Abstract
Inward eutrophic remodeling is the most prevalent structural change of resistance arteries in hypertension. Sympathetic and angiotensin (ANG)-induced vasoconstriction has been associated with hypertension and with the production of matrix metalloproteinases (MMPs) and ROS. Therefore, we hypothesize that prolonged exposure to norepinephrine (NE) and ANG II induces arteriolar inward remodeling dependent on the activation of MMPs and the production of ROS. This hypothesis was tested on rat cremaster arterioles that were isolated, cannulated, pressurized, and exposed to either NE (10−5.5 mol/l) + ANG II (10−7 mol/l) or vehicle (control) for 4 h. The prolonged exposure to NE + ANG II induced inward remodeling, as evidenced by the reduced maximal arteriolar passive diameter observed after versus before exposure to the vasoconstrictor agonists. NE + ANG II also increased the arteriolar expression and activity of MMP-2 and the production of ROS as determined, respectively, by real-time RT-PCR, gel and in situ zymography, and the use of ROS-sensitive dyes with multiphoton microscopy. Inhibition of MMP activation (with GM-6001) or ROS production (with apocynin or tempol) prevented the NE + ANG II-induced inward remodeling. Inhibition of ROS production prevented the activation of MMPs and the remodeling process, whereas inhibition of MMP activation did not affect ROS production. These results indicate that prolonged stimulation of resistance arterioles with NE + ANG II induces a ROS-dependent activation of MMPs necessary for the development of arteriolar inward remodeling. These mechanisms may contribute to the structural narrowing of resistance vessels in hypertension.
Keywords: arterioles, structural narrowing, hypertension, oxidative stress
inward eutrophic remodeling is the most common structural change observed in resistance arteries and arterioles of individuals with essential hypertension (14, 22, 36, 43). Vessels with this type of remodeling have a reduced passive luminal diameter with no significant change in wall cross-sectional area. The presence of inward eutrophic remodeling in hypertensive individuals has been associated with an increased incidence of life-threatening cardiovascular events (36, 43), and although it has been shown to predict cardiovascular risk better than plasma cholesterol levels and pulse pressure amplitude, the stimuli that initiate the remodeling process and the mechanisms that control it remain incompletely understood.
Studies in essential hypertensive individuals, animal models of hypertension, and isolated vascular preparations have suggested that prolonged vasoconstriction rather than prolonged intravascular pressure augmentation is the main stimulus responsible for inducing inward eutrophic remodeling. Hypertensive individuals treated with antihypertensive drugs that reduce cardiac output but do not affect vascular tone demonstrate a successful normalization of arterial blood pressure with no amelioration of the inward remodeling process (46, 47). In comparison, normalization of arterial pressure with drugs that induce vasodilation, thereby reducing vascular tone, successfully eliminates the inward eutrophic remodeling of arterioles (11, 45). Similar results have been reported in animal models of hypertension (9, 10, 13). In vitro, we and others (1, 33) have demonstrated that prolonged exposure to a number of vasoconstrictor agonists is sufficient to induce inward eutrophic remodeling in isolated arterioles. Other in vitro studies (1, 2) have also suggested that prolonged vasoconstriction rather than increased intravascular pressure is the main stimulus responsible for inducing inward eutrophic remodeling.
Essential hypertension is consistently associated with augmented sympathetic outflow and activation of the renin-angiotensin (ANG) system (26). At the vascular level, this implies that cells are exposed to increased levels of stimulation by norepinephrine (NE) and ANG II. These vasoconstrictor agonists also induce the activation of matrix metalloproteinases (MMPs) and the production of ROS (4, 5, 17, 20, 21, 54). In conduit arteries, both MMPs and ROS have been implicated in the hypertrophic remodeling process associated with hypertension, but their role in the inward eutrophic remodeling process that occurs in resistance arteries is not known (6, 7). Therefore, we designed the present study to test the hypothesis that MMPs and ROS play a role in the development of inward eutrophic remodeling of resistance arteries exposed to NE and ANG II.
MATERIALS AND METHODS
Animals
The Animal Care and Use Committee of the University of Missouri (Columbia, MO) approved all procedures involving animals in this study. Male Sprague-Dawley rats (250–350 g) were used in the experiments. Before experimentation, rats had ad libitum access to standard rat chow and water for consumption while housed in pairs under a 12 h/day illumination regimen.
Isolated Vessel Preparation
Rats were anesthetized with an intraperitoneal injection of pentobarbital sodium (100 mg/kg). After surgical plane anesthesia was confirmed by loss of spinal reflexes, the right and/or left cremaster muscles were excised and pinned flat in a refrigerated (4°C) dissecting chamber containing physiological saline solution (PSS) [composed of (in mmol/l) 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 1.0 NaH2PO4, 5.0 dextrose, 3.0 MOPS buffer, 2.0 pyruvate, 0.02 EDTA, and 0.15 BSA; pH 7.4]. A segment of the first-order (1A) feed arteriole from the cremaster muscle was isolated, removed, and placed in a cannulation and observation chamber (Living Systems Instrumentation, Burlington, VE) filled with MOPS-PSS without albumin as previously described (35). Isolated arterioles were cannulated onto glass micropipettes filled with PSS with albumin. The upstream end of the arteriole was tied first onto an open pipette to remove red blood cells within the vascular lumen by application of gentle positive pressure (≤20 mmHg) and flow. The downstream end of the arteriole was then cannulated and tied onto a closed micropipette. Arterioles were gradually pressurized to their in situ pressure of 60 mmHg (38) using a Pressure Servo System (Living Systems Instrumentation) and warmed to 34.5°C over 60 min. The chamber with the cannulated arteriole was transferred to the stage of an inverted microscope equipped with a video display and video caliper to record measurements of vessel luminal diameter (34) or the stage of a confocal-multiphoton microscopy system (Leica TCS SP5).
Experimental Protocols
Effect of prolonged exposure to NE + ANG II.
Isolated arterioles were subjected to a protocol designed to test the effects of prolonged exposure to NE + ANG II on vascular remodeling. Only vessels that developed spontaneous myogenic tone were included in the experiments. The protocol consisted of a first exposure to 10−4 M adenosine to dilate the vessel followed by incubation in Ca2+-free PSS containing 2 mM EGTA and adenosine (10−4 M) to obtain maximum passive diameter. Vessels were then allowed to regain basal spontaneous tone in Ca2+-containing solution followed by a 4-h exposure to NE (10−5.5 M) + ANG II (10−7 M). The protocol was concluded by washing the vessels for 30 min to remove the vasoconstrictor agonists followed by an exposure to adenosine (10−4 M) and then to Ca2+-free solution. A reduction in passive diameter upon the second exposure to Ca2+-free PSS compared with the first exposure was evidence of inward remodeling (33).
