Abstract
Chronic inhibition of phosphodiesterase-5 with sildenafil immediately after permanent occlusion of the left anterior descending coronary artery was shown to limit ischemic heart failure (HF) in mice. To mimic a more clinical scenario, we postulated that treatment with sildenafil beginning at 3 days post-myocardial infarction (MI) would also reduce HF progression through the inhibition of the RhoA/Rho-kinase pathway. Adult male ICR mice with fractional shortening < 25% at day 3 following permanent left anterior descending coronary artery ligation were continuously treated with either saline (volume matched, ip, 2 times/day) or sildenafil (21 mg/kg, ip, 2 times/day) for 25 days. Echocardiography showed fractional shortening preservation and less left ventricular end-diastolic dilatation with sildenafil treatment compared with saline treatment at 7 and 28 days post-MI (P < 0.05). Both fibrosis and apoptosis, determined by Masson's trichrome and terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL), respectively, were attenuated in the sildenafil-treated mice (P < 0.05 vs. saline). Western blot analysis showed enchanced Bcl-2-to-Bax ratio with sildenafil treatment (P < 0.05 vs. saline). Activity assay showed sildenafil-mediated PKG activation 1 day after treatment (P < 0.05 vs. sham and saline). PKG activation was associated with sildenafil-mediated inhibition of Rho kinase (P < 0.05) compared with saline treatment, whereas PKG inhibition with KT-5823 abolished this inhibitory effect of sildenafil. In conclusion, for the first time, our findings show that chronic sildenafil treatment, initiated at 3 days post-MI, attenuates left ventricular dysfunction independent of its infarct-sparing effect, and this cardioprotection involves the inhibition of the RhoA/Rho-kinase pathway. Sildenafil may be a promising therapeutic tool for advanced HF in patients.
Keywords: ischemia-reperfusion injury, protein kinase G, echocardiography
cardiac remodeling following myocardial infarction (MI) contributes to the progression of heart failure (HF) (1), and current therapies strive to limit adverse remodeling to preserve cardiac systolic function (4). Despite medical advances, HF following acute MI has a poor prognosis in patients.
Phosphodiesterase-5 (PDE-5) inhibitors sildenafil, vardenafil, and tadalafil are Food and Drug Administration-approved for the treatment of erectile dysfunction, and recently sildenafil and tadalafil were approved for the treatment of pulmonary arterial hypertension (PAH) (10). In animal models, sildenafil exerted an infarct-sparing effect when given before ischemia (24, 27) or at the time of reperfusion (28). Furthermore, chronic treatment with sildenafil immediately after permanent occlusion of the left anterior descending coronary artery (LAD) in mice attenuated ischemic cardiomyopathy (29). These cardioprotective effects are mediated by activation of protein kinase G (PKG), expression of endothelial and inducible nitric oxide (NO) synthase (eNOS and iNOS, respectively), and increased Bcl-2-to-Bax ratio (5, 7, 27, 29). However, little is known about the effects of PDE-5 inhibition on limiting adverse remodeling independent of its ability to modulate infarct size. This concept is clinically relevant, especially in patients with advanced ischemic HF, because necrosis has a negligible role in a postinfarct setting (1).
Interestingly, a growing body of evidence suggests a role of Rho kinase (RhoK) in the development of cardiovascular disease and PAH (23, 36). RhoK has two known isoforms and is activated by the small GTPase RhoA. RhoK functions as signal transducer in actin cytoskeletal organization, smooth muscular contraction, and gene expression (23). Fasudil, a nonselective inhibitor of RhoK, is currently approved in Japan for the treatment of PAH (36). Moreover, fasudil reduced infarct size 24 h post-MI (2) and left ventricular (LV) adverse remodeling when given chronically in mouse models (13). Interestingly, sildenafil reduced PAH by inhibiting RhoK in a chronic hypoxia model (12) and in a bleomycin-induced pulmonary hypertension model (14). Nonetheless, there is little evidence on the relationship between sildenafil and RhoK in the LV as well as on the ability of sildenafil to reduce LV dysfunction in advanced ischemic cardiomyopathy.
The present study sought to determine whether sildenafil treatment following LV dysfunction, with LV fractional shortening (FS) < 25% at day 3 post-MI, could prevent the progression of HF in a permanent LAD occlusion model. We also investigated whether the sildenafil-induced cardioprotection is mediated by an inhibition of the RhoA/RhoK pathway.
METHODS
Animals.
Adult male ICR mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). The mean body weight was 33.2 ± 0.3 g. The investigation conforms with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The animal protocol was approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
Drugs and chemicals.
Fasudil was purchased from LC Laboratory (Auburn, MA). KT-5823 and 10% formalin were purchased from Sigma-Aldrich (St. Louis, MO). Sildenafil powder was kindly provided by Pfizer.
