Abstract
The slow-releasing hydrogen sulfide (H2S) donor, GYY4137, caused concentration-dependent killing of seven different human cancer cell lines (HeLa, HCT-116, Hep G2, HL-60, MCF-7, MV4-11 and U2OS) but did not affect survival of normal human lung fibroblasts (IMR90, WI-38) as determined by trypan blue exclusion. Sodium hydrosulfide (NaHS) was less potent and not active in all cell lines. A structural analogue of GYY4137 (ZYJ1122) lacking sulfur and thence not able to release H2S was inactive. Similar results were obtained using a clonogenic assay. Incubation of GYY4137 (400 µM) in culture medium led to the generation of low (<20 µM) concentrations of H2S sustained over 7 days. In contrast, incubation of NaHS (400 µM) in the same way led to much higher (up to 400 µM) concentrations of H2S which persisted for only 1 hour. Mechanistic studies revealed that GYY4137 (400 µM) incubated for 5 days with MCF-7 but not IMR90 cells caused the generation of cleaved PARP and cleaved caspase 9, indicative of a pro-apoptotic effect. GYY4137 (but not ZYJ1122) also caused partial G2/M arrest of these cells. Mice xenograft studies using HL-60 and MV4-11 cells showed that GYY4137 (100–300 mg/kg/day for 14 days) significantly reduced tumor growth. We conclude that GYY4137 exhibits anti-cancer activity by releasing H2S over a period of days. We also propose that a combination of apoptosis and cell cycle arrest contributes to this effect and that H2S donors should be investigated further as potential anti-cancer agents.
Introduction
Hydrogen sulfide (H2S) is synthesized naturally from cysteine by several enzymes including cystathionine γ lyase (CSE), cystathionine β synthetase (CBS) and 3-mercaptosulfurtransferase (3-MST) in a wide range of mammalian and non-mammalian cells both in vitro and in vivo. In the last decade, numerous physiological and pathophysiological roles have been proposed for this gas along with a plethora of cellular and molecular targets including a range of ion channels, enzymes and transcription factors [1].
Apart from its potential roles in normal physiology there is also a comprehensive literature describing the toxicity of H2S and its role as an environmental pollutant [2]. A number of studies have investigated the role of H2S in triggering cell death and evidence has been presented that this gas can exert both pro- and anti-apoptotic activity in cultured cells [3], [4]. However, the precise mechanism(s) involved remain unclear.
Perhaps surprisingly, there have been few studies of the effect of H2S on cancer cells in vitro and no reports of its effect on tumor progression in vivo. Several years ago we reported that H2S protected colon cancer cells (HCT-116) from apoptosis due to β-phenylethyl isothiocyanate [5]. Others have subsequently reported that H2S increases human colon cancer cell proliferation and reduces apoptosis in several cell lines (e.g. HCT-116, [6]) whilst decreasing survival in other human colon cell lines (e.g. WiDR, [7]). These disparate observations are difficult to reconcile. However, one explanation may lie in the choice of H2S donor. Sulfide salts such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) have been widely used to study the biological effects of this gas in many cells, tissues and animals. On addition of water, these salts generate a large amount of H2S over a short time period. Since cell culture takes place over a period of hours or days, it is likely that little, if any, H2S is present in medium within a short time of adding either NaHS or Na2S. Thus, whilst no direct measurements have been made up to this point, it seems likely that the concentration of H2S that cancer (or indeed other) non-cancer cells are exposed to during culture with NaHS will be high at the start and not sustained throughout the experiment. Thus, it may be difficult to draw firm conclusions about the ability of H2S to affect cancer cell survival using sulfide salts as donor agents.
With this in mind, we previously reported that GYY4137 releases H2S slowly both in aqueous media and when administered to intact animals over a period of hours to days [8], [9]. We have now compared the effect of GYY4137 and NaHS on survival of a range of cancer and non-cancer cells in culture and correlated their effect with changes in concentration of H2S in the medium. In addition, we have examined the effect of GYY4137 on tumor growth using a xenograft model in immunodeficient mice.
