Abstract
Mechanisms associated with right ventricular (RV) hypertension and arrhythmias are less understood than those in the left ventricle (LV). The aim of our study was to investigate whether and by what mechanisms a proarrhythmic substrate exists in a rat model of RV hypertension and hypertrophy. Rats were injected with monocrotaline (MCT; 60 mg/kg) to induce pulmonary artery hypertension or with saline (CON). Myocardial levels of mRNA for genes expressing ion channels were measured by real-time RT-PCR. Monophasic action potential duration (MAPD) was recorded in isolated Langendorff-perfused hearts. MAPD restitution was measured, and arrhythmias were induced by burst stimulation. Twenty-two to twenty-six days after treatment, MCT animals had RV hypertension, hypertrophy, and decreased ejection fractions compared with CON. A greater proportion of MCT hearts developed sustained ventricular tachycardias/fibrillation (0.83 MCT vs. 0.14 CON). MAPD was prolonged in RV and less so in the LV of MCT hearts. There were decreased levels of mRNA for K+ channels. Restitution curves of MCT RV were steeper than CON RV or either LV. Dispersion of MAPD was greater in MCT hearts and was dependent on stimulation frequency. Computer simulations based on ion channel gene expression closely predicted experimental changes in MAPD and restitution. We have identified a proarrhythmic substrate in the hearts of MCT-treated rats. We conclude that steeper RV electrical restitution and rate-dependant RV-LV action potential duration dispersion may be contributing mechanisms and be implicated in the generation of arrhythmias associated with in RV hypertension and hypertrophy.
Keywords: electrical restitution, fibrillation, action potentials
increased pulmonary arterial pressure increases loading on the right ventricle (RV), which can lead to electrical, mechanical, and structural remodeling. In humans, pulmonary artery hypertension (PAH) is a disease that can be unstable and RV failure is a common outcome (4, 13, 38). The QT interval and QT dispersion of the ECG have been reported to be increased (19, 20), and the shift in ECG derived ventricular gradient [reflecting action potential duration (APD) dispersion] has been related to increased RV load (17). Such changes in electrical activity have been linked to arrhythmias (1, 39), and PAH is associated with arrhythmias in patients (11). RV pathophysiology is in general understudied compared with the left ventricle (LV; Ref. 38), and the mechanisms that underlie RV changes in electrical activity in response to hypertension are not well understood.
The monocrotaline (MCT)-induced model of pulmonary arterial hypertension and RV hypertrophy is well established (e.g., Refs. 14, 23). MCT is a pyrrolizidine alkaloid from the plant Crotalaria spectabilis. A single injection of MCT results in injury to the vascular endothelium of the lung, pulmonary hypertension, and RV hypertrophy and failure occurs within 3–4 wk (15, 24). Heart failure can be identified by clinical symptoms that have been linked to hemodynamic indexes of right heart failure (15). Following MCT treatment, the RV action potential is prolonged (9, 26, 32). This is associated with decreased K+ channel activity, although studies disagree precisely which channels (26, 27, 32, 41). Interestingly, the changes in ventricular gradient and QT interval seen in human PAH (17, 19, 20) are also seen in the MCT model (16, 27, 32). These observations would predict that the MCT model is proarrhythmic, but this has not previously been directly investigated. The purpose of this study was to test the hypothesis that the MCT model of pulmonary hypertension has a proarrhythmic substrate and, if so, to investigate associated mechanisms to better understand arrhythmias associated with RV hypertension and hypertrophy.
MATERIALS AND METHODS
Animal model.
All experiments were conducted in accordance with the Animals (Scientific Procedures) Act 1986, with the approval of the UK Home Office. Male Wistar rats (200 g) received a single intraperitoneal injection of MCT (60 mg/kg in saline) or an equivalent volume of saline. Animals were weighed weekly for 3 wk postinjection and then daily. Ethical permissions required intervention on the observation of clinical signs of heart failure (e.g., dyspnea, cold extremities, lethargy, and weight loss) to prevent unplanned mortality (see Refs. 9, 16). Control (CON) animals were killed on equivalent days, postinjection.
In vivo measurement of RV function.
Rats were anesthetized with isoflurane (5%) and oxygen. Following induction of anesthesia, they were placed on a heated pad to control body temperature. Anesthesia was then maintained using 1.5–2.5% isoflurane. While the animals were under an OPMI pico surgical stereomicroscope (Carl Zeiss), a midline incision was made in the neck and the left and right jugular veins were isolated. A 1.4-F combined pressure-conductance catheter (model SPR-839; Millar Instruments) was advanced into the RV via the right jugular vein and secured in place. The catheter was connected to a pressure-conductance unit (MPVS-300; Millar Instruments), and the data were acquired on a Power Lab 8/30 (AD Instruments) attached to a computer running LabChart Pro (AD Instruments). The pressure-volume loops were analyzed using PVAN 3.6 software (Millar Instruments) to determine the cardiac hemodynamics. A cannula was inserted into the left jugular vein to inject a bolus (20 μl) of hypertonic saline (15%) to determine the parallel conductance of surrounding tissue (30). Before cardiac catheterization, the Millar device was calibrated for pressure using a mercury manometer. At the end of the experiment, fresh heparinized blood was collected from the animal and used to fill a cuvette (Millar P/N 910–1048) to calibrate for volume.
