SUMMARY
Biochemical studies have suggested that certain synaptic proteins associate with lipid rafts to perform key functions within the synapse. However, variability in biochemical preparations raises questions as to which synaptic proteins actually associate with lipid rafts. In this study, we use both electron microscopy and biochemistry to investigate AMPA receptor localization in synaptic membrane subfractions prepared in two different ways, by Triton X-100 detergent treatment or without detergent by sonication at high pH. Immunogold electron microscopy shows that a detergent-resistant synaptosomal membrane subfraction consists of empty vesicles 0.1 – 1.0 μm in diameter. A sub-population of these vesicles labeled for glycosphingolipid GM1 ganglioside, a marker of lipid rafts, and 46 percent of the labeled vesicles also labeled for the AMPA receptor subunit GluR2. This co-segregation into specific vesicles does not depend on effects of detergent because a similar distribution of label was found in vesicles isolated without the use of detergent. Our results imply that AMPA receptors localize within specific regions of synaptic membranes rich in GM1 ganglioside.
INTRODUCTION
Lipid rafts are thought to participate in sequestering proteins in cell membranes. Lipid rafts are implicated in AMPA receptor trafficking [1, 2], and AMPA receptor subunit motility is vital to synaptic plasticity and maintenance. To further define the association of synaptic proteins with lipid rafts, we combine biochemistry and electron microscopy to investigate the association of AMPA receptors with lipid raft marker GM1 ganglioside in synaptic membrane subfractions.
Lipid rafts are characterized by an abundance of sphingolipids, cholesterol, and GPI-anchored protein [1–8]. Treatment of synaptosomes with Triton X-100 detergent and density gradient centrifugation yields a fraction with sphingolipid and cholesterol content similar to that characteristic of lipid rafts [1, 6, 7, 9]. Alternatively, high pH combined with sonication can be used instead of Triton X-100 to isolate a similar fraction from synaptosomes without detergent [3]. The glycosphingolipid GM1 ganglioside is a well-known component of the detergentresistant membrane and marker for lipid rafts [1, 2, 9–13]. The isolated lipid fractions can be assumed to contain lipid rafts because each shows a heavy presence of GM1 ganglioside, but it is unknown how much of the fractions contain GM1 or how it is distributed [1, 2, 9–11].
In the present study, we combine negative stain electron microscopy with immunogold labeling to determine the morphological properties of isolated synaptosomal membrane subfractions as well as the distribution of GM1 ganglioside and AMPA receptors. Our results show that GM1 is not distributed uniformly but is isolated within a subpopulation of lipid vesicles. In comparing the distribution of GM1 with AMPA receptors, it becomes apparent that AMPA receptor subunits are typically located in GM1 containing vesicles, regardless of whether they are isolated with or without detergent.
EXPERIMENTAL PROCEDURES
Antibodies and Immunoblotting
SDS-PAGE was carried out using 7.5% minigels (Bio Rad). Proteins were transferred to nitrocellulose and immunoblots were obtained using the following antibodies. Rabbit polyclonal Anti-GluR 2/3 (Millipore) and rabbit polyclonal Anti-PSD-95 (custom made by New England Peptide) [14] were used at 1:500 for Western blots. Mouse monoclonal extracellular Anti-GluR 2 (Clone 6C4, Millipore) was used at 1:100 dilution for immunocytochemistry and 1:1000 dilution for Western blots. Rabbit polyclonal Anti-Ganglioside GM1 (abcam) was used at 1:100 dilutions for Immunocytochemistry and 1:1000 for dot blots. Anti-rabbit IgG Alkaline Phosphatase Conjugate (Millipore) was used for Western blots at a dilution of 1:5000. Anti-Mouse IgG Alkaline Phosphatase Conjugate (Millipore) was used for Western blots at a dilution of 1:5000. Goat Anti-Mouse 10nm Gold (British BioCell) was used at 1:100 dilution to detect GluR2 and goat Anti-Rabbit 15nm Gold (British BioCell) was used at 1:100 dilution to detect GM1 in all immunocytochemistry. For dot blots, fractions were dotted on a nitrocellulose membrane and allowed to dry before being labeled with antibody.
