Abstract
Ecologists need an empirical understanding of physiological and behavioural adjustments that animals can make in response to seasonal and long-term variations in environmental conditions. Because many species experience trade-offs between timing and duration of one seasonal event versus another and because interacting species may also shift phenologies at different rates, it is possible that, in aggregate, phenological shifts could result in mismatches that disrupt ecological communities. We investigated the timing of seasonal events over 14 years in two Arctic ground squirrel populations living 20 km apart in Northern Alaska. At Atigun River, snow melt occurred 27 days earlier and snow cover began 17 days later than at Toolik Lake. This spatial differential was reflected in significant variation in the timing of most seasonal events in ground squirrels living at the two sites. Although reproductive males ended seasonal torpor on the same date at both sites, Atigun males emerged from hibernation 9 days earlier and entered hibernation 5 days later than Toolik males. Atigun females emerged and bred 13 days earlier and entered hibernation 9 days earlier than those at Toolik. We propose that this variation in phenology over a small spatial scale is likely generated by plasticity of physiological mechanisms that may also provide individuals the ability to respond to variation in environmental conditions over time.
Keywords: climate, Urocitellus parryii, phenotypic flexibility, local phenological variation, Arctic
1. Introduction
Seasonal timing of biological events or ‘phenology’ has advanced recently in certain animals and plants living at temperate and high latitudes, and in some cases these changes have been attributed to climate change [1–8]. Problematically, if the phenology of one species shifts at a different rate or in a different direction from that of an interacting species living in the same ecological community, a mismatch in timing of suitable conditions may result. For example, the timing of caribou migration in the spring has not kept pace with the seasonal advancement in plant growth that has occurred in some caribou calving grounds; this may be contributing to recent declines in reproduction and increases in offspring mortality in arctic caribou populations [7].
Many recent studies describe shifts in the timing of a single phenological event, often related to reproduction, such as the arrival of migrating birds on breeding grounds or egg laying (e.g. [5,7,9–11]). However, animals may experience trade-offs between adjustments of the timing and duration of one seasonal event in relation to another. Such trade-offs may determine the manner in which successive seasonal events and even life-history stages may respond to environmental variability. In migratory birds, changes in the timing of arrival at breeding sites in response to warming may alter the egg-laying dates required to maintain a match with food availability [10,12]. A further complication is that studies often only consider shifts in response to the initiation or termination of an optimal period, e.g. food availability, whereas climatic change may also affect the absolute duration of the period [13].
Here, we investigate an aggregate of seasonal, phenological events in two nearby populations of arctic ground squirrels (Urocitellus parryii; AGS) living above 68° N, north of the Brooks Range, Alaska. The two sites are located only 20 km apart, yet show substantial differences in timing of snow cover and thus biological activities. At Toolik Lake, the tundra is covered by snow approximately two weeks earlier in autumn and the first snow-free day occurs approximately four weeks later in the spring than at Atigun River. Differences in the timing of snow cover have been associated with flowering dates of plants [2], and green-up typically occurs earlier at Atigun River than at Toolik Lake (B. M. Barnes 1997-present, personal observation). Urocitellus parryii is the northernmost ground squirrel species in North America and the largest species in its genus; with a geographical range that reaches the shores of the Arctic Ocean, it is the northernmost hibernating terrestrial mammal species [14,15].
AGSs are an excellent representative species in which to investigate phenological variation, because the annual cycle of these squirrels is comprised of a distinct series of events. These include: entrance into and emergence from hibernation, defined here as seasonal intervals of reduced activity and continuous below-ground existence that includes a period of regulated heterothermy; heterothermy, a series of long bouts of torpor that occurs during hibernation, which are interrupted by regular brief arousal intervals (ca 24 h) when core body temperature (Tb) returns to euthermic level; reproduction, including stages of gonadal development, mating, pregnancy and lactation; and pre-hibernation fattening. The growing season in the Arctic is short, and breeding adults are only above-ground for 3–5 months [16,17]. The timing of reproduction is critical to allow young of the year adequate time for postnatal somatic growth, dispersal and pre-hibernation fattening. Adults must also reach an appropriate body mass and deposit sufficient fat stores or food caches to enter hibernation at an appropriate time and to survive the hibernation period.
We implanted Tb loggers to obtain continuous, records of Tb from 1997 to 2010 in over 200 free-ranging squirrels at the two sites. We were able to detect with high precision the annual timing of the beginning and the end of hibernation and of heterothermy in adult and juvenile AGS, and the dates of conception and parturition in adult and yearling females. Owing to the earlier spring snow-free date at Atigun River, we predicted earlier hibernation and breeding events for this population when compared with the Toolik Lake animals.