Materials And Methods
Protocols were conducted with the approval of National University of Singapore (NUS) Institutional Review Board (IRB, reference code: 09-120E) and NUS Institutional Animal Care and Use Committee (IACUC, protocol number: 804/05).
Chemical synthesis of GYY4137 and ZYJ1122
GYY4137 was synthesized chemically in house as described previously [8]. ZYJ1122 (morpholin-4-ium diphenylphosphinate) was synthesized as follows. To a solution of diphenylphosphinic acid (1.0 mmol, 1.0 equiv.) in dichloromethane (DCM; 2 ml) at room temperature, morpholine (2.0 mmol, 2.0 equiv.) was added drop-wise. The reaction was stirred at the same temperature for 1 hour and the product subsequently collected by suction filtration. The pure product was obtained after washing with cold DCM. White solid was obtained as 56% yield. 1H NMR (500 MHz, CDCl3, ppm): δ = 7.77–7.74 (m, 4H), 7.38–7.32 (m, 6H), 3.77–3.75(m, 4H), 2.95–2.93(m, 4H); LRMS (ESI) m/z 217.2 (M−). The purity and structures of the compounds were verified using Proton Nuclear Magnetic Resonance Spectrometry (1H NMR) and Mass Spectrometry (Figures S1, S2, S3, S4).
Measurement of H2S
The generation of H2S from either NaHS (Sigma), GYY4137 or ZYJ1122 (all 400 µM) was determined in aliquots (100 µl) withdrawn at timed intervals (up to 7 days) from cultured MCF-7 cells maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) as described below. The concentration of H2S (determined as a combination of free H2S, HS− and S2−) was measured spectrophotometrically as described previously [10]. Briefly, medium (100 µl) was mixed with 0.85% w/v zinc acetate/3% NaOH mixture (1:1 ratio, 100 µl). Methylene blue was then formed by the addition of N,N-dimethyl-p-phenylenediamine-dihydrochloride dye and FeCl3 (final concentrations, 2.5 mM and 3.3 mM respectively) and absorbance subsequently monitored at 670 nm. The concentration of H2S (defined as above) was determined using a standard curve of NaHS (0–400 µM; R2 = 0.9987).
Cell culture and cell viability
All the cell lines except HCT-116 were obtained from American Type Culture Collection (ATCC). Human cervical carcinoma (HeLa), colorectal carcinoma (HCT-116), hepatocellular carcinoma (Hep G2), osteosarcoma (U2OS), breast adenocarcinoma (MCF-7) and human diploid lung fibroblasts (IMR90 and WI-38) were cultured in DMEM supplemented with 10% v/v fetal bovine serum (FBS; HyClone), penicillin/streptomycin (100 U/ml; Sigma) and L-glutamine (2 mM; Caisson) at 37°C in an atmosphere of 5% CO2. Human acute promyelocytic leukemia cells (HL-60) were cultured in DMEM containing 20% v/v FBS whilst human myelomonocytic leukemia (MV4-11) cells were cultured in RPMI with 10% v/v FBS under the same incubation conditions. Live cell populations incubated with NaHS, GYY4137 or ZYJ1122 (400 or 800 µM) were collected after 5 days and counted in triplicate after staining with trypan blue using a haemocytometer. Concentration response for GYY4137 (100–1000 µM) were generated in MCF-7, HL-60 and MV4-11 exposed to drugs for 5 days and the ability to reduce survival assessed as IC50 values. Colony formation using MCF-7 cells was also assessed by a clonogenic survival assay as described elsewhere [11]. Briefly, MCF-7 cells (10,000) were seeded in triplicate in 6-well plates in the presence of GYY4137, NaHS or ZYJ1122 (200 to 600 µM) for 10 days until colonies were readily visible. Colonies were then stained with crystal violet (5% w/v) and the representative pictures were captured using a ChemiGenius 2 Bio Imaging System (SynGene Ltd).
