7%) | 0.88 (0.47, 1.65) | 0.46 (0.12, 1.71) | |
| White blood cells in CVL fluid | 261/289 (90.3%) | 1.21 (0.44, 3.33) | 0.98 (0.12, 8.30) |
ASCUS, atypical squamous cells of uncertain significance; SIL, squamous intraepitheliar lesion.
The combination of vaginal discharge on examination and a positive culture for Candida.
Nugent score: intermediate versus normal flora (P = 0.0025).
Nugent score: BV versus normal flora (P < 0.0001).
Vaginal discharge (P = 0.0030).
Trichomonas (P = 0.0064).
pH (P < 0.0001).
Nugent score: intermediate versus normal flora (P = 0.0450).
Nugent score: BV versus normal flora (P = 0.0107).
Nugent score: intermediate versus normal flora (P = 0.171).
Nugent score: BV versus normal flora (P < 0.0001).
CVL supernatant fluid added to U1 cell cultures increased HIV-1 viral expression compared with controls, as measured by p24 antigen in the cell culture supernatant. Antigen levels were significantly greater with unheated CVL fluid (median = 872.0 pg/mL, range: 0–22,311) than with heated CVL fluid (median = 154.5 pg/mL, range: 0–19,145.0; P < 0.0001). The quantitative results for unheated and heated HIF activity were strongly correlated (r = 0.63, P < 0.001). Quantitative HIF was strongly associated with BV by Nugent criteria assessing unheated (P < 0.0001) and heated (P < 0.0001) CVL fluid.
Using a cutoff value of 600 pg/mL of p24 antigen, equivalent to 3 SD above the control mean, HIF was present in unheated CVL fluid samples from 160 (54%) of 295 women and in heated CVL fluid samples from 66 (22%) of 295 women. Using this definition, HIF in unheated CVL fluid was present in 47 (42%) of 111 women with normal vaginal flora, in 10 (30%) of 33 women with intermediate flora (OR compared with normal flora = 0.59, 95% CI: 0.26, 1.36), and in 69 (77%) of 90 women with BV (OR compared with normal flora = 4.47, 95% CI: 2.42, 8.30).
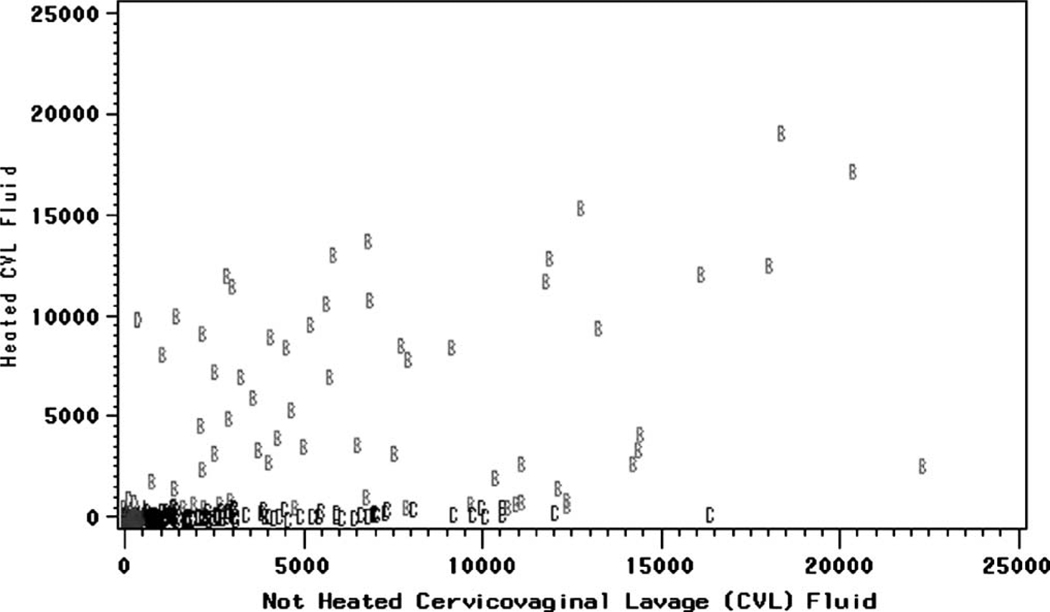
Three different categories of HIF could be distinguished in heated CVL fluid (Fig. 1): activity that persisted in samples from 62 women (21%) and was called heat-stable HIF, activity that decreased below the cutoff in 98 women (33%) and was called heat-labile HIF, and activity that appeared for the first time in 4 women (1%) and was called heat-induced HIF.
