Summary
Multiple cyclin-dependent kinases (CDKs) control eukaryotic cell division, but assigning specific functions to individual CDKs remains a challenge. During the mammalian cell cycle, Cdk2 forms active complexes before Cdk1, but lack of Cdk2 protein does not block cell-cycle progression. To detect requirements and define functions for Cdk2 activity in human cells when normal expression levels are preserved, and non-physiologic compensation by other CDKs is prevented, we replaced the wild-type kinase with a version sensitized to specific inhibition by bulky adenine analogs. The sensitizing mutation also impaired a non-catalytic function of Cdk2 in restricting assembly of cyclin A with Cdk1, but this defect could be corrected by both inhibitory and non-inhibitory analogs. This allowed either chemical rescue or selective antagonism of Cdk2 activity in vivo, to uncover a requirement in cell proliferation, and non-redundant, rate-limiting roles in restriction point passage and S-phase entry.
Introduction
During G1 phase of the division cycle, a eukaryotic cell commits to a round of duplication or enters a quiescent state (G0) (Morgan, 2007). When conditions are appropriate for proliferation, growth factor signaling promotes passage through the restriction (R) point, after which further cell-cycle progression becomes growth factor-independent (Pardee, 1974). This transition coincides with initiation of a transcription program directed by the E2F family of DNA-binding factors, which regulate genes critical for DNA synthesis (S) phase and mitosis (Bracken et al., 2004). Analysis of gene expression in single cells suggests that the switch governing E2F activation is bistable in response to increasing growth factor concentration, attaining an ON or OFF steady state with no intermediate states of partial activation (Yao et al., 2008).
G1/S transcription is controlled by both activator and repressor E2Fs that bind to a family of pocket proteins comprising the retinoblastoma tumor suppressor protein pRb, p107 and p130 (Cobrinik, 2005). In G0 and early G1, gene expression is inhibited by repressor E2Fs, in complexes with p107 or p130 that bind E2F-responsive promoters and recruit histone-modifying enzymes to impose a repressive chromatin state (Frolov and Dyson, 2004). Later in G1 and S phase, repression is relieved and free activator E2Fs, dissociated from pocket proteins, promote transcription. Cyclin-dependent kinases (CDKs) play a decisive role in this switch by phosphorylating pRb, p107 and p130. In vitro, cyclin D-, E- and A-dependent kinases phosphorylate pRb with overlapping but distinct site-specificity (Zarkowska and Mittnacht, 1997), and multiple CDKs contribute to complete pRb inactivation in vivo (Lundberg and Weinberg, 1998). Phosphorylations on distinct sets of residues—targets in vitro of different CDKs—decrease affinity between discrete regions of pRb and E2F (Rubin et al., 2005; Burke et al., 2010). The deregulation of pocket protein function and its control by CDKs is a hallmark of many cancers (Classon and Harlow, 2002).
Cdk2 was originally implicated in this regulatory circuit because its period of cyclin-binding and activity extended from late G1 until just prior to mitosis (Pagano et al., 1992; Rosenblatt et al., 1992; Pagano et al., 1993), and expression of dominant-negative Cdk2 arrested cells in G1, S and G2 phases (van den Heuvel and Harlow, 1993; Hu et al., 2001). This model was challenged by the discovery that Cdk2 is not essential for mouse viability (Berthet et al., 2003; Ortega et al., 2003). In cells lacking a full complement of catalytic subunits, Cdk1 can form complexes with cyclins D and E (Aleem et al., 2005; Santamaria et al., 2007); compensation by Cdk1 may explain how cells lacking Cdk2, Cdk4 and Cdk6 can proliferate in culture (Santamaria et al., 2007). It remained unclear whether events early in the cell cycle depend exclusively on Cdk2 in wild-type cells, or if Cdk1 can perform these functions normally.
To begin to address this question, we previously determined the relative amounts of Cdk1 and Cdk2 bound to various cyclins during the course of a human cell cycle (Merrick et al., 2008). Cyclins E and B bound almost exclusively to Cdk2 and Cdk1, respectively, whereas cyclin A formed complexes with both CDKs in strict temporal order—predominantly with Cdk2 until mid-S phase, and only thereafter with Cdk1. Cdk2 has priority despite being ~10-fold less abundant than Cdk1, possibly as a consequence of different activation mechanisms for the two kinases. In vivo, Cdk1 and Cdk2 follow distinct paths to full activity even though they are ~65% identical in sequence, have overlapping cyclin-binding profiles, and are targets of the same CDK-activating kinase (CAK)—the Cdk7 complex. The primary pathway for Cdk2 comprises two steps: first, phosphorylation of the activation (T-) loop by Cdk7 and then, binding to cyclin (Merrick et al., 2008). Cdk1, conversely, cannot be phosphorylated by Cdk7 in the absence of a cyclin, but cannot form a stable complex without that phosphorylation, meaning that the two steps must occur in concert (Larochelle et al., 2007). In vitro, a yeast CAK that phosphorylates monomeric Cdk1 can force it into the Cdk2 pathway, switching cyclin A-binding preference from Cdk2 to Cdk1 in extracts (Merrick et al., 2008). This suggested that pairing rules for CDK-cyclin assembly derive from kinetic insulation of activation pathways.
Therefore an important function of Cdk2 may be to exclude Cdk1 from cyclin complexes until DNA replication has commenced, restricting Cdk1/cyclin A activity to later in S phase and potentially preventing precocious firing of late replication origins (Katsuno et al., 2009). When that scaffolding function is preserved, moreover, Cdk2 activity might be required for early cell-cycle events, even though there is not a strict requirement for Cdk2 protein. Removing Cdk2 by gene disruption abolishes both catalytic activity and a non-catalytic function in restraining Cdk1 activation. To distinguish and define those roles, we needed an alternative strategy that would allow: 1) specific inhibition of Cdk2 catalytic activity; and 2) control over Cdk2-cyclin pairing and, consequently, Cdk1 complex assembly.
Here we replace wild-type Cdk2 in human epithelial cells with an analog-sensitive (AS) version modified to bind bulky adenine analogs. A Cdk2as allele is hypomorphic; the mutant protein has lost its competitive advantage over Cdk1 in binding cyclin A. That priority can be restored by different analogs, which either promote activation or inhibit activity of Cdk2as. We thus created a chemical “allelic series” that allows both rescue and selective antagonism of Cdk2 functions. Specific inhibition impedes cell proliferation, demonstrating an essential requirement for the catalytic activity of Cdk2. Both catalytic and scaffold functions are required for normal responses to growth factors and timing of R-point passage. Therefore, chemical genetics reveals Cdk2 to be a non-redundant, rate-limiting regulator of G1/S progression in human cells.
Results
Selective inhibition of Cdk2 uncovers a required function in cell proliferation
Gene disruption did not reveal a requirement for Cdk2 in mouse cell proliferation. The removal of Cdk2 protein, however, allows atypical CDK/cyclin complexes to compensate for, and thereby obscure, any exclusive functions it might perform (Aleem et al., 2005; Santamaria et al., 2007). Furthermore, available small molecules effective against Cdk2 also inhibit other CDKs. Therefore, to inactivate Cdk2 specifically while maintaining normal expression levels, we combined chemical genetics (Bishop et al., 1998) with homologous gene replacement in human somatic cells (Papi et al., 2005; Larochelle et al., 2007).
Most protein kinases contain a bulky “gatekeeper” residue in the ATP binding pocket that can be mutated to a smaller Ala or Gly residue, sensitizing them to bulky adenine analogs that do not inhibit wild-type kinases. We targeted the endogenous Cdk2 locus, in human telomerase-expressing retinal pigment epithelial (RPE-hTERT) and HCT116 colon carcinoma cells, with recombinant adeno-associated virus (rAAV) vectors containing a Cdk2F80G AS mutation (Kraybill et al., 2002) (Figures 1A, S1A, S1B). In this way we created an allelic series: Cdk2as/+, which expresses normal levels of Cdk2, half of which is AS; Cdk2as/neo, in which only Cdk2as is expressed but at half the wild-type level due to silencing of one allele by a retained neoR gene; and Cdk2as/as, in which Cdk2as is expressed exclusively and at wild-type levels (Figures 1B, S1C). AS kinases can use bulky ATP analogs as substrates (Kraybill et al., 2002), so to test if Cdk2as expressed in human cells was active, we added radiolabeled N6-(benzyl)-ATP to extracts from the various lines (Figure 1C). As expected, there was no labeling above background in extracts of wild-type cells. In mutant cell extracts, however, there was phosphorylation of discrete proteins, which increased with the number of expressed Cdk2as alleles. The labeling patterns were grossly similar in Cdk2as/as HCT116 and RPE-hTERT extracts, but with differences in intensity of some substrates. We conclude that Cdk2as encodes an active, AS kinase in both human cell types.
