Abstract
The consumption of omega-3 polyunsaturated fatty acids (n-3 PUFAs) has been reported to decrease resting heart rate (HR) and increase heart rate variability (HRV). However, the effects of n-3 PUFAs on these variables in response to a physiological stress (e.g., exercise or acute myocardial ischemia), particularly in postmyocardial infarction (MI) patients, are unknown. Therefore, HR and HRV (high frequency and total R-R interval variability) were evaluated at rest, during submaximal exercise, and during a 2-min coronary artery occlusion at rest and before and 3 mo after n-3 PUFA treatment in dogs with healed MI (n = 59). The dogs were randomly assigned to either placebo (1 g/day corn oil, n = 19) or n-3 PUFA supplement (docosahexaenoic acid + eicosapentaenoic acid ethyl esters; 1 g/day, n = 6; 2 g/day, n = 12; or 4 g/day, n = 22) groups. The treatment elicited significant (P < 0.01) dose-dependent increases in right atrial n-3 PUFA levels but dose-independent reductions in resting HR and increases in resting HRV. In contrast, n-3 PUFAs did not attenuate the large changes in HR or HRV induced by either the coronary occlusion or submaximal exercise. These data demonstrate that dietary n-3 PUFA decreased resting (i.e., preexercise or preocclusion) HR and increased resting HRV but did not alter the cardiac response to physiologic challenges.
Keywords: parasympathetic nervous system, exercise, fish oil, docosahexaenoic acid, eicosapentaenoic acid
alterations in cardiac autonomic regulation have been implicated in an increased risk for sudden cardiac death (5). In particular, reduced cardiac parasympathetic activity coupled with an enhanced sympathetic activation in both patients and animals following myocardial infarction is associated with an increased incidence of malignant ventricular tachyarrhythmias and sudden cardiac death (5). Therefore, considerable effort has been expended in the search for therapeutic interventions that improve cardiac balance and could thereby protect against sudden death in high-risk patient populations.
To be effective, these interventions must not only improve baseline or resting cardiac autonomic balance but also maintain these changes when the heart is challenged by a physiologic stress such as acute myocardial ischemia or exercise. For example, low doses of cholinergic muscarinic antagonists (atropine or scopolamine) have, paradoxically, been shown to enhance resting cardiac vagal regulation in both humans (14, 19, 32) and animals (23, 29). It was therefore proposed that the continuous administration of low doses of muscarinic antagonists could improve cardiac vagal regulation and, as a consequence, would also reduce the incidence of sudden cardiac death in myocardial infarction patients (14, 19). However, these interventions failed to prevent ventricular fibrillation induced by myocardial ischemia (23, 29) despite significant increases in resting time and frequency domain markers of heart rate variability (HRV; indexes of cardiac parasympathetic regulation; Refs. 2, 48). It was subsequently shown that low doses of atropine did not alter the cardiac autonomic response elicited by either submaximal exercise or acute myocardial ischemia (23). Thus the search for effective interventions that improve autonomic balance both at rest and during stress condition remains elusive.
A number of experimental and clinical studies report that dietary omega-3 polyunsaturated fatty acids (n-3 PUFAs; Ref. 16) or the acute intravenous administration (9) of these lipids can both lower resting heart rate (HR) and increase resting HRV, data consistent with an enhanced baseline cardiac parasympathetic tone. It has been proposed that the cardiovascular benefits ascribed to dietary n-3 PUFAs could result, at least in part, from these reductions in HR and the corresponding putative improvements in cardiac autonomic balance (16). However, this concept has been challenged (11, 22, 24) and, to date, the effects of dietary n-3 PUFA on HR and HRV during physiological stress have received little attention. In a similar manner, several lines of evidence suggest that the modulation of cardiac autonomic function may not be solely responsible for the n-3 PUFA-induced reductions in HR. For example, the acute application of n-3 PUFA reduced the contraction rate of neonatal rat myocyte cultures (31) while dietary n-3 PUFA supplements decreased HR of isolated rat atria (33) and cardiac transplant patients (25). A reduced pacemaker current (If) density has also been recorded in sinus nodal myocytes from rabbits fed n-3 PUFAs compared with cells from the hearts of animals given similar amounts of sunflower oil (50). However, whether these changes in intrinsic pacemaker rate are maintained during physiological stress also remains to be determined.
It was, therefore, the purpose of the present study to evaluate the effects of dietary n-3 PUFAs (1–4 g/day for 3 mo) on the HR and the HRV responses to physiological stressors [exercise (submaximal exercise, assessed at its onset and at its termination) and acute myocardial ischemia (2-min coronary artery occlusion)] in dogs with healed myocardial infarctions. In particular, the hypothesis that the dietary n-3 PUFA-mediated reductions in HR and increases in HRV would be maintained during a physiologic challenge was tested.
METHODS
All the animal procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996).
Surgical Preparation
A timeline for the studies may be found in Fig. 1. Eighty heartworm free mixed breed dogs (2- to 3-yr-old male, n = 21, and female, n = 59) weighing 19.3 ± 0.2 kg (range: 13.6–25.9 kg) that were part of an ongoing investigation of the cardiovascular effects of dietary n-3 PUFA (11) were used in this study. The animals were anesthetized and instrumented to measure a ventricular electrogram (from which HR and HRV were subsequently determined) and left circumflex coronary artery blood flow as previously described (3, 11). A hydraulic vascular occluder (model OC3; In Vivo Metric, Healdsburg, CA) was placed around the left circumflex coronary artery and used to induce acute myocardial ischemia for the coronary occlusion experiments described below (see HRV Protocols). The left anterior descending coronary artery was also isolated during the instrumentation surgery, and a two-stage occlusion of this artery was then performed approximately one-third the distance from its origin to produce an anterior wall myocardial infarction (∼16% of left ventricular mass; Ref. 3). This vessel was partially occluded for 20 min and then tied off. Similar instrumentation surgery was performed on six additional sham infarction dogs (i.e., the left anterior descending artery was isolated but not ligated). The dogs were given analgesic, antibiotic, and anti-arrhythmic therapy to alleviate postoperative pain, to prevent postoperative infection, and to reduce acute arrhythmias associated with the myocardial infarction as has been previously described (3, 11).
Fig. 1.
Study timeline. Pre- and posttreatment data collection studies consist of the following: three submaximal exercise studies and one 2-min coronary artery occlusion at rest. Studies were performed at least 24 h apart. MI, myocardial infarction.
HRV Protocols
HRV was calculated using a Delta-Biometrics vagal tone monitor triggering off the ECG R-R interval (Urbana, Champaign, IL). This device employs the time-series signal processing techniques as developed by Porges to estimate the amplitude of respiratory sinus arrhythmia [the high frequency (HF) component of R-R interval variability; Ref. 42]. Details of this analysis have been described previously (6, 28). Data were averaged over 30-s intervals either during exercise or the coronary occlusion. The following indexes of HRV were determined: vagal tone index, the HF (0.24 to 1.04 Hz) component of R-R interval variability, and standard deviation (SD) of the R-R intervals (a marker of total variability) for the same 30-s time periods.
The studies began 3–4 wk after the production of the myocardial infarction. Fifteen (18.8%, 6 males, 9 females) died within 72 h of the myocardial infarction, and reliable ECG recordings could not be obtained in six dogs (1 male and 5 females). Thus HRV studies were completed on 59 (14 male and 45 female) dogs following the myocardial infarction and all six sham-operated dogs. First, over the period of 3–5 days, the dogs learned to run on a motor-driven treadmill. The cardiac response to submaximal (i.e., 60–70% of maximal HR) exercise was then evaluated as follows: exercise lasted a total of 18 min with workload increasing every 3 min. The protocol began with a 3-min “warm-up” period, during which the dogs ran at 4.8 kph at 0% grade. The speed was then increased to 6.4 kph, and the grade was increased every 3 min (0, 4, 8, 12, and 16%). The submaximal exercise test was repeated three times (1/day). On a subsequent day, with the dogs lying quietly unrestrained on a table, a 2-min left circumflex coronary occlusion was made. Left circumflex blood flow, HR, and HRV were monitored continuously throughout the exercise or occlusion studies. The submaximal exercise and the coronary occlusion at rest studies were performed both before and after 3 mo of treatment with either placebo (1 g/day corn oil) or daily n-3 PUFA capsules (1–4 g/day).
