Abstract
The incidence of melanoma is rising in the United States, leading to an estimated 68,720 new diagnoses and 8,650 deaths. The natural history involves metastases to lymph nodes, lung, liver, brain, and often to other sites. Primary treatment for melanoma is surgical excision of the primary tumor and affected lymph nodes. The role of adjuvant or definitive radiation therapy in the treatment of melanoma remains controversial, as melanoma has traditionally been viewed as a prototypical radioresistant cancer. However, recent studies suggest that under certain clinical circumstances, there may be a significant role for radiation therapy in melanoma treatment. Stereotactic radiosurgery for brain metastases has shown effective local control. High dose per fraction radiation therapy has been associated with a lower rate of locoregional recurrence of sinonasal melanoma. Plaque brachytherapy has evolved into a promising alternative to enucleation at the expense of moderate reduction in visual acuity. Adjuvant radiation therapy following lymphadenectomy in node positive melanoma prevents local and regional recurrence. The newer clinical data along with emerging radiobiological data indicate that radiotherapy is likely to play a greater role in melanoma management, and should be considered as a treatment option.
Keywords: hypofractionation, brachytherapy, melanoma cell cycle, radiosurgery, biological agents
Introduction
In their classic 1946 text, McKee and Cipollaro1 wrote a disheartening chapter on melanoma. The authors opine that “in spite of occasionally good results, it is our opinion that irradiation alone by any technique should not be relied on for the cure of these lesions.” This reputation as one of the least radioresponsive and least radiocurable tumors has dogged melanoma since the early days of orthovoltage x-ray treatments for skin conditions. In the modern era, many investigators are questioning whether this reputation is still accurate. Although many published reports show poor melanoma radiation responsiveness, others do not2–6. In fact, in vitro data on melanoma cell lines are generally acknowledged to show high levels of damage repair at conventional fractionation doses and increased cell death with larger doses per fraction. A popular hypo-fractionated regimen involving 3 fractions of 7 Gy each delivered days 1, 7, and 21 was developed for postoperative patients in which most gross disease has been removed.2 Complete remission rates approached 40%, with greater than 90% local control at 4 years.2
Although the trends to utilize radiotherapy were slow to catch on, recent evaluations suggest that the reputation of malignant melanoma as a “radio-insensitive” disease may not be entirely justified. This review summarizes some of the clinical and radiobiological evidence on the role of radiotherapy in melanoma management and focuses on three relatively new areas of clinical growth: intracranial radiosurgery, retinal plaque brachytherapy, and lymphatic field radiotherapy for node-positive, surgically debulked cases. For some of these clinical situations, it appears that moderate dose radiotherapy may be considered a viable option to surgery (intra-cranial metastases, uveal melanoma, mucosal melanoma of the head and neck) and as an adjunct in others.
Epidemiology
In 2009, melanoma will be responsible for 68,720 new cases and 8,650 deaths.7 Melanoma is the 5th and 6th leading cause of cancer in men and women, respectively.7 The age-adjusted annual incidence rate is 19.6 per 100,000, with Caucasian men at highest risk. Melanoma also has the fastest growing incidence of any cancer among men and the second fastest growing incidence in women8. The lifetime risk for development of melanoma is 1 in 39 for men and 1 in 58 for women.7 The median age at diagnosis is 59 years, peaking during the 4th and 5th decades.
At diagnosis, 82–85% have localized disease, 10–13% have regional disease, and 2– 5% have non-regional metastatic disease9. The five year survival rates are 98% if localized, 62% if regional, and 15% if metastatic.8 The 5 yr overall survival rates have increased over the decades: 82% in 1975–77, 87% in 1984–86, and 92% in 2004.7 The median age at death is 68 years. The death rate for melanoma has decreased slightly for women in the past twenty years, but has continued to increase for men.7
The Cell Cycle Pathway and Melanoma Development
Melanomas result from neoplastic growth of melanocytes. Melanocytes originate from the neural crest cells and migrate to the basal layer of the skin’s epidermis and other mucosal surfaces. Accumulation of melanocytes within the basal layer forms a flat brown lesion with regular borders, called a benign nevus.10 Nevi are typically controlled by a pathway of senescence and remain benign. However, mutations which override the normal regulators of the cell cycle result in malignant transformation. Several aberrant gene products are implicated in the pathogenesis:10, 11 BRAF (enhances cell division); N-RAS (promotes cell proliferation); CDKN2A locus loss [encoding two proteins, p16INK4a and ARF (p14 in humans and p19 in mice), involved in cell cycle progression]; MITF over expression (promotes survival and inappropriate cell cycle progression); c-KIT (involved in invasion and metastases); SLUG (involved in metastases); EDNRB (involved in invasiveness); and loss of E-CADHERIN (CDH1; involved in tumor progression). Furthermore, a defective p53 (TP53) gene, which plays a role in response to DNA damage, may also play a role in the radioresistance of some human melanoma cell lines.12 Therefore, in order to understand the latest developments in melanoma, it is necessary to revisit the molecular biology of cell cycle progression.
The movement of cells through the cell cycle is regulated by cyclins and their catalytic partners, cyclin dependent kinases (CDKs). For example, cyclin D, which regulates the transition from G1 to S phase, is activated upon complexing with CDK4 and CDK6. This complex phosphorylates the retinoblastoma protein (Rb), which disassociates from E2F and allows E2F to activate transcription of genes critical for DNA synthesis, and progression into the S-phase of the cell cycle.13, 14 Upon E2F activated transcription, more cyclins are produced, such as cyclin E which allows the cell to progress into the S-phase of the cell cycle.15 Important regulators of this pathway are the inhibitors of CDKs: CDKN1A (p21/WAF1; activated by p53 following DNA damage) and p16INK4a, which complexes with CDKs-4 and 6 and prevents phosphorylation of Rb, as well as ARF, leading to inhibition of p53 degradation via the ubiquitin ligase hDM2.
