Abstract
The role of 5-HT7 receptor has been demonstrated in various animal models of mood disorders; however its function in cognition remains largely speculative. This study evaluates the effects of SB-269970, a selective 5-HT7 antagonist, in a translational model of working memory deficit and investigates whether it modulates cortical glutamate and/or dopamine neurotransmission in rats. The effect of SB-269970 was evaluated in the delayed non-matching to position task alone or in combination with MK-801, a non-competitive NMDA receptor antagonist, and, in separate experiments, with scopolamine, a non-selective muscarinic antagonist. SB-269970 (10 mg/kg) significantly reversed the deficits induced by MK-801 (0.1 mg/kg) but augmented the deficit induced by scopolamine (0.06 mg/kg). The ability of SB-269970 to modulate MK-801-induced glutamate and dopamine extracellular levels was separately evaluated using biosensor technology and microdialysis in the prefrontal cortex of freely moving rats. SB-269970 normalized MK-801 -induced glutamate but not dopamine extracellular levels in the prefrontal cortex. Rat plasma and brain concentrations of MK-801 were not affected by co-administration of SB-269970, arguing for a pharmacodynamic rather than a pharmacokinetic mechanism. These results indicate that 5-HT7 receptor antagonists might reverse cognitive deficits associated with NMDA receptor hypofunction by selectively normalizing glutamatergic neurotransmission.
Introduction
The 5-HT7 receptor, a postsynaptic G protein coupled receptor was identified in 1993 through a homology cloning strategy and found to modulate positive cAMP formation via Gs [1]. In the brain, 5-HT7 receptors are distributed in the suprachiasmatic nucleus of the hypothalamus, hippocampus, cortex, thalamus and raphe nuclei on GABAergic interneurons or on glutamate terminals [1], [2], [3]. 5-HT7 receptor antagonists have been evaluated in several animal models predictive of anxiolytic-and antidepressant-like activity [3], [4], [5]. For example, blockade of 5-HT7 receptors in the rat Vogel test increased the number of shocks [6]. In addition, mice treated with 5-HT7 receptor antagonists as well as mice lacking the 5-HT7 receptors display decreased immobility time in the tail suspension test [7], [8], [9], [10]. These results suggest that blockade of 5-HT7 receptors may have anxiolytic and antidepressant activity in humans. 5-HT7 receptor antagonists have been also evaluated in animal models predictive of antipsychotic-like activity. One hypothesis of schizophrenia is based on N-methyl-D aspartic acid (NMDA) receptor hypofunction. NMDA antagonists such as ketamine and phencyclidine (PCP) induce hyperactivity, stereotypy and sensorimotor gating deficits in multiple species including humans and they can exacerbate positive symptoms of schizophrenia. Treatments with atypical antipsychotic drugs are known to reverse these effects [11]. Preclinical studies have demonstrated that SB-258741, a selective 5-HT7 receptor antagonist, blocked phencyclidine (PCP)-induced hyperactivity in rats [12]. Furthermore, SB-269970, an analog of SB-258741, partially but significantly blocked ketamine -induced hyperactivity in mice [13]. In addition, PCP-induced prepulse inhibition deficits were less pronounced in 5-HT7 knockout mice compared to wild-type mice [14]. However, 5-HT7 receptor antagonists did not reverse prepulse inhibition deficits in rats and mice [14]. Thus, the antipsychotic-like activity of selective 5-HT7 receptor antagonists is weaker than clinically proven antipsychotic drugs[15]. However, current antipsychotics do not show strong efficacy in cognitive deficit associated with psychiatric disorders including depression and schizophrenia.
It is currently debated whether 5-HT7 receptor antagonists can improve cognition [16]. Mice lacking the 5-HT7 receptor showed impaired contextual fear conditioning but no significant deficits in motor and spatial learning or cued and operant conditioning [17]. Animal studies with selective 5-HT7 receptor antagonists have provided mixed results [16]. In one study using the radial arm maze task, 5-HT7 receptor blockade had a pro-cognitive effect, when the learning task implicated a high degree of difficulty [18]. Another study showing that the 5-HT7 antagonist SB-269970 did not have any effect on an associative learning task in normal animals [19]. However, in the later study, SB-269970 reversed the amnesic effect elicited by scopolamine (a non selective muscarinic antagonist), MK-801 (dizocilpine, a non-competitive NMDA receptor antagonist) andmCPP (m-chlorophenylpiperazine, a non selective serotonin ligand) [19].
Altered acetylcholine neurotransmission induced by muscarinic antagonist (e.g. scopolamine) and hypofunction of NMDA receptors induced by NMDA antagonists (e.g. MK-801) are associated with cognitive-related deficits in many species including humans [20], [21]. For example, MK-801 and ketamine increase errors in the 8-arm maze delayed-matching to position and autoshaping learning task in rodents [19], [22]. Similarly, in humans, NMDA receptor antagonists impair performance in the Wisconsin Card Sort task as well as other cognitive models [21], [23], [24], [25]. NMDA antagonists increase both glutamate and dopamine extracellular levels in the rat cortex, a brain region critical in mediating cognitive-related responses, particularly working memory and executive function which are impaired in schizophrenic patients [26], [27]. It is thought that NMDA antagonists produce schizophrenic-like reactions in humans by disrupting cortical networks and enhancing glutamate release. Antipsychotics attenuate MK-801-induced glutamate release in the cortex [28], which may mediate some aspects of their clinical efficacy. It is also known that working memory performance is highly dependent on an optimal amount of dopamine in the prefrontal cortex, which tightly regulates network activity [29]. Thus, too little or too much dopamine interferes with working memory performance. MK-801, which enhances dopamine release in the cortex, would thus be expected to interfere with working memory, in part through an over-stimulation of the cortical dopamine system and subsequent disruption to organized network activity.