Effect of MMP and ROS inhibition on prolonged NE + ANG II exposure and remodeling.
In these experiments, arterioles were subjected to a prolonged exposure to the NE + ANG II protocol except that once arterioles had reestablished a stable basal tone after the initial exposure to adenosine and Ca2+-free solution, they were exposed separately to either GM-6001 (1.5 or 15 μM) to inhibit MMPs (39), apocynin (300 μM) to inhibit NADPH oxidase (56) and ROS (25), tempol (250 μM) to dismutate superoxide and scavenge H2O2 (32), or vehicle control. Any effects on basal tone occurring during the initial incubation period (20 min) with the MMP or ROS inhibitors were recorded. After the initial 20-min incubation with an inhibitor, vessels were subjected to a prolonged exposure to NE + ANG II while in the presence of the inhibitor. The protocol was concluded by washing the vessels for 30 min to remove the vasoconstrictor agonists followed by an exposure to adenosine (10−4 M) and then to Ca2+-free solution.
Detection of MMP activity.
IN SITU ZYMOGRAPHY.
In these experiments, after arterioles had established a stable basal tone, they were exposed for the rest of the experiment to DQ gelatin (10 μg/ml), a highly quenched fluorescein-labeled gelatin that, upon digestion by gelatinases MMP-2 and MMP-9, becomes bright green fluorescent. DQ gelatin was applied alone or in combination with NE + ANG II. Immediately (5 min) after the application of DQ gelatin, arterioles were imaged with a multiphoton microscope (Leica TCS SP5) using a ×63 water-immersion 1.2 numerical aperture objective and the following parameters: excitation wavelength of 800 nm, emission detection with nondescanned detectors at a wavelength of 500–550 nm, and z-sections at 2 μm to cover the whole vessel. Images with the same parameters were taken every hour for 4 h. Four-dimensional reconstructions were performed using Imaris software (Bitplane). The fluorescence intensity was calculated for every three-dimensional image of the same dimensions that included the whole vessel. Changes in fluorescence intensity over time were calculated and expressed as percent changes in fluorescence from the first image taken 5 min after the initial exposure to DQ gelatin. In an additional series of experiments, arterioles were incubated with GM-6001, tempol, apocynin, or vehicle control 20 min before and throughout the exposure to DQ gelatin with and without NE + ANG II to determine the effect of MMP and ROS inhibition on the arteriolar in situ gelatinolytic activity.
GEL ZYMOGRAPHY.
In these experiments, control arterioles and arterioles exposed for 4 h to NE + ANG II with or without additional inhibitors were snap frozen in liquid nitrogen and homogenized in extraction buffer containing 10 mM Tris (pH 6.8), 7 M urea, 10% glycerol, 1% SDS, and protease inhibitors. After extraction, samples with an equal amount of protein were combined with sample buffer (Bio-Rad) under nonreducing conditions in a 1:1 ratio and loaded on a 10% polyacrilamide gel containing 0.1% gelatin (Bio-Rad). The same amount of total protein was loaded in each well. After electrophoresis, gels were incubated first in renaturing buffer (Bio-Rad) for 30 min and second in development buffer (Bio-Rad) at 37°C for 7–9 days. After being developed, gels were stained with brilliant blue and destained with a 50:40:10 mixture of methanol, water, and acetic acid for visualization of the bands where gelatin had been digested. Densitometric analyses of gelatinolytic bands were performed using a Bio-Rad ChemiDoc and Bio-Rad ImageLab software. Data were collected in arbitrary densitometric units and expressed as fold changes from the respective control.
Detection of ROS.
Two ROS-sensitive fluorescent probes were used to assess intracellular ROS activity in isolated arterioles. Dihydroethidium (DHE) was used to detect superoxide, and 5- (and 6-)carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH) was used to detect H2O2 (28). In these experiments, arterioles with spontaneous myogenic tone were exposed for the rest of the experiment to either 5 μM DHE or 30 μM DCFH alone or in combination with NE + ANG II. Immediately (5 min) after the application of DHE or DCFH, arterioles were imaged with a multiphoton microscope (Leica TCS SP5) using a ×63 water-immersion 1.2 numerical aperture objective and the following parameters: excitation wavelength of 800 nm, emission detection wavelength of 600–700 nm for DHE or 500–600 nm for DCFH, and z-sections at 2 μm to cover the whole vessel. Images with the same parameters were taken every hour for 4 h. Four-dimensional reconstructions were performed using Imaris software (Bitplane). The fluorescence intensity was calculated for every three-dimensional image of the same dimensions that included the whole vessel. The background fluorescence intensity, obtained from the areas outside the arteriole, was calculated and subtracted from every three-dimensional image that included the whole vessel. Changes in fluorescence intensity of the arteriole over time were calculated and expressed as percent changes in fluorescence from the first image taken 5 min after the initial exposure to DHE or DCFH. In an additional series of experiments, arterioles were incubated with GM-6001, tempol, apocynin, or vehicle control 20 min before and throughout the exposure to DHE or DCFH to determine the effect of MMP and ROS inhibition on the intracellular activity of ROS.
Detection of the MMP message for expression by real-time RT-PCR.
These experiments were performed to determine the level of message for the expression of gelatinases MMP-2 and MMP-9 in control arterioles and in arterioles exposed for 4 h to NE + ANG II. For this purpose, arterioles were snap frozen in liquid nitrogen immediately after the end of the 4-h incubation protocol. Arteriolar RNA was extracted with an Ambion MELT total nucleic acid isolation system. RNA was reverse transcribed with Advantage RT-for-PCR (Clontech) and amplified by PCR. Real-time PCR assays were performed using a Cepheid Smart Cycler system and SYBR Premix Ex Taq (Takara). Primers were purchased from Realtimeprimers.com. Primer sequences were as follows: MMP-2, forward 5′-AAGATGTGGCAACCCAGATG-3′ and reverse 5′-ACTTTTAAGGCCCGAGCAAA-3′; MMP-9, forward 5′-ACTTCTGGCGTGTGAGTTTC-3′ and reverse 5′-TGTATCCGGCAAACTAGCTC-3′; and GAPDH, forward 5′-AGACAGCCGCATCTTCTTGT-3′ and reverse 5′-CTTGCCGTGGGTAGAGTCAT-3′. MMP expression was normalized to GAPDH expression for each sample. Expression of MMP in the NE + ANG II-treated arterioles was determined relative to expression in control arterioles not exposed to NE + ANG II using the 2−ΔΔCT method for analysis of relative changes in gene expression, where CT is threshold cycle (30).
