Abstract
The class Ascidiacea presents fundamental opportunities for research in the fields of development, evolution, ecology, natural products and more. This review provides a comprehensive overview of the current knowledge regarding the global biodiversity of the class Ascidiacea, focusing in their taxonomy, main regions of biodiversity, and distribution patterns. Based on analysis of the literature and the species registered in the online World Register of Marine Species, we assembled a list of 2815 described species. The highest number of species and families is found in the order Aplousobranchia. Didemnidae and Styelidae families have the highest number of species with more than 500 within each group. Sixty percent of described species are colonial. Species richness is highest in tropical regions, where colonial species predominate. In higher latitudes solitary species gradually contribute more to the total species richness. We emphasize the strong association between species richness and sampling efforts, and discuss the risks of invasive species. Our inventory is certainly incomplete as the ascidian fauna in many areas around the world is relatively poorly known, and many new species continue to be discovered and described each year.
Introduction
Ascidians (Phylum Chordata, Class Ascidiacea), or sea squirts, are the largest and most diverse class of the sub-phylum Tunicata (also known as Urochordata). They comprise approximately 3000 described species found in all marine habitats from shallow water to the deep sea [1]–[3]. The group was initially difficult for zoologists to classify systematically, although ascidians were recognized as a distinct group as early as Aristotle [1]. The first clear description of an ascidian was made by Schlosser in 1756 in a letter entitled “An account of a curious, fleshy, coral-like substance”. This specimen was dredged along the British Islands and was actually what we know now as the widely distributed colonial ascidian Botryllus schlosseri [4].
The name “tunicate” (sub-phylum Tunicata) was first coined by Lamarck [5] for ascidians, pyrosomes, and salps [6], [7]. The name originates from the polysaccharide-containing tunic that envelops the animal and forms a somewhat flexible skeleton [1]. Milne Edwards [8] mistakenly included the Bryozoa in this group, and both, together with the Brachiopoda, were included in the Mollusca by Hancock [9]. Savigny [10] also recognized the Tunicata (ascidians, salps, doliolids, and appendicularians) as a distinct group separate from the Mollusca [6]. Finally, the chordate nature of the ascidian tadpole larva was recognized by Kowalevsky [11], and they were reclassified with chordates [12]. The name Urochordata was not used until Balfour [13] created it as a replacement name for Tunicata, presumably to emphasize the chordate affinity. Indeed, recent phylogenomic studies place the tunicates as the sister group to the vertebrates [14]–[16], suggesting that they are our closest relatives among the invertebrates, which provides a fertile ground for evolutionary and developmental studies [17].
Following the original classification of Lahille [18], the class Ascidiacea is now divided into three orders based on the structure of the adult branchial sac: Aplousobranchia (simple), Phlebobranchia (vascular) and Stolidobranchia (folded) (Fig. 1). This is the current classification used by most ascidian taxonomists that also corresponds to molecular phylogenetic analysis based on the 18S rDNA [7], [19] as opposed to Perrier's [20] division that was based upon the position of the gonads and other morphological considerations and comprised only two orders: Enterogona and PleurogonaAscidians belonging to the order Aplousobranchia are all colonial while the Phlebobranchia and Stolidobranchia include both colonial and solitary species [7].
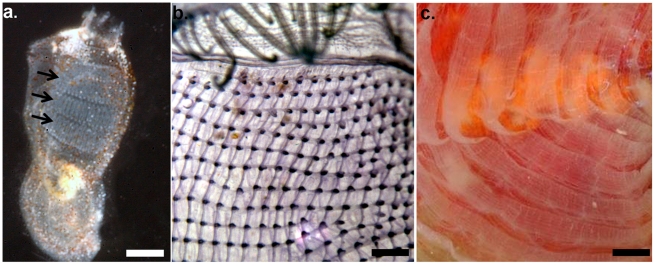
Figure 1. Ascidian branchial sac structure, a distinguishing taxonomic character.
a) A simple branchial arrangement in an aplousobranch (Didemnum sp.). Arrows pointing out the straight stigmata rows. Photo: A. Shoob. Scale bar 1 mm; b) phlebobranch (Ascidia sp.) with longitudinal blood vessels; c) stolidobranch (Herdmania momus) with branchial folds. Photos: N Shenkar. Scale bar 1 mm and 4 mm respectively.
Adult ascidians are sessile, inhabiting a wide variety of habitats such as soft sediments, coral reefs and rocky substrates. They successfully foul various artificial substrata such as jetties, ship hulls, floating docks and other man-made structures all over the world [21], [22]. They remain sessile following larval settlement throughout their adult life, so they cannot avoid salinity or temperature changes, and thus larval behavior is critical [23], [24]. Only a few species can survive in salinities below 20–25‰ [22], [25], or above 44‰ [26], (Shenkar N. unpublished results). The tropical Ecteinascidia thurstoni has been recorded along the Suez Canal in habitats with salinity reaching 46‰ overgrowing metal pilings of jetties [26], while several species inhabit marine lakes in Indonesia with salinity of 28.5‰ [27]. In salinities below 22‰ larval development is severely affected [24], as is the health of adult zooids [28]. Nonetheless, highly tolerant species such as Ciona intestinalis survive a wide range of salinities (12–40‰), and can withstand short periods of lower salinities (<11‰) [29], [30]. In general, ascidians exhibit a wider tolerance to temperature range than salinities [22], [31]. Antarctic species can tolerate temperature as low as -1.9°C [32], while others can survive seawater temperature higher than 35°C in the Arabian Gulf [33].
Both salinity and temperature are among the most important environmental variables influencing ascidian recruitment and reproduction [31], [34]–[36]. Other factors that may affect spatial distribution and recruitment include light, substrate type, hydrodynamics, predation and competition [22], [34], [37]. Understanding the role of these factors in ascidian recruitment, dispersal and survival is crucial to our understanding of ascidian global distribution patterns.
Ascidians are a key ecological group because of their invasive potential and ability to thrive in eutrophic (nutrient-rich) environments. Introductions of non-indigenous ascidians into harbors in both tropical and temperate waters are now commonplace, with the rate of introductions increasing, sometimes creating severe damage to natural fauna by overgrowth [1], [22], [38]–[41] (reviewed in a special issue of Aquatic Invasions January 2009). For example, the solitary ascidians Styela clava and Ciona intestinalis have had an adverse effect on aquaculture along Canada's east coast, mainly on mussel culture [42]–[44]. S. clava, when extremely abundant, may result in significantly decreased mussel growth and also cause severe problems in crop handling, resulting in increased production costs estimated at $4.5 million [45]. In contrast, several species of ascidians are cultured for food primarily in Japan, Korea and France. The solitary ascidian Halocynthia roretzi has long been a popular seafood in Japan and Korea, with a market value of $18 million in 2006 [46]. Recently, a unique infectious agent has been identified as the cause of mass mortality of these cultured ascidians [47].
Ascidians provide a fertile ground for studies in the field of natural products. Similar to sponges and bryozoans, many ascidians avoid predation or fouling by producing noxious secondary metabolites [48]–[52]. Because of these properties, numerous species of ascidians may thus be a potential source of new anti-cancer compounds [53], [54]. Trabectedin (earlier known as ecteinascidin-743, commercial name Yondelis®), a marine-derived alkaloid isolated from extracts of Ecteinascidia turbinate, is now being used in treatment of soft-tissue sarcomas [55], [56]. Antimalarial compounds have been isolated from the solitary ascidians Microcosmus helleri, Ascidia sydneiensis and Phallusia nigra [57], and numerous other compounds with anti-cancer, anti-viral and anti-bacterial capabilities are in various clinical trial stages by the pharmaceutical industry. The management and use of these organisms as sources of natural products is dependent, however, on understanding their taxonomy, the integrative basis of biology.
Ascidians have a poor fossil record [58]. Although calcareous spicules of distinctive shapes are found in some species of the families Polycitoridae, Pyuridae, and especially the Didemnidae [59], [60], their fossils are rarely reported by paleontologists [61]. This is possibly due to their susceptibility to dissolution, and small size; many are less than 0.1 mm [60]. Fossil didemnid ascidian spicules were encountered in rocks from various regions around the world, usually dating to the Late Pliocene-Early Pleistocene period [61], [62]. Eight specimens of a solitary fossil tunicate have been discovered with a body size of 2–4 cm; they resemble the extant Clavelina genus and are presumably ∼520 million years old [63].
Currently there are numerous web-based sources of taxonomic inventories (e.g., Encyclopedia of Life http://www.eol.org, Integrated Taxonomic Information System http://www.itis.gov), but only a few websites are dedicated to ascidians (e.g., The Dutch ascidians Home Page http://www.ascidians.com, Ascidian Home Page for United States http://depts.washington.edu/ascidian/), and they do not aim to provide an inventory list. Unfortunately, most web-based datasets often lack updates due to limitations in funding and expertise. The Ascidiacea World Database (http://www.marinespecies.org/ascidiacea/), which is a part of the World Register of Marine Species (WoRMS), is unique; it contains a comprehensive list of ascidian species, including information on synonymy, taxonomic literature, and distribution [64]. This database is the result of a joint effort of several ascidian taxonomists who constantly update and revise the information. With the aid of this database and the large taxonomic literature, our aim is to provide a systematic review of the class Ascidiacea, describe the main regions of highest biodiversity, discuss the risks of invasive species, and summarize the current trends in ascidian global distribution patterns.
Methods
Biogeographic distribution
Ascidian specimens are held by museums and similar institutions all over the world. However, only a few institutions provide reliable on-line options to search their collections (e.g., Smithsonian Invertebrate Zoology Collections, The Santa Barbara Museum of Natural History, Yale Peabody Museum Catalog Service, The Online Zoological Collections of Australian Museums). In these on-line collections we were able to find invaluable unpublished information regarding species distribution, and verify the occurrence of certain species in their native or introduced range. In addition, a literature search was done to record the number of species identified in various regions of the world in order to provide an estimate of global species richness. It is important to note that the numbers we provide represent the exact number of species mentioned in each citation.
