Abstract
Species that have been introduced to islands experience novel and strong selection pressures after establishment. There is evidence that exotic species diverge from their native source populations; further, a few studies have demonstrated adaptive divergence across multiple exotic populations of a single species. Exotic birds provide a good study system, as they have been introduced to many locations worldwide, and we often know details concerning the propagule origin, time of introduction, and dynamics of establishment and dispersal within the introduced range. These data make them especially conducive to the examination of contemporary evolution. Island faunas have received intense scrutiny, therefore we have expectations concerning the patterns of diversification for exotic species. We examine six passerine bird species that were introduced to the Hawaiian archipelago less than 150 years ago. We find that five of these show morphological divergence among islands from the time since they were established. We demonstrate that some of this divergence cannot be accounted for by genetic drift, and therefore we must consider adaptive evolution to explain it. We also evaluate evolutionary divergence rates and find that these species are diverging at similar rates to those found in published studies of contemporary evolution in native species.
Keywords: contemporary evolution, exotic birds, Hawaii, islands, archipelago
1. Introduction
Naturalists and biologists have long considered islands to be showcases of evolution [1]. The most striking examples of adaptation on oceanic archipelagos are taxa that have diverged in life history and morphology on multiple islands, resulting in many closely related yet taxonomically distinct forms, known as adaptive radiations [2]. Many island species have greatly diverged from their mainland ancestors, in some cases making it difficult to determine the mainland species that is most closely related to the island taxon (e.g. [3]). This lack of information limits our ability to explore rates of morphological diversification and the role of genetic drift in producing trait differences between islands for native taxa, as their history of change cannot be safely inferred from their present day condition [4]. Species that have recently colonized islands (naturally or by human-mediated processes) give us the rare opportunity to directly observe the dynamics of diversification that occur immediately following the arrival of colonizing species onto islands [5]. We examine six species of passerine bird that have been introduced to the Hawaiian archipelago and we determine whether among-island differentiation of morphological features has occurred in the time since initial release. Using the abundant information on each species' introduction history, we evaluate the roles of adaptive and non-adaptive evolution in the generation of between-island population differences in morphology.
Exotic species are useful ‘unplanned experiments’ giving us the opportunity to observe evolutionary processes in real time [5]. Further, we often know many details of the original introductions (e.g. date, geographical and subspecific identity of the source population, exact introduction location) that are unavailable in natural colonizations, and may be important in understanding the dynamics and mechanisms of divergence. Therefore, a focus on the evolution of exotic species can allow us to observe the genesis of insular diversification, and provide insights into the interspecific variability of responses to insularity [5]. In particular, we can produce relatively unbiased evaluation as to whether evolution resulted from genetic drift, founder effects or adaptive selection. We can also test hypotheses concerning correlates of diversification that are impossible to know if the founding taxon is unknown or extinct. In the context of exotic bird introductions to archipelagos, species can display morphological divergence via three mechanisms: phenotypic plasticity, non-adaptive evolution or adaptive evolution.
Phenotypic plasticity is non-genetic morphological shifts due to environmental effects [6–8]. If environments among islands are sufficiently different, and a morphological feature is phenotypically labile, different populations will display differences among islands such that traits are matched to the environmental constraints of each island. Phenotypic plasticity's contributions to observed differences in avian traits (including several of the morphological traits considered here) across populations has seen modest amounts of study [9]. Merilä & Sheldon [9] evaluated 10 analyses that tested the degree to which observed population differences in morphology, behaviour and life history could be attributed to genetic rather than environmental sources. They concluded that, ‘… phenotypic differences among contemporary populations are indeed mostly, although not solely, of genetic, rather than of environmental origin’.
Alternatively, exotic bird populations in an archipelago may diverge in morphology via non-adaptive evolution if either non-random subsets of individuals make up the colonizing propagules on each island (a founder effect; [10]), or if genetic drift sends each population along a unique but random evolutionary trajectory [11]. Either scenario is likely to have occurred for exotic birds introduced to the Hawaiian Islands, and indeed for exotic species overall [12,13]. The number of individuals of exotic birds released is usually below 50 [14], making genetic drift nearly inevitable [15]. This scenario is all the more likely here given that several exotic birds spread to all Hawaiian islands from one initial introduction point on a single island [16]. Although not as common, exotic birds have been released onto multiple islands on independent occasions, the introductions stemming from different native populations. This scenario presents the possibility that any inter-island differences could be due to founder effects, each island population simply reflecting traits from a geographically structured native range [13].
Finally, exotic bird populations can adapt (via natural selection) to the biotic and abiotic conditions present on each island. It is well-documented that introduced species change quickly in their introduced ranges [17], and it has been demonstrated that observed morphological changes in exotic birds are often consistent with adaptive responses to local environments (e.g. [18–20]). Adaptation can occur only for heritable traits that possess genetic variability [21]. Each morphological trait that we examine has been shown to have moderate to high heritability scores within passerines, with some of these measures based on the species we consider [22–24]. Although there is the expectation that the process of introduction should reduce genetic variation, thus far such reductions have not limited the evolutionary potential of a wide variety of exotic species [5] and exotic birds in particular [14]. Thus, the amount of morphological divergence we see here could be determined by the magnitude and the direction of selection pressures experienced by the populations on each island.
We now highlight the introduction history of the six species we consider in order to gauge the likelihood of the three alternative modes of diversification discussed earlier. The house finch (Carpodacus mexicanus), nutmeg mannikin (Lonchura punctulata) and house sparrow (Passer domesticus) were each introduced to the Hawaiian archipelago only once, to a single island and each propagule came from one known source region [25]. From those initial island populations, these species spread to the other main islands under their own power (i.e. there is no indication that people purposefully moved them between islands; [25]). Based on this introduction history, the most parsimonious expectation is that these species continue to exchange individuals today via dispersal and thus will demonstrate little morphological differentiation. If we do observe divergence in morphology between islands, it may have arisen via in situ changes (adaptation or genetic drift), as a single introduction eliminates the possibility of observed differences being the result of morphologically distinct propagules arriving from multiple native source populations.