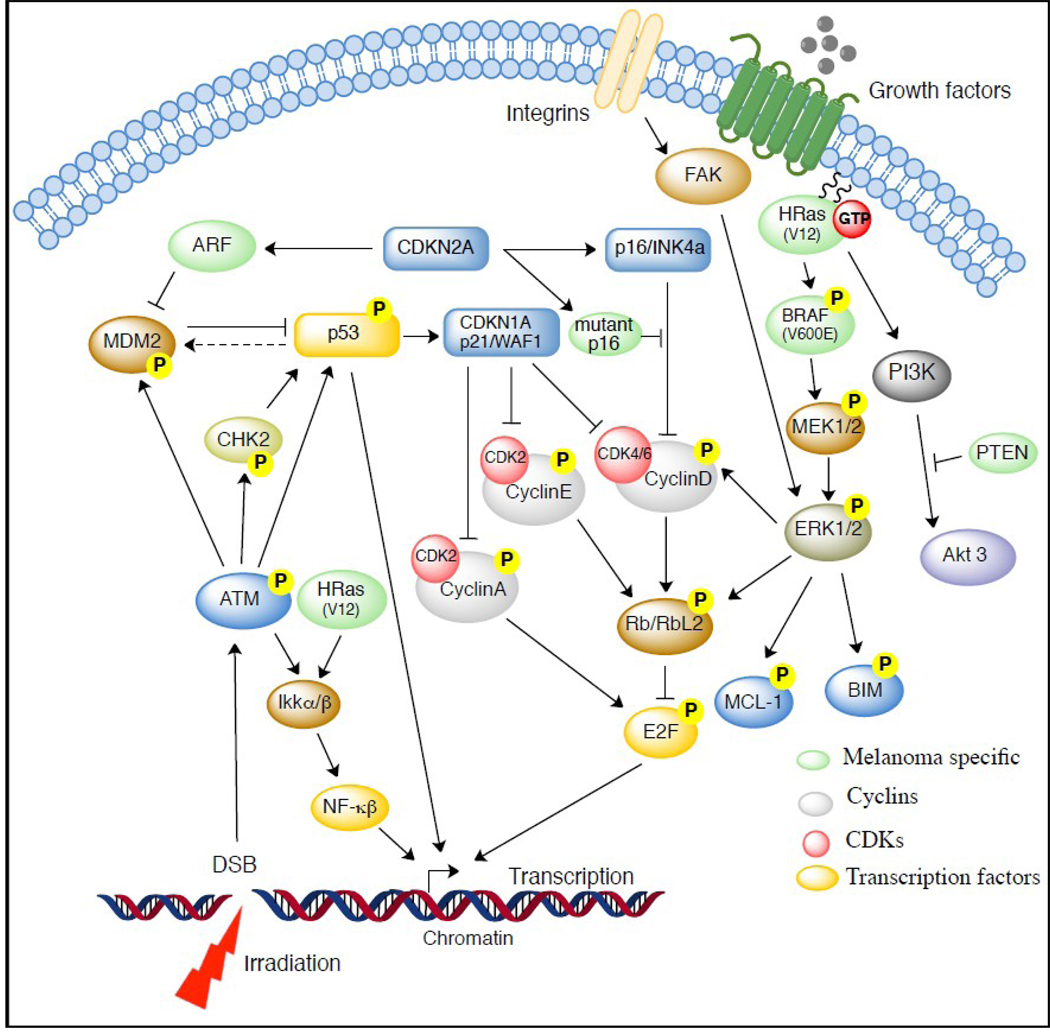
Another pathway involved in cell survival and reproduction is the mitogen-activated protein kinase (MAPK) signaling cascade (Fig. 1). MAPK signaling is initiated at the cell membrane, either by the receptor tyrosine kinase binding ligand or by integrin adhesion to ECM, which transmits activation signals via the RAS GTPase on the inner surface of the cell membrane. The GTP-bound RAS activates effector proteins BRAF and PI3K. BRAF activates MAPK/ERK kinase (MEK 1/2), and the resulting cascade activates ERK 1/2. ERK1/2 then relays the proliferative/cell survival signals via various targets, that provide cross-talk with cell cycle (Cyclin D, RBL2, Myc), or cell survival (Bim, Mcl-1) regulators (Fig. 1), as suggested earlier.12, 16 The PI3K–AKT pathway is also critical, with > 60% of human melanomas exhibiting activated AKT, and inactivation and/or deletion of the PI3K negative regulator (PTEN) occurring in 5–15% of uncultured melanoma specimens and metastasis, 17% of melanoma short-term cultures, and 30–40% of established melanoma cell lines.17
Fig. 1.
Cell Survival Signals in Melanoma Tumorogenesis.
Mutations at various points along this pathway have been implicated in the development of melanoma tumor types by allowing MAPK signaling pathways to escape normal cellular regulation, and stimulate growth.10 In particular, BRAF and N-RAS mutations are associated with melanoma development, especially in patients who have had intermittent exposure to sunlight and UV rays. N-RAS mutations, which predominate in human melanomas, make the protein constitutively active in about 15% of cutaneous melanomas.16 In a murine model, the H-RAS isoform was necessary to maintain melanomas, where as loss of the isoform led to tumor regression. BRAF somatic missense mutations are found in 66% of malignant melanomas.16 Similarly, in human melanoma cells, BRAF knockdown has led to melanoma regression and apoptosis.16
The next step in melanoma progression is the formation of a dysplastic nevus. Dysplastic nevi may be asymmetric, have irregular borders, and may increase in size.16 Molecularly, this step is marked by the activation of oncogenes and the loss of tumor suppressor genes. Two of the most significant genes involved in this pathogenic process are altered by mutations at the 9p21 and 12q14 loci.18 The CDKN2A locus is positioned at 9p21 and encodes two tumor suppressor proteins, p16INK4a and ARF involved in cell cycle regulation. Germline mutations of CDKN2A are responsible for predisposition to melanoma in 20–50% of melanoma families worldwide.19 The CDKN2A gene is considered the main melanoma-predisposing gene, and has been implicated in many human melanomas, particularly familial melanoma.16
The CDKN2A locus is unique in that through overlapping coding regions, it encodes two structurally unrelated proteins involved in growth regulation: p16INK4a and ARF. One study has suggested that ARF could be more frequently inactivated than p16.20 While 9p21 deletions have been discovered in melanoma families, they are otherwise considered to be rare events.18 p16 mutations are noted in 70% of melanoma cases.21 This mutation removes the normal inhibition on the formation of the CDK 4/6 and cyclin D complex required for cell cycle progression into the S phase via the phosphorylation of Rb. Removal of this inhibition therefore leads to increased cellular proliferation.
Mutations at the CDK4 locus (located in the region 12q14) are not as common as the CDKN2A mutations. However, they result in amplification of CDK4 to levels that are no longer inhibited by p16. Consequently, the excess CDK4 complexes with cyclin D and causes increased cellular proliferation via phosphorylation of Rb (or the related pocket protein RBL2/p130). Ongoing research on these specific mutations and their effect on the cell cycle have provided researchers with potential molecular targets to alter uncontrolled growth cycles, as will be discussed in a later section (targets for therapy).