Maps and geographic regions
Species distribution information was compiled based on the geographic regions of the Exclusive Economic Zone division v5 standard map provided by VLIZ Maritime Boundaries Geodatabase [65].
Species names and systematic validation
We followed the taxonomic classification and used the tabular keys of Monniot et al. 1991 [1] (revised by F. Monniot and G. Lambert 2008–2009, unpublished data). Annual check-lists are published on-line by the Catalogue of Life [66], and the Encyclopedia of Life [67]. Both databases are connected to the World Register of Marine Species (WoRMS) check list, to which the Ascidiacea World Database belongs [64]. Therefore, to avoid confusion, only the valid names and classification provided by the Ascidiacea World Database http://www.marinespecies.org/ascidiacea/ were used for systematic analysis of families, genera etc. Division of colonial versus solitary species was based on the Monniot et al. 1991 keys. Taxonomic contribution was analyzed according to the authority index in the World Ascidiacea Database, and only first authors were taken into consideration.
Records of non-indigenous ascidians
In order to compile a current list of non-indigenous ascidians, we gathered data not only from the available literature but also from different governmental reports which often provide the first record of an introduced species. In addition, valuable information was obtained from the proceedings of the International Invasive Sea Squirt conferences (J Exp Mar Biol Ecol 342 (1), 2007 and Aquat Inv 4 (1) 2009). The list includes only species that are mentioned as introduced or non-indigenous. Species that are classified as “cryptogenic” (species that cannot be reliably demonstrated as being either introduced or native, 68) were not included.
Results
Systematic division of ascidian species
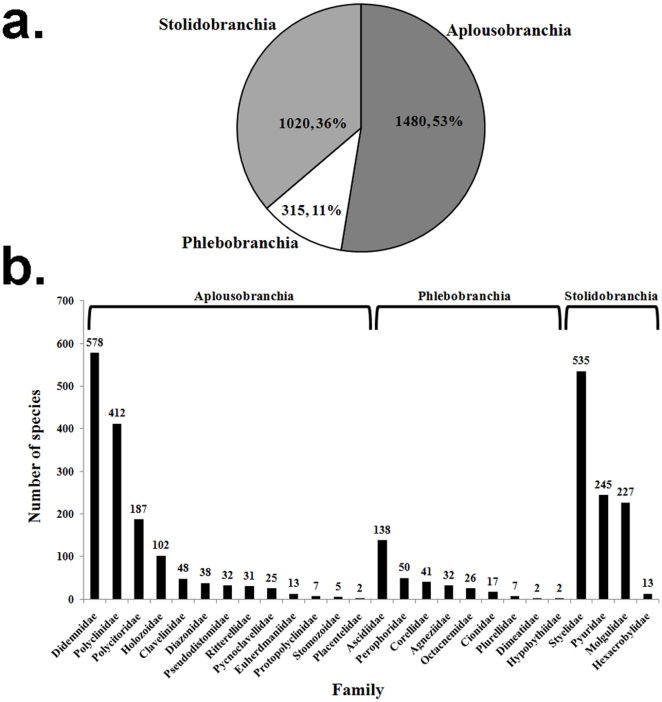
Our systematic list includes 2815 valid species of ascidians. The highest number of species and families is found in the order Aplousobranchia, with approximately 50% of the species (1480) in the class Ascidiacea (Fig. 2a). Based on the classification of the Ascidiacea World Database, there are currently 26 families in the class Ascidiacea, of which 13 belong to the Aplousobranchia, with the Didemnidae having the highest number of species (578). The genera with the highest number of described species are Aplidium (259) and Didemnum (228), in the Aplousobranchia. However, the highest number of genera per family was found in the Styelidae (38), order Stolidobranchia, which also has the second highest number of species (535) (Fig. 2b). The majority of described species in the Ascidiacea are colonial (1730, 61.5%) (Table S1, supporting material).
Figure 2. Systematic division of ascidian species.
a) Number and percentage of species per order; b) number of species by family within each order.
Discovery rate and author contribution
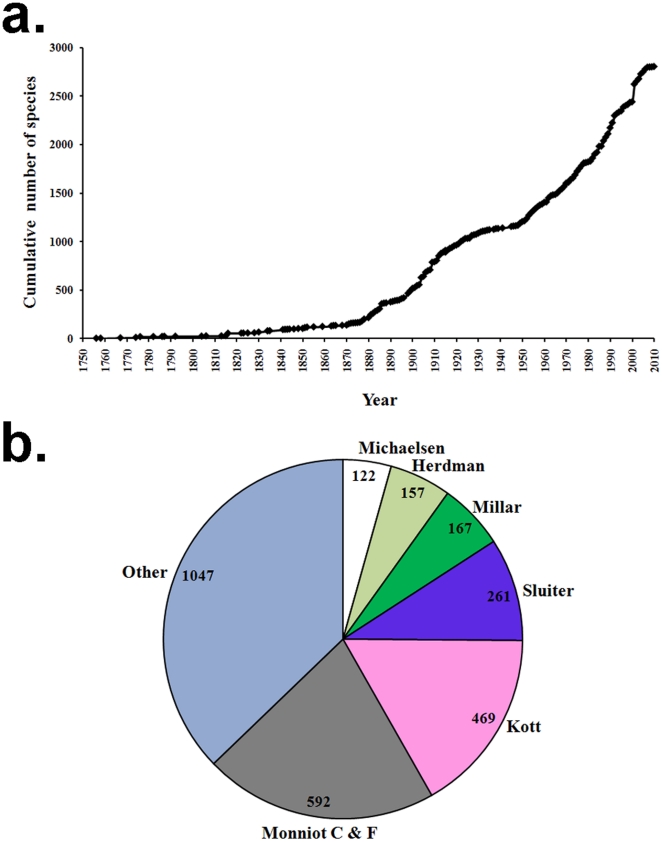
The discovery rate of ascidian species from 1756 until 2010 is presented in Fig. 3a. The rate of discovery has accelerated since 1950, when the major taxonomists of this group, P. Kott, C. and F. Monniot, and R.H. Millar began publishing. Over 1600 species have been described by these experts including the numerous descriptions by C.P. Sluiter and W.A. Herdman from the late 19th century-beginning of the 20th century. Figure 3b summarizes the contribution of the major taxonomists to total ascidian species described. Only authors responsible for more than 100 descriptions are mentioned by name; Claude and Françoise Monniot were grouped together due to their numerous collaborative publications.
Figure 3. Discovery rate and author contribution.
a) Cumulative number of valid ascidian species described between 1750–2010; b) Percentage and number of species described per taxonomic authority. Note: only taxonomic authorities with more than 100 species are mentioned by name.
Non-indigenous ascidians
Review of the literature resulted in records of 64 non-indigenous species (Table 1). Thirty three species are colonial. Half of the introduced species (32) belong to the order Stolidobranchia, the rest divide between the other two orders. Almost half of the species (30) have records only from the northern hemisphere, 13 have records only from the southern hemisphere, and 21 have records from both sections.
Table 1. Documented locations of non-indigenous ascidians.