Dietary Omega-3 Polyunsaturated Fatty Acid Protocol
The dogs were place on a diet that did not contain any n-3 PUFAs (Harlan Teklad; Harlan Laboratories, Indianapolis, IN) beginning 1 wk before the instrumentation surgery and were maintained on this diet until the end of the study (∼4 mo). After the pretreatment data collection (3–4 wk after the surgery) had been collected, the dogs were then randomly assigned to the following groups: placebo (n = 19, 3 male and 16 female); 1 g/day n-3 PUFA (n = 6, all female); 2 g/day n-3 PUFA (n = 12, 7 male and 5 female); 4 g/day n-3 PUFA (n = 22, 4 male and 18 female); and sham 4 g/day sham (n = 6, 1 male and 5 females). The dogs were given supplements similar to those used in the GISSI-Prevenzione study (20). The n-3 PUFA group received 465 mg ethyl eicosapentaenoate (EPA) + 375 mg ethyl docosahexaenoate (DHA) (375 per capsule; Lovaza; GlaxoSmithKline, Research Triangle Park, NC). The placebo was corn oil (1 g, 58% linoleic acid + 28% oleic acid). The capsules were given per os before the daily feeding (between 8:00 and 10:00 AM each day, 7 days per wk for 3 mo).
Red Blood Cell and Cardiac Tissue Fatty Acid Analysis
Fasting blood samples (5 ml) were drawn into EDTA tubes from a cephalic vein between 8:00 and 9:00 AM 1 day before the initiation of the treatment (placebo or n-3 PUFA) and when tissue was harvested at the end of the study (∼14 wk after the treatment began). Right atrial and left ventricular tissues were obtained when the hearts were harvested; the tissue and red blood cells (RBC) were flash frozen in liquid nitrogen and stored at −80°C for future analysis.
RBC and phospholipids from cardiac tissue were analyzed for fatty acid composition using previously described techniques (12, 35). The samples were analyzed by gas chromatography using a GC2010-FID (Shimadzu, Columbia, MD) equipped with a 100-mm capillary column (SP-2560; Supelco, Bellefonte, PA). Fatty acids of interest were identified by comparison with known standards and expressed as a percentage of total fatty acids. The coefficient of variation for the RBC EPA + DHA assays was < 5%.
Data Analysis
All data are reported as means ± SE. The data were digitized (1 kHz) and recorded using a Biopac MP-100 data acquisition system (Biopac Systems, Goleta, CA). The HR and HRV data were averaged over 30-s intervals either during exercise or the coronary occlusion. The following time points were evaluated for the exercise trials: the last 30 s before the onset of exercise, from 0 to 30 s, from 30 to 60 s, and from 90 to 120 s after exercise onset; and for recovery from exercise: the last 30 s of exercise, from 0 to 30 s, from 30 to 60 s, and from 90 to 120 s after the termination of exercise. The values averaged over these time periods are reported as time 0, 30, 60, and 120 s, respectively. ECG variables were averaged over the last five beats before and 60 s after the onset of the coronary occlusion. QT interval was corrected for changes in HR using Van de Water's correction formula [QTc = QT-87(60/HR-1)] (49).
The data were compared using ANOVA for repeated measures (NCSS Statistical Software, Kaysville, UT). For example, the effects the n-3 PUFAs on resting HR and HRV were analyzed using a two-way ANOVA [pre-post (2 levels) × dose (4 levels)] with repeated measures on one factor (pre-post) measures. In a similar fashion, the effects of n-3 PUFAs on the HR and HRV response to either submaximal exercise or the coronary artery occlusion at rest were analyzed using a three-factor ANOVA [pre-post (2 levels) × dose (placebo vs. n-3 PUFA) × time (exercise 7 levels or occlusion 6 levels) with repeated measures on two factors (pre-post and time)]. The effect of n-3 PUFA on ECG variables before and 60 s after coronary occlusion were evaluated using a three factor [pre-post (2 levels), dose (2 levels), and occlusion time (2 levels)] ANOVA with repeated measures on two (pre-post and occlusion time) factors. Homogeneity of covariance (sphericity assumption, equal correlates between the treatments) was tested using Mauchley's test and, if appropriate, adjusted using Huynh-Feldt correction. RBC and cardiac tissue lipid compositions were compared using a two-factor ANOVA with repeated measures on one factor (pre-post) or a one-factor ANOVA, respectively. If the F value exceeded a critical value (P < 0.05), post hoc comparisons of the data were then made using Tukey-Kramer multiple comparison test.
RESULTS
Effect of n-3 PUFA on RBC and Cardiac Tissue Fatty Acid Content
In agreement with our previous study (11), n-3 PUFA supplements elicited a dose-dependent (dose effect, pre-post, and dose × pre-post interaction all; P < 10−6) increases in RBC membrane EPA, DHA, and the omega-3 index (EPA + DHA) compared with the placebo-treated animals (Table 1). Thus n-3 PUFA treatment (all 3 doses) increased RBC n-3 PUFA content while lipid composition did not change during the 3-mo study in the placebo-treated dogs. In a similar manner, cardiac tissue n-3 PUFA content was significantly higher (P < 10−6, all 3 doses) in n-3 PUFA compared with the placebo-treated animals (Table 2).
Table 1.
Red blood cell omega-3 polyunsaturated fatty acid content
| EPA |
DHA |
Omega-3 Index |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Placebo (n = 12) | 0.16 ± 0.01 | 0.25 ± 0.04 | 0.28 ± 0.07 | 0.32 ± 0.06 | 0.43 ± 0.08 | 0.54 ± 0.08 |
| 1 g/day (n = 6) | 0.19 ± 0.02 | 1.20 ± 0.13* | 0.17 ± 0.02 | 2.15 ± 0.22* | 0.36 ± 0.03 | 3.34 ± 0.26* |
| 2 g/day (n = 12) | 0.16 ± 0.01 | 1.71 ± 0.11* | 0.29 ± 0.08 | 2.01 ± 0.13* | 0.56 ± 0.13 | 3.72 ± 0.22* |
| 4 g/day (n = 22) | 0.20 ± 0.02 | 3.92 ± 0.21* | 0.21 ± 0.03 | 3.02 ± 0.17* | 0.41 ± 0.05 | 7.11 ± 0.27* |
| Sham (n = 5) | 0.24 ± 0.03 | 4.41 ± 0.48* | 0.17 ± 0.02 | 3.421 ± 0.33* | 0.40 ± 0.05 | 7.49 ± 0.45* |
All values are means ± SE and are expressed as %total lipid content. Sham (i.e., no myocardial infarction) received 4 g/day; EPA = eicosapentaenoic acid; DHA = docasahexaenoic acid; omega-3 index = EPA + DHA.
P < 0.01, pre vs. post.
Table 2.
