Abstract
Although the induction of myocyte apoptosis by ischemia-reperfusion (I/R) is attenuated by ischemic preconditioning (IPC), the underlying mechanism is not fully understood. Phosphatase and tensin homologs deleted on chromosome 10 (PTEN) promotes apoptosis through Akt-dependent and -independent mechanisms. We tested the hypothesis that IPC attenuates the mitochondrial localization of PTEN in the myocardium induced by I/R. Isolated hearts from wild-type mice were exposed to IPC or normal perfusion followed by 30 min of ischemia and reperfusion. IPC attenuated myocardial infarct size and apoptosis after I/R. Heart fractionation showed that mitochondrial PTEN and Bax protein levels and the physical association between them were increased by 30 min of I/R and that IPC attenuated all of these effects of I/R. Muscle-specific PTEN knockout decreased mitochondrial Bax protein levels in the reperfused myocardium and increased cell survival. To determine whether PTEN relocalization to mitochondria was influenced by I/R-induced production of ROS, hearts were perfused with N-acetylcysteine (NAC) to scavenge ROS or H2O2 to mimic I/R-induced ROS. Mitochondrial PTEN protein levels were decreased by NAC and increased by H2O2. PTEN protein overexpression was generated in mouse hearts by adenoviral gene transfer. PTEN overexpression increased mitochondrial PTEN and Bax protein levels and ROS production, whereas muscle-specific PTEN knockout produced the opposite effects. In conclusion, myocardial I/R causes PTEN localization to the mitochondria, related to the generation of ROS; IPC attenuates the mitochondrial localization of PTEN after I/R, potentially inhibiting the translocation of Bax to the mitochondria and resulting in improved cell viability.
Keywords: Bax, reperfusion injury, apoptosis, phosphatase and tensin homologs deleted on chromosome 10
early reperfusion is an effective strategy to salvage the ischemic myocardium, but it has the potential to cause cardiac injury. Lethal ischemia-reperfusion (I/R) injury can be markedly limited by ischemic preconditioning (IPC), defined as one or several episodes of brief ischemia and reperfusion (30, 31). It has been reported that IPC inhibits the production of ROS and prevents mitochondrial permeability transition pore (MPTP) opening in the heart upon reperfusion (1, 2, 6, 27, 35, 43). Despite the importance of these events in cell death, the molecular mechanism responsible for I/R-induced apoptosis and the protection of IPC in the reperfused myocardium is not fully understood.
Apoptosis and necrosis coexist in the heart after I/R. Although <5% of cells are apoptotic in reperfused hearts, this type of cell death is actively regulated (9, 20). Bax is a necessary mediator of apoptosis (33, 47). In response to various stressors, including I/R, Bax translocates from the cytosol to the outer membrane of mitochondria, promoting the release of cytochrome c (47). Bax deletion decreases myocardial I/R injury (19). Necrotic cell death is caused, at least in part, by MPTP opening. After reperfusion, intracellular Ca2+ elevation and increased ROS production trigger MPTP opening, leading to the depletion of ATP and loss of mitochondrial integrity (8, 14, 16, 18, 48). IPC attenuates the increase in Bax protein levels in the reperfused myocardium and inhibits ROS production induced by I/R (32, 35). However, the connection between ROS and mitochondrial Bax is not well defined.
Phosphatase and tensin homologs deleted on chromosome 10 (PTEN) is well known as a tumor suppressor. It is ubiquitously expressed in animals and humans. Under basal conditions, PTEN is heavily phosphorylated and mainly localized in the cytoplasm (45). After dephosphorylation, PTEN moves to the plasma membrane, where it catalyzes the breakdown of phosphatidylinositol 3,4,5-trisphosphate into phosphatidylinositol 4,5-bisphosphate and Pi (7, 34, 45). PTEN is also found in the mitochondria (49). Its biological function in the subcellular space has not been studied before in the heart. In the present study, we investigated the hypothesis that I/R increases the mitochondrial localization of PTEN and promotes apoptosis through Bax. We found that mitochondrial protein levels of PTEN and Bax were increased in the heart by I/R, that this effect was attenuated by IPC, and that mitochondrial Bax protein levels were associated with PTEN protein levels in reperfused hearts.
MATERIALS AND METHODS
Animals.
All experiments were performed with age-matched male mice. At the time of the experiments, mice were 8–10 wk old. Wild-type (WT; C57BL6) mice were purchased from The Jackson Laboratory. Muscle-specific PTEN knockout (PKO) mice (Ptenloxp/loxp;ckm-Cre+/−) were generated by loxp-cre technology (50). Ptenloxp/loxp;ckm-Cre−/− mice were used as the controls for PKO mice. Their phenotypes were as previously described (7, 50). All procedures were approved by the Institutional Animal Care and Use Committee of The Johns Hopkins University and conformed with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Mouse Langendorff preparation.
Mice were anesthetized by an intraperitoneal injection of pentobarbital (70 mg/kg). The Langendorff preparation was performed as previously described (5). Briefly, the chest was opened, the heart was excised, and the ascending aorta was cannulated with a blunt needle. The heart was perfused at a constant pressure of 100 cmH2O with modified Krebs-Henseleit buffer [containing (in mmol/l) 17 glucose, 120 NaCl, 25 NaHCO3, 2.5 CaCl2, 5.9 KCl, 1.2 MgSO4, and 0.5 EDTA], which was maintained at 37°C and bubbled continuously with a mixture of 95% O2 and 5% CO2. Global ischemia was induced by the cessation of perfusion followed by reperfusion.
Experimental protocol.