| Species | Introduced sites | Lifestyle | Order | Remarks | References |
| 1. Aplidium glabrum | Netherlands | C | A | NH | [69] |
| 2. Aplidium phortax | New Zealand | C | A | SH | [70] |
| 3. Aplidium accarense | Brazil | C | A | SH | [71] |
| 4. Ascidia archaia | Atlantic Panama | S | P | T, NH | [72] |
| 5. Ascidia cannelata | Mediterranean Sea | S | P | NH | [73]–[75] |
| 6. Ascidia sp. | California harbors | S | P | NH | [41], [76] |
| 7. Ascidia sydneiensis | Atlantic Panama, Brazil, Guam, Hawaii, India South America | S | P | T | [39], [40], [77]–[82] |
| 8. Ascidia zara | California harbors | S | P | NH | [38], [41], [76] |
| 9. Ascidiella aspersa | Argentina, New England, New Zealand, South Africa, South Australia, Tasmania | S | P | [82]–[87] | |
| 10. Asterocarpa humilis | Chile, New Zealand | S | S | SH | [70], [88] |
| 11. Bostrichobranchus pilularis | California harbors | S | S | NH | [38] |
| 12. Botrylloides leachi | South Australia and Tasmania | C | S | SH | [85], [86] |
| 13. Botrylloides perspicuum | California harbors | C | S | NH | [41], [76] |
| 14. Botrylloides sp. | New Zealand | C | S | SH | [70] |
| 15. Botrylloides violaceus | Alaska, Atlantic Canada, Belgium, California harbors, England, Mediterranean Sea, Netherlands, New England, San Francisco Bay | C | S | NH | [41], [69], [73], [75], [76], [83], [89]–[95] |
| 16. Botryllus schlosseri | Atlantic Canada, California harbors, India, New England, San Francisco Bay, South Africa, South Australia and Tasmania, US West coast | C | S | [38], [41], [76], [78], [82], [83], [85], [86], [89], [90], [92], [93], [96] | |
| 17. Ciona intestinalis | Atlantic Canada, California harbors, Chile, China/Korea, New Zealand, South Africa, South Australia and Tasmania, Washington | S | P | [38], [41], [70], [76], [82], [85]–[89], [97]–[99] | |
| 18. Ciona savignyi | California harbors, Japana, New Zealand, Washington | S | P | [38], [41], [76], [98], [100], [101] | |
| 19. Clavelina lepadiformis | NW Atlantic, South Africa | C | A | [82], [102] | |
| 20. Cnemidocarpa areolata (C. irene) | Brazil | S | S | T, SH | [80] |
| 21. Cnemidocarpa humilis | South Africa | S | S | SH | [82] |
| 22. Cnemidocarpa irene | Hawaii | S | S | T, NH | [103] |
| 23. Corella eumyota | England, Iberia Atlantic coast, New Zealand, NW France | S | P | [70], [91], [104], [105] | |
| 24. Cystodytes philippinensis | Mediterranean Sea | C | A | NH | [73], [75] |
| 25. Didemnum cineraceum | Atlantic Panama, Brazil | C | A | T | [72], [80] |
| 26. Didemnum perlucidum | Brazil, Caribbean, Guam, Gulf of Mexico | C | A | T | [40], [71], [106], [107] |
| 27. Didemnum sp. | Hawaii | C | A | T, NH | [81] |
| 28. Didemnum vexillum | England, New England, San Francisco Bay, Washington, widely distributed | C | A | [83], [90], [98], [108], [109] | |
| 29. Diplosoma listerianum | Brazil, Guam, Netherlands, New England, South Africa | C | A | T | [40], [69], [71], [82], [83] |
| 30. Distaplia bermudensis | Brazil, Florida, Mediterranean Sea | C | A | T | [71], [73], [75], [110] |
| 31. Distaplia stylifera | Brazil | C | A | SH | [80] |
| 32. Ecteinascidia styeloides | Mediterranean Sea | C | P | NH | [73], [75] |
| 33. Ecteinascidia thurstoni | Mediterranean Sea | C | P | NH | [74], [75] |
| 34. Eudistoma elongatum | New Zealand | C | A | SH | [111] |
| 35. Eusynstyela tincta | India | C | S | NH | [78] |
| 36. Herdmania momus | Hawaii, Mediterranean Sea | S | S | T, NH | [39], [73]–[75] |
| 37. Herdmania pallida | Atlantic Panama, Hawaii | S | S | T, NH | [72] Lambert unpublished data |
| 38. Lissoclinum fragile | Guam | C | A | T, NH | [40] |
| 39. Microcosmus exasperatus | Atlantic Panama, Guam, Hawaii, India, Mediterranean Sea | S | S | T, NH | [39], [40], [72]–[74], [75], [78] |
| 40. Microcosmus squamiger | California harbors, India, Mediterranean Sea, South Africa | S | S | T, NH | [38], [41], [73], [75], [76], [78], [82], [112] |
| 41. Molgula citrina | Alaska | S | S | NH | [113] |
| 42. Molgula ficus | California harbors, Chila | S | S | [88], [114] | |
| 43. Molgula manhattensis | California harbors, China/Korea, Europe, NE Pacific, Netherlands, South Australia and Tasmania | S | S | [38], [41], [69], [76], [86], [94], [115] | |
| 44. Perophora japonica | Atlantic Europe, England, Netherlands, Northern California | C | A | NH | [69], [91], [116], [117] |
| 45. Perophora multiclathrata | Mediterranean Sea | C | A | [73], [75] | |
| 46. Phallusia nigra | Guam, Hawaii, India, Mediterranean Sea | S | P | T, NH | [39], [40], [73]–[75], [78], [81] |
| 47. Polyandrocarpa anguinea | Brazil | C | S | T, SH | [80] |
| 48. Polyandrocarpa sp. | Hawaii | C | S | T, NH | [39] |
| 49. Polyandrocarpa zorritensis | California harbors, Gulf of Mexico, Mediterranean Sea | C | S | T, NH | [38], [41], [73], [75], [76], [107] |
| 50. Polycarpa aurita | Hawaii | S | S | T, NH | [103] |
| 51. Polycarpa spongiabilis | Brazil | S | S | SH | [71] |
| 52. Polycarpa tumida | Brazil | S | S | T, SH | [80] |
| 53. Polyclinum aurantium | Brazil | C | A | SH | [77] |
| 54. Polyclinum constellatum | Guam, Brazil, Pacific Mexico | C | A | T | [40], [71], [118] |
| 55. Pyura praeputialis | Chile | S | S | [88] | |
| 56. Pyura vittata | Atlantic Panama | S | S | T, NH | [72] |
| 57. Rhodosoma turcicum | Mediterranean Sea, Florida | S | P | NH | [74], [75] Lambert unpublished data |
| 58. Styela canopus | Atlantic Panama, Brazil, California harbors, Guam, India | S | S | T | [40], [42], [43], [74], [75], [78], [80], [82] |
| 59. Styela clava | Atlantic Canada, California harbors, China/Korea, Denmark, England, Germany, Mediterranean Sea, Netherlands, New England, New Zealand, San Francisco Bay, South Australia and Tasmania, England, Washington | S | S | [38], [41], [70], [73], [75], [76], [85], [86], [90], [91], [95], [98], [99], [119] | |
| 60. Styela plicata | Brazil, California harbors, China/Korea, Gulf of Mexico, South Africa, South Australia and Tasmania | S | S | T | [38], [41], [76], [77], [79], [82], [85], [86], [99], [107], [120] |
| 61. Symplegma brakenhielmi | California harbors, Guam. Hawaii, Mediterranean Sea | C | S | T, NH | [38]–[40], [73]–[75] |
| 62. Symplegma reptans | California harbors, Hawaii | C | S | T, NH | [38], [39], [41], [76] |
| 63.Symplegma rubra | Brazil, Gulf of Mexico | C | S | T | [71], [107] |
| 64. Trididemnum cf. savignii | Mediterranean Sea | C | A | NH | [75] |
C- Colonial, S- Solitary, Order: A-Aplousobranchia, P-Phlebobranchia, S- Stolidobranchia, Remarks: T- Tropical, NH- Northern Hemisphere only, SH- Southern Hemisphere only.
Records of introduction of ascidians in tropical waters are mainly from Hawaii, Guam and Panama. Of the 64 documented global non-indigenous species, 27 species have records in tropical regions. However, only 14 have records that are restricted to tropical environments (Ascidia archaia, Cnemidocarpa areolata, Cnemidocarpa irene, Didemnum cineraceum, Didemnum sp. (Hawaii), Distaplia stylifera, Herdmania pallida, Lissoclinum fragile, Polyandrocarpa anguinea, Polyandrocarpa sp., Polycarpa aurita, Polycarpa tumida, Polyclinum constellatum, Pyura vittata). The majority of species (50) have introduction records from temperate environments, including both the northern and southern hemispheres. There are no records yet of non-indigenous ascidians from the arctic.
Ascidian global distribution
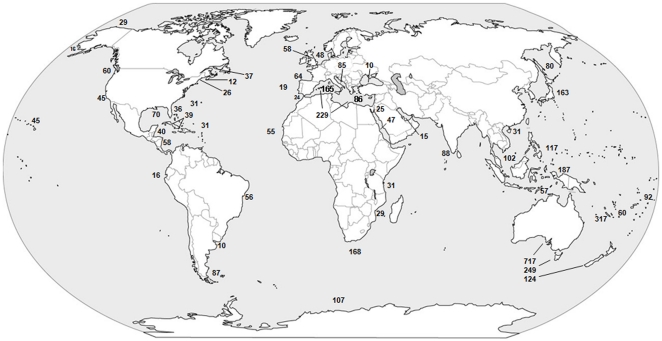
Fig. 4 provides a summary of species richness in different regions of the world. A complete list of sites and references is provided in Table 2. The highest number of ascidian species is found in the Indo-Pacific, with inventory numbers such as 317 species from New-Caledonia, 187 species from the Western Pacific ocean, 117 and 102 species from Guam and Gulf of Siam area (numbers represent the exact number of species mentioned in each citation). The ascidian fauna along the coasts of Australia and New Zealand was studied extensively, resulting in records of 717 species from Australia, 249 species from Tasmania, and 124 species from New Zealand. At higher latitudes, the Mediterranean and Japan each represent areas with high number of species with about 229 species from the Mediterranean and 163 species in Japan. Antarctica and South Africa also have extensive records of ascidian species of 107 and 168 species respectively. The North American coasts have been studied thoroughly with approximately 170 species along both the Atlantic and Pacific coasts.
Figure 4. Ascidian global distribution (abyssal species not included).
Table 2. Ascidian regional species richness.
| Area | Number of species | Reference |
| Australia | 717 | [121] |
| New Caledonia | 317 | [122] |
| Tasmania | 249 | [123] |
| Mediterranean Sea | 229 | [124] |
| Western Pacific Ocean: Palau, The Philippines, Indonesia, and Papua New Guinea | 187 | [125] |
| South Africa | 168 | [126] |
| Western Mediterranean | 165 | [127] |
| Japan | 163 | [128] |
| New Zealand | 124 | [123] |
| Guam | 117 | [129] |
| Antarctica | 107 | [32] |
| Indo West Pacific region | 102 | [130] |
| French Polynesia | 92 | [131] |
| India | 88 | [132] |
| South America | 87 | [133] |
| Eastern Mediterranean | 86 | [127] |
| Adriatic | 85 | [127] |
| North West Pacific (Kamchatka) | 80 | [134]–[140] |
| Gulf of Mexico | 70 | [107] |
| Gibraltar | 66 | [141] |
| Iberia | 64 | [142] |
| Fiji | 60 | [143] |
| Pacific Northwest | 60 | [144] |
| Panama | 58 | [145] |
| British | 58 | [146] |
| Timor and Arafura Sea | 57 | [147] |
| Brazil | 56 | [148] |
| Africa north west coast | 55 | [149] |
| Chile | 55 | [150] |
| Scandinavia | 48 | [151] |
| Red Sea | 47 | [152] |
| Hawaii | 45 | [153] |
| California | 45 | [154] |
| Belize | 40 | [31] |
| Jamaica | 39 | [155] |
| Cuba | 39 | [156] |
| Gulf of Saint Lawrence | 37 | [157] |
| Florida | 36 | [154] |
| Hong Kong | 31 | [158] |
| Bermuda | 31 | [159] |
| West indies | 31 | [160] |
| Tanzania | 31 | [161] |
| Mozambique | 29 | [162], [163] |
| Circumpolar | 29 | [113] |
| Venezuela | 29 | [164] |
| Massachusetts | 26 | [154] |
| Gulf of Aqaba | 25 | [165], [166] |
| Azores Islands | 19 | [167] |
| Galapagos | 16 | [168] |
| Bering Sea | 16 | [154] |
| Bahrain | 15 | [169] |
| Bay of Fundy | 12 | [154] |
| Black Sea | 10 | [127] |
Data sorted by number of species.