2. Methods
(a). Study area
The two sites are separated by about 20 km: Toolik Lake (68°38′ N, 149°38′ W; elevation 719 m) and Atigun River (68°27′ N, 149°21′ W; elevation 812 m). Despite this close proximity, physical and biotic conditions differ. Toolik Lake is categorized as cotton grass (Eriophorum) tussock and dry tundra with great than 98 per cent of the vegetative biomass and productivity attributable to only 10 species [18]. The topography is relatively flat, with gently rolling hills underlain by continuous permafrost with a depth of thaw usually <1 m [17]. Atigun River is composed of a slightly sloping sandy substrate along the northern bank of the Atigun River. The flora is dominated by a mix of low-growing Salix sp. (less than 30 cm), Dryas octopetala, Rhododendron lapponicum, Arctostaphylos alpina and Vaccinium uliginosun. Thaw depth is 1–2 m. Because both sites are relatively flat, entrances in AGS burrows were randomly oriented and showed no differences in either exposure to or shelter from prevailing environmental conditions, such as solar radiation input, shade or wind.
The environment within our general study area has shown no consistent directional changes in temperature, or date of green-up and senescence of plants (Environmental Data Centre, Toolik Field Station; http://toolik.alaska.edu/edc/) during this time. To assess the environmental differences between our two sites, we compared the change in soil temperature and the timing of snow cover. We measured soil temperature using five high-resolution temperature loggers (Hobo Pro Series Temp, ONSET) at each site. Loggers recorded temperature every 4 h from thermistor probes placed at the bottom of a 1 m deep shaft, which was backfilled with sand and tamped to be firm (see [19] for details). We compared the average soil temperature in October (month when males enter hibernation) and April (when males and females emerge from hibernation) between our two sites from 2005 through 2010. To quantify snow cover specific to our Atigun River site, we used a camera (Campbell Scientific, CC640 Digital Camera System mounted inside a Pelco EH4700 Environmental Enclosure) mounted on a 10 m tower facing southeast across the study site, which acquired an image daily at solar noon. At Toolik Lake, we quantified per cent snow cover in the same manner from data provided by the Environmental Data Centre. To describe snow cover differences between our two sites, we recorded the day when the site was first 100 per cent snow covered and remained 100 per cent snow covered for ≥3 d in autumn and the day when the site was first 100 per cent snow-free and remained so for ≥3 d in spring from 2007 to 2010.
(b). Animal trapping and handling
A total of 204 Tb loggers (Toolik Lake: 39 adult males, seven young of the year (juvenile) males, 61 adult females and 19 young of the year (juvenile) females; Atigun River: 21 adult males, six young of the year (juvenile) males, 41 adult females and 10 young of the year (juvenile) females) were implanted and recovered through subsequent surgeries 6–12 months apart from autumn 1997 through spring 2010. Animals were captured using Tomahawk live-traps baited with carrot. Traps were set in the early morning and checked every 1–3 h until closure in the mid-afternoon. Trapped animals were transported to the Toolik Field Station of the University of Alaska, Fairbanks. Animals were anaesthetized by a 3–5 min exposure to methoxyflurane or isoflurane vapours and uniquely tagged (ear tags—Monel no. 1; pit tags—AVID MUSICC), weighed, sexed and assessed for reproductive status. Males with enlarged and descended testes and a pigmented scrotum were scored as reproductive. Females that were palpably pregnant were scored pregnant and those with prominent nipples surrounded by partially hairless skin were scored as lactating. Once every year each animal was implanted/explanted with an abdominal temperature-sensitive data logger (modified TidBit Stowaway model TBICU32-05+44, accuracy of ±0.2°C, Onset Computer Corporation) that was programmed to record Tb at 20 min intervals for up to 18 months [20]. Loggers were coated in Elvax (Dupont), calibrated (to verify accuracy) and sterilized prior to implantation; coated loggers weighed 12–14 g. We have validated that loggers do not drift over time. After surgery, animals were monitored for 12 h prior to release at their site of capture.
(c). Phenology of hibernation and reproduction
We estimated phenological events from patterns of Tb change recorded by the data loggers implanted in free-living ground squirrels. We define the hibernation season as beginning when an animal was continuously sequestered within its burrow or hibernaculum. The beginning of hibernation was estimated as the date when an animal's Tb first became stabilized within a normal resting (normothermic) range that did not increase above resting levels typical of animal activity and movement on the surface. The heterothermic season during hibernation, when animals alternate between long bouts of torpor and short arousal episodes (ca 24 h), begins with the first date an animal's Tb declined below 30°C for more than 24 h and ends with the sustained return to euthermy in spring [21]. Spring emergence from hibernation onto the surface was determined as the date when an animal's Tb began to show diurnal cycles of large amplitude (2–4°C) that corresponds to movement and activity on the surface. Date of parturition was determined from a unique, sharp, upward shift in mean Tb and increased diurnal Tb amplitude that followed a consistently declining average Tb during gestation. The date of mating and conception is 25 days prior to the day of parturition [22]. The methodology of determining phenological events from Tb was validated by direct observation of behaviours coupled with Tb measures in the same individuals.