The other three species, northern cardinal (Cardinalis cardinalis), red-billed leiothrix (Leiothrix lutea) and Japanese white-eye (Zosterops japonicus) were introduced to more than one island, with some introduction events stemming from different native regions [25]. The most parsimonious expectation is that these species will show some divergence in morphology owing to founder effects alone. If genetic drift and/or adaptation come into play, any existing founder-based divergences in morphology may be magnified. Alternatively, if we find no differences in morphological traits between populations, dispersal between islands must be great enough to have overcome founder effects and may be preventing the development of island-specific types.
Finally, we assess the influence of evolutionary potential on the degree of morphological divergence among island populations for our six exotic passerine species on Hawaii. Here we acknowledge that certain lineages seem prone to diversification, whereas others do not appear to have diverged to any extent over long time periods. Good examples of the former are Zosterops species, which are in an avian family (Zosteropidae) that contains the largest number of island colonizers of any passerine group [26]. We should perhaps expect that once Japanese white-eyes (Z. japonicus) established in Hawaii, they would quickly colonize all other islands (which they did; [25]) and diverge into island-specific forms (which we test here). We will examine whether two metrics of evolutionary history predict the degree of divergence found among islands. These two metrics are generic species richness and the number of recognized subspecies.
2. Material and methods
In order to determine whether insular populations of our six exotic passerine birds have morphologically diverged between islands in the Hawaiian archipelago, it was necessary to obtain measurements of body dimensions from individuals on multiple islands. These measurements were taken on field and museum specimens. Only adults were measured, as young individuals are still growing and do not provide accurate measures of adult body dimensions. All measurements were taken by one investigator (B.A.M.). The following characters were measured: mass, tail length, wing chord, head length (from tip of bill to back of head), culmen length, bill depth (at anterior margin of nares), bill width (also at anterior margin of nares) and tarsus length. Mass was measured in grams and all other characters were measured in millimetres. Mass of live individuals was measured using an Ohaus CS200 compact scale (Ohaus Corporation, Pine Brook, NJ, USA), which has one-tenth of a gram precision. Culmen length, head length, bill depth, bill width and tarsus length were measured with a Mitutoyo dial calliper (Mitutoyo America Corporation, Aurora, IL, USA) to one-hundredth of a millimetre precision. Tail length and wing chord were measured with a 15 cm wing rule accurate to 1 mm (Avinet, Inc., Dryden, NY, USA).
We visited Kauai, Oahu and the Big Island (Hawaii) in the summer of 2008 to obtain field measurements. Mist nets were placed in areas that experience regular bird activity. No lures or baits were used in order to prevent bias in the sex ratio of captured individuals. All morphological measurements on field-captured individuals were taken in the same season thus avoiding systematic bias in morphological traits that vary with season (e.g. wing chord [27]).
In addition to live individuals, specimens originally collected on Kauai, Oahu, Maui or the Big Island were measured at the Bishop Museum (Hawaii, USA), US National Museum (Washington, DC, USA), Natural History Museum (Tring, England), University of Kansas (USA) and Louisiana State University (USA). Mass was recorded for specimens when present on the museum tag. Tail length, wing chord, culmen length, bill depth and bill width were measured on museum specimens. The preparation of avian museum specimens involves the removal of the back of the skull, making head length measurement inappropriate for museum specimens. Tarsus length is difficult to measure on museum specimens, and is often impossible to obtain without damage, therefore it was not taken for most museum specimens.
Data from live-captured individuals and museum specimens were combined for each island. Thus, we matched the season of capture of live-caught individuals with the date of collection for museum specimens to reduce any systematic bias. The one exception to this pattern was our morphological data on house sparrows, which were all from museum specimens; however, all of these specimens were collected in the same months (October and November). Population locations and sample sizes are presented in table 1.
Table 1.
Population locations and sample sizes for the six species examined. The last two columns provide data concerning the initial human-mediated introduction(s), including the date of initial introduction and the islands on which individuals were released.
| species | Big Island | Kauai | Maui | Oahu | date of introduction | island(s) released |
|---|---|---|---|---|---|---|
| northern cardinala | 19 | 13 | 22 | ∼1930 | Oahu, Kauai, Big Island | |
| house finch | 26 | 31 | 59 | <1870 | Maui | |
| red-billed leiothrixa | 30 | 11 | 18 | 1918–1928 | all 5 main islands | |
| nutmeg mannikin | 40 | 21 | 37 | 1865 | Big Island | |
| house sparrow | 22 | 176 | 141 | 1869 | Oahu | |
| Japanese white-eyea | 44 | 47 | 11 | 73 | 1929 | Oahu, Big Island, Maui(?) |
aSpecies were introduced to the Hawaiian archipelago more than once.
As a gauge of measurement error (ME) for our morphological characters, we calculated the percentage measurement error (%ME) using type II ANOVA to relate within-individual to among-individual variance [28]. Characters with high values of %ME will render tests of differences among groups more conservative than tests using characters with lower %ME (i.e. we are less likely to detect differences between groups when %ME is high). We measured wing chord, tail length, culmen length, bill depth and bill width on 25 individuals of each species, conducting three repeat measures on each individual [28]. For logistical reasons, we were restricted to using only museum specimens for two of the six species we consider here (house sparrow and house finch). Thus, our %ME results provide a guide to the power of our tests but not a definitive characterization of ME across all characters or species.
It is well-documented that bird specimens experience changes in some mensural characters (e.g. wing chord) after preparation [29–31] owing to drying of the skin. In order to be able to combine the measurements from live individuals with museum specimen measurements, we multiplied field measurements of tail length, wing chord and tarsus length by taxon and character-specific correction factors. Correction factors have been derived for only a handful of species; in the absence of species-specific correction factors, Winker [31] suggested applying an average correction factor for wing chord that he derived from the existing literature. We followed the methods of Winker [31] and calculated an average correction factor for tail and tarsus length based on appropriate analyses published for passerines (table 2). All correction factors were applied to the field measurements prior to the analyses below.