Cardiac tissue omega-3 polyunsaturated fatty acid content
| EPA | DHA | Omega-3 Index | |
|---|---|---|---|
| Right atrium | |||
| placebo (n = 12) | 0.32 ± 0.11 | 0.56 ± 0.14 | 0.85 ± 0.22 |
| 1 g/day (n = 6) | 1.24 ± 0.11* | 3.38 ± 0.20* | 4.63 ± 0.18* |
| 2 g/day (n = 12) | 1.45 ± 0.23* | 2.89 ± 0.41* | 4.34 ± 0.64* |
| 4 g/day (n = 22) | 2.76 ± 0.44* | 3.54 ± 0.50* | 6.31 ± 0.88* |
| Sham (n = 5) | 2.80 ± 0.43* | 3.83 ± 0.23* | 6.63 ± 0.31* |
| Left ventricle | |||
| placebo (n = 12) | 0.31 ± 0.06 | 0.49 ± 0.08 | 0.80 ± 0.11 |
| 1 g/day (n = 6) | 1.25 ± 0.09* | 3.08 ± 0.17* | 4.33 ± 0.21* |
| 2 g/day (n = 12) | 1.69 ± 0.20* | 2.67 ± 0.21* | 4.35 ± 0.64* |
| 4 g/day (n = 22) | 3.81 ± 0.30* | 3.43 ± 0.20* | 7.24 ± 0.45* |
| Sham (n = 5) | 3.71 ± 0.43* | 3.65 ± 0.28* | 7.07 ± 0.70* |
All values are means ± SE and are expressed as %total lipid content. Sham (i.e., no myocardial infarction) received 4 g/day; EPA = eicosapentaenoic acid; DHA = docasahexaenoic acid; omega-3 index = EPA + DHA.
P < 0.01, omega-3 treated vs. placebo.
Effect of n-3 PUFA on Resting HR and HRV
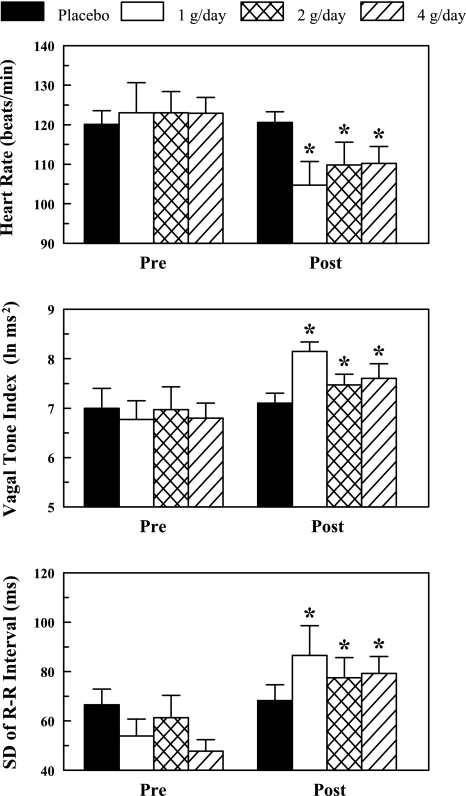
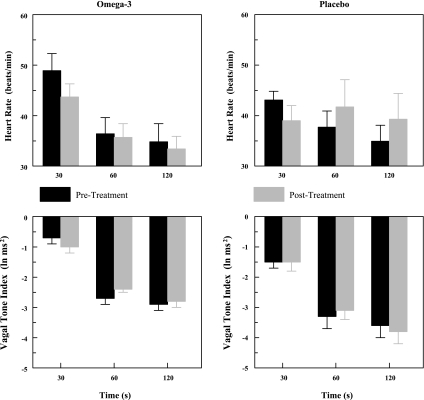
As n-3 PUFA have been reported to elicit different responses in males than in females (17), the effect of gender was first evaluated. There were no significant gender differences in HR (P = 0.69) or HRV (HF, P = 0.91; SD, P = 0.30) before or after the 3-mo treatment period. Therefore, these data have been combined in all subsequent analyses. The effects of placebo and the n-3 PUFA treatment on resting (i.e., obtained while the animals were standing waiting for the onset of exercise) HR and HRV are displayed in Fig. 2. HR decreased (pre-post effect, P < 0.006) and HRV [either HF, P < 0.008, or SD, P < 0.0006] increased after 3 mo of treatment while these variables did not change during the 3-mo duration of the study in the placebo (P = 0.98) treated group. All three n-3 PUFA doses elicited similar reductions in HR, and increases HRV such that there were no significant differences noted between these treatments (i.e., no significant dose effect: HR, P = 0.51; HRV: HF, P = 0.66, SD, P = 0.80 or dose × pre-post interaction: HR, P = 0.28; HRV: HF, P = 0.42, SD, P = 0.10 ).
Fig. 2.
Effect of increasing doses of dietary omega-3 polyunsaturated fatty acids (n-3 PUFAs) on baseline heart rate and heart rate variability. Data were obtained at rest and the last 30 s before the onset of a submaximal exercise test. Vagal tone index is the high frequency component of the R-R interval variability (0.24 to 1.04 Hz). SD, standard deviation of the R-R interval; pre, before placebo or n-3 PUFA treatment began; post, after 3 mo of treatment. Placebo (n = 19), 1 g/day n-3 PUFA (n = 6), 2 g/day n-3 PUFA (n = 12), and 4 g/day n-3 PUFA (n = 22) are shown. Each 1-g capsule of n-3 PUFA contained 465 mg ethyl eicosapentaenoate (EPA) + ethyl docosahexaenoate (DHA) 375 mg while the placebo contained corn oil (1 g, 58% linoleic acid + 28% oleic acid). *P < 0.01, posttreatment vs. the corresponding pretreatment values.
Sham (no infarction) dogs: n-3 PUFA treatment (4 g/day) elicited reductions in HR (pre 124.8 ± 6.3 vs. post 103.2 ± 10.8 beats/min) and increases in HRV (HF: pre 5.2 ± 0.3 vs. post 7.2 ± 0.7 ln ms2; SD: pre 40.2 ± 7.2 vs. post 78.4 ± 20.8 ms) that were similar to those induced in the infarcted dogs. Thus n-3 PUFA provoked changes in resting HR and HRV that were consistent with an enhanced cardiac vagal regulation (2, 48) in both infarcted and noninfarcted animals.
Effect of n-3 PUFA on the ECG Variables, HR, and HRV Response to Acute Myocardial Ischemia
As with resting values, each dose of n-3 PUFA elicited similar changes in the HR and HRV responses to the 2-min coronary artery occlusion (no significant dose effect: HR, P = 0.63; HRV: HF, P = 0.54, SD, P = 0.10). Therefore, the n-3 PUFA data for all three doses have been combined in the subsequent analyses. The effects of placebo and the n-3 PUFA treatment on the HR and HRV (HF only) response to the acute myocardial ischemia are displayed in Fig. 3, while the effects of the coronary occlusion on ECG variables are listed in Table 3. The coronary occlusion provoked significant increases in HR (P < 10−6) and the descending portion of the T wave (P < 10−5), a marker of the dispersion of repolarization (41, 51) while eliciting decreases in PR interval (P < 10−6). No other ECG variable was affected by the ischemia. The n-3 PUFA treatment (but not placebo) elicited significant reductions in HR (P < 0.004) and increases in the PR interval (P < 0.0005), changes that were maintained during the coronary occlusion. Interestingly, preocclusion QTc interval increased in both the placebo and n-3 PUFA treatment (P < 10−5) at the end of the 3-mo treatment period compared with values obtained before the treatment began.
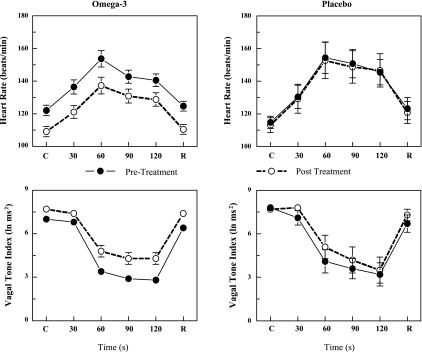
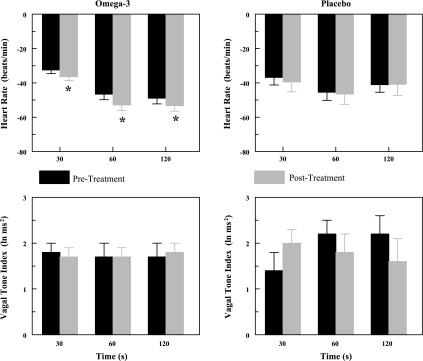
Fig. 3.
