Abstract
The number of current and future vaccines for adults has been steadily increasing. Yet, vaccine coverage rates for adult vaccinations have historically been low, and less is known about how adults in the mid-adult age range make vaccine decisions for themselves. The purpose of this study was to assess which vaccine characteristics affect vaccine decision-making among mid-adult women. Adult women, aged 27–55 (n=258) rated 9 hypothetical vaccine scenarios, each of which was defined along 4 dimensions: mode of transmission (STI or non-STI), severity of infection (curable, chronic, or fatal), vaccine efficacy (50%, 70%, or 90%), and availability of behavioral methods for prevention (available or not available). Ratings ranged from 0 to 100. Conjoint analysis was used to assess the effect of relative preferences for the vaccine scenario characteristics on participant ratings of scenarios. The mean vaccine scenario rating was 78.2. Nearly half (40%, n =104) of participants rated all nine scenarios the same, with the majority of those (84%) holding strongly positive views. Conjoint analysis of the other 154 participants who discriminated between scenarios indicated that the main drivers for vaccine acceptability were severity of the disease and the efficacy of the vaccine to prevent the disease. Mode of transmission and availability of a preventative behavioral measure did not play a significant role. Future studies should further assess how women's understanding of severity of the disease and efficacy of the vaccine to prevent disease may be useful for increasing vaccine acceptability.
Keywords: vaccination, immunization, adult, attitudes
Introduction
The number of current and future vaccines for adults has been steadily increasing. In some cases, this is due to extension of recommendations to the general adult population of existing vaccines licensed for that age group such as the influenza vaccine.1 In other cases, such as for the human papillomavirus (HPV) vaccine, manufacturers are seeking extension of licensure into the mid-adult age range, up to 45–55 years.2 Still more new vaccines are in development for adults, particularly against sexually transmitted infections (STI) such as human immunodeficiency virus, Chlamydia trachomatis, and Neisseria gonorrhoeae.3 Although vaccine coverage rates for childhood vaccinations are, in general, high,4 in large part due to school requirements, and coverage rates for adolescent vaccinations are improving,5 coverage rates for adult vaccinations have historically been lower. For example, only 63.1% of adults 19-49 years old are up to date for tetanus,6 compared to 83.9% of 19-35 month olds.4 Likewise, HPV vaccination coverage (≥1 dose) for adults for whom it is licensed (19-26 year olds) is 17.1% compared to 44.3% of adolescents 13- 17 years old.5, 6
A key to improving vaccination rates among adults and to increasing uptake of future vaccines is to understand factors that motivate adults in the mid-adult range to seek vaccination.7 There are many studies focusing on vaccine choices that adult women make for their children, and some assessing their interest in specific vaccines for themselves.8-11 Yet, little is known about the relative importance women place on different vaccine associated factors when making vaccine decisions for themselves. Using hypothetical vaccine scenarios is a useful method for identifying such factors since there are few current vaccines utilized for this population. Therefore, the goal of this study was to understand what vaccine characteristics affect vaccine decision-making among adult women, using hypothetical vaccine scenarios and conjoint analysis.
Materials and Methods
Mothers accompanying their adolescents to a medical appointment from 2002-2004 at participating urban adolescent health clinics and pediatric private practices located in Indiana were interviewed about their attitudes regarding vaccination of their child and themselves.12 Adolescents were sons or daughters aged 12 to 17. Findings regarding participants' beliefs about adolescent vaccination have previously been reported.12-14
Research assistants approached women in the waiting room, and nearly two thirds (62.8%) of those eligible who were approached provided written consent to participate in the study. The majority of those who declined did so because of lack of time to complete the study. For the purposes of the present study, only mothers 27-55 years old were included, and we evaluated the data regarding their views of vaccination for themselves. This age range was selected because the makers of the quadrivalent and bivalent HPV vaccines are seeking extension of licensure to women 27-45 years old and 27-55 years old, respectively. Of the original 299 parents interviewed who answered questions regarding self-vaccination, 23 were excluded from this analysis since they were male, 5 since they were older than 55 years and 13 since they were younger than 27 years old. After obtaining written informed consent, anonymous, audio, computer-assisted, self-administered interviews (ACASI) were completed. Surveys were conducted in a private room using a notebook computer with a touch sensitive screen. Women received $15 compensation for the time and effort required to complete the survey. The study was approved by the Indiana University Institutional Review Board.