Table 2.
Specimen shrinkage correction factors derived from published values (willow tit (Parus montanus) [30]; house sparrow [29]; bulbuls (Hypsipetes) [59]; Tennessee warbler (Oreothlypis peregrina) and ‘Traill's’ flycatcher (Empidonax) [31]). Winker [31] recommends a wing chord correction value of 0.983 for species lacking the examination of shrinkage. Tail length and tarsus length correction factors are averages derived from published estimates. Sample size is the number of such estimates used in calculating the average correction factor. The taxa from which we derived these correction factors are listed in the last column.
| character | correction factor | sample size | taxa used |
|---|---|---|---|
| wing chord | 0.9830 | — | recommended by Winker [31] |
| tail length | 0.9941 | 5 | house sparrow, bulbuls, willow tit, Tennessee warbler, ‘Traill's’ flycatcher |
| tarsus length | 0.9866 | 3 | house sparrow, bulbuls, Tennessee warbler |
Pearson product–moment correlation coefficients were computed for all eight morphological characters within each species (correlations may differ across species). Most correlations were small (across species mean < 0.5), although wing chord/tail length and head length/culmen length were higher (mean ± 1 s.d. across species: 0.62 ± 0.21 and 0.68 ± 0.09, respectively). However, owing to variability in correlation between species, these characters were evaluated independently.
We used analysis of variance (ANOVA) to determine which species showed morphological differentiation among island populations. Given a statistically significant ANOVA, a Tukey's post hoc test was used to determine which island populations differed from each other within each species. We used the Benjamini–Hochberg [32] α correction at the species level to correct for employing multiple comparisons.
As a first approximation of whether genetic drift could account for differences in morphology that we observed across island populations, we used Lande's [11] constant-heritability equation extended for use with multiple populations [33,34]. Our use of the constant-heritability equation is appropriate here as the number of generations since founding is less than Ne/5 [34,35]. Lande [33] posits that the test statistic
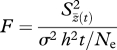 |
2.1 |
serves as an approximate test for the genetic drift hypothesis, where F has an F-distribution with n − 1 degrees of freedom in the numerator and infinite degrees of freedom in the denominator. In this equation, σ2 = phenotypic variance, h2 = heritability of the trait, Ne the effective population size and t the number of generations since founding. The term 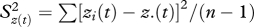 is the between-population mean square differences in phenotype for n replicate populations after t generations, with z(t) = mean of the trait at generation t in a population of effective size Ne, and z.(t) = ∑zt(t)/n. A significant p-value in this test indicates that observed differences in trait values across populations are large enough that they do not probably result from genetic drift.
is the between-population mean square differences in phenotype for n replicate populations after t generations, with z(t) = mean of the trait at generation t in a population of effective size Ne, and z.(t) = ∑zt(t)/n. A significant p-value in this test indicates that observed differences in trait values across populations are large enough that they do not probably result from genetic drift.
Turelli et al. [34] suggest that the denominator in equation (2.1) be a conservatively large estimate. Thus, based on data summarized in Merilä & Sheldon [9], we set h2 as 0.5 in all cases except for house sparrows and house finches where published estimates were available (house sparrow: [24]; house finch: [23]). In these species, estimated value of h2 ranged from 0.31 to 0.54. Population sizes are not known with enough precision to calculate Ne directly. Instead, we followed Baker [35] and performed the calculations for equation (2.1) using Ne = 102 and again using Ne = 103. Based on population growth information summarized in Pyle & Pyle [36] for each species, we consider these upper and lower values of Ne reasonable and conservative approximations. These values also stand in good accord with the recent estimates of Ne derived for other island bird populations (e.g. [37]).
We estimated generation times (T) for the species examined in the same manner as Sæther et al. [38]:
where T is the generation time, α is age at maturity and s is the annual adult survival rate. We assumed α = 1 in all cases, as there is no evidence of delayed breeding in these species. Adult survival rate was gleaned from the literature for three species (northern cardinal: [39]; house finch: [40]; house sparrow: [41]). The average of those generation times was used for the other three species, as adult survival rate data were not available.
We measured the evolutionary rate of change for these characters, comparing the island populations to their native source populations (native data from Mathys & Lockwood in review). Evolutionary rate was measured in Haldanes (H), calculated as
where ln X1 and ln X2 are the means of natural log measurements from the introduced and native populations, respectively; t2 and t1 are time measured in generations and Sln x is the pooled standard deviation from the natural log measurements for both populations [42]. We used standard linear regression to fit a line through our data, and calculated the 95% confidence interval around this line for comparison with that of Stockwell et al. [43].
We performed linear regressions to determine whether a species' evolutionary history predicts the proportion of populations diverging for each species (see previous paragraph). The species richness of the genus and the number of subspecies recognized for each species were used as the predictor variables in linear regressions. These data were derived from field guides, Birds of North America accounts and the primary literature (northern cardinal: [44]; house finch: [45,46]; red-billed leiothrix: [47]; nutmeg mannikin: [48]; house sparrow: [49]; Japanese white-eye: [50,51]). The dependent variable was the number of populations that diverged divided by the total number of population comparisons for each species. We also regressed proportion of populations diverging against time since each species was first introduced, in order to evaluate the possibility that interspecific differences in divergence were simply a result of some species having more time to evolve once established within the Hawaiian archipelago.
3. Results
Measurement error (%ME) for house sparrows and house finches varied across characters from 1.1 (wing chord for house finches) to 35.7 per cent (bill width for house sparrows; table 3). Mean %ME across all characters and species was 12.9 per cent. There are surprisingly few comparable estimates for other passerines in the literature, however, these %ME are considered small by Merilä & Björklund [52] relative to the animal morphology literature that they reviewed. Our observed differences in %ME across characters indicated that differences between groups (i.e. island populations) will be least likely to show significant differences for bill width, and most likely to show differences for wing chord and bill depth, if such differences exist.
