Abstract
Antibodies that mediate human immunodeficiency virus (HIV)-specific antibody-dependent cell-mediated cytotoxicity (ADCC) are present in the cervical fluid of many HIV-positive women; however, the role that these antibodies play in host defense against HIV is not known. To understand the contribution of ADCC in cervical secretions as a protective mechanism against HIV, we evaluated ADCC titers in paired serum and cervical-lavage (CVL) samples from >300 HIV-1–positive women who participated in the multicenter Division of AIDS Treatment Research Initiative Study 009. The present study demonstrates that women with CVL ADCC activity had lower genital viral loads than did women with serum ADCC activity only. Women with CVL ADCC activity were likely to have HIV-1 gp120–specific immunoglobulin (Ig) G, but not IgA, in their cervical fluid. This finding suggests that specific IgG in cervical fluid can mediate ADCC activity that inversely correlates with genital viral load.
Antibody-dependent cell-mediated cytotoxicity (ADCC) combines acquired and innate immunity. The acquired response provides the antibodies; the effector cells are part of innate immunity. Innate ADCC effector cells include NK cells [1], monocytes and macrophages [2], polymorphonuclear leukocytes [3], and eosinophils [4]; they are not antigen specific, but they lyse infected cells after binding to the antibody’s Fc region.
The specific contribution of innate immunity to host defense against HIV is controversial. The importance of innate immunity for viruses such as cytomegalovirus and herpes simplex viruses is clear [5, 6]. Vaccines against lymphocytic choriomeningitis virus are not protective unless they induce ADCC antibodies [7]. Children deficient in CD16+CD56+ NK cells can have life-threatening infections with herpes viruses, such as varicella-zoster virus. Cytotoxic T lymphocytes (CTLs) are more important for other viruses. Antibodies, CTLs, natural immunity, and ADCC all contribute to host defense against HIV infection and delay disease progression. The relative contribution of each to host defense against HIV-1 has not been determined.
Our knowledge of the infective and immune processes in the female genital tract is limited [8]. Antibodies that mediate ADCC against HIV-1 gp120 in an in vitro assay are present in cervical-lavage (CVL) fluid [9], but we have not determined the isotype of these antibodies. ADCC effector cells are primarily specific for IgG1 [10, 11]. Although IgA is predominant in many mucosal secretions [12], cervical fluid normally contains slightly more IgG than IgA [13]; also, when a woman is infected with HIV-1, the HIV-1–specific antibodies are almost exclusively IgG [13]. Although secretory IgA (S-IgA) antibodies may mediate ADCC [14], HIV-1–positive women have relatively high concentrations of HIV-1–specific IgG antibodies in their cervical fluid [15, 16]. Either class of antibody could be responsible for the functional activity reported elsewhere [9].
HIV-1–specific ADCC antibodies are present in the serum [17] and cervical fluid [9] of many HIV-1–positive women. Serum ADCC antibodies contribute to the protective defense against HIV disease progression; rapid progressors in the Multicenter AIDS Cohort Study had low titers, whereas long-term survivors had high titers [17]. When individuals have high CD4+ cell numbers, plasma ADCC activity correlates with lower plasma viral loads [18]. ADCC antibody level correlates with higher CD4+ cell numbers and lower viral loads [19]. Longitudinal in vivo studies in macaques have shown that ADCC activity correlates with delayed progression to AIDS [20]. All of these studies have addressed the role that ADCC plays in the circulation, but they have not addressed the contribution of ADCC to mucosal defense in the female genital tract.
In a previous study, we used very stringent criteria for a positive response and reported that ~16% of HIV-1–positive women had HIV-1–specific CVL ADCC antibodies [9]. Although this demonstrated that infected women can have cervical ADCC, the sample size was too small to draw conclusions about the role that ADCC plays in local host defense against HIV-1. In the present study, we evaluated the ADCC activity of paired serum and CVL samples from 302 women who participated in the Division of AIDS Treatment Research Initiative Study 009 (DATRI 009). In an effort to determine the effect that ADCC activity has on HIV disease, we then compared data on ADCC activity with data from other DATRI 009 investigators on samples collected at the same visit.
SUBJECTS, MATERIALS, AND METHODS
Study population
Paired serum and CVL samples were obtained from 302 HIV-1–positive women enrolled in DATRI 009 between January 1997 and July 1998. Before entry into the study, women were positive for HIV-1 and had been receiving either stable or no antiretroviral therapy (ART) for at least 1 month, were 18–45 years of age, were not pregnant, and had an intact uterus and cervix. ADCC activity was determined for 282 of the 302 women. Clinical, virologic, and immunologic characteristics of this cohort have been described elsewhere [21]. Informed consent was obtained from all women who provided serum and/or CVL samples for these studies. Investigators followed the human-experimentation guidelines of the US Department of Health and Human Services, and protocols were approved by the appropriate institutional review boards.
To establish baseline ADCC activity, HIV-1–negative serum and CVL samples were used. Serum (n = 20) and CVL (n = 15) samples were collected from women in the Chicago cohort of the Women’s Interagency HIV Study (WIHS). The WIHS is a multicenter prospective study designed to determine the effect of HIV-1 infection in women.
ART status of participants
Women were treated with antiretroviral medications on the basis of their disease progression, as determined by their physician. Study participants reported the type and number of medications they were taking at the time of enrollment. The majority of women, 61% (173/282), were receiving ART: 34% were taking 3–4 antiretroviral medications, 21% were taking 2 antiretroviral medications, and 2% were taking only 1 antiretroviral medication.
