Abstract
The mechanisms that drive species coexistence and community dynamics have long puzzled ecologists. Here, we explain species coexistence, size structure and diversity patterns in a phytoplankton community using a combination of four fundamental factors: organism traits, size-based constraints, hydrology and species competition. Using a ‘microscopic’ Lotka–Volterra competition (MLVC) model (i.e. with explicit recipes to compute its parameters), we provide a mechanistic explanation of species coexistence along a niche axis (i.e. organismic volume). We based our model on empirically measured quantities, minimal ecological assumptions and stochastic processes. In nature, we found aggregated patterns of species biovolume (i.e. clumps) along the volume axis and a peak in species richness. Both patterns were reproduced by the MLVC model. Observed clumps corresponded to niche zones (volumes) where species fitness was highest, or where fitness was equal among competing species. The latter implies the action of equalizing processes, which would suggest emergent neutrality as a plausible mechanism to explain community patterns.
Keywords: species coexistence, emergent neutrality, community dynamics, morpho-functional traits
1. Introduction
The mechanisms underlying species diversity have long puzzled ecologists. Theoretical explanations concerning coexistence range from purely niche-based mechanisms [1,2] to complete neutrality [3]. Recently, it has been suggested that a combination of both types of mechanisms may better explain observed patterns [4,5]. Groups of similar, coexisting species arise as the result of ecological and evolutive interactions among species, a mechanism known as emergent neutrality [6,7]. However, empirical evidence on how such processes operate is scarce.
Within Chesson's [4] framework, there are two groups of mechanisms underlying species coexistence, named stabilizing and equalizing processes. The classical niche-based mechanisms, which are included in the first group, assume that the differences in resource use among species facilitate coexistence as they tend to increase negative intraspecific interactions relative to interspecific interactions [4]. The second group includes the less intuitive mechanisms, which tend to decrease average fitness differences among competing species, such as convergent coevolution on the use of non-substitutable resources [8]. Fitness is related to species traits and defines their performance independently of their relative abundance [9]; for example, the ability of a species to draw down resources (R*, [10]). Generally, niche differences promote coexistence, whereas fitness differences among species lead to competitive exclusion and, consequently, to a decrease in diversity [4,9]. It is highly relevant to empirically test the role of such mechanisms as they are deeply involved in biodiversity maintenance [11] and might determine the outcome of biological invasions [9]. However, the relative contribution of niche and neutral mechanisms in natural communities has seldom been quantified.
Recently, a combination of stabilizing (niche) and equalizing (neutral) mechanisms was shown to be responsible for the existence of groups of coexistent species (clumps) in a phytoplankton community [5]. However, a mechanistic explanation of this emergent pattern is still lacking. This is partially owing to the overwhelming number of species that compose phytoplankton communities, and the myriad of physiological variables required to understand individual responses and interactions among coexisting species required to parametrize dynamic models. Individual volume summarizes several important facets of plankton physiology and life history, and is a good surrogate for species niche [12]. Moreover, the use of size scaling of physiological rates has proved useful in the reduction of parameters in plankton models [13]. However, there are departures from allometric scaling caused by individual traits other than volume [14]. Therefore, while volume is a key variable to reducing dimensionality, it is necessary to include other traits to represent relevant differences among functional groups [12]. Within such a framework, morphology-based functional group (MBFG) classification of phytoplankton species [15] effectively synthesizes organism physiology, improves predictability [16] and helps to represent community succession [17]. Moreover, because of the short generation time (less than 5 days), the study of phytoplankton community processes on a monthly to yearly time scale provides an insight into the long-term ecological and evolutive dynamics for plant and animal communities.
Previous studies have suggested that some regions of the niche are more tightly occupied by species relative to other regions [5,7,18]. Furthermore, niche occupancy patterns are mediated by species interactions on evolutive or ecological time scales. It remains an open question as to which mechanisms determine the use of niche space by species, their distribution within it and the typical time scales involved in the emergence of such patterns. We hypothesize that morphological constraints and environmental forces define a fitness landscape. In this landscape and in ecological time scales, the interactions among organisms in a community lead to the forming of groups of species that share a similar use of the niche axis, i.e. clumps of species. Species within a clump will have roughly equal fitness and may persist in the environment for several generations, despite the absence of niche differences. Using individual volume as a proxy of the species' niche, we hypothesize that phytoplankton species will show peaks and troughs in the distribution of biomass, and that the richness of species will be highest in zones where fitness differences are minor.