The goal of the present studies was two-fold: first, to evaluate the effects of the selective 5-HT7 receptor antagonist SB-269970 [30], in a translational behavioral model of working memory. The second goal was to evaluate, at a neurochemical level, the effects of SB-269970 on cortical glutamate and dopamine extracellular levels. Specifically, the effect of SB-269970 was evaluated in rats using delayed non-matching to position (DNMTP), a translational procedure that is thought to measure working memory. In addition, the effects of SB-269970 on glutamate and dopamine extracellular levels were evaluated using biosensor technology (glutamate) and microdialysis (dopamine) in freely moving rats. A pharmacokinetic study was also carried out to determine plasma and brain concentrations of MK-801 after co-administration of SB-269970 to investigate a potential drug-drug interaction.
Methods
Ethics Statements
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978) and were approved by the Institutional Animal Care and Use Committee (IACUC) at Johnson & Johnson Pharmaceutical Research & Development, L.L.C., San Diego (microdialysis protocol #1048, behavior protocol #100063, pharmacokinetics protocol # 1000117).
Drugs
SB-269970 hydrochloride ((2R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]-pyrrolidine) was purchased from Tocris Bioscience (Ellisville, MO). MK-801 ((5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate) and scopolamine were purchased from Sigma Aldrich (St. Louis, MO). All compounds were dissolved in saline (SB-269970, MK-801) or water (scopolamine). Doses are expressed as mg/kg of free base (volume of injection = 1 ml/kg).
Delayed non-matching to position (DNMTP) task
Sprague-Dawley rats (Harlan, Indianapolis, Ind., USA, 250–300 g upon arrival) were maintained at 80–90% of their free feeding weight. All animals were single-housed in plastic cages under a 12h:12hlight:dark schedule in humidity controlled rooms and had ad libitum access to water. Animals were trained to respond for food in sound attenuating operant chambers (Med-Associates, St Albans, VT). Chambers were equipped with two levers with stimulus lights and a food hopper. Each test session lasted 30 min and started with the illumination of the house-light and the random presentation of one lever. When the animal pressed the lever, it was immediately retracted for up to a random period of time (0, 4, 8, 16, or 32 sec delay). At the end of the delay both levers were presented. Pressing the lever which was not initially presented (i.e. correct response) resulted in the illumination of both stimulus lights for 1 s, the delivery a 45 mg food pellet (Bio-Serv, Frenchtown, NJ, USA), and the end of the trial. Pressing the lever that was initially presented (incorrect response) resulted in the retraction of both levers and the end of the trial. At the end of the trial, a new trial with a new random delay was initiated. SB-269970 (10 mg/kg) and scopolamine (0.06 mg/kg), or their vehicles, were administered intraperitoneally at 10 and 30 min, respectively, before the beginning of the session. SB-269970 (10 mg/kg) and MK-801 (0.1 mg/kg), or their vehicles, were co-administered intraperitoneally at 10 min before the beginning of the session. The dose of MK-801 was chosen based on pilot studies showing impaired working memory without producing gross psychostimulant effects that would interfere with lever pressing. Each experiment was conducted in a Latin-square design with washout periods between each drug test (N = 6). Data from each experiment were analyzed for percent correct choices with a two-way analysis of variance (ANOVA) for treatment group x delay, with repeated measures on delay, followed by Duncan's post-hoc tests when appropriate. The number of trials completed during each session was analyzed using a one-way repeated measures ANOVA for treatment group. Statistica (StatSoft Inc., Tulsa OK) was used for data analysis.
Real-time measurements of glutamate efflux in the cortex of freely moving rats
Male Sprague-Dawley rats (Charles River Laboratories) weighing 280–350 grams were used. All animals were single-housed in a 12h:12hlight:dark schedule in humidity controlled rooms and had ad libitum access to water. Each rat was given a subcutaneous 0.05 mL injection of Buprenex (buprenorphine hydrochloride; Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) at 0.06 mg/kg 5 minutes prior to anesthesia. Animals were anesthetized with an Isoflurane/air mixture and stereotaxically implanted with a guide cannula (BAS) in the prefrontal cortex (incisor bar, −3.5 mm, 3.2 mm anterior, 0.9 mm lateral and 2 mm ventral to Bregma, [31]. The guide cannula and bone screws were encased with acrylic dental cement. Animals were allowed at least 5 days to recover from surgery prior to experimentation. The animals were handled each day and experimentation occurred within the animal's home cage.
The glutamate biosensor (wireless model 7001, Pinnacle Technologies, Lawrence KS) specification and hardware setup was described previously [32]. Prior to sensor insertion, each biosensor was calibrated in-vitro to verify glutamate sensitivity and interference rejection. The morning of experimentation, under light isoflurane anesthesia, the biosensor was inserted into the prefrontal cortex. Experimentation began once a stable sensor signal was obtained, approximately 4 hours. At the completion of the experiment, each sensor was removed from the guide cannulae and calibrated in vitro for glutamate sensitivity and interference rejection of ascorbate at 37°C in a circulating water bath.