Sample collection
CVL and endocervical-swab samples were collected from women who had abstained from douching, using spermicides, receiving antimicrobial and antifungal therapy, and having vaginal intercourse for 48 h before the study visit. Abstinence during the prior 48 h was confirmed by a negative assay for seminal-fluid antigen in CVL fluid (SEMA test; Humagen Fertility Diagnostics). CVL samples were collected by irrigating the cervical os with 10 mL of nonbacteriostatic saline that was aspirated from the posterior vaginal fornix. To obtain supernatants, CVL samples were centrifuged at 400 g for 10 min. Endocervical swabs were used to collect samples for quantitation of HIV-1 RNA. A puritan sterile Dacron polyester-tip applicator (Harwood Products) was inserted into the cervical os and rotated 360 degrees. The swabs were placed in a 4 mol/L guanidine isothiocynate solution, mixed with mercaptoethanol. The swabs were then placed in extraction medium before reverse-transcription polymerase chain reaction (RT-PCR) was performed. Plasma used for quantitation of HIV-1 RNA was obtained from peripheral blood collected in acid-citrate dextrose. All samples were frozen at −70°C and were shipped to the central repository.
Target cells
CEM.NKR, a CD4+ NK cell–resistant cloned cell line derived from CEM [22], were used as target cells in the 51Cr-release assay. CEM.NKR cells have <5% NK cell activity. More than 90% of the target cells bind recombinant HIV-1 gp120 after 1 h of incubation, as determined by flow cytometry (data not shown). Target cells (6 × 106) were incubated with 100 μCi of Na2 51CrO4 (DuPont) for 1 h, washed, and incubated with recombinant HIV-1MN gp120 (13 μg/mL; Chiron) for 1 h. Target cells (2000 in a 100-μL volume) were added to each microtiter well.
Effector cells
Peripheral-blood leukocytes (PBLs) were obtained from the blood of HIV-1–negative healthy donors with a history of consistently high ADCC activity, by use of a standardized anti-HIV IgG preparation (HIVIG; North American Biologicals). Peripheral-blood mononuclear cells (PBMCs) were isolated by ficoll-hypaque (Sigma) density-gradient centrifugation and were rotated in RPMI 1640 plus 20% fetal bovine serum with carbonyl iron particles for 1 h at 37°C. A magnet was used to remove monocytes that had ingested the iron particles. PBLs (monocyte-depleted PBMCs) were diluted to yield effector-totarget (E:T) cell ratios of 40:1, 20:1, and 10:1.
Antibodies
Serum and CVL samples were the source of antibodies for ADCC assays. Paired serum and CVL samples from HIV-1–positive women (n = 302) were obtained from the study population. To establish baseline activity, serum (n = 20) and CVL (n = 15) samples were also obtained from HIV-1–negative women in the WIHS. Samples were stored at −70°C before testing and were diluted to final dilutions of 1:50, 1:100, 1:500, 1:1000, and 1:10,000, for serum samples, and 1:10, 1:50, 1:100, 1:500, 1:1000, and 1:10,000, for CVL samples. In each experiment, HIVIG was included as a positive control.
51Cr-release assay
Paired HIV-1–positive serum and CVL samples from each participant were assessed for ADCC activity in the same assay. Dilutions of serum samples, CVL samples, or HIVIG (50 μL/well for each) were incubated in 96-well microtiter plates (VWR) with 2000 target cells. In each assay, HIVIG was used as a positive control for standardization. After 30 min, effector cells from selected healthy women with known levels of ADCC activity were added to give E:T ratios of 40:1, 20:1, and 10:1. Each E:T ratio was assayed in quadruplicate. Background NK cell activity was measured by incubating effector cells with target cells in the absence of any potential source of antibodies. The spontaneous and maximum release of 51Cr were obtained by incubating targets with either medium or 0.5% Triton X-100 (BioWhittaker), respectively. The microtiter plates were incubated for 3.5 h in a humidified 5% CO2 environment at 37°C; 100 μL of supernatant was then harvested, and 51Cr release was measured (Micromedic ME plus; ICN Micromedic Systems).
The percentage of specific 51Cr release was calculated by the following formula: %SR = [(experimental cpm–spontaneous cpm)/(maximum cpm–spontaneous cpm)] × 100, where cpm is counts per minute. The %SR from 3 E:T ratios were converted into lytic units (LU20) [23]. An LU20 is defined as the inverse of the number of effector cells × 106 required for lysis of 20% of the target cells. The ADCC titer is the inverse of the highest dilution that had significant LU20 activity.
HIV-1 RNA loads in serum, endocervical-swab, and CVL samples
HIV-1 RNA loads in serum, endocervical-swab, and CVL samples were determined by RT-PCR (Roche Amplicor HIV-1 assay; Roche Molecular Systems), as described by Mulder et al. [24]. Assays were performed in laboratories that were participating in the Virology Health Quality Assurance Program (Division of AIDS [DAIDS], National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]). Use of this assay and information about the viral loads in the women in this cohort have been reported elsewhere [25, 26]. The lower limit of detection for this assay is 400 copies/mL.
Levels of HIV-1 gp120–specific IgG and IgA
A single dilution of serum or CVL fluid was incubated in microtiter plates coated with a CHO cell–expressed recombinant HIV-1MN gp120 (Genentech), and an ELISA was performed as described elsewhere [27]. The gp120-specific antibodies were quantified by interpolating the optical densities on calibration curves constructed from standardized S-IgA or serum IgG, as described elsewhere [28, 29].
CD4+ and CD8+ T cell numbers
CD4+ and CD8+ T cell numbers were determined by flow cytometry [30] in laboratories participating in the Immunology Health Quality Assurance Program (DAIDS, NIAID, NIH).