Subcellular Fractionation
Isolation of postsynaptic density (PSD) and detergent resistant synaptic membrane (DRSM) fractions using Triton X-100
The method of Carlin et al [15] was used to obtain subcellular fractions from rat brains (Pel-Freez Biologicals) with modifications as detailed in Dosemeci et al [16]. In short, a synaptosome fraction was obtained and treated with 0.5% TritonX-100 at 4°C. Detergent-insoluble material was collected by centrifugation and further fractionated on sucrose gradients. DRSM fraction was collected from the 0.32/1.0M sucrose interface. Crude PSD fraction was collected from the 2.1/1.5M sucrose interface and treated again with 0.5% TritonX-100/75mM KCl and collected on 2.1M sucrose cushion to obtain the final PSD fraction used in this study.
Synaptosomal lipid raft fraction without detergent treatment
The detergent-free preparation was adapted from previously published methods using high pH and sonication [3, 17]. Synaptosomes were obtained as above and pelleted by centrifugation (20,000rpm for 30min using Sorwall SS34 rotor). The synaptosomal pellet was re-suspended in 0.5M Na2CO3 pH11 and sonicated using a probe sonicator, five times for 20sec with 2min intervals (Kontes Micro Ultrononic Cell Disruptor, output control at 60). Resulting particulate material was fractionated using a sucrose gradient. Material from the 0.32/1.0M and 1.0/1.5M sucrose interfaces as well 1.0M sucrose layer were collected. No visible material was present at the 1.5/2.1M sucrose interface. Fractions were stored at −20°C in 33% glycerol [18].
Cholesterol Depletion
The depletion of cholesterol on subfractions previously isolated from synaptosomes was accomplished by treating the sample with 0.5% saponin in HEPES buffer for 15 min at 25°C while shaking.
Electron Microscopy
Negative Stain
Isolated fractions were transferred without fixation or resin embedding to glow discharged, formvar coated, 400 mesh, copper grids (SPI Supplies) by floating the grid on 10 μl drops of each fraction, diluted in 20mM HEPES, for 10min. Grids were then transferred to 30 μl drops of 0.5% casein in PBS (BioFX) block for 1hr before transfer to 30 μl drops of primary antibody diluted in 0.5% casein in PBS block. Primary antibodies in 0.5% casein block were applied in separate drops for 1hr each; controls were merely placed in casein block for this step. All grids were washed in 0.05% TWEEN 20 in casein block for 30min before being placed in 30 μl droplets of secondary antibodies diluted in TWEEN. It has been shown that TWEEN 20 does not significantly solublize membrane [8]. The secondary with the larger gold was applied first, and each secondary was applied for 1hr. Grids were finally washed in three solutions consecutively: 0.05% TWEEN 20 in PBS, 30min; PBS, 20min; 5mM HEPES, 10min. The samples were then passed through three drops of 1% uranyl acetate - 30sec each, dried, and examined at 200KEV with a JOEL 200CV electron microscope. All presented immunocytochemstry data was taken from micrographs of double-immunolabeled samples.
Thin-Section
Thin section microscopy was performed as described in Dosemeci et al [16]. TEM of thin-sectioned pellets was preformed only for morphology and structure investigations. No immunogold labeling was performed on fixed or resin embedded thin-sections.
Counts of gold label
Individual vesicles were measured for cross-sectional area and numbers of gold label, in micrographs of the detergent-resistant synaptosomal membrane fraction that featured multiple vesicles with well-defined membrane boundaries. Cross-sectional area was measured with ImageJ software (NIH) and gold labels were counted manually. Two particles of the same diameter, clustered within 15nm, were counted as one label. In micrographs of detergent-free fractions, where vesicles were in aggregates, a grid with squares of 0.1 μm2 (approximately the average area of a DRSM vesicle) was laid over the micrograph and counts per square were recorded in every square entirely filled by vesicles.
In control samples (primary antibody omitted), secondary antibodies, conjugated to 5 and 10nm gold, labeled only 4.4 and 6.8% respectively of the vesicles in the detergent-resistant synaptosomal membrane fraction, with no vesicles labeled with more than one immunogold in any of the control micrographs.