Initially, all 15 animals received an intraperitoneal vehicle injection. Sixty min later, they received the following intraperitoneal treatments: vehicle + MK-801, 0.1 mg/kg (N = 5), SB-269970, 10 mg/kg + MK-801, 0.1 mg/kg (N = 5) or SB-269970, 10 mg/kg + vehicle (N = 5).
Following experimentation the data was exported to excel and binned into 5 minute current (nA) intervals. These data points were then converted to glutamate concentrations using calibration curve regression data for the corresponding sensor.
Statistical analyses were performed on the change in glutamate concentrations with a two-way ANOVA for treatment x time, with repeated measures on time, followed by Duncan's post-hoc tests. Statistics were calculated using Statistica (StatSoft Inc., Tulsa OK).
Dopamine microdialysis in the cortex of freely moving rats
Male Sprague-Dawley rats (Charles River Laboratories) weighing 280–350 grams were used. All animals were single-housed in a 12h:12hlight:dark schedule in humidity controlled rooms and had ad libitum access to water. Each rat was given a 0.05 ml SC injection of Buprenex 0.06 mg/kg (buprenorphine hydrochloride) 5 mins prior to anesthesia. Animals were anesthetized with an isoflurane/air mixture and stereotaxically implanted with a guide cannulae (Eicom) in the prefrontal cortex (incisor bar −3.5 mm, +3.2 mm anterior, 0.8 mm lateral and 1 mm ventral to Bregma) [31]. The guide cannula was secured in place with skull screws and dental cement. Animals were allowed at least 3 days to recover from surgery prior to experimentation.
Dialysis experiments were conducted as previously described [33]. Dialysate was analyzed by high-performance liquid chromatography with an electrochemical detector (Eicom HPLC/EC). The dopamine concentration for each sample was calculated from the peak area of the chromatographic signal and the slope from the corresponding standard curve.
A between subject design was used. The following conditions were evaluated: vehicle + vehicle (N = 5), vehicle + MK-801, 0.1 mg/kg (N = 6), SB-269970, 10 mg/kg + MK-801, 0.1 mg/kg (N = 5) and SB-269970, 10 mg/kg + vehicle (N = 5). All compounds were administered intraperitoneally.
The percent change from baseline values were calculated from the mean basal value of each neurotransmitter for each animal and presented in the figures as mean ± S.E.M.
Statistical analyses were performed on the percent baseline with a two-way ANOVA for treatment x time, with repeated measures on time, followed by Duncan's post-hoc tests. Statistics were calculated using Statistica (StatSoft Inc., Tulsa OK).
At the end of the experiments, microdialysis and biosensor probe placement was visually verified for each animal. Brains were removed, frozen and sectioned with a microtome to verify probe placement. Only animals with correct placement of the probe were used for data analysis.
Pharmacokinetics and bioanalysis
Male Sprague-Dawley rats (Charles River Laboratories) weighing 300–350 grams were used. Intraperitoneal dosing (vehicle +0.1 mg/kg MK-801 or 10 mg/kg SB-269970 +0.1 mg/kg MK-801; N = 4 per group) was followed by blood sampling via cardiac puncture over a time course. Brains were removed from the animals and homogenized for LC/MS-MS analysis. All blood samples were deproteinized by 1∶4 dilution of the sample with acetonitrile with vigorous mixing. These samples were incubated for 5 minutes, and then centrifuged at 14,000 rpm in a micro-centrifuge for 4 minutes. The supernatant was recovered into auto-sampler vials and diluted 1∶1 with sterile water. Samples were analyzed by LC-MS/MS. A Vydac SP C18 2.1×50 mm analytical column was used for separation.
Statistics (paired t-test) were calculated using Prism software (GraphPad, San Diego, CA). The level of significance was p<0.05. A one compartmental pharmacokinetic model was applied to these data using the software package WinNonlin Version 4.0.1. (Pharsight, Palo Alto, Ca). A one compartment first order, no lag time, first order elimination model (Model 3) was employed. The parameters of the model were optimized using least squares non-linear regression. Pharmacokinetic and blood brain barrier penetration parameters are given as the means ± coefficient of variation. The coefficient of variation is a measure of dispersion of a probability distribution. It is defined as the ratio of the standard deviation to the mean. The coefficient of variation was calculated as the ratio of the standard error for each parameter to its estimated value.
Results
SB-269970 blocks MK-801 induced deficits in the DNMTP task
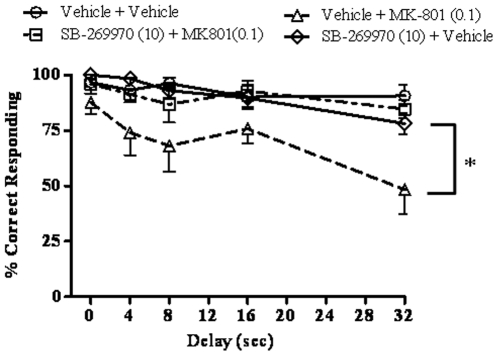
The effect of SB-269970 (10 mg/kg, IP) on MK-801 (0.1 mg/kg, IP) induced deficits was evaluated in the DNMTP task (Fig. 1). The number of trials completed for each group is given in Table 1. A two-way repeated measures ANOVA (delay x treatment group), with repeated measures on group, detected a main effect of delay (F(4, 25) = 5.99, p = 0.0016) and group (F(3, 75) = 15.49, p = 0.000005), but no interaction (F(12, 75) = 1.02, p = 0.44). Duncan's post-hoc tests on group revealed that MK-801 worsened performance (p<0.00005 comparing vehicle + vehicle to vehicle + MK-801). SB-269970 had no effect on its own (p = 0.68, comparing vehicle + vehicle to SB-269970 + vehicle), but reversed the deficit caused by MK-801 (p<0.0002 comparing vehicle + MK-801 to SB-269970 + MK-801) so that the SB-269970 + MK-801 group was no different from the SB-269970 + vehicle group (p = 0.70). There was no difference in the number of trials completed in each session among treatment groups (F(3,15) = 2.42, p = 0.11).