Effect of n-3 PUFAs on the heart rate and heart rate variability response to acute myocardial ischemia (2-min left circumflex coronary artery occlusion). As there were no significant differences noted between the various n-3 PUFA doses, these data have been combined. Dietary n-3 PUFA, but not the placebo, produced a significant downward shift of the heart rate response that was accompanied by an upward shift in the high frequency component of the R-R interval variability (vagal tone index: 0.24–1.04 Hz; i.e., significant pre-post treatment effect). Despite the shifts in the curves, the absolute change in these variables induced by the occlusion was not altered before or after the treatment (i.e., there was no significant pre-post treatment × ischemia interaction). Placebo (n = 15) and n-3 PUFA (1–4 g/day, n = 39) are shown. Pre, before treatment began; post, after 3 mo of treatment; C, control preocclusion; R, recovery postocclusion.
Table 3.
Effect of dietary omega-3 fatty acid on ECG parameters at rest and during coronary artery occlusion
| Pretreatment |
Posttreatment |
|||
|---|---|---|---|---|
| Control | Occlusion | Control | Occlusion | |
| Heart rate, beats/min | ||||
| Placebo | 114.8 ± 4.9 | 154.4 ± 9.7* | 115.0 ± 6.0 | 152.8 ± 11.1* |
| n-3 PUFA | 122.0 ± 3.5 | 153.8 ± 5.0* | 109.3 ± 3.2† | 137.3 ± 5.1*† |
| PR interval, ms | ||||
| Placebo | 95.1 ± 3.2 | 83.6 ± 4.1* | 100.4 ± 3.4 | 91.1 ± 3.3* |
| n-3 PUFA | 97.2 ± 2.4 | 87.8 ± 2.5* | 104.2 ± 2.3† | 96.1 ± 2.7*† |
| QRS duration, ms | ||||
| Placebo | 80.1 ± 2.7 | 79.6 ± 2.8 | 83.7 ± 2.4 | 82.4 ± 1.7 |
| n-3 PUFA | 81.4 ± 1.3 | 79.2 ± 1.5 | 82.2 ± 0.9 | 81.5 ± 1.1 |
| QTc interval, ms | ||||
| Placebo | 248.1 ± 4.3 | 251.3 ± 3.8 | 270.4 ± 3.7† | 258.9 ± 3.7† |
| n-3 PUFA | 254.6 ± 2.4 | 253.8 ± 2.6 | 271.3 ± 2.6† | 265.5 ± 3.0† |
| Tpeak-Tend (corrected), ms | ||||
| Placebo | 88.0 ± 3.8 | 95.4 ± 4.0 | 88.8 ± 2.9 | 101.4 ± 5.5* |
| n-3 PUFA | 93.1 ± 2.3 | 105.4 ± 2.6* | 89.2 ± 2.6 | 105.6 ± 3.2* |
Values are means ± SE. Heart rate correction: QTc, QT-87(60/HR-1); Tpeak-Tend(corrected), Tpeak-Tend-87(60/HR-1) (Ref. 49).
P < 0.01, control vs. coronary artery occlusion (60 s after occlusion onset).
P < 0.01, pretreatment vs. posttreatment.
In agreement with previous studies (3, 18), the coronary occlusion significantly increased HR (occlusion time effect, P < 10−6) and decreased both indexes of HRV (occlusion time effect, P < 10−6) in the placebo and in the n-3 PUFA-treated animals. HR was significantly lower (pre-post, P < 0.0005), and both HF (pre-post, P < 0.00004) and SD (data not shown pre-post, P < 0.0008) were higher during the coronary occlusion after n-3 PUFA treatment compared with values obtained before the treatment. However, the absolute change in these variables induced by myocardial ischemia (ΔHR pretreatment 31.8 ± 4.1 vs. posttreatment 28.1 ± 4.5 beats/min; ΔHF pre −3.6 ± 0.4 vs. post −2.9 ± 0.4 ln ms2; ΔSD pre −43.8 ± 5.1 vs. post −30.3 ± 4.8 ms) was not altered by the n-3 PUFA treatment (pre-post × time interaction: HR, P = 0.70; HF, P = 0.11; SD, P = 0.15). Thus n-3 PUFA treatment elicited a downward shift in resting HR and an upward shift in the resting HRV but did not alter the magnitude of the change in these variables that was induced by the coronary artery occlusion (i.e., the response to the coronary occlusion per se was not affected by the treatment). Similar results were obtained for the sham n-3 PUFA-treated dogs (data not shown).
In contrast to the results from the n-3 PUFA treatment, neither HR (pre-post, P = 0.83), nor the HF component of R-R interval variability (pre-post, P = 0.20), nor the SD of R-R interval (pre-post, P = 0.14) was different before and at the end the placebo treatment period. The change in these variables was also similar before and at the end of the placebo treatment (pre-post × time interaction: HR, P = 0.98; HF, P = 0.57; SD, P = 0.67).
Effect of n-3 PUFA on the HR and HRV Response to Submaximal Exercise
As with resting values, each dose of n-3 PUFA elicited similar changes in the HR and HRV responses to exercise (no significant dose effect: HR, P = 0.88; HRV: HF, P = 0.75, SD, P = 0.83). Therefore, the n-3 PUFA data for all three doses have been combined in all subsequent analyses. As one would predict, exercise provoked large increases in HR (exercise level effect, P < 10−6) that were accompanied by large reductions in HRV (exercise level effect, P < 10−6). As these results confirm previous studies (3), these data are not presented. Rather, the major focus in the subsequent sections has been placed on the analysis of the effects of n-3 PUFA treatment on the HR and HRV response to exercise.
Submaximal exercise.
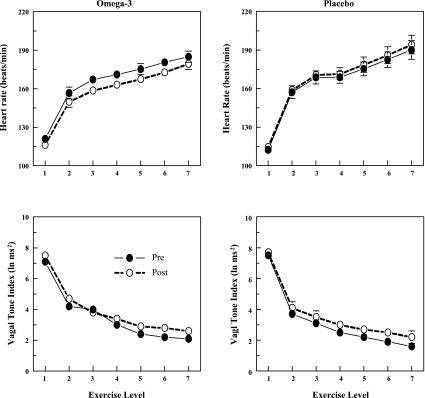
The HR and HRV response to submaximal exercise before and after 3 mo of placebo or n-3 PUFA treatment are displayed in Fig. 4. HR was significantly lower (HR, pre-post effect P < 0.03), and both HF (pre-post P < 0.02) and SD (data not shown pre-post P < 0.00004) were higher during exercise after n-3 PUFA treatment compared with values obtained before the treatment. In contrast, neither HR (pre-post HR, P = 0.41) nor the SD of R-R interval (pre-post SD, P = 0.62) was altered while HF increased at the end the placebo treatment period (pre-post, P < 0.02). The HF increase noted in the placebo-treated animals was similar to that noted in the n-3 PUFA-treated dogs (i.e., HF were not significantly different in placebo and n-3 PUFA-treated animals, P = 0.66) while HR was significantly lower in the n-3 PUFA-treated as compared in the placebo-treated dogs (P < 0.03). The changes in HR (pre 64.1 ± 4.1 vs. post 63.3 ± 45 beats/min) and HF (pre −5.1 ± 0.2 vs. post −5.0 ± 0.2 ln ms2) provoked by exercise were not affected by the n-3 PUFA treatment (no significant pre-post × exercise level interaction: HR, P = 0.72; HF, P = 0.97). In contrast, exercise elicited a significantly (pre-post × exercise level interaction P < 0.02) greater reduction in SD; following n-3 PUFA treatment resting SD increased (pre 54.4 ± 3.6 vs. post 72.9 ± 5.4 ms) but declined to a similar value at the final stage of exercise both before and after the n-3 PUFA treatment (pre 13.2 ± 1.1 vs. post 15.6 ± 1.3 ms). Thus n-3 PUFA produced a shift in the preexercise values of HR, HF, and SD that was maintained throughout the exercise period. This treatment either did not alter (HR and HF) or actually increased (greater change in SD, pre −41.2 ± 3.5 vs. post −57.3 ± 5.0 ms) the magnitude of the change in these variables that was induced by exercise. In other words, the response to exercise per se was not affected by the treatment. These data suggest that the HR reductions induced by n-3 PUFA did not result solely from changes in cardiac parasympathetic regulation. Similar results were obtained for the sham n-3 PUFA-treated dogs. HR was decreased and both HF and SD were increased at rest and during exercise by n-3 PUFA treatment (data not shown).