(d). Statistical analysis
Animals were grouped by location, sex and age, i.e. Toolik adult males, Toolik juvenile males, etc. [17]. The Tb patterns of AGS from the beginning of their second hibernation were not different from those of older (greater than 2 years old) adults (p > 0.05), and thus were grouped with adults for comparison with juveniles. Although certain animals were implanted with Tb loggers multiple times over the years, a mixed-effects model with animal identification included as a random factor showed that it was not significant in any of the comparisons; thus animal identification was dropped from the model. From 1997 to 2010, we found no effect of year or interaction between year and any other variable, and thus no further analysis of the effects of year-to-year variation was included in the results we present here.
All data are expressed as means ± 1 s.e., unless otherwise stated. ANOVAs and Kruskal–Wallis non-parametric tests were performed using the software package Statistica v. 6. The assumption of normality was tested with Shapiro–Wilks test, and the assumption of homogeneity of variances was tested with Levene's test. If these assumptions were not met, data were log-transformed [23] and re-tested. If the assumptions were still not met, a Kruskal–Wallis one-way ANOVA on ranks was performed. Comparisons of the means were considered significant if p < 0.05.
3. Results
(a). Comparison of temperature and snow cover
Soil temperatures at 1 m depth were similar and showed no consistent, overall annual differences between Toolik Lake and Atigun River from 2005 to 2010. The average October soil temperature was −0.59 ± 0.09°C and the average April soil temperature was −10.86 ± 0.29°C.
Duration of snow cover, as indicated on daily camera images, was 44 d longer at Toolik Lake than at Atigun River. From 2007 to 2010 snow cover began in autumn, on average, 17 ± 6 d earlier and the first snow-free day occurred in spring on average 27 ± 5 d later at Toolik than at Atigun. The first day snow cover was 100 per cent ranged from 18 September to 2 October at Toolik and from 28 September to 18 October at Atigun. The first 100 per cent snow-free day ranged from 27 May to 3 June at Toolik and from 30 April to 9 May at Atigun. The lack of overlap in dates of springtime snow-free exposure of the ground, with a 27 days difference, is the most conspicuous difference in environmental conditions between the two sites.
(b). Entrance into hibernation and heterothermy
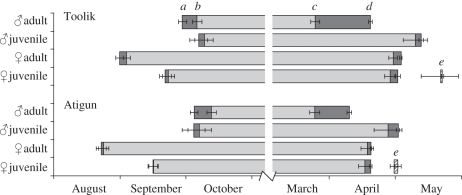
While adult male AGS became sequestered in their hibernacula and subsequently began heterothermy earlier in the population living near Toolik Lake (25 September ± 1.86 d; 2 October ± 2.18 d, respectively) than adult males at Atigun River (30 September ± 1.71 d; 9 October ± 2.11 d, respectively; F1,58 = 3.44, p = 0.07; F1,58 = 3.99, p = 0.05, respectively; figure 1), juvenile males entered hibernacula and became heterothermic at these two sites at the same time (Toolik: 3 October ± 4.00 d; 5 October ± 4.16 d, respectively; Atigun: 1 October ± 5.13 d; 3 October ± 5.39 d, respectively; F1,11 = 0.12, p = 0.74; F1,11 = 0.10, p = 0.76, respectively; figure 1).
Figure 1.
Timing of hibernation seasons (mean dates ± s.e.) in AGSs at two nearby sites in Northern Alaska, approximately 68.5° N: Toolik Lake and Atigun River. Chronologies are shown separately for adult males and females and for young of the year (juveniles) of both sexes. (a) Autumnal entry into the hibernation burrow (hibernaculum), following cessation of above-ground activity, (b) initiation of heterothermy, (c) resumption of euthermy, following last torpor bout of spring, (d) emergence from the hibernaculum and resumption of surface activity, and (e) return to euthermy and emergence onto surface by non-reproductive juvenile females.
The pattern in adult females was opposite to that of males; Toolik females began hibernation and heterothermy later (28 August ± 1.79 d; 31 August ± 1.68 d, respectively) than adult females at Atigun River (19 August ± 2.34 d; 20 August ± 2.33 d, respectively; F1,94 = 8.32, p = 0.005; F1,94 = 15.20, p = 0.0002, respectively; figure 1). Similarly, juvenile females entered hibernacula and became heterothermic later at Toolik (18 September ± 2.72 d; 19 September ± 2.80 d, respectively) than at Atigun (12 September ± 2.19 d; 12 September ± 2.20 d, respectively; H1,28 = 3.99, p = 0.045; H1,28 = 5.73, p = 0.02, respectively; figure 1).
