Abstract
The induction of renal cyclooxygenase-2 (COX-2) in diabetes has been implicated in the renal functional and structural changes in models where hypertension or uninephrectomy was superimposed. We examined the protective effects of 3 mo treatment of streptozotocin-diabetic rats with a highly selective COX-2 inhibitor (SC-58236) in terms of albuminuria, renal hypertrophy, and the excretion of TNF-α and TGF-β, which have also been implicated in the detrimental renal effects of diabetes. SC-58236 treatment (3 mg·kg−1·day−1) of diabetic rats resulted in reduced urinary excretion of PGE2, 6-ketoPGF1α, and thromboxane B2, all of which were increased in the diabetic rat compared with age-matched nondiabetic rats. However, serum thromboxane B2 levels were unchanged, confirming the selectivity of SC-58236 for COX-2. The renal protective effects of treatment of diabetic rats with the COX-2 inhibitor were reflected by a marked reduction in albuminuria, a reduction in kidney weight-to-body weight ratio, and TGF-β excretion and a marked decrease in the urinary excretion of TNF-α. The protective effects of SC-58236 were independent of changes in plasma glucose levels or serum advanced glycation end-product levels, which were not different from those of untreated diabetic rats. In an additional study, the inhibition of COX-2 with SC-58236 for 4 wk in diabetic rats resulted in creatinine clearance rates not different from those of control rats. These results confirm that the inhibition of COX-2 in the streptozotocin-diabetic rat confers renal protection and suggest that the induction of COX-2 precedes the increases in cytokines, TNF-α, and TGF-β.
Keywords: cyclooxygenase-2 inhibition, kidney
there have been numerous reports of increased renal cyclooxygenase (COX) activity and prostaglandin production in diabetes (10, 27), which has been linked to hyperfiltration (8, 21) that precedes renal structural changes and the development of nephropathy (13, 22), although the cause and effect is not unequivocally established. Before the recognition of different COX isoforms, there were some studies linking altered renal arachidonic acid (AA) metabolism to the development of nephropathy by showing that NSAIDs exerted renal protective effects in diabetes (21). Following the discovery of multiple COX isoforms, it was shown that experimental diabetes results in the upregulation of renal COX-2 (17). We confirmed the increase in renal COX-2 in the diabetic rat and showed an association with enhanced prostaglandin release from the perfused kidney challenged with AA, which serves as an index of COX activity but does not distinguish the COX isoforms (24). Nonetheless, our studies have shown that interventions that prevent the upregulation of renal COX-2 in the diabetic rat are, for the most part, associated with a reduction of the exaggerated renal release of prostaglandins in response to AA, suggesting that increased COX-2 expression/activity contributes to the enhanced prostaglandin production in the diabetic rat (2, 3, 18). Subsequently, the upregulation of renal COX-2 was linked to elevated glomerular filtration rate (GFR) using inhibitors of COX-2 (17). We confirmed that an acute inhibition of COX-2 with nimesulide, which reduced the exaggerated renal release of prostaglandins (24), reduced GFR in the diabetic rat using creatinine clearance as an index (unpublished observations). Although COX-2 is considered as an inducible enzyme and is upregulated in inflammatory conditions, it is also constitutively expressed at specific sites in the kidney and vasculature (6, 11) and there is evidence that COX-2 is the source of vascular prostacyclin (20), providing an explanation for the cardiovascular toxicity of COX-2 inhibitors. However, the inhibition of COX-2 has been shown to attenuate the development of glomerulosclerosis in uninephrectomized diabetic rats (16) and to reduce the appearance of markers of renal damage in a hypertensive diabetic rat model (5). Thus the inhibition of COX-2 as a protective strategy against the development of nephropathy has been addressed in models of diabetes superimposed on hypertension or uninephrectomy to induce and accelerate renal damage in the rat (5, 16), which is resistant to diabetic nephropathy. The study reported here was undertaken to assess the protective effects, if any, of chronic administration of a highly selective COX-2 inhibitor in a diabetic model without the complicating influences imposed by hypertension or uninephrectomy. Consequently, diabetic rats were treated with SC-58236 for 12 wk, and the effects on urinary albumin, TNF-α, and TGF-β were determined as markers for renal damage. Thus diabetes is associated with albuminuria and increased renal levels of TNF-α and TGF-β, which have been implicated in the development of diabetic nephropathy (15). To verify the selectivity of COX-2 inhibition by SC-58236, we measured the urinary excretion of PGE2 and 6-ketoPGF1α and serum thromboxane levels as platelet-derived thromboxane is considered to arise solely from COX-1. The effects of chronic inhibition of COX-2 on AA-stimulated release of 6-ketoPGF1α from the isolated perfused kidney were also determined. In a supplementary study, we assessed the influence of chronic inhibition of COX-2 for 4 wk on GFR in diabetic rats using creatinine clearance as an index.