In the current study, we tested the above hypothesis in the well-studied phytoplankton community of Laguna de Rocha, Uruguay (34°33′ S, 54°22′ W). Our findings present empirical evidence pointing to a mixture of equalizing (neutral) and stabilizing (niche) mechanisms as drivers of population abundance and community size structure. Using MBFG, size-based physiological trade-offs and a ‘microscopical’ Lotka–Volterra competition (MLVC) model (i.e. with explicit recipes to compute its parameters, carrying capacities and competition coefficients, from empirical data; sensu [19]), we disentangle the plausible mechanisms behind observed patterns and provide evidence supporting the emergent neutrality hypothesis.
2. Material and methods
(a). General strategy
We first analysed empirical patterns of phytoplankton size structure, taxonomic and MBFG richness in over 2 years of monthly phytoplankton records in two contrasting areas of Laguna de Rocha. MBFG allometric relationships were estimated between individual volumes and growth and sinking rates that were obtained from literature data. Using these estimated relationships, a size-based equation for fitness was derived for each MBFG, and fitness was compared among species in the niche axis. Finally, mechanisms of coexistence were explored among species using an MLVC model.
(b). Study site
Laguna de Rocha (34°33′ S, 54°22′ W) is a choked coastal lagoon (mean depth, z = 0.8 m; surface area = 72 km2) that connects with the Atlantic Ocean several times per year through the opening of a sand bar. As a consequence, cycles of freshwater outflow and marine water intrusion occur in the lagoon, generating north–south gradients of salinity, nutrients, phytoplankton composition and biomass [20,21]. A detailed description of the biological and physico-chemical dynamics of the lagoon can be found in Conde et al. [21] and Bonilla et al. [20] and references therein. The lagoon was sampled monthly from August 1996 to February 1998, and from December 1998 to March 2000. A total of 32 subsurface phytoplankton samples were collected at two sites located at the marine (south) and limnetic (north) influenced areas with a Van Dorn sampler for qualitative and quantitative analyses (see the electronic supplementary material, appendix A for detailed phytoplankton sampling methodology) [20].
(c). Morphology-based functional groups
Species were classified into MBFG according to Kruk et al. [15]. A detailed description of the MBFGs can be found elsewhere (electronic supplementary material, appendix A) [15].
(d). Community description
The individual volume of the species was used as the fundamental niche axis (X = log2 volume). To provide a quantitative framework for the clumping to favourable niches, we used the Shannon–Wiener index or entropy (S). This index has been used to recognize aggregated species distribution in niche axes [22] and is defined by
| 2.1 |
where pi is the fraction of biovolume of species i in the community of n species. Therefore, the niche axis was divided into equally spaced segments. For each segment, S was calculated and represented its species diversity. Clumps were defined as modes in species diversity bracketed by segments of low species diversity. We tested the significance of entropy peaks by comparing observed entropy against an expected uniform distribution under the null hypothesis of homogeneous entropy (electronic supplementary material, appendix A). Then, taxonomic and MBFGs richness was plotted as a function of niche position (X) in octave bins.
(e). Size-dependent physiological constraints
A literature search was carried out for the physiological traits of the species included in the current dataset. Size-dependent growth rates and sinking velocities were compiled from 55 culture experiments [15], but not from size calculations. For each MBFG, relationships were fitted between the niche position and physiological rates (see the electronic supplementary material, appendix B).
