Abstract
Structural coronary microcirculation abnormalities are important prognostic determinants in clinical settings. However, an assessment of microvascular resistance (MR) requires a velocity wire. A first-pass distribution analysis technique to measure volumetric blood flow has been previously validated. The aim of this study was the in vivo validation of the MR measurement technique using first-pass distribution analysis. Twelve anesthetized swine were instrumented with a transit-time ultrasound flow probe on the proximal segment of the left anterior descending coronary artery (LAD). Microspheres were injected into the LAD to create a model of microvascular dysfunction. Adenosine (400 μg·kg−1·min−1) was used to produce maximum hyperemia. A region of interest in the LAD arterial bed was drawn to generate time-density curves using angiographic images. Volumetric blood flow measurements (Qa) were made using a time-density curve and the assumption that blood was momentarily replaced with contrast agent during the injection. Blood flow from the flow probe (Qp), coronary pressure (Pa), and right atrium pressure (Pv) were continuously recorded. Flow probe-based normalized MR (NMRp) and angiography-based normalized MR (NMRa) were calculated using Qp and Qa, respectively. In 258 measurements, Qa showed a strong correlation with the gold standard Qp (Qa = 0.90 Qp + 6.6 ml/min, r2 = 0.91, P < 0.0001). NMRa correlated linearly with NMRp (NMRa = 0.90 NMRp + 0.02 mmHg·ml−1·min−1, r2 = 0.91, P < 0.0001). Additionally, the Bland-Altman analysis showed a close agreement between NMRa and NMRp. In conclusion, a technique based on angiographic image data for quantifying NMR was validated using a swine model. This study provides a method to measure NMR without using a velocity wire, which can potentially be used to evaluate microvascular conditions during coronary arteriography.
Keywords: coronary microvasculation
coronary microvascular dysfunction occurs in a number of myocardial disease states and has important prognostic implications in different clinical settings, including acute coronary syndromes, diabetic coronary disease, hypertrophic cardiomyopathy, and transplant cardiac allograft vasculopathy (25, 26, 39). Intracoronary physiological techniques have been advocated and several indexes for the assessment of changes in the microcirculatory bed have been proposed (2, 11, 51), such as coronary flow reserve (CFR) (9, 13, 34) and microvascular resistance (MR) (12, 39, 49). Many previous study findings suggest that MR is a useful variable for directly assessing microcirculatory function. In 1974, Gould et al. (19, 20, 21) postulated a hypothesis that minimum MR is independent of epicardial stenosis severity. Recently, Fearon and colleagues (1, 7, 12, 14, 39) and Marques et al. (32) proved that the MR specifically interrogates the status of microcirculation and is independent of the presence of an epicardial stenosis using thermodilution method. When compared with CFR, MR is independent of many hemodynamic perturbations. Therefore, measurements of coronary MR play a pivotal role in diagnosing and monitoring the effects of therapeutic interventions.
Resistance is determined by dividing the pressure gradient by flow. In the case of the heart, true microcirculatory resistance (1, 12, 26) is theoretically defined as the mean aortic to coronary back pressure (vide infra) gradient divided by absolute coronary blood flow (in mmHg·ml−1·min−1) at hyperemia. Myocardial perfusion pressure is easy to measure invasively with a catheter or a coronary pressure wire. Therefore, the challenge for calculating MR is to measure the absolute coronary blood flow. Several invasive and noninvasive techniques have been developed for evaluating the status of the coronary microcirculation (24, 26). Some common techniques for evaluating microcirculation include positron emission tomography (PET), cardiovascular magnetic resonance imaging, myocardial contrast echocardiography (56, 57, 59), and the thermodilution method (9, 13, 16, 54). However, a widespread implementation of these techniques has been hampered by several factors including its complexity, irreproducibility, invasiveness, and expense and of the fact that it does not independently interrogate the microcirculation.
A first-pass distribution analysis (FPA) technique to measure absolute coronary blood flow using angiographic image data was validated (22, 31, 37, 38). The studies demonstrated the feasibility and potential utility of the FPA algorithm in conjunction with digital subtraction angiography for measuring volumetric coronary blood flow. When compared with other blood flow measurement techniques, the current angiographic method is simple to implement. It can be done in conjunction with routine coronary angiography, which can be accomplished during diagnostic cardiac catheterization. Furthermore, MR can be easily calculated using angiographic volumetric blood flow. The purpose of the current study is to validate the MR measurement technique in vivo using the FPA technique.
METHODS
Protocol.
In an open-chest swine model, MR measurements were performed at various stages of severity of microvascular disease in the left anterior descending coronary artery (LAD) at hyperemia. Microcirculation was disrupted by embolized microspheres. Both the coronary pressure from the angiographic catheter and blood flow from the flow probe were continuously recorded. Coronary angiograms were acquired for each data set. The study protocol was approved by the University of California, Irvine Institutional Animal Care and Use Committee.
Animal preparation.