Figure 1. Cdk2 activity is required for human cell proliferation.
(A) The Cdk2 locus was targeted by an rAAV vector containing a neomycin resistance (neoR) cassette bounded by loxP sites and homologous arms, as indicated. The F80G mutation and EcoRI site used for genotyping were introduced in exon 3.
(B) RPE-hTERT cells with varying amounts of total and AS Cdk2, quantified by immunoblot.
(C) HCT116 and RPE-hTERT whole-cell extract proteins were labeled by endogenously expressed Cdk2as with [γ-32P]N6-(benzyl)-ATP.
(D) RPE-hTERT cells of the indicated genotype were grown for 7 d in the presence of increasing amounts of 3-MB-PP1. Cells were fixed and stained with crystal violet solution.
(E) RPE-hTERT cells of the indicated genotype were treated with DMSO or 1 μM 3-MB-PP1 for 48 hr prior to seeding. Cells were maintained in DMSO or, if pre-treated with 1 μM 3-MB-PP1, switched to 10 μM 3-MB-PP1, and cell number was determined every 24 hr. Each data point is the mean +/− SEM of 3 independent plates of cells.
We next asked if specific inhibition of Cdk2as affected cell proliferation (Figures 1D, E, S1D, E). In the absence of drugs, all four RPE-hTERT lines grew at similar rates, although Cdk2as/as HCT116 cells grew more slowly than did wild-type cells. Growth of Cdk2as/as cells—both RPE-hTERT and HCT116—was inhibited by the pyrazolopyrimidine-based, allele-specific inhibitor 3-MB-PP1 in a dose-dependent manner. Wild-type cells were impervious to 3-MB-PP1 concentrations up to 10 μM, establishing that effects of the drug were strictly dependent on the Cdk2as mutation. In both drug titrations (Figure 1D) and time courses at 10 μM 3-MB-PP1 (Figure 1E), Cdk2as/+ and Cdk2as/neo RPE-hTERT cells had sensitivities intermediate between those of Cdk2+/+ and Cdk2as/as cells—a dependency on gene dosage that validates the chemical-genetic strategy. The indistinguishable sensitivities of Cdk2as/+ and Cdk2as/neo cells imply that either wild-type Cdk2 itself (in Cdk2as/+), or other CDKs such as Cdk1 (in Cdk2as/neo), can compensate for inhibition of Cdk2as expressed from a single gene copy. Also consistent with this interpretation, mouse embryonic fibrobalasts (MEFs), derived from Cdk2−/− cells but expressing Cdk2as at ~ten times the physiologic level (data not shown), were more sensitive to 3-MB-PP1 than were human cells expressing Cdk2as at 50–100% of normal levels (Figure S1E). [Construction and characterization of Cdk2as MEFs will be described elsewhere (D.H., N.E.H. and A.G., unpublished observations).] Although overexpression of Cdk2as could interfere with Cdk1 functions, specific inhibition of Cdk2as expressed at exactly wild-type levels slowed or blocked proliferation of both non-transformed RPE-hTERT and HCT116 cancer cells. The latter were resistant to reductions in Cdk2 protein or activity levels by dominant-negative or antisense approaches (Tetsu and McCormick, 2003). This difference illustrates the power of chemical genetics to uncover essential functions missed by other methods.
Cdk2as has conformational defects rescued by analogs
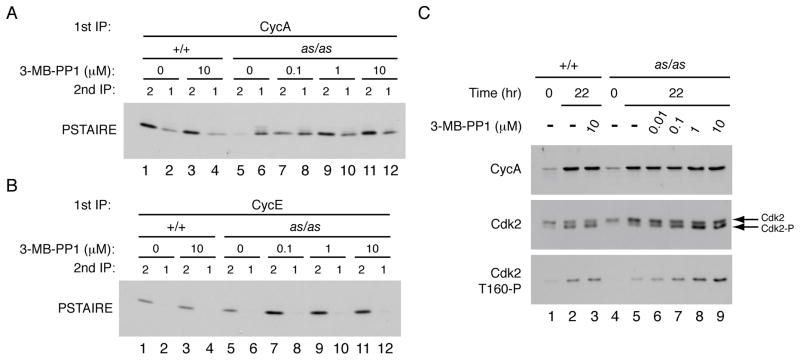
Mammalian cells can tolerate loss of Cdk2 protein, but not acute, selective inhibition of Cdk2as expressed at physiologic levels, perhaps because normal CDK-cyclin pairing was preserved in the latter scenario. We tested this with an assay we developed previously to compare the relative amounts of Cdk1 and Cdk2 bound to each cyclin (Merrick et al., 2008). The nearly exclusive binding of cyclin E to Cdk2 was maintained in Cdk2as/as HCT116 cells, but Cdk2as had a defect in binding cyclin A (Figure 2A, B). In asynchronously growing wild-type cells, cyclin A complexes contained ~2-fold more Cdk2 than Cdk1, but in the mutant cells the ratio was inverted (Figure 2A, compare lanes 1 and 2 with lanes 5 and 6). We obtained similar results in RPE-hTERT cells; introduction of one or two expressed copies of Cdk2as increased cyclin A-binding to Cdk1, at the expense of Cdk2 (Figure S2A). The reduction in Cdk2as/cyclin A correlated with decreased Cdk2as T-loop phosphorylation (Figures 1B, 2C). In vitro, Cdk2as readily formed complexes with cyclin A (data not shown), suggesting that the mutation specifically impaired the ability of Cdk2as to compete with Cdk1 in vivo. These results seemed to indicate that normal CDK-cyclin pairing had not been maintained in the mutant cells as we supposed.
Figure 2. Cdk2as has cyclin A-binding and T-loop phosphorylation defects in vivo.
(A) Wild-type and Cdk2as/as HCT116 cells were treated with DMSO or indicated concentration of 3-MB-PP1 for 96 hr. Cyclin A was immunoprecipitated from extracts and immune complexes were denatured by boiling in 1% SDS. The supernatants were diluted, Cdk1 and Cdk2 were immunoprecipitated, and the amount of each CDK recovered was determined by immunoblotting with PSTAIRE antibody.
(B) Same as (A) except that first immunoprecipitation was of cyclin E.
(C) Wild-type and Cdk2as/as RPE-hTERT cells were synchronized by contact inhibition and released into increasing concentrations of 3-MB-PP1. Amounts of cyclin A, total Cdk2 and Cdk2-Thr160 phosphorylation were determined by immunoblotting—the last both by electrophoretic mobility shift and with Cdk2-Thr160-P specific antibodies.
An active-site mutation that reduced ATP affinity of Cdk2 (Kraybill et al., 2002) disrupted its scaffold function and prevented proper assembly with cyclin A in vivo. We reasoned that a tight-binding ATP-analog inhibitor might correct the conformational defects of the mutant protein (Papa et al., 2003). Indeed, 3-MB-PP1 stimulated cyclin A-Cdk2 binding in Cdk2as/as HCT116 cells in dose-dependent manner—restoring the normal ~2:1 ratio of Cdk2 to Cdk1 in cyclin A complexes (Figure 2A, lanes 11 and 12)—but had no effect on distribution of cyclin A in wild-type cells, or of cyclin E in either cell line (Figure 2B). We next asked if the increase in cyclin A-binding coincided with rescue of T-loop phosphorylation (Figure 2C). To test this, we released G0-arrested RPE-hTERT cells into fresh media containing DMSO or 3-MB-PP1; Cdk2-Thr160 phosphorylation occurred upon cell-cycle reentry in wild-type cells, but this response was diminished in DMSO-treated Cdk2as/as cells (compare lanes 2 and 5). Treatment of Cdk2as/as but not wild-type cells with the analog increased Thr160 phosphorylation in a concentration-dependent manner. The T-loop phosphorylation defect could be complemented by multiple inhibitors of AS kinases (Figure S2B), built on two distinct scaffolds; degree of rescue in vivo correlated with inhibitory potency in vitro (Zhang and Shokat, 2007). The defect in binding cyclin A was accompanied by reduced association with the CDK inhibitor (CKI) p21Cip1, which binds preferentially to CDK/cyclin complexes (Harper et al., 1995); p21Cip1-binding was also restored by 3-MB-PP1 treatment (Figure S2C). Therefore, defects in cyclin A- and CKI-binding, and in phosphorylation by CAK, were rescued by allele-specific inhibitors. In the absence of drugs, impaired complex formation might cause Cdk2as/as cells to resemble Cdk2−/− cells, and could account for their cell-cycle defects (see below). Addition of 3-MB-PP1 restored the normal distribution of cyclin A between its CDK partners, however, thus fulfilling the condition we sought to establish by the AS kinase strategy.