Survey Instrument
A purpose-built survey was developed based on prior vaccine research, and formative semi-structured interviews.15 In addition, the survey was pretested in the study population and the feedback incorporated into the final study instrument. Parents were given nine hypothetical vaccine scenarios. Each of these scenarios was uniquely defined along four dimensions. The first was mode of transmission of infection (sexually transmitted or not). Participants were told that “this vaccine keeps people from getting a disease that can be sexually transmitted” or “this vaccine keeps people from getting a disease that cannot be sexually transmitted”. The second focused on vaccine efficacy (50%, 70% or 90%); for example, participants were told that “the vaccine works for 9 out of 10 people who get it”. The third defined the severity of the infection (curable, chronic or fatal). Participants were told either (a) “the disease can be cured with antibiotics”; (b) “the disease cannot be cured, but people don't die from it”; or (c) “people die from this disease in most cases”. The fourth included whether a behavioral strategy was available that could prevent the infection (hand washing for non-STI and condom use for STI; see Table 1). Examples are: “using condoms will keep a person from getting the disease” or “washing hands several times a day will not keep a person from getting the disease”. After each scenario was presented, the participant was asked: “If this vaccine was available today, and you had time, would you get vaccinated?”
Table 1. Vaccine scenarios presented to participants.
| MODE OF TRANSMISSION | VACCINE EFFICACY | SEVERITY OF INFECTION | BEHAVIORAL PREVENTION | MEAN SCORE |
|---|---|---|---|---|
| Non-STI | 90% | Fatal | No | 85.7 |
| STI | 70% | Fatal | Yes | 81.4 |
| STI | 50% | Fatal | Yes | 79.1 |
| STI | 90% | Chronic | Yes | 78.4 |
| STI | 70% | Chronic | No | 78.3 |
| STI | 90% | Curable | Yes | 77.8 |
| Non-STI | 70% | Curable | Yes | 75.3 |
| STI | 50% | Curable | No | 74.4 |
| Non-STI | 50% | Chronic | Yes | 73.6 |
NOTE: STI: sexually transmitted infection
Participants rated each scenario was rated on an 11-point scale in intervals of 10 points (0 – 100), where 0 represented that they would never get the vaccine, and 100 signified that they would definitely get the vaccine. A full factorial design would have required the presenting of 36 combinations of scenarios, which would have represented an unreasonable response burden. Therefore, we instead used a fractional factorial design with a representative subset of 9 scenarios, which allowed us to examine the main effect of each of the four dimensions, but prevented us from evaluating interaction effects. The nine scenarios were selected using the conjoint analysis procedure available in SPSS Conjoint 8.0 SPSS Inc, Chicago, Ill). The vaccine scenarios were presented in a random order across participants to eliminate bias due to ordering effects. Sociodemographic information was also collected including participant age, race/ethnicity and educational level.
Analysis
We used SPSS 17.0 (SPSS Inc, Chicago, Ill) to describe basic characteristics of the study population. We then used ratings-based conjoint analysis to examine how vaccine scenario characteristics influenced ratings of the scenarios for those participants who did not assign the same ratings to all scenarios. Ratings-based conjoint analysis is a methodological and statistical technique used to understand how product preferences are influenced by product attributes that it has been validated for the use in health related studies.16-18 Unlike a traditional survey, it allows respondents to consider attributes jointly, allowing them to make trade-offs. Conjoint analysis of the nine scenarios was used to reveal the relative preferences participants placed on each of the characteristics within each dimension. These relative preferences are called part-worth utilities. The stronger the preferences within a dimension, the wider the range of the part- worth utility. Within each dimension, the sum of the part-worth utilities must equal zero. For example, mode of transmission compares a vaccine that protects against an STI to a vaccine that does not protect against an infection that is sexually transmitted. If a woman consistently rated vaccines against STI more positively than non-STI vaccines, then her part-worth utility score would be highly positive for the STI attribute, and would be equally negative for non-STI vaccines; therefore the sum of the values would be zero. A negative part-worth utility does not necessarily imply opposition to a vaccine with that attribute (e.g., non-STI vaccine), it simply indicates a relative preference for the alternative attribute (e.g. STI vaccine).