The third step in the development of malignant melanoma is the radial-growth phase, when cells begin proliferating within the epidermal layer. Cells in this layer can proliferate rapidly because of p53 and other mutations, which increase local cell growth by escaping cell cycle regulatory mechanism. The fourth step is the vertical growth phase, in which cells invade the deeper portions into and beyond the dermal layer. These cells have lost E-cadherin and express N-cadherin instead. The final stage is the metastatic phase, when the abnormal cells are able to spread locally and widely.10
Traditional Management of Melanoma: Limited Role for Radiotherapy
For many years, melanoma was considered highly radioresistant. Simpson in 1913 was one of the first to treat a black nevus with radiation. He showed that the nevus could be easily removed with radiation without much damage of surrounding skin.3 This approach was considered unwise due to concerns of toxicity, and controversy3, 22, 23 continued until two other landmark studies provided convincing evidence to support Simpson. Ellis in 1939 treated 38 patients using 5500 to 6000 cGy over 7 to 10 days and demonstrated a good responses in 12 patients.3, 24 Ellis was also one of the first to suggest that melanoma is a highly variable tumor with many factors affecting radiosensitivity.3,24 The second study in 1963 reported on the 25 year experience, and demonstrated that that the 5 yr overall survival was 68% in 95 patients with results that equaled surgery.3,25 Despite the promise of earlier studies, advances in infection control and anesthesia led to improved surgical morbidity and mortality. As such, surgery became the preferred therapy. Thus, the use of radiation for the management of melanoma waned until the 1970s when reports of melanoma radiobiology emerged, which suggested that they were heterogeneous and should not be universally considered radioresistant.3, 6, 26
The first clinical prospectively randomized trial was published in 1991 by the Radiation Therapy Oncology Group (RTOG 83-05).27,28 The trial tested the earlier radiobiological findings that a higher radiation dose per treatment is needed to control melanoma by comparing a hypofractionated group, 8 Gy per fraction in 4 treatments delivered over 21 days, with a standard group, 2.5 Gy per fraction in 20 treatments delivered 5 days a week over 26–28 days. The trial included 137 patients and demonstrated an overall response rate of 57–60%, but failed to show a difference between the different fractionation regimens. Furthermore, the overall response rate of around 60% was much lower than the previously reported response rate of 97%, comparing 9 Gy in 3 fractions with that of 5 Gy in 8 fractions delivered twice a week.27 Therefore, this trial was closed prematurely. The results of RTOG 83–05 are also supported by another small retrospective study29 which concluded that hypofractionation was equivalent to conventional fractionation. However, this was a small retrospective study, with only 15 patients in the conventional fractionation arm.
Others have reported that a large fraction size was linked to improved response rates, confirming earlier radiobiological findings which suggested a small alpha beta ratio30. One study of 35 patients (67 cutaneous or lymph node metastases) showed a complete response of 9% if ≤ 5 Gy per treatment were used. The response rate increased to 50% if radiation doses ≥ 5Gy per treatment were used.31 Another study from 1983–1988 looked at 83 patients with melanoma of the head and neck region and demonstrated a 2 year loco-regional control of 95% in patients without palpable lymph nodes treated with large doses of adjuvant radiation (24 to 30 Gy in 4 to 5 fractions at 5 to 6 Gy per fraction) to the tumor bed and regional lymph nodes. The authors concluded that the locoregional control rates were better than surgery alone for comparable patients and that treatment morbidity with the addition of hypofractionated radiation was minimal.32
Newer Indications for Radiotherapy: Hypofractionation
Radiosurgery and Whole Brain Radiation for Brain Metastasis
The brain is a common site of melanoma metastasis, with brain metastases contributing to 20–54% of all deaths.33 Patients at increased risk for brain metastases include males, patients with head and neck mucosal melanomas, and lesions with metastases to three or more regional lymph nodes.34 These late-stage patients generally have poor prognoses, and treatment options are limited. Chemotherapy is generally ineffective.35 However, radiotherapy can get beyond the meninges and provide targeted, high-dose treatment, making it a valuable weapon in the arsenal against melanoma brain metastases.
Radiotherapy options include stereotactic radiosurgery (SRS) alone, whole brain radiotherapy (WBRT) with or without SRS, and postoperative WBRT. Several retrospective studies have shown the efficacy of both linear-accelerator based SRS (LINAC-SRS) as well as gamma knife based radiosurgery (GKRS). These studies are summarized in table 1 and table 2 and have demonstrated 1 year local control rates of 49–90%.36–39 SRS also improves quality of life40 and may also improve overall survival when combined with WBRT.41 One retrospective review concluded that with surgical excision alone or GKRS alone, the addition of whole brain radiation improved overall survival by 7.3 months.41
Table 1.
The role of LINAC SRS in treatment of melanoma brain metastases.
| Study | Number of patients | 1 year LC | 1 year OS | Comments |
|---|---|---|---|---|
| Mori Y36 | 60 | 90% | 7 months | Lack of active systemic disease, 1 metastasis = improved survival on MV analysis |
| 51 (WBRT +SRS) | ||||
| Selek U37 | 103 | 49% | 6.7 months | 75% LC for tumors <2 cm with initial SRS alone |
| 61 (SRS) | 60% | 7.5 months | ||
| 12 (SRS + WBRT) | 0% | 3.7 months | ||
| 30 (SRS after WBRT) | 37% | 5.4 months | ||
Table 2.
The role of GKRS in treatment of melanoma brain metastases.