FIGURE 1.
HIV-inducing factor (HIF) in heated and unheated cervicovaginal lavage (CVL) fluid from HIV-1–infected women (Spearman rank correlation coefficient: r = 0.63, P = 0.00001). HIF was measured as p24 antigen in HIV cell culture supernatants with heated or unheated CVL fluid added. Each mark represents the results of heated and unheated CVL fluid obtained from a single woman. A indicates HIF with no activity (<600 pg/mL before and after heating CVL fluid). B indicates heat-stable HIF (≥600 pg/mL before and after heating CVL fluid). C indicates heat-labile HIF (≥600 pg/mL before heating but decreases to below HIF cutoff [<600 pg/mL] after heating CVL fluid). D indicates heat-induced HIF (<600 pg/mL before heating but increases to above HIF cutoff (≥600 pg/mL after heating CLV fluid).
Heat-stable HIF was detected in samples from 1 (1%) of 111 women without BV, 5 (15%) of 33 women with intermediate Nugent scores (OR = 19.64, 95% CI: 2.21, 174.94), and 43 (48%) of 90 women with BV (OR = 100.64, 95% CI: 13.46, 752.47) (P for trend <0.0001). Heat-labile HIF had an inverse association with intermediate flora (OR = 0.25, 95% CI: 0.09, 0.70) and no association with BV (OR = 0.57, 95% CI: 0.32, 1.04).
In unadjusted analysis, heat-stable HIF was also associated with vaginal discharge, trichomoniasis, and elevated vaginal pH but not with other genital tract conditions (see Table 2). Including all these genital conditions in the full logistic model for heated HIF, only intermediate flora and BV remained significant (see Table 2). Using backward elimination to create a reduced model containing only variables associated with heated HIF, only intermediate flora and BV remained associated (see Table 2). There were no significant associations between heat-labile HIF and any other genital tract conditions in unadjusted analysis, nor was heat-labile HIF associated with BV or other conditions using adjusted logistic regression or backward elimination (data not shown).
Additional analyses of HIF as a categorical or continuous variable in unheated and heated CVL fluid gave similar results. In bivariate analysis, the (categorical) presence of HIF in unheated CVL fluid was associated with BV, a high pH, and squamous intraepithelial lesions (SILs) on cytology; in the final logistic regression models, unheated HIF was associated only with BV (OR = 3.97, 95% CI: 1.28, 12.32) but not with intermediate flora. The analysis of heated HIF gave results essentially identical to those for heat-stable HIF (the group with heated HIF included 62 women with heat-stable HIF and 4 women with heat-induced HIF). In bivariate analysis of unheated and heated quantitative HIF (p24 antigen in supernatant as a continuous variable), there were strongly significant associations with BV, vaginal fluid pH, and trichomoniasis but not with the other variables. In the full multivariate gamma model, only BV remained significantly associated with unheated (P < 0.0001) or heated (P < 0.0001) HIF. Using backward elimination, the model for unheated quantitative HIF reduced to 2 variables: BV (P < 0.0001) and pH (P = 0.02). For heated quantitative HIF, the model also reduced to 2 variables: BV (P < 0.0001) and a low but not high level of red blood cells (RBCs) in CVL fluid (P = 0.004).
In unadjusted logistic regression models, any genital tract HIV-1 shedding was associated with heat-stable HIF activity (OR = 2.48, 95% CI: 1.21, 5.08; P = 0.01; Table 3). After adjustment for plasma HIV-1 RNA level, there was no significant association between HIF and genital tract HIV-1 shedding. Finally, in Weibull survival models, there were no statistically significant associations between quantitative HIV-1 RNA measures in CVL fluid or endocervical samples with heated or unheated HIF after adjusting for plasma HIV-1 RNA (data not shown).
TABLE 3.