(c). Emergence from hibernation and euthermy
In spring, adult males ended heterothermy at the same time at Toolik Lake (25 March ± 1.25 d) and at Atigun River (25 March ± 1.72 d; F1,55 = 0.001, p = 0.97). However, males at Toolik first emerged from their hibernacula on average 9 d later than those at Atigun (Toolik—19 April ± 1.04 d; Atigun—10 April ± 1.35 d; F1,55 = 28.71, p < 0.0001; figure 1). Thus, adult males at Toolik remained in their hibernacula at normal, high Tb after ending heterothermy for 25 ± 0.88 d versus 15 ± 0.94 d for adult males at Atigun (F1,55 = 46.07, p < 0.0001). Juvenile males became euthermic later and emerged from their hibernacula later at Toolik (9 May ± 2.50 d; 12 May ± 2.11 d, respectively) than at Atigun (27 April ± 2.78 d; 1 May ± 3.41 d, respectively; F1,11 = 11.11, p = 0.007; F1,11 = 7.51, p = 0.02, respectively; figure 1). At both sites, juvenile males that did not become reproductively mature as yearlings became euthermic and remained below-ground after ending heterothermy for only 3–5 d (Toolik: 3 ± 0.89 d; Atigun: 5 ± 0.81 d; F1,11 = 2.14, p = 0.17). However, this pattern is more complex. At Atigun one adult male (greater than 2 year old) did not become reproductively active, and his emergence pattern was similar to that of a juvenile male at Atigun (euthermy: 26 April, emergence: 28 April, time below-ground before emergence: 2 d). Furthermore, 4 juvenile males (3 Toolik; 1 Atigun) became reproductively active after their first hibernation season and had similar emergence patterns to those of older, reproductive adults at their respective sites (Toolik: euthermy: 6 April ± 3.51 d, emergence: 25 April ± 0.88, time below-ground: 19 ± 3.53 d; Atigun: euthermy: 18 March, emergence: 4 April, time below-ground: 17 d).
Adult females ended heterothermy 11 days later and emerged from their hibernacula 13 days later at Toolik Lake (30 April ± 1.23 d; 3 May ± 1.11 d, respectively) than at Atigun River (19 April ± 0.63 d; 21 April ± 0.55 d, respectively; H1,78 = 34.93, p < 0.0001; H1,78 = 46.07, p < 0.0001, respectively; figure 1). Adult females at both sites first emerged on the surface only 2–3 d after ending torpor (Toolik: 3 ± 0.46 d; Atigun: 2 ± 0.22 d; H1,78 = 0.76, p = 0.38). Juvenile females that became pregnant as yearlings became euthermic and emerged from their hibernacula later at Toolik (28 April ± 1.94 d; 1 May ± 1.45 d, respectively) than at Atigun (18 April ± 1.85 d; 20 April ± 2.11 d, respectively; H1,19 = 7.11, p = 0.008; H1,19 = 8.96, p = 0.003, respectively; figure 1), and at both sites first emerged on the surface only 2–3 days after ending torpor (Toolik: 3 ± 0.95 d; Atigun: 2 ± 0.51 d; H1,18 = 0.27, p = 0.61). Juvenile females that did not become pregnant as yearlings became euthermic and emerged from their hibernacula much later than those that became pregnant at their respective sites (Toolik: euthermy: 21 May ± 4.00 d, H1,11 = 4.63, p = 0.03; emergence: 22 May ± 3.5 d, H1,11 = 4.65, p = 0.03; Atigun: euthermy: 30 April ± 1.83 d, H1,9 = 6.10, p = 0.01; emergence: 1 May ± 1.77 d, H1,9 = 6.00, p = 0.01; figure 1). However, the number of days they remained below-ground after ending heterothermy did not differ from those that became pregnant (Toolik: 3 ± 1.25 d, H1,11 = 1.47, p = 0.22; Atigun: 1 ± 0.37 d, H1,9 = 2.31, p = 0.13).
(d). Hibernation and heterothermy
The timing of entrance into and emergence from hibernation and heterothermy varied greatly between sites, and between sexes and age classes (figure 1). Adult males hibernated and remained heterothermic significantly longer at Toolik Lake (205 ± 2.95 d; 174 ± 2.00 d, respectively) than those at Atigun River (191 ± 1.96 d; 167 ± 2.36 d, respectively; F1,55 = 21.07, p < 0.0001; F1,55 = 4.52, p = 0.04, respectively). In juvenile males, the duration of hibernation and heterothermy did not differ significantly between Toolik (221 ± 5.45 d; 216 ± 5.41 d, respectively) and Atigun (213 ± 6.44 d; 205 ± 6.39 d, respectively; F1,11 = 1.01, p = 0.34; F1,11 = 1.54, p = 0.24, respectively).