Uveal Melanoma
Uveal melanomas arise in the choroid, the ciliary body, or the iris (in decreasing order of frequency), and have recently been shown to feature a mutation at GNA11 locus involved in MAP Kinase modulation.42 Historically, these tumors were treated with enucleation. Due to elevated intraocular pressure and disruption of the tumor, it was thought that enucleation could promote seeding of tumor cells into the circulation.43 The Collaborative Ocular Melanoma Study (COMS) randomized 1003 patients with large choroidal melanomas (> 10.0 mm in apical height or > 16.0 mm in largest basal diameter) to enucleation with and without prior radiation (20 Gy in five daily fractions). It found that the 5-year all-cause mortality were comparable for both treatment arms: 57% for enucleation and 62% for pre-enucleation radiation (P = 0.32)44 with similar results at 10 years.45 The COMS study also randomized 1317 patients with medium sized choroidal melanomas (2.5 – 10.0 mm in apical height and 5–16 mm in largest basal diameter) to enucleation or iodine-125 brachytherapy and found no difference in 5-year survival rates (81% for enucleation and 82% for brachytherapy, P=0.48).46
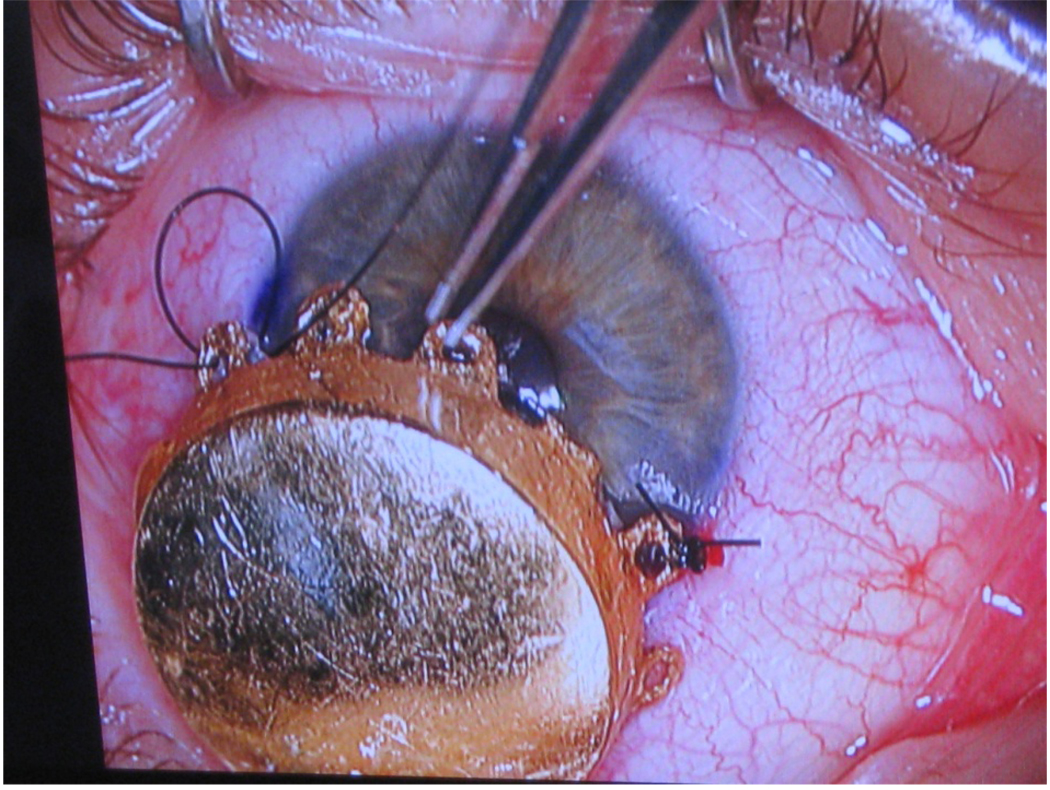
In recent years, novel techniques have emerged for ocular preservation such as brachytherapy, transpupillary thermotherapy, local tumor resection, proton beam irradiation, and stereotactic radiosurgery. Protons using a total dose of 70 cGy cobalt-60 equivalent have reported tumor control rates of 97%.47 Gamma knife radiosurgery offers tumor control and complication rates comparable to brachytherapy.48 Plaque brachytherapy (Fig.2) has evolved into a promising alternative to enucleation as it provides excellent local control with only a moderate reduction in visual acuity.
Fig. 2.
I–125 Plaque Brachytherapy for an Iris Melanoma Patient
Control of Lymph Node Positive Disease
Though earlier studies49 suggested adjuvant radiation therapy for melanoma was ineffective, newer studies now suggest promise in the control of local and regional recurrence after excision of the primary and involved lymph nodes in select high-risk patients. Patients with desmoplastic histology, positive margins, recurrent disease, Breslow > 4.0 mm with ulceration or satellitosis are at high risk for local recurrence and may benefit with adjuvant radiotherapy.50 Similarly, patients with ≥4 lymph nodes, extracapsular extension, lymph node size ≥ 3 cm, cervical lymph node involvement, sentinel lymph node involved but without complete lymph node dissection, and recurrent disease are at high risk for nodal relapse and may also benefit with radiotherapy50, 29, 51 Further progress in this arena would be welcomed, as local recurrence and in the nodal basins after lymphadenectomy is associated with lower survival rates.52
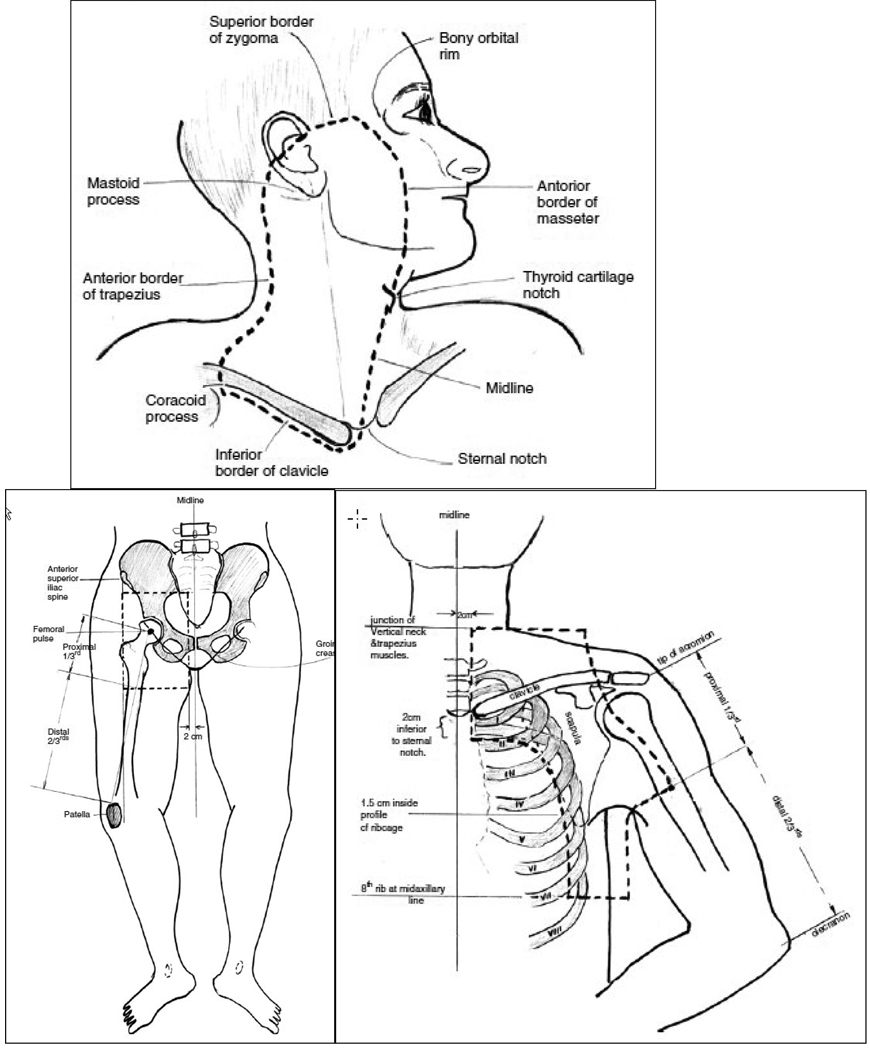
A 2006 phase II study of adjuvant radiation therapy using 48 Gy in 20 fractions to the nodal basins showed an impressive regional control.52 The study enrolled 234 patients from 3 nodal basins (head and neck, axilla/supraclavicular, and ilioinguinal). The authors demonstrated a low in-field recurrence rate (7%), a low adjacent relapse rate (14%), and an impressive 5 yr regional control rate of 91%. However, the 5 yr overall survival rates (36%) and progression-free survival rates (27%) continued to be dismal due to uncontrolled systemic metasteses.52 The authors concluded that the side effects after radiotherapy were minimal and that radiotherapy was well tolerated. The recommended post-operative radiation fields are shown in Fig. 3.