RESULTS AND DISCUSSION
Biochemical analyses of synaptic membrane subfractions have shown that synaptic proteins associate with Triton X-100-insoluble lipids [1, 7, 9]. We use biochemistry and electron microscopy to investigate the association of glycosphingolipid GM1 ganglioside with AMPA receptors in structures isolated in synaptic membrane subfractions.
Biochemical and morphological differences in detergent-isolated fractions
Treatment of membrane fractions with Triton X-100 has been extensively used as a strategy to assess association of neuronal proteins within specialized lipid domains thought to include lipid rafts [1, 7, 9]. In particular, AMPA receptors have been observed in detergent-insoluble fractions from synaptosomal membranes [7, 9] and from a heavy membrane (P2) fraction [1]. The starting material as well as the detergent concentration appears to influence the distribution of other proteins, specifically PSD-95, in the detergent-resistant fractions. Using 0.5% TX-100, Suzuki et. al. (2001) observed relatively little PSD-95 in the cholesterol-rich, light detergent-resistant synaptosomal membrane (DRSM) fraction compared to the heavy detergent-resistant PSD fraction. On the other hand, Suzuki et al (2008) used a lower concentration of TX-100 in a later study and found a relatively higher amount of PSD-95 captured in the synaptosomal raft fraction. Hering et. al. (2003) found higher amounts of PSD-95 in the light, DRSM fractions relative to the heavy, PSD subfraction, when using 0.5% TX-100.
In this study, it was confirmed that GM1 was more abundant in the light fraction isolated from the 0.32/1.0M sucrose interface when compared to the 1.5/2.1M sucrose interface. Western blot analysis showed the presence of GluR2 in both fractions with slightly higher levels in the 1.5/2.1M interface fraction compared to the 0.32/1.0M interface fraction. Western blots also indicated that the 1.5/2.1M interface fraction had more PSD-95 compared to that found in the DRSM fraction (Figure 1, lower right inset). The present study, which uses conditions similar to those of Suzuki et. al. (2001), confirms their findings.
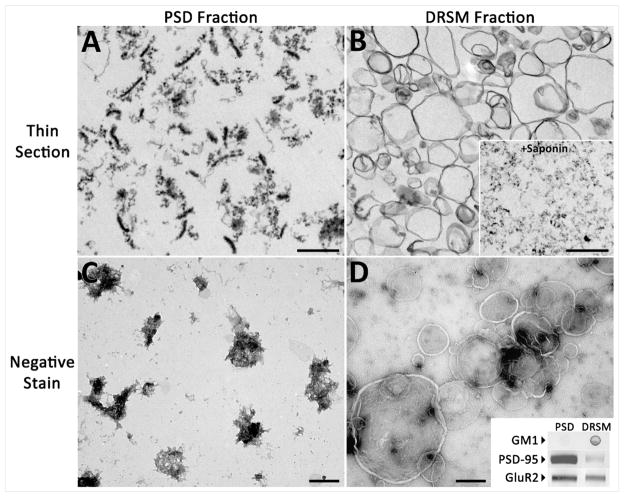
Figure 1. Synaptic GluR2 is present in PSDs as well as detergent-resistant lipid vesicles.
Representative views of particulate material in post synaptic density (PSD) and detergent-resistant synaptosomal membrane (DRSM) fractions. (a) A thin-section TEM micrograph of the PSD fraction shows numerous PSDs as well as other electron dense material. (b) Thin-section TEM of the DRSM fraction shows cross-sections of empty vesicles. Vesicles disappear from the DRSM fraction after saponin treatment (b, Inset). Micrographs of negatively stained PSD (c) and DRSM (d) fractions show en face views of structures in each fraction. Comparison of the two fractions by Western and dot blot (d, Inset) shows that both fractions contain GluR2, but GM1 is higher in the DRSM fraction while PSD-95 is almost exclusive to the PSD fraction. Scale bars, 500nm.