Flipping a three-position switch: chemical complementation of Cdk2as
Although AS-kinase inhibitors could rescue conformational defects of Cdk2as, they would inhibit any active kinase that might form in vivo, and are therefore unlikely to complement Cdk2as phenotypes. We hypothesized that a different small molecule might restore priority in cyclin A-binding to Cdk2as without blocking its activity. Such a compound would occupy the expanded ATP-binding pocket preferentially, but could be competed off by ATP at physiologic concentrations.
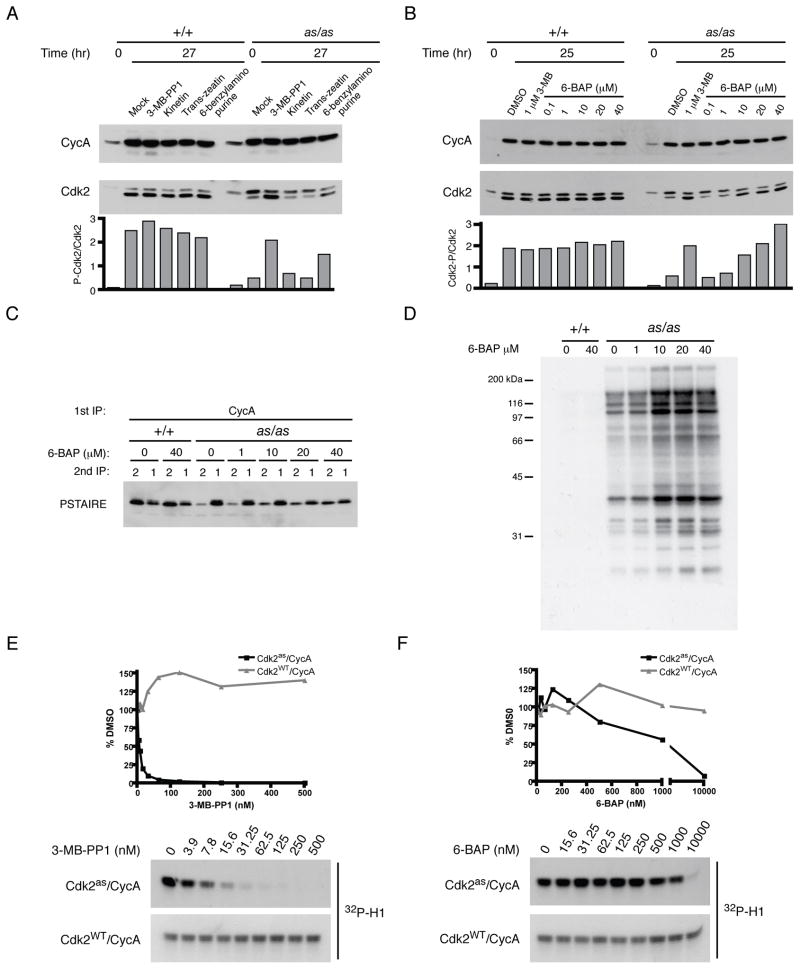
A priori, one class of compounds likely to fulfill both criteria was the N6-modified adenines. These are nucleobase components of ATP analogs used by AS kinases, designed to bind with the N6-substituent group occupying space normally taken up by the gatekeeper side chain (Kraybill et al., 2002), but apt to bind less tightly than the corresponding nucleotides due to their lack of a ribose sugar and phosphates. We screened several adenines by monitoring phosphorylation of the Cdk2as T-loop upon release from contact inhibition (Figure 3A). One compound, 6-benzylaminopurine (6-BAP), rescued phosphorylation in a dose-dependent manner (Figure 3B). Treating cells with 6-BAP also corrected cyclin A- and p21Cip1-binding defects (Figures 3C, S2C), and increased labeling by Cdk2as with [32P]N6-(benzyl)-ATP in extracts (Figure 3D), suggesting a positive effect on Cdk2as activation in vivo. Compared to 3-MB-PP1, 6-BAP was a weak inhibitor of Cdk2as in vitro (IC50 of ~3 nM and ~1 μM, respectively), and neither compound inhibited wild-type Cdk2 (Figure 3E, F). Importantly, 6-BAP, unlike 3-MB-PP1, did not slow Cdk2as/as cell proliferation at the highest dose we tested (80 μM, Figure S3), suggesting that it might be capable of chemical complementation. Cdk2as could then be turned ON or OFF with different small molecules in vivo (see below).
Figure 3. Chemical rescue of Cdk2as by 6-BAP.
(A) Wild-type and Cdk2as/as RPE-hTERT cells were synchronized by contact inhibition and released for 27 hr into medium containing DMSO, 1 μM 3-MB-PP1, or 10 μM of indicated compound. Cyclin A and Cdk2 were detected by immunoblotting, and rescue of Cdk2as was assessed by calculating ratio of phosphorylated-to-unphosphorylated Cdk2 in each extract.
(B) Cells synchronized as in (A) were released into indicated concentration of 3-MB-PP1 or 6-BAP, collected after 25 hr, and phosphorylated-to-unphosphorylated Cdk2 ratio determined.
(C) The ability of 6-BAP to restore normal cyclin A pairing was determined as in Figure 2A.
(D) Extracts from (C) were labeled with [γ-32P]N6-(benzyl)-ATP to determine effect of treating cells with 6-BAP on amount of active Cdk2as in extracts.
(E) Cdk2as/cyclin A and Cdk2WT/cyclin A complexes were tested for sensitivity to 3-MB-PP1 by incubation with increasing concentrations of drug prior to histone H1 kinase assay. IC50 for Cdk2as/cyclin A is ~3 nM.
(F) As in (E) for 3-MB-PP1, IC50 of 6-BAP for Cdk2as/cyclin A was determined to be ~1 μM.
Cdk2as is impaired at two steps of its activation pathway
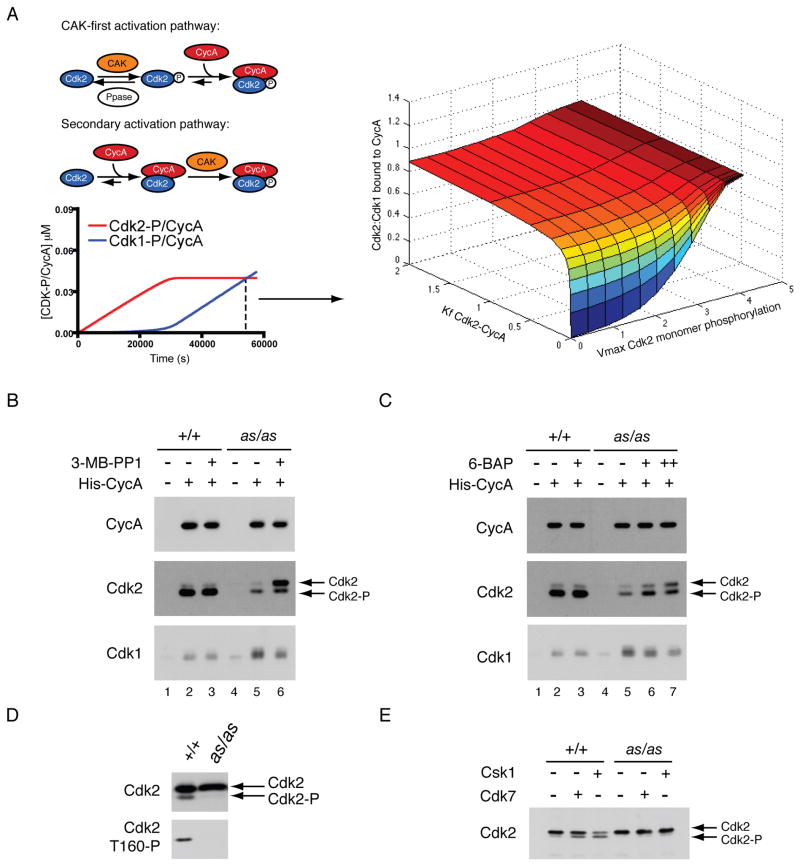
We developed a mathematical model of CDK activation (Merrick and Fisher, 2010), which recapitulates the normal temporal order of Cdk2/ and Cdk1/cyclin A complex formation in vivo (Figures 4A, left, and S4A). To predict how changing kinetic parameters of Cdk2 activation would affect partner selection by cyclin A, we plotted the ratio of Cdk2 to Cdk1 bound to cyclin A at the point (i.e., the cyclin A concentration) when the two complexes are equally abundant under physiologic conditions (Figure 4A, right), which corresponds to late S phase. Deviating from those conditions, either by decreasing the rate of Cdk2 monomer phosphorylation by CAK or reducing affinity of unphosphorylated Cdk2 for cyclin A, did not significantly impair the ability of Cdk2 to compete with Cdk1. Only when both parameters were changed in the same simulation was there a switch to predominant Cdk1-binding, suggesting that reinforcing mechanisms ensure the normal, preferential pairing of cyclin A with Cdk2.