Figure 1. Effects of SB-269970 on MK-801-induced DNMTP deficits.
SB-269970 (10 mg/kg) and MK-801 (0.1 mg/kg), or their vehicles, were co-administered 10 min before the beginning of the session (N = 6 per group). SB-269970 had no effect on its own but reversed the deficit caused by MK-801 (*p<0.05, main effect). For statistical details see the result section.
Table 1. Number of trials completed in the DNMTP task.
| MK-801 (0.1 mg/kg) | Scopolamine (0.06 mg/kg) | |||||||
| Vehicle + Vehicle | Vehicle + MK-801 | SB-269970 + MK-801 | SB-269970 + Vehicle | Vehicle + Vehicle | Vehicle + Scopolamine | SB-269970 + Scopolamine | SB-269970 + Vehicle | |
| Number of trials | 48±6 | 46±8 | 53±5 | 40±5 | 44±5 | 43±4 | 35±8 | 53±5 |
SB-269970 (10 mg/kg) and MK-801 (0.1 mg/kg), or their vehicles, were co-administered 10 min before the beginning of the session. SB-269970 (10 mg/kg) and scopolamine (0.06 mg/kg), or their vehicles, were administered 10 and 30 min, respectively, before the beginning of the session. There was no difference in the number of trials completed in each session among treatment groups. Data are expressed as mean ± S.E.M., N = 6 per group).
SB-269970 does not block scopolamine-induced deficits in the DNMTP task
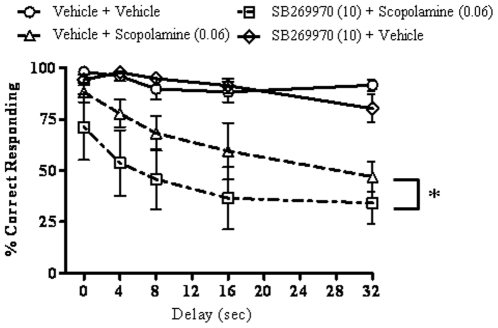
The effect of SB-269970 (10 mg/kg, IP) on scopolamine (0.06 mg/kg, IP) -induced deficits was evaluated in the DNMTP task (Fig. 2). A two-way repeated measures ANOVA (delay x treatment group), with repeated measures on delay, detected a main effect of delay (F(4, 25) = 2.79, p = 0.048) and group (F(3, 75) = 37.29, p = 0.000005), but no interaction (F(12, 75) = 0.88, p = 0.57). Duncan's post-hoc tests on group revealed that scopolamine worsened performance in both the vehicle-pretreated and SB-269970- pretreated groups (p<0.00006 comparing vehicle + vehicle to vehicle + scopolamine and p<0.00005 comparing vehicle + vehicle to SB-269970 + scopolamine). SB-269970 had no effect on its own (p = 0.83, comparing vehicle + vehicle to SB-269970 + vehicle), but augmented the deficit caused by scopolamine (p<0.0003 comparing vehicle + scopolamine to SB-269970 + scopolamine). There was no difference in the number of trials completed in each session among treatment groups (F(3,15) = 2.65, p = 0.086).
Figure 2. Effects of SB-269970 on scopolamine-induced DNMTP deficits.
SB-269970 (10 mg/kg) and scopolamine (0.06 mg/kg), or their vehicles, were administered 10 and 30 min, respectively, before the beginning of the session (N = 6 per group). SB-269970 augmented the deficit caused by scopolamine (*p<0.05, main effect). For statistical details see the result section.
SB-269970 blocks MK-801-induced glutamate efflux in the cortex of freely moving rats
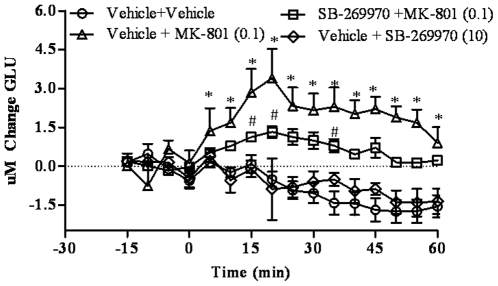
The effect of SB-269970 (10 mg/kg, IP) on MK-801 (0.1 mg/kg, IP) induced glutamate efflux was evaluated in the cortex of conscious rats using biosensor technology (Fig. 3.). A two-way repeated measures ANOVA (group x time) revealed a main effect of group (F(3, 26) = 12.45, p = 0.00003) and time (F(15, 390) = 4.31, p = 0.000005), and an interaction (F(45, 390) = 4.34, p = 0.000005). Post-hoc tests revealed that the vehicle + MK-801 had higher glutamate concentrations compared to the vehicle + vehicle group from 10–60 min (p = 0.000001–0.04 at those time points). SB-269970 did not affect glutamate levels per se (p>0.05 at all time points), but attenuated MK-801-induced increases in glutamate (p<0.05 at 15 min, p<0.02 at 20 min, p<0.04 at 35 min, comparing SB-269970 + MK-801 to vehicle+MK-801). Glutamate levels were only elevated in the SB-269970 + MK-801 group compared to the SB-269970 + vehicle group at 20 and 25 min (p<0.02 and p<0.04, respectively).