Chemicals
All drugs and chemicals were acquired from Sigma unless otherwise indicated. Adenosine was prepared as a stock solution at a concentration of 10−2 M in PSS and added into the vessel bath to reach a concentration of 10−4 M. NE was freshly prepared, first as a stock solution at 10−2 M in light-protected vials and then further diluted to 10−5.5 M as needed throughout the experiment. ANG II was first dissolved in double-distilled H2O to make a stock solution at a concentration of 10−3 M and further diluted to 10−7 M in PSS as needed during the experiment. DQ gelatin (Invitrogen) was diluted in double-distilled H2O to make a stock solution at a concentration of 10 mg/ml and further diluted in PSS to make a final working concentration of 10 μg/ml as needed. GM-6001 was acquired from Chemicon (Millipore) as a 2.5 mM solution in DMSO and further diluted to 1.5 or 15 μM in PSS as needed. Apocynin (Calbiochem) was diluted in DMSO to make a stock solution at 60 mM and further diluted in PSS to make a final working concentration of 300 μM as needed. Tempol was prepared as a stock solution at a concentration of 50 mM in PSS and added into the vessel bath to reach a concentration of 250 μM. DHE (invitrogen) was first diluted in DMSO to make a stock solution of 5 mM, aliquoted in light-protected vials at 25 μl, and immediately lyophilized. On the day of the experiment, each vial was reconstituted with 25 μl DMSO and further diluted to a final concentration of 5 μM in PSS as needed. DCFH (Invitrogen) was first diluted in DMSO at a concentration of 15 mM and further diluted in PSS to make a final working solution at 30 μM.
Data Analyses
Data are presented as means ± SE. Diameters are expressed as percentages of the maximal diameter obtained during the first exposure to Ca2+-free PSS plus adenosine or as percent changes in diameter from the original diameter obtained under the same conditions before the prolonged exposure to NE + ANG II. In situ gelatinolytic activity and in situ ROS activity are expressed as changes in fluorescence from the first fluorescence intensity obtained after 5 min of fluorophore exposure to the fluorescence intensity obtained after 4 h of incubation with the fluorophore. The gelatinolytic activity of MMP-2 in the gel zymograms was obtained in arbitrary densitometric units with a Bio-Rad ChemiDoc XRS and Bio-Rad ImageLab software and expressed as fold changes from its respective control. Paired t-tests were performed to determine the effect of prolonged exposure to NE + ANG II on basal tone, adenosine-mediated dilation, and maximal passive diameter within vessels under the same treatment. Across experimental treatments, comparisons were made by ANOVA followed by Fisher's protected least-significant-difference test. P values of ≤0.05 were considered significant.
RESULTS
Prolonged Exposure to NE + ANG II Induces Inward Eutrophic Remodeling, Activates MMPs, and Generates ROS
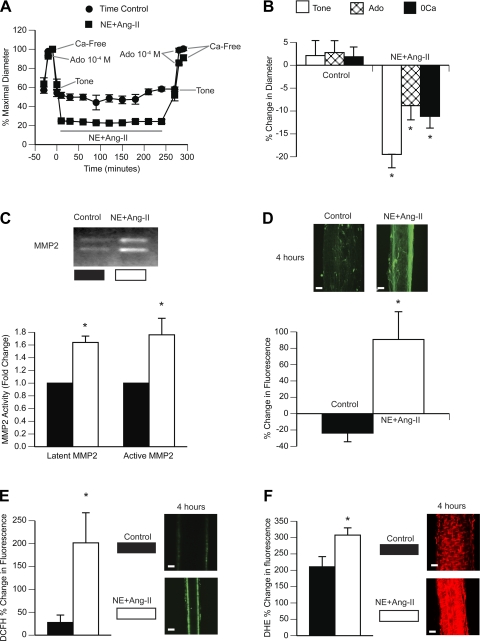
NE + ANG II induced consistent and continuous vasoconstriction of arterioles throughout the 4-h exposure to the agonists (Fig. 1A). When the agonists were washed from the vessels for 30 min, arterioles remained partially constricted, indicating a greater level of spontaneous tone compared with the level of spontaneous tone they had before exposure to the agonists (Fig. 1B). The diameters observed upon exposure to adenosine and Ca2+-free solution were also significantly smaller after the prolonged exposure to NE + ANG II than before exposure to the vasoconstrictor agonists (Fig. 1B). This indicated that 4 h of exposure to NE + ANG II induced inward remodeling in isolated arterioles. Arterioles maintained for 4 h under similar conditions but not exposed to NE + ANG II did not remodel (Fig. 1, A and B).
Fig. 1.
Prolonged exposure to norepinephrine (NE) + ANG II induces inward remodeling, activates matrix metalloproteinases (MMPs), and generates ROS in isolated, cannulated, and pressurized rat cremaster arterioles. A: arterioles were exposed to NE (10−5.5 M) + ANG II (10−7 M) for 4 h (n = 5) or maintained pressurized for the same period (n = 5; time control). Before and after the 4-h incubation with or without NE + ANG II, arterioles were allowed to develop spontaneous myogenic tone and were exposed to adenosine (Ado; 10−4 M) and then to Ca2+-free solution. Data are expressed as percentages of the maximal diameter obtained under Ca2+-free conditions before the 4-h incubation with or without the vasoconstrictor agonists. B: comparisons were made between control (n = 5) and NE + ANG II-exposed (n = 5) arterioles on the percent changes in diameter occurring after versus before the 4-h incubation period at spontaneous tone, Ado dilation, and maximal passive diameter (Ca2+-free conditions; 0Ca). C: pools (n = 3) of four control or four NE + ANG II-treated arterioles were subjected to gel zymography. Bands representing gelatinolytic activity were analyzed by densitometry and expressed as fold changes from the control for the activity of latent (72 kDa) and active (64 kDa) forms of MMP-2. Contrast was optimized for the best visualization of the bands on the digitized image of the zymogram gel. D: arterioles incubated for 4 h with (n = 4) or without (n = 4) NE + ANG II were coincubated with DQ gelatin (10 μg/ml). Three-dimensional (3-D) images of the arterioles were obtained with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in fluorescence intensity of 3-D images from 5 min to 4 h of incubation. E: arterioles with (n = 4) or without (n = 4) NE + ANG II were coincubated with 5- (and 6-)carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCFH; 30 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative images (bars = 20 μm) are optical sections taken at the mid-diameter plane of one control and one NE + ANG II-treated arteriole at 4 h of incubation. These mid-diameter optical sections show that fluorescence comes from within the vascular wall. The bar graph represents the percent changes in DCFH fluorescence intensity from 5 min to 4 h of incubation. F: arterioles with (n = 4) or without (n = 4) NE + ANG II were coincubated with dihydroethidium (DHE; 5 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) are shown of one control and one NE + ANG II-treated arteriole at 4 h of incubation. The bar graph represents the percent changes in DHE fluorescence intensity from 5 min to 4 h of incubation. All data are means ± SE. *P ≤ 0.05 compared with control.