(f). Fitness measures in phytoplankton
One indirect measure of fitness in phytoplankton is the minimum level to which a population is capable of drawing down resources (R*, [10]). In cases where there is competition for one limiting resource, the species with the lowest R* is the best suited to succeed in a stable environment. Within this framework, there are two ways to estimate fitness: either from the cultures of an individual species, or from the growth kinetics of the species [10]. In this study, the latter was applied, assuming the Monod model for nutrient uptake and growth kinetics dependent on volume. In this manner, R* can be expressed as a function of organismic volume as
| 2.2 |
where k is the half-saturation constant for phosphate-limited growth (average k = 0.07 mg l−1; [15]), and the maximum growth rate (μvol) depends on volume and MBFG-specific allometric coefficients. Mvol is the total specific loss rate. Here, loss processes (Mvol) comprise being flushed (f; day−1) out of the system, sinking to the bottom of the lagoon and being grazed (m; day−1). Flushing rate is the rate at which the water is renewed in the lagoon. Sinking loss depends on size-dependent sinking velocity (s; m day−1), the probability of mixing of the water column (p) and the depth of the system (z; m). The depth of the system was set at z = 0.8 m, as it was the average depth in the recorded samples. Finally, grazing mortality was considered to be constant in Laguna de Rocha and unrelated to volume at m = 0.1 day−1 (D. Calliari 2009, unpublished data). Total loss processes were, therefore, expressed as
| 2.3 |
(g). Competition model
Mechanisms behind the observed patterns were explored using an MLVC model [19]. In this study, the model involved only empirically measured quantities, except for the species-carrying capacities, which were derived using minimal ecological assumptions. For a detailed description of model parameters, ecological assumptions, calculations and data sources, see the electronic supplementary material, appendix B. The carrying capacity (Ki) of a species is the biomass a system can support of this particular species. Each species i has its own carrying capacity that depends on MBFG membership (i.e. μ and s allometric coefficients), its niche position (i.e. X; average log2 volume), while the lagoon hydrodynamics (flushing, f and resuspension, p) and zooplankton grazing (m) are shared among all species:
| 2.4 |
where Ro = 0.3 (mg P l−1) is the input of nutrient (phosphate) to the system, Q is the yield of nutrient per unit of phytoplankton (Q = 5.05 × 10−3 mg P mm−3; [23]) and other parameters are as in equations (2.2) and (2.3). The only free parameters are flushing (f = rand[0.032–0.284]; day−1) and probability of resuspension (p = rand[0–1]), which represent system hydrological properties. They were chosen to include the full range of variability observed in the lagoon (see the electronic supplementary material, appendix B).
The MLVC, using X as fundamental niche axis, is thus defined by
| 2.5 |
where Ni denotes the population biomass density of species i, μi its maximum growth rate (as equations (2.2) and (2.3)) and αij the competition coefficient between species i and j. The competition coefficient (αij) was estimated as the probability of overlap between species niches (for a detailed description of statistical procedures, see the electronic supplementary material, appendix B) [7,19]. The rationale is that species which are far apart in the niche axis will interact less than those that are closer, and that species with narrower niches will compete less than those with wider niches.
Fifty competing species were seeded from each MBFG represented in Laguna de Rocha, and were assigned to equally spaced positions on the niche axis, covering the whole volume range. Finally, MLVC model simulations were run using the estimated parameters (i.e. μ, K, α, see above) for each of the seeded species. Then 5000 Monte Carlo simulations were performed, starting with low species initial biovolume (N0 = rand[0,1] mm3 l−1), representing approximately 1 per cent of species carrying capacities (K). In each of the 5000 simulations, species position on the niche, biovolume and MBFG affiliation after 30 days were registered. The elapsed time was chosen similarly to the time between successive sampling events in the field. Modelled size structure and diversity patterns were then analysed.