In addition, we calculated the contribution of each dimension to the overall vaccine ratings using importance scores, defined as the relative ranges of the part-worth utilities across the 4 dimensions. The sum of importance scores across dimensions must equal 100.
Results
Subjects
A total of 258 women were included in this analysis, with a mean age of 39 ± 5.8. Approximately one third (38.7%) were Latina, 24.1% were non-Latina Black, and 35.6% were White, non-Latina. Most (67.8%) were married or living with a partner. A little over one third did not graduate from high school (36.0%), 20.2% solely finished high school and 43.8% had at least some college education.
Across all nine vaccine scenarios, the mean score was 78.2 (SD=24.1; median=85.6). The vaccine scenario with the highest rating was one that was for a non-STI, protected against a fatal disease, was 90% efficacious and for which there was not a preventative behavioral measure available. For that vaccine scenario, the mean score was 85.7 (SD=23.0; median= 100). The distribution of scores was quite skewed, with 61.9% of women giving this scenario a score of 100 and only 1.6% giving it a score of 0. The lowest rated vaccine scenario was also for a non-STI, but for a chronic infection for which the vaccine was only 50% efficacious and there was a preventative behavioral measure available. The mean score for this scenario was 73.6 (SD=28.5; Median=100). Although, less skewed than the highest rated scenario, 42.6% of women still gave a score of 100 and only 2.7% scored it 0.
Nearly half (40%, n =104) of participants rated all nine scenarios the same, with the majority of those (84%) holding strongly positive views (Table 2). Few women (3%) held strongly negative views. Ten women (9.6%) rated all vaccines at the midpoint range. The participants who rated all vaccines the same were more likely to lack a high school education (44.2%) compared to those who did discriminate between scenarios (30.5%; p < .05). They were also more likely to be Latino (50.0% vs. 31.1%; p < .01). After excluding those participants who rated all the vaccines the same, the order of vaccine scenarios by mean score was exactly the same.
Table 2. Number of and ratings by participants who rated all vaccine scenarios with the exact same rating.
| RATING | NUMBER OF PARTICIPANTS N= 104 |
|---|---|
| 100 | 81 (77.9%) |
| 90 | 4 (3.8%) |
| 80 | 2 (1.9%) |
| 60 | 2 (1.9%) |
| 50 | 10 (9.6%) |
| 40 | 2 (1.9%) |
| 0 | 3 (2.9%) |
For the 154 participants that did not rate all vaccine scenarios the same, the most important decisional factors were the severity of the infection that the vaccine prevented and the efficacy of the vaccine (Figure 1). The range between the part-worth utilities for severity of infection illustrated the importance of that factor. In addition, participants showed greatest preference for vaccines that were 90% efficacious, and least preference for those with 50% efficacy. The part-worth utilities for mode of transmission indicated that whether the vaccine protected against an STI or not, had almost no influence on vaccine decisions. Finally, the availability of preventive behavioral measure had only a modest effect on attitudes about vaccination.
Figure 1. Conjoint analysis assessing the effect of relative preferences (part-worth utilities) for different vaccine scenario characteristics on parental ratings of scenarios.