METHODS
The studies were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Male Wistar rats (6 to 7 wk old) were made diabetic with streptozotocin (STZ; 70 mg/kg iv), whereas age-matched control rats received an equivalent volume of the vehicle, citrate buffer (pH 4.5). Forty-eight hours after the administration of STZ, some of the diabetic rats were treated with 4-[5-(4-chlorophenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide (SC-58236; 3 mg·kg−1·day−1) added to the drinking water. SC-58236 was dissolved in 95% polyethylene glycol and 5% Tween 20 before addition to the drinking water. The concentration of SC-58236 in the drinking water was adjusted daily according to the weight of the rats and the water consumption over the previous 24 h. Treatment was continued for 12–14 wk as signs of renal pathology such as albuminuria, glomerular hypertrophy, and mesangial matrix expansion are evident after 3 mo of diabetes (31).
Twelve weeks after starting treatment, rats were placed in metabolic cages, and an 8-h urine collection was obtained. Urine samples were separated into aliquots and frozen before analysis for prostaglandins and thromboxane, albumin, TNF-α, and TGF-β.
Twelve to fourteen weeks after the initiation of treatment with SC-58236, rats were prepared for perfusion of the right kidney. The left kidney was removed and weighed. After anesthesia and before perfusion of the kidney, a blood sample was withdrawn via cardiac puncture for the determination of serum thromboxane B2 (TxB2).
In a subsequent study, to address the effects of chronic inhibition of COX-2 on creatinine clearance as an index of GFR, 6- to 7-wk-old male Wistar rats were made diabetic with STZ or given an equivalent volume of vehicle. Forty-eight hours later, treatment with SC-58236 was initiated and continued for 4 wk, at which time a 24 h urine collection was obtained as well as plasma from a blood sample drawn via cardiac puncture under isoflurane-induced anesthesia. Samples were frozen at −35°C until analysis for creatinine using the Quantichrome Creatinine Assay kit from BioAssay Systems (Hayward, CA).
Isolated perfused kidney.
Rats were anesthetized with pentobarbitone (65 mg/kg ip). After a blood sample via cardiac puncture was obtained, a midline laparotomy was performed and the right renal artery cannulated via the mesenteric artery to prevent an interruption of blood flow to the kidney, which was perfused with oxygenated Krebs' Henseleit buffer at 37°C. Following excision, the kidney was perfused at a constant flow at a rate to obtain a perfusion pressure of ∼60 mmHg. For those kidneys obtained from rats treated with SC-58236, the perfusate was supplemented with 100 nM SC-58236. Once a stable perfusion pressure was obtained, vasoconstrictor responses to 1 and 3 μg AA and 100 ng phenylephrine were determined. The perfusate was collected for 1 min before and 1 min after the administration of 1 μg AA for the determination of 6-ketoPGF1α, the hydrolysis product of prostacyclin as an index of renal COX activity.
Prostanoids.
Urinary PGE2, urinary and perfusate 6-ketoPGF1α, urinary TxB2, and serum TxB2 were measured by ELISA using kits obtained from Cayman Chemical (Ann Arbor, MI). Serum was obtained from blood samples incubated at room temperature for 1 h, and serum TxB2 levels were used as an index of COX-1 activity to evaluate the selectivity of SC-58236 for COX-2.
Nε-(carboxymethyl) lysine.
Nε-(carboxymethyl) lysine (CML) is a major antigenic component of advanced glycation end products (AGEs) and was measured in serum obtained from blood samples incubated at room temperature for 1 h using an ELISA kit from Cell Biolabs (San Diego, CA). Protein concentration of the serum samples was measured with the Bio-Rad protein assay before adjusting the concentration to 10 μg/ml and further diluting the samples for assay of CML.
Albumin.
Urinary albumin was measured using an ELISA for rat albumin with materials obtained from Bethyl Laboratories (Montgomery, TX).
TGF-β.
Urinary TGF-β was measured using the Emax Immunoassay System from Promega (Madisom, WI), following an acid treatment of the samples according to the manufacturer's instructions.
TNF-α.
Urinary TNF-α was measured using an ELISA kit from BD Biosciences (San Diego, CA) according to the manufacturer's instructions.
Analysis of results.
Data are expressed as means ± SE and compared using an unpaired t-test or ANOVA followed by Newman-Keuls test. A P value < 0.05 was considered statistically significant.
RESULTS
Body weight and blood glucose.
At the time of death, the mean body weight for the untreated diabetic group was 291 ± 15 g (n = 7) compared with 363 ± 21 g (n = 4) for the diabetic group treated with SC-58236 (P < 0.05). The mean body weight for the age-matched control group was 576 ± 20 g (n = 5; P < 0.01 vs. the diabetic groups). Mean blood glucose was 104 ± 2 mg/dl for the nondiabetic group compared with 367 ± 15 mg/dl for the untreated diabetic group and 356 ± 22 mg/dl for the diabetic group treated with the COX-2 inhibitor.