Measurement of monophasic action potentials and electrical restitution curves.
Isolated hearts were mounted on a Langendorff perfusion system and perfused at 0.11 ml·s−1·g−1 with a modified Krebs-Henseleit solution containing the following (in mmol/l): 118.5 NaCl, 25 NaHCO3, 11.1 glucose, 4.2 KCl, 1.2 KH2PO4, 1.2 MgSO4,H2O, and 1 CaCl2 continuously gassed with 95% O2-5% CO2 (pH 7.4) at 37°C. Hearts were paced at the right atrial-ventricular junction, and monophasic action potentials (MAPs) were recorded at the epicardial surface of the RV and LV using Franz-type (12) spring-loaded contact electrodes. MAP duration (MAPD) was measured at 25, 50, and 90% repolarization. In one set of hearts, electrical restitution curves were generated by stimulating hearts with a computer-driven S1–S2 protocol whereby 20 S1 pulses with a cycle length of 200 ms were followed by the application of a S2 stimulus at an interval that was reduced from 200 to 100 ms in 20-ms decrements and from 100 to 40 ms by 10-ms decrements. The MAP generated by the S2 stimulation was analyzed, and the shortest S1–S2 interval generating a MAP was assumed to be the effective refractory period.
Stimulation of arrhythmias.
Another set of hearts was stimulated at a frequency of 6 Hz; then, to induce arrhythmias, a period of burst stimulation (50 Hz for 1 s) was applied, and external stimulation was then stopped and the heart's intrinsic pacing allowed to resume. Stimulus intensity during burst pacing was increased until tachycardia or fibrillation was induced (e.g., Ref. 28). Arrhythmias were defined by the presence of tachycardia (VT, a rapid intrinsic heart rate >10 Hz) or ventricular fibrillation (VF). The duration of the VT/VF episode was measured and quantified as nonsustained arrhythmia if it lasted <90 s or as sustained if it lasted 90 s. The dominant frequency of arrhythmias was assessed by fast-Fourier analysis.
Measurement of ion channel mRNA by real-time RT-PCR.
Hearts from 12 CON and 14 MCT animals were removed, and the RV and LV free wall were excised and snap frozen in liquid nitrogen between the large flat ends of a pair of tongs. Then, 25-μm sections were collected on a cryostat from the whole of the RV wall. The thickness of the LV wall was measured with precooled calipers, and 25-μm sections equivalent to one-third of the wall thickness were collected on a cryostat from the epicardial surface. All samples were stored at −80°C until RNA extraction. Total RNA extraction was performed following the Qiagen mini-kit protocol for striated muscle, with the exception that an additional volume of RLT was added to the proteinase K solution before binding of the RNA onto the mini-columns. Integrity, quantity and purity of the isolated RNA were assessed using NanoDrop microspectrophotometry. cDNA was prepared from 4 μg of total RNA with random priming using Superscript III first-strand synthesis system (Invitrogen, Life Technologies, Rockville, MD) and diluted 1:10 in TE (10 mM Tris·HCl pH 7.5 and 1 mM EDTA) before use in real-time PCR.
Real-time RT-PCR was performed using TaqMan low-density arrays (Micro Fluidic Cards; Applied Biosystems, Foster City, CA). Each card consisted of 384 wells, preloaded with predesigned fluorogenic TaqMan probes and primers, configured to allow detection of 48 transcripts for seven experimental and one calibrator sample. Our calibrator sample consisted of a mixture of cDNA from RV and LV epicardial regions of a CON heart. Each of the eight sample lanes in a card was loaded with 100 μl of a 1:1 mixture of cDNA (equivalent to 86.96 ng input RNA) and TaqMan Universal PCR master mix (Applied Biosystems). PCR was performed according to the recommended protocol (50 °C for 2 min and 94.5 °C for 10 min, followed by 40 cycles at 97 °C for 30 s, and 59.7 °C for 1 min) on an ABI PRISM 7900HT Sequence detection system (Applied Biosystems). Data were collected with instrument spectral compensations by the Applied Biosystems SDS 2.2 software and analyzed using the threshold cycle (Ct) relative quantification method. Transcript expression was normalized to the housekeeping gene 18S in the test samples and then made relative to the normalized signal level in the corresponding calibrator sample.
Computer simulations.