Adult females hibernated 41 days longer at Toolik Lake and 50 days longer at Atigun River than adult males at each site. However, the duration of hibernation and heterothermy was not significantly different for adult females between the two populations (Toolik: 246 ± 2.00 d, 241 ± 2.02 d; Atigun River: 242 ± 2.81 d, 240 ± 2.90 d; F1,76 = 1.06, p = 0.31; F1,76 = 0.12, p = 0.73, respectively). Juvenile females that became pregnant as yearlings did not differ in duration of hibernation and heterothermy between Toolik (223 ± 1.83 d; 218 ± 2.47 d, respectively) and Atigun (223 ± 2.77 d; 220 ± 3.11 d, respectively; H1,19 = 0.17, p = 0.67; H1,19 = 0.019, p = 0.88, respectively). However, juvenile females that did not become pregnant as yearlings had significantly longer seasons of hibernation and heterothermy at Toolik (246 ± 8.00 d; 244 ± 8.50 d, respectively) than at Atigun (229 ± 1.94 d; 227 ± 1.95 d, respectively; H1,6 = 4.04, p = 0.04; H1,6 = 3.82, p = 0.05, respectively). Furthermore, at Toolik juvenile females that did not become pregnant hibernated longer and remained heterothermic longer than those that became pregnant (H1,11 = 4.63, p = 0.03, H1,11 = 4.63, p = 0.03, respectively); however, this comparison was not significant at Atigun (H1,6 = 2.97, p = 0.08, H1,11 = 2.16, p = 0.14, respectively).
(e). Reproductive season
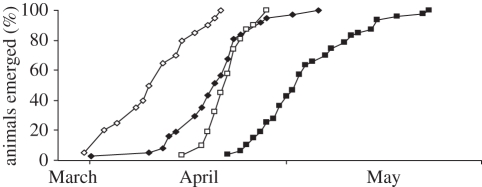
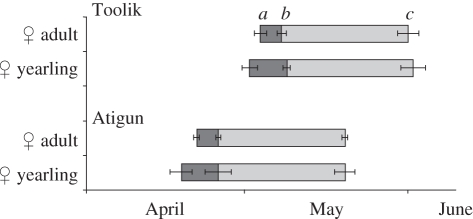
Adult males emerged on average 14 days prior to the average emergence date of females at Toolik Lake and 11 days prior to females at Atigun River (figure 2). At Toolik, 97 per cent of males had emerged at the time 50 per cent of females had emerged (2 May). At Atigun, 100 per cent of males had emerged by the time 50 per cent of females emerged (21 April). Adult females became pregnant within 1–4 d following their emergence, with no significant difference between Toolik and Atigun (Toolik: 4 ± 0.86 d after emergence; Atigun: 4 ± 0.46 d after emergence; H1,27 = 0.04, p = 0.83; figure 3). However, owing to their later date of emergence from hibernation, adult females gave birth 12 d later at Toolik (31 May ± 1.99 d, 20 May to 13 June) than at Atigun (19 May ± 0.49 d, 15–23 May; H1,34 = 21.93, p < 0.0001; figure 3). Yearling females that became pregnant after their first hibernation did so shortly after emergence (Toolik: 7 ± 0.75 d after emergence; Atigun: 7 ± 2.5 d after emergence; F1,8 = 0.005, p = 0.95; figure 3). Similar to older females, yearling females also gave birth later at Toolik (31 May ± 2.33 d, 24 May to 13 June) than at Atigun (18 May ± 2.00 d, 16–20 May; F1,8 = 7.34, p = 0.03; figure 3). The average duration between the conception of and entrance into hibernation in autumn by female young of the year was 135 days at Toolik and 140 days Atigun. This duration was longer in male young of the year, 150 days at Toolik and 160 days Atigun.
Figure 2.
Cumulative proportion of adult males (diamonds) and adult females (squares) that emerged in spring onto the surface following hibernation season in relation to date at two sites in Northern Alaska: Toolik (filled symbols) and Atigun (open symbols).
Figure 3.
Timing of reproductive seasons (mean dates ± s.e.) in female AGSs (adults and yearlings shown separately) at two sites in Northern Alaska: Toolik and Atigun. (a) Emergence from hibernaculum, (b) conception, i.e. the initiation of gestation and (c) parturition (25 days after conception).
4. Discussion
To investigate the impact that changes in patterns of environmental seasonality may have on arctic mammals, we compared the phenologies of two populations of AGS living at sites with different snow cover regimes. Compared with Atigun River, Toolik Lake became 100 per cent snow covered approximately 17 days earlier in autumn and was not 100 per cent snow free until approximately 27 days later in spring. Because of the earlier spring at Atigun, we predicted that AGS living there would show earlier seasonal hibernation and breeding events thus allowing their offspring a greater amount of time to grow and fatten in preparation for hibernation. We found that although the general sequential patterns of hibernation and reproduction were similar in AGS populations at Atigun and Toolik, the annual timing of these events significantly differed between the two populations (figures 1 and 2). Evidence indicates that the Northern Hemisphere is warming [24] and, associated with these changes, patterns of environmental seasonality are being altered, with spring-like conditions occurring earlier and autumn-like conditions being prolonged [25]. Our observation of phenological variation between nearby populations of ground squirrels demonstrates a physiological flexibility in individuals that allows them to respond to differences in local conditions. This flexibility could enable populations to match timing of critical events in their annual cycle to rapid changes in climate and sustained trends in environmental conditions and patterns.