Statistical analysis
Data analysis was performed by use of SPSS software (version 11). Unless stated otherwise, the independent-sample t test or the Mann-Whitney U test was used to compare means and medians, respectively, between 2 groups of continuous variables. Comparison of proportions was performed by the 2-sample z test. For statistical analysis, viral loads were log10 transformed.
RESULTS
Criteria for positive ADCC activity against HIV-1
Serum or CVL samples were considered to be positive for ADCC antibodies if they had an LU20 value greater than both (1) the mean baseline NK activity plus 1 SD (NK effectors and targets without antibody) and (2) the mean baseline LU20 value plus 1 SD of samples from HIV-1–negative women. Mean baseline activity was obtained from serum (n = 20) and CVL (n = 15) samples from HIV-1–negative women. We had previously used more-stringent criteria; however, when these new criteria were employed, women who previously would not have been considered to be positive had activity at multiple dilutions or had LU20 values substantially greater than the mean baseline NK activity, indicating that these women clearly had ADCC activity (table 1). For this reason, we adopted these less-stringent criteria.
Table 1. Determination of antibody-dependent cell-mediated cytotoxicity (ADCC) antibody titer and activity (highest lytic unit [LU20]).
| Serostatus | Background NK cell activity, LU20 ± SD |
CVL dilution, LU20 |
ADCC antibody titer |
Highest LU20 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 1:10 | 1:50 | 1:100 | 1:500 | 1:1000 | 1:10000 | ||||
| Positive (n = 4) | |||||||||
| Patient 1 | 1.7 ± 1.6 | 13.9a | 2.0 | 4.6 | 1.9 | 3.0 | 2.1 | 10 | 13.9 |
| Patient 2 | 5.4 ± 1.1 | 13.6a | 12.2 | 7.8 | 6.3 | 0.9 | 3.4 | 50 | 13.6 |
| Patient 3 | 2.5 ± 1.4 | 58.5a,b,c | 45.9a,b,c | 45.5a,b,c | 15.8a,b | 10.2a | 8.4 | 1000 | 58.5 |
| Patient 4 | 4.6 ± 1.1 | 13.1a | 14.0a,b | 9.9 | 10.0 | 4.5 | 11.9a | 10,000 | 14.0 |
| Negative (n = 15) | |||||||||
| Mean | 5.8 | 6.6 | 6.2 | 6.5 | 6.1 | 6.4 | 6.7 | … | … |
| 1 SD | 4.0 | 4.0 | 3.8 | 3.6 | 4.2 | 3.6 | 3.7 | … | … |
| Mean + 1 SD | … | 10.6 | 10.0 | 10.1 | 10.3 | 10.0 | 10.4 | … | … |
| Mean + 2 SD | … | 14.6 | 13.8 | 13.8 | 14.5 | 13.6 | 14.1 | … | … |
| Mean + 3 SD | … | 18.6 | 17.6 | 17.4 | 18.8 | 17.2 | 17.9 | … | … |
NOTE. Cervical-lavage (CVL) samples were incubated with normal peripheral-blood mononuclear cells and HIV-1 gp120–bearing CEM.NKR in a standard 51Cr-release assay. NK cell background equals activity in the absence of CVL.
Activity is greater than the sum of background NK cell activity and the mean + 1 SD of negative control samples.
Activity is greater than the sum of background NK cell activity and the mean + 2 SD of negative control samples.
Activity is greater than the sum of background NK cell activity and the mean + 3 SD of negative control samples.
Serum and CVL ADCC activity in HIV-1–positive women
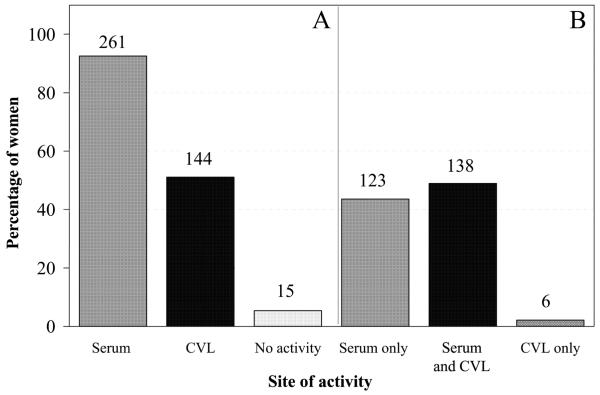
Paired serum and CVL samples from 282 HIV-1–positive women were analyzed for ADCC antibodies against HIV-1 gp120. Figure 1A shows the percentages of women who had ADCC antibodies at each indicated site, regardless of activity at the other site. In the present study, the majority of HIV-1–positive women had serum ADCC antibodies, and ~50% had CVL ADCC antibodies (figure 1A). Most HIV-1–positive women (258/261) had serum ADCC antibody titers ≥1000, whereas CVL ADCC antibody titers were evenly distributed throughout the range evaluated (i.e., 10-10,000 [data not shown]). Figure 1B demonstrates that the majority of the HIV-1–positive women had activity in serum and CVL fluid. Six women who did not have serum ADCC antibodies had CVL ADCC antibodies (figure 1B). Of these, 2 had titers ≥10,000 (highest LU20, 18.8 and 25.7), 1 had a titer of 100 (highest LU20, 13.0), and 3 had titers of 10 (highest LU20, 10.8, 11.8, and 12.5). Fifteen women did not have ADCC antibodies at either site (figure 1A).
Figure 1.