We utilized a mathematical model of cardiac electrophysiology developed by Pandit et al. (31) to investigate the effects of changes in ion channel expression on the action potential in RV heart failure. The Pandit model is based on experimental data obtained from isolated rat LV myocytes at room temperature (22°C) and contains Hodgkin-Huxley formulations for the major transmembrane ion channels (INa, ICaL, Ito,f, Ito,s, Iss, IK1, If, INa,b, ICa,b, and IK,b), as well as a description for sarcolemmal pumps (INaK and ICaATP), the sodium-calcium exchanger (INaCa), and a multicompartment model of intracellular calcium handling (31). Here, a description of IKs was incorporated in the model and the ion channel gating kinetics of ICaL, Ito,f, Ito,s, Iss, and IKs were scaled by Q10 factors of 2.1, 2.66, 2.66, 2.18, and 2.18, respectively, to allow simulation of LV action potentials at 37°C, as recently done by Noujaim et al. (29). A formulation of the T-type calcium current ICaT was incorporated as in Faber and Rudy (10). RV (and MCT-treated LV) action potentials in the CON and MCT group were obtained by scaling the relevant whole cell ion channel conductances according to their respective mRNA expression obtained from our PCR studies and normalized to the control expression in the LV (6). We only retained those changes in ion channel expression between CON and MCT right hearts that were statistically significant and for which the corresponding current was present in the mathematical model. Supplemental Table S2 summarizes the whole cell ion channel conductances of interest in the four ventricular types and the computed APD at 90% repolarization (Supplemental Material for this article is available online at the Am J Physiol Heart Circ Physiol website). The extracellular Na+, K+, and Ca2+ concentrations were assumed to be 143.5, 5.4, and 1.0 mM, respectively, to match the Tyrode solution used in our MAP experiments. All other parameter values are as described in the original model (31). The model equations were solved using a forward Euler method with an adaptive time step varying between 0.001 and 0.01 ms as in a previous study (18) . Action potentials were obtained after pacing the model cell at 5 Hz for 10 s. Restitution curves were simulated using an S1–S2 stimulation protocol consisting of 20 S1 pulses at a 200-ms cycle length and an S2 pulse at a gradually decreasing coupling interval from 200 ms down to 40 ms in decrements of 10 ms.
Statistics.
All data are expressed as means ± SE. Two-way ANOVA, two-way repeated-measures ANOVA, unpaired Student's t-tests, or χ2-tests were performed as appropriate. Statistically significant difference was assumed if P < 0.05.
RESULTS
RV hypertension and hypertrophy.
MCT treated animals were killed upon showing clinical signs of heart failure, 22–26 days postinjection. They displayed significantly greater heart and lung weights, RV (but not LV) weights, and RV-to-LV weight ratios (Table 1). Millar catheter recordings from the RV of anaesthetized animals, on the day of death, showed significantly increased RV diastolic and systolic pressures and volumes in MCT animals, together with a significant reduction in ejection fraction (Table 2).
Table 1.
Whole animal and organ weights in CON and MCT animals
| CON (n = 18) | MCT (n = 14) | |
|---|---|---|
| Body weight, g | 346 ± 8 | 293 ± 7† |
| Heart weight, g | 1.70 ± 0.06 | 1.99 ± 0.06* |
| Lung weight, g | 1.75 ± 0.09 | 2.69 ± 0.06† |
| Heart weight/body weight, mg/g | 4.91 ± 0.10 | 6.83 ± 0.20† |
| RV weight/body weight, mg/g | 0.76 ± 0.02 | 1.54 ± 0.07† |
| LV weight/body weight, mg/g | 1.61 ± 0.03 | 1.65 ± 0.06 (P = 0.835) |
| RV weight/LV weight, g/g | 0.48 ± 0.02 | 0.95 ± 0.05† |
| Lung weight/body weight, mg/g | 5.04 ± 0.24 | 9.17 ± 0.34† |
Values are means ± SE. RV, right ventricle; LV, left ventricle.
P < 0.01,
P < 0.001, control (CON) vs. monocrotaline (MCT).
Table 2.
In vivo measures of cardiac function in anesthetized CON and MCT animals
| CON (n =8) | MCT (n =10) | |
|---|---|---|
| HR, beats/min | 429 ± 9 | 393 ± 15 (P = 0.068) |
| SV, μl | 33.7 ± 8.6 | 36.6 ± 4.3 (P = 0.749) |
| CO, μl/min | 14,683 ± 3,903 | 14,458 ± 1,906 (P = 0.957) |
| EDP, mmHg | 4.73 ± 0.81 | 8.0 ± 1.2* |
| ESP, mmHg | 24.1 ± 3.06 | 79.2 ± 6.2‡ |
| Ved, μl | 47.3 ± 14 | 97.7 ± 14.7* |
| Ves, μl | 24.5 ± 10.1 | 70.6 ± 12.5* |
| EF, % | 66.2 ± 8 | 38.4 ± 3† |
Values are means ± SE. HR, heart rate; SV, RV stroke volume; CO, cardiac output; EDP, RV end diastolic pressure; ESP, RV end systolic pressure; Ved, RV end diastolic volume; Ves, RV end systolic volume; EF, ejection fraction.
P < 0.05,
P < 0.01,
P < 0.001, CON vs. MCT.
MAPs.
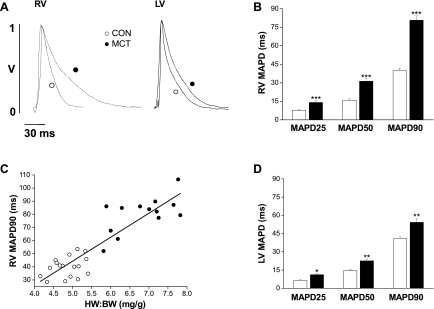
At a stimulation frequency of 5 Hz, the MAPD was significantly longer in the RV of MCT hearts compared with CON hearts at early, mid, and late repolarization (Fig. 1, A and B; P < 0.001). Novel observations were that MAPD90 was correlated with heart weight-to-body weight BW ratio (Fig. 1C), and the LV MAP of MCT-treated hearts was also significantly prolonged but to a lesser extent (Fig. 1, A and D; P < 0.01).