Discussion
Even though the class Ascidiacea has been the object of much scientific interest in the last decade [170], there are extensive regions around the world where very little collecting of ascidians has been done, resulting in very low number of described ascidian species and general lack of data (e.g., South and Central America, Canada, Alaska, and many thousands of islands in the tropical west Pacific). The current study reveals a strong association between species richness and sampling efforts. In addition, there is a clear trend of arrival and spread of non-indigenous species that put the endemic fauna at risk. Both of these issues emphasize the need for additional research in the field of ascidian biodiversity and biogeography.
In geographical areas where taxonomists have long been active, we typically found high numbers of species. The majority of the described ascidian species (more than 60%) are attributed to only seven taxonomic experts. This is demonstrated in the high species richness found in Australia [121], New Caledonia [122], Japan [128], the Caribbean Sea [171], and South Africa [172]–[174]. In contrast, along the coasts of South America, Indian Ocean, and Eastern Atlantic, there are vast areas with only scarce information regarding the occurrence of ascidians, and in some cases the only information comes from studies that may be out of date and not representative of the diversity these areas currently exhibit [154], [175]. For instance, the ascidian fauna of the Western Mediterranean has been studied in great detail and has been recorded in number of publications with an estimate of 165 described species [127]. The nearby Red Sea, which supports one of the most diverse ecosystems in the world [176] is represented by only 47 described species [152]. Thus, this discrepancy appears to be a result of less research and fewer sampling efforts, rather than a decrease in ascidian diversity [177]. Our inventory of 2815 described species of ascidians is certainly incomplete, with an estimation that approximately 3000 species remain to be discovered and described (Appeltans et al. 2011 unpublished data). Applying molecular approaches may further assist in locating cryptic speciation of a single species.
The high diversity of some of the ascidian families is remarkable. With approximately 26 families in the class Ascidiacea, the colonial Didemnidae family contains 20% of the described species, possibly due to highly diverse Didemnum genera, with more than 200 species. The Styelidae family is also highly diverse with 38 genera, and 535 described species, colonial and solitary. Colonial species characterize more than 60% of the described species. The high diversity of colonial ascidians is increasingly important since many contain very active secondary metabolites important to the pharmaceutical industry [178].
In general, it has been shown that in tropical environments colonial species dominate the substrate [179]. This is attributed to their asexual reproduction and indeterminate growth which provide them with a significant advantage for the exploitation of tropical habitats. Thus there are many more colonial ascidian species than solitary species in the tropics, representing about 80% of the species [125], [126], [131], [143], [180]. Although colonial ascidians are generally considered a minor benthic component on exposed surfaces of the natural coral reefs they can rapidly overgrow corals and outcompete them for space during periods of nutrient enrichment [181]–[183]. Since ascidians are able to filter even minute particulate matter [184], [185], any rise in nutrient levels and organic material in coastal waters will have a direct influence on their abundance.
In temperate waters solitary ascidians comprise 52% of the American fauna [154], and 75% in European waters (but this includes abyssal forms, almost all of which are solitary), [125], [186]. In the Antarctic, 58% of the species are solitary [32]. It is possible that solitary ascidians in the Antarctic and the deep sea, many of which are stalked, have an advantage over encrusting colonial species since most of the benthos is composed of soft sediments, so their vertical growth lifts them above the sediment. This three dimensional structure may improve food capture and assimilation during periods of winter inactivity and sedimentation [187]. In addition, since in solitary ascidians fertilization and larval development usually occur in the water column (in contrast to colonial species which are brooders), it is possible that they have a higher potential for dispersal [74]. This may also be advantageous in the Antarctic in cases of anchor ice formation [188], and ice scouring [189] which have a key role in determining marine biodiversity in high latitudes, emphasizing the importance of larval dispersal processes.
Historical baselines for comparison to present day from museum collections and published literature are required in order to understand and respond to changes in global biodiversity [190]. The current study provides a list of 64 non-indigenous ascidians (NIAs) with published records of introduction. This number is likely to be an underestimate, due to difficulty in taxonomic identification of aplousobranch species in particular. In some cases it may be difficult to determine if a certain species record is of a new introduction, or of a previously undiscovered natural population [113]. Lambert [40] suggests two criteria for the designation of NIAs in Guam, following the general guidelines of Chapman and Carlton [68] for determining non-indigenous species: (1) restricted to artificial surfaces and (2) an extra Indo-West Pacific distribution. The first criterion may be especially important especially in tropical environments which may be more resistant to invasion due to their diverse communities [191], [192]. In temperate and cold water environments there are records of rapid spread of NIAs on natural substrates such as Didemnum vexillum (Gulf of Maine) [109], [193] and Microcosmus squamiger (Western Mediterranean) [112]. A molecular approach, therefore, may be more relevant in revealing the status of a certain species [194]–[196].
The majority of records of NIAs are from cold water environments, suggesting this environment may be more favorable to introductions of ascidians. Nonetheless, nearly half of the NIAs have geographical records from tropical environments. Under lab conditions, at high temperature, the growth rate of NIAs was higher compared to that of native species [197], and they were able to tolerate significantly higher temperatures [198]. Thus, there is growing evidence that global warming may facilitate a shift northward by non-native species, accelerating homogenization of the global biota [199]. Nevertheless, high rates of endemism can be found in tropical environments such as the Great Barrier Reef [121], New Caledonia and French Polynesia [122], [131], and also in unique environments such as Southern New Zealand [123], and the Antarctic, with its isolated and homogeneous fauna [32].
The class Ascidiacea presents vast opportunities for research in the fields of evolution and development, physiology, natural products, and marine bioinvasion. Yet, there are many areas around the world that are relatively poorly known, and in others the available data should be updated and revised. Many more species are yet to be discovered, contributing to our accumulating knowledge of this unique group.
Supporting Information
Acknowledgments
We express our gratitude to G. Lambert for her advice and helpful suggestions and to C. Primo for her collaboration. N. Shenkar would like to thank the Israeli Taxonomy Initiative and the Dan-David foundation for financial support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: NS was funded by the Israeli Taxonomy Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Monniot C, Monniot F, Laboute P. Paris: ORSTOM; 1991. Coral Reef Ascidians of New Caledonia. [Google Scholar]
- 2.Cameron CB, Garey JR, Swalla BJ. Evolution of the chordate body plan: new insights from phylogenetic analyses of deuterostome phyla. Proc Natl Acad Sci USA. 2000;97:4469–4474. doi: 10.1073/pnas.97.9.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kott P. New and little-known species of Didemnidae (Ascidiacea, Tunicata) from Australia (Part 3). J Nat Hist. 2005;39:2409–2497. [Google Scholar]
- 4.Forbes E, Hanley SCT. 4 vols. London: John Van Voorst; 1853. A history of British Mollusca and their shells. [Google Scholar]
- 5.Lamarck JB. Paris: Déterville; 1816. Histoire naturelle des animaux sans vertèbres. Tome III. Tuniciers. pp. 80–130C. [Google Scholar]
- 6.Lambert CC. Historical introduction, overview, and reproductive biology of the protochordates. Can J Zool. 2005;83:1–7. [Google Scholar]
- 7.Zeng L, Swalla BJ. Molecular phylogeny of the protochordates: chordate evolution. Can J Zool. 2005;83:24–33. [Google Scholar]
- 8.Milne Edwards H. Éléments de zoologie. Vol. 2. Animaux sans vertèbres. 1843:313–316. [Google Scholar]
- 9.Hancock A. On the anatomy of the fresh water Bryozoa. Ann Nat Hist. 1850;2:173–204. [Google Scholar]
- 10.Savigny JC. Part 2. Paris: G Dufour; 1816. Mémoires sur les Animoux sans Vertèbres. [Google Scholar]
- 11.Kowalevsky AO. Entwicklungsgeschichte der einfachen Ascidien. Mem Acad Sci St Petersb. 1866;10:1–19. [Google Scholar]
- 12.Raff RA, Love AC. Kowalevsky, comparative evolutionary embryology, and the intellectual lineage of evo-devo. J Exp Zool (Mol Dev Evol) 2004;302B:19–34. doi: 10.1002/jez.b.20004. [DOI] [PubMed] [Google Scholar]