In vivo efficacy of GYY4137
The animal experimental protocol has been described previously [12]. Briefly, female, severe combined immunodeficiency (SCID) mice (17–20 g, 4–6 weeks old) were bred in house and maintained throughout in specific pathogen-free (SPF) isolators. Exponentially growing HL-60 and MV4-11 cells (1×107) (>95% viability) were washed twice in phosphate-buffered saline and subcutaneously injected into the loose skin between the shoulder blades and the left front leg of recipient mice. Animals were treated with either GYY4137 (100, 200 and 300 mg/kg/day, i.p.) or saline (1 ml/kg/day, i.p.) for 14 days commencing 14 days after injection of cells at which point mice had palpable tumors of 100 mm3 average size All animals were closely monitored. Weight and tumor size were measured at daily intervals. For tumor size measurement, length (L) and width (W) of the tumor were measured with a caliper, and tumor volume (TV) was calculated as TV = (L×W2)/2.
Cell Cycle Analysis and Western Blotting
MCF-7 cells (40,000) were incubated in 6-well plates in the presence or absence of GYY4137 (400 µM) for either 5 or 8 days. Cells treated with ZYJ1122 were used as a control. To analyze the cell cycle profile, cells were fixed with 70% v/v ethanol on ice for at least 2 hours and then stained in propidium iodide solution (20 µg/ml propidium iodide, 100 µg/ml RNase A and 0.1% v/v Triton X-100) for 15 min at 37°C. Stained cells were then subject to DNA content analysis by flow cytometry (Dako CyAn ADP) and the data obtained was processed using Summit software (Beckman Coulter). Cell lysates of MCF-7 were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked in Tris buffered saline (TBS) containing non-fat dry milk (5% w/v) and thereafter incubated with the relevant primary antibody (1 µg/ml) at 4°C overnight. Antibodies used were α-PARP, α-cleaved-PARP, α-cleaved-caspase 9 (Cell Signaling Ltd.) and α-tubulin (Sigma).
Statistical analysis
Cell survival, IC50 and tumor volumes were expressed as mean ± standard error (SEM). For in vitro studies, cell survival of both non-treatment (NT) and treatment groups was analyzed using one-way ANOVA followed by a post-hoc t test. For in vivo studies, the comparisons between vehicle control group and different dosage treatments was analyzed using linear mixed model for longitudinal data analysis by SPSS software (IBM). P<0.05 was considered significant.
Results
Release of H2S from NaHS and GYY4137 in culture medium
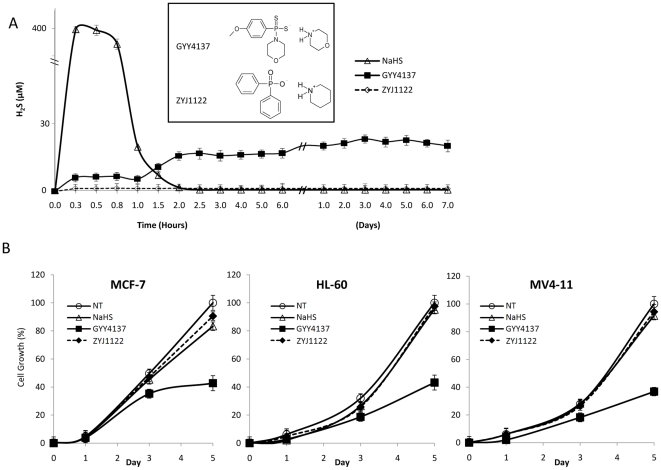
Incubation of either NaHS or GYY4137 in culture medium resulted in the release of detectable amounts of H2S as reflected by an increase in concentration of H2S (µM) following removal of aliquots and assay for methylene blue formation. Release of H2S from NaHS was rapid - peaking at or before 20 min and declining to undetectable levels by 90 min. In stark contrast, H2S release from GYY4137 was much lower (<10% of that observed with NaHS) but was sustained, remaining higher than baseline for up to 7 days. No release of H2S was apparent from ZYJ1122, a control for GYY4137 lacking sulfur and thus unable to form H2S, under the same experimental conditions for up to 7 days (Figure 1A). The chemical structures of GYY4137 and ZYJ1122 are shown in the inset to Figure 1A.