MI protocol.
Adult ICR mice underwent permanent occlusion of the LAD coronary artery as previously described (29). In brief, the animals were anesthetized (pentobarbital sodium, 70 mg/kg ip), intubated orotracheally, and ventilated on a positive-pressure ventilator. The tidal volume was set at 0.2 ml, and the respiratory rate was adjusted to 133 cycles/min. All surgical procedures were carried out under sterile conditions. A left thoracotomy was performed at the fourth intercostal space, and the heart was exposed by stripping the pericardium. The LAD was then identified and permanently occluded by placing a 7.0-silk ligature around it. The air was expelled from the chest and the animals were extubated, which was followed by injection of intramuscular doses of analgesia (buprenex, 0.02 mg/kg) and antibiotic (gentamicin, 0.7 mg/kg for 3 days).
Experimental groups.
At 3 days post-MI, mice with LV FS < 25% were randomly assigned to one of six treatment groups: 1) saline (vehicle, volume-matched, ip, 2 times/day); 2) sildenafil (21 mg/kg, ip, 2 times/day); 3) fasudil (10 mg/kg, ip, 2 times/day), a nonselective RhoK inhibitor; 4) PKG inhibitor KT-5823 (1 mg/kg, ip, 2 times/day) + saline: KT-5823 was given 10 min before saline as in group 1; 5) KT-5823 + sildenafil: KT-5823 was also administered 10 min before sildenafil as in group 2; and 6) sham: animals received a left thoracotomy (as control for the surgical procedure) but no MI or treatment until heart collection. Treatments began at 3 days post-MI and continued until death. In groups 1–3, LV function (n = 10–12/group) was reassessed via echocardiography at 4, 7, and 28 days post-MI. In groups 1–6, hearts (n = 3–6/group) were collected at 4 days post-MI (or 1 day after treatment initiation) for molecular analyses. Hearts (n = 4–6/group) from groups 1–3 were sampled on days 7 and 28 after echocardiography for histological examinations.
Survival.
Animals that survived starting from the time of enrollment into the study until death were included in the calculation of survival rate.
Echocardiography and hemodynamics.
M-mode echocardiography was performed using the Vevo770 imaging system (VisualSonics; Toronto, Canada) before surgery (baseline) and on days 3, 4, 7, and 28 post-MI before the animals were euthanized as previously detailed (29). Heart rate (HR), LV end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), anterior wall diastolic thickness (AWDT), and anterior wall systolic thickness were measured. LV FS was calculated as (LVEDD − LVESD)/LVEDD·100.
Systolic, diastolic, mean blood pressures, and HR were measured using a noninvasive tail-cuff volume pressure recording system (CODA-2, Kent Scientific; Torrington, CT) in anesthetized mice (pentobarbital sodium; 30 mg/kg ip) at 1 h after treatment on day 7 according to manufacturer's instructions.
Protein kinase G activity.
Cardiac protein kinase G activity was examined using a commercially available PKG activity kit (Cyclex; Nagano, Japan) in whole heart samples (n = 6/group). Activity was measured according to the manufacturer's instructions. Spectrophotometric absorbance was measured at 450 nm. Results were normalized as per milligram of protein.
RhoK activity.
RhoK activity was measured by phosphorylation of ezrin-radixin-moesin (pERM) family protein, a substrate of RhoK (19), using Western blot analysis (n = 3/group). The activity was also confirmed using a commercially available activity kit (Cyclex; Nagano, Japan). Briefly, plates are precoated with a substrate corresponding to the recombinant COOH-terminus of myosin-binding subunit of myosin phosphatase, which contains a threonine residue that may be phosphorylated by RhoK. The tissue extracts were allowed to phosphorylate the bound substrate in the presence of Mg2+ and ATP. The amount of phosphorylated substrate was measured by binding it with a horseradish peroxidase conjugate of AF20, a anti-phospho-myosin-binding subunit of myosin phosphatase threonine-696-specific antibody, which then catalyzes the conversion of the chromogenic substrate tetramethylbenzidine from a colorless solution to a blue solution. The color was quantified by spectrophotometric measurement of absorbance at 450 nm. The results were normalized as per milligrams of protein.
Western blot analysis.
Isolated LV samples (n = 3/group) were collected at 4 days post-MI. Samples were homogenized, sonicated, and centrifuged at 10,000 g for 15 min at 4°C. Total proteins (100 μg) from each sample were separated by SDS-PAGE on 10% acrylamide gels, transferred onto a nitrocellulose membrane, and blocked with 5% nonfat dry milk in Tris-buffered saline (27, 29). Membranes were incubated overnight with rabbit polyclonal antibodies (dilution 1:500, Santa Cruz) specific for Bcl-2, Bax, or α-tubulin and rabbit polyclonal antibodies (dilution 1:1,000, Cell Signaling) specific for pERM or ERM. A horseradish peroxidase-conjugated anti-rabbit IgG (dilution 1:2,000, Amersham) or anti-goat IgG (dilution 1:2,000, Santa Cruz) served as the secondary antibody. Membranes were developed with enhanced chemiluminescence and exposed to X-ray film. Protein level was quantified using densitometry.