Isolated hearts from WT mice were exposed to IPC, which consisted of 10 min of ischemia and 5 min of reperfusion (I-10/R-5) or an equivalent period of normal perfusion (NIS) followed by 30 min of ischemia and 120 min of reperfusion (I-30/R-120). Isolated hearts from PKO and control mice were directly exposed to I-30/R-120 after an initial 15 min of perfusion. At the end of the experiment, myocardial infarct size (IS) was measured by triphenyltetrazolium chloride (TTC) staining. Since apoptosis is progressively increased after reperfusion (37), we measured apoptotic cells and analyzed the accompanying molecular events at I-30/R-30. Because ROS levels decrease rapidly after reperfusion (22), we assessed ROS production at 1 min of reperfusion after 30 min of ischemia. To inhibit the burst of ROS after reperfusion, isolated hearts were perfused with N-acetylcysteine (NAC) before 5 min of ischemia and during 30 min of reperfusion. In other experiments, H2O2 was added into the perfusion line by a syringe pump.
Assessment of myocardial IS.
At the end of the experiments, mouse hearts were perfused with 1% TTC (dissolved in 0.9% NaCl) for 1 min and then incubated with 1% TTC at 37°C for 15 min. After being frozen at −80°C, hearts were transected into five pieces. Cardiac sections were incubated with 10% formalin for 30 min. Both sides (A and B) of each section were photographed. The areas of red and white color were measured by computerized planimetry (ImageJ, NIH, Bethesda, MD). IS was calculated as a percentage of the left ventricle (LV) as follows: .
Heart fractionation.
Cytosolic, mitochondrial, and nuclear protein fractions were extracted as previously described (25). Heart tissue was homogenized and then centrifuged at 2,000 g at 4°C. The supernatant was collected and centrifuged for 10 min at 12,000 g, producing a cytosolic fraction in the supernatant and a mitochondrial fraction in the pellet. To obtain the nuclear-enriched fraction, the loose pellet from the first centrifugation was homogenized and suspended. This suspension underwent ultracentrifugation at 30,000 g for 45 min at 4°C. The pellet containing the nuclear fraction was resuspended in glycerol storage buffer. All subcellular fractions were stored at −80°C.
Measurement of ROS production.
ROS production in perfused hearts was measured using the fluorescent probes dihydroethidium (DHE) and dihydrofluorescein (DHF) diacetate (DHFDA) as previously described (22, 42). DHFDA passes through cell membranes and is converted to DHF by intracellular esterase and then oxidized to green fluorescein. DHF is trapped in the cell and is sensitive to oxidants (22). DHE enters cells and reacts with oxidants to become ethidium, which binds to DNA and produces red fluorescence of the nuclei. Extracellular residential probes are washed out by Krebs-Henseleit perfusion buffer before I/R. Hearts were loaded with DHE (5 μmol/l) or DHFDA (20 μmol/l) at a temperature of 37°C for 20 min followed by 5 min of washout. At the end of the experiment, hearts were homogenized in fresh Krebs-Henseleit buffer. Protein (50 μg) from each sample was loaded into each well. Fluorescence intensity was measured in a 96-well plate by a multifunction plate reader (DHE: 540-nm excitation and 590-nm emission; DHF: 490-nm excitation and 535-nm emission).
TUNEL assay.
A TUNEL assay was performed with the commercially available ApopTag® Plus In Situ Apoptosis Fluorescein Detection Kit according to the manufacturer's instructions. Briefly, heart tissue sections were fixed in 4% paraformaldehyde solution for 15 min. Proteinase K (100 μl) was added to each slide. After fixation, sections were incubated with rTdT buffer at 37°C for 60 min. Reactions were terminated with 2× SSC. After being washed, slides were mounted in Vectashield + 4',6-diamidino-2-phenylindole to stain nuclei. All samples were analyzed under a fluorescence microscope.
PTEN overexpression by adenoviral gene transfer.
PTEN-overexpressing hearts were generated as previously described (29). WT mice were anesthetized by an intraperitoneal injection of pentobarbital (60 mg/kg). The heart was exposed under a stereomicroscope. A dose of 108 plaque-forming units (pfu)/30 μl of Pten adenoviruses (adPten), which contained human Pten-expressing sequence, or empty vectors (adNull) (Vector Biolabs, Philadelphia, PA) was injected into the LV wall at three equidistant points by a 30-gauge syringe. Seven days later, hearts were isolated for measurements of protein levels and ROS production.
Immunoblot assay.
Cardiac tissues were homogenized in lysis buffer [containing (in mM) 20 Tris (pH 7.5), 150 NaCl, 1 EDTA, 1 EGTA, 1 PMSF, and 1 Na3VO4 with 1% Triton X-100]. Proteins were detected using primary antibodies followed by horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence. Antibodies against PTEN (rabbit), Bax, p53, phosphorylated (p-)Akt (Ser473), and total Akt were purchased from Cell Signaling Technology (Danvers, MA).
Statistical analysis.
Data are presented as means ± SE. Differences among groups were analyzed using Student's t-test or one-way ANOVA with the Tukey post hoc test. Differences were considered significant if P < 0.05.
RESULTS
IPC and PTEN deletion decreases cell death after I/R.