Fig. 3.
Post Surgical Radiation Fields for Node Positive Patients (*Reprinted with permission from Burmeister52 et al)
The phase II study was followed by a multicenter phase III trial.53 The trial included post-lymphadenectomy patients with isolated regional recurrence who were deemed to be high risk (> 25%) for further regional recurrence (≥1 parotid lymph node, ≥2 cervical or axillary lymph node, ≥ 3 groin nodes, any extranodal spread of melanoma, or maximum metastatic node diameter ≥ 3 cm in neck or axilla, or ≥ 4 cm node in the groin). 250 patients were randomly assigned to observation versus regional radiation therapy using 48 Gy in 20 fractions delivered at 2.4 Gy per fraction . The study showed that postoperative radiation resulted in improved disease free survival (hazard ratio (HR) of 1.77 with p=0.041).53 Others have also recommended the use of adjuvant radiotherapy after therapeutic lymphadenectomy for lymph node-metastatic melanoma.54, 55
Mucosal Melanoma in H&N
Adjuvant radiotherapy may be particularly useful in treating mucosal melanomas of the head and neck. Primary mucosal melanomas of the head and neck region have < 30% 5 year survival rate.56 A review of 48 patients treated with surgery alone, surgery and adjuvant radiotherapy or surgery and biochemotherapy (with or without adjuvant radiotherapy) demonstrated that radiation therapy decreased local failure rates, but without impacting overall survival. The lack of benefit in overall survival was due to the high rate of distant metastases.57 Another review of 69 patients, with 23% reporting lymph node involvement, reviewed the results of 30 patients who had surgery alone and 39 patients who had post-operative radiotherapy (70 Gy in 29 patients and 50 Gy in 10 patients). Local control was improved in patients who received adjuvant treatment, but survival rates were worse. The patients who had received radiotherapy developed significantly more systemic metastases, but on multivariate this was ascribed to a more advanced tumor and nodal classification in the radiotherapy group.56
Recent case reports have addressed the differences in molecular mutations between melanomas occurring in chronic sun exposed regions (infrequent BRAF & NRAS and increased KIT mutation) compared to those in non-sun exposed regions58 and have suggested that treatment with imatinib, a c-KIT inhibitor may provide additional optimism.59 Using molecular biology to develop a tailored multidisciplinary approach is an area of ongoing research.
Melanoma Mutations – Targets for Therapy
The perception of radioresistance has changed over time. In the 1970s and 1980s, researchers began focusing on radiosensitivity.3, 60 Cell culture studies, studies using spheroids, and in-vivo studies have demonstrated that melanoma cells are heterogeneous in radio-responsiveness and should not be considered uniformly radioresistant.4,6,5,26 An improved understanding of the cell cycle blocks and the role CDKs play is particularly important , as these molecules are also involved in controlling and modifying the radio-response of melanoma cells. If one can control CDKs, then one can develop a mechanism to explain the relative radioresistance of melanoma and provide radiobiological evidence as to why higher doses of radiation may overcome potentially lethal damage repair (PLDR).
The p16INK4a-Rb tumor suppressor pathway is required for the initiation and maintenance of cellular senescence. Senescence can be overcome if the pathway is not fully engaged, as may occur when p16INK4a is inactivated.61 p16INK4a can initiate a CDK4/6-dependent autonomous senescence program that is disabled by inherited melanoma-associated mutations.61
As more knowledge is obtained regarding aberrant components of the cell cycle regulatory circuit leading to melanoma development, therapeutic trials targeting specific mutant proteins are underway. For example, one study using human melanoma cancer cell lines in-vivo and in mice in-vitro showed that selective and structurally distinct small molecular inhibitors of CDK4 and CDK6 resulted in increased cellular-radioresistance, especially in those cancer cell lines dependent on CDK4/6 pathway for proliferation. In contrast, CDK4/6 inhibitors did not protect cell lines that proliferated independently of CDK4/6 activity.62
Moreover, sorafenib is a Raf tyrosine kinase inhibitor, which inhibits the MAPK kinase pathway in vitro and in vivo. It has anti-angiogenic effects and has effectively treated cancers with and without BRAF mutations, making it a promising adjuvant therapy for melanoma.16 As most melanomas harbor an activating missense mutation (V600E) in the BRAF oncogene, targeted inhibition of the V600E gene product is a particularly rational therapeutic goal in this otherwise therapy-resistant tumor type.63 Pharmacologic inhibition of oncogenic BRAF blocks proliferation and causes tumor regression. A phase 1 trial of PLX4032, an oral inhibitor of V600E mutated BRAF, demonstrated complete or partial tumor regression in 81% of patients and improved median progression-free survival from 2 months to 6.2 months for patients with metastatic melanoma.64
Current research is exploring the use of adjuvant therapies targeted at normal cells to reduce the toxicity of cancer treatments.65 These therapies focus on suppressing apoptosis in healthy cells by inhibiting p53 and activating NF-kB and preventing cell cycle progression by inhibiting the CDKs from complexing with cyclin D.65
Conversely, targeting the cell cycle control pathway components in tumor cells, in addition to hypo-fractionated radiotherapy protocols, may further improve the radio responsiveness of melanoma. In addition to being highly active in melanoma cells, the COX-2, P13K–AKT, and NF-kB pathways are also involved in the radioprotective response. It has been shown recently that Ink4a/Arf–/– mice with melanocyte-specific deletion of Ikkβ were protected from H-RasV12-initiated melanoma when p53 was expressed, providing genetic and mechanistic evidence that mutant H-Ras initiation of tumorigenesis requires Ikkβ-mediated NF-κB activity.66 Suppressing those pathways pushes higher numbers of melanoma cells into the mitotic phase, which is the most radiosensitive phase in the cell cycle leading to decreased overall melanoma cell survival.67 One study compared the radiosensitivity of cells expressing wild-type p16 to those with the mutant p16 found in melanomas, and found that the melanoma cells were less sensitive to X-ray irradiation if they had a p16 mutation.68 Therefore, an adjuvant therapy targeting the expression of mutant p16 would not only lead to better cell cycle regulation, but leave the melanoma cells more radiosensitive.