Percentage of HIV-1–positive women with antibody-dependent cell-mediated cytotoxic (ADCC) antibodies. Serum or cervical-lavage (CVL) samples were incubated with normal peripheral-blood mononuclear cells in a standard 4-h 51Cr-release assay against HIV-1 gp120–bearing CEM.NKR target cells. Women were considered to have ADCC antibodies if the lytic units (LU20) of any serum or CVL dilution was greater than both (1) the mean baseline NK activity plus 1 SD (NK effectors and targets without antibody) and (2) the mean baseline LU20 value plus 1 SD of negative control samples. A, Percentage of women with ADCC antibodies in serum or CVL fluid, regardless of whether they had antibodies at the other site; B, Percentage of women with ADCC antibodies in serum or CVL fluid, separated on the basis of whether the women had activity in a single site or in both serum and CVL fluid. The nos. above the bars indicate the no. of women in each group.
Effect of ART status on ADCC activity in HIV-1–positive women
Women who had not been receiving ART for at least 1 month before enrollment were considered to be not receiving ART. Women who were taking ≥1 antiretroviral medication were considered to be receiving ART. We wanted to determine whether the presence of ADCC activity was associated with the ART status of HIV-1–positive women.
Overall, HIV-1–positive women were equally likely to have ADCC activity, regardless of their ART status. Women taking ≥3 antiretroviral medications (n = 102) were as likely to have ADCC activity in serum or CVL fluid as were women who were taking <3 antiretroviral medications (n = 71) (data not shown). Also, women receiving a combination of nucleoside/nucleotide reverse-transcriptase inhibitor (NRTI) and nonnucleoside reverse-transcriptase inhibitor (NNRTI) with a protease inhibitor (PI) were as likely to have ADCC activity in their serum (93% vs. 90%) or CVL fluid (53% vs. 51%) as were women receiving an NRTI/NNRTI combination without a PI.
HIV-1–specific CVL ADCC antibodies correlate with lower genital HIV-1 load
Approximately 50% of the HIV-1–positive women in the present study had CVL ADCC antibodies of any titer (figure 1A); ~25% had titers of CVL ADCC antibodies ≥500. Because CVL ADCC antibodies could penetrate the surrounding tissues of the vaginal canal, interact with local ADCC effector cells, and potentially reduce genital HIV-1 RNA load, we compared CVL ADCC activity with genital HIV-1 RNA load. ADCC antibody titers were determined by a standard 4-h 51Cr-release assay. HIV-1 RNA loads in endocervical-swab samples were determined by the Roche Amplicor HIV-1 assay.
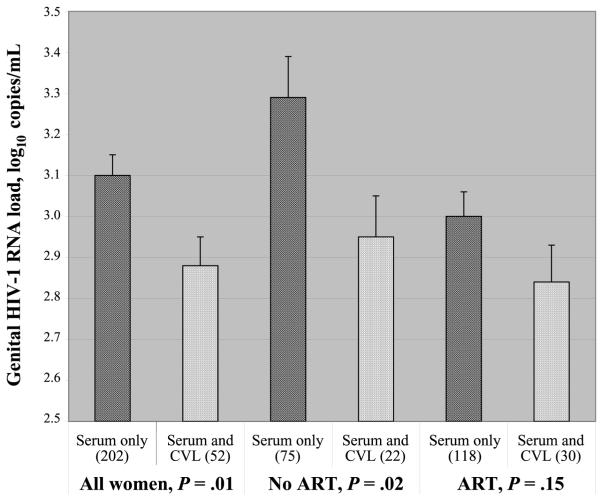
Women were separated into 2 groups, on the basis of the presence or absence of CVL ADCC activity. Overall, women with a CVL ADCC antibody titer ≥500 had genital HIV-1 RNA loads that were lower than those of women who did not have CVL ADCC antibodies. This difference was significant when all women were considered, regardless of whether parametric analysis (figure 2) or nonparametric analysis was performed. This difference was significant both in a Spearman’s correlation analysis (P = .04) and in a Mann-Whitney U test analysis (P = .08). That these P values were not smaller indicates that these statistically significant differences were not robust. Genital viral loads were also significantly higher in women who were not receiving ART (P = .02, for not receiving ART; figure 2). In women receiving ART, the difference in viral load between women with and those without ADCC antibodies was not significant (P = .15, for receiving ART; figure 2), presumably because antiretroviral medications reduce viral load in the genital tract.
Figure 2.
Antibody-dependent cell-mediated cytotoxicity (ADCC) antibodies in cervical-lavage (CVL) fluid correlate with lower mean genital HIV-1 RNA loads. ADCC activity in CVL fluid was determined by use of a standard 4-h 51Cr-release assay against CEM.NKR cells adsorbed with recombinant HIV-1MN gp120. Genital viral loads in women with CVL and serum ADCC antibody titers ≥500 were compared with those in women with serum ADCC antibodies only. The Roche Amplicor HIV-1 assay was used to determine genital HIV-1 RNA loads. Because the lower limit of detection of this assay is 400 copies/mL, women with undetectable virus were scored as having a viral load of 399 copies/mL. Bars provide the SE of each group. The nos. in parentheses indicate the no. of women in each group. ART, antiretroviral therapy.
Genital viral load was also compared with CVL ADCC activity. Women with CVL ADCC antibody titers ≥500 had significantly less genital viral load than did women with serum ADCC activity and CVL ADCC titers <500 (P = .03, Student’s t test). This correlation held for women not receiving ART (P = .05, Student’s t test), but it was not significant for women receiving ART.