Serum CML.
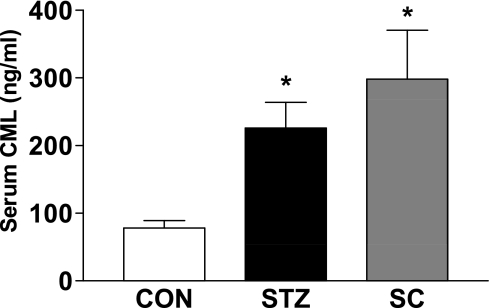
As treatment of diabetic rats with the COX-2 inhibitor did not affect blood glucose levels, we evaluated any effect of SC-58236 treatment on the formation of AGEs, which have been linked to the development of diabetic complications including nephropathy. A measurement of serum CML levels revealed an almost threefold increase in the untreated diabetic rats compared with the nondiabetic rats (Fig. 1). Consistent with the lack of effect of COX-2 inhibition on plasma glucose levels, SC-58236 was without effect on CML levels.
Fig. 1.
Effect of treatment of diabetic rats with SC-58236 (SC; 3 mg·kg−1·day−1) for 12 wk on serum levels of Nε-(carboxymethyl) lysine (CML) where n = 8 for the untreated diabetic group, n = 3 for the treated diabetic group, and n = 5 for the control group. STZ, streptozotocin. *P < 0.05 vs. the control group (CON).
Serum TxB2.
Levels of TxB2 in serum samples from the two diabetic groups and the control group were not significantly different. Thus, in the untreated diabetic group (n = 8), mean TxB2 was 269 ± 50 ng/ml compared with 232 ± 45 ng/ml for the diabetic group treated with SC-58236 (n = 3) and 199 ± 42 ng/ml for the control group (n = 5). The lack of difference between the two diabetic groups indicates that SC-58236 was without effect on platelet COX-1 activity.
Urinary prostanoids.
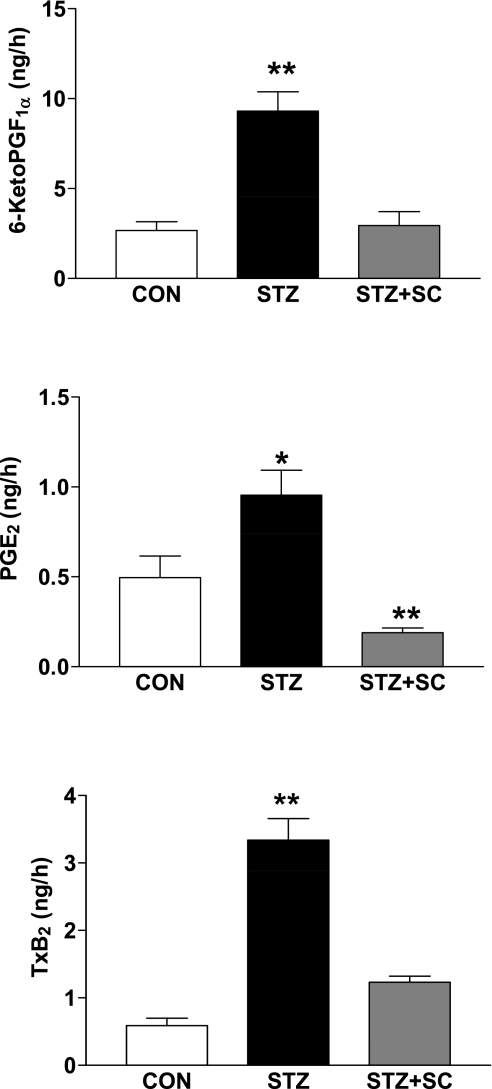
We determined 6-ketoPGF1α, PGE2, and TxB2 in urine samples obtained from the three groups of rats. The volumes for the 8-h urine collections were 9.1 ± 1.1, 20.9 ± 2.9, and 19.7 ± 1.2 ml for the control, diabetic, and SC-58236-treated diabetic groups, respectively. The excretion rates for all three prostanoids were increased in the diabetic rats compared with the age-matched control rats. Thus 6-ketoPGF1α was increased more than threefold, PGE2 almost twofold, and TxB2 more than fivefold (Fig. 2). In the diabetic group that was treated with the COX-2 inhibitor, the excretion of 6-ketoPGF1α was not different from that of the control group, whereas PGE2 excretion was reduced to a level lower than that of the control group and TxB2 was reduced to approximately twofold that of the control group (Fig. 2). These results, coupled with those showing no effect of SC-58236 treatment on serum TxB2 levels, indicate the selectivity of the COX-2 inhibitor and suggest that the increases in urinary excretion of 6-ketoPGF1α, PGE2, and TxB2 in the diabetic rat derive, in large part, from COX-2 activity.