- 13.Balfour FM. London: Vol. 2.Macmillan; 1881. A treatise of comparative embryology. [Google Scholar]
- 14.Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–88. doi: 10.1038/nature05241. [DOI] [PubMed] [Google Scholar]
- 15.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 16.Swalla BJ, Smith AB. Deciphering deuterostome phylogeny: molecular, morphological and palaeontological perspectives. Phil Trans R Soc B. 2008;363:1557–1568. doi: 10.1098/rstb.2007.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishida H, Sawada K. Macho-1 encodes a localized mRNA in ascidian eggs that specifies muscle fate during embryogenesis. Nature. 2001;409:724–729. doi: 10.1038/35055568. [DOI] [PubMed] [Google Scholar]
- 18.Lahille F. Sur la classification des Tuniciers. CR Acad Sci Paris. 1886;102:1573–1575. [Google Scholar]
- 19.Tsagkogeorga G, Turon X, Hopcroft RR, Tilak M, Feldstein T, et al. An updated 18S rRNA phylogeny of tunicates based on mixture and secondary structure models. BMC Evol Biol. 2009;9:187. doi: 10.1186/1471-2148-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perrier JO. Note sur la classification des Tuniciers. CR Acad Sci Paris. 1898;126:1758–1762. [Google Scholar]
- 21.Lambert G. A global overview of ascidian introductions and their possible impact on the endemic fauna. In: Sawada H, Yokosawa H and Lambert CC (ed.), The Biology of Ascidians. Tokyo, Springer-Verlag, 2001:249–257. [Google Scholar]
- 22.Lambert G. Ecology and natural history of the protochordates. Can J Zool. 2005;83:34–50. [Google Scholar]
- 23.Svane I, Young CM. The ecology and behavior of ascidian larvae. Oceanogr Mar Biol Ann Rev. 1989;27:45–90. [Google Scholar]
- 24.Vazquez E, Young CM. Responses of compound ascidian larvae to haloclines. Mar Ecol Prog Ser. 1996;133:179–190. [Google Scholar]
- 25.Sims LL. Osmoregulatory capabilities of three macrosympatric stolidobranch ascidians, Styela clava Herdman, S. plicata (Lesueur) and S. montereyensis (Dall). J Exp Mar Biol Ecol. 1984;82:117–129. [Google Scholar]
- 26.Gab Alla AAFA. Distribution of the sea squirt Ecteinascidia thurstoni Herdman, 1890 (Ascidiacea: Perophoridae) along Suez Canal and Egyptian Red Sea Coasts. Oceanologia. 2008;50:239–253. [Google Scholar]
- 27.Monniot F. Some ascidians from Indonesian marine lakes (Raja Ampat Islands, West Papua). Zootaxa. 2009;2106:13–40. [Google Scholar]
- 28.Dijkstra J, Dutton A, Westerman E, Harris L. Heart rate reflects osmotic stress levels in two introduced colonial ascidians Botryllus schlosseri and Botrylloides violaceus. Mar Biol. 2008;154:805–811. [Google Scholar]
- 29.Dybern BI. Settlement of sessile animals on eternite slabs in two polls near Bergen. Sarsia. 1967;29:137–180. [Google Scholar]
- 30.Therriault TW, Herborg LM. Predicting the potential distribution of the vase tunicate Ciona intestinalis in Canadian waters: informing a risk assessment. ICES J Mar Sci. 2008;65:788–794. [Google Scholar]
- 31.Goodbody I. Diversity and distribution of ascidians (Tunicata) at Twin Cays, Belize. Atoll Res Bull. 2004;524:1–20. [Google Scholar]
- 32.Primo C, Vazquez E. Antarctic ascidians: an isolated and homogeneous fauna. Polar Res. 2009;28:403–414. [Google Scholar]
- 33.Monniot C, Monniot F. Records of ascidians from Bahrain, Arabian Gulf with three new species. J Nat Hist. 1997;31:1623–1643. [Google Scholar]
- 34.Millar RH. The biology of ascidians. Adv Mar Biol. 1971;9:1–100. [Google Scholar]
- 35.Auker LA, Oviatt CA. Factors influencing the recruitment and abundance of Didemnum in Narragansett Bay, RhodeIsland. ICES J Mar Sci. 2008;65:765–769. [Google Scholar]
- 36.Shenkar N, Loya Y. The solitary ascidian Herdmania momus: native (Red Sea) vs. non-indigenous (Mediterranean) populations. Biol Inv. 2008;10:1431–1439. [Google Scholar]
- 37.Shenkar N, Zeldman Y, Loya Y. Ascidian recruitment patterns on an artificial reef in Eilat (Red Sea). Biofouling. 2008;24:119–128. doi: 10.1080/08927010801902083. [DOI] [PubMed] [Google Scholar]
- 38.Lambert CC, Lambert G. Non-indigenous ascidians in southern California harbours and marinas. Mar Biol. 1998;130:675–68. [Google Scholar]
- 39.Coles SL, DeFelice RC, Eldredge LG, Carlton JT. Historical and recent introductions of non-indigenous marine species into Pearl Harbor, Oahu, Hawaiian Islands. Mar Biol. 1999;135:147–158. [Google Scholar]
- 40.Lambert G. Nonindigenous ascidians in tropical waters. Pac Sci. 2002;56:291–298. [Google Scholar]
- 41.Cohen AN, Harris LH, Bingham BL, Carlton JT, Chapman JW, et al. Rapid assessment survey for exotic organisms in southern California bays and harbors, and abundance in port and non-port areas. Biol Inv. 2005;7:995–1002. [Google Scholar]
- 42.Thompson R, MacNair N. PEI Department of Agriculture, Fisheries, Aquaculture and Forestry Technical Report 234, VIII +; 2004. An overview of the clubbed tunicate (Styela clava) in Prince Edward Island.29 [Google Scholar]
- 43.Bourque D, Davidson J, MacNair NG, Arsenault G, LeBlanc AR, et al. Reproduction and early life history of an invasive ascidian Styela clava Herdman in Prince Edward Island, Canada. J Exp Mar Biol Ecol. 2007;342:78–84. [Google Scholar]
- 44.Howes S, Herbinger CM, Darnell P, Vercaemer B. Spatial and temporal patterns of recruitment of the tunicate Ciona intestinalis on a mussel farm in Nova Scotia, Canada. J Exp Mar Biol Ecol. 2007;342(1):85–92. [Google Scholar]
- 45.Raynolds C, Fortune R. What is the view from the boat? Atlantic Canadian Tunicate Workshop Proceedings, Atlantic Veterinary College, PEI Canada March 2003. 2003:23. [Google Scholar]
- 46.Nguyen TTT, Taniguchi N, Nakajima M, Na-Nakorn U, Sukumasavin N, et al. Aquaculture of sea-pineapple, Halocynthia roretzi in Japan. Aquac Asia Mag. 2007;12:21–23. [Google Scholar]
- 47.Kumagai A, Suto A, Ito H, Tanabe T, Takahashi K, et al. Mass mortality of cultured ascidians Halocynthia roretzi associated with softening of the tunic and flagellate-like cells. Dis Aquat Organ. 2010;90:223–2343. doi: 10.3354/dao02228. [DOI] [PubMed] [Google Scholar]
- 48.Teo SLM, Ryland JS. Toxicity and palatability of some British ascidians. Mar Biol. 1994;120:297–303. [Google Scholar]
- 49.Teo SLM, Ryland JS. Potential antifouling mechanisms using toxic chemicals in some British ascidians. J Exp Mar Biol Ecol. 1995;188:49–62. [Google Scholar]
- 50.Davis AR. Antifouling defense in a subtidal guild of temperate zone encrusting invertebrates. Biofouling. 1998;12:305–320. [Google Scholar]
- 51.Pisut DP, Pawlik JR. Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids? J Exp Mar Biol Ecol. 2002;270:203–214. [Google Scholar]
- 52.McClintock JB, Amsler MO, Amsler CD, Southworth KJ, Petrie C, et al. Biochemical composition, energy content and chemical antifeedant and antifoulant defenses of the colonial Antarctic ascidian Distaplia cylindrica. . Mar Biol. 2004;145:885–894. [Google Scholar]
- 53.Wright AE, Forleo DA, Gunawardana GP, Gunasekera SP, Koehn FE, et al. Antitumor tetrahydroisoquinoline alkaloids from the colonial ascidian Ecteinascidia turbinata. J Org Chem. 1990;55:4508–4512. [Google Scholar]
- 54.Scotto KW. ET-743: more than an innovative mechanism of action. Anti-Cancer Drugs. 2002;13(suppl. 1):S3–6. [PubMed] [Google Scholar]
- 55.Casali PG, Sanfilippo R, D'Incalci M. Trabectedin therapy for sarcomas. Curr Opin Oncol. 2010;22:342–346. doi: 10.1097/CCO.0b013e32833aaac1. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Trufero J, Alfaro J, Felipo F, Alvarez M, Madani J, et al. Response to trabectedin treatment in a highly pretreated patient with an advanced meningeal hemangiopericytoma. Anti-Cancer Drug. 2010;21:795–79. doi: 10.1097/CAD.0b013e32833d19f0. [DOI] [PubMed] [Google Scholar]
- 57.Mendiola J, Hernández H, Sariego I, Rojas L, Otero A, et al. Antimalarial activity from three ascidians: an exploration of different marine invertebrate phyla. Trans Roy Soc Trop Med H. 2006;100:909–916. doi: 10.1016/j.trstmh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 58.Brookfield ME. Where are all the fossil sea squirts? Micropaleontology. 1988;34:277–283. [Google Scholar]
- 59.Lambert G, Lambert CC, Lowenstam HA. Protochordate biomineralization. In Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. In: Carter JG, editor. NY: Van Nostrand Reinhold; 1990. pp. 461–469. [Google Scholar]
- 60.Kott P. The Australian Ascidiacea. Part 4: Aplousobranchia (3), Didemnidae. Mem Queensland Mus. 2001;47(1):1–407. [Google Scholar]
- 61.Varol O. A review and classification of fossil didemnid ascidian spicules. J Micropalaeontol. 1996;15:135–149. [Google Scholar]
- 62.Sagular EK. Fossil didemnid ascidian spicule records in the Plio-Quaternary marine clastics of the Antalya Basin (Eastern Mediterranean) and their stratigraphic calibration to new nannofossil data. Geosci J. 2009;13:121–131. [Google Scholar]
- 63.Chen JY, Huang DY, Peng QQ, Chi HM, Wang XQ, et al. The first tunicate from the Early Cambrian of South China. P Natl Acad Sci USA. 2003;100:8314–8318. doi: 10.1073/pnas.1431177100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shenkar N, Gittenberger A, Lambert G, Rius M, Moreira Da Rocha R, et al. World Ascidiacea Database. 2010 http://www.marinespecies.org/ascidiacea. Consulted on 10-28-2010. [Google Scholar]
- 65.VLIZ. 2010 Maritime Boundaries Geodatabase, version X. http://www.vliz.be/vmdcdata/marbound. Consulted on 11-03-2010. [Google Scholar]
- 66.Bisby FA, Roskov YR, Orrell TM, Nicolson D, Paglinawan LE, et al. 2010. Species 2000 & ITIS Catalogue of Life, 2nd October 2010. Digital resource at http://www.catalogueoflife.org/col. Species 2000: Reading, UK.