Figure 4. Two defects in Cdk2as activation lead to aberrant CDK/cyclin pairing.
(A) A mathematical model of CDK activation (see Figure S4 and Supplemental Methods for details) was used to test effects of perturbing different parameters on cyclin pairing. The ratio of Cdk2/cyclin A to Cdk1/cyclin A at the end of a simulation (dashed line on left) was calculated and plotted as activation parameters were varied.
(B) To test cycin A selectivity in vitro, an ATP-regenerating system and a limiting amount of purified His-tagged cyclin A were added to extracts of wild-type or Cdk2as/as HCT116 cells. After a 1-hr incubation, His-cyclin A-associated proteins were recovered by Ni2+-affinity, and amounts of bound Cdk1 and Cdk2 determined by immunoblotting. Where indicated, 1 μM 3-MB-PP1 was added to extracts 1 hr prior to cyclin A and ATP.
(C) Cyclin A selectivity was tested as in (B), except where indicated 1 μM 6-BAP was added to extracts 1 hr prior to cyclin A and ATP. In lane 7, 1 μM 6-BAP was also included in buffers for pull-down and wash steps.
(D) Extracts from wild-type and Cdk2as/as HCT116 cells were fractionated in a Superdex 200 sizing column, and Cdk2 monomer fractions were pooled and immunoblotted with antibodies for Cdk2 and Cdk2-Thr160P.
(E) Cdk2 monomer populations from (D) were immunoprecipitated and treated with either Cdk7 complex or S. pombe Csk1. Increased phosphorylation was detected by mobility shift of Cdk2.
Because the Cdk2as mutation abrogated normal pairing rules, it presented an opportunity to test predictions of the model: that, in the absence of analogs, both affinity of Cdk2as for cyclin A and phosphorylation of monomeric Cdk2as should be reduced, relative to wild-type Cdk2. To test the first prediction, we incubated wild-type and Cdk2as/as HCT116 cell extracts with ATP and a limiting amount of recombinant, His-tagged cyclin A, and isolated exogenous cyclin A on Ni2+ beads to determine its relative binding to endogenous Cdk2 and Cdk1. This assay recapitulated the loss of Cdk2- and gain of Cdk1-binding in the mutant cells (Figure 4B, compare lanes 2 and 5). When 3-MB-PP1 was added to Cdk2as/as extracts prior to cyclin A, Cdk2-cyclin A assembly increased whereas the amount of bound Cdk1 decreased nearly to wild-type levels (lane 6). The Cdk2as we recovered was mostly unphosphorylated on the T-loop, indicating that 3-MB-PP1 restored cyclin-binding independent of activating phosphorylation, consistent with the predicted decrease in Cdk2as-cyclin A affinity. The results suggest, moreover, that 3-MB-PP1 directly altered Cdk2as conformation to restore a competitive advantage over Cdk1. Compared to 3-MB-PP1, 6-BAP was less effective at promoting Cdk2as-cyclin A pairing unless it was also included at pull-down and wash steps (Figure 4C, compare lanes 6 and 7), indicating a lower affinity of this compound for the AS kinase. When we omitted 6-BAP from post-incubation steps, we preferentially recovered phosphorylated Cdk2, suggesting that continued presence of the drug was needed to stabilize binding of cyclin A to unphosphorylated Cdk2as, but dispensable for stability of phosphorylated complexes, once they had formed.
To test the second prediction of the model, we asked if Cdk2as was able to follow the primary pathway of activation and become phosphorylated as a monomer in vivo (Figure 4A). Size exclusion chromatography of wild-type and Cdk2as/as HCT116 cell extracts revealed similar profiles of total Cdk2 (Figure S4B, C), but relatively less Thr160 phosphorylation in monomer fractions from Cdk2as/as extracts (Figure 4D). This could arise from inability of CAK to phosphorylate Cdk2as or increased susceptibility of Cdk2as to phosphatases. In support of the first explanation, Cdk2 monomer from wild-type cells could be phosphorylated in vitro by human Cdk7 or by the fission yeast CAK Csk1, whereas Cdk2as was not efficiently phosphorylated by either CAK (Figure 4E). Therefore, as predicted by a mathematical model, Cdk2as is impaired in two distinctive features of its activation pathway that play reinforcing roles in ensuring selectivity of cyclin A for wild-type Cdk2 over Cdk1.
A heightened requirement for Cdk2 in G1/S when growth factors are limiting
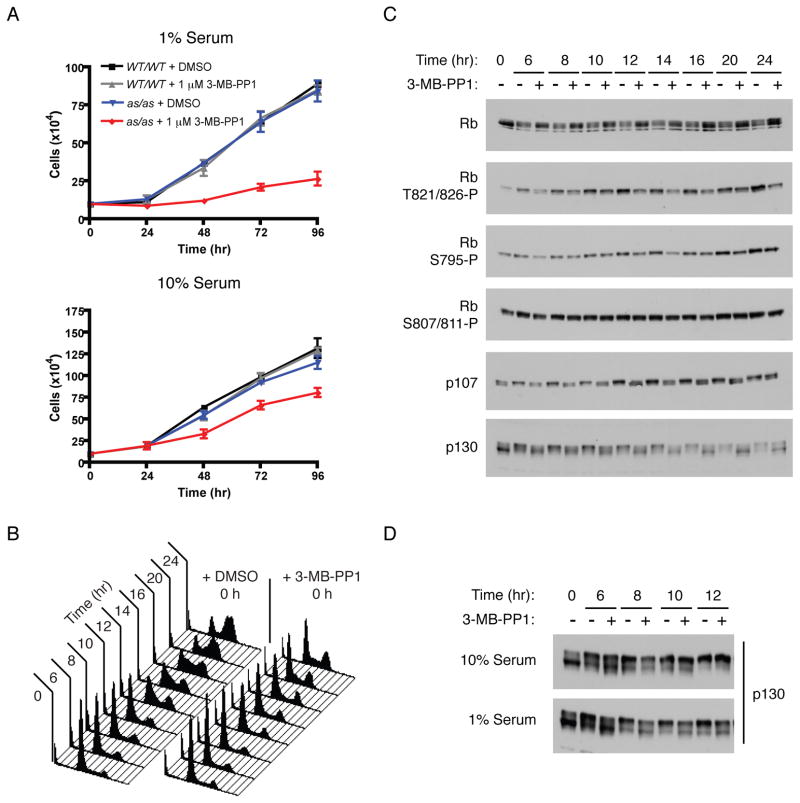
During exit from G0, E2F activation is bistable in response to serum concentration (Yao et al., 2008), suggesting that early cell-cycle events might be more dependent on CDK activity when mitogens are limiting (Lee et al., 2010). Treatment of cells grown in 10% serum with 10 μM 3-MB-PP1 increased doubling time from ~26 to ~40 hr, whereas 1 μM 3-MB-PP1 was less effective at slowing proliferation (doubling time ~33 hr). In 1% serum, however, 1 or 10 μM 3-MB-PP1 was equally effective at prolonging doubling time, to 96 hr, compared to ~28 hr for mock-treated cells (Figure 5A and data not shown). Therefore, growth factor limitation sensitized RPE-hTERT cells to specific inhibition of Cdk2.
Figure 5. A more stringent Cdk2 activity requirement when growth factors are limiting.
(A) Wild-type or Cdk2as/as RPE-hTERT cells were grown in 1% or 10% serum-containing medium with DMSO or 1 μM 3-MB-PP1, and cell number was determined every 24 hr. Cells were pre-treated with DMSO or 1 μM 3-MB-PP1 for 48 hr as in Figure 1E. Each data point is the mean +/− SEM of 3 independent plates of cells.
(B) Cdk2as/as HCT116 cells were synchronized in G0/G1 by serum starvation and released into medium containing 1% serum, nocodazole, and DMSO or 10 μM 3-MB-PP1. Cells were collected at indicated times, and cell-cycle distribution determined by flow-cytometric analysis of DNA content.
(C) Cdk2as/as HCT116 cells synchronized as in (B) were analyzed for pocket protein phosphorylation by immunoblotting with indicated antibodies.
(D) Cdk2as/as HCT116 cells were synchronized as in (B) and released into medium containing 1% or 10% serum and DMSO or 10 μM 3-MB-PP1. Phosphorylation of p130 was detected by mobility shift in immunoblots with pan-specific antibodies.