Twelve fasted domestic Yorkshire swine (36.6 ± 6.0 kg, male, S&S Farms) were sedated and premedicated with Telazol-ketamine-xylazine (4.4, 2.2, and 2.2 mg drug/kg body wt, respectively) (27, 28) and atropine (0.05 mg/kg). Anesthesia was maintained with ∼1 to 2% isoflurane (Highland Medical Equipment Vaporizer; Temecula, CA), and supplemental oxygen was provided via endotracheal intubation. The arterial partial pressure of CO2 was maintained within normal limits (∼40–45 mmHg). Heart rate and percent oxygen saturation were continuously monitored (Nellcor N-200 Pulse Oximeter; Hayward, CA). The carotid artery and jugular vein were cut down for sheath placement. An intravenous drip of adenosine (400 μg·kg−1·min−1) (17, 50, 61) was used to induce maximum hyperemia. Electrocardiogram, arterial blood pressure, X-ray pulse signal, and other relative physiological parameters were continuously recorded (MP100, Biopac Systems, Santa Barbara, CA). An example of recorded signals during baseline and hyperemia is shown in Fig. 1.
Fig. 1.
All recorded parameters during baseline and hyperemia. Continuous recording of ECG, coronary pressure (Pa), distal coronary pressure (Pd), right atrium pressure (Pv), and volumetric blood flow from the flow probe (Qp) is shown.
Surgery and catheterization.
Lidocaine (50 μg·kg−1·min−1 for total ∼4 to 5 ml) was administered intravenously to prevent arrhythmia before the chest was opened. A lateral thoracotomy was performed using standard surgical techniques. The fourth and fifth ribs were spread apart, and the heart was fully exposed. The pericardium was opened, and the proximal segment of the LAD was dissected free. A transit-time ultrasound flow probe (Transonic Systems, Ithaca, NY) was placed on the proximal segment of the LAD. An extravascular occluder (IVM In Vivo Metric, Healdsburg, CA) was also placed around the LAD just distal to the flow probe to produce a 100% occlusion for reactive maximum hyperemia.
Before catheterization, heparin was administered (10,000-unit bolus followed by additional ∼4,000–5,000 U/h). Activating clotting time was tested every 30 min (model 801, Hemochron, Edison, NJ) to monitor the coagulation. The left main ostium was cannulated with a 6-Fr hockey-stick catheter through the left carotid artery under fluoroscopic guidance. Another 4-Fr hockey-stick catheter was placed in the right atrium to measure the coronary venous pressure. Microcirculation was disrupted by gradually injecting ∼50–100 μm (1.8 × 104 microspheres/ml) microspheres (Polysciences, Warrington, PA) (2, 3, 5, 12, 48, 52) down the LAD through the catheter. This procedure was repeated for the different degrees of severity of microcirculatory embolism.
Image acquisition and processing.
All images were acquired using a conventional X-ray tube with a constant potential X-ray generator (Optimus M200, Philips Medical Systems, Shelton, CT). A cesium iodide-based flat panel detector (PaxScan 4030A, Varian Medical, Palo Alto, CA) was used for image acquisition. The flat panel detector has a 40 × 30 cm2 field of view and pixel size of 0.194 × 0.194 mm2. The zoom-center mode was used to acquire images with 1,024 × 768 pixels. Gain and flat-field corrections were performed before image acquisition. The flat panel detector has a dynamic range >8,000 with no pincushion distortions. Images were acquired at 30 frames/s. The detector signal in each pixel was digitized with 14-bit precision. All images were corrected for X-ray scatter before logarithmic transformation (10). A publicly available software (ImageJ, National Institutes of Health, Bethesda, MD) was used for image analysis.
Each swine was positioned on its right side under the flat panel detector. The projection angle was optimized for separation of the LAD and left circumflex artery perfusion beds. Pancuronium (0.1 mg/kg) was administered intravenously. Coronary angiograms were acquired when the blood flow reached maximum hyperemia. The ventilator was turned off at the end of a full expiration to minimize respiratory motion. Images of at least one full heart cycle were acquired before contrast injection for selecting a cardiac phase-matched mask image for temporal subtraction. Contrast material (Omnipaque-350; Princeton, NJ) was power injected (Leibel-Flarsheim Angiomat 6000; Cincinnati, OH) at 3 ml/s for 3 s.
An image of a calibration phantom positioned over the heart was also acquired to determine the correlation between image gray level and iodine mass. The iodine calibration phantom (35), which consisted of different cylindrical cells filled with iodine, was used to quantify iodine mass from X-ray densitometric signal. Correction was made for differential magnification of the phantom and the heart. The calibration curve and the known iodine concentration of the contrast agent were used to convert the integrated densitometric iodine signal to contrast volume, as shown in Fig. 2.
Fig. 2.
Calibration phantoms. Image of the 2 calibration phantoms is shown (A) along with an example of a calibration curve showing the relationship between iodine volumes (in ml) and measured integrated gray levels (B).
Blood flow measurement using angiographic images.
Several previous studies have evaluated coronary blood flow by using angiographic images. Pijls et al. (40, 41, 42) measured the mean transit time for the assessment of myocardial perfusion by videodensitometry. Molloi et al. (35, 37) have shown that the FPA analysis technique can be used to measure absolute coronary blood flow by analyzing the propagation of a contrast material signal in the coronary system. In the FPA theory, the volume of the vascular bed supplied by a major coronary artery is modeled as a reservoir with a single input. The model does not require any assumptions regarding the internal structure of the vascular bed or the nature of exit conduits. This technique combines the densitometric analysis of spatial and temporal aspects concerning the contrast propagation through the myocardium. Flow measurements are made by summing up the pixel values in the regions of interest (ROIs) using temporal subtraction images.