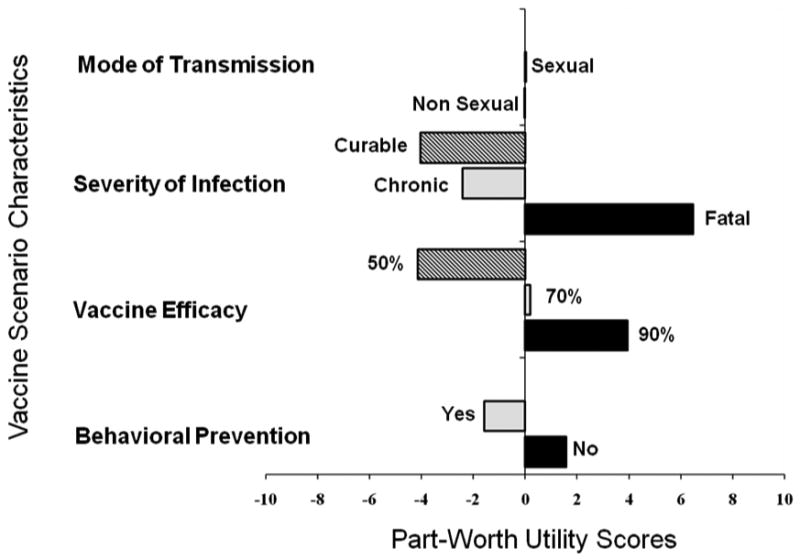
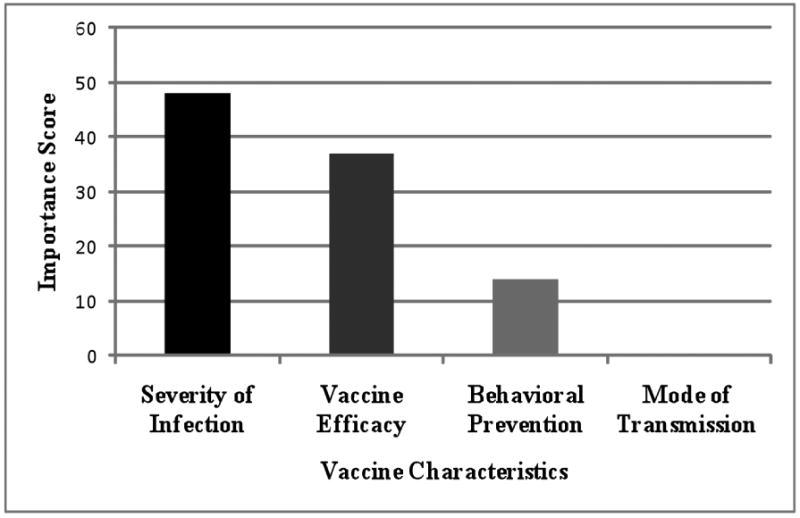
Severity of infection was ranked as the most important factor with a score of 48, followed by vaccine efficacy at 37, presence of a behavioral preventive measure had an importance score of 14, and mode of transmission was 0 (Figure 2).
Figure 2. Contribution of each vaccine scenario characteristic to overall vaccine ratings using importance scores.
Discussion
In this study, we found that participants were for the most part positive about vaccination for themselves. Even the vaccine scenario with the lowest rating had a mean rating of 73 out of 100. A strong minority of participants had categorical vaccine attitudes, and rated all vaccines exactly the same i.e. 40% of the population did not discriminate at all based on vaccine scenario specification, particularly with categorically positive attitudes. This finding of overall positive vaccination views as well as non-discrimination, mostly positive, is in itself important in illustrating that women in this study were interested in vaccines targeted for their age group. Additionally, mid-adult women seemed most interested in a vaccine that prevents a serious infection and that is efficacious. Future studies could assess whether vaccine education targeting severity of infection and vaccine efficacy, along with improving general vaccine attitudes, would be helpful in this population. Health education that focuses on how the disease that the vaccine prevents is transmitted may be less fruitful. Of note, while women in this study were generally interested in vaccines for themselves, other barriers to adult vaccination exist which could affect effective delivery.19
Although many participants had categorical vaccine attitudes, the majority did discriminate between the vaccine scenarios, and these differences may be used to identify targets for interventions. According to the Health Belief Model (HBM), a person would seek vaccination if that person feels that the infection that the vaccine protects against can indeed be avoided, has a positive expectation that the vaccine will be efficacious in preventing the infection; and that they can successfully be vaccinated.20 The model includes perceived susceptibility, perceived severity, perceived benefits, and perceived barriers. The main drivers for vaccine acceptability in this study were severity of the infection and the efficacy of the vaccine to prevent the disease (perceived benefit). Not surprisingly if the disease was fatal rather than curable, vaccine ratings were higher, which confirms previous findings in other studies that mid-adult women who understand the connection between cervical cancer and HPV are more accepting of the HPV vaccine.11 This information may be important for understanding how best to increase adults' acceptance of vaccines for conditions they consider mild, such as influenza but which can, in reality, have significant morbidity and mortality. For example, there is a link between influenza vaccination during times of increased media reports of influenza deaths.21, 22