Figure 3. Effects of SB-269970 on MK-801-induced glutamate efflux in the prefrontal cortex.
Real-time measurements of glutamate were conducted before and after compound administration. Results are expressed as as mean ± S.E.M of change in glutamate concentrations (N = 5–15 per group). SB-269970(10 mg/kg) did not affect glutamate levels per se but attenuated MK-801(0.1 mg/kg)-induced increases in glutamate efflux. *p<0.05, at each time point comparing vehicle + MK-801 to vehicle + vehicle. # p<0.05, at each time point comparing SB-269970 + MK-801 to vehicle + MK-801.
SB-269970 does not block MK-801-induced dopamine extracellular levels in the cortex of freely moving rats
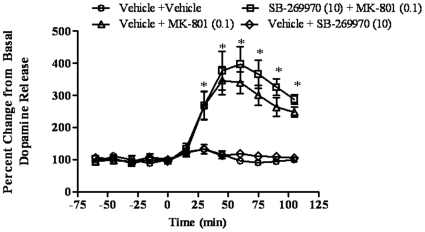
The effect of SB-269970 (10 mg/kg, IP) on MK-801 (0.1 mg/kg, IP) induced dopamine extracellular levels was evaluated in the cortex of conscious rats using microdialysis (Fig. 4.). A two-way repeated measures ANOVA revealed a main effect of group (F(3, 17) = 21.18, p = 0.00001) and time (F(11, 187) = 45.27, p = 0.00005), and an interaction (F(33, 187) = 13.89, p = 0.00005). Post-hoc tests revealed that the vehicle + MK-801 had higher dopamine levels compared to the vehicle + vehicle group from 30 – 105 min (p = 0.000004–0.000075 at those time points). SB-269970 did not affect dopamine levels by itself, nor did it alter the effect of MK-801 on dopamine levels (p>0.05 at all time points).
Figure 4. Effects of SB-269970 on MK-801-induced dopamine release.
Results are expressed as percentage ofbaseline (mean ± S.E.M., N = 5-6 per group). SB-269970 (10 mg/kg) did not affect dopamine levels by itself, nor did it alter the effect of MK-801 (0.1 mg/kg) on dopamine levels.*p<0.001, at each time point compared to control.
SB-269970 does not change MK-801 plasma and brain concentrations
MK-801 plasma and brain concentrations were determined following intraperitoneal dosing of vehicle + MK-801 (0.1 mg/kg) or co-administration of MK-801 (0.1 mg/kg) with SB-269970 (10 mg/kg). The concentration data was analyzed using a one compartment model with WinNonlin to determine pharmacokinetic parameters (Table 2). A t-test was performed on the pharmacokinetic parameters showed that SB-269970 did not affect the kinetics of MK-801 (p>0.05 Table 2).
Table 2. Pharmacokinetic and blood brain barrier parameters of MK-801 (0.1 mg/kg, IP) co-administered with vehicle or with SB-269970 (10 mg/kg, IP).
| Vehicle + MK-801 | SB-269970 + MK-801 | |
| Plasma | ||
| Tmax (h) | 0.31±32% | 0.31±35% |
| Cmax (µM) | 0.04±14% | 0.03±19% |
| AUCinf (h µmol/L) | 0.08±33% | 0.04±25% |
| Brain | ||
| Tmax (h) | 0.40±20% | 0.37±28% |
| Cmax (µM) | 0.28±11% | 0.20±15% |
| AUCinf (h µmol/L) | 0.60±23% | 0.33±21% |
Data are expressed as mean ± coefficient variation (%) (N = 4 per group).
Discussion
The results of the present work indicated that the selective 5-HT7 receptor antagonist SB-269970 reversed MK-801 but not scopolamine -induced cognitive deficit in the DNMTP task. In addition, the 5-HT7 receptor antagonist normalized MK-801-induced glutamate efflux but not MK-801-induced dopamine extracellular levels in the cortex of freely moving rats. The pharmacokinetic study showed that rat plasma and brain concentrations of MK-801 were not affected by co-administration of SB-269970.
The 5-HT7 receptor is expressed in brain regions involved in learning and memory such as hippocampal formation and frontal cortex [3]. Several reports in the literature suggest that it may play a role in the control of learning and memory processes [16], [34], [35], [36]. In this study we evaluated the effect of a selective 5-HT7 receptor antagonist in the DNMTP task, a translational assay of working memory. As previously reported, MK-801 and scopolamine were found to decrease percent correct responding without affecting the number of trials [20], [37], [38], [39], [40]. The selective 5-HT7 receptor antagonist, SB-269970, had no effect by itself on percentage correct responding and number of trials. However, SB-269970 significantly reversed MK-801 but not scopolamine-induced memory deficits. The dose and pretreatment time of SB-269970 selected for this study were based on previous studies where it was demonstrated that the compound is biologically active in other behavioral assays [3], [7], [13], [30]. For example, SB-269970 at a dose of 10 mg/kg was found to reduce immobility time in the tail suspension test and decrease REM sleep duration [7]. In the present study we carried out a pharmacokinetic study to show that rat plasma and brain concentrations of MK-801 were not affected by co-administration of SB-269970, demonstrating that the effect observed in the DNMTP task is not confounded by a drug-drug interaction.
