Abstract
End-stage kidney disease is a terminal stage of chronic kidney disease, which is associated with a high incidence of cardiovascular disease. Cardiovascular disease frequently results from endothelial injury caused by carbamylated LDL (cLDL), the product of LDL modification by urea-derived cyanate. Our previous data suggested that cLDL induces mitogen-activated protein kinase-dependent mitotic DNA fragmentation and cell death. However, the mechanism of this pathway is unknown. The current study demonstrated that cLDL-induced endothelial mitotic cell death is independent of caspase-3. The expression of endonuclease G (EndoG), the nuclease implicated in caspase-independent DNA fragmentation, was significantly increased in response to cLDL exposure to the cells. The inhibition of EndoG by RNAi protected cLDL-induced DNA fragmentation, whereas the overexpression of EndoG induced more DNA fragmentation in endothelial cells. Ex vivo experiments with primary endothelial cells isolated from wild-type (WT) and EndoG knockout (KO) mice demonstrated that EndoG KO cells are partially protected against cLDL toxicity compared with WT cells. To determine cLDL toxicity in vivo, we administered cLDL or native LDL (nLDL) intravenously to the WT and EndoG KO mice and then measured floating endothelial cells in blood using flow cytometry. The results showed an increased number of floating endothelial cells after cLDL versus nLDL injection in WT mice but not in EndoG KO mice. Finally, the inhibitors of MEK-ERK1/2 and JNK-c-jun pathways decreased cLDL-induced EndoG overexpression and DNA fragmentation. In summary, our data suggest that cLDL-induced endothelial toxicity is caspase independent and results from EndoG-dependent DNA fragmentation.
Keywords: carbamylated low-density lipoprotein, endonuclease G, mitotic cell death, mitogen-activated protein kinase, atherosclerosis
cardiovascular disease (CVD) is the major complication in patients with end-stage kidney disease (ESKD) (15, 40). The risk of CVD among patients with ESKD is about 10–30 times of that among the general population, and CVD remains the major cause of mortality among patients with ESKD (1, 18, 38). Endothelial injury plays a crucial role in the development of CVD (22, 37). Recent studies identified carbamylated low-density lipoprotein (cLDL) as an important factor that is detected in healthy individuals and patients with chronic kidney disease (CKD), causes endothelial injury in vitro, and leads to atherosclerosis in vivo (3, 5, 10, 19, 23, 36). cLDL is produced by chemical modification of low-density lipoprotein (LDL) by cyanate derived from urea or thiocyanate (11, 28). Because of the chronic elevation of urea, patients with CKD have more protein carbamylation (28) and increased plasma cLDL (5). cLDL uses several scavenger receptors on endothelial cells and causes the induction of adhesion molecules and monocyte adhesion, suggesting its importance for atherosclerosis (4, 6, 8, 36). cLDL was also shown to induce endothelial cell proliferation followed by cell death that occurred predominantly in mitotic cells (2). The prevention of endothelial proliferation by DNA polymerase or mitogen-activated protein kinase (MAPK) inhibitors protected cells from cLDL-induced cell death. The cLDL-induced cell death was associated with DNA fragmentation measured by using the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). The nuclease that produced cLDL-induced DNA fragmentation and death of endothelial cells is unknown; however, one potential candidate is endonuclease G (EndoG). It was previously described as a nuclear DNA-coded mitochondrial manganese-dependent endonuclease that has a unique site selectivity, initially attacking poly(dG).poly(dC) sequences in double-stranded DNA (39). EndoG is known of being released from mitochondria during apoptosis and cleaving DNA without sequence specificity at higher degrees of DNA degradation (30). It can be localized in nuclei during apoptotic DNA fragmentation confirmed by the TUNEL assay (29). EndoG is an important participant of the caspase-independent mitotic cell death (27, 34), which was detected in several models of endothelial injury (32, 42).
Therefore, in this study, we hypothesized that EndoG is the mediator of cLDL-induced mitotic death of endothelial cells.
MATERIALS AND METHODS
LDLs.
Human native LDL (nLDL) was purchased from Intracel (Frederick, MD), and all other chemicals were purchased from Sigma (St. Louis, MO), unless stated otherwise. cLDL was prepared by chemical modification, as previously described (2). Carbamylation of LDL was verified by the colorimetric method using diacetyl monoxime (41). Electrophoretic mobility of nLDL and cLDL was determined in 0.5% agarose gel and 0.2% bovine serum albumin (wt/vol), as described by Noble (35). All LDLs were diluted with phosphate-buffered saline (PBS) containing 200 μM EDTA, kept at 4°C away from light, and used within 3 wk after preparation.