Fig. 1.
A: representative monophasic action potentials (MAPs) from the right ventricular (RV) and left ventricular (LV) epicardial surface of a control (CON; ○) and MCT (●) heart. RV (B) and LV (D) MAP duration (MAPD) at 25, 50, and 90% repolarization in CON (open bars) and MCT (closed bars) hearts. B: RV MAP duration of MCT hearts was significantly prolonged at each level of repolarization (***P < 0.001, CON vs. MCT). C: MAP duration at 90% repolarization was significantly correlated with the heart weight-to-body weight ratio (HW:BW; R2 = 0.90; P < 0.0001) for CON (○) and MCT (●) hearts. D: LV MAP duration in failing hearts was significantly prolonged at each level of repolarization (*P < 0.05, **P < 0.01, CON vs. failing; n = 17 CON and 14 MCT hearts).
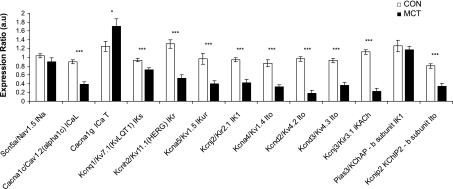
Altered ion channel expression in MCT hearts.
Gene expression of ion channels that contribute to the rat action potential was measured using real-time RT-PCR (Fig. 2 and Supplemental Table S1). In the RV, the expression of the main gene encoding for Na+ channels (Nav1.5) was unchanged. Gene expression of the major Ca2+ channels subtype (l-type) was decreased but was increased for the minor (T-type) Ca2+ channel. mRNA expression for the genes encoding for many K+ channels was reduced. In contrast, markers for heart failure (atrial and brain natriuretic peptide, collagen type 1, ratio of β- to α-myosin heavy chain) were all increased. Changes in MCT LV gene expression were broadly similar to RV but less numerous, such that of 18 mRNAs measured, 16 were significantly changed in the RV but only 10 in the LV, relative to the corresponding CON chamber (see Supplemental Table S1).
Fig. 2.
Relative expression of mRNA for genes encoding for the major Na+, Ca2+, and K+ channels associated with the rat action potential in samples from the RV of normal (open bars) and MCT (closed bars) hearts. Transcript expression is normalized to the housekeeper gene 18S and relative to the normalized transcript expression level in a calibrator sample (***P < 0.001, *P < 0.05, CON vs. failing, statistical significance for two-way ANOVA; n = 12 CON and 14 MCT hearts ). Expression of K+ channel genes in the RV of MCT hearts is generally depressed. See Supplemental Table S1 for additional RV and all LV data.
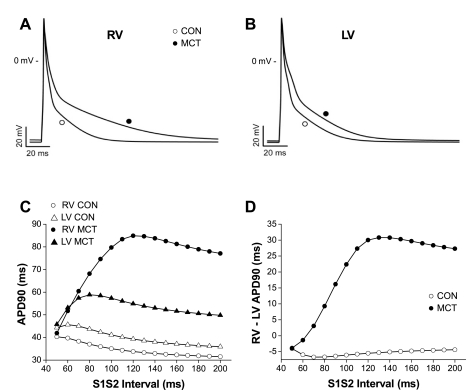
Predictive computer simulations based on the significant changes in Ca2+ and K+ ion channel gene expression (see Supplemental Table S2) were able to closely model action potential prolongation in the RV (Fig. 3A) and the lesser prolongation in LV(Fig. 3B). Analysis of the contribution of individual ion channels predicted that the differential lengthening of the RV and LV action potential in MCT myocytes was primarily due to the differential depression of Ito and IK1 currents. By altering currents in isolation we observed that those giving the closest responses to the ensemble changes, and thus the most influential in the simulated APD changes were Ito and IK1. When an S1–S2 type APD restitution experiment was simulated, it was observed that the RV, MCT restitution curve had a steeper slope than the three other simulations (Fig. 3C) and that the RV-LV dispersion of APD was greater in MCT hearts (Fig. 3D). Similar observations in vivo might contribute to a proarrhythmic substrate.
Fig. 3.
Computer modeling of the intracellular action potential of rat ventricular myocytes using the model of Pandit et al. (31). When the relevant ion channels in the model are scaled from control levels (○) with respect to significant changes in gene expression in MCT hearts (●) (see Ref. 6 and Supplemental Table S2), there is a prolongation of the RV (A) and LV (B) action potential consistent with changes in MAPD measured in MCT hearts. C: simulated S1–S2 APD restitution curves for CON RV (○) and LV (▵) and MCT RV (●) and LV(▴). D: simulated differences in RV and LV APD in MCT (●) and CON (○) hearts. C and D are based on the parameters that generated APDs shown in A and B. Simulation predicts steep APD restitution in MCT RV compared with CON and a greater APD dispersion between ventricles in MCT hearts.