Coronary blood flow was determined from the change in volume during one cardiac cycle. An ROI for flow measurement was drawn around the LAD vascular bed that encompassed both the visible arteries and the microcirculatory blush (43, 60) (Fig. 3). Power injection of contrast material was assumed to momentarily replace blood with contrast material. The known iodine concentration in the contrast material and a linear regression analysis between the measured integrated gray levels in the calibration phantom were used to convert the gray level to volume. Therefore, the difference of densitometric signal in the vascular bed can be converted to the volume of contrast bolus entering the vascular bed between successive images using system iodine calibration. The time period of the cardiac cycle was calculated from the image acquisition rate (30 frames/s). The ratio of the measured volume change to the time period of the cardiac cycle yields volumetric coronary blood flow.
Fig. 3.
Regions of interest for flow measurement. An example of a global region of interest used for angiographic measurement of coronary volume flow is shown.
Blood flow measurement using flow probe.
Since volumetric blood flow from the transit time flow probe was used as our gold standard, the flow probe was recalibrated before every experiment. Flow measurement was performed with the flow probe around a calibration tubing (Transonic Systems, Ithaca, NY) during the saline injection. A direct volume collection for 30 s was used for flow probe calibration. The correlation between flow measurements from flow probe and volume collection was calculated using linear regression. The linear least-squares fits shows Qflowprobe = 0.820 Qcollection + 3.540 ml/min (r2 = 0.994, P < 0.0001).
MR calculation.
MR is defined as myocardial perfusion pressure divided by volumetric coronary flow (in mmHg·ml−1·min−1) at hyperemia:
| (1) |
Myocardial perfusion pressure was calculated as aortic pressure (Pa) minus venous pressure (Pv). Both Pa and Pv were measured continuously with pressure transducers using a sheath in the carotid artery and a catheter in the right atrium, respectively. However, volumetric coronary blood flow (in ml/min) and the calculated resistance (in mmHg·ml−1·min−1) are dependent on the arterial perfusion bed size. Therefore, it is not possible to establish a standard value for normal MR. Measured flow using PET is normalized by the myocardial mass (in ml·min−1·g−1). It is not possible to directly measure regional myocardial mass with angiography. However, it is possible to measure the dependent arterial lumen volume using densitometry (35), which can also be related to the myocardial mass (29, 30). Therefore, arterial lumen volume can be used to account for the dependent arterial bed size. Previous reports (36, 37, 60) have shown that a power law relationship exists between the hyperemic blood flow (Q) through a stem and the corresponding crown volume (V):
| (2) |
Vref (1 ml) is a reference volume to make V raised to the power of 3/4 unitless. Normalized MR (NMR) can be calculated by combining Eqs. 1 and 2:
| (3) |
All the MR measurements were calculated using Eq. 3 where Pa is aortic pressure (in mmHg), Pv is coronary back pressure (in mmHg), Q is the hyperemic blood flow (in ml/min) through a stem, V is the corresponding crown arterial volume (in ml), and Vref is the reference arterial lumen volume (1 ml). Therefore, angiography-based NMR (NMRa) was calculated using the angiographic flow using the FPA technique (Qa), whereas the reference NMR (NMRp) was calculated using the flow from the flow probe (Qp). Pressure and flow probe data were measured over five cardiac cycles before contrast injection.
Microcirculation evaluation involving epicardial stenosis.
Microspheres were gradually injected in the LAD to disrupt the microcirculation. The extravascular occluder was placed around the LAD just distal to the flow probe to produce an intermediate epicardial stenosis (∼75% area stenosis). NMRp and NMRa measurements were made for various stages of severity of microvascular disruption with and without the epicardial stenosis.
Diagnosis of microcirculation disruption involving epicardial stenosis.
Diagnostic abilities for microcirculation disruption using NMRp and NMRa were tested in two models: 1) normal epicardial artery model (N model), which included the normal condition and different severities of microvascular disruption with normal epicardial arteries, and 2) intermediate epicardial stenosis model (S model), which included intermediate coronary epicardial stenosis (∼75% area stenosis) and different severities of microvascular disruption with the same epicardial stenosis. The gold standard used in detecting microvascular disease was determined by the fact of whether microspheres were injected.
Statistical analysis.
Linear regression analysis was performed between angiographic flow probe measurements to determine the coefficient in the regression equation. The correlation coefficient (r) and standard error of estimate (SEE) were determined by a linear regression analysis. SEE is defined as the standard deviation of the measured values from the regression line. The degree of agreement between the different methods was also assessed using the Bland-Altman analysis (4). Paired samples t-test were made for NMRp and NMRa measurements at various degrees of severity of the microvascular disruption with and without epicardial stenosis. Two series of receiver-operating characteristic curves were made for both the N model and the S model. The areas under each curve (AUCs) were calculated to compare the diagnostic abilities of NMRp and NMRa. A P < 0.05 was considered to be statistically significant for all statistical analyses.