Clinical data also indicates that hypo-fractionation may overcome the traditional PLDR capability of melanoma. One study looking at 618 malignant melanomas treated with radiotherapy showed that 48% of the treated tumors achieved a complete response in 87% and that the response was persistent even after 5 yrs post irradiation.5, 69 The authors concluded that neither total dose, treatment time, nor various modifications of the nominal standard dose (NSD) showed any significant relationship to tumor response post irradiation.5,27, 69 The only parameter that predicted increased response was higher dose per fraction (> 4 Gy/fraction). The alpha/beta ratio was 2.5 Gy and more consistent with late responding tissue. The authors also developed an iso-effect formula to compare different radiation fractionation schedules: extrapolated total-dose (ETD) corrected for tumor volume = Dose x [(dose per fraction +2.5)/2.5] * mean tumor diameter (cm) – 0.33.5,27,69 Another in-vivo study of 121 patients reported on the tumor-control probability using fractionated radiotherapy with different fraction sizes, total doses and overall treatment time and reported an alpha/beta ratio of 0.57, implying that fraction size is important.30 The authors in this study suggested that overall treatment time did not influence tumor control since melanoma cells behave like late-responding tissue and that a dose per fraction of > 6 Gy per fraction is warranted.
A recent randomized trial of ipilimumab evaluated the role of an antibody targeting CTLA-4 in HLA-A*0201-positive unresectable stage III/IV melanoma patients who progressed on chemotherapy showed a significant improvement in median overall survival (10.0 vs. 6.4 months, p < 0.0001) 70 Radiotherapy effects on the cellular immune system, or aboscopal effects, may be partially responsible for the apparent positive impact on node positive melanoma. Irradiated tumor tissues may develop inflammation leading to immune system activation, and is an area of ongoing research 71
Conclusions
Although it appears true that malignant melanoma is a relatively radio-insensitive tumor type, the use of favorable fractionation schemes and delivery plans combined with greater radiobiological understanding may provide excellent control for certain clinical situations. Intracranial radiosurgery and stereotactic radiotherapy appear to provide control rates for melanoma on par with that seen for other more sensitive epithelial tumors. Small and medium choroidal melanomas show a natural history in which metastasis outside the eye is unusual, thus validating the apparent safety of protracted tumor response which produces acceptable levels of visual field sparing as an alternative to surgical enucleation. Finally, recent Phase III data have shown significant improvements in local control after lymphatic radiotherapy for recurrent node positive post lymphadenectomy patients. For all of these clinical scenarios, it appears that the concentrated highly targeted radiation dose, with minimal fractionation and normal tissue inclusion represents some of the keys to restoring confidence in the importance of this therapeutic modality for malignant melanoma.
Recent molecular biological understanding suggests that malignant melanoma deposits, long known to proliferate primarily in UV-exposed body surfaces, have evolved multiple overlapping protective mechanisms capable of activating or suppressing master gene panels controlling the dynamics of cell cycle progression in the face of cellular or chromosomal damage. Among these top-line damage sensor and repair pathways are p53, ATM, NF-kB and components of the Cyclin/CDK regulatory network. The reversal or slowing of some of these protective pathways via genetic modifications (e.g. targeted mutations) or pharmacologic inhibitors (e.g. p53 or p16 small molecule inhibitors) perhaps in combination with appropriately fractionated radiotherapy and perhaps immunotherapy and chemotherapy may finally transform melanoma from the most lethal to one of the most curable of the primary cutaneous malignancies.
Acknowledgements
We would like to thank Mr. Gaurav Choudhary (Case Western Reserve University) for help with illustrations and NIH ( CA127264-02 to A.A) for partial support.
*Fig 4. “Reprinted from Radiotherapy Oncology. Vol 81. Edition 2. Burmeister BH, Mark Smithers B, Burmeister E, et al. A prospective phase II study of adjuvant postoperative radiation therapy following nodal surgery in malignant melanoma-Trans Tasman Radiation Oncology Group (TROG) Study 96.06. Nov 2006 with permission from Elsevier”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Notification
The authors have no conflicts of interest to disclose.
Bibliography
- 1.MacKee GM, Cipollaro AC, Montgomery H. X-rays and radium in the treatment of diseases of the skin. 4th ed. Philadelphia: Lea & Febiger; 1946. [Google Scholar]
- 2.Million RR, Cassisi NJ. Management of head and neck cancer : a multidisciplinary approach. Philadelphia: Lippincott; 1984. [Google Scholar]
- 3.Jenrette JM. Malignant melanoma: the role of radiation therapy revisited. Semin Oncol. 1996 Dec;23(6):759–762. [PubMed] [Google Scholar]
- 4.Stevens G, McKay MJ. Dispelling the myths surrounding radiotherapy for treatment of cutaneous melanoma. Lancet Oncol. 2006 Jul;7(7):575–583. doi: 10.1016/S1470-2045(06)70758-6. [DOI] [PubMed] [Google Scholar]
- 5.Overgaard J, Overgaard M, Hansen PV, von der Maase H. Some factors of importance in the radiation treatment of malignant melanoma. Radiother Oncol. 1986 Mar;5(3):183–192. doi: 10.1016/s0167-8140(86)80048-2. [DOI] [PubMed] [Google Scholar]
- 6.Rofstad EK. Radiation biology of malignant melanoma. Acta Radiol Oncol. 1986 Jan-Feb;25(1):1–10. doi: 10.3109/02841868609136368. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009 Jul-Aug;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 8.Homer MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006. Bethseda, MD: National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975_2006/. Accessed April 3, 2010. [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006 Jul 6;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 11.Uong A, Zon LI. Melanocytes in development and cancer. J Cell Physiol. 2010 Jan;222(1):38–41. doi: 10.1002/jcp.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satyamoorthy K, Chehab NH, Waterman MJ, et al. Aberrant regulation and function of wild-type p53 in radioresistant melanoma cells. Cell Growth Differ. 2000 Sep;11(9):467–474. [PubMed] [Google Scholar]