Thin section EM indicates structural differences between the two synaptosomal raft fractions defined by Suzuki et. al. While the fraction obtained using 0.5% TX-100 consists mainly of smooth vesicles (Figure 1, [7]), the fraction derived with 0.15% detergent has an abundance of electron dense material [9]. Our micrographs of thin-sectioned 1.5/2.1M interface, or PSD fraction, showed typical PSDs and other electron dense aggregates. The 0.32/1.0M interface, or detergent-resistant synaptosomal membrane (DRSM), subfraction contained lipid vesicles that vary greatly in size but do not encapsulate PSDs or other, smaller electron dense aggregates (Figure 1B). Similarly, en face views of negatively stained DRSM vesicles show nearly transparent vesicles that do not encapsulate PSDs or PSD like aggregates (Figure 1D). This is significantly different from the vesicles shown in Suzuki et. al. (2008) in which 0.15% TX-100 was used. Thus, rather than being embedded into lipid vesicles together with AMPA receptors, PSD-95 may be associated with electron-dense protein complexes. The raft fraction obtained by Hering et. al. (2003) by treatment of a P2 fraction with 0.5% TX-100, on the other hand, may contain intracellular trafficking complexes in addition to synaptic rafts, which may be responsible for its relatively high content of PSD-95.
Co-segregation of synaptic GM1 ganglioside with AMPA receptor subunits in detergent-derived membrane subfractions
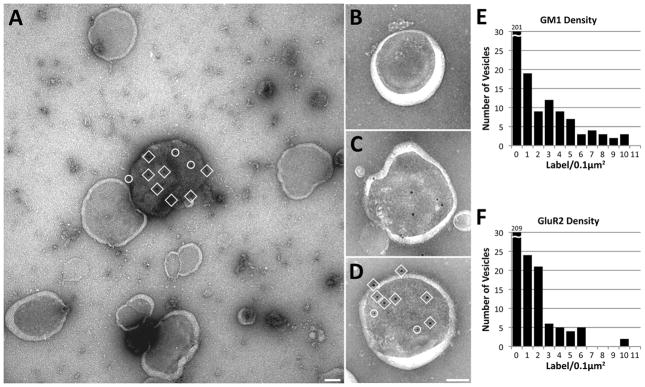
The resolution afforded by electron microscopy is essential for determining the distribution of individual components in isolated lipid fractions. In this study, we obtained electron micrographs of double-immunolabeled Detergent-Resistant Synaptosomal Membrane (DRSM) that show that GM1 ganglioside and AMPA receptors aggregate within a subset of vesicles (Figure 2). Sixty percent of vesicles in the DRSM fraction did not label for GM1 or AMPA receptors (Table 1), showing that the majority of vesicles label at a frequency distinguishably less than background (0.061 ± 0.029 and 0.149 ± 0.028 label/0.1 μm2 respectively). The results indicate a biased distribution of lipids and proteins after detergent separation, with GM1 tagged vesicles accounting for only 27% of the detergent-derived lipid vesicles. A subpopulation of vesicles labeled for the AMPA receptor subunit GluR2 accounted for 24% of vesicles in the detergent-derived fraction, and 46% of GluR2 labeled vesicles also contained label for GM1 (Table 1). Since GM1 is regarded as a marker for lipid rafts [1, 2, 9–13], these data agree with previous biochemical and light microscopy evidence suggesting that AMPA receptors associate with lipid rafts [1, 2, 7].
Figure 2. GM1 and GluR2 co-segregate in a small number of DRSM vesicles.
Negative stain immunogold labeling of vesicles in DSRM fraction. (a) Single vesicle in DSRM fraction is co-labeled for GM1 (10nm gold, in circles) and GluR2 (15nm gold, in squares). Other single vesicles (b–d) show density of co-labeling varying from zero (b) to heavy (d, label indicated as in a). (e,f) These graphs indicate the density of GM1 and GluR2 immunogold label for 276 vesicles in DSRM fraction. The average background on the substrate between vesicles for GM1 and GluR2 labeling was 0.061 ± 0.029 and 0.149 ± 0.028 label/0.1 μm2 respectively. Scale bars, 100nm.