Histology.
Transverse sections of the median third of the LV (n = 4–6/group) were fixed in 10% formalin for at least 48 h, embedded in parrafin, and sectioned (5 μm). Apoptosis was examined using the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay (ApopTag; Millipore, Billerica, MA) according to manufacturer's instructions. Apoptotic rate within in the peri-infarct regions was calculated at ×40 magnification under light microscopy.
Myocardial fibrosis was examined to address prevalence of scar formation within the LV. Heart sections (5 μm) were stained with Masson's trichrome (Sigma-Aldrich; St. Louis, MO). Fibrotic area was computed using computer morphometry (Bioquant 98) and expressed as a percentage of LV.
Cardiac hypertrophy.
On day 28 post-MI, the mice were weighed before death. The hearts were weighed immediately after collection. Cardiac hypertrophy was calculated as the ratio of heart weight to tibia length.
Statistical analysis.
The data were expressed as group means ± SE. Changes in echocardiography, myocardial fibrosis, and TUNEL were analyzed using two-way repeated-measures ANOVA to determine the main effect of time, group, and time-by-group interaction. Western blot results, including PKG and RhoK activity, and hypertrophy were analyzed using a one-way ANOVA. If global tests showed major interactions, Bonferroni's post hoc analysis was conducted for multiple comparisons. Statistical difference was set at P < 0.05. Kaplan-Meyer analysis was used to test for differences in survival.
RESULTS
Survival.
A total of 113 mice were enrolled into this study. The sildenafil-treated group had 18 of 18 (100%) mice survive, which was greater (but not statistically significant) than 15 of 19 (76%) mice that survived in the saline-treated group (P > 0.05). Fasudil-treated group had 17 of 18 (94%) mice survived (P > 0.05 vs. saline). Sham mice had 100% survival rate.
LV remodeling and function.
Representative M-mode images from 3 days post-MI and sham-operated, saline, sildenafil, and fasudil-treated mice at 28 days post-MI are shown in Fig. 1A. At 3 days post-MI, mice receiving sildenafil, saline, or fasudil treatment had similar FS (18 ± 1, 19 ± 1, and 17 ± 1%, respectively, P > 0.05, Fig. 1B) compared with baseline value of 47 ± 1%. At days 7 and 28 post-MI, sildenafil-treated group had a significantly higher FS than saline-treated mice (P < 0.05, Fig. 1B). Fasudil treatment resulted in a higher FS at day 28 compared with saline treatment (P < 0.05, Fig. 1B). Both LVEDD and LVESD were increased in saline-treated mice compared with sildenafil-treated and fasudil-treated mice (P < 0.05, Fig. 1, D and E), indicating more dilatation. Moreover, AWDT was greater in sildenafil-treated and fasudil-treated animals versus saline-treated animals (P < 0.05, Fig. 1F) on day 28 post-MI.
Fig. 1.
M-mode echocardiography. A: representative echocardiography images of mice at 3 day (d) post-myocardial infarction (MI), sham-operated, saline-treated, sildenafil-treated, and fasudil-treated mice at 28 day post-MI. B: fractional shortening. C: ejection fraction. D: left ventricular (LV) end-diastolic diameter. E: LV end-systolic diameter. F: anterior wall diastolic thickness (AWDT). G: heart rate. bpm, Beats/min. *P < 0.05 vs. saline at 7 days; #P < 0.05 vs. saline at 28 days; n = 10–12/group.
FS of sham-operated mice was 43 ± 1.0% at 28 days post-left thoracotomy. Baseline LVEDD was 3.5 ± 0.1 mm. An increase in LVEDD and a decrease in FS in saline-, sildenafil-, and fasudil-treated mice compared with baseline and sham-operated mice (P < 0.05) were observed on days 3 and 28 (data not shown). HR of sildenafil-treated and fasudil-treated mice was comparable with saline-treated mice at each time point (Fig. 1G).
Hemodynamics.
Sildenafil-treated mice had a slight drop in systolic, diastolic, and mean arterial blood pressures (P > 0.05) compared with saline-treated mice at 1 h posttreatment on day 7 post-MI. HR was comparable between groups (Table 1).
Table 1.