The reversal of MK-801 induced deficits by the 5-HT7 receptor antagonist in the DNMTP task observed in the present study is consistent with the study of Meneses showing that SB-269970 reversed MK-801-induced amnesia in the autoshapingPavlovian/instrumental learning task [19]. In the later study, SB-269970 was also found to reverse scopolamine -induced deficit in the same procedure. In our study, using a different experimental paradigm, SB-269970 was found to augment scopolamine -induced deficits in the DNMTP task. It should be noted that the experimental conditions were different between our study and Meneses's study and could have contributed to the contrasting findings. For example, the dose of scopolamine was different (0.17 vs 0.06 mg/kg in our study) and a different strain of rat was used (Wistar in Meneses's study vs Sprague-Dawley in our study).In addition, different forms of memory were being measured (consolidation memory in the Meneses's study, with drug effects measured 24 hours later, and working memory in the present study, with drug effects measured during the test while the drugs were on board). Interestingly, in the present study, SB-269970 shifted the scolopamine delay-response curve straight down, even at the longer delay, where the scolopamine animals were already performing at 50%. Fifty percent represents chance performance, but the SB-269970+scolopamine treated animals were performing at 36.5 and 34.1% at the two longest delays. Performance below chance suggests the animals were specifically choosing the same lever as presented at the start of thetrial, instead of the opposite lever or choosing randomly. Such performance could represent an inability to disengage attention from the first presented lever and might represent an over-compensation for the attention-impairing effects of scolopamine. Such effects would not be observed in the Meneses's study, where consolidation memory was measured and neither drug was on board during the subsequent test. Further neurochemical investigations on the effect of SB-269970 on the level of acetylcholine are needed to shed light on this finding. Potential drug-drug interaction between SB-269970 and scopolamine should be investigated as well. On the other hand, it is also possible that the 5-HT7 receptor has more of a modulatory function, with positive or negative effects on memory performance depending on the background state. Noteworthy, 5-HT7 receptor antagonists were found to decrease the memory enhancing effects of the 5-HT1A/7 agonist 8-OH-DPAT in the autoshapingPavlovian/instrumental learning task to levels far below that of controls, such that performance was even worse than in the vehicle-treated controls [19]. While the 5-HT7 receptor antagonists had no effect by themselves per se, they were able to worsen memory performance, but only under the background of 5-HT1A/7 activation (which by itself improved performance). Interestingly, in the same study the 5-HT7 receptor antagonists were also able to reverse amnesic-inducing effects while being inactive alone [19]. It was hypothesized that under procognitive or amnesic conditions 5-HT7 receptor activity would be unmasked, leading to a modulatory role in memory formation [19]. In fact, the mRNA expression of the 5-HT7 receptor has been shown to be enhanced during memory consolidation [36], suggesting that 5-HT7 receptor activity can be important to normal memory functions. In this study it was also shown that an agonist of the 5-HT7 receptor (AS-19) improved memory consolidation, while partially downregulating 5-HT7 mRNA expression, whereas amnesic-drugs strongly downregulated 5-HT7 mRNA expression. AS-19 reversed the effects of the amnesic-drugs on both memory performance and mRNA expression. Thus, it is possible that a nominal amount of 5-HT7 receptor activity is required for normal memory processes and that 5-HT7 receptor ligands can have procognitive or amnesic effects (depending on the background level of 5-HT/5-HT7 activity) once 5-HT7 receptors are unmasked.
A recent study by Horiguchi and Meltzer [41] showed that selective 5-HT7 antagonism had a procognitive effect on the PCP-induced impairment in novel object recognition test, a rodent model of declarative memory deficit in schizophrenia. In addition, preferential 5-HT7 antagonism was also found to contribute to the ability of the atypical antipsychotics drugs lurasidone and amisulpride to ameliorate the PCP-induced novel object recognition deficit. Another recent study by Horisawa and colleagues also showed that the selective 5-HT7 receptor antagonist (SB-656104) and lurasidone reversed MK-801-induced learning impairment in the Morris Water Maze test [42]. Consistent with these finding, Gasbarri and colleagues demonstrated that 5-HT7 receptor blockade enhanced memory retention in the radial maze task [18]. In contrast to their previous results [19], Perez-Garcia and Meneses suggested that 5-HT7agonism may promote memory in the hypoglutamatergic rat brain [34]. However, information about the selectivity and brain penetration of the 5-HT7 agonist (AS-19) used in the later study is lacking, hampering the full interpretation of these data [34]. Another study by Ballaz and colleagues showed that a high dose of SB-269970 caused impairment in novel object recognition in low but not high responder animals suggesting that 5-HT7 blockade removed phenotypic differences in novel object discrimination performance [43]. The authors concluded that these findings might indicate different levels of 5-HT7 receptor activity, suggesting that the manner in which rats adapt to the environment may be under the influence of the 5-HT7 receptor.