RESULTS
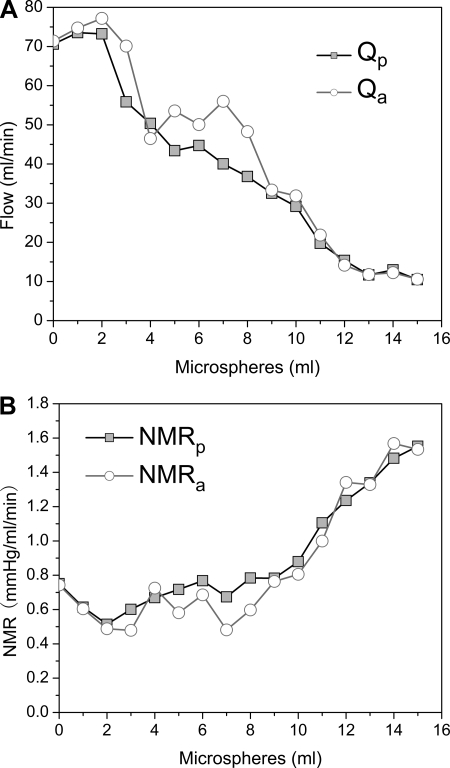
A total of 258 measurements were made at hyperemia. The mean heart rate was 93.41 ± 16.40 beats/min. The mean Qp and Qa were 40.90 ± 27.63 and 43.38 ± 26.02 ml/min, respectively. The mean NMRp and NMRa were 1.15 ± 0.69 and 1.06 ± 0.65 mmHg·ml−1·min−1, respectively. Qa showed a strong correlation with the reference standard Qp (r = 0.956, SEE = 7.627 ml/min). The equation of the regression line was determined to be Qa = 0.90 Qp + 6.6 ml/min (P < 0.001) (Fig. 4). NMRa correlated linearly with NMRp as NMRa = 0.90 NMRp + 0.02 mmHg·ml−1·min−1 (P < 0.001) with a good correlation coefficient (r = 0.956, SEE = 0.019 mmHg·ml−1·min−1) (Fig. 5). Additionally, in the Bland-Altman plot, the mean differences between the two measurements were 2.5 ± 15.9 ml/min for flow measurement and −0.09 ± 0.39 mmHg·ml−1·min−1 for NMR measurement. There was no statistically significant difference from zero, implying a lack of bias between the two techniques. Figure 6 shows the changes of Q and NMR in one animal while microspheres were gradually injected. Figure 7 compared the MR and NMR for the LAD and its branch in one animal. The results indicated that the measured NMR was nearly the same regardless of the vascular bed size.
Fig. 4.
Linear regression and Bland-Altman analysis of angiography-based flow (Qa) and flow probe-based flow (Qp). A linear regression analysis (A) and the Bland-Altman analysis (B) of Qa and Qp measurements are shown. The solid line represents the regression line (Qa = 0.900 Qp + 6.566 ml/min, r2 = 0.914, SEE = 7.627 ml/min, P < 0.0001). All the data points were divided into 4 groups according to the amount of microspheres injection: group A (normal condition); group B (mild microvascular disruption, <1.5 × 105 microspheres); group C (moderate microvascular disruption, ∼1.5 × 105-3.0 × 105 microspheres); and group D (severe microvascular disruption, >3.0 × 105 microspheres).
Fig. 5.
Linear regression and Bland-Altman analysis of angiography-based (NMRa) and probe-based (NMRp) normalized microvascular resistance. A linear regression analysis (A) and the Bland-Altman analysis (B) of NMRa and NMRp measurements are shown. The solid line represents the regression line (NMRa = 0.904 NMRp + 0.022 mmHg·ml−1·min−1, r2 = 0.915, standard error of estimate = 0.190 mmHg·ml−1·min−1, P < 0.0001).
Fig. 6.
The changes of Q and NMR in 1 animal. When microspheres were gradually injected into the left anterior descending coronary artery (LAD), Q (A) dropped while NMR (B) slowly increased. There were 1.8 × 104 microspheres/ml in suspension.
Fig. 7.
A: NMR for the LAD and its branch in 1 animal. An example of microvascular resistance (MR) and NMR comparison for the LAD and its branch in one animal is shown. NMR can correct the variation of MR in different size arteries. B: the results indicated that the measured NMR was nearly the same regardless of the vascular bed size.
Microcirculation evaluation involving epicardial stenosis.
From the results of the paired samples t-test for 33 pairs of NMR measurements at various degrees of severity of the microvascular disruption with and without intermediate epicardial stenosis, there were no significant differences between NMRp (P = 0.073) and NMRa (P = 0.057) measurements.
Diagnosis of microcirculation disruption involving epicardial stenosis.
In 167 measurements in normal model, the AUCs for NMRp and NMRa were 0.964 and 0.960. And in 170 measurements in the intermediate severity stenosis model, the AUCs were 0.921 and 0.920, respectively (Fig. 8). There were no significant reductions in AUCs for NMRp and NMRa between normal and intermediate severity stenosis models (P > 0.05).
Fig. 8.
Receiver-operating characteristic curves for NMR involving epicardial stenosis. In 167 measurements in normal model, the areas under each curve (AUCs) for NMRp and NMRa were 0.964 and 0.960, and in 170 measurements in intermediate severity epicardial stenosis (∼75% area stenosis) model, the AUCs were 0.921 and 0.920, respectively. There were no significant reductions in AUCs for NMRp and NMRa between normal and intermediate stenosis models (P > 0.05).
DISCUSSION
This study demonstrated that both the angiographic Q and NMR based on the FPA technique have strong correlations with Q and NMR from the flow probe. Bland-Altman analysis also showed that there was no significant bias between these two methods. However, when we compared our measurement of NMR, we found that NMR has a slightly lower correlation (r = 0.9562) than Qa (r = 0.9564). This is because every data set had a different (Pa − Pv) to calculate NMR. In summary, NMR can be accurately measured using the FPA technique in a swine coronary microvascular disruption model.
Relationship between thrombolysis in myocardial infarction frame count and FPA.