Table 1.
Distribution of GluR2 and GM1 among vesicles in the detergent-resistant synaptosomal membrane (DRSM) fraction
| GluR2 Label | GM1 Label | Number of Vesicles | Percentage of Vesicles |
|---|---|---|---|
| − | − | 165 | 60 % |
| − | + | 44 | 16 % |
| + | − | 36 | 13 % |
| + | + | 31 | 11 % |
The cross-sectional area and gold label was measured for 276 vesicles in micrographs of double-immunogold labeled vesicles.
Saponin Treatment Destroys Nearly All Vesicles in the Detergent-derived Fraction
To further characterize the detergent-derived subfraction from synaptosomes, the fractions were treated with saponin, a cholesterol-depletion reagent. This strategy defines the role of cholesterol in maintaining the vesicles and helps assess whether the GM1 and GluR2 positive vesicles are different with respect to cholesterol content. Saponin, although considered a relatively strong detergent, acts specifically on cholesterol [19, 20]. For example, while a mild non-ionic detergent such as Triton X-100 can practically destroy the cell membrane, saponin treatment merely produces holes and leaves morphology relatively intact [20].
After saptonin treatment of the detergent-resistant synaptosomal membrane fraction, thin-section electron microscopy of saponin-insoluble material reveals an almost complete disappearance of lipid vesicles (Figure 1B, inset). This result indicates that cholesterol is a major constituent of all lipid vesicles in the detergent-derived subfraction, and that segregation of GluR2/GM1 in a subset of vesicles is not related to cholesterol.
Co-segregation of GM1 and GluR2 in Subfractions Isolated without Detergent
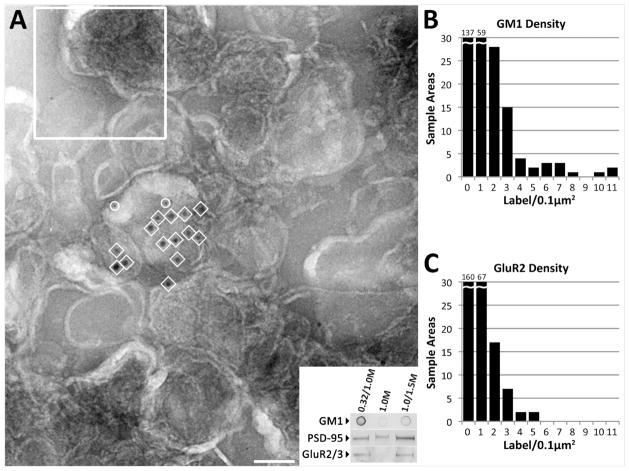
Whether co-segregation of GM1 ganglioside and AMPA receptors in fractions isolated with detergent reflects their original state in the cell is open to question. Therefore, we isolated an equivalent lipid fraction [5] without detergent to confirm that the segregation observed in the detergent-derived lipid fraction did not depend on detergent (Figure 3). The detergent-free disruption of synaptic membrane using high pH combined with sonication does not result in a fraction identical to detergent isolation (Figure 3A, inset). Isolated fractions from the 0.32/1.0M and 1.0/1.5M sucrose interfaces as well as the 1.0M sucrose layer were examined, by Western and dot blots as well as electron microscopy with immunocytochemistry. Our results indicate that the light vesicles collected at the 0.32/1.0M sucrose interface have similar properties to the detergent-derived fraction collected at the same density interface. Dot blots show that GM1 ganglioside had a heavier presence in the 0.32/1.0M sucrose interface than in either 1.0M or 1.0/1.5M fractions (Figure 3A, Inset). The detergent-free fraction from the 0.32/1.0M interface has a composition and distribution of PSD-95, GluR2, and GM1 similar to that of the detergent-derived fraction, as evidenced by immunoblots and electron microscopy (Figure 3). Electron micrographs of immunolabeled vesicle fraction show that vesicles are more aggregated than those in the DRSM fraction (Figure 3A). GM1 and GluR2 are clustered within these aggregates at concentrations higher than one gold label/0.1 μm2 (Figure 3B–C). Forty-nine percent of regions with high GluR2 concentration included clusters of GM1 (Table 2). Different stereological methods were required to determine label densities due to the aggregation, but the pattern of co-segregation in both samples appear to be the same.