In addition to inducing inward remodeling, prolonged exposure to NE + ANG II also induced the production and activation of MMP-2, as detected by real-time RT-PCR and gel zymography. Compared with control arterioles maintained cannulated and pressurized for 4 h but not exposed to any agonists, arterioles exposed to NE + ANG II had a 2.19 ± 0.02-fold (n = 3) increase in message for MMP-2 expression. MMP-9 was not detectable. Arterioles exposed to NE + ANG II also had greater activities of both latent (72 kDa) and active (64 kDa) MMP-2 bands in zymogram gels compared with control arterioles (Fig. 1C). To determine the net activity of the gelatin metalloproteinases in the intact arteriolar wall, we also performed in situ zymography. In situ zymography, arterioles exposed to NE + ANG II had greater gelatinolytic activity than controls, as measured by the increased lysis and fluorescence emission of DQ gelatin during the 4-h exposure to the vasoconstrictor agonists (Fig. 1D).
Production of ROS also increased in response to the prolonged exposure to NE + ANG II. The intracellular fluorescence emitted by DCFH in arterioles exposed for 4 h to NE + ANG II increased at a rate nearly 100 times greater than in control arterioles not exposed to the vasoconstrictor agonists (Fig. 1E). This suggests that NE + ANG II significantly augmented the intracellular production of H2O2. The rate of increase in intracellular fluorescence emitted by arterioles loaded with DHE and exposed to NE + ANG II was also greater than that in arterioles not exposed to NE + ANG II (Fig. 1F). Because fluorescence emitted by DHE is predominantly related to the production of superoxide, these results indicate that prolonged exposure to NE + ANG II increased the production of superoxide anions in the wall of isolated arterioles.
MMP Inhibition Prevents the Development of Inward Remodeling Induced by Prolonged Exposure to NE + ANG II
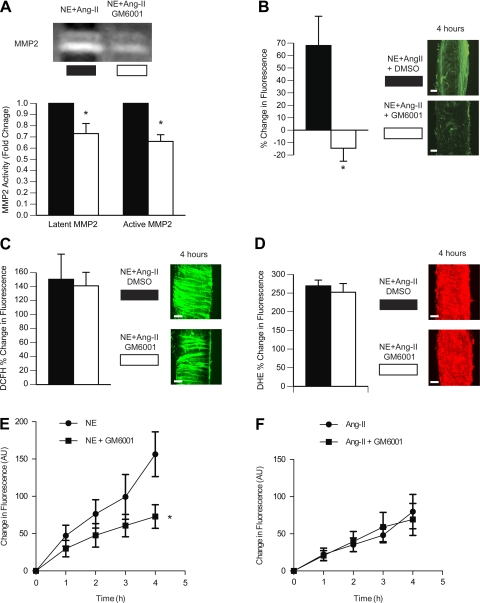
Incubation of arterioles with a broad-spectrum inhibitor of MMPs, GM-6001 (1.5 and 15 μmol/l), prevented the 4-h incubation with NE + ANG II from inducing inward remodeling (Fig. 2). At a concentration of 15 μmol/l, GM-6001 also prevented the augmented level of tone and the reduced level of vasodilation to adenosine observed in arterioles exposed to the vasoconstrictor agonists without GM-6001. The initial maximal (within 5 min) vasoconstrictor response to NE + ANG II was reduced (∼50%) by GM-6001 at a concentration of 15 μmol/l but not at 1.5 μmol/l. However, at 1.5 μmol/l, GM-6001 caused the vasoconstriction to NE + ANG II to wane over time. After 4 h of incubation with NE + ANG II while in the presence of the vasoconstrictor agonists, only arterioles exposed to 0.06% DMSO (vehicle control) remained fully constricted. All other arterioles had significantly greater diameters.
Fig. 2.
MMP inhibition prevents the development of inward remodeling induced by prolonged exposure to NE + ANG II. A: arterioles were incubated with NE (10−5.5 M) + ANG II (10−7 M) for 4 h in the presence of 0.06% DMSO (vehicle control; n = 6) or 1.5 μM GM-6001 (n = 7). Before and after the 4-h incubation, arterioles were allowed to develop spontaneous myogenic tone and exposed to Ado (10−4 M) and then to Ca2+-free solution. Data are expressed as percentages of the maximal diameter obtained under Ca2+-free conditions before the 4-h incubation period. B: comparisons were made between incubation treatments (NE + ANG II + 0.06% DMSO, n = 6; NE + ANG II + 1.5 μM GM6001, n = 7) on the percent changes in diameter occurring after versus before the 4-h incubation period at spontaneous tone, Ado dilation, and maximal passive diameter (Ca2+-free conditions). *P ≤ 0.05 compared with DMSO 0.06%. C: arterioles were incubated with NE (10−5.5 M) + ANG II (10−7 M) for 4 h in the presence of 0.6% DMSO (n = 7) or 15 μM GM-6001 (n = 6). Before and after the 4-h incubation, arterioles were allowed to develop spontaneous myogenic tone and exposed to Ado (10−4 M) and then to Ca2+-free solution. Data are expressed as percentages of the maximal diameter obtained under Ca2+-free conditions before the 4-h incubation period. D: comparisons were made between incubation treatments (NE + ANG II + 0.6% DMSO, n = 7; NE + ANG II + 15 μM GM-6001, n = 6) on the percent changes in diameter occurring after versus before the 4-h incubation period at spontaneous tone, Ado dilation, and maximal passive diameter (Ca2+-free conditions). *P ≤ 0.05 compared with DMSO 0.6%.
Incubation of arterioles with GM-6001 prevented the activation of MMPs, as indicated by the reduced activity of MMP-2 in gel zymography and the reduced fluorescence of DQ gelatin (i.e., gelatinolytic activity) in situ (Fig. 3, A and B). Importantly, incubation with GM-6001 or its vehicle did not significantly affect the increased production of ROS induced by the prolonged incubation with NE + ANG II (Fig. 3, C and D). As NE and ANG II exert their actions in part via an MMP-dependent transactivation of growth factor receptors and downstream production of superoxide (21, 55), we performed a series of experiments to determine the effect of MMP inhibition with GM-6001 on the production of superoxide induced by incubation with either ANG II or NE alone. DHE-dependent fluorescence indicated that, at the concentrations used, NE caused a 19% greater increase in superoxide production than ANG II (Fig. 3, E and F). Inhibition of MMP activity with 15 μmol/l GM-6001 significantly blunted the DHE-dependent fluorescence in NE-exposed arterioles (Fig. 3E) but not in ANG II-exposed arterioles (Fig. 3F).