The seasonal phenology of male AGS was not extended forward as predicted (figure 1). At Atigun River, adult males entered hibernation later than adult males at Toolik Lake. We attribute this difference in timing to differences in food availability and effects on foraging and caching behaviour. Because snow cover in autumn occurs later at Atigun River, males have a prolonged opportunity to cache food but may also need to stay above-ground longer to defend these caches from other males. Indeed, agonistic interactions among AGS males attain frequencies and intensities in autumn that are as great as those during the breeding season in spring [26]. At Toolik Lake, the cost of remaining on the surface to increase cache size likely begins to outweigh the benefits when snow covers the ground and the access to food becomes limited.
Adult and yearling males that became reproductively active ended heterothermy and resumed euthermy approximately 15–25 d before emergence from hibernation. A prolonged euthermic interval in spring prior to emergence is necessary for gonadal growth and maturation; in terms of energy demands, this period is probably supported by reserves of body fat and food that was cached the previous autumn [27–29]. Although there was no difference between the sites in the date that males ended heterothermy, those at Atigun River emerged from their hibernacula on average 9 days earlier than those at Toolik Lake (figure 1). Several studies have shown that emergence of hibernating ground squirrels is generally correlated with local factors, such as snow cover, snow depth, air temperature and soil temperature [30–33], although Buck & Barnes [19] found that timing of emergence of males at a single site did not vary between two successive years despite spring air temperatures that differed by 20°C and snow depth that differed by 50 per cent. We suggest that, on an ultimate level, the earlier emergence of males at Atigun is timed to match the earlier emergence of females at this site. However, on a proximate level, we are uncertain of the cues used by AGS to time their emergence from hibernation. It is possible that males assess local differences in snow cover in spring, but their patterns of Tb change after ending heterothermy suggest that they remain sequestered within their hibernacula until first emergence and the onset of continuing above-ground activity [21]. Moreover, we detected no differences in soil temperature between Atigun and Toolik that may be used by males to cue differences in the timing of their emergence.
Unlike male AGS, adult and yearling females at Atigun River began hibernation and reproduction 1–2 weeks earlier than females at Toolik Lake, as we predicted (figures 1 and 3). However, the duration of both hibernation and reproduction did not differ between the two sites. We suggest that the earlier phenological sequence at Atigun River is driven by an attempt to match production and subsequent growth of young to the timing of optimal food availability [34].
Variation in the timing of hibernation and reproduction has been previously demonstrated in hibernating golden-mantled ground squirrels (Callospermophilus lateralis) over short distances associated with altitude [31] or nearby sites that experience differences in amount and timing of snowfall [33]. As with our contrasting patterns of timing at Toolik Lake and Atigun River, these are powerful demonstrations of the role of physiology and phenotypic plasticity in providing local populations with the ability to adjust their seasonal hibernation and breeding phenology to match optimal conditions of the entire annual environmental cycle.
The earlier breeding of AGS at Atigun River is not temporally matched to the earlier snow-free day, i.e. the duration from the first snow-free day and AGS emergence is greater at Atigun than at Toolik. This observation is similar to reports for other species that are beginning to show earlier migratory or breeding events in response to climatic shifts [6,11]. For example, from 2002 to 2006 the timing of caribou calving has advanced 1.3 days while the onset of the plant-growing season has advanced 14.8 d [7]. Pied flycatchers (Ficedula hypoleuca) have advanced their egg-laying date over the past 20 years; however, this temporal shift has been insufficient to match environmental changes over this time [35]. Furthermore, many species that have adjusted seasonal timing to correspond with shifts in environmental conditions have only advanced some parts or a single stage in the overall sequence of events. Pied flycatchers have not advanced their migratory arrival date on breeding grounds [35]. In the plant, alpine chickweed (Cerastium alpinum), the timing of emergence was not different between warmed and control plots; however, the duration of the emergence stage was significantly shortened in warmed plots and thus flower set occurred earlier [8].
Our results and the cited examples demonstrate the critical value of examining all major stages of the natural temporal sequence of phenological events in both males and females in relation to spatial variation in environmental conditions. Species may not only become mismatched against other species in their ecological community, but they may also show differential responses to environmental change between the sexes. Assuming that a changing environmental regime will impact only a single phenological event in a single sex may fail to accurately predict how climate change could affect a species. While shifts in the timing of major seasonal events, such as migration, hibernation, breeding and postnatal growth may be largely driven by attempts to match offspring production with optimal food availability, the aggregate of all seasonal events must be considered. The ability of an organism to adjust the timing and duration of various stages in its natural phenological sequence may ultimately predict the resilience of a species to environmental change.