Figure 1. Differential H2S-releasing manner of NaHS and GYY4137.
(A) H2S-releasing profile of NaHS, GYY4137 and ZYJ1122. H2S released from NaHS, and GYY4137 (400 µM) was determined in aliquots (100 µl) of medium withdrawn at timed intervals (up to 7 days) from cultured MCF-7 cells. Concentration of H2S amounts was assessed spectrophotometrically using N,N-dimethyl-p-phenylenediamine-dihydrochloride and results showed the H2S concentration (µM). H2S release from GYY4137 was significantly different (P<0.05) from T = 0 at all time points from 0.3 hour to 7 days. H2S release from NaHS was significantly different (P<0.05) from T = 0 at all time points up to 1.5 hours. No detectable H2S was released from ZYJ1122 (400 µM) under identical experimental conditons. Results show mean ± s.e. mean, n = 3. The chemical structures of GYY4137 and ZYJ1122 are shown in the inset. (B) Growth curve analyses of MCF-7, HL-60 and MV4-11 cells treated with NaHS, GYY4137 and ZYJ1122 (400 µM) over 5 days. Cell survival was determined by trypan blue staining. Results show cell growth as a percentage relative to NT cell numbers at day 5 and are mean ± s.e. mean, n = 3.
Effect of NaHS and GYY4137 on cell growth and viability
The effect of NaHS, GYY4137 and ZYJ1122 on growth of three cancer cell lines i.e. MCF-7 (breast adenocarcinoma), MV4–11 (acute promyelocytic leukemia) and HL-60 (myelomonocytic leukemia), was monitored for 5 days. At each indicated interval, the number of live cells from each treatment group was recorded in triplicate. GYY4137 (400 µM) significantly reduced cell proliferation of all three cancer lines whereas both NaHS and ZYJ1122 were inactive (Figure 1B).
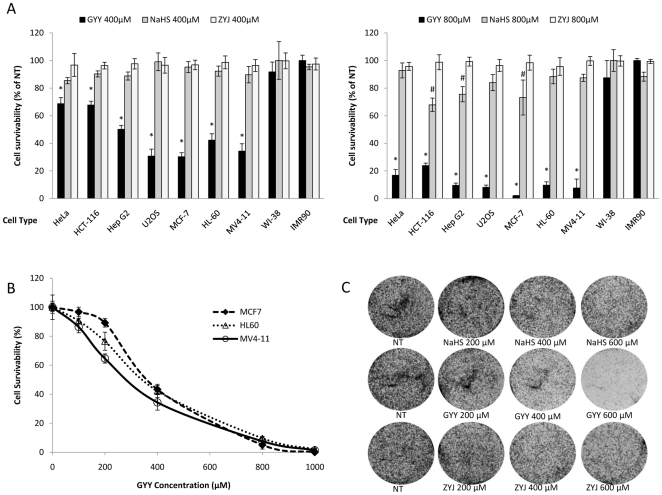
To determine the effect of two different concentrations (400 µM and 800 µM) of GYY4137, NaHS and ZYJ1122 on a wider panel of human cancer cell lines, cell survival of a further four cancer cell lines of different origins i.e. cervical carcinoma (HeLa), colorectal carcinoma (HCT-116), hepatocellular carcinoma (Hep G2) and osteosarcoma (U2OS) cells was determined in comparison with two normal human diploid fibroblasts (WI-38 and IMR90) (Figure 2A). Over a 5-day culture period, NaHS (400 µM) failed to influence the survival of any of the seven cancer lines tested. In contrast, the effect of GYY4137 on cell survival was much more profound with 30–70% (P<0.01) death in all cancer cell lines at the same concentration. A higher concentration of NaHS (800 µM) resulted in a further, albeit small, reduction in HCT-116, Hep G2 and MCF-7 cell survival (approximately 15–30%) although again no significant difference in cell survival was apparent in HeLa, HL-60, U2OS and MV4-11 cells. In contrast, GYY4137 at the same concentration, markedly reduced survival by approximately 75–95% in all cancer cell lines. The absolute degree of cell death caused by GYY4137 varied between cancer cell lines with greatest effect in HepG2, HL-60, MV4–11, MCF-7 and U2OS cells and least effect in HCT-116 and HeLa cells. For this reason, subsequent experiments were conducted using one or more of HL-60, MCF-7 and MV4-11 cancer cells. Importantly, neither NaHS nor GYY4137 significantly changed the survival of human non-cancer WI-38 and IMR90 fibroblasts. The sulfur-lacking control compound, ZYJ1122, was without significant effect on the survival of any cell line, suggesting that the observed effects of GYY4137 on cancer cells are likely due to H2S release. The concentration response relationship for GYY4137 (100–1000 µM) to reduce cell survival was also examined in MCF-7, HL-60 and MV4-11 cells. The IC50 values for this compound were 337.1±15.4, 389.3±16.8 and 341.8±21.2 µM (all n = 3) respectively (Figure 2B).