Fig. 2.
Urinary excretion (in ng/h) of 6-ketoPGF1α (**P < 0.01 vs. other groups), PGE2 (*P < 0.05 vs. CON; **P < 0.01 vs. STZ), and thromboxane B2 (TxB2; **P < 0.01 vs. other groups) in untreated diabetic rats (STZ; n = 7), diabetic rats treated with SC-58236 (3 mg·kg−1·day−1; STZ + SC; n = 4) for 3 mo, and age-matched control rats (CON; n = 5).
Isolated perfused kidney.
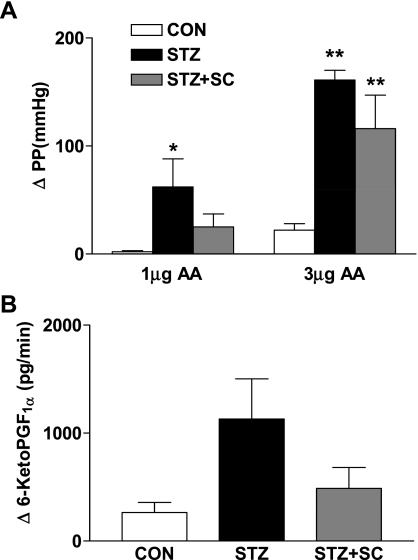
Perfusion pressures and flow rates in the perfused kidneys from the different groups of rats were not different. Thus perfusion pressure in the control group was 64 ± 2 mmHg and perfusate flow was 13.2 ± 0.6 ml/min when compared with 60 ± 5 mmHg and 12.1 ± 1.0 ml/min, respectively, for the diabetic group and 59 ± 5 mmHg and 12.9 ± 1.6 ml/min, respectively, for the treated diabetic group. As we have previously reported, the renal vasoconstrictor effect of AA was markedly enhanced in kidneys from the diabetic group (n = 5) compared with kidneys from nondiabetic rats (n = 4) (Fig. 3). For example, 3 μg AA increased perfusion pressure by 161 ± 9 mmHg in the diabetic rat kidney compared with 22 ± 6 mmHg in the control rat kidneys. Treatment of diabetic rats with SC-58236 (n = 4) tended to reduce the vasoconstrictor effect of AA, but the difference did not achieve significance (Fig. 3). In previous studies, we found that the increased renal vasoconstrictor response to AA that is mediated by endoperoxides (25) was associated with increased release of prostaglandins from the perfused kidney. In the present study, the increase in the release of 6-ketoPGF1α from the diabetic rat kidney following challenge with 1 μg AA was threefold that of the control kidney but the difference did not achieve significance because of the high variability. Treatment of diabetic rats with SC-58236 was associated with a smaller increase in the release of 6-ketoPGF1α compared with that from untreated diabetic rat kidneys, but, again, the difference was not significant (Fig. 3).
Fig. 3.
A: increases in perfusion pressure (PP; in mmHg) in response to arachidonic acid (AA) in isolated perfused kidneys from age-matched control rats (CON; n = 4), diabetic rats (STZ; n = 5), and diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 4). *P < 0.05 vs. control; **P < 0.01 vs. control. B: release of 6-ketoPGF1α (in pg/min) from isolated perfused kidneys of age-matched control rats (CON; n = 4), untreated diabetic rats (STZ; n = 5), and diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 4).
Renal vasoconstrictor responses to 100 ng phenylephrine were not different among the groups, and increases in perfusion pressure were 15 ± 9, 17 ± 5, and 33 ± 14 mmHg for the control, diabetic, and treated diabetic groups, respectively.
Urinary TNF-α.
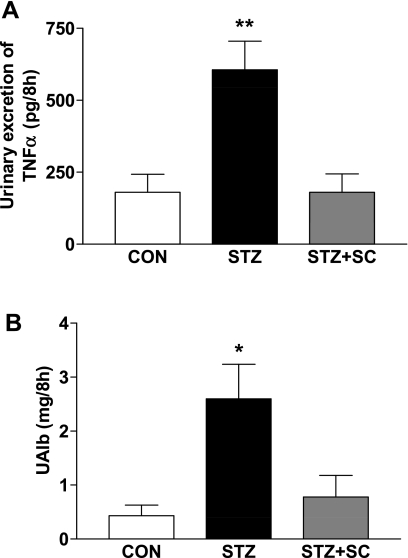
Excretion of TNF-α was threefold greater in the diabetic (n = 6) compared with the age-matched control (n = 5) rats (P < 0.01; Fig. 4). This increase in TNF-α excretion was abolished when diabetic rats were treated with SC-58236 (n = 5) for 12 wk (P < 0.01).
Fig. 4.