No relationship between plasma viral load and CVL ADCC antibodies
A higher plasma viral load may lead to increased production of antibodies in serum and CVL fluid, therefore making it more likely for HIV-1–positive women to have CVL ADCC activity. We compared the mean plasma HIV-1 RNA load in HIV-1–positive women with CVL ADCC antibodies to those in HIV-1–positive women without CVL ADCC antibodies. All women with results for both plasma HIV-1 RNA and CVL ADCC antibodies were evaluated. There was no significant difference between the plasma HIV-1 RNA loads of HIV-1–positive women with and those without CVL ADCC antibodies. This was the case regardless of whether results were analyzed by parametric tests (table 2) or the nonparametric Mann-Whitney U test (P = .88). ART status did not alter this observation.
Table 2. Plasma HIV-1 RNA load in HIV-1–positive women with or without antibody-dependent cell-mediated cytotoxicity (ADCC) activity in cervical-lavage (CVL) fluid, by antiretroviral therapy (ART) status.
| Plasma HIV-1 RNA load, mean ± SD (no.), log10 copies/mL |
|||
|---|---|---|---|
| Group | Women with CVL ADCC activity |
Women without CVL ADCC activity |
Pa |
| All women | 3.42 ± 0.76 (56) | 3.50 ± 0.87 (208) | .56 |
| Women receiving ART | 3.18 ± 0.76 (33) | 3.45 ± 0.87 (121) | .11 |
| Women not receiving ART | 3.76 ± 0.65 (23) | 3.62 ± 0.89 (79) | .40 |
NOTE. CVL ADCC activity against HIV-1 gp120 was measured by use of a standard 51Cr-release assay. Women with both serum and CVL ADCC titers ≥500 were grouped as women with CVL ADCC activity; women with a serum ADCC titer ≥500 and a CVL ADCC titer <500 were grouped as women without CVL ADCC activity. Plasma HIV-1 RNA load was determined by use of the Roche Amplicor HIV-1 assay. Viral loads were converted to log10 of the no. of copies/mL. The effect of ART was also evaluated.
The independent-sample t mean plasma HIV-1 RNA load test was used to compare the log10 between the 2 groups of women. P ≤ .05 was considered to be statistically significant.
Relationships between antibody concentrations and the presence of CVL ADCC activity
To determine whether women with CVL ADCC activity had more HIV-1–specific CVL ADCC antibodies than did women without CVL ADCC activity, we compared the mean concentrations of HIV-1 gp120–specific IgG and IgA. All women with data on CVL ADCC activity and genital viral load (252/282) were analyzed. Women with CVL ADCC activity had a 2-fold higher concentration of HIV-1 gp120–specific IgG in their CVL samples (P = .02, Mann-Whitney U test). However, the difference in HIV-1 gp120–specific IgA concentrations at this site was not significant (P = .58, Mann-Whitney U test).
It is possible that antibodies from the plasma cross mucosal barriers and are responsible for CVL ADCC activity. Therefore, we determined whether women with CVL ADCC activity had higher HIV-1 gp120–specific antibodies in their blood—women with CVL ADCC activity did not have higher levels of HIV-1 gp120–specific IgG in their blood (P = .56, Mann-Whitney U test). Together, the last 2 sections show that the presence or absence of CVL ADCC activity is associated with the concentration of HIV-1–specific IgG in CVL fluid and is not associated with plasma viral load or the concentration of antibody in the circulation.
Relationships between CVL ADCC activity and HIV-1–specific IgG and IgA in CVL fluid
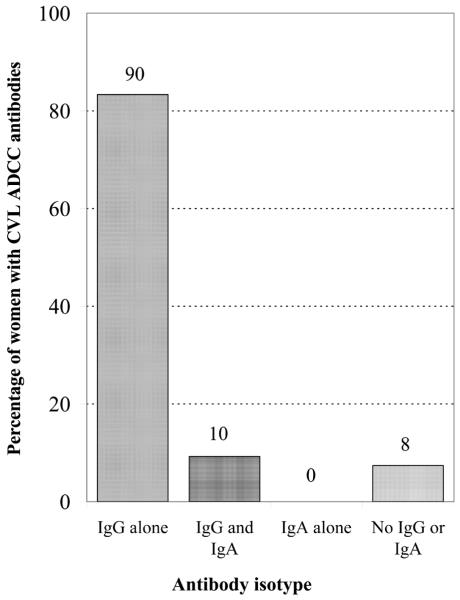
ADCC activity is usually mediated by immunoglobulins of the IgG isotype. Some evidence suggests that IgA may contribute to ADCC activity at mucosal sites [14]. We wanted to determine whether IgG, IgA, or both were responsible for CVL ADCC activity. We evaluated women with CVL ADCC activity to determine the proportion of women with HIV-1 gp120–specific IgG and IgA in their CVL fluid. All women with CVL ADCC activity and data on HIV-1–specific IgG and IgA were studied (n = 108). Figure 3 illustrates that the majority of women (100/108) with CVL ADCC activity had HIV-1 gp120–specific IgG in their CVL fluid. Ninety (82%) of 108 women did not have any HIV-1 gp120–specific IgA. Furthermore, none of the women with CVL ADCC activity had HIV-1 gp120–specific antibody restricted exclusively to the IgA isotype. In women with CVL ADCC activity, a greater proportion of women had HIV-1 gp120–specific IgG than IgA (P < .001) (figure 3). In summary, the majority of women with CVL ADCC activity had HIV-1 gp120–specific IgG, whereas none of the women with CVL ADCC activity had HIV-1 gp120–specific IgA alone.
Figure 3.