Figure 2. GYY4137 but not NaHS significantly affected cancer cell survivability.
(A) The effect of treatment (5 days) of a range of cancer and non-cancer cells with NaHS, GYY4137 and ZYJ1122 (400 µM or 800 µM) as determined by trypan blue staining. Results show percentage of cell viability to non-treatment (NT) following incubation in the absence of drug treatment and are mean ± s.e. mean, n = 3, (# P<0.05; *P<0.01). (B) Concentration-response curves showing the effect of GYY4137 treatment for 5 days on survival of MCF-7, HL-60 and MV4-11 cells. Results show mean ± s.e. mean, n = 3. (C) Representative photographs showing clonogenic survival assays of MCF-7 cells following exposure (5 days, 200–600 µM) to either NaHS (top row), GYY4137 (middle row) and ZYJ1122 (bottom row). NT = non-treatment.
The effect of NaHS, GYY4137 and ZYJ1122 (200–600 µM) on survival of MCF-7 cells was also assessed in vitro using a clonogenic assay. Representative photographs are shown in Figure 2C. For these experiments, MCF-7 cells were plated in the presence or absence of drugs and cultured over a 10-day period. GYY4137 caused a concentration dependent loss of cell colony formation which was close to maximal at a concentration of 600 µM. Cell loss was not apparent in both NaHS and ZYJ1122 treated samples.
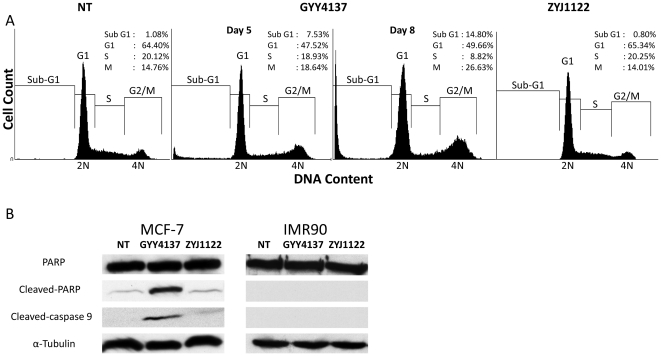
The effect of GYY4137 (400 µM, 5 or 8 days) on MCF-7 cells was also examined using cell cycle analysis. The sub-G1 population of MCF-7 cells exposed to GYY4137 was significantly higher (P<0.05) compared either to non-treated cells or cells exposed to the same concentration of ZYJ1122 on day 5 (Figure 3A). Thus, the sub-G1 population of cells treated with GYY4137 represented 7.5% of the total cell population at day 5 and 14.8% at day 8 of treatment compared with approximately 1% of cells which either did not receive treatment or were exposed to ZYJ1122 (Figure 3A). In addition, there was a significant accumulation of 4N-DNA cell population in cells treated with GYY4137 (to 18.6% and 26.6% after 5 and 8 days of incubation respectively) as compared to either untreated (14.8%) or ZYJ1122-treated (14%) cells (Figure 3A). In further experiments, the possibility that GYY4127 triggers cancer cell death by promoting apoptosis was also studied. A strong signal for cleaved-PARP and activated caspase 9 was detected in MCF-7 samples treated with GYY4137 (400 µM, 5 days) with a greatly reduced signal in cells treated with ZYJ1122 (Figure 3B). Interestingly, no cleavage of PARP and no activation of caspase 9 were observed in IMR90 cells incubated with either GYY4137 or ZYJ1122.