A: urinary excretion of TNF-α (in pg/8 h) in age-matched control rats (CON; n = 5), untreated diabetic rats (STZ; n = 6), and diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 5). **P < 0.01 vs. citrate and STZ + SC. B: urinary excretion of albumin (UAlb; in μg/8 h) in age-matched control rats (CON; n = 4), untreated diabetic rats (STZ; n = 6), and diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 5). *P < 0.05 vs. CON and STZ + SC.
Urinary albumin.
Diabetes resulted in more than a fivefold increase in the urinary excretion of albumin compared with that of age-matched control rats (Fig. 4). In diabetic rats treated with SC-58236 to inhibit COX-2, the increase in urinary albumin excretion was prevented, providing further evidence for a renal protective effect of inhibition of COX-2 in the diabetic rat.
Urinary TGF-β.
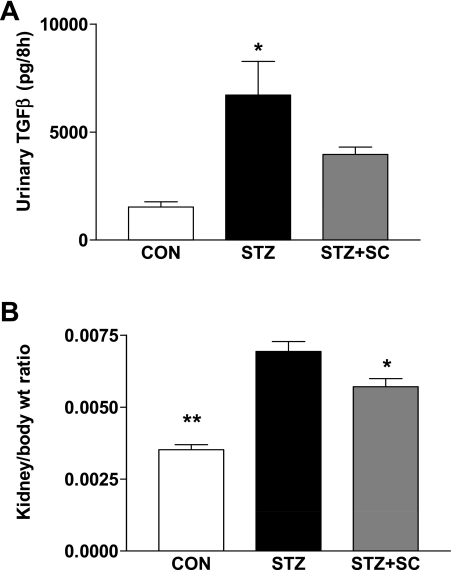
Urinary excretion of TGF-β was increased more than fourfold (P < 0.01) in diabetic rats (n = 6) compared with age-matched control rats (n = 5; Fig. 5). Treatment of diabetic rats with SC-58236 (n = 4) attenuated the increase in the excretion of TGF-β observed in the diabetic rat (not significant), although the excretion remained greater than twofold that of the nondiabetic control group (not significant).
Fig. 5.
A: urinary excretion of TGF-β (in pg/8 h) in age-matched control rats (CON; n = 5), untreated diabetic rats (STZ; n = 6), and diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 4). *P < 0.01 vs. control. B: left kidney weight-to-body weight ratio in untreated diabetic rats (STZ; n = 5), diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 12 wk (STZ + SC; n = 3), and nondiabetic rats (CON; n = 5). *P < 0.05 vs. STZ; **P < 0.01 vs. STZ and STZ + SC.
Ratio of kidney weight to body weight.
Associated with the attenuation of urinary excretion of TGF-β in the diabetic rats treated with the COX-2 inhibitor, we found a reduction in the left kidney weight-to-body weight ratio compared with that in untreated diabetic rats (Fig. 5). Thus, in the untreated diabetic group (n = 5), the kidney weight-to-body weight ratio was almost double that of the control group (n = 6), and this ratio was significantly less in the diabetic group treated with SC-58236 (n = 3). However, there were no significant differences in the mean weights of the left kidney from the 3 groups; these were 2.013 ± 0.13, 1.96 ± 0.11, and 2.02 ± 0.12 g for the control, diabetic, and SC-58236-treated diabetic groups, respectively. The reduction of kidney weight-to-body weight ratio in the treated versus the untreated diabetic groups, coupled with the attenuation of the increases in the urinary excretion of the cytokines TNF-α and TGF-β, suggests a protective effect of the COX-2 inhibitor.
Creatinine clearance.
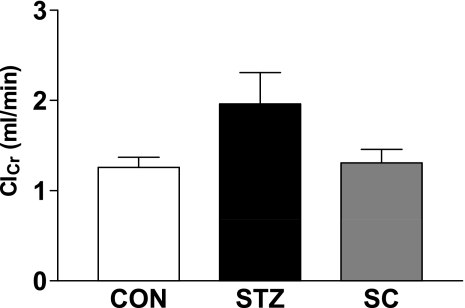
To further evaluate any potential renal protective effect of the inhibition of COX-2 in the diabetic rat, we conducted an additional study where diabetic rats were treated with SC-58236 (n = 5) for ∼4 wk. Creatinine clearance was determined as an index of GFR and compared with that for the untreated diabetic rats (n = 4) and the age-matched control rats (n = 6). As shown in Fig. 6, creatinine clearance was greater in the diabetic rats than the nondiabetic rats but the difference did not quite achieve significance (P = 0.053). In the diabetic rats treated with SC-58236, creatinine clearance was very similar to that of the nondiabetic rats, indicating that a chronic inhibition of COX-2 may reduce the hyperfiltration of diabetes and supporting earlier results where we found that an acute inhibition of COX-2 with nimesulide reduced the hyperfiltration of diabetes.
Fig. 6.