Hemodynamics
| Sham | Saline | Sildenafil | |
|---|---|---|---|
| SBP, mmHg | 95 ± 3 | 90 ± 5 | 79 ± 5 |
| DBP, mmHg | 71 ± 4 | 68 ± 4 | 58 ± 5 |
| MAP, mmHg | 80 ± 2 | 75 ± 5 | 65 ± 5 |
| HR, beats/min | 581 ± 13 | 663 ± 59 | 661 ± 41 |
Parameters (means ± SE) were measured at 1 h posttreatment on day 7 post-myocardial infarction. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial blood pressure; HR, heart rate.
Protein kinase G activity.
Cardiac PKG activity (A450/mg protein) was increased one day after treatment with sildenafil (2.93 ± 0.42, P < 0.05) compared with saline and sham treatment (1.20 ± 0.27 and 0.51 ± 0.11, respectively, Fig. 2).
Fig. 2.
Cardiac PKG activity (A450/mg protein) at 4 days postinfarction (or 1 day after treatment) in sildenafil-treated or saline-treated mice compared with sham-operated mice. *P < 0.05 vs. other groups; n = 6/group.
RhoK activity.
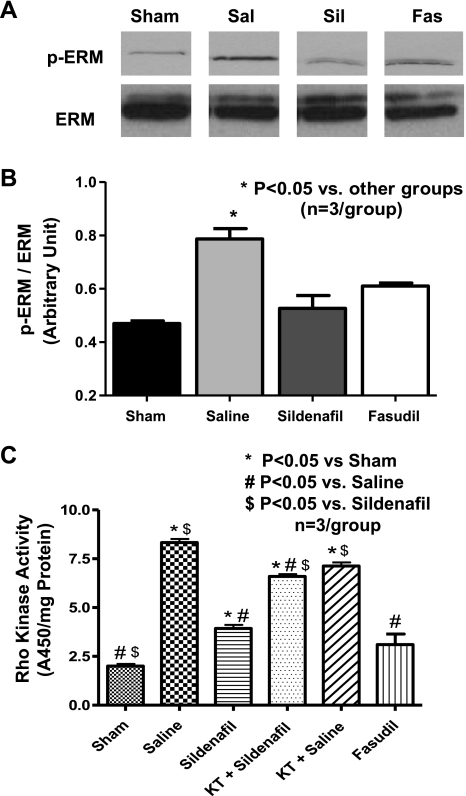
The extent of pERM to total ERM was increased at day 4 post-MI in saline-treated mice (P < 0.05 vs. sham). Both sildenafil and fasudil treatment (P < 0.05 vs. saline) inhibited the increase in pERM (Fig. 3, A and B). Moreover, the activity assay showed an increase in RhoK activity (A450/mg protein) at day 4 post-MI with saline treatment (8.3 ± 0.3, P < 0.05), compared with sham (2.0 ± 0.2), sildenafil (3.86 ± 0.2), and fasudil (3.10 ± 0.55, Fig. 3C) treatment. Administration of KT-5823, a PKG inhibitor, abolished the inhibitory effect of sildenafil on Rho-kinase activity (P < 0.05 vs. sildenafil, Fig. 3C).
Fig. 3.
LV Rho kinase activity at 4 days postinfarction (or 1 day after treatment). A: representative Western blots of ezrin-radixin-moesin (ERM) phosphorylation (pERM), substrates of Rho kinase. A representative lane was chosen from replicates of 3 per group. Sal, saline; Sil, sildenafil; Fas, fasudil. B: densitometric quantification of the ratio of pERM to total ERM. C: activity assay results of Rho kinase activity (A450/mg protein). *P < 0.05 vs. other groups; n = 3/group.
Apoptosis.
Apoptotic rate was higher in saline (7.9 ± 1.4%)- compared with sildenafil (2.8 ± 0.7%, P < 0.05)- and fasudil (4.2 ± 0.6%, P < 0.05)-treated mice at 7 days post-MI. Similarly, apoptotic rate was significantly lower in both sildenafil (2.1 ± 0.4%, P < 0.05)- and fasudil (3.7 ± 0.8%, P < 0.05)-treated mice compared with saline-treated mice (6.5 ± 0.4%) at 28 days post-MI (Fig. 4A).
Fig. 4.
Myocardial apoptosis and Bcl-2-to-Bax ratio. A: apoptotic rate [percentage of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive nuclei compared with total nuclei]. *P < 0.05 vs. saline at 7 days; #P < 0.05 vs. saline at 28 days; n = 4–6/group. B: representative Western blots showing Bcl-2 and Bax expression. A representative lane was chosen from replicates of 3 per group. C: densitometric quantification of the ratio of Bcl-2 to tubulin. *P < 0.05 vs. other groups; n = 3/group. D: densitometric quantification of the ratio of Bax to tubulin. *P < 0.05 vs. other groups. E: densitometric quantification of the ratio of Bcl-2 to Bax. *P < 0.05 vs. other groups; #P < 0.05 vs. sildenafil.