3. Results
(a). Community patterns
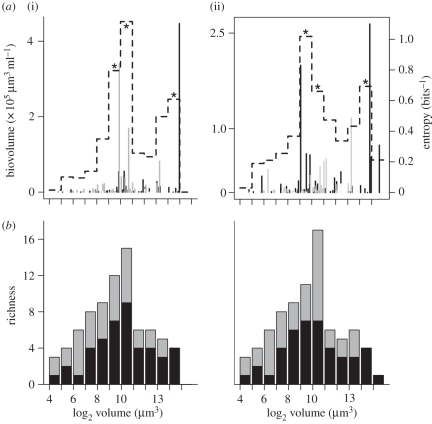
From the seven MBFGs, only two groups (groups V and VI) were clearly dominant in terms of biovolume and richness in Laguna de Rocha. Group V included flagellated unicells of diverse taxonomic groups (dinoflagellates, euglenophytes, chlorophytes and cryptophytes), while group VI included non-flagellated organisms with siliceous exoskeletons mainly unicellular and chain-forming diatoms. In terms of richness, from a total of 95 recorded taxa, group VI and V comprised 93.7 per cent of species richness, with 50.5 and 43.2 per cent, respectively. These groups accounted for 98.9 per cent of the biovolume in the marine area and 96.7 per cent in the limnetic area. Therefore, only these groups were considered further in the analyses. Both groups encompassed species with a wide range of individual volumes covering almost the whole niche axis (figure 1). The smallest species average volume found for group V was 27 µm3 and the largest 10 087 µm3, whereas group VI included species ranging from 27 to 51 229 µm3, which represent class 4–16 in octaves, respectively.
Figure 1.
Phytoplankton size structure and diversity patterns in Laguna de Rocha (i) marine and (ii) limnetic influenced areas. (a) Stems represent species average biovolume and dashed line is the entropy showing clump (*p < 0.05) and gap region (see text for explanation) Grey lines, group V; black lines, group VI. (b) Taxonomic richness against size classes within each morphology-based functional group. Grey bars, group V; black bars, group VI.
In both the limnetic- and the marine-influenced areas, overall average phytoplankton biovolume tended to accumulate in two regions of the niche axis (figure 1). Those two peaks were clearly evidenced by significant (p < 0.05) entropy peaks at size classes 9, 10 and 14 in both marine and limnetic zones (figure 1). Moreover, the aggregated pattern remained conspicuous when considering individual samples (electronic supplementary material, figure S3 in appendix C). Taxonomic richness showed a single dominant peak in the species richness–size curve, located in middle-sized species in marine- and limnetic-influenced areas (figure 1). Group V had a higher number of taxa at smaller sizes, whereas group VI showed the opposite trend, with a higher richness at larger sizes (figure 1).
(b). Physiological size constraints and fitness (R*)
Growth rate and sinking velocity as functions of volume showed different behaviour depending on the MBFG (electronic supplementary material, table S1 in appendix B). Group V (flagellated unicells with medium to large size) growth rate decreased with volume, whereas sinking rate showed the opposite trend. Group VI (non-flagellated organisms with siliceous structures) growth rates increased with volume, whereas no significant relationship with sinking velocity was found (electronic supplementary material, table S1).
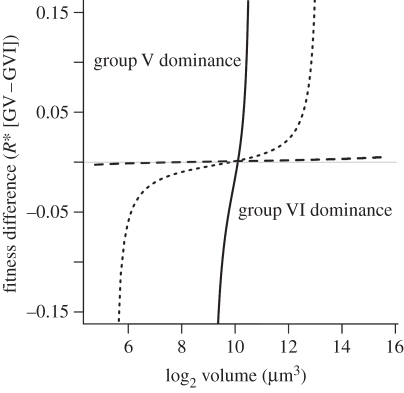
Size-dependent fitness (Rvol*) estimated from the volume-dependent growth kinetics showed contrasting patterns between the groups. Group V showed the highest fitness at small sizes and its competitive ability decreased (i.e. increased R*) markedly at volumes larger than 10 000 µm3. By contrast, group VI showed minimum fitness at small sizes (less than 200 µm3), which monotonically increased with larger volumes. We then considered the fitness differences between the MBFGs and observed that minimal fitness differences were found at approximately 1000 µm3 (figure 2). Furthermore, the shape of the curve strongly depended on the probability of sinking (p). As expected, a decrease in the sinking probability led to an improvement in the overall fitness of group VI species and a decrease in the absolute fitness differences between species of both MBFGs (figure 2).
Figure 2.
Fitness difference in relation to individual volume between MBFG V and VI species. Fitness was estimated as the break-even resource concentration (R*; equation (2.2); table 1). Parameters in equation (2.2) were z = 0.8 m; f = 0.071 day−1 (residence time of the system approx. two weeks). Solid line, p = 0, full sinking; small-dashed line, p = 0.5, half sinking; large-dashed line, p = 1, no sinking.