Creatinine clearance (ClCr; ml/min) in untreated diabetic rats (STZ; n = 4), diabetic rats treated with SC-58236 (3 mg·kg−1·day−1) for 4 wk (STZ + SC; n = 5), and age-matched control rats (CON; n = 6). P = 0.053 STZ vs. CON.
DISCUSSION
The results of the studies presented here suggest that the upregulation of renal COX-2 and the associated increases in renal prostaglandin formation in diabetes are detrimental, contributing to the hypertrophy and albuminuria, which may be linked to increases in TNF-α and TGF-β. Thus treatment of diabetic rats with a highly selective inhibitor of COX-2 to reduce renal prostaglandin formation provided a renoprotective effect that was reflected by decreased albuminuria and renal TNF-α and TGF-β production.
There are numerous reports of increased renal COX activity and the conversion of AA to prostaglandins in diabetes. The increase in renal prostaglandins was linked to the characteristic hyperfiltration of diabetes by showing that the inhibition of COX with NSAIDs reduced GFR in human and experimental diabetes. Following the recognition of multiple COX isoforms, it was shown that the inducible form, COX-2, which is increased in inflammatory conditions, possibly in response to oxidative stress (18), was upregulated in kidneys of diabetic rats (17, 24). Thus we have shown that Tempol treatment of diabetic rats prevented the induction of renal COX-2 (18). Similarly, the inhibition of NO formation in diabetic rats prevented the upregulation of COX-2 (2), suggesting a possible role of peroxynitrite, and our recent study revealed that treatment of diabetic rats with a peroxynitrite decomposition catalyst also prevented the induction of renal COX-2 as well as the increase in creatinine clearance (4). The report by Komers et al. (17) also revealed that an acute inhibition of COX-2 reduced the elevation of GFR in the STZ-diabetic rat. In subsequent studies in diabetic rat models with the superimposition of hypertension or uninephrectomy to induce and accelerate renal damage, it was shown that the inhibition of COX-2 provided a protective effect (5, 16). Thus the inhibition of COX-2 reduced mesangial matrix expansion in hypertensive diabetic rats (5) and glomerulosclerosis in uninephrectomized diabetic rats (16). The COX-2 product or products responsible for the detrimental renal effects in diabetes were not addressed in these studies, but Makino et al. (19) reported that selective antagonism of the E-prostanoid type 1 receptor was protective in the STZ-diabetic rat model. More recently, these findings were extended to a mouse model of type 1 diabetes where inhibition of COX-2 was shown to exert a renoprotective effect and reduce glomerular hypertrophy (23). The results of the present study confirm these findings by showing that the inhibition of COX-2 with SC-58236 in the STZ-diabetic rat reduced the increase in urinary albumin, a marker for renal damage. A histological examination of renal sections from two rats from each group revealed glomerular hypertrophy and mesangial expansion in the diabetic rats, whereas treatment of diabetic rats with SC-58236 reduced glomerular hypertrophy. Moreover, we found that a chronic treatment of the diabetic rat for 1 mo with SC-58236 resulted in a reduction of creatinine clearance, although the increase in the diabetic rat did not achieve statistical significance. Nonetheless, there is considerable evidence for a contribution of prostaglandins in the hyperfiltration of diabetes that precedes the onset of diabetic nephropathy and may play a causative role (12). The identity of the prostaglandin(s) that contributes to hyperfiltration in diabetes remains unknown, but reported increases in the urinary excretion of PGE2 and the 6-ketoPGF1α (17, 19), which we have confirmed in this study, implicate PGE2 and/or PGI2. Treatment of diabetic rats with SC-58236 also resulted in a slight but significant decrease in the kidney weight-to-body weight ratio, indicative of an effect to reduce renal hypertrophy and consistent with results of the study in the type 1 diabetic mouse model (23). It should be noted that the body weight of the diabetic rats treated with the COX-2 inhibitor was greater than that of the untreated diabetic rats, suggesting a beneficial effect that was unrelated to the improvement of glucose control as SC-58236 was without effect on blood glucose levels. Consistent with the lack of effect of COX-2 inhibition on plasma glucose levels, we found no effect of SC-58236 on the increased serum levels of CML, a major component of AGEs, in diabetic rats. This observation does not exclude a role for AGEs in the development of diabetic nephropathy, and it is possible that AGEs contribute to the induction of COX-2 in diabetes.