Figure 3. GYY4137 induced sub-G1 population and apoptosis in MCF-7.
(A) Cell cycle analysis of MCF-7 cells after 5 days (NT and ZYJ1122) and 5 and 8 days (GYY4137) drug treatment. Insets show percentage distribution of cells in each cell cycle phase. Results shown are indicative of 3 individual experiments. NT: non-treatment. (B) Western blot analysis of apoptosis markers (α-cleaved-PARP, α-cleaved-caspase 9) of MCF-7 and IMR90 treated (5 days) with GYY4137 or ZYJ1122 (both 400 µM). The anti-cleaved caspase-9 antibody used detects the large fragment of caspase-9 following cleavage but does not recognize uncleaved procaspase-9. α-tubulin was used as a loading control. Results shown are indicative of 3 individual experiments.
Effect of GYY4137 on tumor growth in vivo
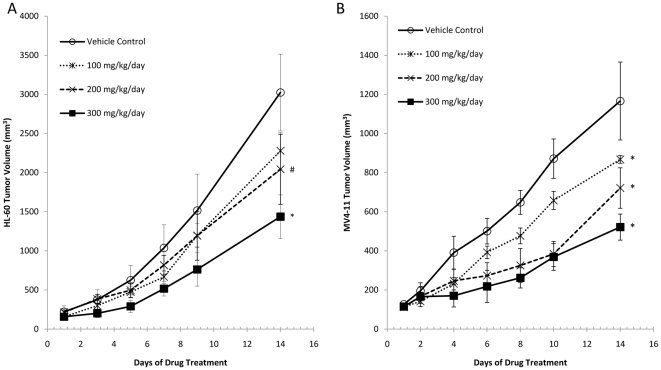
Subcutaneous transplantation of either HL-60 or MV4–11 cells resulted in time-dependent tumor growth in the SCID mouse (Figure 4A, B). Tumor volume at the end of the experiment was 3024±220 mm3 and 1166±199 mm3 (n = 4–6) in animals receiving daily vehicle injection and administered HL-60 and MV4-11 cells respectively. Administration of GYY4137 on a daily basis resulted in a significant (P<0.05) dose related inhibition of tumor growth in both sets of animals. GYY4137 (at the highest dose used i.e. 300 mg/kg) administered daily for 14 days reduced tumor volume by 52.5±9.2% (n = 6) and 55.3±5.7% (n = 4) in HL-60 and MV4–11 injected animals. Although not measured objectively in these experiments, GYY4137 treatment did not affect animal weight or gross behavior.
Figure 4. Effect of GYY4137 on tumor growth in vivo.
Changes in volume of established tumors in, (A) HL-60 xenograft mice (B) MV4–11 xenograft mice treated daily with either GYY4137 (100, 200 and 300 mg/kg, i.p.) or vehicle control. Treatment with GYY4137 significantly reduced the tumor volume in both animal models, in a dose-dependent manner. Results show changes of tumor volume in mean ± s.e. mean (n = 4–6, # P<0.05; * P<0.01).
Discussion
We report here that, (i) GYY4137 (but not NaHS) causes a concentration-dependent reduction in cancer cell survival, (ii) neither GYY4137 nor NaHS, using identical concentrations and experimental conditions, affected the survival of normal i.e. non-cancer cells, (iii) GYY4137 promoted cancer cell (MCF-7) but not normal cell (IMR90) apoptosis as indicated by measurement of sub-G1 population and by observation of cleaved PARP and cleaved caspase 9 and triggered cell cycle arrest of MCF-7 cells in the G2/M phase, (iv) the H2S concentration detected in medium containing MCF-7 cells exceeded ‘basal’ levels for up to 7 days after exposure to GYY4137 but for less than 2 h after exposure to NaHS, (v) ZYJ1122, a control for GYY4137 lacking sulfur and thus unable to form H2S, was inactive in all cases and, (vi) GYY4137 administered daily to immunodeficient mice for 14 days caused a dose-dependent reduction in tumor growth elicited by prior injection of one of two human leukemia cell lines.