(c). MLVC model output
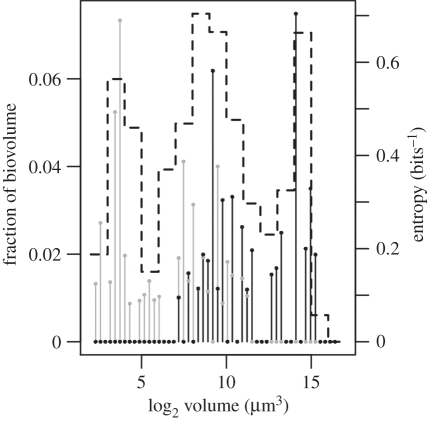
Niche width presented a V-shaped relationship with average species volume (electronic supplementary material, table S2 in appendix B). The MLVC model with volume as the fundamental niche axis reproduced several patterns found in the phytoplankton community of the lagoon (figure 3). These included the coexistence of species of both groups at middle sizes (approx. 1000 µm3), peaks in biovolume at middle and large sizes and the dominance of group VI at large body sizes (figure 3). The model produced another peak in biomass composed of species from group V at small sizes. It is remarkable that size structure was reached quickly (less than 30 days), and was robust to variation in the specific functional form chosen to describe the relation of niche position (X) to the niche width (σ).
Figure 3.
Emergent size structure of phytoplankton from a microscopic Lotka–Volterra competition (MLVC) model (equation (2.3)) after 30 days of simulation. Stems represent individual species of MBFGs V (grey) and VI (black) from a selected simulation. Dashed line represents entropy from average biovolume of 5000 simulations at day 30, started with random initial biovolume, flushing rate and sinking probability.
4. Discussion
Species coexistence, size structure and diversity patterns observed at Laguna de Rocha were explained by a combination of four fundamental factors: MBFG classification, size-based constraints, system hydrology and species competition. The first three factors provided realism and precision in the model, whereas the fourth is fundamental for the clumping of species as previously demonstrated [19,22]. This provides a mechanistic explanation for species coexistence along a niche axis and community dynamics based on empirical measured quantities (e.g. growth rates), minimal ecological assumptions (e.g. derivation of carrying capacities) and stochastic processes (e.g. resuspension). The present results support emergent neutrality as a plausible mechanism for driving community dynamics in ecological time scales.
(a). Aggregated distribution of species in a niche axes
Clumps representing the aggregated distribution of species in niche axes have been found in other communities (birds and mammals [18]; phytoplankton [5]). This suggests that certain ranges of the niche axis are more favourable for species coexistence than others under particular environmental conditions. The empirical dataset from Laguna de Rocha showed two conspicuous clumps in diversity and biovolume along the niche axis. The fact that clumps were observed in both marine- and limnetic-influenced areas and over 2 years of sampling (figure 1) suggests that it is a common phenomenon. Moreover, it means that clumpy distribution of phytoplankton biomass in the lagoon is not dependent on light availability or salinity, which have been shown to differ between marine- and limnetic-influenced areas [20,24]. The observed clumps correspond to large- and medium-sized organisms. The large-sized clump was dominated by species of group VI (non-flagellated siliceous organisms, mainly diatoms). Such a finding could be expected from model results showing that this group reached its highest fitness at large individual sizes. These large organisms occur alternatively in the water column and on the surface sediments, being resuspended on a daily basis. There is a sharp difference in the use of habitat between large, rapidly sinking species in group VI, which constitute the dominant biomass fraction on the surface sediments of Laguna de Rocha [24], and middle-sized species (approx. 1000 µm3) that are permanently suspended in the water column. In these middle sizes, the occurrence of a biovolume clump and richness peak is less straightforward, as fitness curves indicate that no middle-sized species from either MBFG exhibit maximum fitness. However, in that middle-sized zone, fitness differences among species from both groups are at a minimum, implying that coexistence is facilitated for organisms with similar fitness. Highest richness at the region of minimum fitness difference strongly suggests the action of equalizing processes, i.e. those processes that tend to decrease average fitness differences among competing species, as stated in our working hypothesis. Finally, the expected peak in biomass for small-sized species in group V was not found in the empirical data. In fact, this clump could be an artefact caused by the assumption made by the MLVC model that there is no phytoplankton beyond the limits of the niche axis, which in reality is not true. Small picoplankton (maximum linear dimension less than 3 µm; not accounted for in this study) can reach high biomasses in this lagoon [25] and so competition can be intense at small sizes. Future studies, including picoplankton biomass and their kinetic responses, will resolve the smaller sized region of the niche axis. Indeed, the appearance of peaks close to the niche boundaries is a robust result for an MLVC model along a continuous one-dimensional niche axis [22]. Size structure and richness patterns together with size-dependent fitness suggest equalizing processes might cause the observed patterns. Moreover, the MLVC model provided a mechanistic explanation of these patterns. In this sense, our results showed that emergent neutrality acting on an ecological time scale is a plausible mechanism for shaping the Laguna de Rocha phytoplankton community. Coexistence among medium-sized species is favoured by their equal fitness, and these species are able to coexist with large-sized species because of different niche use.