In the present study, biosensor and microdialysis studies in the cortex of freely moving rats were conducted to further investigate the neurochemical interaction between 5-HT7 and NMDA receptors. The biosensor technique is very comparable to the microdialysis technique and both are able to selectively monitor glutamate levels in the extracellular space. Compared to microdialysis, biosensors are slightly smaller, and may result in less damage around the recording area, and offer a much greater time resolution. Theoretically, both microdialysis and biosensors decrease the concentration of glutamate around the recording area – microdialysis by sampling and removing glutamate from the extracellular fluid and biosensors by converting glutamate to alpha-ketoglutarate. Another difference between the biosensor methods and conventional microdialysis methods is that in microdialysis, the animal is tethered so that tubing can deliver the CSF. In biosensor studies, the animals are completely free moving and untethered, and the biosensor signal is transmitted wirelessly. This may result in less stress to the animal during testing. Using the biosensor technology, MK-801 was found to increase glutamate efflux in the cortex in agreement with the literature [32], [44]. By itself SB-269970 did not modify glutamate or dopamine extracellular levels in cortex. SB-269970 was found to significantly reduce MK-801-induced glutamate efflux. These effect were qualitatively similar to the results obtained with the mGluR2/3 receptor agonist LY-379268 [44].
In contrast, SB-269970 did not modify MK-801 induced dopamine extracellular levels in cortex indicating that blockade of 5-HT7 receptors selectively reduced cortical glutamate outflow.
Collectively, the results from the present study indicated that a selective 5-HT7 receptor antagonist might reverse cognitive deficits, particularly those associated with NMDA receptor hypofunction, by selectively normalizing glutamatergic neurotransmission. Selective 5-HT7 antagonism may represent a novel approach for improvement of cognitive impairments in schizophrenia, particularly working memory.
Acknowledgments
The assistance of Dr. Kevin Sharp, Dr. Tatiana Koudriakova and their staff at Johnson & Johnson Pharmaceutical Research and Development L.L.C. (San Diego, CA), is gratefully acknowledged. We thank Dr. Christine Dugovic for her helpful comments.
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: This work was funded by Johnson & Johnson Pharmaceutical Research and Development, L.L.C. All the authors were employees of Johnson & Johnson Pharmaceutical Research and Development, L.L.C. when the work was done. The funders had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not alter the authors' adherence to all the PLoS ONE policies.
Funding: This work was funded by Johnson & Johnson Pharmaceutical Research and Development, L.L.C.. The funders had a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not alter the authors' adherence to all the PLoS ONE policies.
References
- 1.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 2.Harsing LG, Jr, Prauda I, Barkoczy J, Matyus P, Juranyi Z. A 5-HT7 Heteroreceptor-Mediated Inhibition of [3H]Serotonin Release in Raphe Nuclei Slices of the Rat: Evidence for a Serotonergic-Glutamatergic Interaction. Neurochem Res. 2004;29:1487–1497. doi: 10.1023/b:nere.0000029560.14262.39. [DOI] [PubMed] [Google Scholar]
- 3.Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology. 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. PharmacolTher. 2011;129:120–148. doi: 10.1016/j.pharmthera.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mnie-Filali O, Faure C, Lambas-Senas L, Mansari ME, Belblidia H, et al. Pharmacological Blockade of 5-HT(7) Receptors as a Putative Fast Acting Antidepressant Strategy. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wesolowska A, Nikiforuk A, Stachowicz K, Tatarczynska E. Effect of the selective 5-HT7 receptor antagonist SB 269970 in animal models of anxiety and depression. Neuropharmacology. 2006;51:578–586. doi: 10.1016/j.neuropharm.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 Receptor Inhibition and Inactivation Induce Antidepressantlike Behavior and Sleep Pattern. Biol Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Wesolowska A, Nikiforuk A, Stachowicz K. Potential anxiolytic and antidepressant effects of the selective 5-HT7 receptor antagonist SB 269970 after intrahippocampal administration to rats. Eur J of Pharmacol. 2006;553:185–190. doi: 10.1016/j.ejphar.2006.09.064. [DOI] [PubMed] [Google Scholar]
- 10.Sarkisyan G, Roberts AJ, Hedlund PB. The 5-HT(7) receptor as a mediator and modulator of antidepressant-like behavior. Behav Brain Res. 2010;209:99–108. doi: 10.1016/j.bbr.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in Neuro-Psychopharmacology and Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Pouzet B, Didriksen M, Arnt J. Effects of the 5-HT(7) receptor antagonist SB-258741 in animal models for schizophrenia. PharmacolBiochemBehav. 2002;71:655–665. doi: 10.1016/s0091-3057(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 13.Galici R, Boggs JD, Miller KL, Bonaventure P, Atack JR. Effects of SB-269970, a 5-HT7 receptor antagonist, in mouse models predictive of antipsychotic-like activity. BehavPharmacol. 2008;19:153–159. doi: 10.1097/FBP.0b013e3282f62d8c. [DOI] [PubMed] [Google Scholar]