Fig. 3.
MMP inhibition diminished the superoxide generated by NE stimulation and the gelatinolytic activity induced by prolonged exposure to NE + ANG II but not the superoxide generated by stimulation with ANG II alone or in combination with NE. A: pools (n = 3) of arterioles from NE + ANG II + 0.6% DMSO or NE + ANG II + 1.5 μM GM-6001 incubation treatments were subjected to gel zymography. Bands were analyzed by densitometry and expressed as fold changes from the DMSO (vehicle control) treatment for the activity of latent (72 kDa) and active (64 kDa) forms of MMP-2. Contrast was optimized for the best visualization of the bands on the digitized image of the zymogram gel. B: arterioles incubated for 4 h with NE + ANG II + 0.6% DMSO (n = 4) or NE + ANG II + 15 μM GM-6001 (n = 4) were coincubated with DQ gelatin (10 μg/ml). 3-D images of the arterioles were obtained with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in fluorescence intensity of 3-D images from 5 min to 4 h of incubation. C: arterioles incubated with NE + ANG II + 0.6% DMSO (n = 4) or NE + ANG II + 15 μM GM-6001 (n = 4) were coincubated with DCFH (30 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in DCFH fluorescence intensity from 5 min to 4 h of incubation. D: arterioles incubated with NE + ANG II + 0.6% DMSO (n = 4) or NE + ANG II + 15 μM GM-6001 (n = 4) were coincubated with DHE (5 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative 3-D images of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in DHE fluorescence intensity from 5 min to 4 h of incubation. All data are means ± SE. *P ≤ 0.05 compared with control. E: arterioles incubated with 10−5.5 M NE (n = 5) or NE + 15 μM GM-6001 (n = 6) were coincubated with DHE (5 μM) and imaged with a multiphoton microscope every hour for 4 h. The line graph represents the changes in DHE fluorescence intensity [in arbitrary units (AU)] from 5 min to 4 h of incubation. All data are means ± SE. *P ≤ 0.05 compared with NE without GM-6001. F: arterioles incubated with 10−7 M ANG II (n = 8) or ANG II + 15 μM GM-6001 (n = 6) were coincubated with DHE (5 μM) and imaged with a multiphoton microscope every hour for 4 h. The line graph represents the changes in DHE fluorescence intensity (in AU) from 5 min to 4 h of incubation. All data are means ± SE.
ROS Inhibition Prevents the Development of Inward Remodeling and the Activation of MMPs Induced by Prolonged Exposure to NE + ANG II
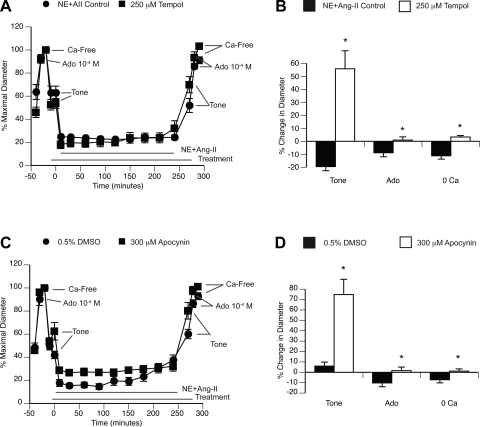
The inward remodeling induced by prolonged exposure to NE + ANG II was prevented by incubation of arterioles with the NADPH oxidase inhibitor and antioxidant apocynin (300 μmol/l) as well as with the SOD mimetic tempol (250 μmol/l). Both apocynin and tempol also significantly reduced the level of spontaneous myogenic tone observed in arterioles and prevented the impaired relaxation response to adenosine that occurred after the prolonged exposure to NE + ANG II (Fig. 4).
Fig. 4.
ROS inhibition with apocynin or tempol prevents the development of inward remodeling induced by prolonged exposure to NE + ANG II. A: arterioles were incubated with NE (10−5.5 M) + ANG II (10−7 M) for 4 h in the absence (n = 5) or presence (n = 9) of 250 μM tempol. Before and after the 4-h incubation, arterioles were allowed to develop spontaneous myogenic tone and exposed to Ado (10−4 M) and then to Ca2+-free solution. Data are expressed as percentages of the maximal diameter obtained under Ca2+-free conditions before the 4-h incubation period. B: comparisons were made between incubation treatments (NE + ANG II, n = 5; NE + ANG II + tempol, n = 9) on the percent changes in diameter occurring after versus before the 4-h incubation period at spontaneous tone, Ado dilation, and maximal passive diameter (Ca2+-free conditions). *P ≤ 0.05 compared with NE + ANG-II control. C: arterioles were incubated with NE (10−5.5 M) + ANG II (10−7 M) for 4 h in the presence of 0.5% DMSO (vehicle control; n = 8) or 300 μM apocynin (n = 7). Before and after the 4-h incubation, arterioles were allowed to develop spontaneous myogenic tone and exposed to Ado (10−4 M) and then to Ca2+-free solution. Data are expressed as percentages of the maximal diameter obtained under Ca2+-free conditions before the 4-h incubation period. D: comparisons were made between incubation treatments (NE + ANG II + DMSO, n = 8; NE + ANG II + apocynin, n = 7) on the percent changes in diameter occurring after versus before the 4-h incubation period at spontaneous tone, Ado dilation, and maximal passive diameter (Ca2+-free conditions). *P ≤ 0.05 compared with DMSO 0.5%.
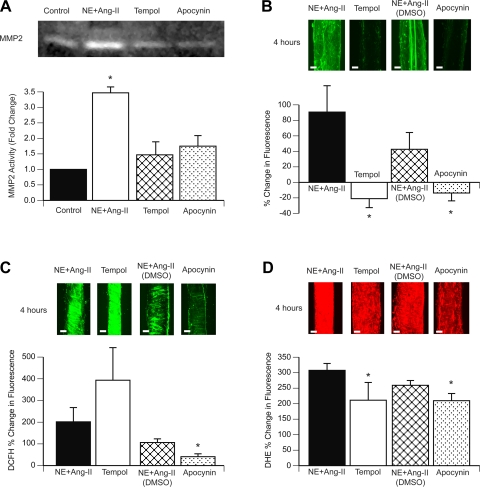
Incubation of arterioles with apocynin or tempol prevented the activation of MMP-2 induced by NE + ANG II, as detected by gel zymography, and reduced the overall gelatinolytic activity of arterioles in situ (Fig. 5, A and B). Whereas apocynin reduced the rate of the DCFH fluorescence increase caused by NE + ANG II, tempol increased it (Fig. 5C). In comparison, both tempol and apocynin reduced the rate of DHE fluorescence caused by the prolonged exposure to NE + ANG II (Fig. 5D).