Bcl-2-to-Bax ratio.
Bcl-2 expression was increased with sildenafil treatment (P < 0.05) at day 4 post-MI relative to sham and saline treatment (Fig. 4, B and C). Although Bax was also increased in the saline- and sildenafil-treated mice compared with sham-operated mice (Fig. 4D, P < 0.05), the Bcl-2-to-Bax ratio was decreased after MI in the saline-treated mice. Sildenafil treatment attenuated the decrease in Bcl-2-to-Bax ratio (Fig. 4E, P < 0.05), whereas fasudil treatment had no effect on Bcl-2-to-Bax ratio compared with saline (P > 0.05).
Myocardial fibrosis.
Myocardial fibrosis (%LV) was higher in saline (19.0 ± 5.3%)- compared with sildenafil (4.7 ± 0.8%, P < 0.05)- and fasudil (8.9 ± 0.8%, P < 0.05)-treated mice on day 7 post-MI. Fibrosis was lower in both sildenafil (5.8 ± 1.3%, P < 0.05)- and fasudil (9.5 ± 1.6%, P < 0.05)-treated mice compared with saline-treated mice (18.9 ± 3.4%) on day 28 post-MI (Fig. 5, A and B).
Fig. 5.
Myocardial fibrosis and hypertrophy. A: representative images of Masson's trichrome-stained heart sections showing myocardial fibrosis at 28 days in saline-, sildenafil-treated, and fasudil-treated mice. B: myocardial fibrosis (percentage of LV). *P < 0.05 vs. saline at 7 days; #P < 0.05 vs. saline at 28 days; n = 4–6/group. C: cardiac hypertrophy at 28 days postinfarction. *P < 0.05 vs. sham; #P < 0.05 vs. saline; n = 10–12/group. D: mean body weight in the various experimental groups.
Cardiac hypertrophy.
The cardiac hypertrophy (expressed as ratio of heart weight to tibia length) was higher in saline (110.9 ± 6.1 mg/cm)-, sildenafil (86.4 ± 3.8 mg/cm)-, and fasudil (91.5 ± 3.4 mg/cm)-treated groups compared with the sham-operated group (65.9 ± 3.6 mg/cm, P < 0.05) at 28 days post-MI. However, hypertrophy in the sildenafil-treated and fasudil-treated mice was lower compared with the saline-treated mice (P < 0.05, Fig. 5C). Also, there was no significant difference in the total body weight between the groups at the time of death (Fig. 5D).
DISCUSSION
Chronic treatment with sildenafil has been shown to attenuate MI-induced HF when administered immediately after permanent coronary artery ligation in mice (29). In the present study, we demonstrated that sildenafil, when given at 3 days post-MI, also limited the progression of HF. We further demonstrated that the inhibition of RhoA/Rho-kinase pathway mediated sildenafil-induced cardioprotection and restriction of HF.
Delayed treatment with sildenafil preserved LV function and reduced adverse LV remodeling. Preservation of FS, attenuation of LV dilatation, and conservation of AWDT were observed with sildenafil treatment compared with saline. One reasonable explanation for this protection could be partially attributed to the significant attenuation of myocardial apoptosis and fibrosis with sildenafil treatment on days 7 and 28 post-MI. Cardiac hypertrophy, a hallmark of HF, was also attenuated with sildenafil treatment. These findings indicate a protective effect within the infarct and peri-infarct regions and support our earlier studies showing cardioprotection with sildenafil when given at the onset of permanent ischemia (29) or at the onset of reperfusion in an ischemia-reperfusion (I/R) model (28). These data are also consistent with a recent study showing the antihypertrophic/remodeling effect of sildenafil when initiated at 3 wk after transverse aortic constriction in mice with preexisting advanced hypertrophy caused by pressure overload (20). Our data indicate that sildenafil attenuates progression of LV dysfunction by limiting cardiac hypertrophy, apoptosis, fibrosis, and LV dilatation.
PDE-5 inhibition prevents the degradation of NO-driven cGMP and can thus increase PKG activity, leading to increased penile blood flow and pulmonary vascular relaxation. The enzyme PDE-5 is found abundantly in the corpus cavernosum and pulmonary artery smooth muscle. It is also expressed in adult murine (5) and canine (35) hearts. Although the role of PDE-5 in the human heart is questioned (16, 39), elevated PDE-5 expression was observed in the LV of patients with advanced HF (25). Overexpression of PDE-5 in transgenic mice was associated with increased LV remodeling in MI-induced HF (25). These findings strengthen the concept of PDE-5 inhibition as a potential treatment for ischemic HF. Nevertheless, the role of sildenafil in the human myocardium remains to be elucidated, although clinical studies have shown that sildenafil increased oxygen capacity in patients with HF (11, 17).