The hypertrophy of the kidney in the diabetic rat was associated with a marked increase in the urinary excretion of TGF-β, a factor that is involved in growth and proliferation and cellular differentiation and which has been implicated in some of the renal manifestations of diabetes (26, 28). Increased TGF-β gene expression has been reported for the BB rat and the NOD mouse, models of type 1 diabetes (29), and TGF-β protein expression was increased in glomeruli from a patient with diabetes (30). Moreover, the inhibition of TGF-β reduced renal hypertrophy in STZ-diabetic mice (28) and reduced urinary albumin excretion in STZ-diabetic rats (26). In the present study, we found that the treatment of diabetic rats with SC-58236 attenuated the renal hypertrophy, and this effect was associated with a reduction, albeit insignificant, in the urinary excretion of TGF-β, which was greatly increased in diabetic compared with nondiabetic rats. This observation suggests a possible role for COX-2 in the increase in TGF-β but provides no insight into the mechanism. The interactions of TGF-β and COX appear complex with reports that TGF-β can lead to the induction of COX-2, as well as reports that inhibitors of COX-2 reduce renal TGF-β. Thus aspirin treatment of the STZ-diabetic rat inhibited the upregulation of TGF-β and attenuated mesangial expansion (19), and Cheng et al. (5) reported that the inhibition of COX-2 with SC-58236 decreased renal TGF-β expression in a hypertensive diabetic model.
We also found that the urinary excretion of TNF-α was increased in the diabetic rat, confirming the results of Kalantarinia et al. (15). The increase in TNF-α, which is produced by glomeruli, mesangial, endothelial, and renal tubular cells, as well as immune cells, has been linked to the development of diabetic nephropathy. Thus the increase in TNF-α has been associated with albuminuria, and a TNF-α antagonist prevented renal hypertrophy in the STZ-diabetic rat (7). We found that the treatment of diabetic rats with a COX-2 inhibitor reduced both the albuminuria and the increase in TNF-α, supporting the observations of Kalantarinia et al. (15) that increased urinary and renal interstitial TNF-α levels preceded the onset of albuminuria. Based on our results, it would seem that the increase in COX-2 is proximal to the increase in TNF-α, although it is well established that TNF-α can lead to the upregulation of COX-2 (1). However, our observations are not without precedent as aspirin and meloxicam reduced retinal TNF-α levels in diabetic rats (14) and indomethacin was shown to reduce the gene expression for TNF-α in mice subjected to ischemia-reperfusion (9). The reduction of TNF-α may reflect an anti-inflammatory effect of SC-58236, whereby macrophage infiltration of the kidney is reduced, a possibility not addressed in this study.
We have shown that a chronic administration of SC-58236 to diabetic rats reduced the urinary excretion of 6-ketoPGF1α, PGE2, and TxB2, suggesting that these prostanoids derive, in part, from the activity of COX-2. Alternatively, SC-58236 may exhibit inhibitory effects on COX-1, although it has been reported to have 1,000-fold greater activity against COX-2. To evaluate a potential effect of SC-58236 on COX-1, we measured serum TxB2 levels in treated and untreated rats as platelet-derived thromboxane is considered to arise solely from the activity of COX-1. There was no effect of SC-58236 on serum TxB2 levels in diabetic rats, suggesting that COX-1 activity was unaffected at the dosage used. As we have previously reported, urinary 6-ketoPGF1α and TxB2 were increased in diabetic rats and returned to control levels with treatment with the COX-2 inhibitor. Similarly, in this study, we found that urinary PGE2 excretion was increased in the diabetic rat in agreement with the results of Komers et al. (17) and Makino et al. (19), and the treatment of the diabetic rats with SC-58236 greatly reduced the excretion to levels below those observed in the age-matched control rats. As SC-58236 treatment of diabetic rats also prevented an increase in GFR, it is likely that COX-2-derived prostaglandins play a role in the renal functional manifestations of diabetes.
In summary, treatment of diabetic rats with a highly selective COX-2 inhibitor reduced albuminuria and hypertrophy, signifying a renal protective effect that was accompanied by reductions in TNF-α and TGF-β. These results confirm and extend those from other studies that showed protective effects of COX-2 inhibition in hypertensive or uninephrectomized models of diabetes. It is not feasible to treat patients with diabetes with COX-2 inhibitors because of the cardiovascular risks associated with the inhibition of vascular prostacyclin production. However, the removal of the stimulus for the induction of renal COX-2 (without affecting vascular COX-2 and prostacyclin production) may be a feasible goal and should prevent downstream events such as the increase in TNF-α, which have been linked to diabetic nephropathy.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-25394.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1. Bakhle YS, Botting RM. Cyclooxygenase-2 and its regulation in inflammation. Mediators Inflamm 5: 305–323, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen YJ, Li J, Quilley J. Effect of inhibition of nitric oxide synthase on renal cyclooxygenase in the diabetic rat. Eur J Pharmacol 541: 80–86, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Chen YJ, Quilley J. Fenofibrate treatment of diabetic rats reduces nitrosative stress, renal COX-2 expression and enhanced renal prostaglandin release. J Pharmacol Exp Ther 324: 658–663, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Chen YJ, Santos M, Quilley J. Treatment of diabetic rats with a peroxynitrite decomposition catalyst prevents induction of renal COX-2. Am J Physiol Heart Circ Physiol 300: H1125–H1132, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int 62: 929–939, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Davidge ST. Prostaglandin H synthase and vascular function. Circ Res 89: 650–660, 2001 [DOI] [PubMed] [Google Scholar]