Fig. 4.
Effect of n-3 PUFAs on the heart rate and heart rate variability response to submaximal exercise. As there were no significant differences noted between the various n-3 PUFA doses, these data have been combined. Dietary n-3 PUFA, but not the placebo, produced a significant downward shift of the heart rate response curve that was accompanied by an upward shift in the high frequency component of the R-R interval variability (vagal tone index: 0.24 to 1.04 Hz; i.e., significant pre-post effect). Despite the shifts in the curves, the absolute change in these variables induced by the exercise was not altered before or after the treatment (i.e., there was no significant pre-post treatment × exercise interaction). Data were averaged over the last 30 s of given exercise level. Exercise levels were as follows: 1 = 0 kph and 0% grade; 2 = 4.8 kph and 0% grade; 3 = 6.4 kph and 0% grade; 4 = 6.4 kph and 4% grade; 5 = 6.4 kph and 8% grade; 6 = 6.4 kph and 12% grade; 7 = 6.4 kph and 16% grade. Pre, before placebo (n = 16) or n-3 PUFA (1–4 g/day, n = 35) treatment began; post, after 3 mo of treatment.
Exercise onset.
The changes (from preexercise levels) in HR and HRV (HF component) provoked by onset of exercise before and after 3 mo treatment with either the placebo or n-3 PUFA are displayed in Fig. 5. HR was significantly lower (HR, pre-post effect P < 0.04), and both HF (pre-post, P < 0.02) and SD (data not shown pre-post, P < 0.0002) were higher during the onset of exercise after n-3 PUFA treatment compared with values obtained before the initiation of the treatment. In contrast, neither HR (pre-post, P = 0.27), HF (pre-post, P = 0.77), nor the SD of R-R interval (pre-post, P = 0.74) was different before and at the end of the placebo treatment period. In a similar manner, the change in HR, HF, and SD provoked by exercise were not affected by either the n-3 PUFA treatment (no significant pre-post × exercise level interaction: HR, P = 0.39; HF, P = 0.16; SD, P = 0.10) or the placebo treatment (no significant pre-post × exercise: HR, P = 0.28; HF, P = 0.70; and SD, P = 0.78). Thus, although n-3 PUFA produced a shift in the baseline (preexercise onset) values of HR, HF, and SD, this treatment did not alter the change in these variables induced by the onset of exercise. Similar results were obtained for the sham n-3 PUFA-treated dogs (data not shown).
Fig. 5.
Effect of n-3 PUFAs on the change in heart rate and heart rate variability in response to the onset of submaximal exercise. As there were no significant differences noted between the various n-3 PUFA doses, these data have been combined. As indicated in Fig. 4, dietary n-3 PUFA, but not the placebo, produced a significant downward shift of the heart rate response that was accompanied by an upward shift in the high frequency component of the R-R interval variability (vagal tone index: 0.24–1.04 Hz; i.e., significant pre-post treatment effect). Despite the shifts in the curves, the absolute change in these variables induced by the exercise was not altered before or after the treatment (i.e., there was no significant pre-post treatment × exercise onset interaction). Placebo (n = 16) and n-3 PUFA (1–4 g/day, n = 35) are shown. Pre, before treatment began; post, after 3 mo of treatment.
Exercise recovery.
The changes in HR and HRV (HF) recovery following the termination of exercise before and after 3-mo treatment with either the placebo or n-3 PUFA are displayed in Fig. 6. Placebo treatment did not affect the recovery of HR (pre-post, P = 0.89), HF (P = 0.11), or SD (P = 0.74) following the termination of exercise. In contrast, HR declined more rapidly (i.e., a faster recovery, HR pre-post P < 0.001) after n-3 PUFA treatment compared with values obtained before the initiation of the treatment. The faster HR recovery from exercise after n-3 PUFA treatment was accompanied by a greater increase in SD (pre-post, P < 0.001) but not HF (pre-post, P = 0.10). In a similar manner, absolute change in HR (pre-post × time interaction P < 0.05) and in SD (pre-post × time interaction, P < 0.03) following the termination of exercise were greater, while HF (no significant pre-post × time interaction, P = 0.99) was not altered by n-3 PUFA treatment compared with values obtained before n-3 PUFA treatment. Similar results were obtained for the sham n-3 PUFA-treated dogs (data not shown).
Fig. 6.
Effect of n-3 PUFAs on the change in heart rate and heart rate variability following the termination of submaximal exercise. As there were no significant differences noted between the various n-3 PUFA doses, these data have been combined. Heart rate more rapidly declined following the termination of exercise after the n-3 PUFA but not in the placebo-treated dogs. In contrast to what was noted during exercise onset, the absolute changes in heart rate following the termination of exercise were greater after dietary n-3 PUFA treatment (i.e., significant pre-post treatment × exercise recovery interaction). However, the more rapid decline in heart rate was not accompanied by an upward shift in the high frequency component of the R-R interval variability (vagal tone index: 0.24–1.04 Hz; i.e., no significant pre-post treatment × exercise recovery interaction). Placebo (n = 16) and n-3 PUFA (1–4 g/day, n = 35) are shown. Pre, before treatment began; post, after 3 mo of treatment. *Posttreatment vs. the corresponding pretreatment time interval.
DISCUSSION
The present study investigated the effects of dietary n-3 PUFA (1–4 g/day for 3 mo) on HR and HRV both at rest (baseline conditions) and during physiological stress (exercise or acute myocardial ischemia). The major findings of the study are as follows: first, and in agreement with previous studies (11, 26), the n-3 PUFA treatment elicited dose-dependent increases in both RBC and cardiac (right atrial and left ventricular) tissue DHA and EPA content. Second, n-3 PUFA treatment induced reductions in resting HR that were accompanied by increases in HRV. Third, these changes were maintained during a physiological challenge (the peak values obtained during the stimulus were lower after n-3 PUFA treatment compared with values reached before the treatment began). However, the absolute magnitude of the change in HR and HRV provoked by either exercise or acute myocardial ischemia was not altered by n-3 PUFA treatment and was similar to that recorded for the placebo. In other words, the n-3 PUFA treatment produced parallel shifts in the response to either exercise or the coronary occlusion due to changes in resting (prechallenge) HR and HRV. These data suggest that reductions in intrinsic pacemaker rate rather than enhanced cardiac parasympathetic activity may be responsible for the n-3 PUFA-induced changes in HR. Fourth, a more rapid HR recovery following the termination of exercise was noted after n-3 PUFA treatment compared with placebo-treated animals. However, this faster HR recovery was not accompanied by corresponding increases in the HF component of R-R interval variability, data that would also suggest that cardiac vagal regulation was not affected by the n-3 PUFA treatment. Finally, similar HR and HRV changes were noted in sham (noninfarcted animals), and as such, the n-3 PUFA changes are not restricted to individuals with preexisting cardiac damage.