Thus, the present data reveals, for the first time, an anti-cancer effect of the slow-releasing H2S donor, GYY4137. All cancer cells tested were susceptible to this compound albeit to different extents. It is now well established that NaHS releases large amounts of H2S over a short time period. In the present experiments, we show that GYY4137, like NaHS, also releases H2S following incubation in culture medium containing MCF-7 cells thereby confirming our previous observation of spontaneous H2S generation in aqueous media [8]. Since ZYJ1122 exhibited no anti-cancer activity in any of the in vitro models we conclude that the anti-cancer activity of both GYY4137 and NaHS (at high concentration) is very likely to be H2S-dependent. Perhaps surprisingly, GYY4137 exhibited greater cancer cell killing activity than did NaHS in vitro even though it leads to markedly lower concentrations of H2S in the cell medium. Thus, optimal killing of cancer cells by H2S under these experimental conditions would appear to occur at low concentrations of the gas spread over a period of several days as opposed to a much higher concentration achieved over a shorter time frame following exposure of cells to NaHS. It should be noted that whilst relatively high concentrations of GYY4137 (i.e. 400–800 µM) are required for this effect, the effective concentration of H2S generated is much less i.e. <20 µM based on the measurements in culture medium. However, we cannot rule out the possibility that GYY4137 may accumulate inside cancer cells and thereby release larger amounts of H2S intracellularly. With this proviso in mind, the present data implies that the rate at which cells are exposed to H2S as well as the concentration of H2S encountered is critical in determining the ability of this gas to promote cell killing.
Interestingly, neither GYY4137 nor NaHS caused significant killing of normal i.e. non-cancer cells suggesting that the effect of both H2S donors is specific for cancer cells. The mechanism of action of the cancer cell killing effect of GYY4137 has also been investigated. Treatment of MCF-7 cells with GYY4137 resulted in cell cycle arrest in the G2/M phase and promotion of apoptosis as evidence by increased sub-G1 population as well as the presence of both cleaved PARP and cleaved caspase 9. No significant effect either on cell cycle or on apoptosis was apparent in ZYJ1122-treated cells again suggesting that both of these effects were secondary to the sustained exposure of cells to low levels of H2S. The ability of H2S to cause cancer cell killing in this way has not previously been reported. However, H2S has previously been shown to affect both cell cycle and apoptosis. For example, H2S promotes cell cycle entry and proliferation of intestinal IEC-18 cell in vitro by activating MAPK [13]. H2S can also exhibit both pro- (e.g. [14]) and anti- (e.g. [15]) apoptotic activity depending on the cell type studied and the experimental conditions, particularly the concentration of H2S used. A number of potential molecular targets have been implicated in the effect of H2S on apoptosis including p38 and caspase-3 [15], [16], other MAPK such as MEK and JNK [17] as well as augmented production of heat shock protein (HSP-90) [18]. Whilst the precise cellular mechanism of action of GYY4137 remains unclear, it seems not unreasonable that H2S, released slowly over a period of several days, may affect redox mechanisms within the cell to bring about the anti-cancer effect. In this light, it might be of interest to assess the effect of GYY4137 on reactive oxygen species generation and anti-oxidant enzyme levels in cancer cells.
In conclusion, GYY4137 exhibits anti-cancer cell activity both in vitro and in vitro. We propose that GYY4137 breaks down slowly to yield H2S which, by a combination of cell cycle arrest and promoting apoptosis, inhibits tumor growth. No cell death was apparent in non-cancer cells. Whether such cells simply break down H2S at a faster rate or whether cancer cells are uniquely sensitive to the killing effect of this gas requires further study. The finding that cancer cells can be killed selectively when exposed to relatively small amounts of H2S over a relatively long time period is the key. This observation needs to be borne in mind in any future work examining the part played by H2S in cancer cell survival and also in the development of novel H2S-based anti-tumor agents.