Figure 3. Detergent-Free Fraction shows DRSM-like segregation.
Negative stain immunogold labeling of vesicles in detergent-free fraction (a). Vesicles in this fraction clump, obscuring their borders. Label for GM1 (10nm gold, in circles) and GluR2 (15nm gold, in squares) is concentrated over one or a few vesicles. Density of labeling was measured by overlaying images with a 0.1 μm2 grid, close the average vesicle size measured in the detergent fraction (sample size indicated in upper left). (b,c) Graphs indicate the label density frequencies for GM1 and GluR2 in 255 sample areas. (Inset) Dot and Western immunoblots of synaptosomal subfractions were obtained for the detergent-free procedure showing relative levels of GM1, PSD-95, and GluR2/3. Scale bar, 100nm.
Table 2.
Distribution of GluR2 and GM1 within sample areas of lipid vesicles obtained from synaptosomes by the detergent-free procedure
| GluR2 Label | GM1 Label | Number of Sample Areas | Percentage of Sample Areas |
|---|---|---|---|
| − | − | 89 | 35 % |
| − | + | 71 | 28 % |
| + | − | 48 | 19 % |
| + | + | 47 | 18 % |
A sample area consists of a 0.1 μm2 grid square containing aggregated lipid vesicles. A total of 255 sample areas were counted in micrographs of double-immunogold labeled vesicles.
Though we have determined that co-segregation of GM1 ganglioside with AMPA receptors is not an effect of detergent, the temperature at which both isolations were preformed could influence properties of the resulting fractions. One clear artifact is vesicle size of 0.1 to 1.0 μm in the detergent-derived fraction – lipid membrane domains of this size are not likely to exist at the synapse under physiological conditions [10].
Biochemical and light microscopy studies have pointed to a connection between specialized lipid domains and AMPA receptors [1, 2, 7]. Examining lipid fractions from synaptic membranes by immunoelectron microscopy supports this idea. Our results indicate that the distributions of GM1 and AMPA receptors are coupled, independent of detergent interaction with the membranes. The association of AMPA, a major PSD receptor, with specialized lipid domains implies that neuronal communication could be influenced by the lipid composition of the synapse. GM1 ganglioside and AMPA receptor subunits co-segregate into the same vesicles, but do not strongly co-localize. The lack of co-localization indicates that there may be a property of co-segregated vesicles that attracts or traps specific lipids and proteins. For instance, Eggeling et al (2009) showed that areas of sphingomyelin and cholesterol hinder certain protein diffusion rates. It is possible that AMPA receptor subunits linger in lipid rafts without being specifically bound to any particular component.
Acknowledgments
The authors would like to thank Dr. Jung-Hwa Tao-Cheng and the National Institutes of Neurological Disorders and Stroke Electron Microscopy Facility for thin section electron microscopy. Also, we thank Drs. Carolyn Smith, Jung-Hwa Tao-Cheng, and Christine Winters for their critical reading of this manuscript and discussion.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
Footnotes
Author Contribution
Andy Cole designed and carried out experiments, analyzed the data, and wrote the manuscript. Ayse Dosemeci assisted in the design and performance of biochemical aspects of the research, analysis of the data, and in the preparation of the manuscript. Thomas Reese assisted with electron microscopy and morphological aspects of the research as well as the analysis of data and preparation of the manuscript.