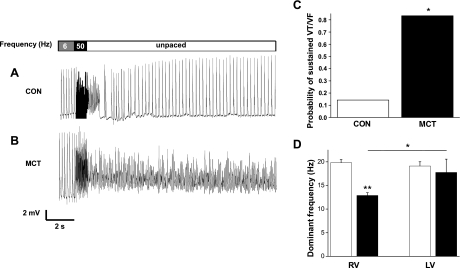
Sustained arrhythmias in MCT hearts.
When isolated hearts underwent burst pacing, CON hearts usually restored intrinsic rhythm after a brief period of tachycardia (e.g., Fig. 4A), the mean duration of these events was 1.90 ± 0.53 s. In contrast, MCT hearts suffered sustained arrhythmias that did not terminate spontaneously (e.g., Fig. 4B). Arrhythmias appeared more easily provoked in MCT hearts as there was a tendency (P = 0.11, data not shown) for MCT hearts to require a smaller increase in stimulation intensity to provoke arrhythmias. Once initiated, the proportion of MCT hearts showing sustained arrhythmias (90 s before intervention) was eightfold greater than CON hearts. Thus sustained tachycardia or fibrillation was significantly more common in MCT hearts (P < 0.05; Fig. 4C). The dominant frequency of the arrhythmias in RV MCT was significantly lower than the other ventricles (Fig. 4D).
Fig. 4.
Arrhythmias in Langendorff-perfused hearts. MAPs from a CON (A) and MCT (B) heart. Hearts were initially paced at 6 Hz, then a 1-s burst stimulus was applied to induce ventricular tachycardia (VT) or fibrillation (VF), and external stimulation was then stopped. After a brief period of arrhythmia the CON heart recovers a stable, intrinsic rhythm; however, the VF generated in the MCT heart is sustained. C: probability that CON and MCT hearts show sustained VT or VF (*P < 0.05, CON vs. MCT). D: dominant frequency of arrhythmias from CON (open bars) and MCT (closed bars) RV and LV (**P < 0.01, CON RV vs. MCT RV; *P < 0.05, MCT LV vs. MCT RV; n = 7 CON and 6 MCT).
MAP restitution and dispersion.
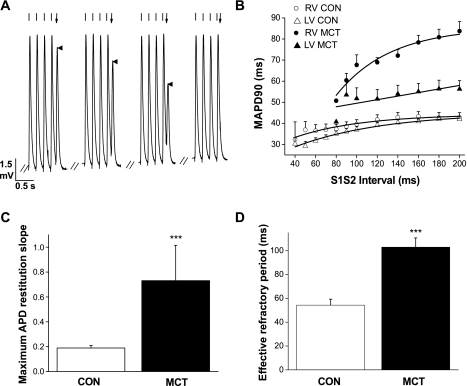
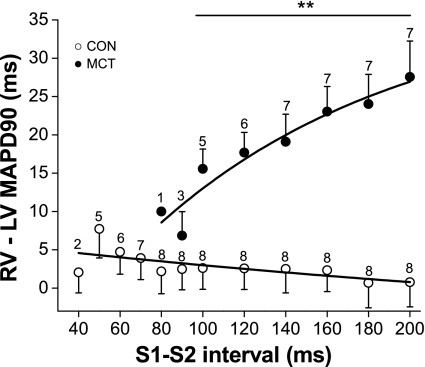
When S1–S2 protocols were applied to generate electrical restitution curves (Fig. 5; A and B), it was observed that the slope of the restitution curve was significantly steeper and the effective refractory period significantly longer in the RV of MCT hearts (Fig. 5, C and D, P < 0.001). In contrast, the slope of the restitution curve of the LV of MCT hearts was similar to CON (Fig. 5B). This heterogenic response in the RV and LV of MCT hearts caused a large MAPD dispersion between the ventricles which altered dramatically with S1–S2 interval (Fig. 6). Using dynamic restitution protocols, we found similar behavior (data not shown).
Fig. 5.
Electrical restitution in CON and MCT hearts. A: example of a S1–S2 electrical restitution experiment. MAPs were recorded from the epicardial surface of the RV, and the heart was paced at 5 Hz (indicated by regularly spaced lines above each MAP). Following the final (S1) stimulus at 5 Hz, an S2 stimulus was given with varying delay (indicated by arrow above the final MAP); this is the S1–S2 interval. When the S2 stimulus falls within the absolute refractory period of the tissue, the S2 stimulus fails to elicit a MAP. B: restitution curves showing mean MAP duration at 90% repolarization for various S1–S2 intervals in CON (RV, ○; LV, ▵) and MCT (RV, ●; LV ▴) hearts. MCT LV MAPs demonstrated a shallow restitution curve compared with MCT RV. C: maximum slope of the restitution curves for RV CON and MCT hearts. D: effective refractory period (shortest S1–S2 interval to elicit a MAP) in RV of CON and MCT hearts. RV of MCT hearts had significantly steeper restitution curves and longer effective refractory periods than CON hearts. (***P < 0.001, CON vs. MCT; n = 8 CON and 7 MCT hearts).
Fig. 6.