Table 3.
Measurement error (%ME) for five morphological characters in house sparrows and house finches. Larger values of %ME represent a relatively larger within-individual variance in measurement relative to variance within groups [28]. We measured 25 individuals of each species, with each individual measured three times (n = 75 per character).
| species | wing chord | tail length | culmen length | bill depth | bill width |
|---|---|---|---|---|---|
| house sparrow | 10.3 | 15.1 | 7.5 | 2.6 | 35.7 |
| house finch | 1.1 | 9.0 | 18.3 | 2.9 | 26.1 |
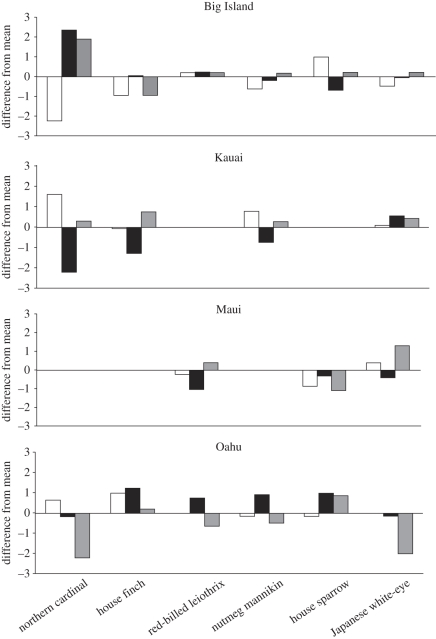
Out of 48 ANOVA performed, testing for differences in a morphological trait across island populations within species, 20 were significant. Out of 132 between-island population comparisons using Tukey's post hoc test, 38 showed divergence for the morphological trait in question (see electronic supplementary material). The extent of between-island divergence varied markedly, from all eight characters showing at least some divergence among islands (house sparrow, with 15 out of 18 between-island comparisons indicating divergence) to no character showing any divergence among islands (red-billed leiothrix showed no differences for 16 comparisons; electronic supplementary material). Northern cardinal showed the largest differences between islands, but only for wing chord, tail length and mass (figure 1). The characters diverging the most among islands were mass and wing chord (figure 1; electronic supplementary material). We found mass differences among islands for 10 out of 15 between-island comparisons. Wing chord differences were found in nine out of 21 comparisons (electronic supplementary material). Head length and tarsus length diverged at the lowest frequency. Individual islands had similar proportions of morphological traits that showed divergence, with no single island emerging as a leader in the number of times the traits diverged (figure 1). Similarly, there was no clear pattern of divergence for any single character across species or across islands (figure 1). Nevertheless, there were three instances of morphological characters consistently being larger than the overall mean: red-billed leiothrix on the Big Island, Japanese white-eye on Kauai and house finch on Oahu (figure 1).
Figure 1.
Divergence pattern for three morphological characters measured across island populations of the six exotic bird species we considered. White bars represent mass (g), black bars represent length (mm) and grey bars wing chord (mm). All other characters did not diverge substantially across islands. Differences are calculated as across-island mean − within-island mean, and thus the zero line represents the across-island (overall) mean value for each morphological trait. Positive values represent characters that are larger than the overall mean for that species (x-axis) on that island (panels), and vice versa for negative values.
If we assume Ne = 102, three morphological character differences across island populations are considered unlikely to be the result of genetic drift (table 4). These characters were mass, wing chord and tail length for northern cardinals. That number rises to 18 if we assume Ne = 103 (table 4). The degree of differences observed in mass, tail length and wing chord were considered to be outside the bounds predicted by genetic drift for all six species when Ne = 103 (table 4). Note that this includes some differences in mean trait values that were considered non-significant in our ANOVA (e.g. red-billed leiothrix; electronic supplementary material). Differences across island populations for tarsus were considered unlikely owing to drift for northern cardinals, and culmen length differences were considered outside of drift expectations for house sparrows and house finches; each of these results pertains only when Ne = 103 (table 4).
Table 4.
F-values that indicate whether observed divergence rates in morphology across islands within species can be attributed to genetic drift. Calculations of F follow Lande [11] as updated in Turelli et al. [34]. See text for details of how each morphological trait was measured. Values of effective population sizes (Ne, are upper and lower bounds derived from the literature. Italic type indicates statistical significance at the α = 0.05 level, which in this test indicates that genetic drift is unlikely to explain the rate of morphological divergence observed across island populations. The degrees of freedom for each test are given as n−1, where n is the number of island populations examined.