Effect of n-3 PUFA on Resting HR and HRV
In agreement with the present study, a number of clinical (15–17) and experimental studies (11, 31, 33, 34) report that n-3 PUFA ingestion or acute intravenous administration (9) lower HR and increase HRV, suggestive of an increase in cardiac parasympathetic regulation. The PR interval also increased following n-3 PUFA treatment in the present study, data also consistent with a parasympathetically mediated reduction in atrioventricular nodal conduction. However, there are also studies in which n-3 PUFA failed to alter either HRV or other measures of autonomic function (22, 24, 45), such as baroreceptor sensitivity (22). Furthermore, even in the studies that reported a positive action of n-3 PUFAs on HR or HRV, the effect was often quite small (36–38). For example, a meta-analysis of 30 trials found that fish oil supplements (∼3.5 g/day of EPA + DHA) reduced baseline HR by 2.5 beats/min (38), while Mozaffarian et al. (37) reported that individuals with the highest fish consumption (≥5 meals per weak) only exhibited 1.5-ms greater HRV compared with those with the lowest fish consumption. Although this difference was statistically significant, such a small change in resting HRV is not likely to be physiologically relevant. Indeed, these investigators calculated that only a 1.1% reduction in the relative risk for sudden cardiac death could be associated with this very modest increase in HRV (37). However, these small changes could have important consequences if they are maintained during a physiological stressor such as exercise or acute myocardial ischemia. Reductions in HR would reduce metabolic demand placed on the heart particularly when oxygen supply is compromised by coronary artery lesions/obstructions. The resulting better match between oxygen supply and oxygen demand would, indirectly, decrease the risk for adverse cardiac events associated with myocardial ischemia. In fact, individuals with the lowest resting HRs also exhibited the lowest long-term (>20 yr) mortality rate (30). Furthermore, the beneficial effects of β-adrenergic receptor antagonists, the most effective anti-arrhythmic medication, have been attributed to the negative chronotropic actions of these drugs (27).
Effect of n-3 PUFA on HR and Heart Variability Response to Myocardial Ischemia
As far as we are aware, the present study provides the first description of the effects of dietary n-3 PUFAs on the HR and HRV response to acute myocardial ischemia in intact unanesthetized preparations. As expected from previous studies (3, 18), the coronary artery occlusion elicited a robust HR increase that was accompanied by a rapid withdrawal in parasympathetic regulation as indicated by the decline in both total R-R interval variability (SD) and the HF component of R-R interval variability. However, despite alterations in preocclusion HR and HRV, the cardiac response to coronary artery occlusion was not altered by the n-3 PUFA treatment. The n-3 PUFA treatment produced parallel shifts in the coronary occlusion response curves (due to changes in the preocclusion HR and HRV), but the magnitude of the change in these variables was not altered by the n-3 PUFA treatment. As the robust autonomic response to the coronary occlusion was not altered, the changes in preischemic HR and HRV induced by the n-3 PUFA may be insufficient to prevent malignant changes in the cardiac rhythm. Indeed, the results from the present study are analogous to those obtained following treatment with low doses of the cholinergic antagonist atropine (23). With the use of the same canine model of myocardial infarction, low-dose atropine decreased baseline HR and increased HRV but, as in the present study, did not alter the response to myocardial ischemia (23). This intervention also failed to prevent the induction of ventricular fibrillation (23). In marked contrast, however, exercise training not only improved resting HR and HRV but also dramatically reduced the response to coronary occlusion and completely suppressed the formation of malignant ventricular tachyarrhythmias (7). When considered together, these results strongly suggest that, to be effective, an intervention must enhance cardiac parasympathetic regulation during myocardial ischemia; resting changes alone may be insufficient to protect against malignant arrhythmias.
Effect of n-3 PUFA on HR and Heart Variability Response to Exercise
In the present study, dietary n-3 PUFA treatment also elicited similar parallel shifts in the HR and HRV in the exercise HR and HF response curves as was noted during the coronary occlusion. However, exercise elicited a greater reduction in the SD in R-R interval following n-3 PUFA treatment, and preexercise SD increased but declined to a similar value at the final stage of exercise both before and after the n-3 PUFA treatment. Interestingly, the HF component of R-R interval variability exhibited a small but significant increase at the end of the 3-mo placebo period, an increase similar to that noted after n-3 PUFA treatment. Despite these similar changes in HF, the HR response to exercise was reduced in n-3 PUFA compared with placebo-treated dogs. These data suggest that the HR reductions induced by n-3 PUFA did not result solely from changes in cardiac parasympathetic regulation. In contrast to these n-3 PUFA findings, an endurance exercise training program (10-wk treadmill running) both improved resting HR and HRV and attenuated the cardiac response to submaximal exercise in the same canine model as was used in the present study (7, 8) .
There are relatively few studies that evaluated the effects of n-3 PUFA on the response to exercise in humans (13, 39, 40, 43). In agreement with the present study, dietary fish oil supplements (8-wk, 8 g/day) produced HR reductions both at rest and at peak workload in well-trained men (bicyclists) during submaximal exercise (43). In a similar manner, fish oil (5-wks, 6 g/day) reduced HR during exercise in a group of Australian rule football players (13). Finally, fish oil supplements (12 wk, 6 g/day DHA-rich tuna oil) produced small reductions in baseline HR and larger reductions in HR with increasing workload in sedentary overweight adults (39). Unlike the previous clinical studies (13, 40, 43), these investigators also evaluated the effects of the interventions on HRV (39). The fish oil supplement also increased the HF component of HRV both at rest and during exercise. The changes in HR and HRV induced by the fish oil supplements were similar to those recorded in a group of exercise-trained subjects (39). The authors concluded that fish oil supplements “reduced HR and modulated HRV in keeping with an improved parasympathetic-sympathetic balance in overweight adults” (39). These latter results contrast somewhat with the present study, in that the tuna oil supplements induced modest changes in baseline HRV and HR but also attenuated both the HR and the HRV responses to exercise. Species (dog vs. human) may account for the seemingly disparate results.
Analysis of the HR and HRV response to the onset and recovery from exercise can also provide insight into cardiac autonomic regulation (4, 47). Although preexercise onset HR decreased and HRV increased at the end of the 3-mo n-3 PUFA treatment, the changes in these variables that were induced by exercise onset were similar both before and after treatment. As far as we are aware, no other studies (either in humans or animals) have evaluated the effects of n-3 PUFA on the cardiac response to the onset of exercise. Once again, the findings reported in the present study contrast with the results obtained following a 10-wk exercise training program (8). Exercise training significantly attenuated the increase in HR but did not alter the reductions in HRV, elicited by the onset of exercise, data that suggest that cardiac sympathetic rather than cardiac parasympathetic response to exercise onset was reduced following exercise training (8).
In contrast to the response to exercise onset, n-3 PUFA treatment altered both the HR and SD of R-R interval recovery following termination of exercise. In agreement with human studies (40), HR declined more rapidly (i.e., a faster recovery), which was accompanied by a greater increase in total R-R interval variability but not the HF component of this variability (an index of cardiac parasympathetic activity) after n-3 PUFA treatment compared with values obtained before the initiation of the treatment. Thus the absolute changes in HR and the SD of R-R interval following the termination of exercise were greater, while the HF component of HRV was not altered by n-3 PUFA treatment compared with values obtained before n-3 PUFA treatment. Since the HF component of R-R interval variability was not altered by n-3 PUFA treatment, the faster return to baseline after termination of exercise in the n-3 PUFA-treated animals probably did not result as a consequence of a faster reactivation of cardiac parasympathetic activity but rather may reflect either changes in the intrinsic pacemaker rate or cardiac sympathetic activity.
Possible Mechanism of Action
The mechanisms responsible for the n-3 PUFA-induced decreases in HR and increases in HRV were not investigated in the present study and remain to be determined. However, there are at least two possible explanations for these observations: HR reductions could result from changes in cardiac autonomic regulation (enhanced parasympathetic and/or reduced sympathetic activity) and/or from alterations in intrinsic pacemaker rate. In the present study, the prechallenge (exercise or coronary occlusion) HF component of the R-R interval variability increased following 3 mo of n-3 PUFA treatment. Although there are limitations with this index (2, 28, 42), it is now generally accepted that it can provide an indirect and qualitative assessment of cardiac parasympathetic regulation (2, 48). As such, the reduction in HR that accompanies n-3 PUFA treatment could reflect an enhanced parasympathetic efferent (either of central or peripheral origin) regulation of the heart. However, as has been noted, both exercise and coronary artery occlusion elicited similar changes in HRV before and after n-3 PUFA treatment. The parallel shifts in the HRV response curves after n-3 PUFA treatment are more consistent with reductions in the intrinsic pacemaker rate than with alterations in autonomic neural regulation. If parasympathetic activity had been enhanced or sympathetic activity reduced following n-3 PUFA treatment, then one would expect smaller changes in HR and HRV during physiological interventions (as was the case following endurance exercise training; Refs. 7, 8) rather than similar changes as noted in the present study. Furthermore, an accelerated HR recovery was noted after n-3 PUFA without corresponding changes in HF power. Finally, despite similar changes in HRV, the peak HR response to exercise was attenuated in the n-3 PUFA treated but not in the placebo-treated dogs. When considered together, these data suggest that intrinsic changes in pacemaker rate may play a more important role in n-3 PUFA-induced HR reductions than do changes in cardiac autonomic regulation. Indeed, n-3 PUFA have been recently reported to reduce the pacemaker current (If) in sinoatrial node cells obtained from rabbits fed diets enriched with fish oil but not in cells isolated from the hearts of animals fed sunflower oil (50).