Cell cultures.
Human coronary artery endothelial cells (HCAECs) were purchased from Lonza (Walkersville, MD) and propagated in EGM-2 medium (Lonza) for up to three passages. Unless stated otherwise, HCAECs were treated with 200 μg/ml cLDL for 24 h, and control HCAECs were treated with either equal amount/volume of nLDL or vehicle (PBS, 200 μM EDTA), as previously described (36). The vehicle did not show any cellular toxicity and did not induce any DNA fragmentation. For all experiments, HCAECs were plated 4–6×103 cells/cm2.
Cell death was tested by lactate dehydrogenase (LDH) release assay, for which cells were plated in 96-well plates and treated with LDLs for 24 h. The experiments were performed using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI), as previously described (2).
For experiments with MAPK inhibitors, 2 h before the LDL exposure, HCAECs were pretreated with U-0126 (MEK inhibitor) or SP-600125 (JNK inhibitor) at final concentrations of 10 and 1 μM, respectively, as previously elaborated (2).
Primary mouse aortic endothelial cells were isolated, as previously reported (31). Briefly, mice were exsanguinated and perfused through the heart with ice-cold Hanks' balanced saline solution. The thoracic aorta was isolated from the body, separated from heart, and incubated with 0.1% collagenase IV in molecular, cellular, and developmental biology 131 (MCDB-131) complete medium from Vec Technologies (Rensselaer, NY) for 3 min. The aorta was then segmented and immobilized in 34-mm petri dishes coated with 0.2% gelatin. Endothelial cells were allowed to proliferate for 72 to 96 h in complete MCDB-131, after which aortic segments were removed and endothelial cells were isolated by fluorescein-activated cell sorting (FACS) after staining with 1,1-dioctadecyl-3,3,3′,3′-tetramethylinocarbocyanine perchlorate acelated LDL (Dil-Ac-LDL; Biomedical Technologies, Stoughton, MA) (31).
Animal experiments.
All experiments with animals were approved by the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System. EndoG knockout (KO) mice (C57BL6/J background) were obtained from Lieber and collaborators (24). Wild-type (WT) and EndoG KO mice were subjected to a single intravenous administration of cLDL or nLDL (40 mg/kg). Control mice were injected with an equal volume of vehicle (200 μM EDTA in saline). Twenty-four hours later, mice were euthanized and exsanguinated. From each animal, 1 ml of blood was collected in 1-ml lithium heparin minicollect vials from Greiner Bio-One (Monroe, NC) and used for flow cytometry.
Cytochemistry and image analyses.
TUNEL assay and immunocytochemical double staining of LDL-treated cells with anti-bromodeoxyuridine (BrdU) (Oncogene, Cambridge, MA) and anti-EndoG antibody (Millipore, Billerica, MA) were performed, as previously described (7). HCAECs were plated in eight-well slide chambers and treated with 200 μg/ml LDL, as described in Cell cultures. BrdU label (Oncogene) was added to the medium 4 h after the start of cLDL treatment. In 24 h, the cells were washed with ice-cold PBS, fixed with 4% formaldehyde (pH 7.0), and probed overnight with the mouse anti-BrdU and rabbit anti-EndoG at 1:100 and 1:300 dilution, respectively. The primary antibodies were detected with 1:400-diluted anti-mouse IgG-AlexaFluor 594 and anti-rabbit IgG-AlexaFluor 647 conjugates (Invitrogen, Carlsbad, CA), respectively. Subsequently, DNA fragmentation was measured with TUNEL assay using an in situ cell death detection kit (Roche). Cells were then washed, counterstained with 4′,6-diamidino-2-phenylindol, mounted under coverslips with Prolong Antifade kit (Invitrogen), and acquired using the Olympus IX-81 inverted microscope (Olympus America, Center Valley, PA), equipped with Hamamatsu ORCA-ER monochrome camera (Hamamatsu Photonics, Hamamatsu City, Japan). Image analysis was performed using the SlideBook 4.2 software (SciTech). Quantification was done as previously described (44).
Small interfering RNA and EndoG-cyan fluorescent protein overexpression.
EndoG silencing by small interfering RNA (siRNA) was performed, as described earlier (9). HCAECs were grown in 96-well plates to 70–80% confluence and transfected with designed siRNA duplexes (sense 5′-AUGCCUGGAACAACCUGGAdTdT-3′, and antisense 3′-UCCAGGUUGUUCCAGGCAUdTdT-5′) or Control Non-Targeting siRNA No. 1 from Dharmacon (Lafayette, CO) in mixtures containing 50 nM siRNA and TransIT-TKO transfection reagent (Mirus, Houston, TX) in serum-free medium for 48 h. The cells were then washed and exposed with 200 μg/ml cLDL for an additional 24 h. Control cells were treated with nLDL or vehicle (PBS, 200 μM EDTA). After the treatment, cell death was assessed using the LDH release assay.