| Ne | mass | tail | wing | head length | culmen | bill depth | bill width | tarsus | |
|---|---|---|---|---|---|---|---|---|---|
| northern cardinal | 100 | 7.66 | 8.77 | 9.52 | 0.22 | 0.19 | 0.38 | 0.03 | 1.66 |
| 1000 | 76.62 | 87.74 | 95.25 | 2.19 | 1.89 | 3.78 | 0.30 | 16.59 | |
| n−1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | |
| house finch | 100 | 6.13 | 1.74 | 1.38 | 0.03 | 0.91 | 0.13 | 0.22 | 0.20 |
| 1000 | 61.31 | 17.36 | 13.77 | 0.35 | 9.07 | 1.33 | 2.19 | 2.03 | |
| n−1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | |
| red-billed leiothrix | 100 | 0.30 | 2.05 | 0.78 | 0.51 | 0.00 | 0.01 | ||
| 1000 | 2.96 | 20.49 | 7.81 | 5.06 | 0.04 | 0.14 | |||
| n−1 | 1 | 2 | 2 | 2 | 1 | 2 | |||
| nutmeg mannikin | 100 | 1.56 | 0.94 | 0.36 | 0.18 | 0.10 | 0.05 | 0.08 | 0.21 |
| 1000 | 15.56 | 9.43 | 3.55 | 1.84 | 0.95 | 0.53 | 0.78 | 2.07 | |
| n−1 | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | |
| house sparrow | 100 | 2.08 | 1.40 | 2.15 | 3.45 | 0.30 | 0.35 | ||
| 1000 | 20.84 | 13.99 | 21.52 | 34.48 | 2.98 | 3.55 | |||
| n−1 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| Japanese white-eye | 100 | 1.22 | 0.47 | 6.01 | 0.21 | 0.37 | 0.04 | 0.04 | 0.05 |
| 1000 | 12.22 | 4.71 | 60.06 | 2.07 | 3.68 | 0.42 | 0.36 | 0.55 | |
| n−1 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 2 |
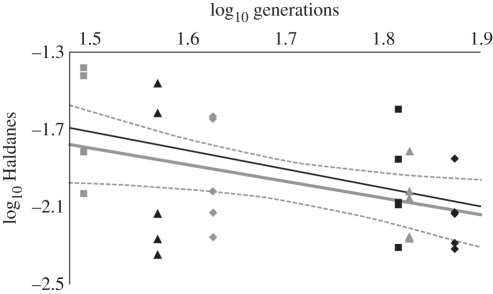
We found the average evolutionary rate measurements to range from 0.004 to 0.042 Haldanes for the five characters with native range data available (table 5). In figure 2, we graph our rates around the trend line reported in Stockwell et al. [43] in order to evaluate how our results compare with other estimates of contemporary evolutionary rates. The 95% confidence interval for our regression line easily encompasses that of Stockwell et al. [43].
Table 5.
Evolutionary rates (in Haldanes) for the six species examined in this study, comparing Hawaiian populations to native source populations. Data points are averages across all populations of that species. Native range data were only available for these five morphological characters.
| tail length | wing chord | culmen length | bill depth | bill width | |
|---|---|---|---|---|---|
| northern cardinal | 0.015 | 0.042 | 0.009 | 0.038 | |
| house finch | 0.014 | 0.005 | 0.007 | 0.007 | 0.005 |
| red-billed leiothrix | 0.005 | 0.023 | 0.022 | 0.009 | 0.007 |
| nutmeg mannikin | 0.005 | 0.015 | 0.009 | 0.005 | 0.009 |
| house sparrow | 0.005 | 0.008 | 0.025 | 0.014 | 0.008 |
| Japanese white-eye | 0.034 | 0.024 | 0.004 | 0.005 | 0.007 |
Figure 2.
Evolutionary rates in Haldanes, graphed versus generations for six exotic bird species on the Hawaiian Islands. Each data point represents one morphological character (table 5 for actual rate values). The black line is the trend line taken from Stockwell et al. [43], and is based on other published studies of evolutionary rates. The grey lines represent the trend line and 95% CI for our data. The trend line from Stockwell et al. [43] falls within our 95% CI. Grey squares, Cardinalis cardinalis; black triangles, Zosterops japonicus; grey diamonds, Leiothrix lutea; black squares, Passer domesticus; grey triangles, Lonchura punctulata; black diamonds, Carpodacus mexicanus.
The linear regression of the proportion of between-island comparisons showing divergence for each species versus generic species richness was not statistically significant (r2 = 0.013, p = 0.830). Similarly, a regression of the number of subspecies versus population divergence proportion was not significant (r2 = 0.076, p = 0.597). We found no relationship between time since introduction and proportion of populations that have diverged (r2 = 0.202, p = 0.371).
4. Discussion
Five out of the six non-native bird species that we considered here show measurable morphological divergence in at least one trait across islands in the Hawaiian archipelago after only 70–140 years of putative isolation from each other. In particular, we show substantial divergence in wing chord and body mass across island populations of the same species. We also show that many of these differences are unlikely to result from genetic drift alone. The frequency with which we observe character divergence is striking, especially given the various introduction scenarios. Although there have been several examples of such divergence between non-native populations for other taxonomic groups, our results represent one of the few to demonstrate substantial phenotypic divergence in a non-native vertebrate group [53].
Wing chord and body mass diverged most frequently, and often to a degree that is unlikely owing to genetic drift alone. In contrast, bill dimensions did not often diverge between populations, although our calculations of ME suggest that our power to detect such changes was less than that for other characters. Nevertheless, the failure to find differences in bill dimensions run somewhat counter to the well-known examples of divergence in avian groups (e.g. [1]). However, wing chord and body mass are known to be strongly selected based on their role in avian flight dynamics [54], and thus could be responding to biotic and abiotic conditions on each island. There is an allometric relationship between these characters, whereby wing chord increases in response to an increase in body mass (e.g. [55]), although in our data this correlation was weak. We documented only three instances of characters diverging in the same direction within a single island population. More commonly wing length and mass, and the other characters examined, diverged in an idiosyncratic manner within species and within islands.
Two illustrative examples of idiosyncratic divergence patterns, and the difficulty of deciphering causation, come from the northern cardinal and Japanese white-eye. The various island populations of northern cardinals showed strong divergences between each other in wing chord, tail length and body mass. Japanese white-eye populations diverged across several traits, with tail length showing the most exceptional differences. It is possible that these differences reflect adaptation to island-specific biotic and abiotic environments, perhaps in particular conditions related to flight dynamics. However, for these two species, this explanation is confounded with possible founder effects. These species are two of the three (the third is red-billed leiothrix) that were released multiple times across islands, thus allowing for the possible release of individuals from morphologically distinct native populations. Differences between islands could thus be due to founder effects, admixture, selection or a mixture of the three [13].