Limitations of the Study
In the present study, cardiac vagal nerve activity was not directly recorded. Cardiac parasympathetic regulation was only indirectly evaluated using noninvasive markers of HRV. Although a number of studies provide strong evidence that beat-to-beat fluctuation in HR reflect corresponding changes in cardiac parasympathetic regulation (2, 48), an accurate assessment of nerve activity can only be obtained from direct nerve recordings. As such, HRV data should always be interpreted with care. Second, previous studies (10, 11) demonstrate that ventricular function is not altered by myocardial infarction in the canine model used in the present study. As such, one might speculate that the potential benefits of dietary n-3 PUFA on cardiac autonomic regulation could be more obvious in individuals with more severe cardiac impairment. To the best of our knowledge, there have not been any studies that have evaluated the effects of dietary n-3 PUFA on the HRV response to exercise or acute myocardial ischemia in patients with impaired cardiac function. Future investigation will be required to determine if n-3 PUFA exhibit greater effects in these patient populations. Third, selecting an appropriate dose can prove to be problematic when comparing across species of different sizes. In the present study, if one adjusts dose by body surface area, then the 1, 2, and 4 g/day dose would be equivalent to ∼1.5, 4.9, and 9.4 g/day in human subjects. As such, these doses are somewhat higher than the dose (1 g/day) most commonly used in secondary prevention trials. However, doses up to 8 g/day n-3 PUFA have been used to evaluate the effect of n-3 PUFA on HRV in human subjects (43). In addition, n-3 PUFA treatment in the present study yielded RBC membrane concentrations within the range that were associated with a significant reduction in the risk for sudden cardiac death in epidemiological studies (1). Specifically, Albert et al. (1) found that a mean RBC concentration of 6.9% was associated with a 90% reduction in the risk for sudden death, a value that compares favorably to that obtained in the present study (after 3 mo, the 4 g/day treatment RBC concentration was ∼7.1%). Finally, although dogs and humans exhibit a similar cardiac autonomic regulation (46), species difference could also contribute to responses differences. One must always use caution when extrapolating results between species.
Conclusions and Future Directions
In the present study, dietary n-3 PUFA (DHA + EPA ethyl esters, 1–4 g/day for 3 mo) elicited dose-independent reductions in baseline HR that were accompanied by increases in HRV in dogs with and without healed myocardial infarctions. However, n-3 PUFA treatment did not alter the robust autonomic response (identical increases in HR and decreases in HRV before and after treatment) induced by either exercise or, more importantly, by myocardial ischemia. Furthermore, HR recovery following exercise was faster after n-3 PUFA without corresponding changes HF power and the peak HR obtained during exercise was lower in n-3 PUFA-treated dogs compared with placebo-treated animals despite similar HRV changes. When considered together, these data suggest that changes in cardiac autonomic regulation are not solely responsible for n-3 PUFA-induced changes in HR; alterations in the intrinsic pacemaker rate also contribute to the chronotropic actions of these fatty acids.
If n-3 PUFA-induced changes in intrinsic pacemaker rate are responsible for (or at least contribute to) the HR reductions associated with this treatment, then one can make several predictions. In vivo: 1) One would predict that selective antagonists of the pacemaker current (If) should elicit parallel shifts in the HR response to exercise or coronary occlusion similar to those noted in the present study. In fact, Zatebradine reduced both the resting HR and the HR at the end of each stage of an exercise stress test (21). 2) One would predict that after complete cardiac autonomic neural blockade, HR would be lower in n-3 PUFA treated than in placebo-treated subjects. If, however, intrinsic rate is not altered by this intervention, then alterations in autonomic regulation could play an important role in mediating the HR reduction following n-3 PUFA treatment. In vitro: 3) One would predict a reduced If current density in sinus nodal myocytes obtained from n-3 PUFA-treated dogs similar to that which has been reported in rabbit preparations (50). 4) This reduced pacemaker current could result from either reduced expression of the pacemaker channel (i.e., reduced HCN protein content) or from the posttranslational modification of this protein (trafficking, phosphorylation, oxidation, etc). 5) In contrast, if the HR reduction resulted from increased parasympathetic activity, then one would expect an increase in the acetylcholine-activated potassium current (IK-ach) accompanied by an increased expression of this channel protein and/or the muscarinic (M2) receptors (and associated signaling pathway proteins). The determination of the relative contribution of putative changes in intrinsic pacemaker rate and cardiac autonomic regulation to n-3 PUFA-mediated reductions in HR will require further investigation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-086700 (to G. E. Billman).
DISCLOSURES
William S. Harris is a scientific advisor to GlaxoSmithKline, Monsanto, and Unilever; a speaker for GlaxoSmithKline; and the owner of OmegaQuant, a company offering blood omega-3 testing. There are no other conflicts to declare.
ACKNOWLEDGMENTS
We thank Raven Morgan, Anita McKenzie, and Andrew Christianson for excellent technical assistance during the course of the studies. We also thank GlaxoSmithKline for generously providing the n-3 PUFA ethyl ester and placebo capsules for this study.