Supporting Information
1H NMR spectrum of GYY 4137.
(TIF)
Mass spectrometry spectrum of GYY 4137.
(TIF)
1H NMR spectrum of ZYJ1122.
(TIF)
Mass spectrometry spectrum of ZYJ1122.
(TIF)
Acknowledgments
We are very grateful to Dr. Thilo Hagen for his valuable suggestions concerning experimental designs, and Dr. Zhao Yudong for his advice on in vivo data analysis. We thank Dr. Bert Vogelstein for providing HCT-116 cell line.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The work is supported by a National University of Singapore research grant to PKM, LWD and CHT (R-183-000-240-720, R183-000-240-101, R183-000-268-733). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol. 2011;51:169–187. doi: 10.1146/annurev-pharmtox-010510-100505. [DOI] [PubMed] [Google Scholar]
- 2.Fiedler N, Kipen H, Ohman-Strickland P, Zhang J, Weisel C, et al. Sensory and cognitive effects of acute exposure to hydrogen sulfide. Environ Health Perspect. 2008;116:78–85. doi: 10.1289/ehp.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu LF, Wong PTH, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi S, Kang L, Kimura T, Niki I. Hydrogen sulphide protects mouse pancreatic β-cells from cell death induced by oxidative stress, but not by endoplasmic reticulum stress. Br J Pharmacol. 2011;162:1171–1178. doi: 10.1111/j.1476-5381.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, et al. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J Gastroenterol. 2005;11:3990–3997. doi: 10.3748/wjg.v11.i26.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai WJ, Wang MJ, Ju LH, Wang C, Zhu YC. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol Int. 2010;34:565–572. doi: 10.1042/CBI20090368. [DOI] [PubMed] [Google Scholar]
- 7.Cao Q, Zhang L, Yang G, Xu C, Wang R. Butyrate-stimulated H2S production in colon cancer cells. Antioxid Redox Signal. 2010;12:1101–1109. doi: 10.1089/ars.2009.2915. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 9.Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, et al. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid Redox Signal. 2010;12:1147–1154. doi: 10.1089/ars.2009.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Chua JH, Yew WS, Sivaraman J, Moore PK, et al. Site-directed mutagenesis on human cystathionine-gamma-lyase reveals insights into the modulation of H2S production. J Mol Biol. 2010;396:708–718. doi: 10.1016/j.jmb.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 11.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 12.Shen J, Tai YC, Zhou J, Stephen Wong CH, Cheang PTS, et al. Synergistic antileukemia effect of genistein and chemotherapy in mouse xenograft model and potential mechanism through MAPK signaling. Exp Hematol. 2007;35:75–83. doi: 10.1016/j.exphem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Deplancke B, Gaskins HR. Hydrogen sulfide induces serum-independent cell cycle entry in nontransformed rat intestinal epithelial cells. FASEB J. 2003;17:1310–1312. doi: 10.1096/fj.02-0883fje. [DOI] [PubMed] [Google Scholar]
- 14.Yang G, Sun X, Wang R. Hydrogen sulfide-induced apoptosis of human aorta smooth muscle cells via the activation of mitogen-activated protein kinases and caspase-3. FASEB J. 2004;18:1782–1794. doi: 10.1096/fj.04-2279fje. [DOI] [PubMed] [Google Scholar]
- 15.Hu LF, Lu M, Wu ZY, Wong PTH, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, et al. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest. 2006;86:391–397. doi: 10.1038/labinvest.3700391. [DOI] [PubMed] [Google Scholar]
- 17.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology. 2009;241:11–18. doi: 10.1016/j.tox.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Jha S, Calvert JW, Duranski MR, Ramachandran A, Lefer DJ. Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am J Physiol Heart Circ Physiol. 2008;295:H801–H806. doi: 10.1152/ajpheart.00377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H NMR spectrum of GYY 4137.
(TIF)
Mass spectrometry spectrum of GYY 4137.
(TIF)
1H NMR spectrum of ZYJ1122.
(TIF)
Mass spectrometry spectrum of ZYJ1122.
(TIF)