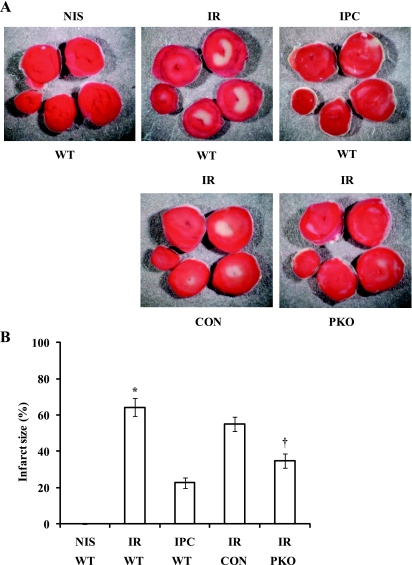
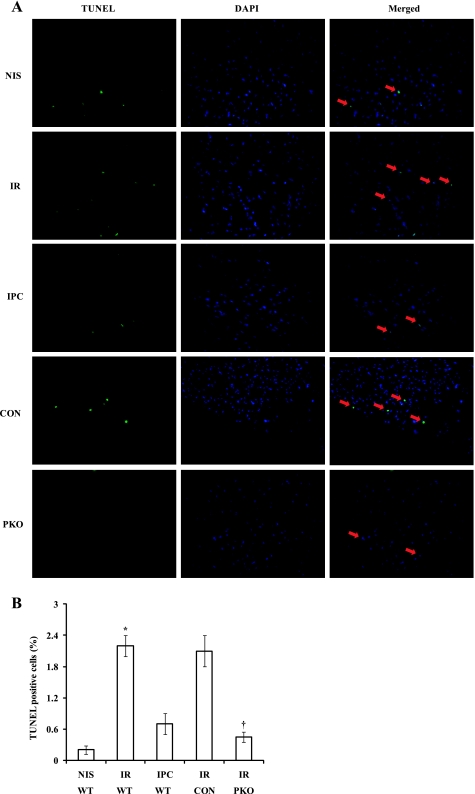
To demonstrate that both IPC and PTEN inactivation induce cardioprotection in isolated mouse hearts, we exposed isolated hearts from WT mice to NIS, I-30/R-120 (I/R), or I-10/R-5 + I/R (IPC) and hearts from PKO and control mice to I/R. IPC significantly decreased myocardial IS compared with I/R (IS: 23 ± 3% with IPC vs. 64 ± 5% with I/R, P < 0.01; Fig. 1). PTEN deletion produced an IPC-like effect on myocardial IS (IS: 35 ± 4% in PKO mice vs. 55 ± 4% in control mice, P < 0.01; Fig. 1). We measured apoptosis after I-30/R-30. Most apoptotic cells were located at the border area between living and necrotic tissues. Both IPC and PTEN deletion significantly decreased TUNEL-positive cells (TUNEL: 0.7 ± 0.2% with IPC vs. 2.2 ± 0.2% with I/R, P < 0.05, and 0.45 ± 0.1% in PKO mice vs. 2.1 ± 0.3% in control mice, P < 0.05; Fig. 2). These results demonstrate that both IPC and PTEN deletion induces cardioprotection against I/R injury.
Fig. 1.
Ischemic preconditioning (IPC) and phosphatase and tensin homologs deleted on chromosome 10 (PTEN) inactivation decreases myocardial infarct size (IS). A: isolated hearts from wild-type (WT) mice were exposed to normal perfusion (NIS), 30 min of ischemia/120 min of reperfusion [I-30/R-120; ischemia-reperfusion (I/R)], or 10 min of ischemia/5 min of reperfusion + I/R (I-10/R-5 + I/R; IPC), and hearts from muscle-specific PTEN knockout (PKO) mice and control (CON) mice were exposed to I/R. I/R caused myocardial infarction. B: IS was decreased by IPC. Myocardial IS was decreased in PKO hearts compared with control hearts. n = 6. *P < 0.01, I/R vs. NIS or IPC. n = 5. †P < 0.05, PKO vs. control hearts.
Fig. 2.
Both IPC and PTEN inactivation inhibit apoptosis in the reperfused myocardium. A: a TUNEL assay was performed in the hearts after they had been exposed to NIS, I-30/R-30 (I/R), or I-10/R-5 + I/R (IPC). IPC decreased apoptotic cells compared with I/R. PKO and control hearts were exposed to I/R. There were fewer apoptotic cells in PKO hearts. Arrows indicate apoptotic cells. DAPI, 4′,6-diamidino-2-phenylindole. B: percentages of TUNEL-positive cells. n = 6. *P < 0.01, I/R vs. NIS or IPC. n = 5. †P < 0.01, PKO vs. control hearts.
IPC preserves whole cell and cytosolic PTEN protein levels in reperfused hearts.