We next asked if the Cdk2 activity requirement for S-phase entry was sensitive to changing serum concentration. Cdk2as/as HCT116 cells were synchronized by serum starvation and released into media containing varying concentrations of serum with or without 10 μM 3-MB-PP1. Nocodazole was added, to trap any cells that progressed through S and G2 phases in mitosis, and cells were collected for flow cytometry 24 hr after release. In 10% serum, 3-MB-PP1 caused a delay in accumulation of cells with 4N DNA content, which became more pronounced as serum concentration was lowered (Figure S5). Upon release into a medium containing 1% serum, selective inhibition of Cdk2 caused a fraction of cells to arrest with 2N DNA content (Figure 5B).
In parallel, we asked if specific inhibition of Cdk2 affected phosphorylation of pRb, p107, and p130 (Figure 5C). Treatment with 3-MB-PP1 reduced phosphorylation of pRb on residues recognized preferentially by Cdk2 (Thr821) or by both Cdk2 and Cdk4 (Ser795), but not on those targeted exclusively by Cdk4 (Ser807/811) in vitro (Zarkowska and Mittnacht, 1997). In addition, Cdk2 inhibition limited accumulation of hyperphosphorylated isoforms of p107 and p130, indicating that Cdk2 activity is required for phosphorylation of all three pocket proteins in vivo. In 10% serum, the effects of Cdk2 inhibition on pocket protein phosphorylation were less severe (Figure 5D and data not shown), consistent with its diminished impact on G1/S progression (Figure S5). Therefore, requirements for Cdk2 activity to phosphorylate pocket proteins and drive S-phase entry become more stringent when growth factors are limiting.
Cdk2 activity is required for normal R-point timing
Activation of E2F-dependent transcription correlates with passage through the R point, so we tested whether inhibition of Cdk2 affected timing of this irreversible transition. We first asked how RPE-hTERT cells respond to stimulation with different concentrations of serum, by measuring the fraction that passed into S phase after release from contact inhibition (Figure 6A). Even in the absence of drugs, Cdk2as/as cells were more sensitive than wild-type cells to decreased serum concentration. In the presence of 3-MB-PP1, Cdk2as/as cells became more sensitive still, and a large fraction remained arrested in G0/G1 even at saturating serum concentrations. In contrast, releasing Cdk2as/as cells into media containing 6-BAP shifted their serum response closer to that of wild-type cells, suggesting that this compound could indeed complement Cdk2as in vivo. Neither drug affected serum-responsiveness of wild-type RPE-hTERT cells (Figure S6A). These results, together with the increased sensitivity of HCT116 cells to Cdk2 inhibition in low serum (Figures 5A, B), suggest a general requirement for Cdk2 activity in the response to mitogens.
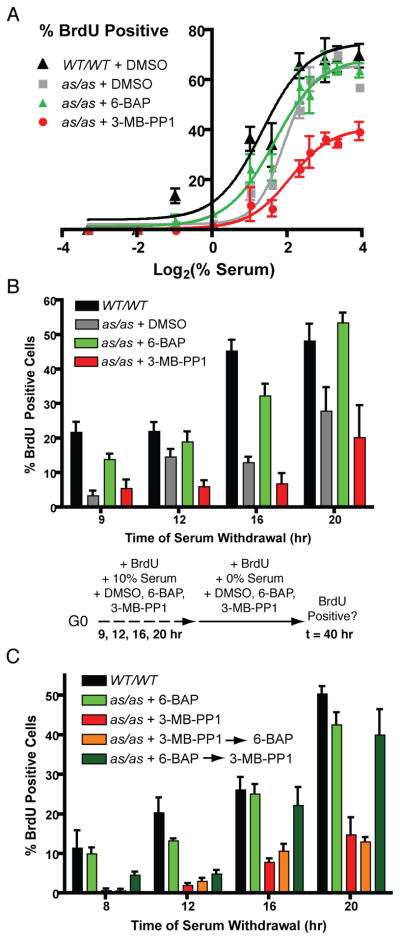
Figure 6. Manipulating Cdk2 with small molecules uncovers a non-redundant role in R-point control.
(A) Wild-type and Cdk2as/as RPE-hTERT cells were synchronized in G0 by contact inhibition and released into medium containing nocodazole, BrdU, the indicated concentration of serum, and DMSO, 0.5 μM 6-BAP or 10 μM 3-MB-PP1. Cells were fixed 40 hr after release and stained to detect BrdU incorporation.
(B) Wild-type and Cdk2as/as RPE-hTERT cells were synchronized in G0 by contact inhibition and released into 10% serum-containing medium supplemented with BrdU and DMSO, 0.5 μM 6-BAP or 10 μM 3-MB-PP1, for 9, 12, 16, or 20 hr. Cells were washed and changed to serum-free medium containing BrdU while maintaining drug treatment. Cells were fixed 40 hr after release and stained to detect BrdU incorporation.
(C) R-point passage was determined as in (B), except that when cells were transferred from 10% to 0% serum, drug treatment was switched from 3-MB-PP1 to 6-BAP (orange bars) or from 6-BAP to 3-MB-PP1 (dark green bars).
Each data point is the mean +/− SEM of 4 fields of cells.
We next asked if modulating Cdk2 activity with small molecules affected the timing of R-point passage in response to saturating levels of growth factors. Cells were released from contact inhibition into media containing 10% serum and either DMSO, 6-BAP or 3-MB-PP1. The medium was removed at various times, and replaced by a serum-free medium containing the same compound present during the first incubation. Finally, we scored R-point passage by measuring BrdU incorporation 40 hr after release. Compared to wild-type cells, Cdk2as/as cells were delayed in passing the R point, suggesting that the mutation per se hindered the ability to respond to mitogens. Treatment with 3-MB-PP1 caused a further delay in passage of the R point, whereas 6-BAP advanced the timing of R-point passage to near that of wild-type cells (Figure 6B). Neither drug had any effect on R-point timing in wild-type cells (Figure S6B). Therefore, Cdk2 activity is rate-limiting for cell-cycle commitment.
We sought to determine when Cdk2 activity is needed: during growth factor stimulation, to pass the R point; after serum removal, to execute a post-R-point function required for S-phase entry; or both. We repeated the R-point experiment with a variation: when 6-BAP or 3-MB-PP1-treated cells were transferred to serum-free media, they were either maintained in the same compound or switched into a medium containing the other drug (Figure 6C). There was no difference in the BrdU-positive fraction between cells kept in 3-MB-PP1 for the entire experiment and those switched from 3-MB-PP1 to 6-BAP. This indicates that Cdk2 activity is required to drive cells past the R point, and that establishment of permissive conditions only after serum withdrawal is not sufficient for continued progression. The reciprocal treatment—6-BAP during stimulation and 3-MB-PP1 upon withdrawal—revealed time-dependent effects: at 8 and 12 hr, the number of BrdU-positive cells was reduced compared to the population maintained in 6-BAP; but this effect was lost at 16 and 20 hr, possibly due to breakdown of cell synchrony. This suggests a window of time, after the R point, during which Cdk2 activity is still required to negotiate the G1/S transition. Therefore, Cdk2 is a non-redundant regulator of both R-point passage and S-phase entry in human cells.
Discussion
Chemical genetics reveal a requirement for Cdk2 activity in human cell division
Gene disruptions revealed that Cdk2 protein is dispensable for viability (Berthet et al., 2003; Ortega et al., 2003), and that Cdk1 can perform all essential functions of interphase CDKs when it is able to form complexes with the relevant cyclins, i.e., when their usual partners are missing (Santamaria et al., 2007). These studies sidestepped whether Cdk2 performs essential functions, exclusively, in wild-type cells. This question is important for understanding how different CDK/cyclin complexes collaborate to make metazoan cell cycles run smoothly, and for efforts to target CDKs therapeutically. The viability of Cdk2−/− animals has been invoked both to discourage and to justify attempts to inhibit Cdk2 in cancer cells (Malumbres and Barbacid, 2009). One line of reasoning holds, simply, that drugs selective for Cdk2 are unlikely to be effective because absence of Cdk2 protein fails to block cell proliferation. A counter-argument—that certain tumors might be more dependent on Cdk2 and therefore susceptible to its inhibition, whereas normal cells might be relatively resistant—gained support from studies implicating Cdk2 in suppression of oncogene-dependent cellular senescence (Campaner et al., 2010; Hydbring et al., 2010). That role was inferred, however, from comparisons of Cdk2−/− and wild-type cells, and from responses to drugs that target other CDKs besides Cdk2.