Effect of stimulation frequency on MAP dispersion in CON and MCT hearts. Duration of the epicardial RV and LV MAP was simultaneously measured in CON (○) and MCT (●) hearts at different stimulation frequencies. Difference in RV and LV MAP duration at 90% repolarization measures the MAP dispersion. In CON hearts, there was no significant difference between RV and LV MAPD at any basic cycle length. In contrast, there was a large RV and LV difference in MCT hearts that changed steeply with stimulation frequency. (**P < 0.01, CON vs. MCT for S1–S2 intervals 200–100 ms inclusive; n = 8 CON and 7 MCT hearts).
DISCUSSION
The principal novel findings of our study are as follows: 1) the MCT model of pulmonary hypertension and RV hypertrophy has a proarrhythmic substrate; 2) electrical remodeling occurs in the LV of MCT hearts as well as RV, but to a lesser degree, resulting in increased electrical heterogeneity; and 3) MCT hearts display steeper RV action potential restitution and enhanced RV-LV dispersion of APD. We predict that these mechanisms contribute to the proarrhythmic state.
Our in vivo and whole heart measurements revealed substantial RV hypertension, hypertrophy, dilation, and an almost halved ejection fraction. These findings together with observed clinical signs and increased expression of heart failure marker genes indicate heart failure, as previously reported (5, 15, 24). Our findings show that the predominant effect of MCT was in the RV but that there were also effects in the LV. This is consistent with recent studies (e.g., Refs. 8, 25) that have found mechanical properties of the LV were altered in this model, possibly via neurohormonal and biomechanical mechanisms.
MAP changes.
Our observation that the RV MAP duration was prolonged is consistent with action potential and QT interval measurements in previous studies (9, 26, 32). The correlation between the degree of cardiac hypertrophy and APD is a novel observation in this model and is similar to that between impaired mechanical function and hypertrophy recently reported in MCT hearts (40). We also observed prolongation of LV action potentials by MCT, contrary to the findings of Lee et al. (26). This was perhaps because the previous study (26) did not account for regional variations in LV APD (7) (our own LV data was only taken from the subepicardial myocardium) and because their recordings were made with 10 mM EGTA in the recording pipette, which would be expected to greatly influence Ca2+-activated currents and APD.
Gene expression of ion channels and computer simulations.
Previous studies have investigated gene expression of selected K+ (27, 32, 41) and Ca2+ channels (36), principally in the MCT-treated RV. Ours is the first to combine measurement of Na+, Ca2+, and K+ channels in both the RV and (subepicardial) LV within a single study. When measured, studies agree that mRNA levels for Kv1.2, 1.5, 4.2, and 4.3 are decreased (27, 32, 41, and present study) but Kv1.4 (27, 32) and Kv 2.1 (32) are not always depressed. There is evidence that MAPD and K+ channel remodeling is linked to a metabolic shift associated with pyruvate dehydrogenase kinase activity (32). Our finding that the RV of MCT hearts has increased expression of T-type Ca2+ channels is consistent with the observations of Takebayashi et al. (36) and suggests its role in the proarrhythmic state of MCT hearts warrants further investigation given the upregulation of this ion channel and its role in arrythmogenesis in other types of heart failure (22).
Computer simulations of electrical activity were based on changes in gene expression, which may not always reflect ion channel activity and may thus be a limitation of our study (see Ref. 35 for discussion). However, there is good agreement in the differences between CON and MCT hearts in Kv4.2/4.3 mRNA (27, 32, and present study) and Kv4.2/4.3 protein (32) and changes in Ito current (26). In the present study, the simple scaling of changes in ionic conductance to changes in mRNA expression, initially described by Chandler et al. (6) was remarkably effective at predicting the scale of changes in RV and LV APD and the APD restitution characteristics we measured experimentally and suggest that changes in both Ito and IK1 are important for action potential prolongation.
Proarrhythmic consequences of altered electrical properties.
We have demonstrated that the MCT model is proarrhythmic; there was a tendency for arrhythmias to be more easily triggered and, once triggered, they were much more likely to be sustained. In MCT-treated hearts, the RV has a dramatically prolonged action potential with steep restitution characteristics while the LV APD is only slightly prolonged with more normal restitution characteristics; thus the RV-LV APD dispersion is highly variable at physiological heart rates. This observation is consistent with increased APD dispersion predicted from changes in ECG-derived ventricular gradients in MCT hearts (16). Action potential prolongation (39), a steeper restitution curve (21, 34), and increased dispersion of APD (1) are known to be mechanisms that predispose to arrhythmias by generating a substrate for reentry and were all displayed in MCT hearts. The lower dominant frequency of arrhythmias (Fig. 4D) is consistent with the increased refractory period (Fig. 5D) we measured in the MCT RV. However, the cycle length of the dominant frequencies is shorter than the measured refractory periods; this may reflect the rapid and chaotic pacing history of the arrhythmic events. Comparisons of the dominant frequencies between MCT and CON should be made cautiously due to the different timescales over which they were measured.