The pathway to understanding mechanistic explanations for our observed differences is less obstructed for house sparrows and house finches. Both species were introduced to a single island from one known source location, thus eliminating the possibility of a founder effect. Both have diverged substantially across island populations, and the divergence is not likely to be a result of genetic drift. House sparrows and house finches have been the subject of extensive study within other parts of their exotic ranges, and in all cases, significant morphological divergence has been documented and often attributed to adaptation to local conditions (reviewed in [14]). For example, the morphological differences between islands that we document here are within the range of those reported by Badyaev & Hill [18] for differences among exotic populations in North America, and even sometimes exceed those differences (e.g. mass).
At the other end of the spectrum, nutmeg manikins and red-billed leiothrix had only one character diverge out of the eight measured. These species have not been studied elsewhere in their exotic range, so we cannot judge the uniqueness of this failure to diverge in Hawaii. We did test whether the previous history of divergence (i.e. generic species richness and number of subspecies) in the native range was a predictor of the proportion of populations diverging in our study. We expected that greater generic species richness and more subspecies in the native range would correlate with an increased divergence in the species that we examined. Our results did not support this expectation.
Turelli et al. [34] suggest that the drift equation we used is conservative and should be considered a qualitative assessment of agreement with the null hypothesis (i.e. drift explains observed phenotypic divergence rates). Drift is facilitated when there are very few individuals in a population [15]. Population sizes were probably very low (<50 individuals) right after their initial introduction for all the species considered here, which must have reduced the effective population sizes, albeit to an unknown degree [14,25]. Since their initial release, however, four of the six species (house sparrows, house finches, Japanese white-eyes and northern cardinals) quickly grew in population size and are now commonly found across all six main islands [36]. In each of these, effective population sizes must be near 103 (used as a benchmark here) if not quite a bit higher. For example, the Japanese white-eye is now considered the most common species across all the main Hawaiian Islands numbering in (at least) the hundreds of thousands on each island [36]. We thus consider our test for the effects of drift on these four species to be quite conservative, even when we consider the effective population size to be 103.
Our estimates of evolutionary rates closely match those found in other studies, as reviewed in Stockwell et al. [43]. In particular, we show that the species that have been established as exotics for the shortest period show the highest rates of evolution as measured by Haldanes (table 5). The scaling of evolutionary rate such that ‘fast’ rates are associated with shorter time intervals seems to be a general trend across taxa, and our results add further support to this conclusion [56]. This trend is probably the result of periods of stasis and evolutionary reversals being averaged across longer time periods; in our study and other similar examinations of evolutionary changes over short time scales, evolution is probably unidirectional and constant, leading to a higher average rate.
One advantage to examining exotic species as we do here is that we can compare our results to what has already happened among native species. Do these exotic passerines mimic patterns in divergence found among the native passerines of Hawaii? Divergence in naturally occurring Hawaiian forms is quite variable, with some bird families showing extensive divergence and others not diverging at all. As an example, the family Mohoidae is endemic to the Hawaiian archipelago and contains only five total living and extinct species [57]. This can be contrasted with the Hawaiian honeycreepers, another endemic taxon, which has 29 or more living and extinct members [58]. These two taxa have had a similar amount of time to diverge in Hawaii (approx. 16 Myr) [57], yet the latter group contains more than five times as many species. We cannot, of course, predict if and to what extent exotic passerines will continue to diverge in Hawaii; however, we did find that some species (e.g. house sparrow) have diverged much more than other species (e.g. nutmeg mannikin). It remains to be seen whether adaptive and non-adaptive evolution will continue to build differences in some exotic birds while leaving others largely unchanged, as it did with the native passerines of Hawaii.
Acknowledgements
We would like to thank the Rutgers School of Environmental and Biological Sciences for funding to J.L.L. and the National Geographic Society Committee for Research and Exploration for grant number 8261-07. B.A.M. would like to thank Carla Kishinami (Bishop Museum, Hawaii), James Dean (US National Museum), Nate Rice (Academy of Natural Sciences, Philadelphia), Eric VanderWerf, Lindsay C. Young, Jim Denny, Thane K. Pratt, Caleb Slemmons and Judy Bird for their assistance and advice during field and museum research.
References
- 1.Grant P. R. 1998. Evolution on islands. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Schluter D. 2000. The ecology of adaptive radiation. New York, NY: Oxford University Press [Google Scholar]