The thrombolysis in myocardial infarction frame count (TFC) is a simple clinical tool used for assessing quantitative indexes of coronary blood flow. This measurement has been significantly correlated with flow velocity measurements during both baseline and hyperemia, especially after the corrected TFC was developed (46, 55). TFC is a useful angiographic assessment of both epicardial and myocardial perfusion. In the TFC technique, the number of cine frames required for contrast material to reach the standardized distal landmarks is counted (18). Essentially, it uses an estimate of the contrast material transit time as a surrogate for coronary blood flow. On the other hand, the FPA technique uses the time-density curve, assuming that blood is momentarily replaced with contrast agent to measure absolute volumetric blood flow. Instead of a frame count index, the FPA technique can measure absolute coronary blood flow (in ml/min).
Advantages of NMRa.
Previous reports indicate that techniques used for evaluating coronary microcirculation are limited because they are complicated, not reproducible, too invasive, and expensive and do not independently interrogate the microcirculation (9, 26, 54). For example, perfusion PET suffers from its limited availability and high cost, which is mainly related to the necessity of an onsite cyclotron and expertise regarding the preparation and administration of the involved radiotracers. For cardiovascular magnetic resonance imaging, large doses of gadolinium are administered to achieve an adequate signal-to-noise ratio of the myocardial tissue enhancement. At these concentrations, however, the linear relationship between the measured signal intensity in the left ventricular cavity and the actual contrast concentrations levels off. Echocardiography suffers from a poor acoustic window in a limited number of patients and safety issues of microbubble contrast agents. Thermodilution has been routinely used for NMR measurement. However, it requires a known infusion rate for saline with a known temperature and precise infusion rate. The angiographic NMR based on FPA technique has important advantages compared with the previously reported methods for NMR measurement. The angiographic NMR measurement requires no wires and reduces the cost and procedure time associated with these techniques. All the angiographic NMR procedures can be accomplished during routine diagnostic cardiac catheterization. Therefore, both anatomical and physiological information can be derived from the same angiographic images. Furthermore, the angiographic method for NMR measurement could potentially be used for evaluating the microcirculatory system of patients with stable chest pain in the cardiac catheterization laboratory.
MR and CFR.
With the growing awareness that coronary microcirculatory dysfunction is an important pathophysiological component in many cardiac conditions, several different physiological dynamic indexes, such as CFR and MR, have been introduced. Some authors have used the concept of CFR as the theoretical framework to study microcirculation invasively. However, CFR is highly affected by epicardial stenosis and is very sensitive to hemodynamic changes. Other studies have reported that compared with other indexes, the advantages of MR allow it to be reliably applied for the interrogation of microcirculatory resistance in the catheterization laboratory (12). These advantages are twofold. First, MR is reproducible and largely independent of variations in the hemodynamic state (39). Second, MR calculated from pressure distal to stenosis (Pd) appears to be independent of epicardial artery disease. Previous reports (6, 33, 44, 47) have documented an increase in MR in the presence of an epicardial artery stenosis. However, if collateral flow is accounted for, the minimum achievable MR is not significantly affected by increasing epicardial artery stenosis (1, 14). Additionally, MR is an independent predictor of acute and short-term myocardial damage in patients undergoing primary percutaneous coronary intervention. It may allow us to determine the efficacy of therapeutic strategies for microvascular protection in patients with ST-segment elevation myocardial infarction (15, 23).
Pa and Pd.
The current study used (Pa − Pv) to calculate NMR for normal epicardial arteries because Pa is equal to Pd when there is no epicardial disease (8). However, NMR calculated from Pa combines the total resistance from the epicardial arteries and the microcirculation. Therefore, an abnormally high resistance can be due to epicardial stenosis, diffuse disease, or microvascular disease. The important question is the relative importance or balance of epicardial stenosis, diffuse disease, and microvascular disease. Therefore, NMR measurements were made with and without intermediate severity epicardial stenosis (∼75% area stenosis). From the results of the current in vivo study, our NMRa, which is calculated from Pa, can be applicable to screen the microvascular condition with normal and intermediate severity epicardial stenosis (∼75% area stenosis). In cases of severe epicardial stenosis, when NMR calculated from Pa will be an overestimate, the NMRa can be used to assess the MR after angioplasty.
Study limitations.
The use of microspheres to create a swine animal model for microvascular disruption was shown to be successful. When microspheres were gradually injected into the LAD, the blood flow dropped while NMR slowly increased. However, continued injections of microspheres may cause heterogeneous microinfarcts, which is not the same pathological change as normal myocardial infarction or diffused microvascular disease (5). The microspheres are not chemoattractant and thus are different from in vivo human microcirculations. In human microcirculation, dysfunction is usually caused by physical obstruction and active thrombogenic, vasoconstrictive, and inflammatory affections and their interactions with the vascular wall. Moreover, the experimental animal model only simulated a focal microcirculatory disruption. Only intermediate severity epicardial stenosis (∼75% area stenosis) was studied along with the microvascular disease. Other disease conditions or risk factors, such as ventricular hypertrophy, hypercholesterolemia, diabetes mellitus, and diffuse coronary artery disease could affect NMR. The impact of other disease conditions on NMR requires additional study.
One of the advantages of the FPA (35, 60) technique is the ability to evaluate both global and regional blood flow simultaneously. In this study, only the global blood flow and NMR of LAD were measured. For future studies, different regional blood perfusion beds need to be studied with the selective injection of microspheres.