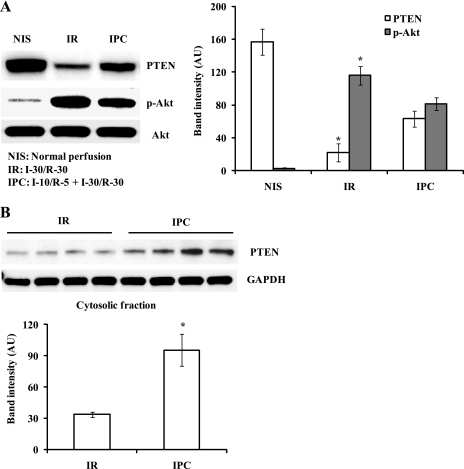
To determine whether the PTEN/Akt signaling pathway plays a role in IPC cardioprotection, we analyzed the protein levels of PTEN, p-Akt, and total Akt in whole cell lysates after I-30/R-30. I/R significantly decreased PTEN protein levels [PTEN: 22 ± 11 arbitrary units (AU) with I/R vs. 157 ± 16 AU with NIS, P < 0.05; Fig. 3A] and increased Akt phosphorylation (p-Akt: 116 ± 11 AU with I/R vs. 2 ± 1 AU with NIS, P < 0.05; Fig. 3). IPC attenuated the loss of PTEN protein (PTEN: 63 ± 10 AU with IPC vs. 22 ± 11 AU with I/R, P < 0.05; Fig. 3A) and slightly inhibited the increase in Akt phosphorylation (p-Akt: 81 ± 8 AU with IPC vs. 116 ± 11 AU with I/R, P < 0.05; Fig. 3A). Total Akt protein levels were unchanged. The ratio of p-Akt to Akt was increased in I/R and IPC hearts compared with NIS hearts (p-Akt/Akt: 0.96 ± 0.10 with I/R vs. 0.01 ± 0.00 with NIS, P < 0.05, and 0.66 ± 0.06 with IPC vs. 0.01 ± 0.00 with NIS, P < 0.05). To determine the effect of IPC on cytosolic PTEN protein levels, we analyzed PTEN protein levels in cytosolic fractions. IPC preserved cytosolic PTEN protein levels in reperfused hearts (PTEN: 96 ± 16 AU with IPC vs. 34 ± 3 AU with I/R, P < 0.01; Fig. 3B).
Fig. 3.
IPC preserves cytosolic PTEN protein levels. Isolated hearts from WT mice were exposed to NIS, I-30/R-30 (I/R), or I-10/R-5 + I/R (IPC). A: representative Western blots of PTEN, phosphorylated (p-)Akt, and total Akt. Whole cell lysates were used for Western blot analysis. PTEN protein levels were decreased in I/R hearts, but this I/R effect was attenuated by IPC. p-Akt protein levels were increased in I/R hearts, but IPC attenuated the increase in Akt phosphorylation compared with I/R. B: PTEN protein levels were analyzed in cytosolic fractions from I/R and IPC hearts. PTEN protein levels were higher in IPC hearts than in I/R hearts. AU, arbitrary units. n = 4. *P < 0.05, I/R vs. NIS or IPC.
IPC attenuates the mitochondrial localization of PTEN and Bax induced by I/R.
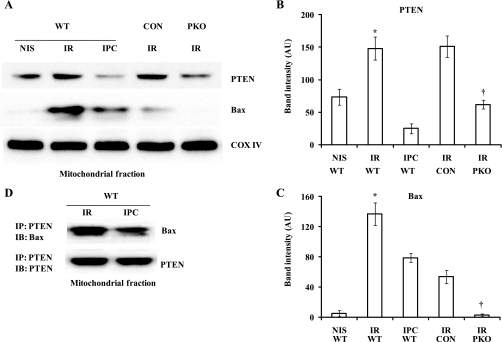
The mitochondria play a critical role in cell death (14, 30, 48). We wondered whether IPC affects mitochondrial PTEN and Bax protein levels in the heart after I/R. We analyzed the protein levels of PTEN and Bax in the mitochondrial fraction of the heart after exposure to I-30/R-30 with or without IPC. I/R significantly increased mitochondrial PTEN (PTEN: 148 ± 17 AU with I/R vs. 73 ± 12 AU with NIS, P < 0.01; Fig. 4, A and B) and Bax (Bax: 137 ± 15 AU with I/R vs. 5 ± 4 AU with NIS, P < 0.01; Fig. 4, A and C). Opposite to the effect on cytosolic PTEN levels, IPC significantly inhibited the increase in mitochondrial PTEN (PTEN: 24 ± 7 AU with IPC vs. 148 ± 17 AU with I/R, P < 0.01; Fig. 4, A and B) and Bax (Bax: 79 ± 6 AU with IPC vs. 137 ± 15 AU with NIS, P < 0.01; Fig. 4, A and C). The levels of mitochondrial protein cytochrome c oxidase IV were unchanged. Although Akt phosphorylation was increased in the mitochondrial fractions of I/R and IPC hearts compared with NIS hearts, there was no significant difference between IPC and I/R (Supplemental Material, Supplemental Fig. S1).1 These results suggest that I/R increased PTEN and Bax translocation from the cytosol to the mitochondria. To determine whether Bax is related to PTEN in the mitochondria, we measured PTEN and Bax protein levels in the mitochondrial fraction from PKO and control hearts after exposure to I-30/R-30. A decrease in mitochondrial PTEN protein levels was associated with a decrease in mitochondrial Bax protein levels (Bax: 3 ± 2 AU in PKO hearts vs. 54 ± 9 AU in control hearts, P < 0.01; Fig. 4, A and C). We measured Akt phosphorylation in control and PKO hearts after I/R. Akt phosphorylation was modestly decreased in PKO hearts compared with control hearts (Supplemental Fig. S2). It has been reported that PTEN is physically associated with Bax in the mitochondria (49). To determine whether IPC affects the physical association between PTEN and Bax in the mitochondria, we first pulled down PTEN protein with anti-mouse PTEN antibody from the mitochondrial fraction of WT hearts exposed to I/R or I-10/R-5 + I/R (IPC). Immunoprecipitates were transferred to a membrane and blotted with anti-rabbit antibodies against Bax or PTEN. IPC attenuated the physical association between Bax and PTEN in the mitochondria (Fig. 4D).
Fig. 4.