- 14.Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB. Inactivation of the 5-HT7 Receptor Partially Blocks Phencyclidine-Induced Disruption of Prepulse Inhibition. Biol Psychiatry. 2008;63:98–105. doi: 10.1016/j.biopsych.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas DR, Hagan JJ. 5-HT7 receptors. Curr Drug Target CNS NeurolDisord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- 16.Cifariello A, Pompili A, Gasbarri A. 5-HT7 receptors in the modulation of cognitive processes. Behav Brain Res. 2008;195:171–179. doi: 10.1016/j.bbr.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Roberts C, Thomas DR, Bate ST, Kew JNC. GABAergic modulation of 5-HT7 receptor-mediated effects on 5-HT efflux in the guinea-pig dorsal raphe nucleus. Neuropharmacology. 2004;46:935–941. doi: 10.1016/j.neuropharm.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Gasbarri A, Cifariello A, Pompili A, Meneses A. Effect of 5-HT7 antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav Brain Res. 2008;195:164–170. doi: 10.1016/j.bbr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 19.Meneses A. Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshapingPavlovian/instrumental learning task. Behav Brain Res. 2004;155:275–282. doi: 10.1016/j.bbr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Chudasama Y, Muir JL. Abehavioural analysis of the delayed non-matching to position task: the effects of scopolamine, lesions of the fornix and of the prelimbic region on mediating behaviours by rats. Psychopharmacology. 1997;134:73–82. doi: 10.1007/s002130050427. [DOI] [PubMed] [Google Scholar]
- 21.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 22.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krystal JH, D'Souza DC, Mathalon D, Perry E, Belger A, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology. 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 24.Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. CurrOpinPharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 26.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Gil X, Babot Z, Amargos-Bosch M, Sunol C, Artigas F, et al. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- 29.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nature Neurosci. 2007;10:376–384. doi: 10.1038/nn1846. [DOI] [PubMed] [Google Scholar]
- 30.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, et al. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. San Diego: Academic Press; 1997. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 32.Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, et al. T-type calcium channel antagonism produces antipsychotic-like effects and reduces stimulant-induced glutamate release in the nucleus accumbens of rats. Neuropharmacology In Press. doi: 10.1016/j.neuropharm.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Barbier AJ, Aluisio L, Lord B, Qu Y, Wilson SJ, et al. Pharmacological characterization of JNJ-28583867, a histamine H3 receptor antagonist and serotonin reuptake inhibitor. Eur JPharmacol. 2007;576:43–54. doi: 10.1016/j.ejphar.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Perez-Garcia GS, Meneses A. Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behavioural Brain Research. 2005;163:136–140. doi: 10.1016/j.bbr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 35.Stahl SM. The serotonin-7 receptor as a novel therapeutic target. J ClinPsychiatry. 2010;71:1414–1415. doi: 10.4088/JCP.10bs06601gry. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Garcia G, Gonzalez-Espinosa C, Meneses A. An mRNA expression analysis of stimulation and blockade of 5-HT7 receptors during memory consolidation. Behav Brain Res. 2006;169:83–92. doi: 10.1016/j.bbr.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Galici R, Boggs JD, Aluisio L, Fraser IC, Bonaventure P, et al. JNJ-10181457, a selective non-imidazole histamine H3 receptor antagonist, normalizes acetylcholine neurotransmission and has efficacy in translational rat models of cognition. Neuropharmacology. 2009;56:1131–1137. doi: 10.1016/j.neuropharm.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 38.Dunnett SB. Comparative effects of cholinergic drugs and lesions of nucleus basalis or fimbria-fornix on delayed matching in rats. Psychopharmacology. 1985;87:357–363. doi: 10.1007/BF00432721. [DOI] [PubMed] [Google Scholar]
- 39.Robinson JK, Mao JB. Differential effects on delayed non-matching-to-position in rats of microinjections of muscarinic receptor antagonist scopolamine or NMDA receptor antagonist MK-801 into the dorsal or ventral extent of the hippocampus. Brain Res. 1997;765:51–60. doi: 10.1016/s0006-8993(97)00426-5. [DOI] [PubMed] [Google Scholar]
- 40.Doyle KM, Feerick S, Kirkby DL, Eddleston A, Higgins GA. Comparison of various N-methyl-D-aspartate receptor antagonists in a model of short-term memory and on overt behaviour. BehavPharmacol. 1998;9:671–681. doi: 10.1097/00008877-199812000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Horiguchi M, Meltzer H. 5-HT7 Receptor Antagonism Contributes to the Ability of Atypical Antipsychotic Drugs (APDs), Including Lurasidone and Amisulpride, to Reverse the Phencyclidine (PCP)-induced Deficit in Novel Object Recognition in Rats. Neuropsychopharmacology. 2010;35 [Google Scholar]
- 42.Horisawa T, Ishibashi T, Nishikawa H, Enomoto T, Toma S, et al. Behav Brain ResIn Press; The effects of selective antagonists of serotonin 5-HT7 and 5-HT1A receptors on MK-801-induced impairment of learning and memory in the passive avoidance and Morris water maze tests in rats: Mechanistic implications for the beneficial effects of the novel atypical antipsychotic lurasidone. [DOI] [PubMed] [Google Scholar]
- 43.Ballaz SJ, Akil H, Watson SJ. The 5-HT7 receptor: Role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience. 2007;149:192–202. doi: 10.1016/j.neuroscience.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 44.Smith SM, Pachmerhiwala R, Hinchliffe RM, Yao L. 13th International Conference on In Vivo Methods: Monitoring Molecules in Neuroscience. Brussels: 2010. Characterization of the central neurochemical effects of the mGluR2/3 receptor agonist LLY379268 and the psychostimulant MK-801. [Google Scholar]