PKG phosphorylates various effectors that are important in cellular survival, proliferation, and relaxation of vascular smooth muscle (3, 6). Our previous studies have shown that sildenafil induces a preconditioning-like effect in reducing simulated ischemia/reoxygenation injury through a PKG-dependent mechanism in mouse cardiomyocytes (7). These effects were also reproduced by overexpression of PKG1α in the absence of sildenafil treatment (6). Moreover, we also demonstrated that tadalafil triggered an infarct-sparing effect against I/R in a PKG/hydrogen sulfide-dependent pathway in mice (31). Our results show that sildenafil treatment was able to increase the PKG activity even on day 1 after initiation of treatment (or at 4 days post-MI), which could potentially contribute toward the inhibition in the progression of HF after permanent ischemia.
Previous studies have shown that RhoK activation is involved in the pathogenesis of PAH in various animal models, and inhibition of RhoK with sildenafil attenuates the disease process (12, 19). Furthermore, RhoK inhibition ameliorated atherosclerosis, LV remodeling post-MI, cardiac hypertrophy, and myocardial I/R injury (23). Clinically, fasudil, a putative RhoK inhibitor, improved the ischemic threshold of stable angina (38) and endothelial function in patients with coronary artery disease (22). Our results showed that sildenafil caused inhibition in pERM (Fig. 3, A and B) and RhoK activity in the heart, which was significantly increased on day 4 post-MI in the saline-treated control mice. Interestingly, sildenafil inhibited this increase in pERM one day after treatment to the same level as fasudil. We confirmed RhoK activation with sildenafil using a RhoK activity assay. In addition, PKG inhibition with KT-5823 abolished the inhibitory effect of sildenafil on RhoK, suggesting that sildenafil-mediated PKG activation negatively regulates RhoK activity. Delayed fasudil treatment limited the progression of HF by preserving FS and reducing LV dilatation, cardiac fibrosis, and hypertrophy compared with saline treatment at 28 days post-MI. These data indicate that RhoK inhibition may be a downstream effector of sildenafil-induced cardioprotection within the LV, which is mediated by PKG.
RhoK is a serine/threonine kinase that is activated by its upstream effector RhoA. The small GTPase RhoA is inactivated when bound to guanosine dissociation inhibitor (GDI), which prevents RhoA from exchanging GDP for GTP (23). RhoK is highly concentrated in the brain, heart, lung, and kidney (21). It plays an important role in regulating vascular smooth muscle contraction, cytoskeletal organization, cellular apoptosis and survival, and gene transcription (23). Increased RhoK activity was associated with upregulation of NADPH oxidase, elevation of hypertrophic growth factors, and apoptotic signaling cascade (36). It is plausible that sildenafil exerted its cardioprotection through inhibition of RhoK and the subsequent adverse signaling.
RhoK activation has been shown to cause eNOS and iNOS mRNA instability and decrease the synthesis of these enzymes (37, 41). RhoK can also reduce eNOS phosphorylation by inhibiting the phosphotidylinositol 3-kinase/Akt pathway, thus lowering the enzymatic capacity to generate NO (42). By inhibiting RhoA/RhoK pathway, sildenafil treatment could subsequently increase NO production in the perfused region of the heart. The generated NO could then diffuse into the ischemic zone and restore the cGMP/PKG pathway. This concept is consistent with our previous findings showing increased eNOS and iNOS expression in the heart at 24 h post-MI with sildenafil treatment and abrogation of sildenafil-induced cardioprotection with NG-nitro-l-arginine methyl ester, an inhibitor of NO synthase (29).
Exactly how sildenafil inhibited RhoK activation is unclear in this study. However, Ellerbroek et al. (9) demonstrated that phosphorylation of RhoA at the Ser188 residue in vivo negatively regulated RhoA activity by increasing RhoA affinity toward GDI. This sequestration of RhoA by GDI would prevent RhoA from binding to and activating RhoK. Other studies have shown that PKG can phosphorylate RhoA at the Ser188 residue, thus leading to RhoK inhibition (33, 34). Moreover, Guilluy et al. (12) established that sildenafil increased RhoA phosphorylation and RhoA binding to GDI in pulmonary vascular smooth muscle, thus resulting in RhoK inhibition. In the present study, increased PKG activity with sildenafil treatment was associated with the ability of sildenafil to inhibit RhoK activation. The enhanced cardiac PKG activity observed with sildenafil treatment may have negatively regulated RhoA and thus inhibited RhoK activity in the heart. Indeed, our results clearly indicate that pharmacological inhibition of PKG with KT-5823 abrogated the attenuation of RhoK activity with sildenafil.