IPC attenuates the mitochondrial localization of PTEN and Bax in reperfused hearts. Isolated hearts from WT mice were exposed to NIS, I-30/R-30 (I/R), or I-10/R-5 + I/R (IPC); isolated hearts from PKO and CON mice were exposed to I/R. Heart fractionation was performed, and the mitochondrial fraction was then used for immunoblot assays. A: representative Western blots of PTEN, Bax, and cytochrome c oxidase (COX) IV. B and C: PTEN (B) and Bax (C) protein levels were increased in I/R hearts; the increase was attenuated by IPC and PTEN deletion. n = 4. *P < 0.05, I/R vs. NIS or IPC; †P < 0.01, control vs. PKO hearts. D: the association between PTEN and Bax was attenuated in the mitochondria by IPC. Shown is a representative Western blot from 3 independent experiments.
ROS promotes the mitochondrial localization of PTEN.
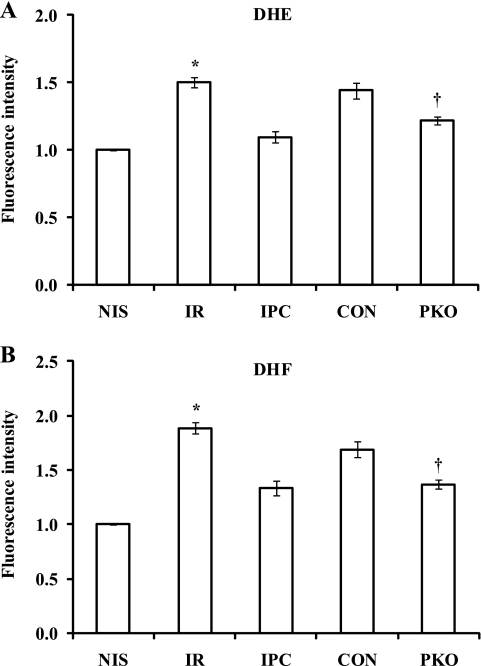
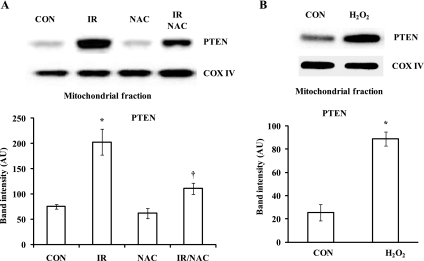
Since mitochondrial PTEN protein levels were increased by I/R and attenuated by IPC, we wondered whether the changes were related to increased ROS levels. We measured ROS production in isolated hearts after they were exposed to I-30/R-1 with or without IPC by DHE and DHF fluorescence assays. I/R increased fluorescence intensity (DHE: 1.5 ± 0.0 ratio with I/R vs. 1.0 ± 0.0 ratio with NIS, P < 0.05, Fig. 5A; DHF: 1.8 ± 0.1 ratio with I/R vs. 1.0 ± 0.0 ratio with NIS, P < 0.05, Fig. 5B). The increases were attenuated by IPC (DHE: 1.1 ± 0.0 ratio with IPC vs. 1.5 ± 0.0 ratio with I/R, P < 0.05, Fig. 5A; DHF: 1.3 ± 0.1 ratio with IPC vs. 1.8 ± 0.1 ratio with I/R, P < 0.05, Fig. 5B). In a similar manner, muscle-specific PKO attenuated the fluorescence intensity of DHE and DHF compared with controls (DHE: 1.2 ± 0.0 ratio in PKO vs. 1.4 ± 0.1 ratio in control, P < 0.05, Fig. 5A; DHF: 1.4 ± 0.0 ratio in PKO vs. 1.7 ± 0.1 ratio in control, P < 0.05, Fig. 5B). Since the changes in ROS levels with I/R and IPC were directionally similar to mitochondrial PTEN protein levels in WT mice, we wondered whether ROS promote the mitochondrial localization of PTEN in the heart. Isolated hearts were treated with 1 mmol/l NAC 5 min before ischemia and during 30 min of reperfusion after 30 min of ischemia (I/R-NAC). Hearts perfused with 1 mmol/l NAC for 35 min were used as controls. NAC attenuated the increase in mitochondrial PTEN levels caused by I/R (PTEN: 111 ± 11 AU with I/R-NAC vs. 203 ± 26 AU with I/R, P < 0.01; Fig. 6A). Additionally, isolated hearts were treated with 50 μmol/l H2O2 for 30 min. H2O2 significantly increased mitochondrial PTEN protein levels compared with nontreated hearts (PTEN: 89 ± 6% in H2O2-treated hearts vs. 25 ± 7% in control hearts, P < 0.01; Fig. 6B). Therefore, these results suggest that mitochondrial PTEN protein levels are affected by ROS production after reperfusion.
Fig. 5.
ROS production is attenuated by IPC or PTEN knockout in the heart after I/R. Hearts from WT mice were exposed to NIS, I-30/R-1 (I/R), I-10/R-5 + I/R (IPC). ROS production was measured by dihydroethidium (DHE; A) and dihydrofluorescein (DHF; B) fluorescence assays. ROS production was increased after I/R. The effect was attenuated by IPC. ROS production was decreased after I/R in PKO hearts compared with control hearts. n = 4. *P < 0.05, I/R vs. NIS or IPC; †P < 0.05, PKO vs. control hearts.
Fig. 6.
Mitochondrial PTEN protein levels are regulated by ROS in reperfused hearts. A: hearts from WT mice were exposed to NIS, I-30/R-1 (I/R), N-acetylcysteine (NAC), or I/R + NAC. Heart fractionation was performed. I/R increased mitochondrial PTEN protein levels. The effect was attenuated by NAC. n = 4. *P < 0.01, I/R vs. NIS; †P < 0.05, I/R + NAC vs. I/R. B: isolated hearts were treated with H2O2 for 30 min. PTEN protein levels were analyzed in the mitochondrial fraction. n = 4. *P < 0.01, H2O2 vs. control.