Crude and Adjusted Association of Cervicovaginal HIV-1 Shedding with HIF in CVL Fluid from HIV-1–Infected Women
| Cervicovaginal HIV-1 Shedding* | ||
|---|---|---|
| Association | OR (95% CI) | P |
| Unadjusted | ||
| HIF activity† | ||
| Heat stable‡ | 2.48 (1.21–5.08) | 0.0134 |
| Heat labile§ | 1.44 (0.81–2.56) | 0.2167 |
| Heat induced¶ | 1.82 (0.16–20.72) | 0.6277 |
| No activity | 1.00 | |
| Adjusted | ||
| HIF activity† | ||
| Heat stable‡ | 1.52 (0.65–3.52) | 0.3324 |
| Heat labile§ | 1.09 (0.55–2.16) | 0.8024 |
| Heat induced¶ | 1.37 (0.10–19.10) | 0.8164 |
| No activity | 1.00 | |
| Log10 Plasma HIV-1 RNA | 5.32 (3.28–8.64) | 0.0001 |
At least 1 genital tract specimen (CVL fluid, swab, or cytobrush) positive for HIV-1 (by RNA detection or culture) indicated genital tract HIV-1 shedding.
HIF activity as ≥600 pg/mL of p24 antigen in supernatant fluids of HIV-1 cultures after the addition of CVL fluid sample.
HIF activity that persists after CVL fluid heating compared with CVL fluid samples lacking HIF activity before and after heating.
HIF activity that decreases below the cutoff value after CVL fluid heating compared with CVL fluid lacking HIF activity before and after heating.
HIF activity that appeared only after CVL fluid heating compared with CVL fluid samples lacking HIF activity before and after heating.
DISCUSSION
In CVL fluid of HIV-1–infected women, HIF is associated with BV, confirming earlier observations made on a small number of women.15 This association is attributable to HIF activity that is present in samples before and after heating CVL fluid (heat-stable). HIF activity that is eliminated by heating (heat-labile) is not associated with BV.
We found a significant increase in heat-stable HIF with increasing alterations in vaginal flora. Heat-stable HIF is strongly associated with Nugent scores diagnostic of BV (range: 7–10) and is less strongly associated with intermediate Nugent scores (range: 4–6) when either is compared with normal scores (range: 0–3). Previously published evidence suggests that bacterial products have in vitro HIV-1–inducing activity and that this activity is heat-stable or even enhanced by heat treatment.15,21–23 Other in vitro studies suggest that a cytokine, myeloid-related protein (MRP-8), is a source of HIF.24,25 Our results support the interpretation that products of BV-associated bacteria are the source of heat-stable HIF, whereas MRP-8 may be the source of heat-labile HIF.
We explored possible associations of HIF with other vaginal conditions. In multivariate logistic regression and the reduced logistic model, heat-stable HIF remained associated only with BV and intermediate flora. In the reduced logistic regression model, heat-labile HIF was associated neither with Nugent scores nor with any other vaginal condition. In a reduced gamma model, unheated quantitative HIF was associated with BV and pH. If the association with pH were real, it could be a result of the increased vaginal pH from altered vaginal flora and BV. Trichomoniasis, which may raise vaginal pH, and which we may have underdiagnosed (DATRI 009 relied on microscopy rather than on culture), might also have contributed. Trichomonas infection itself has been associated with abnormal vaginal flora and BV in HIV-1–infected women.10,26 Heated quantitative HIF was associated with BV and RBCs in a reduced gamma model. The statistical association with RBCs is not likely of biologic importance. It was present in only one of many models, and the effect was noted for a low level of CVL fluid RBCs but not for a high level of RBCs when both were compared with the absence of CVL fluid RBCs. Hence, the strong and specific associations of heat-stable and/or heated HIF with BV and intermediate flora are unequivocal. A possible association of unheated HIF with pH may also be an effect of alterations in vaginal bacteria not fulfilling the Nugent criteria for intermediate flora or BV. No other vaginal condition is associated with HIF.