In the present study, sildenafil treatment led to an attenuation of apoptosis in the heart. Myocardial apoptosis within the peri-infarct region contributes to the progression of MI-induced HF, possibly because of its involvement in impaired systolic function and chronic cardiac remodeling (18, 32). We observed an increase in Bcl-2-to-Bax ratio with delayed sildenafil treatment in the LV region, indicating an antiapoptotic effect of sildenafil in a permanent ischemia setting. The apoptotic rate was also lower in the sildenafil-treated mice than in saline-treated mice at 7 and 28 days post-MI. Our current findings are in agreement with the reduction of apoptosis following permanent ischemia (29) and simulated ischemia-reoxygenation (5, 7) observed with sildenafil in previous studies. Sildenafil-mediated PKG activation could trigger a signaling cascade that leads to an increase in Bcl-2-to-Bax ratio and subsequent attenuation of apoptosis (6, 23, 29). Even though we observed that fasudil treatment yielded a similar Bcl-2-to-Bax ratio (at 4 day post-MI) to saline treatment, there was a significant decrease in the apoptotic rate at 7 and 28 days post-MI with fasudil. RhoK inhibition has been shown to result in increased Bcl-2 expression 4 h after myocardial I/R (2), and RhoK overexpression was associated with increased Bax expression in neonatal rat cardiomyocytes (8). These prior studies clearly implicate the role of RhoK in myocardial apoptosis. It is possible, however, that the severity of ischemia in our permanent coronary artery occlusion model prevented such early (1 day after starting treatment) changes in Bcl-2 and Bax. Nevertheless, at 7 and 28 days, there was a clear attenuation in apoptosis with fasudil compared with saline control.
Along with apoptosis, cardiac fibrosis contributes to HF by exacerbating systolic dysfunction, hypertrophy, and chronic adverse remodeling (1, 4). The present study demonstrated that delayed sildenafil treatment limited the extent of LV scar formation, compared with saline treatment, at 7 and 28 days post-MI. The data signify that PDE-5 inhibition impeded adverse cardiac remodeling independent of its known infarct-sparing effect (24, 27–29). In addition, the lower cardiac hypertrophy index in sildenafil-treated mice may be due to the protective effect of sildenafil against myocardial fibrosis. PKG activation has been shown to inhibit transforming growth factor (TGF)-β-dependent diabetic renal fibrosis (40) and advanced glycation end product-induced renal fibroblast proliferation (15). Sildenafil could potentially act through a similar PKG-dependent mechanism to attenuate fibrosis in the ischemic myocardium. Furthermore, fasudil treatment limited fibrosis and myocyte enlargement following myocardial ischemia (26). This fasudil-mediated cardioprotection against LV remodeling was associated with the suppression of prohypertrophic and fibrogenic cytokines TGF-β2 and -β3 in a permanent LAD occlusion model (13). Similar to these studies, we also observed that fasudil reduced cardiac fibrosis and hypertrophy. Hence, sildenafil treatment could also act through its inhibitory effect on RhoK to reduce myocardial fibrosis.
In the present study, we administered two doses of sildenafil (21 mg·kg−1·day−1), which is 30 times higher than our previous sildenafil dosage of 0.71 mg/kg. We recently reported that while both high- and low-dose sildenafil preserved FS when initiated 3 days post-MI, the higher dosage conferred less LV dilatation in mice than the lower dosage (30). LV dilatation signifies the progression of HF from the compensated to decompensated stage (1). Accordingly, we chose the higher dose to examine the cardioprotective effect of delayed sildenafil treatment. The present hemodynamic data are similar to our previous findings, showing little change following intravenous and oral administration of sildenafil (24) as well as intraperitoneal injection of tadalafil (31). The hypotensive effects of our current sildenafil dose may be marginal, and thus with careful dosing, delayed treatment with sildenafil could be safe in a clinical setting without concomitant nitrate administration.
In summary, we demonstrated that delayed chronic treatment with sildenafil beginning at day 3 post-MI preserved LV function by reducing LV dilatation, apoptosis, fibrosis, and hypertrophy. The attenuation of LV dysfunction was shown to be independent of the infarct-sparing effect of sildenafil, and interestingly, the inhibition of RhoA/RhoK pathway was involved in sildenafil-induced cardioprotection. Therefore, we propose that PDE-5 inhibition may be a potential therapeutic strategy to limit the progression of advanced HF in patients.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-51045, HL-59469, and HL-79424 (to R. C. Kukreja) and from the American Heart Association National Scientist Development Grant 10SDG3770011 (to F. N. Salloum).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Sildenafil powder was kindly provided by Pfizer, Inc.