Other studies have also highlighted efficacy as an important factor in women's vaccination decisions for their daughters.23 Understanding efficacy involves comfort with percentages and ratios. Innumeracy, defined as having limited knowledge of mathematics, is a problem in the United States.24 Therefore, those who wish to use vaccine efficacy as a motivating force for vaccine education should make sure to explain the concept in easy to understand terms or use visual aids to assist in communication.25 Interestingly, there was a vaccine with a 50% efficacy that received a higher mean score than vaccines with 90% efficacy, indicating that efficacy was not of paramount importance.
Mode of transmission did not affect vaccine decision making either negatively or positively. In addition, the possibility of preventing the disease in another behavioral way was not a major driver of acceptability; this may be affected by participants' attitudes towards the effectiveness or lack thereof of preventive measures. Mode of transmission and presence of other preventive measures may function as proxies for the health belief model's construct of sense of perceived susceptibility, suggesting a less important role for this construct in vaccination among these participants. Other studies have found that women with a history of an STI are more likely to accept HPV vaccination, suggesting the potential importance of more personal experience of susceptibility.26 The findings in this study may have been affected by the relationship status of the participants; if participants were in a monogamous relationship they may have felt less personally susceptible.27 Interestingly, because mode of transmission was not important, uptake of current vaccines including Tdap and influenza or other new future vaccines, that are not sexually transmitted, may perhaps be affected by similar vaccine decision making processes as found in this study.
The positive general attitudes regarding vaccination and the drivers of vaccination were similar in both this study and two others analyzing the larger data set of parental attitudes about childhood vaccination in parents who accompanied their children to appointments at the study sites.12, 14 In all studies, the main drivers were vaccine efficacy and severity of infection, while the mode of transmission being sexual or not had little impact, and the presence of a preventative behavioral measure only had a small impact. Interestingly, while severity of the infection was most important for how parents ranked vaccines for themselves followed by efficacy, the order was switched in how parents ranked vaccines for their child with efficacy being the most important; for the study focusing on Latino parents, efficacy and severity were of equal importance. These comparative findings suggest that parents, at least in this study, have, for the most part, similar vaccine decision-making processes for themselves as for their children, but that efficacy and severity have varying importance.