- 13.Crosby ME, Almasan A. Opposing roles of E2Fs in cell proliferation and death. Cancer Biol Ther. 2004 Dec;3(12):1208–1211. doi: 10.4161/cbt.3.12.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almasan A, Yin Y, Kelly RE, et al. Deficiency of retinoblastoma protein leads to inappropriate S-phase entry, activation of E2F–responsive genes, and apoptosis. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5436–5440. doi: 10.1073/pnas.92.12.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazumder S, DuPree EL, Almasan A. A dual role of cyclin E in cell proliferation and apoptosis may provide a target for cancer therapy. Curr Cancer Drug Targets. 2004 Feb;4(1):65–75. doi: 10.2174/1568009043481669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fecher LA, Cummings SD, Keefe MJ, Alani RM. Toward a molecular classification of melanoma. J Clin Oncol. 2007 Apr 20;25(12):1606–1620. doi: 10.1200/JCO.2006.06.0442. [DOI] [PubMed] [Google Scholar]
- 17.Nogueira C, Kim KH, Sung H, et al. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010 Aug 16; doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helsing P, Nymoen DA, Ariansen S, et al. Population-based prevalence of CDKN2A and CDK4 mutations in patients with multiple primary melanomas. Genes Chromosomes Cancer. 2008 Feb;47(2):175–184. doi: 10.1002/gcc.20518. [DOI] [PubMed] [Google Scholar]
- 19.Bisio A, Nasti S, Jordan JJ, et al. Functional analysis of CDKN2A/p16INK4a 5'-UTR variants predisposing to melanoma. Hum Mol Genet. Apr 15;19(8):1479–1491. doi: 10.1093/hmg/ddq022. [DOI] [PubMed] [Google Scholar]
- 20.Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008 Jun 4;100(11):784–795. doi: 10.1093/jnci/djn157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaini MC, Rossi E, de Siqueira Torres PL, et al. Functional impairment of p16(INK4A) due to CDKN2A p.Gly23Asp missense mutation. Mutat Res. 2009 Dec 1;671(1–2):26–32. doi: 10.1016/j.mrfmmm.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Coley WB, Hoquet JP. Metastatic cancer, with a report of 91 patients. Ann Surg. 1916;64:206–241. doi: 10.1097/00000658-191608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coley WB, Leucutia T. The treatment of metastatic tumors of the skin: Pigmented moles and melanomas. Am J Roentgen. 1924;11:335–336. [Google Scholar]
- 24.Ellis F. Radiosensitivity of malignant melanomata. Br J Radiol. 1939;12:327–352. [Google Scholar]
- 25.Hellriegel W. Radiation therapy for primary and metastatic melanoma. Ann N Y Acad Sci. 1963;100:131–141. doi: 10.1111/j.1749-6632.1963.tb57118.x. [DOI] [PubMed] [Google Scholar]
- 26.Barranco SC, Romsdahl MM, Humphrey RM. The radiation response of human malignant melanoma cells grown in vitro. Cancer Res. 1971 Jun;31(6):830–833. [PubMed] [Google Scholar]
- 27.Overgaard J, von der Maase H, Overgaard M. A randomized study comparing two high-dose per fraction radiation schedules in recurrent or metastatic malignant melanoma. Int J Radiat Oncol Biol Phys. 1985 Oct;11(10):1837–1839. doi: 10.1016/0360-3016(85)90042-2. [DOI] [PubMed] [Google Scholar]
- 28.Sause WT, Cooper JS, Rush S, et al. Fraction size in external beam radiation therapy in the treatment of melanoma. Int J Radiat Oncol Biol Phys. 1991 Mar;20(3):429–432. doi: 10.1016/0360-3016(91)90053-7. [DOI] [PubMed] [Google Scholar]
- 29.Chang DT, Amdur RJ, Morris CG, Mendenhall WM. Adjuvant radiotherapy for cutaneous melanoma: comparing hypofractionation to conventional fractionation. Int J Radiat Oncol Biol Phys. 2006 Nov 15;66(4):1051–1055. doi: 10.1016/j.ijrobp.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 30.Bentzen SM, Overgaard J, Thames HD, et al. Clinical radiobiology of malignant melanoma. Radiother Oncol. 1989 Nov;16(3):169–182. doi: 10.1016/0167-8140(89)90017-0. [DOI] [PubMed] [Google Scholar]
- 31.Konefal JB, Emami B, Pilepich MV. Malignant melanoma: analysis of dose fractionation in radiation therapy. Radiology. 1987 Sep;164(3):607–610. doi: 10.1148/radiology.164.3.3112864. [DOI] [PubMed] [Google Scholar]
- 32.Ang KK, Byers RM, Peters LJ, et al. Regional radiotherapy as adjuvant treatment for head and neck malignant melanoma. Preliminary results. Arch Otolaryngol Head Neck Surg. 1990 Feb;116(2):169–172. doi: 10.1001/archotol.1990.01870020045012. [DOI] [PubMed] [Google Scholar]
- 33.Skibber JM, Soong SJ, Austin L, Balch CM, Sawaya RE. Cranial irradiation after surgical excision of brain metastases in melanoma patients. Ann Surg Oncol. 1996;3(2):118–123. doi: 10.1007/BF02305789. [DOI] [PubMed] [Google Scholar]
- 34.Sampson JH, Carter JHJ, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88(1):11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 35.Atkins MB, Sosman JA, Agarwala S, et al. Temozolomide, thalidomide, and whole brain radiation therapy for patients with brain metastasis from metastatic melanoma: a phase II Cytokine Working Group study. Cancer. 2008;113(8):2139–2145. doi: 10.1002/cncr.23805. [DOI] [PubMed] [Google Scholar]
- 36.Mori Y, Kondziolka D, Flickinger JC, Kirkwood JM, Agarwala S, Lunsford LD. Stereotactic radiosurgery for cerebral metastatic melanoma: factors affecting local disease control and survival. Int J Radiat Oncol Biol Phys. 1998;42(3):581–589. doi: 10.1016/s0360-3016(98)00272-7. [DOI] [PubMed] [Google Scholar]
- 37.Selek U, Chang EL, Hassenbusch S, Jr, et al. Stereotactic radiosurgical treatment in 103 patients for 153 cerebral melanoma metastases. Int J Radiat Oncol Biol Phys. 2004;59(4):1097–1106. doi: 10.1016/j.ijrobp.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Yu C, Chen JC, Apuzzo ML, et al. Metastatic melanoma to the brain: prognostic factors after gamma knife radiosurgery. Int J Radiat Oncol Biol Phys. 2002 Apr 1;52(5):1277–1287. doi: 10.1016/s0360-3016(01)02772-9. [DOI] [PubMed] [Google Scholar]
- 39.Radbill AE, Fiveash JF, Falkenberg ET, et al. Initial treatment of melanoma brain metastases using gamma knife radiosurgery: an evaluation of efficacy and toxicity. Cancer. 2004 Aug 15;101(4):825–833. doi: 10.1002/cncr.20447. [DOI] [PubMed] [Google Scholar]
- 40.Scorsetti M, Facoetti A, Navarria P, et al. Hypofractionated stereotactic radiotherapy and radiosurgery for the treatment of patients with radioresistant brain metastases. Anticancer Res. 2009 Oct;29(10):4259–4263. [PubMed] [Google Scholar]
- 41.Stone A, Cooper J, Koenig KL, Golfinos JG, Oratz R. A comparison of survival rates for treatment of melanoma metastatic to the brain. Cancer Invest. 2004;22(4):492–497. doi: 10.1081/cnv-200026387. [DOI] [PubMed] [Google Scholar]