REFERENCES
- 1. Anversa P, Li P, Zhang X, Olivetti G, Capasso JM. Ischaemic myocardial injury and ventricular remodelling. Cardiovasc Res 27: 145–157, 1993 [DOI] [PubMed] [Google Scholar]
- 2. Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma X, Willette RN, Yue T. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res 61: 548–558, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol 152: 855–869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 35: 569–582, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Das A, Xi L, Kukreja RC. Phosphodiesterase-5 inhibitor, sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: essential role of NO signaling. J Biol Chem 280: 12944–12955, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Das A, Smolenski A, Lohmann SM, Kukreja RC. Cyclic GMP-dependent protein kinase Ialpha attenuates necrosis and apoptosis following ischemia/reoxygenation in adult cardiomyocyte. J Biol Chem 281: 38644–38652, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283: 29572–29585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem 282: 8069–8078, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Ghiadoni L, Versari D, Taddei S. Phosphodiesterase 5 inhibition in essential hypertension. Curr Hypertens Rep 10: 52–57, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Guazzi M, Tumminello G, Di Marco F, Fiorentini C, Guazzi MD. The effects of phosphodiesterase-5 inhibition with sildenafil on pulmonary hemodynamics and diffusion capacity, exercise ventilatory efficiency, and oxygen uptake kinetics in chronic heart failure. J Am Coll Cardiol 44: 2339–2348, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Guilluy C, Sauzeau V, Rolli-Derkinderen M, Guérin P, Sagan C, Pacaud P, Loirand G. Inhibition of RhoA/Rho kinase pathway is involved in the beneficial effect of sildenafil on pulmonary hypertension. Br J Pharmacol 146: 1010–1018, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation 109: 2234–2239, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol 294: L24–L33, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Huang JS, Chuang LY, Guh JY, Chen CJ, Yang YL, Chiang TA, Hung MY, Liao TN. Effect of nitric oxide-cGMP-dependent protein kinase activation on advanced glycation end-product-induced proliferation in renal fibroblasts. J Am Soc Nephrol 16: 2318–2329, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Ito M, Nishikawa M, Fujioka M, Miyahara M, Isaka N, Shiku H, Nakano T. Characterization of the isoenzymes of cyclic nucleotide phosphodiesterase in human platelets and the effects of E4021. Cell Signal 8: 575–581, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Lewis GD, Lachmann J, Camuso J, Lepore JJ, Shin J, Martinovic ME, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise hemodynamics and oxygen uptake in patients with systolic heart failure. Circulation 115: 59–66, 2007 [DOI] [PubMed] [Google Scholar]
- 18. MacLellan WR, Schneider MD. Death by design. Programmed cell death in cardiovascular biology and disease. Circ Res 81: 137–144, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 53: 207–215, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumi S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett 392: 189–193, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 99: 1426–1432, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290: C661–C668, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ockaili R, Salloum F, Hawkins J, Kukreja RC. Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial KATP channels in rabbits. Am J Physiol Heart Circ Physiol 283: H1263–H1269, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, Liu X, Gillijns H, Pellens M, Van Lommel A, Buys E, Schoonjans L, Vanhaecke J, Verbeken E, Sipido K, Herijgers P, Bloch KD, Janssens SP. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119: 408–416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren J, Fang CX. Small guanine nucleotide-binding protein Rho and myocardial function. Acta Pharmacol Sin 26: 279–285, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Salloum F, Yin C, Xi L, Kukreja RC. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92: 595–607, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Salloum FN, Takenoshita Y, Ockaili RA, Daoud VP, Chou E, Yoshida K, Kukreja RC. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol 42: 453–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, Kukreja RC. Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294: H1398–H1406, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Salloum FN, Abbate A, Toldo S, Chau VQ, Prabhakar S, Kukreja RC. Chronic treatment with sildenafil three days following myocardial infarction improves survival and left ventricular function in mice (Abstract). Circulation 118: S286, 2008 [Google Scholar]
- 31. Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. Phosphodiesterase-5 inhibitor, Tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase G dependent generation of hydrogen sulfide. Circulation 120: S31–S36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, Amin J, Apstein CS, Colucci WS. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol 279: H422–H428, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun 280: 798–805, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J 15: 1718–1726, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 25: 1767–1775, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 106: 57–62, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U; Fasudil Study Group Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol 46: 1803–1811, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol 83: 3C–12C, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Wang S, Wu X, Lincoln TM, Murphy-Ullrich JE. Expression of constitutively active cGMP-dependent protein kinase prevents glucose stimulation of thrombospondin 1 expression and TGF-beta activity. Diabetes 52: 2144–2150, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Wei CY, Huang KC, Chou YH, Hsieh PF, Lin KH, Lin WW. The role of Rho-associated kinase in differential regulation by statins of interleukin-1beta- and lipopolysaccharide-mediated nuclear factor kappaB activation and inducible nitric-oxide synthase gene expression in vascular smooth muscle cells. Mol Pharmacol 69: 960–967, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 24: 1842–1847, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]