Myocardial perfusion flow is a combination of coronary and collateral flow, so calculations using it tend to overestimate MR if they neglect collateral flow (53, 58) . In our current angiographic flow measurement, the ROI was drawn large enough to ensure that the LAD vascular bed will be within the ROI (35). As a consequence, this technique ensured that any visible potential collateral flow perfusion will be included in the angiographic NMR measurement. However, in cases of severe stenosis, coronary angiography still has only limited sensitivity for quantifying collateral circulation capacity (45, 53). The existence of collateral flow might lead to NMR overestimation because myocardial perfusion combines coronary and collateral flow. However, as pointed out above, NMR measurement is only clinically indicated in cases of mild or no epicardial stenosis. In these cases, collateral flow is not expected to be significant.
Another limitation of the current study is that only coronary angiograms without respiratory motion were analyzed for NMR measurements. Respiratory motion can introduce misregistration artifacts in phase-matched subtracted images and increase measurement error in coronary flow. However, motion artifacts can be minimized with breath holding since only a short time interval is required for blood flow measurement (∼3–5 s).
Conclusion.
This study demonstrated that the angiographic flow measurement based on the FPA technique can be used to assess the severity of microvascular disruption even in the presence of intermediate severity epicardial stenosis (∼75% area stenosis). This new method is accurate, quantitative, and easy to perform, requiring only angiographic images. The application of angiographic NMR in humans could potentially provide a method to assess both anatomically and physiologically effects of coronary artery disease using angiographic images.
GRANTS
This work was supported in part by the National Heart, Lung, and Blood Institute Grant R01-HL-89941 and the Department of Health and Human Services. Partial funding for Z. Zhang was from China National Natural Science Foundation Grant 30870698 and Tianjin Medical University Graduate Innovation Fund 2009GSLI13.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Jerry Wong and Charles Dang for technical support.
REFERENCES
- 1. Aarnoudse W, Fearon WF, Manoharan G, Geven M, van de Vosse F, Rutten M, De Bruyne B, Pijls NH. Epicardial stenosis severity does not affect minimal microcirculatory resistance. Circulation 110: 2137–2142, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Banerjee RK, Ashtekar KD, Effat MA, Helmy TA, Kim E, Schneeberger EW, Sinha RA, Gottliebson WM, Back LH. Concurrent assessment of epicardial coronary artery stenosis and microvascular dysfunction using diagnostic endpoints derived from fundamental fluid dynamics principles. J Invasive Cardiol 21: 511–517, 2009 [PubMed] [Google Scholar]
- 3. Bartoli CR, Okabe K, Akiyama I, Coull B, Godleski JJ. Repeat microsphere delivery for serial measurement of regional blood perfusion in the chronically instrumented, conscious canine. J Surg Res 145: 135–141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud 1: 307–310, 1986 [PubMed] [Google Scholar]
- 5. Carlsson M, Saloner D, Martin AJ, Ursell PC, Saeed M. Heterogeneous microinfarcts caused by coronary microemboli: evaluation with multidetector CT and MR imaging in a swine model. Radiology 254: 718–728, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chamuleau SA, Siebes M, Meuwissen M, Koch KT, Spaan JA, Piek JJ. Association between coronary lesion severity and distal microvascular resistance in patients with coronary artery disease. Am J Physiol Heart Circ Physiol 285: H2194–H2200, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Daniels DV, Fearon WF. The index of microcirculatory resistance (IMR) in takotsubo cardiomyopathy. Catheter Cardiovasc Interv 77: 128–131, 2011 [DOI] [PubMed] [Google Scholar]
- 8. De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart 94: 949–959, 2008 [DOI] [PubMed] [Google Scholar]
- 9. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation 104: 2003–2006, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Ducote JL, Molloi S. Scatter correction in digital mammography based on image deconvolution. Phys Med Biol 55: 1295–1309, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Escaned J, Flores A, Garcia-Pavia P, Segovia J, Jimenez J, Aragoncillo P, Salas C, Alfonso F, Hernandez R, Angiolillo DJ, Jimenez-Quevedo P, Banuelos C, Alonso-Pulpon L, Macaya C. Assessment of microcirculatory remodeling with intracoronary flow velocity and pressure measurements: validation with endomyocardial sampling in cardiac allografts. Circulation 120: 1561–1568, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation 107: 3129–3132, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Fearon WF, Farouque HM, Balsam LB, Caffarelli AD, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Comparison of coronary thermodilution and Doppler velocity for assessing coronary flow reserve. Circulation 108: 2198–2200, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Fearon WF, Aarnoudse W, Pijls NH, De Bruyne B, Balsam LB, Cooke DT, Robbins RC, Fitzgerald PJ, Yeung AC, Yock PG. Microvascular resistance is not influenced by epicardial coronary artery stenosis severity: experimental validation. Circulation 109: 2269–2272, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Fearon WF, Shah M, Ng M, Brinton T, Wilson A, Tremmel JA, Schnittger I, Lee DP, Vagelos RH, Fitzgerald PJ, Yock PG, Yeung AC. Predictive value of the index of microcirculatory resistance in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 51: 560–565, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Ganz W, Tamura K, Marcus HS, Donoso R, Yoshida S, Swan HJ. Measurement of coronary sinus blood flow by continuous thermodilution in man. Circulation 44: 181–195, 1971 [DOI] [PubMed] [Google Scholar]