Our previous work, showing that Cdk2 excludes Cdk1 from active complexes throughout G1 and early S phase (Merrick et al., 2008), suggested a unique function for Cdk2 during the interval when cells commit to a round of division. That role is likely to be general, i.e., not restricted to particular cell types; and to depend critically on Cdk2 levels, because lowering the amount of Cdk2 would advance onset of Cdk1-cyclin assembly and reduce dependency on Cdk2 (as it might, for example, in Cdk2as/neo cells), whereas increasing it would interfere with normal activation of Cdk1 (as it might in MEFs overexpressing Cdk2as).
Here, by a chemical genetic strategy that maintained physiologic expression levels, we showed that Cdk2 activity is required for proliferation even though Cdk2 protein is not essential. Drug sensitivity was dependent on mutant gene dosage (Cdk2as/as > Cdk2as/neo ≈ Cdk2as/+ > Cdk2+/+), implying that ~50% inhibition is sufficient to slow, but complete blockade is needed to arrest, cell division. We probably never achieved 100% inhibition of Cdk2as with 3-MB-PP1 (or complete rescue of function with 6-BAP), so cannot rule out a more stringent dependence on Cdk2 in vivo. We can be certain, however, that conventional CDK inhibitors could not have uncovered this requirement. In vitro, CVT-313 and -2584 are ~8–10-fold more potent towards Cdk2 than towards Cdk1, but ~100-fold less potent than is 3-MB-PP1 towards Cdk2as (Brooks et al., 1997; Campaner et al., 2010). Thus, concentrations required for equivalent inhibition of Cdk2 in vivo would also inhibit other kinases. Consistent with this interpretation, 10 μM CVT-313 blocked S phase more effectively in wild-type cells than did 10 μM 3-MB-PP1 in Cdk2as/as cells (Figure S6C). Therefore, even the most Cdk2-selective drugs available probably have off-target effects that contribute to their phenotypes—a problem obviated by the AS kinase strategy.
A gatekeeper mutant holds keys to CDK activation
A mathematical model indicated that cyclin A pairing rules are enforced by: 1) ability of Cdk7 to phosphorylate Cdk2 monomer and 2) high affinity of unphosphorylated Cdk2 for cyclin A. Introduction of Cdk2as into human cells allowed us to confirm the model’s prediction that both properties would need to be disrupted for Cdk1 to become the preferred partner in vivo.
The order of Cdk2 and Cdk1 activation may coordinate the temporal program of replication origin-firing (Katsuno et al., 2009). In addition, Cdk1/cyclin A has been specifically implicated in attenuating expression of histone genes at the end of S phase (Koseoglu et al., 2008); premature onset of Cdk1/cyclin A assembly could therefore lead to histone depletion and genomic instability. Cdk1 activity is normally kept low until mid-S phase, both by preferential formation of Cdk2/cyclin A complexes (Merrick et al., 2008) and by inhibitory phosphorylation of Cdk1 (Katsuno et al., 2009). Therefore, reinforcing mechanisms evolved to ensure that activation of Cdk2 precedes that of Cdk1 in mammalian cells. Consistent with strong selection to maintain that order, Cdk2as/as cells, in which the barrier to Cdk1/cyclin A activation is lowered, have cell-cycle delays and defective responses to mitogens in the absence of analogs.
The biochemical defects caused by a gatekeeper mutation, and their rescue by small molecules, suggest an important role of active-site structure in normal Cdk2 activation. Similar observations were made for the Ser/Thr kinases Akt, B- and C-Raf, and PKC isoforms α and ε. For each kinase, an ATP-competitive inhibitor induced T-loop phosphorylation in vivo, but by a different mechanism in each case. Drug-binding had two distinct effects on Akt—enhanced membrane localization and a conformational change—both of which favored phosphorylation (Okuzumi et al., 2009). In contrast, Raf inhibitors promoted activating phosphorylation through kinase dimerization (Poulikakos et al., 2010). Cdk2as appears most similar to a PKC gatekeeper mutant with weakened nucleotide binding and reduced T-loop phosphorylation, which was complemented by ATP analogs (Cameron et al., 2009), but may be unique in its impaired binding to an allosteric regulator, cyclin A. This might be analogous to effects of a gatekeeper mutation on interactions between the plant kinase Pto and its bacterial effector proteins, which were also corrected by PP1-based analogs (Salomon et al., 2009).
Here we recapitulated the Cdk2as-activation defect and its rescue by small molecules in vitro, to yield insights into how active-site conformation influences the steps of Cdk2 activation. Cdk2as is a poor substrate for both Cdk7 and Csk1, which was surprising because Csk1, unlike Cdk7, readily phosphorylates monomeric Cdk1 (Merrick et al., 2008). This suggests the T-loop is generally unavailable for protein-protein interactions in the Cdk2as monomer. Because the T-loop contributes to cyclin-binding (Jeffrey et al., 1995; Russo et al., 1996), its mal-orientation could also explain a lower affinity for cyclin A.
We were able to correct these defects in vivo with 3-MB-PP1, a strong inhibitor, or 6-BAP, which is weakly inhibitory in vitro and had no negative effect on cell proliferation. Cdk2as is therefore a three-position switch (Figure 7): defective for activation in the absence of drugs; bound normally to cyclins, but inhibited, in the presence of 3-MB-PP1; and closer to wild-type Cdk2 in both cyclin-binding and activity in the presence of 6-BAP. Chemical rescue of a kinase hypomorph has been described previously; active-site mutations in the Tyr kinases Src and Csk impaired their activity, but imidazole could act as a prosthetic group to restore catalytic function (Williams et al., 2000; Qiao et al., 2006). 6-BAP is likely to rescue Cdk2as by a different mechanism—transient binding to allow complex formation and phosphorylation, and subsequent displacement by ATP to permit catalytic activity. It will be interesting to see if 6-BAP can rescue other AS kinases in which gatekeeper mutations impair function.
Figure 7. Cdk2as: a 3-position switch to regulate R-point passage with small molecules.
Cdk2as has activation defects in untreated cells, leading to lower abundance of Cdk2/cyclin A complexes and impaired R-point control. From this partially active state, Cdk2as can be turned ON or OFF, respectively, with the small molecules 6-BAP or 3-MB-PP1—both of which correct conformation, but with opposite effects on catalytic activity—to uncover a rate-limiting role of Cdk2 in pocket protein phosphorylation, R-point passage and S-phase entry.
Cdk2 is a non-redundant regulator of R-point passage and the G1/S transition
In response to growth-factor signaling, Myc drives expression of cyclin D, which activates Cdk4 and Cdk6, leading to partial phosphorylation of pocket proteins and derepression of E2F targets including cyclin E, which promotes further phosphorylation of Rb family members and full E2F activation (Yao et al., 2008). Inhibition of Cdk2 disrupted normal R-point control, possibly by interrupting this positive feedback loop. Even in the absence of drugs, S-phase entry was more sensitive to lowered serum concentration in Cdk2as/as than in wild-type cells; that sensitivity could be enhanced by 3-MB-PP1 or suppressed by 6-BAP. Similarly, E2F expression in rat fibroblasts was more sensitive to CDK inhibition by CVT-313 at low serum concentrations (Lee et al., 2010). Manipulating Cdk2as activity with 3-MB-PP1 or 6-BAP delayed or accelerated R-point passage, respectively, revealing a rate-limiting function of Cdk2 and, possibly, of positive feedback. When mitogens are limiting, blocking that function arrests cells in G1, but in 10% serum, signaling through other pathways can override inhibition of Cdk2 to allow progression, albeit delayed, through the cell cycle. Indeed, this result might have been predicted by a mathematical model, in which increased amplitude of Myc expression converted the R-point switch from a bistable to a monostable one (Yao et al., 2008).
Once budding yeast cells pass Start—analogous to the mammalian R point—they proceed through division independent of environmental cues (Johnston et al., 1977). Also like the R point, Start depends on CDK/cyclin complexes that activate a transcription program important for later cell-cycle events (Spellman et al., 1998; Skotheim et al., 2008). The cyclin Cln3 initiates the transition by promoting transcription dependent on the activators SBF and MBF, targets of which include CLN1 and CLN2, which also encode cyclins that act, through Cdk1 and SBF/MBF, to promote their own expression (Dirick et al., 1995). Removal of this positive feedback by deletion of CLN1 and CLN2 had two main effects on G1/S transcription. First, activation of the program took longer and occurred with greater cell-to-cell variability. Second, there was a variable delay between onset and maximal expression of target genes. The aberrant timing and incoherence of gene expression in cln1Δ cln2Δ cells led to increased frequency of cell-cycle arrest, demonstrating the importance of positive feedback for normal cell division (Skotheim et al., 2008).