(b). Clumps of species with similar fitness
The occurrence of clumps of coexisting species in the niche axis is in agreement with theoretical models, including neutral- and niche-based processes related to an organism's traits [26]. However, clumps of coexisting species in Laguna de Rocha might be explained following the high-dimensionality hypothesis [1]. This hypothesis states that coexistence can be mediated by other ‘hidden’ niche differences. This might be particularly evident for medium-sized species, as MBFG V and VI are different in several traits. While group V is constituted mainly by flagellates that can actively swim and includes facultatively mixotrophic organisms, group VI is mainly composed of diatoms with heavy siliceous walls and higher sinking rates. However, niche differences between MBFGs cannot explain coexistence of species from the same MBFG with similar traits and similar size (electronic supplementary material, figure S2 in appendix C). At least in these cases, niche differences are not operating or are only minor drivers of this phytoplankton community. Another force that can promote species coexistence is predation [11]. However, we believe this is not the main force in shaping this community. Coexisting phytoplankton species in the observed size range differ in shape and structure, but not so much as to result in important differences in grazing edibility. These algae are well inside the preferred size range of Acartia tonsa [27]. Acartia tonsa is the dominant mesozooplanktonic grazer in the system and has been shown to not exert a high grazing pressure [28]. Moreover, density-dependent grazing is not believed to generate discontinuous biomass distributions, thus excluding predation as an alternative explanation for species clumping and coexistence in Laguna de Rocha. Density-dependent grazing could promote coexistence within clumps once they emerge, by eating the most abundant species, leading to a stabilization of the size structure, as has been shown theoretically [7]. The present evidence suggests that fitness similarity is promoting species coexistence within clumps, whereas niche differences favour coexistence between different clumps. This contention does not rule out the effect of potential density-dependent mechanisms that promote the maintenance of the emergent size structure.
Fitness differences without niche differences lead to the competitive exclusion of poorer competitors [4,9]. In a constant environment, a population excluded in specific environmental conditions would not be able to recover, leading to permanent extinction. However, in Laguna de Rocha, we found alternate patterns of either one dominant species or several coexisting species in terms of community biomass (electronic supplementary material, figure S2 in appendix C). What are the mechanisms that allow these alternate patterns of dominance and coexistence? Here, a high-dimensional explanation might be plausible, which can be represented by the wide amplitude in the scales of environmental variability of the hydrological processes in Laguna de Rocha. Environmental variation is mainly caused by resuspension and flushing rates, which can vary in a time scale of hours (e.g. marine breeze) to months (e.g. marine intrusion). This variability would modulate size-dependent fitness, impeding species dominance if shifts in hydrological conditions are frequent, or leading to the dominance of a particular species if conditions remain stable for a long period. These processes are very difficult to parametrize empirically in a deterministic way. However, the model correctly captured its main effect by using stochastic parameter variation, as has been suggested previously [1].