- 67.Wilson EO. The encyclopedia of life. Trends Ecol Evol. 2003;18:77–80. [Google Scholar]
- 68.Chapman JW, Carlton JT. A test of criteria for introduced species: the global invasion by the isopod Synidotea laevidorsalis (Miers, (1881). J Crustacean Biol. 1991;11:386–400. [Google Scholar]
- 69.Gittenberger A. Invasive tunicates on Zeeland and Prince Edward Island mussels, and management practices in The Netherlandss. Aquat Inv. 2009;4:279–281. [Google Scholar]
- 70.Coutts ADM, Dodgshun TJ. The nature and extent of organisms in vessel sea-chests: A protected mechanism for marine bioinvasions. Mar Pollut Bull. 2007;54:875–886. doi: 10.1016/j.marpolbul.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Rocha RM, Kremer LP, Baptista MS, Metri R. Bivalve cultures provide habitat for exotic tunicates in southern Brazil. Aquat Inv. 2009;4:195–205. [Google Scholar]
- 72.Rocha RM. III IISSC Woods Hole, April; 2010. Monitoring ascidians on natural and anthropogenic habitats in Bocas del Toro, Panama. [Google Scholar]
- 73.Izquierdo-Muñoz A, Diaz-Valdés M, Ramos-Esplá AA. Recent non-indigenous ascidians in the Mediterranean Sea. Aquat Inv. 2009;4:59–64. [Google Scholar]
- 74.Shenkar N, Loya Y. Non-indigenous ascidians (Chordata: Tunicata) along the Mediterranean coast of Israel. Marine Biodiversity Records. 2009;2:166. doi: 10.1017/S1755267209990753. [Google Scholar]
- 75.Zenetos A, Gofas S, Verlaque M, Inar ME, Garci'a Raso JE, et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union's Marine Strategy Framework Directive (MSFD). Part I Spatial distribution. Medit Mar Sci. 2010;11:381–493. [Google Scholar]
- 76.Lambert CC, Lambert G. Persistence and differential distribution of nonindigenous ascidians in harbors of the Southern California Bight. Mar Ecol Prog Ser. 2003;259:145–161. [Google Scholar]
- 77.Marins FO, Novaes RLM, Rocha RM, Junqueira AOR. Non indigenous ascidians in port and natural environments in a tropical Brazilian bay. Zoologia. 2010;27:213–221. [Google Scholar]
- 78.Jaffar Ali HA, Sivakumar V, Tamilselvi M. Distribution of Alien and Cryptogenic Ascidians along the Southern Coasts of Indian Peninsula. World J Fish Mar Sci. 2009;1:305–312. [Google Scholar]
- 79.Rocha RM, Kremer LP. Introduced ascidians in Paranaguá Bay, Paraná, Southern Brazil. Revista Brasileira de Zoologia. 2005;22:1170–1184. [Google Scholar]
- 80.Oliveira FRR, Lotufo TMC. III IISSC Woods Hole, April; 2010. New records of introduced ascidians at Ceara State harbors, Northern Brazil. [Google Scholar]
- 81.DeFelice RC, Eldredge LG, Carlton JT. Nonindigenous marine invertebrates. In: A Guidebook of Introduced Marine Species in Hawaii , Eldredge LG, Smith CM, editors. Honolulu: Bishop Museum; 2001. [Google Scholar]
- 82.Mead A, Carlton JT, Griffiths CL, Rius M. J Nat Hist In Press; 2011. Introduced and cryptogenic marine and estuarine species of South Africa. [Google Scholar]
- 83.Carman MR, Grunden DW. First occurrence of the invasive tunicate Didemnum vexillum in eelgrass habitat. Aquat Inv. 2010;5:23–29. [Google Scholar]
- 84.Brewin BI. Ascidians in the vicinity of the Portobello Marine Biological Station, Otago Harbour. T Roy Soc NZ. 1946;76:87–131. [Google Scholar]
- 85.Wiltshire K, Rowling K, Deveney M. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2010/000305-1. SARDI Research Report Series No. 468; 2010. Introduced marine species in South Australia: a review of records and distribution mapping.232 [Google Scholar]
- 86.Hewitt CL, Campbell ML, Thresher RE, Martin RB, Boyd S, et al. Introduced and cryptogenic species in Port Phillip Bay, Victoria, Australia. Mar Biol. 2004;144:183–202. [Google Scholar]
- 87.Tatian M, Schwindt E, Lagger C, Varela MM. Colonization of Patagonian harbours (SW Atlantic) by an invasive sea squirt (Chordata, Ascidiacea). Spixiana. 2010;33:111–117. [Google Scholar]
- 88.Castilla JC, Neill PE. Marine bioinvasions in the Southern Pacific: status, ecology, economic impacts, conservation and management. In Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives. In: Rilov G, Crooks J, editors. Berlin Springer-Verlag Heidelberg; 2009. pp. 439–458. [Google Scholar]
- 89.Callahan AG, Deibel D, McKenzie CH, Hall JR, Rise ML. Survey of harbours in Newfoundland for indigenous and non-indigenous ascidians and an analysis of their cytochrome c oxidase I gene sequences. Aquat Inv. 2010;5:31–39. [Google Scholar]
- 90.Cohen AN. 2005. Guide to Exotic Species of San Francisco Bay. San Francisco Estuary Institute. Oakland, California. www.exoticsguide.org.
- 91.Arenas F, Bishop JDD, Carlton JT, Dyrynda PJ, Farnham WF, et al. Alien species and other notable records from a rapid assessment survey of marinas on the south coast of England. J Mar Biol Assoc UK. 2006;86:1329–1337. [Google Scholar]
- 92.Carver CE, Mallet AL, Vercaemer B. Canadian Manuscript Report of Fisheries and Aquatic Sciences; 2006. Biological synopsis of the colonial tunicates, Botryllus schlosseri and Botrylloides violaceus.2747 [Google Scholar]
- 93.Lejeusne C, Bock DG, Therriault TW, MacIsaac HJ, Cristescu ME. Comparative phylogeography of two colonial ascidians reveals contrasting invasion histories in North America. Biol Inv. 2010;13:635–650. [Google Scholar]
- 94.Lambert G. New records of ascidians from the NE Pacific: a new species of Trididemnum, range extension and redescription of Aplidiopsis pannosum (Ritter, 1899) including its larva, and several nonindigenous species. Zoosystema. 2003;25:665–679. [Google Scholar]
- 95.Gollasch S, Haydar D, Minchin D, Wolff WJ, Reise K. Introduced aquatic species of the North Sea coasts and adjacent brackish waters. In: Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives , Rilov G, Crooks J, editors. Berlin Springer-Verlag Heidelberg; 2009. pp. 507–528. [Google Scholar]
- 96.Stoner DS, Ben-Shlomo R, Rinkevich B, Weisman IL. Genetic Variability of Botryllus schlosseri invasions to the East and West Coasts of USA. Mar Ecol Prog Ser. 2002;243:93–100. [Google Scholar]
- 97.Brewin BI. Ascidians of New Zealand. Part IV. Ascidians in the vicinity of Christchurch. T Roy Soc NZ. 1950;78:344–353. [Google Scholar]
- 98.Lambert G. Updated October 2010. Prepared for Washington Dept. of Fish and Wildlife; 2007. Washington state 2006 survey for invasive tunicates with records from previous surveys. [Google Scholar]
- 99.Seo KS, Yoon L. A first assessment of invasive marine species on Chinese and Korean Coasts. In: Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives , Rilov G, Crooks J, editors. Berlin Springer-Verlag Heidelberg; 2009. pp. 577–586. [Google Scholar]
- 100.Smith KF, Cahill PL, Fidler AE. First record of the solitary ascidian Ciona savignyi Herdman, 1882 in the Southern Hemisphere. Aquat Inv. 2010;5:363–368. [Google Scholar]
- 101.Zvyagintsev D, Sanamyan ÊE, Kashenko SD. On the Introduction of the Ascidian Ciona savignyi Herdman, 1882 into Peter the Great Bay, Sea of Japan. Russ J Mar Biol. 2007;33:133–136. [Google Scholar]
- 102.Reinhardt JF, Stefaniak LM, Hudson DM, Mangiafico J, Gladych R, et al. First record of the non-native light bulb tunicate Clavelina lepadiformis (Müller, 1776) in the northwest Atlantic. Aquat Inv. 2010;5:185–190. [Google Scholar]
- 103.Godwin S, Harris L, Charette A, Moffitt R. Kaneohe, HI: Hawaii Institute for Marine Biology; 2008. The marine invertebrate species associated with the biofouling of derelict fishing gear in the Papahanoumokuakea–Marine National Monument. A focus on marine non-native species transport.26 [Google Scholar]
- 104.Lambert G. The south temperate and Antarctic ascidian Corella eumyota reported in two harbours in northwestern France. J Mar Biol Assoc UK. 2004;84:239–241. [Google Scholar]
- 105.Nagar AE, Huys R, Bishop JDD. Widespread occurrence of the southern hemisphere ascidian Corella eumyota Traustedt, 1882 on the Atlantic coast of Iberia. Aquat Inv. 2010;5:169–173. [Google Scholar]
- 106.Monniot F. Ascidies littorales de Guadeloupe I. Didemnidae. Bull Mus Natl Hist Nat, Paris 4e ser, 1983;5:5–49. [Google Scholar]