Isotype of HIV-1 gp120–specific antibodies in the cervicolavage (CVL) fluid of women with CVL antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC activity was determined by use of a standard 4-h 51Cr-release assay against CEM.NKR cells adsorbed with recombinant HIV-1MN gp120. Concentrations of HIV-1 gp120–specific IgG and IgA in CVL fluid were determined by ELISA. All women with CVL ADCC activity and data on HIV-1–specific IgG and IgA were studied (n = 108). The nos. above the bars indicate the no. of women in each group.
Relationship between CVL ADCC activity and CD4+ and CD8+ T cell numbers
We compared the number of CD4+ and CD8+ T cells in women with and those without CVL ADCC activity [30]. Women with CVL ADCC activity did not have higher CD4+ T cell numbers (mean ± SD, 382 ± 220 vs. 433 ± 275 cells/mL; P = .10) or higher CD8+ T cell numbers (mean ± SD, 836 ± 491 vs. 969 ± 718 cells/mL; P = .89) than did women without CVL ADCC activity. This was true even when women were separated into groups on the basis of their ART status (data not shown).
DISCUSSION
The effector functions of protective immunity work together in host defense against disease. Components of innate immunity come into play first, because they do not require antigen-driven proliferation and differentiation of effector cells. Mechanisms of acquired immunity join the defense if innate immunity is insufficient. Primary induction of the antigen-specific response requires at least 1 week, and reactivation takes 2–3 days. In the absence of repeated exposure, antibody remains in the system, but activated CTLs disappear. If antigen-specific CTLs were the only immune effector population stimulated by vaccination, on exposure, the virus would have ≥2 days to establish infection before CTLs could mount a defense. If an individual were vaccinated, the antibodies generated after the initial exposure would enable ADCC effector cells to directly kill virus-infected cells immediately after natural exposure to the virus. Therefore, ADCC could potentially be a very important immune effector function in protection after vaccination.
In most women, the first site of HIV exposure is the vagina. ADCC antibodies are found in the CVL fluid of some HIV-1–positive women [9]. The experiments described in the present study were designed to determine whether these ADCC antibodies affect HIV-1. We have studied paired serum and CVL samples from 311 HIV-1–positive women in DATRI 009. Aliquots of these samples were sent to all DATRI investigators and substudy investigators. The data from all studies were available for comparative analysis, which greatly increased our ability to evaluate the importance of ADCC in host defense against HIV-1 infection and disease progression.
In previous studies, our goal was to demonstrate the presence of CVL ADCC activity, so high cutoff values were used. Consequently, women who had activity were not counted. We have, therefore, modified the criteria for positive ADCC activity. When the new cutoff was used, ~50% of the women studied had CVL ADCC activity, and most exhibited measurable serum activity (figure 1). The data in table 1 support this. Further support comes from testing of multiple samples from the same women; when this was done, ADCC status was invariably confirmed (data not shown). Multiple values from the same woman were not included in our analysis.
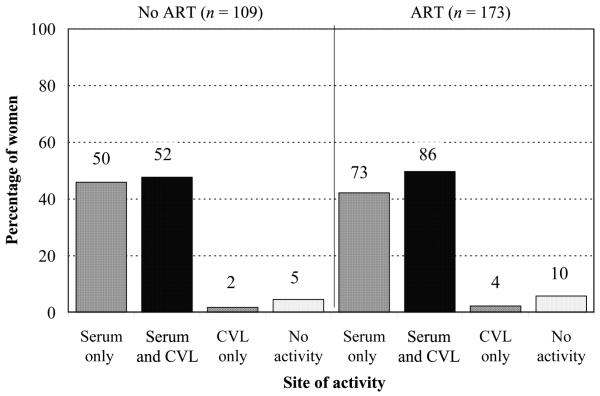
We were concerned that drug treatment might alter ADCC activity. We, therefore, evaluated the effect of therapy. There was no standardized therapy regimen; all women who were receiving any ART were considered to be receiving ART. Figure 4 demonstrates that ART status did not affect the presence or site of ADCC activity, but it did affect correlations where viral load was a factor (figure 2).
Figure 4.
Effect of antiretroviral therapy (ART) on antibody-dependent cell-mediated cytotoxicity (ADCC) activity. ADCC activity was determined by use of a standard 4-h 51Cr-release assay against CEM.NKR cells adsorbed with recombinant HIV-1MN gp120. Women were considered to have ADCC activity if the lytic units (LU20) of any serum or CVL dilution was greater than both (1) the mean baseline NK activity plus 1 SD (NK effectors and targets without antibody) and (2) the mean baseline LU20 value plus 1 SD of negative control samples. The nos. above the bars indicate the no. of women in each group.
The most significant finding of the present study was that women with CVL ADCC antibodies had significantly lower genital HIV-1 RNA loads than did women without CVL ADCC antibodies (figure 2). Genital viral load in these women may be caused by CVL ADCC activity; alternatively, higher CVL ADCC activity and lower genital viral load may be caused by a third parameter. The most striking difference in viral load was seen in the group of women who were not receiving ART. Because ART reduces viral load, differences between those who had CVL ADCC activity and those who did not were less apparent. CVL ADCC activity also had an effect on genital viral load; however, this comparison was not as robust, probably because CVL fluid are diluted during collection. Genital viral loads for the women in the present study are published elsewhere [25].
Although some evidence suggests that HIV-1 in the genital tract evolves independently from HIV-1 in the plasma [31], in the present study, it was possible that plasma viral load might affect genital viral load. Had this been the case, women with CVL ADCC activity might have had lower genital viral loads because they had lower plasma viral loads. It has been demonstrated in the present study that the women with lower plasma viral loads were not the women with lower genital viral loads; this makes it more likely that the reduced viral loads in women with CVL ADCC antibodies resulted from ADCC activity in tissues of the genital tract.