Other studies have explicitly studied vaccine intentions of a parent for their child compared to themselves. Kahn et al found that while up to 86% would vaccinate their older daughter against HPV, only 48% would get the vaccine themselves even if recommended.23 In another study, women who felt it was important for their children to get the HPV vaccine, or were at least neutral about it, were more likely to want the vaccine for themselves compared to those who did not feel it was important for their child.11
There were several limitations to the study. This was a convenience sample of parents visiting a health care site with their adolescent. Therefore, they may be more familiar with vaccine decision-making, having done so vis-à-vis their child compared to non-parent adults or parents who do not accompany their adolescents to health care. In addition, over half of the sample self-identified as a member of racial/ethnic minority groups and half had a high school education or less, therefore the findings may not be generalizable to other groups. Yet, this sample may be reflective of populations at higher risk for vaccine preventable diseases such as HPV 28, 29 and cervical cancer, 30, 31 as well as non-STI such as 2009 H1N1 influenza32 and pertussis,33 and therefore are an important population to study. Second, the use of a fractional factorial design reduces the burden of rating on the subject such that participants can rate 9 instead of 36 scenarios. The SPSS conjoint analysis procedure that generates the fractional factorial design creates an orthogonal set of scenarios such that there is no confounding of interaction effects with main effects. This design, however, limits the analysis to the main effects of each dimension and precludes studying potential interaction effects. Furthermore, by its nature, we could only include in the conjoint analysis participants who discriminated among vaccine scenarios for the dimensions that were included. It is possible that if other vaccine characteristics, such as cost or provider recommendation, had been included, that women may have rated the vaccine scenarios differently. Future studies could assess the impact of other vaccine parameters such as cost on vaccine decision-making. We were unable to assess precisely why certain participants did not discriminate among scenarios, but those who did not were more likely to be Latino and/or lack a high school education. Other studies have shown that mothers lacking a high school education were more accepting of at least HPV vaccination for their daughters,10 and that Latinos were particularly more accepting of HPV vaccination for themselves.34 Although not all parents discriminated, the study sample was adequate for a conjoint analysis.35 These data were collected in 2002-2004, which was pre-licensure of HPV vaccines. Experiences with HPV vaccine as well as media reports could have affected scenario ratings if the study had taken place post-licensure. Finally, this study focused on hypothetical vaccine decisions that may differ from actual vaccine decision-making. For instance, although pre-licensure studies of HPV vaccine attitudes showed strong support among parents for adolescent vaccination (typically around 70%-80% endorsement), subsequent post-licensure acceptance rates in the U.S. have remained relatively modest, at around 44% for one or more doses.5, 36-38 Although conjoint analysis does rely on hypothetical scenarios, it has been validated for the use in health related studies.16-18
Conclusion
In summary, participants in this study were most interested in a potential vaccine that prevents a serious infection and that is efficacious. Sexual transmissibility of infection and presence of a preventative behavioral measure did not influence vaccine attitudes. When future vaccines for adult women are available, targeting women's understanding of severity of the disease and efficacy of the vaccine to prevent disease may be useful for increasing acceptability.
Acknowledgments
This study was supported by grant U19 AI31494 from the National Institute of Allergy and Infectious Diseases, Bethesda, MD (Zimet), and also by an investigator initiated grant from Merck (Zimet/Rosenthal).
Abbreviations
- HPV
Human Papillomavirus
- STI
Sexually Transmitted Infections
- ACASI
Audio-Computer Assisted Self-Interview
Footnotes
Disclosure: Drs. Zimet and Rosenthal are investigators on investigator-initiated research grants related to HPV vaccination, funded by Merck, Inc. Drs. Rosenthal and Mays have served on a Merck and GlaxoSmithKline advisory board, respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010 Aug 6;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.Castellsague X, Schneider A, Kaufmann AM, Bosch FX. HPV vaccination against cervical cancer in women above 25 years of age: key considerations and current perspectives. Gynecol Oncol. 2009 Dec;115(3 Suppl):S15–23. doi: 10.1016/j.ygyno.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 3.Stanberry LR, Bernstein DI. Sexually Transmitted Diseases: Vaccines, Prevention and Control. San Diego, Calif: Academic Press; 2000. [Google Scholar]
- 4.National, state, and local area vaccination coverage among children aged 19-35 months --- United States. MMWR Morb Mortal Wkly Rep. 2009 Sep 17;59(36):1171–1177. [PubMed] [Google Scholar]
- 5.National, State, and Local Area Vaccination Coverage among Adolescents Aged 13--17 Years --- United States. MMWR Morb Mortal Wkly Rep. 2009 Aug 20;59(32):1018–1023. [PubMed] [Google Scholar]
- 6.2009 Adult Vaccination Coverage, The National Health Interview Survey (NHIS) available at http://www.cdc.gov/vaccines/stats-surv/nhis/2009-nhis.htm.