- 3.Burns K. J., Hackett S. J., Klein N. K. 2002. Phylogenetic relationships and morphological diversity in Darwin's finches and their relatives. Evolution 56, 1240–1252 [DOI] [PubMed] [Google Scholar]
- 4.Grant P. R. 2001. Reconstructing the evolution of birds on islands: 100 years of research. Oikos 92, 385–403 10.1034/j.1600-0706.2001.920301.x (doi:10.1034/j.1600-0706.2001.920301.x) [DOI] [Google Scholar]
- 5.Sax D. F., et al. 2007. Ecological and evolutionary insights from invasive species. Trends Ecol. Evol. 22, 465–471 10.1016/j.tree.2007.06.009 (doi:10.1016/j.tree.2007.06.009) [DOI] [PubMed] [Google Scholar]
- 6.DeWitt T. J., Scheiner S. M. 2004. Phenotypic plasticity. New York, NY: Oxford University Press [Google Scholar]
- 7.Falconer D. S. 1952. The problem of environment and selection. Am. Nat. 86, 293–298 10.1086/281736 (doi:10.1086/281736) [DOI] [Google Scholar]
- 8.Stearns S. C. 1989. The evolutionary significance of phenotypic plasticity. BioScience 7, 436–445 10.2307/1311135 (doi:10.2307/1311135) [DOI] [Google Scholar]
- 9.Merilä J., Sheldon B. C. 2001. Avian quantitative genetics. Current ornithology, vol. 16 (eds Nolan V. R., Jr, et al.), pp. 179–255 New York, NY: Kluwer Academic/Plenum Publishers [Google Scholar]
- 10.Mayr E. 1942. Systematics and the origin of species. New York, NY: Columbia University Press [Google Scholar]
- 11.Lande R. 1976. Natural selection and random genetic drift in phenotypic evolution. Evolution 30, 314–334 10.2307/2407703 (doi:10.2307/2407703) [DOI] [PubMed] [Google Scholar]
- 12.Facon B., Pointier J., Jarne P., Sarda P., David P. 2008. High genetic variance in life-history strategies within invasive populations by way of multiple introduction. Curr. Biol. 18, 363–367 10.1016/j.cub.2008.01.063 (doi:10.1016/j.cub.2008.01.063) [DOI] [PubMed] [Google Scholar]
- 13.Keller S. R., Taylor D. R. 2008. History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol. Lett. 11, 852–866 10.1111/j.1461-0248.2008.01188.x (doi:10.1111/j.1461-0248.2008.01188.x) [DOI] [PubMed] [Google Scholar]
- 14.Blackburn T. M., Lockwood J. L., Cassey P. 2009. Avian invaders: the ecology and evolution of exotic birds. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Connor J. K., Hartl D. L. 2004. A primer of ecological genetics. Sunderland, MA: Sinauer Associates Inc [Google Scholar]
- 16.Lockwood J. L. 2006. Life in a double-hotspot: the transformation of Hawaiian bird diversity following invasion and extinction. Biol. Inv. 8, 449–457 10.1007/s10530-005-6415-z (doi:10.1007/s10530-005-6415-z) [DOI] [Google Scholar]
- 17.Lee C. E. 2002. Evolutionary genetics of invasive species. Trends Ecol. Evol. 17, 386–391 10.1016/S0169-5347(02)02554-5 (doi:10.1016/S0169-5347(02)02554-5) [DOI] [Google Scholar]
- 18.Badyaev A. V., Hill G. E. 2000. The evolution of sexual dimorphism in the house finch. I. Population divergence in morphological covariance structure. Evolution 54, 1784–1794 [DOI] [PubMed] [Google Scholar]
- 19.Johnston R. F., Selander R. K. 1971. Evolution in the house sparrow. II. Adaptive differentiation in North American populations. Evolution 25, 1–28 10.2307/2406496 (doi:10.2307/2406496) [DOI] [PubMed] [Google Scholar]
- 20.Mathys B. A., Lockwood J. L. 2009. Rapid evolution of great kiskadees on Bermuda: an assessment of the ability of the island rule to predict the direction of contemporary evolution in exotic vertebrates. J. Biogeogr. 36, 2204–2211 10.1111/j.1365-2699.2009.02169.x (doi:10.1111/j.1365-2699.2009.02169.x) [DOI] [Google Scholar]
- 21.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766–1774 10.2307/2410734 (doi:10.2307/2410734) [DOI] [PubMed] [Google Scholar]
- 22.Badyaev A. V., Martin T. E. 2000. Sexual dimorphism in relation to current selection in the house finch. Evolution 54, 987–997 [DOI] [PubMed] [Google Scholar]
- 23.Badyaev A. V., Martin T. E. 2000. Individual variation in growth trajectories: phenotypic and genetic correlations in ontogeny of the house finch (Carpodacus mexicanus). J. Evol. Biol. 13, 290–302 10.1046/j.1420-9101.2000.00172.x (doi:10.1046/j.1420-9101.2000.00172.x) [DOI] [Google Scholar]
- 24.Jensen H., Sæther B. E., Ringsby T. H., Tufto J., Griffiths S. C., Ellegren H. 2003. Sexual variation in heritability and genetic correlations of morphological traits in house sparrow (Passer domesticus). J. Evol. Biol. 16, 1296–1307 10.1046/j.1420-9101.2003.00614.x (doi:10.1046/j.1420-9101.2003.00614.x) [DOI] [PubMed] [Google Scholar]
- 25.Long J. L. 1981. Introduced birds of the world. London, UK: David and Charles [Google Scholar]
- 26.Clegg S. M., Degnan S. M., Moritz C., Estoup A., Kikkawa J., Owens I. P. F. 2002. Microevolution in island forms: the roles of drift and directional selection in morphological divergence of a passerine bird. Evolution 56, 2090–2099 [DOI] [PubMed] [Google Scholar]
- 27.Arendt W. J., Faaborg J. 1989. Sources of variation in measurements of birds in a Puerto Rican dry forest. J. Field Ornithol. 60, 1–11 [Google Scholar]
- 28.Bailey R. C., Byrnes J. 1990. A new, old method for assessing measurement error in both univariate and multivariate morphometric studies. Syst. Zool. 39, 124–130 10.2307/2992450 (doi:10.2307/2992450) [DOI] [Google Scholar]
- 29.Bjordal H. 1983. Effects of deep freezing, freeze-drying and skinning on body dimensions of house sparrows (Passer domesticus). Cinclus 6, 105–108 [Google Scholar]
- 30.Haftorn S. 1982. Variation in body measurements of the willow tit (Parus montanus), together with a method for sexing live birds and data on the degree of shrinkage in size after skinning. Cinclus 5, 16–26 [Google Scholar]
- 31.Winker K. 1993. Specimen shrinkage in Tennessee warblers and ‘Traill's’ flycatchers. J. Field Ornithol. 64, 331–336 [Google Scholar]