Because both IgG and IgA can mediate ADCC [11, 14], we asked whether CVL ADCC was mediated by IgG or IgA. Antibodies in the majority of mucosal secretions are of the IgA isotype, except for genital tract secretions, in which both IgG and IgA occur at approximately the same levels [13]. The same CVL samples that were tested for ADCC activity were also tested for HIV-1 gp120–specific IgG and IgA. The data in figure 3 clearly demonstrate that the presence of HIV-1 gp120–specific IgG antibodies in the CVL fluid of these women could account for the ADCC activity observed in the present study, whereas HIV-1 gp120–specific IgA antibodies could not. This finding is further supported by data that show that women with CVL ADCC activity have a 2-fold higher concentration of HIV-1 gp120–specific IgG in CVL fluid but not an increased concentration of HIV-1 gp120–specific IgA (P = .02). Also of note, women with CVL ADCC activity who had high concentrations of HIV-1 gp120–specific IgG in CVL fluid did not have high concentrations of HIV-1 gp120–specific IgG in serum (data not shown). This finding suggests that antibodies are produced in CVL fluid independent of serum antibody production.
In summary, most HIV-1–positive women have serum ADCC activity, and ~50% have CVL ADCC activity. Women with CVL ADCC activity are more likely to have lower genital viral loads and, as a result, may be less likely to transmit virus to sex partners. CVL ADCC appears to be mediated primarily by HIV-1 gp120–specific IgG in CVL fluid. These results suggest that ADCC is important in host defense against HIV-1 in infected women. Therefore, an effective HIV-1 vaccine should stimulate the production of CVL ADCC antibodies.
Acknowledgments
We thank the following individuals for their contributions to this report: S. Beckner, V. Goveia, S. Lewis, A. Soloviov, J. Bykoski, K. Balfe, and M. Camarca (Westat, Rockville, MD); S. Holman and B. Driscoll (State University of New York Health Science Center, Brooklyn, NY); D. Mai (Georgetown University Medical Center, Washington, DC); J. Matchett and Y. DeSouza (University of California at San Francisco, San Francisco, CA); Y. Barranday, B. Heikes, and M. Nowicki (Los Angeles County and University of Southern California Medical Center, Los Angeles, CA); S. Micci, M. Romo, and G. Meredith (Cook County Hospital, Chicago, IL); C. Jennings (Rush-Presbyterian/St. Luke’s Medical Center, Chicago, IL); P. Baron (Memorial Sloan Kettering Cancer Center, New York, NY); B. Meyers and B. Rush (Quest Laboratory, Baltimore, MD); and G. Peterson, A. Cent, E. Peterson, and A. Ryncarz (University of Washington, Seattle, WA).
Financial support: Division of AIDS Treatment Research Initiative, National Institute of Allergy and Infectious Diseases, National Institutes of Health (contract NO1-AI-15123); Program Support Center, Department of Health and Human Services (contract 282-98-0015, task order 21); National Institute of Child Health and Human Development (contract PO1-HD40539).
Footnotes
Presented in part: 9th Annual Conference on Retroviruses and Opportunistic Infections, Seattle, 24–28 February 2002 (abstract 783).
The Journal of Infectious Diseases 2004; 190:1970-8
References
- 1.Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 2.Jewett A, Giorgi JV, Bonavida B. Antibody-dependent cellular cytotoxicity against HIV-coated target cells by peripheral blood monocytes from HIV seropositive asymptomatic patients. J Immunol. 1990;145:4065–71. [PubMed] [Google Scholar]
- 3.Gale RP, Zighelboim J. Polymorphonuclear leukocytes in antibody-dependent cellular cytotoxicity. J Immunol. 1975;114:1047–51. [PubMed] [Google Scholar]
- 4.Lopez AF, Begley G, Andrews P, Butterworth AE, Vadas MA. Identification of a human granulocyte functional antigen (GFA-2) involved in antibody-dependent cell-mediated cytotoxicity and phagocytosis. J Immunol. 1985;134:3969–77. [PubMed] [Google Scholar]
- 5.Kohl S. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev Infect Dis. 1991;13(Suppl 11):S950–2. doi: 10.1093/clind/13.supplement_11.s950. [DOI] [PubMed] [Google Scholar]
- 6.Kohl S. Role of antibody-dependent cellular cytotoxicity in defense against herpes simplex virus infections. Rev Infect Dis. 1991;13:108–14. doi: 10.1093/clinids/13.1.108. [DOI] [PubMed] [Google Scholar]
- 7.Kohl S, Charlebois ED, Sigouroudinia M, et al. Limited antibody-dependent cellular cytotoxicity antibody response induced by a herpes simplex virus type 2 subunit vaccine. J Infect Dis. 2000;181:335–9. doi: 10.1086/315208. [DOI] [PubMed] [Google Scholar]
- 8.Smith PR, Wahl SM. Immunobiology of mucosal HIV-1 infection. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. 2nd ed. Academic Press; New York: 1999. pp. 977–89. [Google Scholar]
- 9.Battle-Miller K, Eby CA, Landay AL, Cohen MH, Sha BE, Baum LL. Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1–infected women. J Infect Dis. 2002;185:439–47. doi: 10.1086/338828. [DOI] [PubMed] [Google Scholar]