Mitochondrial Bax protein levels and ROS production were associated with mitochondrial PTEN protein levels in mouse hearts.
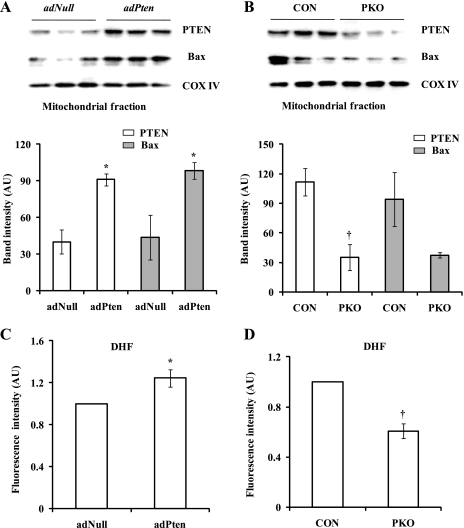
To determine the role of mitochondrial PTEN in the regulation of Bax protein levels and ROS production, we first overexpressed PTEN in mouse hearts by adPten transfer. PTEN-overexpressing hearts were isolated for measurements of PTEN and Bax protein levels and ROS production. Whole PTEN protein levels were increased in adPten hearts (Supplemental Fig. S3A) compared with adNull hearts. Moreover, mitochondrial PTEN protein levels were also increased in adPten hearts (PTEN: 91 ± 5 AU in adPten hearts vs. 40 ± 10 AU in adNull hearts, P < 0.01; Fig. 7A). As expected, mitochondrial PTEN protein levels were decreased in PKO mice, which expressed less PTEN protein in the heart (Supplemental Fig. S3B), compared with control mice (PTEN: 35 ± 13 AU in PKO hearts vs. 111 ± 14 AU in control hearts, P < 0.01; Fig. 7B). Mitochondrial Bax protein levels were increased in PTEN-overexpressing hearts (Bax: 98 ± 6 AU in adPten hearts vs. 43 ± 18 AU in adNull hearts, P < 0.05; Fig. 7A) and were decreased in PKO hearts (Bax: 37 ± 2 AU in PKO hearts vs. 94 ± 27 AU in control hearts, P = 0.06; Fig. 7B). Consistent with these results, ROS production was increased in PTEN-overexpressing hearts (DHF: 1.2 ± 0.1 ratio in adPten hearts vs. 1.0 ± 0.0 ratio in adNull hearts, P < 0.05; Fig. 7C) and decreased in PKO hearts (DHF: 0.6 ± 0.1 ratio in PKO hearts vs. 1.0 ± 0.0 ratio in control hearts, P < 0.05; Fig. 7D). These results suggest that an increase in mitochondrial PTEN may result in an increase in mitochondrial Bax through a physical association between the two proteins and promote ROS production in the heart.
Fig. 7.
Mitochondrial Bax protein levels and ROS production are associated with mitochondrial PTEN protein levels in mouse hearts. A: PTEN was overexpressed in WT hearts by adenoviral Pten (adPten) transfer. Empty vectors (adNull) were used as controls. PTEN and Bax protein levels were analyzed in mitochondrial fractions. B: mitochondrial PTEN and Bax protein levels were measured in muscle-specific PKO and control mouse hearts. C and D: ROS production was measured in adPten and adNull (C) and PKO and control (D) hearts by a DHF fluorescence assay. n = 3. *P < 0.05, adPten vs. adNull hearts; †P < 0.05, PKO vs. control hearts.
DISCUSSION
Here, we report that IPC attenuates the mitochondrial localization of PTEN induced by I/R. We analyzed PTEN protein levels in mitochondrial and cytosolic fractions in hearts after they had been exposed to I/R in the absence or presence of IPC. Although PTEN protein levels were decreased in whole cell lysates after I/R, mitochondrial PTEN levels were increased, suggesting that I/R promotes the translocation of PTEN from the cytosol to the mitochondria. IPC preserved cytosolic PTEN protein levels and attenuated its mitochondrial localization. Furthermore, we showed that I/R causes an increase in mitochondrial Bax protein levels, which is inhibited by IPC. Importantly, we showed that mitochondrial Bax protein levels are physically associated with mitochondrial PTEN levels in reperfused hearts. Because Bax plays an essential role in mediating I/R injury, our study suggests that IPC may mediate cardioprotection through inhibition of the mitochondrial localization of PTEN.
Although the phenomenon of IPC has been known for more than two decades, its underlying mechanisms are still not fully understood. Murry et al. (31) reported that there was a 75% reduction in myocardial IS after dogs had been exposed to IPC consisting of four cycles of 5 min of ischemia and 5 min of reperfusion (I-5/R-5). Later, it was reported that a single cycle of I-5/R-5 can be as effective as multiple cycles in cardioprotection (24). However, the protective effect of IPC appears to be dependent on the duration of ischemia. A 10-min period of ischemia produced better protection than 3 min of ischemia in pigs (39). Adenosine, bradykinin, and opioids, which are generated in the myocardium during ischemia, have been shown to trigger cardioprotection when infused before prolonged I/R (12, 13, 26, 38, 46). These substances activate several protein kinases, including Akt, PKC, and Erk1/2, before prolonged ischemia (10, 11, 28, 44). Because of their prosurvival characteristics, they have been termed survival protein kinases. However, it is still controversial whether IPC increases the phosphorylation of survival protein kinases in the heart after I/R. Hausenloy et al. (15) showed that the phosphorylation of Akt and Erk1/2 was increased in reperfused hearts after IPC. Recently, Clarke et al. (6) reported that IPC did not increase the phosphorylation of survival protein kinases in the heart after I/R, and no change in mitochondrial protein phosphorylation was found. The present study shows that Akt phosphorylation is modestly decreased in preconditioned hearts after I/R, and this result is consistent with the change in PTEN protein levels in whole cell lysates. It has been reported that IPC mediates cardioprotection by decreasing Bax protein levels in reperfused hearts (32). Our study demonstrates, for the first time, that IPC decreases Bax protein levels in the mitochondria and that decreased Bax may result from attenuated mitochondrial localization of PTEN.