- 17. Gewirtz H, Ohley W, Williams DO, Sun Y, Most AS. Effect of intraaortic balloon counterpulsation on regional myocardial blood flow and oxygen consumption in the presence of coronary artery stenosis: observations in an awake animal model. Am J Cardiol 50: 829–837, 1982 [DOI] [PubMed] [Google Scholar]
- 18. Gibson CM, Schomig A. Coronary and myocardial angiography: angiographic assessment of both epicardial and myocardial perfusion. Circulation 109: 3096–3105, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 34: 48–55, 1974 [DOI] [PubMed] [Google Scholar]
- 20. Gould KL, Lipscomb K, Hamilton GW. Physiologic basis for assessing critical coronary stenosis. Instantaneous flow response and regional distribution during coronary hyperemia as measures of coronary flow reserve. Am J Cardiol 33: 87–94, 1974 [DOI] [PubMed] [Google Scholar]
- 21. Gould KL, Lipscomb K, Calvert C. Compensatory changes of the distal coronary vascular bed during progressive coronary constriction. Circulation 51: 1085–1094, 1975 [DOI] [PubMed] [Google Scholar]
- 22. Hangiandreou NJ, Folts JD, Peppler WW, Mistretta CA. Coronary blood flow measurement using an angiographic first pass distribution technique: a feasibility study. Med Phys 18: 947–954, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Ito N, Nanto S, Doi Y, Sawano H, Masuda D, Yamashita S, Okada K, Kaibe S, Hayashi Y, Kai T, Hayashi T. High index of microcirculatory resistance level after successful primary percutaneous coronary intervention can be improved by intracoronary administration of nicorandil. Circ J 74: 909–915, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation 114: 1321–1341, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Kitabata H, Imanishi T, Kubo T, Takarada S, Kashiwagi M, Matsumoto H, Tsujioka H, Ikejima H, Arita Y, Okochi K, Kuroi A, Ueno S, Kataiwa H, Tanimoto T, Yamano T, Hirata K, Nakamura N, Tanaka A, Mizukoshi M, Akasaka T. Coronary microvascular resistance index immediately after primary percutaneous coronary intervention as a predictor of the transmural extent of infarction in patients with ST-segment elevation anterior acute myocardial infarction. JACC Cardiovasc Imaging 2: 263–272, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Knaapen P, Camici PG, Marques KM, Nijveldt R, Bax JJ, Westerhof N, Gotte MJ, Jerosch-Herold M, Schelbert HR, Lammertsma AA, van Rossum AC. Coronary microvascular resistance: methods for its quantification in humans. Basic Res Cardiol 104: 485–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ko JC, Williams BL, Smith VL, McGrath CJ, Jacobson JD. Comparison of Telazol, Telazol-ketamine, Telazol-xylazine, and Telazol-ketamine-xylazine as chemical restraint and anesthetic induction combination in swine. Lab Anim Sci 43: 476–480, 1993 [PubMed] [Google Scholar]
- 28. Ko JC, Williams BL, Rogers ER, Pablo LS, McCaine WC, McGrath CJ. Increasing xylazine dose-enhanced anesthetic properties of telazol-xylazine combination in swine. Lab Anim Sci 45: 290–294, 1995 [PubMed] [Google Scholar]
- 29. Le H, Wong JT, Molloi S. Estimation of regional myocardial mass at risk based on distal arterial lumen volume and length using 3D micro-CT images. Comput Med Imaging Graph 32: 488–501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Le HQ, Wong JT, Molloi S. Allometric scaling in the coronary arterial system. Int J Cardiovasc Imaging 24: 771–781, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Marinus H, Buis B, van Benthem A. Pulsatile coronary flow determination by digital angiography. Int J Card Imaging 5: 173–182, 1990 [DOI] [PubMed] [Google Scholar]
- 32. Marques KM, Knaapen P, Boellaard R, Lammertsma AA, Westerhof N, Visser FC. Microvascular function in viable myocardium after chronic infarction does not influence fractional flow reserve measurements. J Nucl Med 48: 1987–1992, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Marzilli M, Sambuceti G, Fedele S, L'Abbate A. Coronary microcirculatory vasoconstriction during ischemia in patients with unstable angina. J Am Coll Cardiol 35: 327–334, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Meuwissen M, Chamuleau SA, Siebes M, Schotborgh CE, Koch KT, de Winter RJ, Bax M, de Jong A, Spaan JA, Piek JJ. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation 103: 184–187, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Molloi S, Kassab GS, Zhou Y. Quantification of coronary artery lumen volume by digital angiography: in vivo validation. Circulation 104: 2351–2357, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Molloi S, Chalyan D, Le H, Wong JT. Estimation of coronary artery hyperemic blood flow based on arterial lumen volume using angiographic images. Int J Cardiovasc Imaging. 2011. January 7 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molloi S, Ersahin A, Tang J, Hicks J, Leung CY. Quantification of volumetric coronary blood flow with dual-energy digital subtraction angiography. Circulation 93: 1919–1927, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Molloi S, Bednarz G, Tang J, Zhou Y, Mathur T. Absolute volumetric coronary blood flow measurement with digital subtraction angiography. Int J Card Imaging 14: 137–145, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Ng MK, Yeung AC, Fearon WF. Invasive assessment of the coronary microcirculation: superior reproducibility and less hemodynamic dependence of index of microcirculatory resistance compared with coronary flow reserve. Circulation 113: 2054–2061, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Pijls NH, Uijen GJ, Hoevelaken A, Arts T, Aengevaeren WR, Bos HS, Fast JH, van Leeuwen KL, van der Werf T. Mean transit time for the assessment of myocardial perfusion by videodensitometry. Circulation 81: 1331–1340, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Pijls NH, Uijen GJ, Pijnenburg T, van Leeuwen K, Aengevaeren WR, Barth JD, den Arend J, Hoevelaken A, van der Werf T. Reproducibility of mean transit time for maximal myocardial flow assessment by videodensitometry. Int J Card Imaging 6: 101–108, 1990 [DOI] [PubMed] [Google Scholar]