We hypothesize that Cdk2 activity is specifically required for timely and coherent expression of E2F-dependent genes—functions analogous to those of G1 cyclin/CDK complexes in yeast. Selective inhibition of Cdk2as in human cells dampened the response to growth factor signaling in G1, impeded passage of the R point and impaired proliferation. This is consistent with the previous finding that S-phase entry was defective in MEFs exiting G0 without cyclin E (Geng et al., 2003), which binds nearly exclusively to Cdk2 in human cells (Merrick et al., 2008). The ability to escape quiescence and proliferate, often under suboptimal growth conditions, is a feature of oncogenic transformation. Therefore, specific functions of Cdk2 uncovered by chemical genetics could yield new insights into how CDKs in general, and R-point control in particular, might be effectively targeted by anticancer therapy.
Experimental Procedures
Construction of Cdk2as cell lines
To replace wild-type Cdk2 with Cdk2as, we followed the strategy used to create Cdk7as/as cells (Larochelle et al., 2007). We constructed an rAAV vector containing two contiguous regions of the Cdk2 locus amplified from BAC clone RP11-973D8 (Children’s Hospital of Oakland Research Institute) flanking a loxP-neoR-loxP cassette, mutated exon 3 to change Phe80 to Gly and create an EcoRI restriction site for screening purposes, and performed two rounds of targeting as follows: 1) rAAV infection and selection for G418-resistance, PCR screening for correct integration and verification of mutations by sequencing; 2) Cre recombinase-mediated excision of neoR to yield Cdk2as/+ clones, verified by PCR and reversion to G418-sensitivity; and 3) repetition of steps 1 and 2 to yield homozygous Cdk2as/as clones.
Biochemical and Immunological Methods
Extract preparation, analyses of CDK assembly and activation, Superdex 200 chromatography, immunoblotting, and immunoprecipitation were carried out as described (Merrick et al., 2008).
Chemical Genetic Methods
Labeling with [γ-32P]N6-(benzyl)-ATP was performed as previously described (Larochelle et al., 2007; Merrick et al., 2008). 3-MB-PP1, 6-BAP and other N6-modified adenines were dissolved in DMSO and used at indicated concentrations. IC50 was measured by incubating purified Cdk2/cyclin A with varying concentrations of 3-MB-PP1 or 6-BAP, prior to kinase reactions containing 25 mM HEPES pH 7.4, 150 mM NaCl, 10 mM MgCl2, 200 μM ATP, 5 μCi [γ-32P]ATP, 5 μg histone H1, ~3 nM Cdk2/cyclin A. Incorporation was visualized by autoradiography and quantified by phosphorimager.
Cell-based analysis of Cdk2 function
To test a requirement for Cdk2 activity we seeded 100 cells/well in 96-well dishes with varying amounts of 3-MB-PP1. After 7 d, medium was removed and cells were stained with crystal violet. For proliferation curves, asynchronously growing cells pre-treated for 48 hr with DMSO or 3-MB-PP1 (to allow correction of the cyclin A-binding defect) were plated at 105 cells/well in a 6-well dish and cell number determined in triplicate every 24 hr with a hemacytometer. To analyze Cdk2 functions in G1/S, we synchronized cells by serum starvation for 72 hr (HCT116) or by contact inhibition (RPE-hTERT). HCT116 cell-cycle progression was monitored by analyzing DNA content with propidium iodide-staining and flow cytometry. To quantify cells that entered S phase, we released RPE-hTERT cells from G0 in chamber slides containing medium + 10 μg/mL BrdU. Cells were fixed in 70% ethanol, DNA was denatured with 1 M HCl for 10 min, and the rest of the staining protocol was performed as described (Terret et al., 2009). Incorporation was detected with anti-BrdU antibody (1:100) and visualized with secondary antibody conjugated to DyLight488 or Cy5. Cells were counterstained with DAPI to determine total numbers. R-point assays were performed in the same way except that, at various times after release, serum-containing medium was removed, cells were washed twice, and serum-free medium containing BrdU was added.
Supplementary Material
Figure S1, Related to Figure 1: This figure contains details of the screening and genotyping we performed in the course of generating Cdk2as/as HCT116 and RPE-hTERT cells used in this study (Figure S1A, B). Additionally, it shows that Cdk2as/as HCT116 cells have the same amount of Cdk2 protein as wild-type cells (Figure S1C), that Cdk2as/as HCT116 cells are sensitive to 3-MB-PP1 (S1D), and that Cdk2−/− MEFs ectopically expressing Cdk2as are sensitive to 3-MB-PP1.
Figure S2, Related to Figure 2: Here we show that substitution of Cdk1 for Cdk2 in cyclin A complexes occurs in cells expressing one or two copies of Cdk2as ((S1A), that AS inhibitors built on a two distinct scaffolds can restore Cdk2as T-loop phosphorylation to wild-type levels, and chemical rescue correlates with inhibitor potency (S1B), and that the gatekeeper mutation impairs, and ATP analogs restore, binding of Cdk2 to the CDK inhibitor p21 (S2C).
Figure S3, Related to Figure 3: This figure demonstrates that a high dose of 6-BAP (80 μM) does not inhibit proliferation of wild-type or Cdk2as/as RPE-hTERT cells.
Figure S4, Related to Figure 4: Figure S4A shows how the temporal dynamics of CDK-cyclin complex formation is affected by altering parameters of Cdk2’s activation pathways, thus providing a more complete view of the modeling results presented in Figure 4A. Here we also show the complete sizing column profiles from wild-type (Figure S4B) and Cdk2as/as (Figure S4C) HCT116 extracts and indicate the fractions we pooled for our experiments with monomeric Cdk2 (Figure 4D and E).
Figure S5, Related to Figure 5: In this figure we release Cdk2as/as HCT116 from serum starvation into medium with varying serum concentration. We find that decreasing the serum concentration sensitizes cells to Cdk2 inhibition justifying the experiments in Figure 5C-D, performed with cells grown in 1% serum.
Figure S6, Related to Figure 6: Here we show that 6-BAP and 3-MB-PP1 do not affect the serum-response curve of RPE-hTERT wild-type cells (Figure S6A), and these compounds do not affect R point timing in wild-type cells (Figure S6B). Figure S6C provides evidence that the phenotype produced by CVT-313 treatment depends on inhibition of other kinases besides Cdk2.