References
- 1.Hering H, Lin CC, Sheng M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J Neurosci. 2003;23:3262–3271. doi: 10.1523/JNEUROSCI.23-08-03262.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou Q, Huang Y, Amato S, Snyder SH, Huganir RL, Man HY. Regulation of AMPA receptor localization in lipid rafts. Mol Cell Neurosci. 2008;38:213–223. doi: 10.1016/j.mcn.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckert GP, Igbavboa U, Muller WE, Wood WG. Lipid rafts of purified mouse brain synaptosomes prepared with or without detergent reveal different lipid and protein domains. Brain Res. 2003;962:144–150. doi: 10.1016/s0006-8993(02)03986-0. [DOI] [PubMed] [Google Scholar]
- 4.Garner AE, Smith DA, Hooper NM. Visualization of detergent solubilization of membranes: implications for the isolation of rafts. Biophys J. 2008;94:1326–1340. doi: 10.1529/biophysj.107.114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gil C, Cubi R, Blasi J, Aguilera J. Synaptic proteins associate with a subset of lipid rafts when isolated from nerve endings at physiological temperature. Biochem Biophys Res Commun. 2006;348:1334–1342. doi: 10.1016/j.bbrc.2006.07.201. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki T. Lipid rafts at postsynaptic sites: distribution, function and linkage to postsynaptic density. Neurosci Res. 2002;44:1–9. doi: 10.1016/s0168-0102(02)00080-9. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Ito J, Takagi H, Saitoh F, Nawa H, Shimizu H. Biochemical evidence for localization of AMPA-type glutamate receptor subunits in the dendritic raft. Brain Res Mol Brain Res. 2001;89:20–28. doi: 10.1016/s0169-328x(01)00051-1. [DOI] [PubMed] [Google Scholar]
- 8.Pike LJ, Han X, Gross RW. Epidermal growth factor receptors are localized to lipid rafts that contain a balance of inner and outer leaflet lipids: a shotgun lipidomics study. J Biol Chem. 2005;280:26796–26804. doi: 10.1074/jbc.M503805200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki T, Du F, Tian QB, Zhang J, Endo S. Ca2+/calmodulin-dependent protein kinase IIalpha clusters are associated with stable lipid rafts and their formation traps PSD-95. J Neurochem. 2008;104:596–610. doi: 10.1111/j.1471-4159.2007.05035.x. [DOI] [PubMed] [Google Scholar]
- 10.Eggeling C, Ringemann C, Medda R, Schwarzmann G, Sandhoff K, Polyakova S, Belov VN, Hein B, von Middendorff C, Schonle A, Hell SW. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature. 2009;457:1159–1162. doi: 10.1038/nature07596. [DOI] [PubMed] [Google Scholar]
- 11.Gomez-Mouton C, Abad JL, Mira E, Lacalle RA, Gallardo E, Jimenez-Baranda S, Illa I, Bernad A, Manes S, Martinez AC. Segregation of leading-edge and uropod components into specific lipid rafts during T cell polarization. Proc Natl Acad Sci U S A. 2001;98:9642–9647. doi: 10.1073/pnas.171160298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mocchetti I. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cell Mol Life Sci. 2005;62:2283–2294. doi: 10.1007/s00018-005-5188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang HD, Ding JX, Tian WQ, Wang LJ, Huang LX, Ruan Y, Lu TL, Sha YL, Zhang D. Ganglioside GM1 binding the N-terminus of amyloid precursor protein. Neurobiol Aging. 2009;30:1245–1253. doi: 10.1016/j.neurobiolaging.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Winters C, Azzam R, Li X, Galbraith JA, Leapman RD, Reese TS. Organization of the core structure of the postsynaptic density. Proc Natl Acad Sci U S A. 2008;105:4453–4458. doi: 10.1073/pnas.0800897105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosemeci A, Reese TS, Petersen J, Tao-Cheng JH. A novel particulate form of Ca(2+)/calmodulin-dependent [correction of Ca(2+)/CaMKII-dependent] protein kinase II in neurons. J Neurosci. 2000;20:3076–3084. doi: 10.1523/JNEUROSCI.20-09-03076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah MB, Sehgal PB. Nondetergent isolation of rafts. Methods Mol Biol. 2007;398:21–28. doi: 10.1007/978-1-59745-513-8_3. [DOI] [PubMed] [Google Scholar]
- 18.Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder RJ, Ahmed SN, Zhu Y, London E, Brown DA. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 20.Jamur MC, Oliver C. Permeabilization of cell membranes. Methods Mol Biol. 588:63–66. doi: 10.1007/978-1-59745-324-0_9. [DOI] [PubMed] [Google Scholar]