- 7.Armitage CJ, Conner M. Efficacy of the Theory of Planned Behaviour: a meta-analytic review. Br J Soc Psychol. 2001 Dec;40(Pt 4):471–499. doi: 10.1348/014466601164939. [DOI] [PubMed] [Google Scholar]
- 8.Yeganeh N, Curtis D, Kuo A. Factors influencing HPV vaccination status in a Latino population; and parental attitudes towards vaccine mandates. Vaccine. Jun 7;28(25):4186–4191. doi: 10.1016/j.vaccine.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Black LL, Zimet GD, Short MB, Sturm L, Rosenthal SL. Literature review of human papillomavirus vaccine acceptability among women over 26 years. Vaccine. 2009 Mar 10;27(11):1668–1673. doi: 10.1016/j.vaccine.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal SL, Rupp R, Zimet GD, et al. Uptake of HPV vaccine: demographics, sexual history and values, parenting style, and vaccine attitudes. J Adolesc Health. 2008 Sep;43(3):239–245. doi: 10.1016/j.jadohealth.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Ferris DG, Waller JL, Owen A, Smith J. HPV vaccine acceptance among mid-adult women. J Am Board Fam Med. 2008 Jan-Feb;21(1):31–37. doi: 10.3122/jabfm.2008.01.070103. [DOI] [PubMed] [Google Scholar]
- 12.Zimet GD, Mays RM, Sturm LA, Ravert AA, Perkins SM, Juliar BE. Parental attitudes about sexually transmitted infection vaccination for their adolescent children. Arch Pediatr Adolesc Med. 2005 Feb;159(2):132–137. doi: 10.1001/archpedi.159.2.132. [DOI] [PubMed] [Google Scholar]
- 13.Zimet GD, Perkins SM, Sturm LA, Bair RM, Juliar BE, Mays RM. Predictors of STI vaccine acceptability among parents and their adolescent children. J Adolesc Health. 2005 Sep;37(3):179–186. doi: 10.1016/j.jadohealth.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Bair RM, Mays RM, Sturm LA, Perkins SM, Juliar BE, Zimet GD. Acceptability to Latino parents of sexually transmitted infection vaccination. Ambul Pediatr. 2008 Mar-Apr;8(2):98–103. doi: 10.1016/j.ambp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Mays RM, Sturm LA, Zimet GD. Parental perspectives on vaccinating children against sexually transmitted infections. Soc Sci Med. 2004 Apr;58(7):1405–1413. doi: 10.1016/S0277-9536(03)00335-6. [DOI] [PubMed] [Google Scholar]
- 16.Ryan M, Farrar S. Using conjoint analysis to elicit preferences for health care. BMJ. 2000 Jun 3;320(7248):1530–1533. doi: 10.1136/bmj.320.7248.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan M, McIntosh E, Shackley P. Methodological issues in the application of conjoint analysis in health care. Health Econ. 1998 Jun;7(4):373–378. doi: 10.1002/(sici)1099-1050(199806)7:4<373::aid-hec348>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Bouma BJ, van der Meulen JH, van den Brink RB, et al. Validity of conjoint analysis to study clinical decision making in elderly patients with aortic stenosis. J Clin Epidemiol. 2004 Aug;57(8):815–823. doi: 10.1016/j.jclinepi.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011 Feb 17;29(9):1850–1854. doi: 10.1016/j.vaccine.2010.12.097. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock I. Historical origins of the health belief model. Health Educ Monogr. 1974;2:332. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- 21.Yoo BK, Holland ML, Bhattacharya J, Phelps CE, Szilagyi PG. Effects of mass media coverage on timing and annual receipt of influenza vaccination among Medicare elderly. Health Serv Res. Oct;45(5 Pt 1):1287–1309. doi: 10.1111/j.1475-6773.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kempe A, Daley MF, Barrow J, et al. Implementation of universal influenza immunization recommendations for healthy young children: results of a randomized, controlled trial with registry-based recall. Pediatrics. 2005 Jan;115(1):146–154. doi: 10.1542/peds.2004-1804. [DOI] [PubMed] [Google Scholar]