We have demonstrated that phenotypic flexibility can be manifest over a very short amount of ecological space, as evidenced by the differences in annual timing of hibernation and reproduction in AGSs living in two nearby populations. The patterns we have documented and compared offer a useful indication of the power of phenotypic flexibility to provide animals with the ability to respond, via physiological mechanisms, to environmental changes that might occur over the course of time.
Acknowledgements
Animal protocols were approved by the University of Alaska Fairbanks Institutional Animal Care and Use Committee and DOD Animal Care and Use Review.
Funding was provided by the National Science Foundation (EF-0732763 and -0732755) to C.L.B and B.M.B. and US Army Medical Research and Materiel Command grant no. 05178001 to B.M.B.
References
- 1.Hughes L. 2000. Biological consequences of global warming: is the signal already apparent? Trends Ecol. Evol. 15, 56–61 10.1016/S0169-5347(99)01764-4 (doi:10.1016/S0169-5347(99)01764-4) [DOI] [PubMed] [Google Scholar]
- 2.Inouye D. W., Barr B., Armitage K. B., Inouye B. D. 2000. Climate change is affecting altitudinal migrants and hibernating species. Proc. Natl Acad. Sci. USA 97, 1630–1633 10.1073/pnas.97.4.1630 (doi:10.1073/pnas.97.4.1630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-M., Høgh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 4.Parmesan C., Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 10.1038/nature01286 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 5.Root T. L., Price J. L., Hall K. R., Schneider S. H., Rosenzweig C., Pounds A. J. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 10.1038/nature01333 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 6.Dunn P. 2004. Breeding dates and reproductive performance. In Effects of climatic change on birds (eds Møller A. P., Fiedler W., Berthold P.), pp. 69–87 Amsterdam, The Netherlands: Elselvier [Google Scholar]
- 7.Post E., Forchhammer M. C. 2008. Climate change reduces reproductive success of an Arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375 10.1098/rstb.2007.2207 (doi:10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Post E. C., Pedersen C., Wilmers C. C., Forchhammer M. C. 2008. Phenological sequences reveal aggregate life history response to climatic warming. Ecology 89, 363–370 10.1890/06-2138.1 (doi:10.1890/06-2138.1) [DOI] [PubMed] [Google Scholar]
- 9.Winkler D. W., Dunn P. O., McCulloch C. E. 2002. Predicting the effects of climate change on avian life-history traits. Proc. Natl Acad. Sci. USA 99, 13 595–13 599 10.1073/pnas.212251999 (doi:10.1073/pnas.212251999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Both C., Visser M. E. 2005. The effect of climate change on the correlation between avian life-history traits. Glob. Change Biol. 11, 1606–1613 10.1111/j.1365-2486.2005.01038.x (doi:10.1111/j.1365-2486.2005.01038.x) [DOI] [Google Scholar]
- 11.Møller A. P., Flensted-Jensen E., Klarborg K., Mardal W., Nielsen J. T. 2010. Climate change affects the duration of reproductive season in birds. J. Anim. Ecol. 79, 777–784 [DOI] [PubMed] [Google Scholar]
- 12.Cresswell W., McCleery R. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 72, 356–366 10.1046/j.1365-2656.2003.00701.x (doi:10.1046/j.1365-2656.2003.00701.x) [DOI] [Google Scholar]
- 13.Visser M. E., Both C. 2005. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 10.1098/rspb.2005.3356 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helgen K. M., Cole F. R., Helgen L. E., Wilson D. E. 2009. Generic revision in the holarctic ground squirrel genus Spermophilus. J. Mammal. 90, 270–305 10.1644/07-MAMM-A-309.1 (doi:10.1644/07-MAMM-A-309.1) [DOI] [Google Scholar]
- 15.MacDonald S. O., Cook J. A. 2009. Recent mammals of Alaska. Fairbanks, AK: University of Alaska Press [Google Scholar]
- 16.McLean I. G., Towns A. J. 1981. Differences in weight changes and the annual cycle of male and female arctic ground squirrels. Arctic 34, 249–254 [Google Scholar]
- 17.Buck C. L., Barnes B. M. 1999. Annual cycle of body composition and hibernation in free-living arctic ground squirrels. J. Mammal. 80, 430–442 10.2307/1383291 (doi:10.2307/1383291) [DOI] [Google Scholar]