A steeper restitution curve on its own could promote wave breaks and sustain VF, and while APD dispersion and altered refractoriness could be important in creating the initial conduction block leading to reentry, it should be noted that the RV-LV APD dispersion in MCT hearts is very unstable with regard to stimulation frequency. Therefore, in general, it seems reasonable to conclude that the APD dispersion and restitution characteristics we observe in MCT hearts are at least in part associated with the proarrhythmic substrate. However, the initiation and stability of VT/VF would seem also to depend on mechanisms operating at short cycle lengths where our data suggest APD dispersion is less pronounced. It should also be noted that changes in the anisotropic properties of the intact heart due to the amount and location of the gap junction protein connexin 43 have been reported in MCT-treated hearts (37). These together with alterations in Ca2+ handling (e.g., Refs 9, 23) are likely to also contribute to electrical remodeling. Therefore, although we have identified a proarrhythmic substrate, further studies are required to fully clarify mechanisms.
Study implications.
Heart failure is characterized by compromised pumping function, particularly when demand is increased (33), and this phenomena is displayed in the MCT model (24). It was elegantly shown that the rate-dependant decline in force is associated with a frequency-dependent fall in the myofilament Ca2+ sensitivity related to increased levels of phosphorylation of troponin-I (24). However, a frequency-dependant fall in Ca2+ transient amplitude was also reported (9) that may be directly related to the marked frequency-dependant fall in APD we now report in the RV of MCT hearts by abbreviating Ca2+ entry through L-type Ca2+ channels and promoting the extrusion of Ca2+ via NCX (33).
Our RT-PCR investigation has highlighted potential targets for further detailed electrophysiological study. Future studies of ion channel kinetics in single myocytes are likely to be required to fully explain the alterations in restitution characteristics we have observed. Cardiac arrhythmias are associated with several RV conditions such as arrhythmogenic RV cardiomyopathy (3) and the Brugada syndrome (2) in addition to PAH (11). However, investigating underlying mechanisms in humans is difficult. We show proarrhythmic behavior in an animal model of hypertensive RV failure and hypothesize that heterogeneous alterations in Ca2+ and K+ channel activity and electrical restitution are among the potential causes of these behaviors and thus are topics for further study and potential therapeutic targets for electrical disturbance.
GRANTS
This work was funded by British Heart Foundation Grant PG/08/027/24774 (to E. White and M. Drinkhill), Medical Research Council Grant G900524 (to E. White and O. Bernus), and an Emma and Leslie Reid endowed PhD Studentship (to D. Benoist).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
REFERENCES
- 1. Antzelevitch C. Role of spatial dispersion of repolarization in inherited and acquired sudden cardiac death syndromes. Am J Physiol Heart Circ Physiol 293: H2024–H2038, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antzelevitch C, Nof E. Brugada syndrome: recent advances and controversies. Curr Cardiol Rep 10: 376–383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 373: 1289–1300, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bogaard HJ, Abe K, Vonk NA, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 135: 794–804, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, van HC, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 21: 314–323, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Chandler NJ, Greener ID, Tellez JO, Inada S, Musa H, Molenaar P, DiFrancesco D, Baruscotti M, Longhi R, Anderson RH, Billeter R, Sharma V, Sigg DC, Boyett MR, Dobrzynski H. Molecular architecture of the human sinus node: insights into the function of the cardiac pacemaker. Circulation 119: 1562–1575, 2009 [DOI] [PubMed] [Google Scholar]
- 7. Clark RB, Bouchard RA, Salinas-Stefanon E, Sanchez-Chapula J, Giles WR. Heterogeneity of action potential waveforms and potassium currents in rat ventricle. Cardiovasc Res 27: 1795–1799, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Correia-Pinto J, Henriques-Coelho T, Roncon-Albuquerque R, Jr, Lourenco AP, Melo-Rocha G, Vasques-Novoa F, Gillebert TC, Leite-Moreira AF. Time course and mechanisms of left ventricular systolic and diastolic dysfunction in monocrotaline-induced pulmonary hypertension. Basic Res Cardiol 104: 535–545, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Endo H, Miura M, Hirose M, Takahashi J, Nakano M, Wakayama Y, Sugai Y, Kagaya Y, Watanabe J, Shirato K, Shimokawa H. Reduced inotropic effect of nifekalant in failing hearts in rats. J Pharmacol Exp Ther 318: 1102–1107, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Faber GM, Rudy Y. Action potential and contractility changes in [Na(+)](i) overloaded cardiac myocytes: a simulation study. Biophys J 78: 2392–2404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folino AF, Bobbo F, Schiraldi C, Tona F, Romano S, Buja G, Bellotto F. Ventricular arrhythmias and autonomic profile in patients with primary pulmonary hypertension. Lung 181: 321–328, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res 41: 25–40, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 117: 1717–1731, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJ, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 120: 42–49, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Hardziyenka M, Campian ME, de Bruin-Bon HA, Michel MC, Tan HL. Sequence of echocardiographic changes during development of right ventricular failure in rat. J Am Soc Echocardiogr 19: 1272–1279, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Henkens IR, Mouchaers KT, Vliegen HW, van der Laarse WJ, Swenne