For EndoG expression experiments, an EndoG-cyan fluorescent protein (CFP) vector was prepared. Human mature EndoG (amino acids 45–294) coding cDNA was cloned in mammalian expression vector pECFP.N1 (BD Biosciences Clontech, Franklin Lakes, NJ) with restriction sites Bgl II and Age I. The primers used for the construction were 5′-TCAGATCTCGAGATGGCCGATCTTCCCGC and 5′-GGTGGCGACCGGTGGCTTGCTGCCAGCAGTG. Seventy percent confluent HCAECs in eight-well chamber slides were transfected with the EndoG-CFP using Lipofectamin LTX Plus (Invitrogen) for 16 h. Control cells were treated with the vector that expresses CFP only. The cells were next exposed with either cLDL or nLDL (200 μg/ml) for 24 h and then fixed in 4% formaldehyde and used for TUNEL staining, cytochemistry, and microscopy.
Real-time reverse transcriptase PCR.
Total RNA was isolated using the RNeasy Mini kit from Qiagen (Valencia, CA). The reverse transcription reaction was performed using 0.5 μg total RNA, Oligo d(T)16, and the GeneAmp Gold RNA PCR core kit (Applied Biosystems, Carlsbad, CA). The real-time PCR was performed as previously elaborated (43). Briefly, cDNA samples were diluted 1:5 and 1:200 for EndoG and 18S, respectively. The reaction mix was prepared using Platinum SYBR Green qPCR Supermix-UDG (Invitrogen) according to the manufacturer's recommendations. The primers were 5′-CTACCTGAGCAACGTCGCG-3′ and 5′-TCCAGGTTGTTCCAGGCATT-3′. 18S ribosomal subunit RNA was amplified in parallel reaction using primers 5′-TTCGAACGTCTGCCCTATCAA-3′ and 5′-ATGGTAGGCACGGCGACTA-3′. Two-temperature cycles with annealing/extension temperature at 62°C for EndoG and 64°C for 18S were applied to samples using SmartCycler (Cepheid, Sunnyvale, CA). The melting curve analysis was performed between 60 and 95°C to assess the quality of final PCR products. The calculation of the relative RNA concentration was performed using Cepheid SmartCycle software (version 2.0d). Data are presented as a ratio of EndoG to 18S mRNA.
Western blot analysis.
Western blot analysis was performed as previously described (44). Briefly, proteins were separated in 12% gel, transferred onto the nitrocellulose membrane in Novex-transferring buffer (Invitrogen) at 40 V for 3 h. Blocked membrane was incubated with polyclonal anti-EndoG (Millipore) diluted 1:800 and washed in Tris-buffered saline, and primary antibodies were detected with anti-rabbit IgG-horseradish peroxidase (HRP) using a SuperSignal chemiluminescent kit (Thermo Fisher Scientific, Rockford, IL). The load control was performed by stripping the membranes using Restore Plus Western Blot Stripping Buffer (Thermo Fisher Scientific) and probing with anti-β-actin (1:1,000) antibody (Santa Cruz Biotechnology, Santa Cruz, CA).
Cell ELISA.
Cell ELISA was performed as previously described (6). Briefly, HCAECs were seeded in a 96-well plate, treated with MAPK inhibitors and cLDL/nLDL, washed, and fixed (4% wt/vol formaldehyde, 0.012% saponin, PBS) for 10 min at room temperature. After blocking, cells were probed with anti-EndoG antibody (1:800) (Millipore). The primary antibodies were detected with anti-rabbit antibody (1:1,000) conjugated with HRP (Invitrogen). The HRP activity was measured using 3,3′,5,5′-tetramethylbenzidine substrate solution (Sigma) at Δ450–540 nm. Total protein was normalized by β-actin expression measured with the rabbit polyclonal anti-β-actin antibody conjugated with FITC (Santa Cruz Biotechnology). All measurements were done in quadruplicates per one marker per one time/concentration point and were repeated at least three times in different plates. For negative control, the primary antibody was substituted with blocking buffer.
Whole blood FACS analysis.