In summary, the present results support the contention that equalizing (fitness) and stabilizing (niche) processes mediated by environmental forcings drive Laguna de Rocha phytoplankton dynamics. The MLVC model showed that community structure can be reached on ecological time scales, which posits emergent neutrality as a plausible mechanism for shaping natural communities. Moreover, we provide, to the authors' knowledge, the first mechanistic evidence and explanation of the role of equalizing and stabilizing processes in facilitating species coexistence in natural communities.
Acknowledgements
We would like to thank economic support from PEDECIBA BIOLOGÍA & FÍSICA, ANII (BE_POS_2009_1229) and project PDT_71-06. Dr Guan-Mou and S. Mac for inspiring ideas, D. Antoniades for English revision and Inchausti and Arim for statistical advice. We appreciate the feedback of the two anonymous referees whose suggestions improved the quality of this article.
References
- 1.Clark J. S., Dietze M., Chakraborty S., Agarwal P. K., Ibañezz I., LaDeau S., Wolosin M. 2007. Resolving the diversity paradox. Ecol. Lett. 10, 647–662 10.1111/j.1461-0248.2007.01041.x (doi:10.1111/j.1461-0248.2007.01041.x) [DOI] [PubMed] [Google Scholar]
- 2.Hutchinson G. E. 1961. The paradox of plankton. Am. Nat. 882, 137–145 10.1086/282171 (doi:10.1086/282171) [DOI] [Google Scholar]
- 3.Hubbell S. P. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press [Google Scholar]
- 4.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 10.1146/annurev.ecolsys.31.1.343 (doi:10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 5.Vergnon R., Dulvy N. K., Freckleton R. P. 2009. Niches versus neutrality: uncovering the drivers of diversity in a species-rich community. Ecol. Lett. 12, 1079–1090 10.1111/j.1461-0248.2009.01364.x (doi:10.1111/j.1461-0248.2009.01364.x) [DOI] [PubMed] [Google Scholar]
- 6.Holt R. D. 2006. Emergent neutrality. Trends Ecol. Evol. 21, 531–533 10.1016/j.tree.2006.08.003 (doi:10.1016/j.tree.2006.08.003) [DOI] [PubMed] [Google Scholar]
- 7.Scheffer M., Van Nes H. E. 2006. Self-organized similarity, the evolutionary emergence of groups of similar species. Proc. Natl Acad. Sci. USA 103, 6230–6235 10.1073/pnas.0508024103 (doi:10.1073/pnas.0508024103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams P. A. 1987. Alternative models of character displacement and niche shift. I. Adaptive shifts in resource use when there is competition for nutritionally nonsubstitutable resources. Evolution 41, 651–661 10.2307/2409267 (doi:10.2307/2409267) [DOI] [PubMed] [Google Scholar]
- 9.Mac Dougall A. S., Gilbert B., Levine J. M. 2009. Plant invasions and the niche. J. Ecol. 97, 609–615 10.1111/j.1365-2745.2009.01514.x (doi:10.1111/j.1365-2745.2009.01514.x) [DOI] [Google Scholar]
- 10.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press; [PubMed] [Google Scholar]
- 11.Chesson P., Kuang J. J. 2008. The interaction between predation and competition. Nature 456, 235–238 10.1038/nature07248 (doi:10.1038/nature07248) [DOI] [PubMed] [Google Scholar]
- 12.Litchman E., Klausmeier C. A. 2008. Trait-based community ecology of phytoplankton. Annu. Rev. Ecol. Evol. Syst. 39, 615–639 10.1146/annurev.ecolsys.39.110707.173549 (doi:10.1146/annurev.ecolsys.39.110707.173549) [DOI] [Google Scholar]
- 13.Reynolds C. S., Irish A. E., Elliott J. A. 2001. The ecological basis for simulating phytoplankton responses to environmental change (PROTECH). Ecol. Model. 140, 271–291 10.1016/S0304-3800(01)00330-1 (doi:10.1016/S0304-3800(01)00330-1) [DOI] [Google Scholar]