We had hypothesized that the presence of HIF could increase genital tract HIV-1 shedding, which could lead to more efficient transmission of HIV-1 from women with BV. Although heat-stable HIF was associated with genital tract HIV-1 shedding in unadjusted analysis, it was not significantly associated after controlling for plasma HIV-1 RNA levels in this cohort of women. In DATRI 009, the parent study, no association was noted between BV and cervicovaginal HIV-1 shedding.16 Analyzing 123 samples from HIV-1–infected women, Cu-Uvin et al27 found a statistically significant association between BV and HIV-1 RNA by RNA PCR in CVL fluid (OR = 5.9, 95% CI: 1.4, 25.0) after controlling for plasma HIV-1 RNA, antiretroviral therapy, and CD4 lymphocyte count. In a study of 122 HIV-1–infected women, Spinillo et al28 found that BV was associated with cell-associated (OR = 3.58, 95% CI: 1.22, 10.54) and cell-free (OR = 2.94, 95% CI: 1.0, 8.7) HIV-1 RNA in CVL fluid after controlling for plasma viremia. Most recently, Sha et al29 presented data demonstrating an association of quantitative PCR of BV-associated bacteria with genital tract HIV-1 shedding among women with detectable plasma HIV-1 RNA levels. Our results may differ from the observations of Sha and her colleagues because a large proportion of the women in our study had undetectable plasma and genital tract HIV-1 RNA and the study by Sha and her colleagues analyzed a larger number of women with BV and intermediate flora using more specific quantitative measures of vaginal bacteria.
In conducting this substudy, we performed a large number of biologic and statistical analyses on a large number of specimens obtained for different, although related, purposes. Our investigation was designed while study visits for DATRI 009 were in progress and preceded laboratory and statistical analysis within DATRI 009. All our laboratory assays were performed without knowledge of participants’ clinical status or the results from other laboratory procedures. All in vitro work was performed in quality-assured laboratories using standard methods, except for the HIF assays that were performed according to previously published methods.24 In prior results, HIF activity was found in CVL fluid from HIV-1–infected and uninfected women and was demonstrated with clinical HIV-1 isolates in primary human-derived cell lines as well as in laboratory-adapted cells and virus.14 HIF was not associated with the use of antiretroviral therapy in our study (data not shown). Finally, statistical analyses of our data using varied approaches provided consistent results.
In conclusion, we found a strong association, and a dose effect, between heat-stable HIF and alterations in vaginal flora, including BV. Further laboratory studies to characterize the contribution of bacterial products and MRP-8 to HIF are underway. The association between BV-associated bacterial products and genital HIV-1 shedding are being further investigated. A more sophisticated understanding of the influence of cervicovaginal cytokines and vaginal microbial flora on genital HIV-1 quantity and dynamics should help to develop new strategies, such as topical microbicides, to reduce heterosexual HIV transmission.
ACKNOWLEDGMENTS
The authors thank Anna Soloviov and Michael Parloglou of Westat, Rockville, MD, for their contributions to this manuscript. They also thank the women enrolled at the University of California–San Francisco, University of Southern California, Georgetown University, and The Cook County Hospital whose specimens were used in this study.
Supported by the Division of AIDS Treatment Research Initiative, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD (contract NO1-AI-15123); Program Support Center, Department of Health and Human Services (contract 282-98-0015, Task Order Number 21); and PO1 HD40539.
REFERENCES
- 1.del Rio C, Curran JW. Epidemiology and prevention of acquired immunodeficiency syndrome and human immunodeficiency virus infection. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. Philadelphia: Churchill Livingstone; 2000. pp. 1352–1354. [Google Scholar]
- 2.Cohen CR, Duerr A, Pruithithada N, et al. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS. 1995;9:1093–1097. doi: 10.1097/00002030-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Gwanzura L, Mason PR, Latif AS, et al. Vaginal lactobacilli and HIV transmission in African women [abstract ThC4509]. Presented at the 11th International AIDS Conference; July 1996; Vancouver. [Google Scholar]
- 4.Sewankambo N, Gray RH, Wawer MH, et al. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 5.Gray RH, Serwadda D, Wawer M, et al. Treatable STDs account for modest proportion of HIV acquisition in a population-based study: relative and attributable risk estimates from Rakai, Uganda [abstract 23602]. Presented at the 12th International AIDS Conference; June–July 1998; Geneva. [Google Scholar]