Freshly collected blood was treated with red blood cell lysis buffer (containing 0.83% NH4Cl, 0.1% KHCO3, and 0.01% EDTA) for 15 min at room temperature, and cells were precipitated at 220 g for 5 min. After two subsequent washings with PBS, the blood cells were resuspended in an original volume of 2% BSA in PBS containing 1:500-diluted anti-CD31-FITC (Millipore) for 1 h. To control cell labeling, a separate batch of blood cells from WT mice was treated with the same solution without antibody. After incubation, cells were precipitated at 220 g for 5 min, and the hybridization solution was removed. After two washings with PBS, the cells were fixed with 4% formaldehyde and analyzed using the Becton Dickinson BD FACSCalibur flow cytometer (San Jose, CA). The cutoff limit for nonnuclear cells and nonspecific autofluorescence (based on the negative control) was applied to cell sorting setting, which resulted in an exclusion of more than 99.9% of cells. The percentage of CD-31-positive cells was calculated using the Becton Dickinson CELLQUEST software package.
Statistics.
Statistical analysis was performed using ANOVA and Student's t-test. Results were expressed as means ± SE. P < 0.05 was considered significant.
RESULTS
cLDL induces EndoG overexpression and mitotic death in endothelial cells.
Our previous reports suggested that cLDL induces endothelial injury that preferentially occurs in the proliferating cells (2, 43). In our first experimental setting, we tested whether the cLDL-induced DNA fragmentation (detectable by TUNEL) occurred in proliferating cells (detectable by BrdU incorporation). Our secondary goal was to determine whether the cLDL-induced DNA fragmentation was dependent on EndoG or caspase-3. Our results showed that the vast majority of the TUNEL-positive cells had BrdU label, suggesting that mitotic cell death occurred in proliferating cells in response to cLDL treatment (Fig. 1, A and B). In most of the cases, the TUNEL-positive/BrdU-positive cells had a strong association with EndoG overexpression rather than with cleaved caspase-3. Overall, EndoG expression was significantly higher in all cLDL-treated endothelial cells compared with nLDL- or vehicle-treated cells. To rule out the possible problem with cleaved caspase-3 immunostaining, positive control (HCAECs treated with 200 μM H2O2) was successfully used during the immunocytochemical staining (Fig. 1C). cLDL-induced elevation of EndoG mRNA and protein expression were confirmed by real-time RT-PCR and Western blot analysis, respectively (Fig. 1, D and E). Therefore, our data suggest that EndoG and caspase-independent pathway of cell death are likely the mechanisms of cLDL-induced endothelial injury.
Fig. 1.
Endonuclease G (EndoG) is upregulated in the carbamylated LDL (cLDL)-induced mitotic death of endothelial cells. Colocalization of bromodeoxyuridine (BrdU; red color), terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL; green color), and EndoG (A) or cleaved caspase-3 (B) (purple color) in human coronary artery endothelial cells (HCAECs) were treated for 24 h with vehicle, native LDL (nLDL), or cLDL (200 μg/ml each) in serum-free medium. Counterstaining with 4′,6-diamidino-2-phenylindol (DAPI; blue color) is shown. Scales = 20 μm. Immunocytochemical reaction with cleaved caspase-3 was positively controlled using the same endothelial cells treated with 200 μM H2O2 (C). EndoG mRNA expression (D) and protein expression (E) in HCAECs were treated with nLDL or cLDL (200 μg/ml each) by real-time RT-PCR and Western blot analysis, respectively; n = 4 per point. *P < 0.05 compared with nLDL.
EndoG mediates cLDL-induced endothelial cell death in vitro.