- 14.Marañón E. 2008. Inter-specific scaling of phytoplankton production and cell size in the field. J. Plankton Res. 30, 157–163 10.1093/plankt/fbp046 (doi:10.1093/plankt/fbp046) [DOI] [Google Scholar]
- 15.Kruk C., Huszar V. L. M., Peeters E. T. H. M., Bonilla S., Costa L., Lürling M., Reynolds C. S., Scheffer M. 2010. A morphological classification capturing functional variation in phytoplankton. Freshw. Biol. 55, 614–627 10.1111/j.1365-2427.2009.02298.x (doi:10.1111/j.1365-2427.2009.02298.x) [DOI] [Google Scholar]
- 16.Kruk C., Peeters E. T. H. M., Van Nes E. H., Huszar V. L. M., Costa L. S., Scheffer M. In press Phytoplankton community composition can be predicted best in terms of morphological groups. Limnol. Oceanogr. 56 [Google Scholar]
- 17.Segura A. M., Kruk C., Calliari D., Fort H. 2010. Trait-based approach disentangles core features of phytoplankton succession. Morphology captures function in phytoplankton. A large-scale analysis of phytoplankton communities in relation to their environment. PhD thesis, pp. 75–90 University of Wageningen, Wageningen, The Netherlands [Google Scholar]
- 18.Holling C. S. 1992. Cross-scale morphology, geometry, and dynamics of ecosystems. Ecol. Monogr. 62, 447–502 10.2307/2937313 (doi:10.2307/2937313) [DOI] [Google Scholar]
- 19.May R. M. 1973. Stability and complexity in model ecosystems. Princeton Landmarks in Biology Princeton, NJ: Princeton University Press [Google Scholar]
- 20.Bonilla S., Conde D., Aubriot L., Pérez M. C. 2005. Influence of hydrology on phytoplankton species composition and life strategies in a subtropical coastal lagoon periodically connected with the Atlantic Ocean. Estuaries 28, 884–895 10.1007/BF02696017 (doi:10.1007/BF02696017) [DOI] [Google Scholar]
- 21.Conde D., Aubriot L., Bonilla S., Sommaruga R. 2002. Marine intrusions in a coastal lagoon enhances the effects of UV radiation on the phytoplankton photosynthetic rate. Mar. Ecol. Prog. Ser. 204, 57–70 10.3354/meps240057 (doi:10.3354/meps240057) [DOI] [Google Scholar]
- 22.Fort H., Van Nes H. E., Scheffer M. 2010. The clumping transition in niche competition: a robust critical phenomenon. J. Stat. Mech. Theory Exp. 2010, 1–16 10.1088/1742-5468/2010/05/P05005 (doi:10.1088/1742-5468/2010/05/P05005) [DOI] [Google Scholar]
- 23.Reynolds C. S. 1984. The ecology of freshwater phytoplankton. Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Conde D., Bonilla S., Aubriot L., de León R., Pintos W. 1999. Comparison of the areal amount of chlorophyll a of planktonic and attached microalgae in a shallow coastal lagoon. Hydrobiologia 408, 285–291 10.1023/A:1017086513787 (doi:10.1023/A:1017086513787) [DOI] [Google Scholar]
- 25.Vidal L., Bonilla S., Rodriguez-Gallego L., Conde D., Martínez-López W. 2007. Biomass of autotrophic picoplankton in subtropical coastal lagoons: is it relevant? Limnetica 26, 441–452 [Google Scholar]
- 26.Etienne R. S., Olff H. 2004. How dispersal limitation shapes species-body size distributions in local communities. Am. Nat. 163, 69–83 10.1086/380582 (doi:10.1086/380582) [DOI] [PubMed] [Google Scholar]
- 27.Berggreen U., Hansen B., Kiorboe T. 1988. Food size spectra, ingestion and growth of the copepod during development: implications for determination of copepod production Acartia tonsa. Mar. Biol. 99, 341–352 10.1007/BF02112126 (doi:10.1007/BF02112126) [DOI] [Google Scholar]
- 28.Calliari D., Britos A., Conde D. 2009. Testing the relationship between primary production and Acartia tonsa grazing pressure in an estuarine lagoon. J. Plankton Res. 31, 1045–1058 10.1093/plankt/fbp049 (doi:10.1093/plankt/fbp049) [DOI] [Google Scholar]