Mitochondria are located beneath the sarcolemma and between myofibrils in cardiomyocytes. Subsarcolemmal mitochondria can be separated from interfibrillar mitochondria by 10 min of centrifugation at 800 g (3). In the present study, the mitochondria were obtained from the supernatant after the centrifugation at 2,000 g. Therefore, the mitochondria used in this study were subsarcolemmal mitochondria. It has been shown that subsarcolemmal mitochondria contain connexin43, which is required for IPC (3, 40). Although PTEN physically interacts with Bax and likely stabilizes it in the mitochondria after I/R, more studies are needed to determine whether mitochondrial localization of PTEN has an effect on connexin43 protein levels. In the present study, the role of Akt phosphorylation is not clear in reperfused hearts. Both IPC and I/R increased Akt phosphorylation in whole cell lysates and mitochondrial fractions compared with basal conditions, but IPC failed to further raise the level of Akt phosphorylation compared with I/R alone. A similar observation has been reported in postconditioned pig hearts (41). In that study, postconditioning increased Akt phosphorylation in pig hearts, but the effect was not different from that of immediate reperfusion. Cardioprotection can be mediated by Akt-independent pathways, such as the TNF-α pathway (11, 17, 21). Further studies are needed to determine whether PTEN regulates TNF-α signaling in reperfused hearts.
It is widely known that ROS play an important role in mediating reperfusion injury (4, 43). Reperfusion after ischemia results in a burst of ROS, including H2O2. In the present study, we demonstrated that H2O2 increases the mitochondrial localization of PTEN in isolated hearts. Furthermore, it is inhibited by the ROS scavenger NAC. Therefore, the mitochondrial localization of PTEN by I/R is attributed to increased ROS production. It has been reported that IPC attenuates ROS production after I/R (22). Decreased ROS production by IPC may attenuate the localization of PTEN in the mitochondria, leading to a decrease in Bax translocation and apoptosis. Indeed, the present study showed PTEN protein levels are associated with Bax protein levels in the mitochondria. It has been reported that mitochondrial Bax promotes ROS production (23). It appears that IPC attenuates ROS production after reperfusion and breaks a vicious cycle in which increased ROS production precipitates PTEN localization to the mitochondria and then increased mitochondrial PTEN promotes Bax translocation and ROS production.
Although more necrotic cells are present in nonpreconditioned hearts, increased PTEN and Bax protein levels in the mitochondria after I/R cannot be simply attributed to membrane damage. The mitochondrial fraction was collected from the pellet after high-speed centrifugation. Damaged cells would release PTEN and Bax proteins into the buffer, which would be recovered in the cytosolic fraction. Moreover, control protein cytochrome c oxidase IV levels were not different in the mitochondrial fraction.
In conclusion, IPC attenuates the mitochondrial localization of PTEN protein in reperfused hearts, likely through a decrease in ROS production. The IPC-mediated attenuation of PTEN relocalization may decrease mitochondrial Bax protein levels and limit apoptosis. Therefore, inhibition of mitochondrial localization of PTEN during reperfusion may be a novel approach for limiting myocardial I/R injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-65608 and HL-88071.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1. An J, Varadarajan SG, Novalija E, Stowe DF. Ischemic and anesthetic preconditioning reduces cytosolic Ca2+ and improves Ca2+ responses in intact hearts. Am J Physiol Heart Circ Physiol 281: H1508–H1523, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Argaud L, Gateau-Roesch O, Chalabreysse L, Gomez L, Loufouat J, Thivolet-Béjui F, Robert D, Ovize M. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res 61: 115–22, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Boengler K, Stahlhofen S, van de Sand A, Gres P, Ruiz-Meana M, Garcia-Dorado D, Heusch G, Schulz R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res Cardiol 104: 141–147, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Bolli R, Marbán E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1α. Cardiovasc Res 77: 463–470, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res 102: 1082–1090, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, nSuzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell 110: 737–749, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Crompton M, Costi A. Kinetic evidence for a heart mitochondrial pore activated by Ca2+, inorganic phosphate and oxidative stress. A potential mechanism for mitochondrial dysfunction during cellular Ca2+ overload. Eur J Biochem 178: 489–501, 1988 [DOI] [PubMed] [Google Scholar]
- 9. Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 95: 957–970, 2004 [DOI] [PubMed] [Google Scholar]
- 10. da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Differential activation of mitogen-activated protein kinases in ischemic and anesthetic preconditioning. Anesthesiology 100: 59–69, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Deuchar GA, Opie LH, Lecour S. TNFα is required to confer protection in an in vivo model of classical ischaemic preconditioning. Life Sci 80: 1686–1691, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Fryer RM, Pratt PF, Hsu AK, Gross GJ. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther 296: 642–649, 2001 [PubMed] [Google Scholar]
- 13. Goto M, Liu Y, Yang XM, Ardell JL, Cohen MV, Downey JM. Role of bradykinin in protection of ischemic preconditioning in rabbit hearts. Circ Res 77: 611–621, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38: 841–860, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Hausenloy DJ, Tsang A, Mocanu MM, Yellon DM. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol 288: H971–H976, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria. II. Nature of the Ca2+ trigger site. Arch Biochem Biophys 195: 460–467, 1979 [DOI] [PubMed] [Google Scholar]