CA, Maan AC, Draisma HH, Schalij I, van der Wall EE, Schalij MJ, Vonk-Noordegraaf A. Early changes in rat hearts with developing pulmonary arterial hypertension can be detected with three-dimensional electrocardiography. Am J Physiol Heart Circ Physiol 293: H1300–H1307, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Henkens IR, Mouchaers KT, Vonk-Noordegraaf A, Boonstra A, Swenne CA, Maan AC, Man SC, Twisk JW, van der Wall EE, Schalij MJ, Vliegen HW. Improved ECG detection of presence and severity of right ventricular pressure load validated with cardiac magnetic resonance imaging. Am J Physiol Heart Circ Physiol 294: H2150–H2157, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Hillman EM, Bernus O, Pease E, Bouchard MB, Pertsov A. Depth-resolved optical imaging of transmural electrical propagation in perfused heart. Opt Express 15: 17827–17841, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hlaing T, Guo D, Zhao X, DiMino T, Greenspon L, Kowey PR, Yan GX. The QT and Tp-e intervals in left and right chest leads: comparison between patients with systemic and pulmonary hypertension. J Electrocardiol 38: 154–158, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Hong-liang Z, Qin L, Zhi-hong L, Zhi-hui Z, Chang-ming X, Xin-hai N, Jian-guo H, Ying-jie W, Shu Z. Heart rate-corrected QT interval and QT dispersion in patients with pulmonary hypertension. Wien Klin Wochenschr 121: 330–333, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Keldermann RH, Ten Tusscher KH, Nash MP, Hren R, Taggart P, Panfilov AV. Effect of heterogeneous APD restitution on VF organization in a model of the human ventricles. Am J Physiol Heart Circ Physiol 294: H764–H774, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Kinoshita H, Kuwahara K, Takano M, Arai Y, Kuwabara Y, Yasuno S, Nakagawa Y, Nakanishi M, Harada M, Fujiwara M, Murakami M, Ueshima K, Nakao K. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation 120: 743–752, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Kogler H, Hartmann O, Leineweber K, Nguyen vP, Schott P, Brodde OE, Hasenfuss G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ Res 93: 230–237, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, de Tombe PP, van der Velden J, Stienen GJ. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol 582: 695–709, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamberts RR, Vaessen RJ, Westerhof N, Stienen GJ. Right ventricular hypertrophy causes impairment of left ventricular diastolic function in the rat. Basic Res Cardiol 102: 19–27, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Lee JK, Kodama I, Honjo H, Anno T, Kamiya K, Toyama J. Stage-dependent changes in membrane currents in rats with monocrotaline-induced right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 272: H2833–H2842, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Lee JK, Nishiyama A, Kambe F, Seo H, Takeuchi S, Kamiya K, Kodama I, Toyama J. Downregulation of voltage-gated K+ channels in rat heart with right ventricular hypertrophy. Am J Physiol Heart Circ Physiol 277: H1725–H1731, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Ng GA, Brack KE, Patel VH, Coote JH. Autonomic modulation of electrical restitution, alternans and ventricular fibrillation initiation in the isolated heart. Cardiovasc Res 73: 750–760, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN, Jalife J. Up-regulation of the inward rectifier K+ current (I K1) in the mouse heart accelerates and stabilizes rotors. J Physiol 578: 315–326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc 3: 1422–1434, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophys J 81: 3029–3051, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med 88: 47–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pieske B, Kretschmann B, Meyer M, Holubarsch C, Weirich J, Posival H, Minami K, Just H, Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation 92: 1169–1178, 1995 [DOI] [PubMed] [Google Scholar]
- 34. Selvaraj RJ, Picton P, Nanthakumar K, Chauhan VS. Steeper restitution slopes across right ventricular endocardium in patients with cardiomyopathy at high risk of ventricular arrhythmias. Am J Physiol Heart Circ Physiol 292: H1262–H1268, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Stones R, Billeter R, Zhang H, Harrison S, White E. The role of transient outward K+ current in electrical remodelling induced by voluntary exercise in female rat hearts. Basic Res Cardiol 104: 643–652, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takebayashi S, Li Y, Kaku T, Inagaki S, Hashimoto Y, Kimura K, Miyamoto S, Hadama T, Ono K. Remodeling excitation-contraction coupling of hypertrophied ventricular myocytes is dependent on T-type calcium channels expression. Biochem Biophys Res Commun 345: 766–773, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Uzzaman M, Honjo H, Takagishi Y, Emdad L, Magee AI, Severs NJ, Kodama I. Remodeling of gap junctional coupling in hypertrophied right ventricles of rats with monocrotaline-induced pulmonary hypertension. Circ Res 86: 871–878, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation 114: 1883–1891, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Wickenden AD, Kaprielian R, Kassiri Z, Tsoporis JN, Tsushima R, Fishman GI, Backx PH. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovasc Res 37: 312–323, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Wong YY, Handoko ML, Mouchaers KT, de Man FS, Vonk-Noordegraaf A, van der Laarse WJ. Reduced mechanical efficiency of rat papillary muscle related to degree of hypertrophy of cardiomyocytes. Am J Physiol Heart Circ Physiol 298: H1190–H1197, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Zhang TT, Cui B, Dai DZ. Downregulation of Kv4.2 and Kv4.3 channel gene expression in right ventricular hypertrophy induced by monocrotaline in rat. Acta Pharmacol Sin 25: 226–230, 2004 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.