- 7. DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol 284: F113–F121, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Esmatjes E, Fernandez MR, Halperin I, Camps J, Gaya J, Arroyo V, Rivera F, Figuerola D. Renal hemodynamic abnormalities in patients with short term insulin-dependent diabetes mellitus: role of renal prostaglandins. J Clin Endocrinol Metab 60: 1231–1236, 1985 [DOI] [PubMed] [Google Scholar]
- 9. Feitoza CQ, Goncalves GM, Semedo P, Cenedeze MA, Pinheiro HS, Beraldo FC, dos Santos OF, Teixeira VP, dos Reis MA, Mazzali M, Pacheco-Silva A, Camara NO. Inhibition of COX 1 and 2 prior to renal ischemia/reperfusion injury decreases the development of fibrosis. Mol Med 14: 724–730, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerrard JM, Stuart MJ, Rao GH, Steffes MW, Mauer SM, Brown DM, White JG. Alteration in the balance of prostaglandin and thromboxane synthesis in diabetic rats. J Lab Clin Med 95: 950–958, 1980 [PubMed] [Google Scholar]
- 11. Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest 94: 2504–2510, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hostetter TH. Pathogenesis of diabetic glomerulopathy: hemodynamic considerations. Semin Nephrol 10: 219–227, 1990 [PubMed] [Google Scholar]
- 13. Jensen PK, Christiansen JS, Steven K, Parving HH. Renal function in streptozotocin-diabetic rats. Diabetologia 21: 409–414, 1981 [PubMed] [Google Scholar]
- 14. Joussen AM, Poulaki V, Mitsiades N, Kirchhof B, Koizumi K, Dohmen S, Adamis AP. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J 16: 438–440, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Kalantarinia K, Awad AS, Siragy HM. Urinary and renal interstitial concentrations of TNF-alpha increase prior to the rise in albuminuria in diabetic rats. Kidney Int 64: 1208–1213, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Komers R, Lindsley JN, Oyama TT, Anderson S. Cyclo-oxygenase-2 inhibition attenuates the progression of nephropathy in uninephrectomized diabetic rats. Clin Exp Pharmacol Physiol 34: 36–41, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Komers R, Lindsley JN, Oyama TT, Schutzer WE, Reed JF, Mader SL, Anderson S. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. J Clin Invest 107: 889–898, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Chen YJ, Quilley J. Effect of tempol on renal cyclooxygenase expression and activity in experimental diabetes in the rat. J Pharmacol Exp Ther 314: 818–824, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Makino H, Tanaka I, Mukoyama M, Sugawara A, Mori K, Muro S, Suganami T, Yahata K, Ishibashi R, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol 13: 1757–1765, 2002 [DOI] [PubMed] [Google Scholar]
- 20. McAdam BF, Catella-Lawson F, Mardini IA, Kapoor S, Lawson JA, FitzGerald GA. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: The human pharmacology of a selective inhibitor of COX-2. Proc Natl Acad Sci USA 96: 272–277, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moel DI, Safirstein RL, McEvoy RC, Hsueh W. Effect of aspirin on experimental diabetic nephropathy. J Lab Clin Med 110: 300–307, 1987 [PubMed] [Google Scholar]
- 22. Mogensen CE, Steffes MW, Deckert T, Christiansen JS. Functional and morphological renal manifestations in diabetes mellitus. Diabetologia 21: 89–93, 1981 [DOI] [PubMed] [Google Scholar]
- 23. Nasrallah R, Robertson SJ, Hebert RL. Chronic COX inhibition reduces diabetes-induced hyperfiltration, proteinuria, and renal pathological markers in 36-week B6-Ins2 (Akita) mice. Am J Nephrol 30: 346–353, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Quilley J, Chen YJ. Role of COX-2 in the enhanced vasoconstrictor effect of arachidonic acid in the diabetic rat kidney. Hypertension 42: 837–843, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Quilley J, McGiff JC, Nasjletti A. Role of endoperoxides in arachidonic acid-induced vasoconstriction in the isolated perfused kidney of the rat. Br J Pharmacol 96: 111–116, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Russo LM, del RE, Brown D, Lin HY. Evidence for a role of transforming growth factor (TGF)-beta1 in the induction of postglomerular albuminuria in diabetic nephropathy: amelioration by soluble TGF-beta type II receptor. Diabetes 56: 380–388, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Schambelan M, Blake S, Sraer J, Bens M, Nivez MP, Wahbe F. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest 75: 404–412, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-beta 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1094–F1101, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor beta is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 90: 1814–1818, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yong LC, Bleasel AF. Pathological changes in streptozotocin induced diabetes mellitus in the rat. Exp Pathol 30: 97–107, 1986 [DOI] [PubMed] [Google Scholar]