- 42. Pijls NH, Uijen GJ, Hoevelaken A, Pijnenburg T, van Leeuwen KL, Fast JH, Bos HS, Aengevaeren WR, van der Werf T. Mean transit time for videodensitometric assessment of myocardial perfusion and the concept of maximal flow ratio: a validation study in the intact dog and a pilot study in man. Int J Card Imaging 5: 191–202, 1990 [DOI] [PubMed] [Google Scholar]
- 43. Rittger H, Kuper A, Breithardt OA, Kuon E, Schmidt M, Sinha AM, Blum B, Jakob A, Brachmann J. A new angiographic method to assess coronary flow reserve-validation in humans. Catheter Cardiovasc Interv 75: 167–173, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Sambuceti G, Marzilli M, Fedele S, Marini C, L'Abbate A. Paradoxical increase in microvascular resistance during tachycardia downstream from a severe stenosis in patients with coronary artery disease : reversal by angioplasty. Circulation 103: 2352–2360, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Seiler C. The human coronary collateral circulation. Eur J Clin Invest 40: 465–476, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Senen K, Yetkin E, Turhan H, Atak R, Sivri N, Battaloglu B, Tandogan I, Ileri M, Kosar F, Ozdemir R, Cehreli S. Increased thrombolysis in myocardial infarction frame counts in patients with isolated coronary artery ectasia. Heart Vessels 19: 23–26, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Siebes M, Verhoeff BJ, Meuwissen M, de Winter RJ, Spaan JA, Piek JJ. Single-wire pressure and flow velocity measurement to quantify coronary stenosis hemodynamics and effects of percutaneous interventions. Circulation 109: 756–762, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Skyschally A, Haude M, Dorge H, Thielmann M, Duschin A, van de Sand A, Konietzka I, Buchert A, Aker S, Massoudy P, Schulz R, Erbel R, Heusch G. Glucocorticoid treatment prevents progressive myocardial dysfunction resulting from experimental coronary microembolization. Circulation 109: 2337–2342, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Spaan JA, Piek JJ, Hoffman JI, Siebes M. Physiological basis of clinically used coronary hemodynamic indices. Circulation 113: 446–455, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Spinale FG, Tanaka R, Crawford FA, Zile MR. Changes in myocardial blood flow during development of and recovery from tachycardia-induced cardiomyopathy. Circulation 85: 717–729, 1992 [DOI] [PubMed] [Google Scholar]
- 51. Tanaka N, Takazawa K, Takeda K, Aikawa M, Shindo N, Amaya K, Kobori Y, Yamashina A. Coronary flow-pressure relationship distal to epicardial stenosis. Circ J 67: 525–529, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Thielmann M, Dorge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Buchert A, Kruger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res 90: 807–813, 2002 [DOI] [PubMed] [Google Scholar]
- 53. van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. J Am Coll Cardiol 55: 17–25, 2009 [DOI] [PubMed] [Google Scholar]
- 54. van't Veer M, Geven MC, Rutten MC, van der Horst A, Aarnoudse WH, Pijls NH, van de Vosse FN. Continuous infusion thermodilution for assessment of coronary flow: theoretical background and in vitro validation. Med Eng Phys 31: 688–694, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Vijayalakshmi K, De Belder MA. Angiographic and physiologic assessment of coronary flow and myocardial perfusion in the cardiac catheterization laboratory. Acute Card Care 10: 69–78, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Vogel R, Indermuhle A, Seiler C. Determination of the absolute perfusion threshold preventing myocardial ischaemia in humans. Heart 93: 115–116, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vogel R, Indermuhle A, Meier P, Seiler C. Quantitative stress echocardiography in coronary artery disease using contrast-based myocardial blood flow measurements: prospective comparison with coronary angiography. Heart 95: 377–384, 2009 [DOI] [PubMed] [Google Scholar]
- 58. Vogel R, Zbinden R, Indermuhle A, Windecker S, Meier B, Seiler C. Collateral-flow measurements in humans by myocardial contrast echocardiography: validation of coronary pressure-derived collateral-flow assessment. Eur Heart J 27: 157–165, 2006 [DOI] [PubMed] [Google Scholar]
- 59. Vogel R, Indermuhle A, Reinhardt J, Meier P, Siegrist PT, Namdar M, Kaufmann PA, Seiler C. The quantification of absolute myocardial perfusion in humans by contrast echocardiography: algorithm and validation. J Am Coll Cardiol 45: 754–762, 2005 [DOI] [PubMed] [Google Scholar]
- 60. Wong JT, Molloi S. Determination of fractional flow reserve (FFR) based on scaling laws: a simulation study. Phys Med Biol 53: 3995–4011, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Zhu XY, Daghini E, Chade AR, Versari D, Krier JD, Textor KB, Lerman A, Lerman LO. Myocardial microvascular function during acute coronary artery stenosis: effect of hypertension and hypercholesterolaemia. Cardiovasc Res 83: 371–380, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]