- 17. Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118: 1915–1919, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Heusch G, Boengler K, Schulz R. Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105: 151–154, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Hochhauser E, Kivity S, Offen D, Maulik N, Otani H, Barhum Y, Pannet H, Shneyvays V, Shainberg A, Goldshtaub V, Tobar A, Vidne BA. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol 284: H2351–H2359, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res 95: 734–741, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lecour S, Suleman N, Deuchar GA, Somers S, Lacerda L, Huisamen B, Opie LH. Pharmacological preconditioning with tumor necrosis factor-alpha activates signal transducer and activator of transcription-3 at reperfusion without involving classic prosurvival kinases (Akt and extracellular signal-regulated kinase). Circulation 112: 3911–3918, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol 284: H566–H574, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kirkland RA, Windelborn JA, Kasprzak JM, Franklin JL. A Bax-induced pro-oxidant state is critical for cytochrome c release during programmed neuronal death. J Neurosci 22: 6480–6490, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li GC, Vasquez JA, Gallagher KP, Lucchesi BR. Myocardial protection with preconditioning. Circulation 82: 609–619, 1990 [DOI] [PubMed] [Google Scholar]
- 25. Li X, Nemoto M, Xu Z, Yu SW, Shimoji M, Andrabi SA, Haince JF, Poirier GG, Dawson TM, Dawson VL, Koehler RC. Influence of duration of focal cerebral ischemia and neuronal nitric oxide synthase on translocation of apoptosis-inducing factor to the nucleus. Neuroscience 144: 56–65, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation 84: 350–356, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Liu H, Cala PM, Anderson SE. Ischemic preconditioning: effects on pH, Na and Ca in newborn rabbit hearts during ischemia/reperfusion. J Mol Cell Cardiol 30: 685–697, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Ytrehus K, Downey JM. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J Mol Cell Cardiol 26: 661–668, 1994 [DOI] [PubMed] [Google Scholar]
- 29. Meloni M, Caporali A, Graiani G, Lagrasta C, Katare R, Van Linthout S, Spillmann F, Campesi I, Madeddu P, Quaini F, Emanueli C. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ Res 106: 1275–1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murphy E. Primary and secondary signaling pathways in early preconditioning that converge on the mitochondria to produce cardioprotection. Circ Res 94: 7–16, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal injury in ischemic myocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Nakamura M, Wang NP, Zhao ZQ, Wilcox JN, Thourani V, Guyton RA, Vinten-Johansen J. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat heart. Cardiovasc Res 45: 661–670, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619, 1993 [DOI] [PubMed] [Google Scholar]
- 34. Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol 37: 449–471, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Rickover O, Zinman T, Kaplan D, Shainberg A. Exogenous nitric oxide triggers classic ischemic preconditioning by preventing intracellular Ca2+ overload in cardiomyocytes. Cell Calcium 43: 324–333, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17 degrees C ischemia in intact hearts. Cardiovasc Res 61: 580–590, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Scarabelli T, Stephanou A, Rayment N, Pasini E, Comini L, Curello S, Ferrari R, Knight R, Latchman D. Apoptosis of endothelial cells precedes myocyte cell apoptosis in ischemia/reperfusion injury. Circulation 104: 253–256, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol Heart Circ Physiol 268: H2157–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Schulz R, Post H, Vahlhaus C, Heusch G. Ischemic preconditioning in pigs: a graded phenomenon: its relation to adenosine and bradykinin. Circulation 98: 1022–1029, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Schwanke U, Konietzka I, Duschin A, Li X, Schulz R, Heusch G. No ischemic preconditioning in heterozygous connexin43-deficient mice. Am J Physiol Heart Circ Physiol 283: H1740–H1742, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res 104: 15–18, 2009 [DOI] [PubMed] [Google Scholar]
- 42. Stoner JD, Clanton TL, Aune SE, Angelos MG. O2 delivery and redox state are determinants of compartment-specific reactive O2 species in myocardial reperfusion. Am J Physiol Heart Circ Physiol 292: H109–H116, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Sun HY, Wang NP, Kerendi F, Halkos M, Kin H, Guyton RA, Vinten-Johansen J, Zhao ZQ. Hypoxic postconditioning reduces cardiomyocyte loss by inhibiting ROS generation and intracellular Ca2+ overload. Am J Physiol Heart Circ Physiol 288: H1900–H1908, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Tong H, Chen W, Steenbergen C, Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res 87: 309–315, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Vazquez F, Matsuoka S, Sellers WR, Yanagida T, Ueda M, Devreotes PN. Tumor suppressor PTEN acts through dynamic interaction with the plasma membrane. Proc Natl Acad Sci USA 103: 3633–3638, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther 270: 681–689, 1994 [PubMed] [Google Scholar]
- 47. Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72: 19–44, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Zhu Y, Hoell P, Ahlemeyer B, Krieglstein J. PTEN: a crucial mediator of mitochondria-dependent apoptosis. Apoptosis 11: 197–207, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Zu L, Bedja D, Fox-Talbot K, Gabrielson KL, Van Kaer L, Becker LC, Cai ZP. Evidence for a role of immunoproteasomes in regulating cardiac muscle mass in diabetic mice. J Mol Cell Cardiol 49: 5–15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]