- 42.Van Raamsdonk JM, Meng Y, Camp D, et al. Decreased energy metabolism extends life span in Caenorhabditis elegans without reducing oxidative damage. Genetics. 2010 Jun;185(2):559–571. doi: 10.1534/genetics.110.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmerman LE, McLean IW, Foster WD. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br J Ophthalmol. 1978 Jun;62(6):420–425. doi: 10.1136/bjo.62.6.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma III: local complications and observations following enucleation COMS report no. 11. Am J Ophthalmol. 1998 Sep;126(3):362–372. doi: 10.1016/s0002-9394(98)00091-9. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins BS. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am J Ophthalmol. 2004 Dec;138(6):936–951. doi: 10.1016/j.ajo.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Diener-West M, Earle JD, Fine SL, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: initial mortality findings. COMS Report No. 18. Arch Ophthalmol. 2001 Jul;119(7):969–982. doi: 10.1001/archopht.119.7.969. [DOI] [PubMed] [Google Scholar]
- 47.Gragoudas ES. Proton beam irradiation of uveal melanomas: the first 30 years. The Weisenfeld Lecture. Invest Ophthalmol Vis Sci. 2006 Nov;47(11):4666–4673. doi: 10.1167/iovs.06-0659. [DOI] [PubMed] [Google Scholar]
- 48.Toktas ZO, Bicer A, Demirci G, et al. Gamma knife stereotactic radiosurgery yields good long-term outcomes for low-volume uveal melanomas without intraocular complications. J Clin Neurosci. 2010 Apr;17(4):441–445. doi: 10.1016/j.jocn.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Moncrieff MD, Martin R, O’Brien CJ, et al. Adjuvant postoperative radiotherapy to the cervical lymph nodes in cutaneous melanoma: is there any benefit for high-risk patients? Ann Surg Oncol. 2008 Nov;15(11):3022–3027. doi: 10.1245/s10434-008-0087-8. [DOI] [PubMed] [Google Scholar]
- 50.Ballo MT, Ang KK. Radiotherapy for cutaneous malignant melanoma: rationale and indications. Oncology (Williston Park) 2004 Jan;18(1):99–107. discussion 107–110, 113-104. [PubMed] [Google Scholar]
- 51.Verma S, Quirt I, McCready D, Bak K, Charette M, Iscoe N. Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer. 2006 Apr 1;106(7):1431–1442. doi: 10.1002/cncr.21760. [DOI] [PubMed] [Google Scholar]
- 52.Burmeister BH, Mark Smithers B, Burmeister E, et al. A prospective phase II study of adjuvant postoperative radiation therapy following nodal surgery in malignant melanoma-Trans Tasman Radiation Oncology Group (TROG) Study 96.06. Radiother Oncol. 2006 Nov;81(2):136–142. doi: 10.1016/j.radonc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 53.Henderson MA, Burmeister B, Thompson JF, et al. Adjuvant radiotherapy and regional lymph node field control in melanoma patients after lymphadenectomy: Results of an intergroup randomized trial (ANZMTG 01.02/TROG 02.01) J Clin Oncol. 2009;27(18s) [Google Scholar]
- 54.Agrawal S, Kane JM, 3rd, Guadagnolo BA, Kraybill WG, Ballo MT. The benefits of adjuvant radiation therapy after therapeutic lymphadenectomy for clinically advanced, high-risk, lymph node-metastatic melanoma. Cancer. 2009 Dec 15;115(24):5836–5844. doi: 10.1002/cncr.24627. [DOI] [PubMed] [Google Scholar]
- 55.Ballo MT, Ross MI, Cormier JN, et al. Combined-modality therapy for patients with regional nodal metastases from melanoma. Int J Radiat Oncol Biol Phys. 2006 Jan 1;64(1):106–113. doi: 10.1016/j.ijrobp.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 56.Temam S, Mamelle G, Marandas P, et al. Postoperative radiotherapy for primary mucosal melanoma of the head and neck. Cancer. 2005 Jan 15;103(2):313–319. doi: 10.1002/cncr.20775. [DOI] [PubMed] [Google Scholar]
- 57.Owens JM, Roberts DB, Myers JN. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch Otolaryngol Head Neck Surg. 2003 Aug;129(8):864–868. doi: 10.1001/archotol.129.8.864. [DOI] [PubMed] [Google Scholar]
- 58.Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol. 2006 Sep 10;24(26):4340–4346. doi: 10.1200/JCO.2006.06.2984. [DOI] [PubMed] [Google Scholar]
- 59.Handolias D, Hamilton AL, Salemi R, et al. Clinical responses observed with imatinib or sorafenib in melanoma patients expressing mutations in KIT. Br J Cancer. 2010 Apr 13;102(8):1219–1223. doi: 10.1038/sj.bjc.6605635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berk LB. Radiation therapy as primary and adjuvant treatment for local and regional melanoma. Cancer Control. 2008 Jul;15(3):233–238. doi: 10.1177/107327480801500306. [DOI] [PubMed] [Google Scholar]
- 61.Haferkamp S, Becker TM, Scurr LL, Kefford RF, Rizos H. p16INK4a–induced senescence is disabled by melanoma-associated mutations. Aging Cell. 2008 Oct;7(5):733–745. doi: 10.1111/j.1474-9726.2008.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson SM, Torrice CD, Bell JF, et al. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J Clin Invest. 2010 Jun 23; doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008 Feb 26;105(8):3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010 Aug 26;363(9):809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gudkov AV, Komarova EA. Radioprotection: smart games with death. J Clin Invest. 2010 Jun 23; doi: 10.1172/JCI43794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang J, Splittgerber R, Yull FE, et al. Conditional ablation of Ikkb inhibits melanoma tumor development in mice. J Clin Invest. 2010 Jul 1;120(7):2563–2574. doi: 10.1172/JCI42358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson GE, Ivanov VN, Hei TK. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis. 2008 Jun;13(6):790–802. doi: 10.1007/s10495-008-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsumura Y, Yamagishi N, Miyakoshi J, Imamura S, Takebe H. Increase in radiation sensitivity of human malignant melanoma cells by expression of wild-type p16 gene. Cancer Lett. 1997 May 1;115(1):91–96. doi: 10.1016/s0304-3835(97)04714-9. [DOI] [PubMed] [Google Scholar]
- 69.Overgaard J. The role of radiotherapy in recurrent and metastatic malignant melanoma: a clinical radiobiological study. Int J Radiat Oncol Biol Phys. 1986 Jun;12(6):867–872. doi: 10.1016/0360-3016(86)90378-0. [DOI] [PubMed] [Google Scholar]
- 70.Hodi FS, O'Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 Jun 14; doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005 May;31(3):159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]