Fig. 5.
ROS inhibition with apocynin or tempol prevents the activation of MMP-2 induced by prolonged exposure to NE + ANG II. A: pools (n = 3) of control untreated arterioles or arterioles treated with NE + ANG II alone, NE + ANG II + 300 μM apocynin, or NE + ANG II + 250 μM tempol were subjected to gel zymography. Bands were analyzed by densitometry and expressed as fold changes from the untreated control for the activity of the active (64 kDa) form of MMP-2. Contrast was optimized for the best visualization of the bands on the digitized image of the zymogram gel. B: arterioles incubated for 4 h with NE + ANG II alone (n = 4) or in combination with 250 μM tempol (n = 4), 0.5% DMSO (n = 4), or 300 μM apocynin (n = 4) were coincubated with DQ gelatin (10 μg/ml). 3-D images of the arterioles were obtained with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) of vessels from each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in fluorescence intensity of 3-D images from 5 min to 4 h of incubation. C: arterioles incubated with NE + ANG II alone (n = 4), NE + ANG II + tempol (n = 4), NE + ANG II + DMSO (n = 4), or NE + ANG II + apocynin (n = 4) were coincubated with DCFH (30 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative 3-D images (bars = 20 μm) of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in DCFH fluorescence intensity from 5 min to 4 h of incubation. D: arterioles incubated with NE + ANG II alone (n = 4), NE + ANG II + tempol (n = 4), NE + ANG II + DMSO (n = 4), or NE + ANG II + apocynin (n = 4) were coincubated with DHE (5 μM) and imaged with a multiphoton microscope every hour for 4 h. Representative 3-D images of each treatment at 4 h of incubation are shown. The bar graph represents the percent changes in DHE fluorescence intensity from 5 min to 4 h of incubation. All data are means ± SE. *P ≤ 0.05 compared with control.
DISCUSSION
The primary finding of the present study is that prolonged exposure to NE + ANG II induced inward remodeling associated with a ROS-dependent activation of MMPs in isolated arterioles. We previously showed (33) and corroborated in the present study that prolonged exposure (4 h) of isolated, cannulated, and pressurized arterioles to NE + ANG II is sufficient to induce inward eutrophic remodeling. In this study, we used this model of arteriolar remodeling to examine the mechanisms involved in the initial stages of the remodeling process. Prolonged sympathetic stimulation and activation of the renin-ANG system have been implicated with hypertension and arteriolar remodeling as well as with increased activity of MMPs and ROS in vascular tissues (4, 5, 17, 20, 21, 23, 26, 54). To determine the roles of MMPs and ROS in the remodeling process, we first determined that in isolated skeletal muscle arterioles, prolonged exposure to NE + ANG II indeed induced MMP activation and ROS production. This was indicated by the increased activity of MMP-2 in arterioles exposed to NE + ANG II, as observed in gel zymography. Furthermore, the increased gelatinolytic activity observed in in situ zymography as well as the increase message for MMP-2 detected with real-time RT-PCR indicated that exposure to NE + ANG II augmented both the production of the enzyme as well as its net activity at the tissue level. Of the gelatinases, only MMP-2 and not MMP-9 was expressed and activated upon exposure of arterioles to NE + ANG II. A previous study (8) has shown a relationship between MMP-2 activation and activity of membrane-bound MMPs. In addition, adrenergic stimulation of rat mesenteric resistance arteries is known to promote the activation of MMP-7 (20). Therefore, activation of additional MMPs other than MMP-2 by NE + ANG II in arterioles is likely, but their identity remains to be determined. That prolonged exposure to NE + ANG II induced ROS production was corroborated by the increased DCFH- and DHE-dependent fluorescence of arterioles exposed to the vasoconstrictor agonists for 4 h. As DCFH and DHE have selectivity for the detection of H2O2 and superoxide, respectively (28), this indicated that both ROS were increased upon exposure of the arterioles to NE + ANG II. Hypertension as well as sympathetic stimulation and ANG II have been shown to increase the activity of a number of pathways that promote the production of ROS in vascular tissues and cells (51, 52). Although the most likely contributor of ROS in arterioles is the enzyme NADPH oxidase, other potential ROS-producing pathways may also be activated by the prolonged exposure to NE + ANG II (21).
Inhibition of MMP activation or ROS production in isolated arterioles prevented the development of inward remodeling, suggesting that both MMPs and ROS are needed in the remodeling process. In addition to preventing inward remodeling, MMP inhibition also reduced the level of vasoconstriction induced by exposure to NE + ANG II. Whereas 15 μmol/l GM-6001 diminished the initial NE + ANG II-induced constriction by ∼50%, 1.5 μmol/l GM-6001 did not prevent the initial maximal contraction but caused a gradual loss of vasoconstriction over the 4-h exposure to the agonists. Importantly, this reduced vasoconstrictor response to NE + ANG II caused by MMP inhibition is not the mechanism responsible for the remodeling blockade. This is evidenced by the fact that DMSO used at a concentration of 0.6%, as a vehicle control for the 15 μmol/l GM-6001 treatment, also caused a gradual reduction in vasoconstriction without affecting the inward remodeling process. In addition, we (33) have previously demonstrated that a prolonged (4 h) exposure to ANG II alone induces inward eutrophic remodeling while only causing a marginal and transient vasoconstriction beyond the level of spontaneous myogenic tone normally observed in resistance arterioles. Collectively, this indicates that MMP activation is an essential component of the arteriolar inward remodeling process associated with prolonged stimulation with NE + ANG II independent from the level of vasoconstriction. Moreover, these data are congruent with reports (16, 53) indicating that hypertension is associated with increased plasma levels of MMPs.