Acknowledgments
We thank K. George and P. Jallepalli for advice and assistance in gene targeting, D. Morgan for baculovirus encoding Cdk2as, and members of the Fisher lab for helpful discussions and critical review of the manuscript. The work was supported by fellowship support of the Deutsche Forschungsgemeinschaft to L.W. (WO1456) and the California Breast Cancer Research Program to D. H. and N.E.H., and by NIH grants GM056985 to R.P.F., EB001987 to K.M.S. and CA136717 to A.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleem E, Kiyokawa H, Kaldis P. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat Cell Biol. 2005;7:831–836. doi: 10.1038/ncb1284. [DOI] [PubMed] [Google Scholar]
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol. 2003;13:1775–1785. doi: 10.1016/j.cub.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Bishop AC, Shah K, Liu Y, Witucki L, Kung C-y, Shokat KM. Design of allele-specific inhibitors to probe protein kinase signaling. Curr Biol. 1998;8:257–266. doi: 10.1016/s0960-9822(98)70198-8. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Ciro M, Cocito A, Helin K. E2F target genes: unraveling the biology. Trends Biochem Sci. 2004;29:409–417. doi: 10.1016/j.tibs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Brooks EE, Gray NS, Joly A, Kerwar SS, Lum R, Mackman RL, Norman TC, Rosete J, Rowe M, Schow SR, et al. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J Biol Chem. 1997;272:29207–29211. doi: 10.1074/jbc.272.46.29207. [DOI] [PubMed] [Google Scholar]
- Burke JR, Deshong AJ, Pelton JG, Rubin SM. Phosphorylation-induced conformational changes in the retinoblastoma protein inhibit E2F transactivation domain binding. J Biol Chem. 2010;285:16286–16293. doi: 10.1074/jbc.M110.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AJ, Escribano C, Saurin AT, Kostelecky B, Parker PJ. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat Struct Mol Biol. 2009;16:624–630. doi: 10.1038/nsmb.1606. [DOI] [PubMed] [Google Scholar]
- Campaner S, Doni M, Hydbring P, Verrecchia A, Bianchi L, Sardella D, Schleker T, Perna D, Tronnersjo S, Murga M, et al. Cdk2 suppresses cellular senescence induced by the c-myc oncogene. Nat Cell Biol. 2010;12:54–59. doi: 10.1038/ncb2004. sup pp 51–14. [DOI] [PubMed] [Google Scholar]
- Classon M, Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat Rev Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- Dirick L, Bohm T, Nasmyth K. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. Embo J. 1995;14:4803–4813. doi: 10.1002/j.1460-2075.1995.tb00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov MV, Dyson NJ. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J Cell Sci. 2004;117:2173–2181. doi: 10.1242/jcs.01227. [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ, Keyomarski K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Mitra J, van den Heuvel S, Enders GH. S and G2 phase roles for Cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol. 2001;21:2755–2766. doi: 10.1128/MCB.21.8.2755-2766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hydbring P, Bahram F, Su Y, Tronnersjo S, Hogstrand K, von der Lehr N, Sharifi HR, Lilischkis R, Hein N, Wu S, et al. Phosphorylation by Cdk2 is required for Myc to repress Ras-induced senescence in cotransformation. Proc Natl Acad Sci U S A. 2010;107:58–63. doi: 10.1073/pnas.0900121106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey PD, Russo AA, Polyak K, Gibbs E, Hurwitz J, Massagué J, Pavletich NP. Mechanism of CDK activation revealed by the structure of a cyclinA-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- Johnston GC, Pringle JR, Hartwell LH. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977;105:79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Katsuno Y, Suzuki A, Sugimura K, Okumura K, Zineldeen DH, Shimada M, Niida H, Mizuno T, Hanaoka F, Nakanishi M. Cyclin A-Cdk1 regulates the origin firing program in mammalian cells. Proc Natl Acad Sci U S A. 2009;106:3184–3189. doi: 10.1073/pnas.0809350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koseoglu MM, Graves LM, Marzluff WF. Phosphorylation of threonine 61 by cyclin a/Cdk1 triggers degradation of stem-loop binding protein at the end of S phase. Mol Cell Biol. 2008;28:4469–4479. doi: 10.1128/MCB.01416-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraybill BC, Elkin LL, Blethrow JD, Morgan DO, Shokat KM. Inhibitor scaffolds as new allele specific kinase substrates. J Am Chem Soc. 2002;124:12118–12128. doi: 10.1021/ja0264798. [DOI] [PubMed] [Google Scholar]
- Larochelle S, Merrick KA, Terret ME, Wohlbold L, Barboza NM, Zhang C, Shokat KM, Jallepalli PV, Fisher RP. Requirements for Cdk7 in the assembly of Cdk1/cyclin B and activation of Cdk2 revealed by chemical genetics in human cells. Mol Cell. 2007;25:839–850. doi: 10.1016/j.molcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TJ, Yao G, Bennett DC, Nevins JR, You L. Stochastic E2F activation and reconciliation of phenomenological cell-cycle models. PLoS Biol. 2010;8:e1000488. doi: 10.1371/journal.pbio.1000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- Merrick KA, Fisher RP. Putting one step before the other: distinct activation pathways for Cdk1 and Cdk2 bring order to the mammalian cell cycle. Cell Cycle. 2010;9:706–714. doi: 10.4161/cc.9.4.10732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick KA, Larochelle S, Zhang C, Allen JJ, Shokat KM, Fisher RP. Distinct activation pathways confer cyclin binding selectivity on Cdk1 and Cdk2 in human cells. Mol Cell. 2008;32:662–672. doi: 10.1016/j.molcel.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DO. The Cell Cycle: Principles of Control. London: New Science Press Ltd; 2007. [Google Scholar]
- Okuzumi T, Fiedler D, Zhang C, Gray DC, Aizenstein B, Hoffman R, Shokat KM. Inhibitor hijacking of Akt activation. Nat Chem Biol. 2009;5:484–493. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Lukas J, Baldin V, Ansorge W, Bartek J, Draetta G. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa FR, Zhang C, Shokat K, Walter P. Bypassing a kinase activity with an ATP-competitive drug. Science. 2003;302:1533–1537. doi: 10.1126/science.1090031. [DOI] [PubMed] [Google Scholar]
- Papi M, Berdougo E, Randall CL, Ganguly S, Jallepalli PV. Multiple roles for separase auto-cleavage during the G2/M transition. Nat Cell Biol. 2005;7:1029–1035. doi: 10.1038/ncb1303. [DOI] [PubMed] [Google Scholar]
- Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Molina H, Pandey A, Zhang J, Cole PA. Chemical rescue of a mutant enzyme in living cells. Science. 2006;311:1293–1297. doi: 10.1126/science.1122224. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Gu Y, Morgan DO. Human cyclin-dependent kinase 2 (CDK2) is activated during the S and G2 phases of the cell cycle and associates with Cyclin A. Proc Natl Acad Sci USA. 1992;89:2824–2828. doi: 10.1073/pnas.89.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin SM, Gall AL, Zheng N, Pavletich NP. Structure of the Rb C-terminal domain bound to E2F1-DP1: a mechanism for phosphorylation-induced E2F release. Cell. 2005;123:1093–1106. doi: 10.1016/j.cell.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Russo AA, Jeffrey PD, Pavletich NP. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nature Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- Salomon D, Bonshtien A, Mayrose M, Zhang C, Shokat KM, Sessa G. Bypassing kinase activity of the tomato Pto resistance protein with small molecule ligands. J Biol Chem. 2009;284:15289–15298. doi: 10.1074/jbc.M809724200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria D, Barriere C, Cerqueira A, Hunt S, Tardy C, Newton K, Caceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448:811–815. doi: 10.1038/nature06046. [DOI] [PubMed] [Google Scholar]
- Skotheim JM, Di Talia S, Siggia ED, Cross FR. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature. 2008;454:291–296. doi: 10.1038/nature07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B. Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell. 1998;9:3273–3297. doi: 10.1091/mbc.9.12.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terret ME, Sherwood R, Rahman S, Qin J, Jallepalli PV. Cohesin acetylation speeds the replication fork. Nature. 2009;462:231–234. doi: 10.1038/nature08550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi: 10.1016/s1535-6108(03)00053-9. [DOI] [PubMed] [Google Scholar]
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- Williams DM, Wang D, Cole PA. Chemical rescue of a mutant protein-tyrosine kinase. J Biol Chem. 2000;275:38127–38130. doi: 10.1074/jbc.C000606200. [DOI] [PubMed] [Google Scholar]
- Yao G, Lee TJ, Mori S, Nevins JR, You L. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol. 2008;10:476–482. doi: 10.1038/ncb1711. [DOI] [PubMed] [Google Scholar]
- Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- Zhang C, Shokat K. Enhanced selectivity for inhibiton of analog-sensitive protein kinases through scaffold optimization. Tetrahedron. 2007;63:5832–5838. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, Related to Figure 1: This figure contains details of the screening and genotyping we performed in the course of generating Cdk2as/as HCT116 and RPE-hTERT cells used in this study (Figure S1A, B). Additionally, it shows that Cdk2as/as HCT116 cells have the same amount of Cdk2 protein as wild-type cells (Figure S1C), that Cdk2as/as HCT116 cells are sensitive to 3-MB-PP1 (S1D), and that Cdk2−/− MEFs ectopically expressing Cdk2as are sensitive to 3-MB-PP1.
Figure S2, Related to Figure 2: Here we show that substitution of Cdk1 for Cdk2 in cyclin A complexes occurs in cells expressing one or two copies of Cdk2as ((S1A), that AS inhibitors built on a two distinct scaffolds can restore Cdk2as T-loop phosphorylation to wild-type levels, and chemical rescue correlates with inhibitor potency (S1B), and that the gatekeeper mutation impairs, and ATP analogs restore, binding of Cdk2 to the CDK inhibitor p21 (S2C).
Figure S3, Related to Figure 3: This figure demonstrates that a high dose of 6-BAP (80 μM) does not inhibit proliferation of wild-type or Cdk2as/as RPE-hTERT cells.
Figure S4, Related to Figure 4: Figure S4A shows how the temporal dynamics of CDK-cyclin complex formation is affected by altering parameters of Cdk2’s activation pathways, thus providing a more complete view of the modeling results presented in Figure 4A. Here we also show the complete sizing column profiles from wild-type (Figure S4B) and Cdk2as/as (Figure S4C) HCT116 extracts and indicate the fractions we pooled for our experiments with monomeric Cdk2 (Figure 4D and E).
Figure S5, Related to Figure 5: In this figure we release Cdk2as/as HCT116 from serum starvation into medium with varying serum concentration. We find that decreasing the serum concentration sensitizes cells to Cdk2 inhibition justifying the experiments in Figure 5C-D, performed with cells grown in 1% serum.
Figure S6, Related to Figure 6: Here we show that 6-BAP and 3-MB-PP1 do not affect the serum-response curve of RPE-hTERT wild-type cells (Figure S6A), and these compounds do not affect R point timing in wild-type cells (Figure S6B). Figure S6C provides evidence that the phenotype produced by CVT-313 treatment depends on inhibition of other kinases besides Cdk2.