- 32.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Met. 57, 289–300 [Google Scholar]
- 33.Lande R. 1977. Statistical tests for natural selection on quantitative characters. Evolution 31, 442–444 10.2307/2407764 (doi:10.2307/2407764) [DOI] [PubMed] [Google Scholar]
- 34.Turelli M., Gillespie J. H., Lande R. 1988. Rate tests for selection on quantitative characters during macroevolution and microevolution. Evolution 42, 1085–1089 10.2307/2408923 (doi:10.2307/2408923) [DOI] [PubMed] [Google Scholar]
- 35.Baker A. J. 1992. Genetic and morphometric divergence in ancestral European and descendent New Zealand populations of chaffinches (Fringilla coelebs). Evolution 46, 1784–1800 10.2307/2410031 (doi:10.2307/2410031) [DOI] [PubMed] [Google Scholar]
- 36.Pyle R. L., Pyle P. 2009. The birds of the Hawaiian islands: occurrence, history, distribution, and status. B.P. Bishop Museum, Honolulu, HI, USA. Version 1 (31 December 2009). See http://hbs.bishopmuseum.org/birds/rlp-monograph
- 37.Hoeck P. E. A., Bollmer J. L., Parker P. G., Keller L. F. 2010. Differentiation with drift: a spatio-temporal genetic analysis of Galapagos mockingbird populations (Mimus spp.). Phil. Trans. R. Soc. B 365, 1127–1138 10.1098/rstb.2009.0311 (doi:10.1098/rstb.2009.0311) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sæther B., et al. 2005. Generation time and temporal scaling of bird population dynamics. Nature 436, 99–102 10.1038/nature03666 (doi:10.1038/nature03666) [DOI] [PubMed] [Google Scholar]
- 39.Karr J. R., Nichols J. D., Klimkiewicz M. K., Brawn J. D. 1990. Survival rates of birds of tropical and temperate forests: will the dogma survive? Am. Nat. 136, 277–291 10.1086/285098 (doi:10.1086/285098) [DOI] [Google Scholar]
- 40.DeSante D. F., Kaschube D. R. 2006. The Monitoring and avian productivity and survivorship (MAPS) program 1999, 2000, and 2001 report. Bird Popul. 7, 23–89 [Google Scholar]
- 41.Jensen H., Steinsland I., Ringsby T. H., Sæther B. E. 2008. Evolutionary dynamics of a sexual ornament in the house sparrow (Passer domesticus): the role of indirect selection within and between sexes. Evolution 62, 1275–1293 10.1111/j.1558-5646.2008.00395.x (doi:10.1111/j.1558-5646.2008.00395.x) [DOI] [PubMed] [Google Scholar]
- 42.Gingerich P. D. 2001. Rates of evolution on the time scale of the evolutionary process. Genetica 112–113, 127–144 10.1023/A:1013311015886 (doi:10.1023/A:1013311015886) [DOI] [PubMed] [Google Scholar]
- 43.Stockwell C. A., Hendry A. P., Kinnison M. T. 2003. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 18, 94–101 10.1016/S0169-5347(02)00044-7 (doi:10.1016/S0169-5347(02)00044-7) [DOI] [Google Scholar]
- 44.Halkin S. L., Linville S. U. 1999. Northern cardinal (Cardinalis cardinalis). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; retrieved from the birds of North America online; See http://bna.birds.cornell.edu/bna/species/440 [Google Scholar]
- 45.Clement P. 1993. Finches and sparrows. Princeton, NJ: Princeton University Press [Google Scholar]
- 46.Hill G. E. 1996. Subadult plumage in the house finch and tests of models for the evolution of delayed plumage maturation. Auk 113, 858–874 [Google Scholar]
- 47.Male T. D., Fancy S. G., Ralph C. J. 1998. Red-billed leiothrix (Leiothrix lutea). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; retrieved from the birds of North America online; See http://bna.birds.cornell.edu/bna/species/359 [Google Scholar]
- 48.Restall R. 1996. In Munias and mannikins Tonbridge, UK: Pica Press [Google Scholar]
- 49.Lowther P. E., Cink C. L. 2006. House sparrow (Passer domesticus). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; retrieved from the birds of North America online; See http://bna.birds.cornell.edu/bna/species/012 [Google Scholar]
- 50.Monroe B. M., Sibley C. G. 1997. A world checklist of birds. Yale, CT: Yale University Press [Google Scholar]
- 51.Van Riper S. G. 2000. Japanese white-eye (Zosterops japonicus). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology; Retrieved from the birds of North America online. See http://bna.birds.cornell.edu/bna/species/487 [Google Scholar]
- 52.Merilä J., Björklund M. 1995. Fluctuating asymmetry and measurement error. Syst. Biol. 44, 97–101 [Google Scholar]
- 53.Vellend M., Harmon L. J., Lockwood J. L., Mayfield M. M., Hughes A. R., Wares J. P., Sax D. F. 2007. Effects of exotic species on evolutionary diversification. Trends Ecol. Evol. 22, 481–488 10.1016/j.tree.2007.02.017 (doi:10.1016/j.tree.2007.02.017) [DOI] [PubMed] [Google Scholar]
- 54.Norberg U. M. 1995. How a long tail and changes in mass and wing shape affect the cost of flight in animals. Funct. Ecol. 9, 48–54 10.2307/2390089 (doi:10.2307/2390089) [DOI] [Google Scholar]
- 55.Björklund M. 1994. Allometric relations in 3 species of finches (Aves, Fringillidae). J. Zool. 233, 657–668 10.1111/j.1469-7998.1994.tb05372.x (doi:10.1111/j.1469-7998.1994.tb05372.x) [DOI] [Google Scholar]
- 56.Kinnison M. T., Hendry A. P. 2001. The pace of modern life II: from rates of contemporary microevolution to pattern and process. Genetica 112–113, 145–164 10.1023/A:1013375419520 (doi:10.1023/A:1013375419520) [DOI] [PubMed] [Google Scholar]
- 57.Fleischer R., James H., Olson S. 2008. Convergent evolution of Hawaiian and Australo-Pacific honeyeaters from distant songbird ancestors. Curr. Biol. 18, 1927–1931 10.1016/j.cub.2008.10.051 (doi:10.1016/j.cub.2008.10.051) [DOI] [PubMed] [Google Scholar]
- 58.Pratt H. D., Bruner P. L., Berrett D. G. 1987. A field guide to the birds of Hawaii and the tropical Pacific. Princeton, NJ: Princeton University Press [Google Scholar]
- 59.Herremans M. 1985. Post-mortem changes in morphology and its relevance to biometrical studies. Bull. Brit. Orn. Club 105, 89–91 [Google Scholar]