NE and ANG II exert their actions on cells in part via an MMP-dependent transactivation of growth factor receptors (55). This transactivation pathway results in the downstream production of ROS (21). Therefore, it was intriguing that MMP inhibition in the present study did not result in a significant reduction in ROS production when arterioles were stimulated with both vasoconstrictor agonists. Stimulation of arterioles with NE or ANG II alone indicated that MMP inhibition only blunted superoxide production in NE-stimulated arterioles. Thus, a potential explanation for the lack of reduction in ROS production when arterioles were stimulated with both vasoconstrictors is that ANG II can generate ROS through pathways independent of MMPs that involve the activation of the enzyme NADPH oxidase (12, 24). ROS produced by either NADPH oxidase or mitochondria can stimulate ROS production by both mitochondria and NADPH oxidase, which generates a positive and vicious feedback loop (12). This vicious loop may account for the small effect that MMP blockade had on reducing ROS production when both vasoconstrictors were applied together as NADPH oxidase activation by ANG II may induce mitochondrial ROS production in the absence of MMP involvement.
As mentioned above, inhibition of ROS production prevented the development of inward eutrophic remodeling. Both the NADPH oxidase inhibitor and antioxidant apocynin as well as the SOD and catalase mimetic tempol prevented arterioles exposed for 4 h to NE + ANG II from developing inward remodeling. Neither apocynin nor tempol significantly affected the level of vasoconstriction induced by NE + ANG II throughout the 4 h of exposure to the vasoconstrictor agonists. They did, however, significantly reduce the level of spontaneous myogenic tone observed in pressurized vessels, as previously reported (31, 42). Importantly, ROS inhibition by either apocynin or tempol reduced the arteriolar activity of MMP-2 in gel zymography and reduced the overall gelatinolytic activity of arterioles in situ, thus indicating that ROS production is upstream from MMP activation in the inward remodeling process associated with prolonged exposure to NE + ANG II. Treatment with tempol or apocynin reduced the rate of the DHE fluorescence increase induced by the prolonged exposure of arterioles to the vasoconstrictor agonists. This indicated that either treatment reduced the production of superoxide induced by NE + ANG II. In comparison, only apocynin significantly reduced the rate of the DCFH fluorescence increase associated with NE + ANG II exposure. Tempol increased it. This implies that tempol facilitated the dismutation of superoxide into H2O2 and suggests that superoxide, rather than H2O2, is the main ROS associated with the activation of MMPs during the process of NE + ANG II-induced inward remodeling of arterioles.
A reduced level of nitric oxide (NO)-dependent signaling has been shown to augment the inward remodeling process (41), and ROS production during prolonged ANG II-induced vasoconstriction reduces the bioavailability of NO. Thus, it is likely that the NO-scavenging actions of ROS favor the inward remodeling process. However, in our in vitro model of isolated arterioles without luminal flow, the endothelial contribution of NO is minimal (40), which suggests that a mere reduction in NO bioavailability is not sufficient to induce inward remodeling in the time frame of this study's experimental conditions. On the other hand, ROS have been shown to induce the synthesis and modulate the activity of a number of MMPs, tissue inhibitors of MMPs, and a desintegrins and metalloproteases (ADAMs) (18, 27, 48, 50). One mechanism responsible for the activation of MMPs by ROS includes modulation of the thiol interaction between the prodomain and catalytic domain of the enzymes (27). Because ROS do not induce the synthesis of all MMPs and appear to modulate the activities of multiple MMPs in different fashions (27, 49), it remains to be determined which specific MMPs in addition to MMP-2 are produced and activated by ROS during the inward remodeling process. However, as MMP-7 and ADAM-12 have been implicated in the induction of cardiac hypertrophy and remodeling in hypertension (55), these enzymes are likely candidates to be involved in the inward remodeling process associated with NE + ANG II stimulation in resistance vessels.
MMPs and ROS have been previously associated with vascular remodeling, but only with the structural change observed in conduit arteries in response to a number of pathological conditions or the outward remodeling associated with increased flow in resistance arteries (3, 7, 15, 19, 29, 37). To our knowledge, this study is the first direct evidence that ROS-dependent activation of MMPs is implicated in the inward remodeling of resistance arterioles. Previously, we (35) have shown that vascular smooth muscle cells change their position during prolonged agonist-induced vasoconstriction. Thus, a potential contribution of MMP activation to the inward remodeling process may include the partial degradation of extracellular matrix proteins and cellular attachments to allow for vascular smooth muscle cells to change their position during prolonged agonist-induced vasoconstriction. The activation of MMPs may also favor the inward remodeling process by cleaving cellular receptors needed for agonist-dependent vasodilation (44), as impairment of vasodilatory pathways has been shown to induce inward eutrophic remodeling in resistance vessels (41). In summary, the association of ROS and MMPs with remodeling suggests that mechanisms related to their activity, such as cytoskeletal turnover, release of cytokines or chemokines from extracellular matrix components, cleavage of cellular receptors, and/or extracellular matrix degradation, are needed in the early stages of the inward remodeling process.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-088105-02 and American Heart Association Grant 0530031N.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bakker EN, Buus CL, VanBavel E, Mulvany MJ. Activation of resistance arteries with endothelin-1: from vasoconstriction to functional adaptation and remodeling. J Vasc Res 41: 174–182, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Bakker EN, Sorop O, Spaan JA, VanBavel E. Remodeling of resistance arteries in organoid culture is modulated by pressure and pressure pulsation and depends on vasomotion. Am J Physiol Heart Circ Physiol 286: H2052–H2056, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Belin de Chantemele EJ, Vessieres E, Dumont O, Guihot AL, Toutain B, Loufrani L, Henrion D. Reactive oxygen species are necessary for high flow (shear stress)-induced diameter enlargement of rat resistance arteries. Microcirculation 16: 391–402, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res 94: 37–45, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Briest W, Holzl A, Rassler B, Deten A, Baba HA, Zimmer HG. Significance of matrix metalloproteinases in norepinephrine-induced remodelling of rat hearts. Cardiovasc Res 57: 379–387, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Castro MM, Rizzi E, Prado CM, Rossi MA, Tanus-Santos JE, Gerlach RF. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol 29: 194–201, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, Tanus-Santos JE. Antioxidant treatment reduces matrix metalloproteinase-2-induced vascular changes in renovascular hypertension. Free Radic Biol Med 46: 1298–1307, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Chang DI, Hosomi N, Lucero J, Heo JH, Abumiya T, Mazar AP, del Zoppo GJ. Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab 23: 1408–1419, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a beta-blocker on cerebral arterioles in rats. Hypertension 33: 856–861, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Chillon JM, Baumbach GL. Effects of indapamide, a thiazide-like diuretic, on structure of cerebral arterioles in hypertensive rats. Hypertension 43: 1092–1097, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Christensen KL, Mulvany MJ. Vasodilatation, not hypotension, improves resistance vessel design during treatment of essential hypertension: a literature survey. J Hypertens 19: 1001–1006, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dupuis F, Atkinson J, Liminana P, Chillon JM. Comparative effects of the angiotensin II receptor blocker, telmisartan, and the angiotensin-converting enzyme inhibitor, ramipril, on cerebrovascular structure in spontaneously hypertensive rats. J Hypertens 23: 1061–1066, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep 11: 182–189, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Flamant M, Placier S, Dubroca C, Esposito B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC, Lehoux S. Role of matrix metalloproteinases in early hypertensive vascular remodeling. Hypertension 50: 212–218, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Friese RS, Rao F, Khandrika S, Thomas B, Ziegler MG, Schmid-Schonbein GW, O'Connor DT. Matrix metalloproteinases: discrete elevations in essential hypertension and hypertensive end-stage renal disease. Clin Exp Hypertens 31: 521–533, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994 [DOI] [PubMed] [Google Scholar]
- 18. Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res 92: e80–86, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Haas TL, Doyle JL, Distasi MR, Norton LE, Sheridan KM, Unthank JL. Involvement of MMPs in the outward remodeling of collateral mesenteric arteries. Am J Physiol Heart Circ Physiol 293: H2429–H2437, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Hao L, Du M, Lopez-Campistrous A, Fernandez-Patron C. Agonist-induced activation of matrix metalloproteinase-7 promotes vasoconstriction through the epidermal growth factor-receptor pathway. Circ Res 94: 68–76, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to alpha1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol 26: 819–825, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension 21: 391–397, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Heerkens EH, Shaw L, Ryding A, Brooker G, Mullins JJ, Austin C, Ohanian V, Heagerty AM. αV-Integrins are necessary for eutrophic inward remodeling of small arteries in hypertension. Hypertension 47: 281–287, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Herrera M, Silva GB, Garvin JL. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase Cα-dependent NADPH oxidase activation. J Biol Chem 285: 21323–21328, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Johnson RJ, Rodriguez-Iturbe B, Kang DH, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens 18: 431–440, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res 85: 413–423, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 29: 739–745, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lehoux S, Lemarie CA, Esposito B, Lijnen HR, Tedgui A. Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling. Circulation 109: 1041–1047, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Lodi F, Cogolludo A, Duarte J, Moreno L, Coviello A, Peral De Bruno M, Vera R, Galisteo M, Jimenez R, Tamargo J, Perez-Vizcaino F. Increased NADPH oxidase activity mediates spontaneous aortic tone in genetically hypertensive rats. Eur J Pharmacol 544: 97–103, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Luo Z, Chen Y, Chen S, Welch WJ, Andresen BT, Jose PA, Wilcox CS. Comparison of inhibitors of superoxide generation in vascular smooth muscle cells. Br J Pharmacol 157: 935–943, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martinez-Lemus LA. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J Vasc Res 45: 211–221, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Martinez-Lemus LA, Crow T, Davis MJ, Meininger GA. αvβ3- and α5β1-integrin blockade inhibits myogenic constriction of skeletal muscle resistance arterioles. Am J Physiol Heart Circ Physiol 289: H322–H329, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Martinez-Lemus LA, Hill MA, Bolz SS, Pohl U, Meininger GA. Acute mechanoadaptation of vascular smooth muscle cells in response to continuous arteriolar vasoconstriction: implications for functional remodeling. FASEB J 18: 708–710, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Mathiassen ON, Buus NH, Sihm I, Thybo NK, Morn B, Schroeder AP, Thygesen K, Aalkjaer C, Lederballe O, Mulvany MJ, Christensen KL. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J Hypertens 25: 1021–1026, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Matlung HL, Bakker EN, Vanbavel E. Shear stress, reactive oxygen species and arterial structure and function. Antioxid Redox Signal 11: 1699–1709, 2009 [DOI] [PubMed] [Google Scholar]
- 38. Meininger GA, Fehr KL, Yates MB. Anatomic and hemodynamic characteristics of the blood vessels feeding the cremaster skeletal muscle in the rat. Microvasc Res 33: 81–97, 1987 [DOI] [PubMed] [Google Scholar]
- 39. Merchant SJ, Davidge ST. The role of matrix metalloproteinases in vascular function: implications for normal pregnancy and pre-eclampsia. BJOG 111: 931–939, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Murphy TV, Kotecha N, Hill MA. Endothelium-independent constriction of isolated, pressurized arterioles by Nω-nitro-l-arginine methyl ester (l-NAME). Br J Pharmacol 151: 602–609, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pistea A, Bakker EN, Spaan JA, VanBavel E. Flow inhibits inward remodeling in cannulated porcine small coronary arteries. Am J Physiol Heart Circ Physiol 289: H2632–H2640, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation 108: 2230–2235, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Rodrigues SF, Tran ED, Fortes ZB, Schmid-Schonbein GW. Matrix metalloproteinases cleave the β2-adrenergic receptor in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 299: H25–H35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens 17: 1192–1200, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Schiffrin EL, Deng LY. Structure and function of resistance arteries of hypertensive patients treated with a beta-blocker or a calcium channel antagonist. J Hypertens 14: 1247–1255, 1996 [DOI] [PubMed] [Google Scholar]
- 47. Schiffrin EL, Deng LY, Larochelle P. Effects of a β-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension 23: 83–91, 1994 [DOI] [PubMed] [Google Scholar]
- 48. Shigemura K, Sung SY, Kubo H, Arnold RS, Fujisawa M, Gotoh A, Zhau HE, Chung LW. Reactive oxygen species mediate androgen receptor- and serum starvation-elicited downstream signaling of ADAM9 expression in human prostate cancer cells. Prostate 67: 722–731, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Shin MH, Moon YJ, Seo JE, Lee Y, Kim KH, Chung JH. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic Biol Med 44: 635–645, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev 9: 43–51, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19: 1245–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol 122: 339–352, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Tran ED, Delano FA, Schmid-Schonbein GW. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J Vasc Res 47: 423–431, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walter A, Etienne-Selloum N, Sarr M, Kane MO, Beretz A, Schini-Kerth VB. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: preventive effect of red wine polyphenols. J Vasc Res 45: 386–394, 2008 [DOI] [PubMed] [Google Scholar]
- 55. Wang X, Chow FL, Oka T, Hao L, Lopez-Campistrous A, Kelly S, Cooper S, Odenbach J, Finegan BA, Schulz R, Kassiri Z, Lopaschuk GD, Fernandez-Patron C. Matrix metalloproteinase-7 and ADAM-12 (a disintegrin and metalloproteinase-12) define a signaling axis in agonist-induced hypertension and cardiac hypertrophy. Circulation 119: 2480–2489, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol 50: 9–16, 2007 [DOI] [PubMed] [Google Scholar]