- 107.Cole L, Lambert G. Tunicata (Urochordata) of the Gulf of Mexico. Chapt. 73, 1209-1216. In: Felder D.L., Camp D.K., editors. Gulf of Mexico: Origin, Waters, and Biota. Vol. 1, Biodiversity. Texas A&M Univ. Press; 2009. [Google Scholar]
- 108.Griffith K, Mowat S, Holt RHF, Ramsay K, Bishop JDD, et al. First records in Great Britain of the invasive colonial ascidian Didemnum vexillum Kott, 2002. Aquat Inv. 2009;4:581–590. [Google Scholar]
- 109.Lambert G. Adventures of a sea squirt sleuth: unraveling the identity of Didemnum vexillum, a global ascidian invader. Aquat Inv. 2009;4:5–28. [Google Scholar]
- 110.Mastrototaro F, Brunetti R. The non-indigenous ascidian Distaplia bermudensis in the Mediterranean: comparison with the native species Distaplia magnilarva and Distaplia lucillae sp nov. J Mar Biol Assoc UK. 2006;86:181–185. [Google Scholar]
- 111.Page MJ, Morrisey DJ, Handley SJ. III IISSC Woods Hole, April; 2010. Biology, ecology and trials of potential methods for control of the introduced ascidian Eudistoma elongatum in Northland, New Zealand. [Google Scholar]
- 112.Turon X, Nishikawa T, Rius M. Spread of Microcosmus squamiger (Ascidiacea: Pyuridae) in the Mediterranean Sea and adjacent waters. J Exp Mar Biol Ecol. 2007;342:185–188. [Google Scholar]
- 113.Lambert G, Shenkar N, Swalla BJS. First Pacific record of the north Atlantic ascidian Molgula citrina- bioinvasion or circumpolar distribution? Aquatic Inv. 2010;5:369–378. [Google Scholar]
- 114.Lambert G. The nonindigenous ascidian Molgula ficus in California. Cah Biol Marine. 2007;48:95–102. [Google Scholar]
- 115.Haydar D. The scale and consequences of marine bioinvasions in the North Atlantic Ocean. Van Denderen BV, Groningen, The Netherlandss; 2010. What is natural?184 [Google Scholar]
- 116.Pérez-Portela R, Bishop JDD, Turon X. III IISSC Woods Hole, April; 2010. A recent invader, Perophora japonica, in Europe: Temporal genetic change and spatial distribution of genetic diversity in the English Channel. [Google Scholar]
- 117.Lambert G. First North American record of the ascidian Perophora japonica. J Mar Biol Assoc UK. 2005;85:1011–1012. [Google Scholar]
- 118.Tovar-Hernández M, Suárez-Morales E, Yáñez-Rivera B. The parasitic copepod Haplostomides hawaiiensis (Cyclopoida) from the invasive ascidian Polyclinum constellatum in the southern Gulf of California. Bull Mar Sci. 2010;86:637–648. [Google Scholar]
- 119.Davis MH, Davis ME. Styela clava (Tunicata, Ascidiacea) - a new threat to the Mediterranean shellfish industry? Aquat Inv. 2009;4:283–289. [Google Scholar]
- 120.Pineda MC, López-Legentil S, Turon X. III IISSC Woods Hole, April; 2010. Global phylogeography of the solitary ascidian Styela plicata. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kott P. Observations on non-didemnid ascidians in Australian waters. J Nat Hist. 2006;40:169–234. [Google Scholar]
- 122.Monniot F. Some comments on ascidians of New Caledonia. In: Payri CE, Richer de Forges B, editors. Compendium of marine species of New Caledonia. Vol. 117. Doc Sci Tech; 2007. pp. 349–356. [Google Scholar]
- 123.Primo C, Vazquez E. Zoogeography of the southern New Zealand, Tasmanian and southern African ascidian fauna. New Zeal J Mar Fresh. 2008;42:233–256. [Google Scholar]
- 124.Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, et al. The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One. 2010;5(8):e11842. doi: 10.1371/journal.pone.0011842. doi: 10.1371/journal.pone.0011842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Monniot F, Monniot C. Ascidians from the tropical western Pacific. Zoosystema. 2001;23:201–383. [Google Scholar]
- 126.Primo C, Vazquez E. Zoogeography of the southern African ascidian fauna. J Biogeogr. 2004;31:1987–2009. [Google Scholar]
- 127.Koukouras A, Voultsiadou-Koukoura E, Kevrekidis T, Vafidis D. Ascidian fauna of the Aegean sea with a check list of the Eastern Mediterranean and Black Sea species. Annales de l'Institut Oceanographique. 1995;71:19–34. [Google Scholar]
- 128.Nishikawa T. The ascidians of the Japan Sea. I. Publications of the Seto Marine Biological Laboratory. 1990;34:73–148. [Google Scholar]
- 129.Lambert G. Marine biodiversity of Guam: the Ascidiacea. Micronesica. 2003;35-36:588–597. [Google Scholar]
- 130.Millar RH. Ascidians from the Indo-West-Pacific region in the Zoologlcal Museum, Copenhagen (Tunicata, Ascidiacea). Steenstrupia. 1975;20:205–336. [Google Scholar]
- 131.Monniot C, Monniot F. Les ascidies de Polynesie francaise. Memoires du Museum national d'Histoire naturelle, Paris (A) 1987;136:1–155. [Google Scholar]
- 132.Abdul JAH, Sivakumar V. Occurrence and distribution of ascidians in Vizhinjam Bay (south west coast of India). J Exp Mar Biol Ecol. 2007;342:189–190. [Google Scholar]
- 133.Primo C, Vazquez E. Zoogeography of the Antarctic ascidian fauna in relation to the sub-Antarctic and South America. Antarct Sci. 2007;19:321–336. [Google Scholar]
- 134.Sanamyan K. Ascidians from the NW Pacific region. 1. Polycitoridae. Ophelia. 1993a;37:163–173. [Google Scholar]
- 135.Sanamyan K. Ascidians from the NW Pacific region. 2. Molgulidae. Ophelia. 1993b;38:127–135. [Google Scholar]
- 136.Sanamyan K. Ascidians from the NW Pacific region. 3. Pyuridae. Ophelia. 1996;45:199–209. [Google Scholar]
- 137.Sanamyan K. Ascidians from the NW Pacific region. 4. Polyclinidae and Placentelidae. Ophelia. 1998;48:103–135. [Google Scholar]
- 138.Sanamyan K. Ascidians from the NW Pacific region. 5. Phlebobranchia. Ophelia, 1998;49:97–116. [Google Scholar]
- 139.Sanamyan K. Ascidians from the NW Pacific region. 6. Didemnidae. Ophelia. 1999;51:143–161. [Google Scholar]
- 140.Sanamyan K. Ascidians from the North-Western Pacific region. 7. Styelidae. Ophelia. 2000;53:67–78. [Google Scholar]
- 141.Ramos Espla AA, Buencuerpo V, Vasquez E, Lafargue F. Some biogeographical remarks about the ascidian littoral fauna of the Straits of Gibraltar (Iberian sector). Bull de l'Inst Océan Monaco. 1992;9:125–132. [Google Scholar]
- 142.Vazquez E, Ramos-Espla A. Eudistoma roseum n.sp (Ascidiacea, Polycitoridae) from the Iberian Atlantic coast. Ophelia. 1993;37:95–100. [Google Scholar]
- 143.Kott P. The ascidians of the reef flats of Fiji. Proc Linn Soc NSW. 1981;105:147–212. [Google Scholar]
- 144.Lambert CC, Lambert G, Kozloff EN. Phylum Urochordata, Class Ascidiacea. . In: Kozloff EN, editor. Vol. 23. U of Wash Press; 1987. pp. 467–479. In: Marine Invertebrates of the Pacific Northwest. [Google Scholar]
- 145.Rocha RM Faria SB, Moreno TR. Ascidians from Bocas del Toro, Panama. I. Biodiversity. Caribb J Sci. 2005;41:600–612. [Google Scholar]
- 146.Berrill NJ. London: The Ray Soc.; 1950. The Tunicata with an Account of the British Species.354 [Google Scholar]
- 147.Kott P. Ascidiacea (Tunicata) in Australian waters of the Timor and Arafura Seas. Beagle Rec Mus Art Galleries NT. 2004;20:37–81. [Google Scholar]
- 148.Rodrigues SA, Rocha RM, Lotufo TMC. FAPESP, São Paulo; 1998. Guia Ilustrado para Identificação das Ascídias do Estado de São Paulo. [Google Scholar]
- 149.Monniot C, Monniot F. Additions to the inventory of eastern tropical Atlantic ascidians; arrival of cosmopolitan species. Bull Mar Sci. 1994;54:71–93. [Google Scholar]
- 150.Lee MR, Castilla JC, Fernandez M, Clarke M, Gonzalez C, Hermosilla C, Prado L, Rozbaczylo N, Valdovinos C. Free-living benthic marine invertebrates in Chile. Revista Chliena De Historia Natural. 2008;81:51–67. [Google Scholar]
- 151.Millar RH. 1. Tunicata. Ascidiacea. Scandinavian University books, Oslo; 1966. Marine invertebrates of Scandinavia. [Google Scholar]
- 152.Monniot C. Stolidobranch ascidians from the tropical western Indian Ocean. Zool J Linn Soc. 2002;135:65–120. [Google Scholar]
- 153.Abbott, DP, Newberry AT, Morris KM. Section 6B: Ascidians (Urochordata). Reef and Shore Fauna of Hawaii. Bishop Museum Special Publ. 1997;64(6B) [Google Scholar]
- 154.Van Name WG. The North and South American ascidians. Bull Am Mus Nat Hist. 1945;84:1–476. [Google Scholar]
- 155.Goodbody I. The Ascidian fauna of Port Royal, Jamaica In Harbour and mangrove dwelling species. Bull Mar Sci. 2003;73:457–476. [Google Scholar]