- 18.Shaver G. R., Chapin F., III, Gartner B. L. 1986. Factors limiting seasonal growth and peak biomass accumulation in Eriophorum vaginatum in Alaska tussock tundra. J. Ecol. 74, 257–278 10.2307/2260362 (doi:10.2307/2260362) [DOI] [Google Scholar]
- 19.Buck C. L., Barnes B. M. 1999. Temperatures of hibernacula and changes in body composition of arctic ground squirrels over winter. J. Mammal. 80, 1264–1276 10.2307/1383177 (doi:10.2307/1383177) [DOI] [Google Scholar]
- 20.Long R. A., Hut R. A., Barnes B. M. 2007. Simultaneous collection of body temperature and activity data in burrowing mammals: a new technique. J. Wildl. Manage. 71, 1375–1379 10.2193/2006-399 (doi:10.2193/2006-399) [DOI] [Google Scholar]
- 21.Buck C. L., Breton A., Kohl F., Toien O., Barnes B. M. 2008. Overwinter body temperature patterns in free-living Arctic squirrels (Spermophilus parryii). In Proc. 13th Int. Hibernation Symp. of Hypometabolism in Animals: Hibernation, Torpor, and Cryobiology (eds Lovegrove B. G., McKechnie A. E.), pp. 317–326 Pietermaritzburg: University of KwaZulu-Natal [Google Scholar]
- 22.Lacey E. A. 1991. Reproductive and dispersal strategies of male Artic ground squirrels (Spermophilus parryii plesius). PhD thesis, University of Michigan, Ann Arbor, Michigan [Google Scholar]
- 23.Quinn G. P., Keough M. J. 2003. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge University Press [Google Scholar]
- 24.Easterling D. R., Meehl G. A., Parmesan C., Changnon S. A., Karl T. R., Mearns L. O. 2000. Climate extremes: observations, modeling, and impacts. Science 289, 2068–2074 10.1126/science.289.5487.2068 (doi:10.1126/science.289.5487.2068) [DOI] [PubMed] [Google Scholar]
- 25.Menzel A., Fabina P. 1999. Growing season extended in Europe. Nature 397, 659. 10.1038/17709 (doi:10.1038/17709) [DOI] [Google Scholar]
- 26.Buck C. L., Barnes B. M. 2003. Androgen in free-living arctic ground squirrels: seasonal changes and influence of staged male–male aggressive encounters. Horm. Behav. 43, 318–326 10.1016/S0018-506X(02)00050-8 (doi:10.1016/S0018-506X(02)00050-8) [DOI] [PubMed] [Google Scholar]
- 27.Barnes B. M. 1984. Influence of energy stores on activation of reproductive function in male golden-mantled ground squirrels. J. Comp. Physiol. B 154, 421–425 10.1007/BF00684449 (doi:10.1007/BF00684449) [DOI] [Google Scholar]
- 28.Barnes B. M. 1996. Relationships between hibernation and reproduction in male ground squirrels. In Adaptations to the cold: 10th Int. Hibernation Symp. (eds Geiser F., Hulbert A. J., Nicol S. C.), pp. 71–80 Armidale: University of New England Press [Google Scholar]
- 29.Barnes B. M., Licht P., Zucker I. 1987. Temperature dependence of in vitro androgen production in testes from hibernating ground squirrels, Spermophilus lateralis. Can. J. Zool. 65, 3020–3023 10.1139/z87-457 (doi:10.1139/z87-457) [DOI] [Google Scholar]
- 30.Michener G. R. 1978. Effect of age and parity on weight gain and entry into hibernation in Richardson's ground squirrels. Can. J. Zool. 56, 2573–2577 10.1139/z78-345 (doi:10.1139/z78-345) [DOI] [PubMed] [Google Scholar]
- 31.Bronson M. T. 1979. Altitudinal variation in the life history of the golden-mantled ground squirrel (Spermophilus lateralis). Ecology 60, 272–279 10.2307/1937655 (doi:10.2307/1937655) [DOI] [Google Scholar]
- 32.Murie J. O., Harris M. A. 1982. Annual variation of spring emergence and breeding in Columbian ground squirrels (Spermophilus columbianus). J. Mammal. 63, 431–439 10.2307/1380440 (doi:10.2307/1380440) [DOI] [Google Scholar]
- 33.Phillips J. A. 1984. Environmental influences on reproduction in the golden-mantled ground squirrel. In The biology of ground dwelling squirrels: annual cycles, behavioral ecology, and sociality (eds Murie J. O., Michener G. R.), pp. 81–107 Lincoln: University of Nebraska Press [Google Scholar]
- 34.Stenseth N. C., Mysterud A. 2002. Climate, changing phenology, and other life history traits: nonlinearity and match–mismatch to the environment. Proc. Natl Acad. Sci. USA 99, 13 379–13 381 10.1073/pnas.212519399 (doi:10.1073/pnas.212519399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Both C., Visser M. E. 2001. Adjustment of climate change is constrained by arrival date in a long-distance migrant bird. Nature 411, 296–298 10.1038/35077063 (doi:10.1038/35077063) [DOI] [PubMed] [Google Scholar]