REFERENCES
- 1. Albert CM, Campos H, Stamfer MJ, Ridker PM, Mason JE, Willet WC, Ma J. Blood levels of long-chin n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113–1118, 2002 [DOI] [PubMed] [Google Scholar]
- 2. Berntson GG, Bigger JT, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HK, Proges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Pyschophysiology 34: 623–648, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Therap 111: 808–835, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Billman GE. Heart rate response to the onset of exercise: evidence for enhanced cardiac sympathetic activity in animals susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol 291: H429–H435, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Billman GE. Cardiac autonomic neural “remodeling” and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol 297: H1171–H1193, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Billman GE, Dujardin J. Dynamic changes in cardiac vagal tone as measured by time-series analysis. Am J Physiol Heart Circ Physiol 258: H896–H902, 1990 [DOI] [PubMed] [Google Scholar]
- 7. Billman GE, Kukielka M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: protection is not due to enhanced cardiac vagal regulation. J Appl Physiol 100: 896–906, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Billman GE, Kukielka M. Effect of endurance exercise training on the heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol 102: 231–240, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega-3 fatty acids. Proc Natl Acad Sci USA 91: 4427–4430, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Billman GE, Schwartz PJ, Gagnol JP, Stone HL. The cardiac response to submaximal exercise in dogs susceptible to sudden cardiac death. J Appl Physiol 59: 890–897, 1985 [DOI] [PubMed] [Google Scholar]
- 11. Billman GE, Nishijima Y, Belevych AE, Terentyev D, Xu Y, Haizlip KM, Monasky MM, Hiranandani N, Harris WS, Gyorke S, Carnes CA, Janssen PML. Effects of dietary omega-3 fatty acids on ventricular function in dogs with healed myocardial infarctions. Am J Physiol Heart Circ Physiol 298: H1219–H1228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bligh EG, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 13. Buckley JD, Burgess S, Murphy KJ, Howe PRC. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J Sci Med Sport 12: 503–507, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Casadei B, Pipillis A, Sessa F, Conway J, Sleight P. Low doses of scopolamine increase cardiac vagal tone in the acute phase of myocardial infarction. Circulation 88: 353–357, 1993 [DOI] [PubMed] [Google Scholar]
- 15. Carney RM, Freedland KE, Stein PK, Steinmeyer BC, Harris WS, Rubin EH, Krone RJ, Rich WW. Effect of omega-3 fatty acids on heart rate variability in depressed patients with coronary heart disease. Psychosom Med 72: 748–754, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christensen JH, Schmidt EB. Autonomic nervous system, heart rate variability and n-3 fatty acids. J Cardiovasc Med 8, Suppl 1: S19–S22, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Christensen JH, Christensen MS, Dyerberg J, Schmidt EB. Heart rate variability and fatty acid content in blood cell membranes: a dose-response study with n-3 fatty acids. Am J Clin Nutr 70: 331–337, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Collins MN, Billman GE. Autonomic response to coronary occlusion in animals susceptible to ventricular fibrillation. Am J Physiol Heart Circ Physiol 257: H1886–H1894, 1989 [DOI] [PubMed] [Google Scholar]
- 19. De Ferrari GM, Mantica M, Vanoli E, Hull SS, Jr, Schwartz PJ. Scopolamine increases vagal tone and vagal reflexes in patients after myocardial infarction. J Am Coll Cardiol 22: 1327–1334, 1993 [DOI] [PubMed] [Google Scholar]
- 20. Di Stasi D, Bernasconi R, Marchioli R, Marfisi RM, Rossi G, Tognoni G, Tacconi MT. Early modifications of fatty acid composition in plasma phospholipids, platelets and mononucleates of healthy volunteers after low doses of n-3 polyunsaturated fatty acids. Eur J Clin Pharmacol 60: 183–190, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Frishman WH, Pepine CJ, Weiss RJ, Baiker WM, for the Zatebradine Study Group Addition of Zatebradine, a direct sinus node inhibitor, provides no greater exercise tolerance benefit in patients with angina taking extended-release nifedipine: results of multicenter, randomized double-blind, placebo-controlled, parallel-group study. J Am Coll Cardiol 26: 305–312, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Geelen A, Zock PL, Swenne CA, Brouwer IA, Schouten EG, Katan MB. Effect of n-3 fatty acids on heart rate variability and baroreflex sensitivity in middle-aged subjects. Am Heart J 146: E4, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Halliwill JR, Billman GE, Eckberg DL. Effect of a vagomimetic atropine dose on canine cardiac vagal tone and susceptibility to sudden cardiac death. Clin Auton Res 8: 155–164, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Hamaad A, Lee WK, Lip GYH, MacFadyen RJ. Oral omega-3 PUFA therapy (Omacor) has no impact on indices of heart rate variability in stable post myocardial infarction patients. Cardiovasc Drug Ther 20: 359–364, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Harris WS, Gonzles M, Laney N, Sastre A, Borkon AM. Effects of omega-3 fatty acids on heart rate in cardiac transplant recipients. Am J Cardiol 98: 1393–1395, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 110: 1645–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Held PH, Yusuf S. Effects of beta-blockers and Ca2+ channel blockers in acute myocardial infarction. Eur Heart J 14, Suppl F: 18–25, 1993 [DOI] [PubMed] [Google Scholar]
- 28. Houle MS, Billman GE. Low-frequency component of the heart rate variability spectrum: a poor marker of sympathetic activity. Am J Physiol Heart Circ Physiol 276: H215–H223, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Hull SS, Jr, Vanoli E, Adamson PB, De Ferrari GM, Foreman RD, Schwartz PJ. Do increases in markers of vagal activity imply protection from sudden death? The case of scopolamine. Circulation 91: 2516–2519, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Jouven X, Empana JP, Escolano S, Buyck JF, Tafflet M, Desnos M, Ducimetiere P. Relation of heart rate at rest and long-term (>20 years) death rate in initially healthy middle-aged men. Am J Cardiol 103: 279–283, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Kang Leaf A JX. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proc Natl Acad Sci USA 91: 9886–9890, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kottmeier CA, Gravenstein JS. The parasympathomimetic activity of atropine and atropine methylbromide. Anesthesiology 29: 1125–1133, 1968 [DOI] [PubMed] [Google Scholar]
- 33. Laustiola K, Salo MK, Metsä-Ketelä T. Altered physiological responses and decreased cyclic AMP levels in rat atria after dietary cod liver oil supplementation and its possible association with n3/n6 fatty acid ratio. Biochim Biophys Acta 889: 59–79, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Mayyas F, Sakurai S, Ram R, Rennison JH, Hwang ES, Castel L, Lovano B, Brennan ML, Bibus D, Lands B, Barnard J, Chung MK, Van Wagoner DR. Dietary ω3 fatty acids modulate the substrate for post-operative atrial fibrillation in a canine cardiac surgery model. Cardiovasc Res 89: 852–861, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J Lipid Res 5: 600–608, 1964 [PubMed] [Google Scholar]
- 36. Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol 48: 478–484, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Mozaffarian D, Stein PK, Prineas RJ, Siscovick DS. Dietary fish ω−3 fatty acid consumption and heart rate variability in US adults. Circulation 117: 1130–1137, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: meta-analysis of randomized controlled trials. Circulation 112: 1945–1952, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Ninio DM, Hill AM, Howe PR, Buckley JD, Saint DA. Docosahexaenoic acid-rich fish oil improves heart variability and heart rate responses to exercise in overweight adults. Br J Nutr 100: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 40. O'Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fraction. Am J Cardiol 97: 1127–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Opthof T, Coronel R, Wilms-Schopman FJG, Plotnikov AN, Shlapakova IN, Danilo P, Jr, Rosen MR, Janse MJ. Dispersion of repolarization in canine ventricle and electrocardiographic T wav: Tp-e interval does not reflect transmural dispersion. Heart Rhythm 4: 341–348, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Parati G, di Rienzo M, Castiglioni P, Mancia G, Taylor JA, Studinger P. Point: cardiovascular variability is/is not an index of autonomic control of circulation. J Appl Physiol 101: 676–682, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Peoples GE, McLennan PL, Howe PRC, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol 52: 540–547, 2008 [DOI] [PubMed] [Google Scholar]
- 44. Porges SW. Respiratory sinus arrhythmia: physiological basis, quantitative methods and clinical implications. In: Cardiac, Respiratory, and Cardiosomatic Psychophysiology. New York: Plenum, 1986, p. 101–115 [Google Scholar]
- 45. Russo C, Olivieri O, Girelli D, Azzini M, Stanzial AM, Guarini P, Friso S, De Franceschi L, Corrocher R. Omega-3 polyunsaturated fatty acid supplements and ambulatory blood pressure monitoring parameters in patients with mild hypertension. J Hypertens 13: 1823–1826, 1995 [PubMed] [Google Scholar]
- 46. Scher AM, Ohm WW, Bumgarner K, Boynton R, Young AC. Sympathetic and parasympathetic control of heart rate in the dog, baboon and man. Fed Proc 31: 1219–1225, 1972 [PubMed] [Google Scholar]
- 47. Smith LL, Kukielka M, Billman GE. Heart rate recovery after exercise: a predictor of ventricular fibrillation susceptibility after myocardial infarction. Am J Physiol Heart Circ Physiol 288: H1763–H1769, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Task Force of the European Society of Cardiology, and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93: 1043–1065, 1996 [PubMed] [Google Scholar]
- 49. Van de Water A, Verheyen J, Xhonnex R, Reneman RS. An improved method to correct QT intrerval of the electrocardiogram from changes in heart rate. J Pharmacol Methods 22: 207–217, 1989 [DOI] [PubMed] [Google Scholar]
- 50. Verkerk AO, den Ruijter HM, Bourier J, Boukens BJ, Brouwer IA, Wilders R, Coronel R. Dietary fish oil reduces pacemaker current and heart rate in rabbit. Heart Rhythm 6: 1485–1492, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation 98: 1928–1936, 1998 [DOI] [PubMed] [Google Scholar]