- 10.Walker M, Woof J, Bruggemann M, Jefferis R, Burton D. Interaction of human IgG chimeric antibodies with the human FcRI and FcRII receptors: requirements for antibody-mediated host cell–target cell interaction. Mol Immunol. 1989;26:403–11. doi: 10.1016/0161-5890(89)90129-6. [DOI] [PubMed] [Google Scholar]
- 11.Rozsnyay Z, Sarmay G, Walker M, et al. Distinctive role of IgG1 and IgG3 isotypes in FcγR-mediated functions. Immunology. 1989;66:491–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson S, Mestecky J, Moldoveanu Z, Spearmen P. Collection and processing of human mucosal sectretions. In: Ogra PL, Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, editors. Mucosal immunology. 2nd ed. Academic Press; New York: 1999. pp. 1567–76. [Google Scholar]
- 13.Russell M, Mestecky J. Humoral immune responses to microbial infections in the genital tract. Microbes Infect. 2002;4:667–77. doi: 10.1016/s1286-4579(02)01585-x. [DOI] [PubMed] [Google Scholar]
- 14.Black KP, Cummins JE, Jr, Jackson S. Serum and secretory IgA from HIV-infected individuals mediate antibody-dependent cellular cytotoxicity. Clin Immunol Immunopathol. 1996;81:182–90. doi: 10.1006/clin.1996.0175. [DOI] [PubMed] [Google Scholar]
- 15.Belec L, Tevi BC, Lu XS, Prazuck T, Pillot J. Local synthesis of IgG antibodies to HIV within the female and male genital tracts during asymptomatic and pre-AIDS stages of HIV infection. Aids Res Hum Retroviruses. 1995;11:719–29. doi: 10.1089/aid.1995.11.719. [DOI] [PubMed] [Google Scholar]
- 16.Archibald DW, Witt DJ, Craven DE, Vogt MW, Hirsch MS, Essex M. Antibodies to human immunodeficiency virus in cervical secretions from women at risk for AIDS. J Infect Dis. 1987;156:240–1. doi: 10.1093/infdis/156.1.240. [DOI] [PubMed] [Google Scholar]
- 17.Baum LL, Cassutt KJ, Knigge K, et al. HIV-1 gp120–specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol. 1996;157:2168–73. [PubMed] [Google Scholar]
- 18.Forthal DN, Landucci G, Keenan B. Relationship between antibody-dependent cellular cytotoxicity, plasma HIV type 1 RNA, and CD4+ lymphocyte count. AIDS Res Hum Retroviruses. 2001;17:553–61. doi: 10.1089/08892220151126661. [DOI] [PubMed] [Google Scholar]
- 19.Ahmad R, Sindhu ST, Toma E, et al. Evidence for a correlation between antibody-dependent cellular cytotoxicity–mediating anti–HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol. 2001;21:227–33. doi: 10.1023/a:1011087132180. [DOI] [PubMed] [Google Scholar]
- 20.Banks ND, Kinsey N, Clements J, Hildreth JE. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses. 2002;18:1197–205. doi: 10.1089/08892220260387940. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 22.Howell DN, Andreotti PE, Dawson JR, Cresswell P. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing–resistant human T lymphoblastoid cell line. J Immunol. 1985;134:971–6. [PubMed] [Google Scholar]
- 23.Pross HF, Maroun JA. The standardization of NK cell assays for use in studies of biological response modifiers. J Immunol Methods. 1984;68:235–49. doi: 10.1016/0022-1759(84)90154-6. [DOI] [PubMed] [Google Scholar]
- 24.Mulder J, McKinney N, Christopherson C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bremer J, Nowicki M, Beckner S, et al. Division of AIDS Treatment Research Initiative 009 Study Team Comparison of two amplification technologies for detection and quantitation of human immunodeficiency virus type 1 RNA in the female genital tract. J Clin Microbiol. 2000;38:2665–9. doi: 10.1128/jcm.38.7.2665-2669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baron P, Bremer J, Wasserman SS, et al. Division of AIDS Treatment Research Initiative 009 Study Team Detection and quantitation of human immunodeficiency virus type 1 in the female genital tract. J Clin Microbiol. 2000;38:3822–4. doi: 10.1128/jcm.38.10.3822-3824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham BS, Matthews TJ, Belshe RB, et al. The NIAID AIDS Vaccine Clinical Trials Network Augmentation of human immunodeficiency virus type 1 neutralizing antibody by priming with gp160 recombinant vaccinia and boosting with rgp160 in vaccinianaive adults. J Infect Dis. 1993;167:533–7. doi: 10.1093/infdis/167.3.533. [DOI] [PubMed] [Google Scholar]
- 28.Russell MW, Brown TA, Radl J, Haaijman JJ, Mestecky J. Assay of human IgA subclass antibodies in serum and secretions by means of monoclonal antibodies. J Immunol Methods. 1986;87:87–93. doi: 10.1016/0022-1759(86)90347-9. [DOI] [PubMed] [Google Scholar]
- 29.Mestecky J, Kilian M. Immunoglobulin A (IgA) Methods Enzymol. 1985;116:37–75. doi: 10.1016/s0076-6879(85)16005-2. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson J, Kidd P, Mandy F, Livnat D, Kagan J. Three-color supplement to the NIAID DAIDS guideline for flow cytometric immunophenotyping. Cytometry. 1996;26:227–30. doi: 10.1002/(SICI)1097-0320(19960915)26:3<227::AID-CYTO8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 31.Kemal KS, Foley B, Burger H, et al. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycosylation. Proc Natl Acad Sci USA. 2003;100:12972–7. doi: 10.1073/pnas.2134064100. [DOI] [PMC free article] [PubMed] [Google Scholar]