- 6.Taha TE, Hoover DR, Dallabetta GA, et al. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–1706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Taha TE, Gray RH, Kumwenda NI, et al. HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr. 1999;20:52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]
- 8.Royce RA, Thorp J, Granados JL, et al. Bacterial vaginosis associated with HIV infection in pregnant women from North Carolina. J Acquir Immune Defic Syndr. 1999;20:382–386. doi: 10.1097/00042560-199904010-00009. [DOI] [PubMed] [Google Scholar]
- 9.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 10.Moodley P, Connolly C, Sturm AW. Interrelationships among human immunodeficiency virus type 1 infection, bacterial vaginosis, trichomoniasis, and the presence of yeasts. J Infect Dis. 2001;185:69–73. doi: 10.1086/338027. [DOI] [PubMed] [Google Scholar]
- 11.Warren D, Klein RS, Sobel J, et al. A multicenter study of bacterial vaginosis in women with or at risk for human immunodeficiency virus infection. Infect Dis Obstet Gynecol. 2001;9:133–141. doi: 10.1155/S1064744901000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Hoffman IR, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 13.Schacker T, Ryncarz AJ, Goddart J, et al. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1 infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 14.Spear GT, Al-Harthi L, Sha B, et al. A potent activator of HIV-1 replication is present in the genital tract of a subset of HIV-1 infected and uninfected women. AIDS. 1997;11:1319–1326. doi: 10.1097/00002030-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Olinger GG, Hashemi FB, Sha BE, et al. Association of indicators of bacterial vaginosis with a female genital tract factor that induces expression of HIV-1. AIDS. 1999;13:1905–1912. doi: 10.1097/00002030-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 16.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-shedding in the genital tract of women. Lancet. 2001;358:1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 17.Baron P, Bremer J, Wasserman SS, et al. Detection and quantitation of human immunodeficiency virus type 1 in the female genital tract. J Clin Microbiol. 2000;38:3822–3824. doi: 10.1128/jcm.38.10.3822-3824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremer J, Nowicki M, Beckner S, et al. Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. J Clin Microbiol. 2000;38:2665–2669. doi: 10.1128/jcm.38.7.2665-2669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawless FJ. Statistical Models and Methods for Lifetime Data. New York: John Wiley and Sons; 1982. [Google Scholar]
- 21.Hashemi FB, Ghassemi M, Roebuck KA, et al. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J Infect Dis. 1999;179:924–930. doi: 10.1086/314674. [DOI] [PubMed] [Google Scholar]
- 22.Al-Harthi L, Roebuck KA, Olinger GG, et al. Bacterial vaginosis-associated microflora isolated from the female genital tract activates HIV-1 expression. J Acquir Immune Defic Syndr. 1999;21:194–202. doi: 10.1097/00126334-199907010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi FB, Ghassemi M, Faro S, et al. Induction of human immunodeficiency virus type-1 expression by anaerobes associated with bacterial vaginosis. J Infect Dis. 2000;181:1574–1580. doi: 10.1086/315455. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi FB, Mollenhauer J, Madsen LD, et al. Myeloid-related protein (MRP)-8 from cervico-vaginal secretions activates HIV replication. AIDS. 2001;15:441–449. doi: 10.1097/00002030-200103090-00002. [DOI] [PubMed] [Google Scholar]
- 25.Ryckman C, Robichaud GA, Roy J, et al. HIV-1 transcription and virus production are both accentuated by the proinflammatory myeloid-related proteins in human CD4+ lymphocytes. J Immunol. 2002;169:3307–3313. doi: 10.4049/jimmunol.169.6.3307. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson DJ, Duerr A, Klein RS, et al. Longitudinal analysis of bacterial vaginosis: findings from the HIV Epidemiology Research Study. Obstet Gynecol. 2001;98:656–663. doi: 10.1016/s0029-7844(01)01525-3. [DOI] [PubMed] [Google Scholar]
- 27.Cu-Uvin S, Hogan JW, Caliendo AM, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–896. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 28.Spinillo A, Debiaggi M, Zara F, et al. Factors associated with nucleic acids related to human immunodeficiency virus type 1 in cervicovaginal secretions. BJOG. 2001;108:634–641. doi: 10.1111/j.1471-0528.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 29.Sha B, Zariffard R, Wang Q, et al. Analysis of cervical vaginal lavage fluid by quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus is a sensitive indicator of bacterial vaginosis and correlates with genital tract HIV viral load [abstract 955]. Presented at the 11th Conference on Retroviruses and Opportunistic Infections; February 2004; San Francisco. [Google Scholar]