- 23.Kahn JA, Ding L, Huang B, Zimet GD, Rosenthal SL, Frazier AL. Mothers' intention for their daughters and themselves to receive the human papillomavirus vaccine: a national study of nurses. Pediatrics. 2009 Jun;123(6):1439–1445. doi: 10.1542/peds.2008-1536. [DOI] [PubMed] [Google Scholar]
- 24.Paulos JA. Innumeracy: Mathematical Illiteracy and its Consequences. 1990 [Google Scholar]
- 25.Cox DS, Cox AD, Sturm L, Zimet G. Behavioral interventions to increase HPV vaccination acceptability among mothers of young girls. Health Psychol. 2010 Jan;29(1):29–39. doi: 10.1037/a0016942. [DOI] [PubMed] [Google Scholar]
- 26.Chao C, Velicer C, Slezak JM, Jacobsen SJ. Correlates for human papillomavirus vaccination of adolescent girls and young women in a managed care organization. Am J Epidemiol. 2010 Feb 1;171(3):357–367. doi: 10.1093/aje/kwp365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimet GD, Weiss TW, Rosenthal SL, Good MB, Vichnin MD. Reasons for non-vaccination against HPV and future vaccination intentions among 19-26 year-old women. BMC Womens Health. 2010;10:27. doi: 10.1186/1472-6874-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shikary T, Bernstein DI, Jin Y, Zimet GD, Rosenthal SL, Kahn JA. Epidemiology and risk factors for human papillomavirus infection in a diverse sample of low-income young women. J Clin Virol. 2009 Oct;46(2):107–111. doi: 10.1016/j.jcv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahn JA, Lan D, Kahn RS. Sociodemographic factors associated with high-risk human papillomavirus infection. Obstet Gynecol. 2007 Jul;110(1):87–95. doi: 10.1097/01.AOG.0000266984.23445.9c. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2006 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2010. Available at: http://www.cdc.gov/uscs. [Google Scholar]
- 31.Krieger N, Quesenberry C, Jr, Peng T, et al. Social class, race/ethnicity, and incidence of breast, cervix, colon, lung, and prostate cancer among Asian, Black, Hispanic, and White residents of the San Francisco Bay Area, 1988-92 (United States) Cancer Causes Control. 1999 Dec;10(6):525–537. doi: 10.1023/a:1008950210967. [DOI] [PubMed] [Google Scholar]
- 32.Quinn SC, Kumar S, Freimuth VS, Musa D, Casteneda-Angarita N, Kidwell K. Racial Disparities in Exposure, Susceptibility, and Access to Health Care in the US H1N1 Influenza Pandemic. Am J Public Health. 2011 Feb;101(2):285–293. doi: 10.2105/AJPH.2009.188029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schafer S, Gillette H, Hedberg K, Cieslak P. A community-wide pertussis outbreak: an argument for universal booster vaccination. Arch Intern Med. 2006 Jun 26;166(12):1317–1321. doi: 10.1001/archinte.166.12.1317. [DOI] [PubMed] [Google Scholar]
- 34.Scarinci IC, Garces-Palacio IC, Partridge EE. An examination of acceptability of HPV vaccination among African American women and Latina immigrants. J Womens Health (Larchmt) 2007 Oct;16(8):1224–1233. doi: 10.1089/jwh.2006.0175. [DOI] [PubMed] [Google Scholar]
- 35.Orme B. Getting Started with Conjoint Analysis: Strategies for Project Design and Pricing Research. Second. Madison, Wis: Research Publishers LLC; 2010. [Google Scholar]
- 36.Ogilvie GS, Remple VP, Marra F, et al. Parental intention to have daughters receive the human papillomavirus vaccine. CMAJ. 2007 Dec 4;177(12):1506–1512. doi: 10.1503/cmaj.071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007 Feb;40(2):108–115. doi: 10.1016/j.jadohealth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Zimet GD. Improving adolescent health: focus on HPV vaccine acceptance. J Adolesc Health. 2005 Dec;37(6 Suppl):S17–23. doi: 10.1016/j.jadohealth.2005.09.010. [DOI] [PubMed] [Google Scholar]