- 156.Hernández-Zanuy AC, Carballo JL. Distribution and abundance of ascidian assemblages in Caribbean reef zones of the Golfo de Batabanó (Cuba). Coral Reefs. 2001;20:159–162. [Google Scholar]
- 157.Brunel P, Bossé L, Lamarche G. Catalogue of the Marine Invertebrates of the Estuary and Gulf of Saint Lawrence. Canadian Special Publication of Fisheries and Aquatic Sciences. 1998;126:405. [Google Scholar]
- 158.Kott P, Goodbody I. Hong Kong: Hong Kong University Press; 1982. Ascidians of Hong Kong. In: The Marine Flora and Fauna of Hong Kong and Southern China. pp. 503–554. [Google Scholar]
- 159.Berrill NJ. Ascidians of the Bermudas. Biological Bulletin. 1932;62:77–88. [Google Scholar]
- 160.Goodbody I. Ascidians from Caribbean shallow water localities. Studies on the Fauna of Curaçao and Other Caribbean Islands. 1984;67:62–76. [Google Scholar]
- 161.Monniot F, Monniot C. Ascidians collected in Tanzania. J East Afr Nat Hist. 1997;86:1–35. [Google Scholar]
- 162.Millar RH. Ascidians from Mozambique, East Africa. Ann Mag Nat Hist Nat Sci. 1956;9:913–932. [Google Scholar]
- 163.Millar RH. Ascidians collected during the International Indian Ocean Expedition. J Nat Hist. 1988;22:823–848. [Google Scholar]
- 164.Rocha RM, Guerra-Castro E, Lira C, Paul SM, Hernández I, et al. Inventory of ascidians (Tunicata, Ascidiacea) from the National Park La Restinga, Isla Margarita, Venezuela. Biota Neotrop. 2010;10:210–218. [Google Scholar]
- 165.Pérès JM. Sur une collection d'ascidies de la cote israelienne de la mer rouge et de la peninsula du Sinai. Bull Sea Fish Res Haifa. 1962;30:39–47. [Google Scholar]
- 166.Monniot C. Redescription de six ascidies du golfe d'Elat récoltées par H. Schumacher. Israel Journal of Zoology. 1973;22:51–62. [Google Scholar]
- 167.Monniot C. Ascidies littorales et bathyales recoltees au cours de la campagne Biacores: Phlebobranches et Stolidobranches. Bull Mus Natn Hist Nat Paris. 1974;3173(251):1327–1352. [Google Scholar]
- 168.Iturralde M. Taxonomy, abundance, and vertical distribution of the ascidians from Tagus Cove, Isabela Island. Charles Darwin Research Station Annual Report. 1996;1988-1989:120–123. [Google Scholar]
- 169.Monniot C, Monniot F. Records of ascidians from Bahrain, Arabian Gulf, with three new species. J Nat Hist. 1997;31:1623–1643. [Google Scholar]
- 170.Pourquié, O A macho way to make muscles. Nature. 2001;409:679–680. doi: 10.1038/35055657. [DOI] [PubMed] [Google Scholar]
- 171.Goodbody I. The Mona Institute of Applied Sciences, Jamaica; 2006. Caribbean Sea Squirts: The Goodbody collection. [Google Scholar]
- 172.Millar RH. A collection of ascidians from South Africa. P Zool Soc Lond. 1955;125:169–221. [Google Scholar]
- 173.Millar RH. Further descriptions of South African ascidians. Annals of the South African Museum. 1962;46:113–221. [Google Scholar]
- 174.Millar RH. South African ascidians collected by Th. Mortensen, with some additional material. Videnskabelige Meddelelser fra Dansk Naturhistorisk Forening, bd. 1964;127:159–180. [Google Scholar]
- 175.Millar RH. Ascidians from the tropical coast of west Africa. Atlantide Rep. 1965;8:247–255. [Google Scholar]
- 176.Loya Y. Changes in a Red Sea Coral Community Structure: A Long-Term Case History Study. In: Woodwell, editor. The earth in transition Patterns and Processes of Biotic Impoverishment. Woods Hole Oceanographic Institution, Massachusetts; 1991. [Google Scholar]
- 177.Shenkar N, Loya Y. Ecology and systematics of the ascidian fauna in the Gulf of Eilat (Aqaba). In “Aqaba-Eilat, the Improbable Gulf. Environment, Biodiversity and Preservation” Editor FD Por, Magnes, Jerusalem. 2008:197–208. [Google Scholar]
- 178.Menna M. Antitumor potential of natural products from Mediterranean ascidians. Phytochem Rev. 2009;8:461–472. [Google Scholar]
- 179.Jackson JBC. Competition on marine and hard substrata: the adaptive significance of solitary and colonial strategies. Am Nat. 1977;11:743–767. [Google Scholar]
- 180.Monniot C, Monniot F. Ascidies littorals de Guadeloupe. IX: Caractéristiques des populations, écologie, rapports avec la faune mondiale. Tethys. 1985;11:203–213. [Google Scholar]
- 181.Bak RPM, Lambrechts DYM, Joenje M, Nieuwland G, Van Veghel MLJ. Long-term changes on coral reefs in booming populations of a competitive colonial ascidian. Mar Ecol Prog Ser. 1996;133:303–306. [Google Scholar]
- 182.Shenkar N, Bronstein O, Loya Y. Population dynamics of a coral reef ascidian in a deteriorating environment. Mar Ecol Prog Ser. 2008;367:163–171. [Google Scholar]
- 183.Vargas-Ángel B, Godwin LS, Asher J, Brainard R. Invasive didemnid tunicate spreading across coral reefs at remote Swains Island, American Sāmoa. Coral Reefs. 2009;28:53. [Google Scholar]
- 184.Bak RPM, Joenje M, de Jong I, Lambrechts DYM, Nieuwland G. Bacterial suspension feeding by coral reef benthic organisms. Mar Ecol Prog Ser. 1998;175:285–288. [Google Scholar]
- 185.Bone Q, Carre C, Chang P. Tunicate feeding filters. J Mar Biol Ass UK. 2003;83:907–919. [Google Scholar]
- 186.Monniot F, Monniot C. Ascidians from the outer slope and bathyal western Pacific. Zoosystema. 2003;25:681–749. [Google Scholar]
- 187.Gili JM, Coma R, Orejas C, López-González PJ, Zabala M. Are Antarctic suspension-feeding communities different from those elsewhere in the world? Polar Biol. 2001;24:473–485. [Google Scholar]
- 188.Dayton PK, Robilliard GA, Devries AL. Anchor Ice Formation in McMurdo Sound, Antarctica, and Its Biological Effects. Science. 1969;163(3864):273–274. doi: 10.1126/science.163.3864.273. [DOI] [PubMed] [Google Scholar]
- 189.Smale DA, Brown KM, Barnes DKA, Fraser KPP, Clarke A. Ice Scour Disturbance in Antarctic Waters. Science. 2008;321(5887):371. doi: 10.1126/science.1158647. doi: 10.1126/science.1158647. [DOI] [PubMed] [Google Scholar]
- 190.Boakes EH, McGowan PJK, Fuller RA, Chang-qing D, Clark NE, et al. Distorted Views of Biodiversity: Spatial and Temporal Bias in Species Occurrence Data. PLoS Biol. 2010;8(6):e1000385. doi: 10.1371/journal.pbio.1000385. doi: 10.1371/journal.pbio.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stachowicz JJ, Whitatch RB, Osman RW. Species diversity and invasion resistance in a marine ecosystem. Science. 1999;286:1577–1579. doi: 10.1126/science.286.5444.1577. [DOI] [PubMed] [Google Scholar]
- 192.Kennedy TA, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P. Biodiversity as a barrier to ecological invasion. Nature. 2002;417(6889):636–638. doi: 10.1038/nature00776. [DOI] [PubMed] [Google Scholar]
- 193.Bullard SG, Lambert G, Carman MR, Byrnes J, Whitlatch RB, et al. The colonial ascidian Didemnum sp. A: Current distribution, basic biology and potential threat to marine communities of the northeast and west coasts of North America. J Exp Mar Biol Ecol. 2007;342:99–108. [Google Scholar]
- 194.Rius M, Pascual M, Turon X. Phylogeography of the widespread marine invader Microcosmus squamiger (Ascidiacea) reveals high genetic diversity of introduced populations and non-independent colonisations. Divers Distrib. 2008;14:818–828. [Google Scholar]
- 195.Hess JE, Swalla BJ, Moran P. New molecular markers to genetically differentiate populations of Didemnum vexillum - an invasive ascidian species. Aquat Inv. 2009;3:367–378. [Google Scholar]
- 196.Stefaniak L, Lambert G, Gittenberger A, Zhang H, Lin S, et al. Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat Inv. 2009;4:29–44. [Google Scholar]
- 197.Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. Linking climate change and biological invasions: Ocean warming facilitates nonindigenous species invasions. Proc Natl Acad Sci USA. 2002;99:15497–15500. doi: 10.1073/pnas.242437499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Sorte CJB, Williams SL, Zerebecki RA. Ocean warming increases threat of invasive species in a marine fouling community. Ecology. 2010;91:2198–2204. doi: 10.1890/10-0238.1. doi: 10.1890/10-0238.1. [DOI] [PubMed] [Google Scholar]
- 199.Carlton JT, Geller JB. Ecological roulette: the global transport and invasion of nonindigenous marine organisms. Science. 1993;261:78–82. doi: 10.1126/science.261.5117.78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.