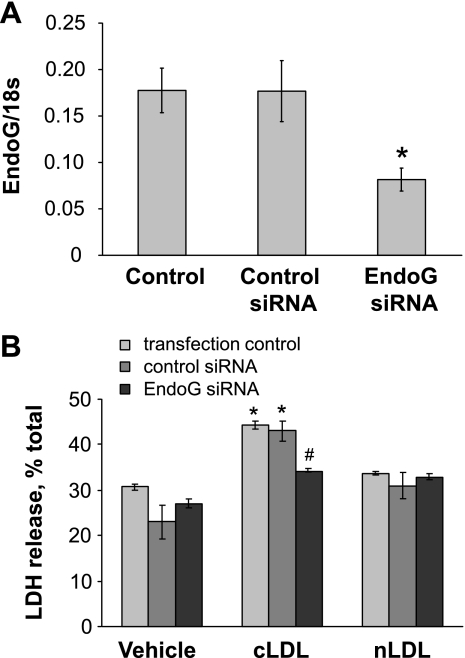
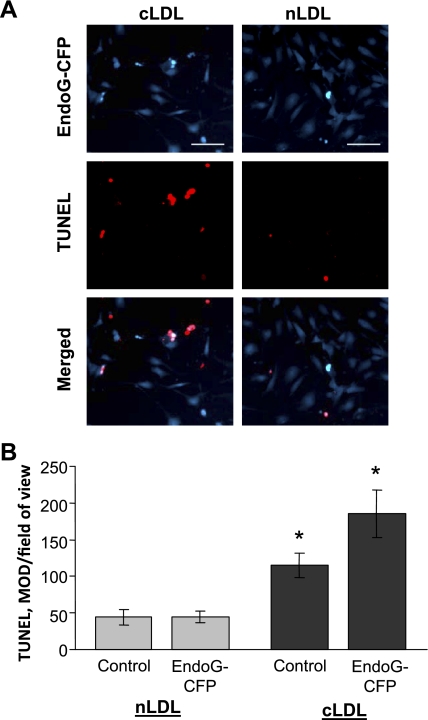
To study whether the observed EndoG induction plays a causative role in cLDL induced in DNA fragmentation and endothelial toxicity, we inhibited EndoG expression using specific siRNA. The efficiency of anti-EndoG siRNA was initially tested in HCAECs, and the results suggested that EndoG is silenced by ∼50% (Fig. 2A). In subsequent experiments, EndoG silencing was shown to be significantly protective against cLDL-induced cell death (Fig. 2B). We then tested whether EndoG overexpression would make HCAECs more susceptible to cLDL toxicity. HCAECs were transfected with pECFP-N1 that expresses EndoG-CFP-fused protein. As determined by TUNEL assay, endothelial cells that overexpressed EndoG were more sensitive to cLDL (Fig. 3A). Quantification of the TUNEL data suggested that EndoG overexpression significantly exacerbated the cLDL-induced DNA fragmentation and cell death (Fig. 3B). Hence, both in vitro models suggested a strong positive link between EndoG expression and cLDL toxicity toward the endothelial cells.
Fig. 2.
EndoG inactivation protects endothelial cells from the cell death induced by cLDL. Effect of EndoG silencing by anti-EndoG small interfering RNA (siRNA) (A) and endothelial protection from cLDL toxicity as measured by lactate dehydrogenase (LDH) release assay (B) is shown; n = 3 per point. *P < 0.05 compared with nLDL; #P < 0.05 compared with control siRNA- and vehicle-treated cells.
Fig. 3.
EndoG overexpression (cyan color) induces DNA fragmentation assessed by TUNEL (red color) in the cLDL- or nLDL-treated (200 μg/ml, 24 h) HCAECs. Representative images (A) and quantification (B) are shown. CFP, cyan fluorescent protein; MOD, mean optical density. Scales = 20 μm; n = 4 per point. *P < 0.05 compared with nLDL.
EndoG KO protects endothelial cells from cLDL-induced cell death ex vivo and in vivo.
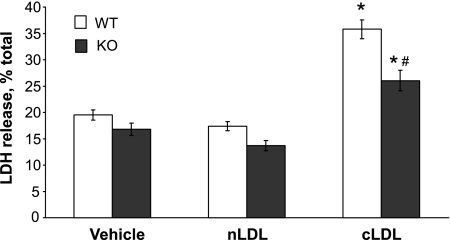
We next examined whether or not primary EndoG KO endothelial cells are protected against cLDL toxicity. For this, primary mouse aortic endothelial cells were prepared from WT and EndoG KO mice, and their injury after cLDL treatment ex vivo was assessed by LDH release. Our data suggested that there was significant induction of endothelial cell death after cLDL exposure compared with nLDL in both WT and EndoG KO cells (Fig. 4). However, when compared with WT cells, EndoG KO cells were partially protected from the injury induced by cLDL. Afterward, our experiments were extended to live animals. For this, WT and EndoG KO mice were subjected to single tail vein administration of cLDL or nLDL. Twenty-four hours later, floating CD31+ (endothelial) nuclear cells were assessed by FACS analysis of the whole blood. Our data suggested a significant increase of floating endothelial cells in circulation of WT mice, whereas EndoG KO mice were insensitive to cLDL (Fig. 5). Thus our in vivo results confirmed in vitro and ex vivo data, suggesting that EndoG plays a critical role in mediating the cLDL-induced endothelial cell death.
Fig. 4.
Endothelial cells with genetic lack of EndoG are less sensitive to cLDL toxicity. Primary mouse aortic endothelial cells isolated from wild-type (WT) and EndoG knockout (KO) mice were treated with cLDL (200 μg/ml, 24 h) and cell death was measured by LDH release assay; n = 6 per point. *P < 0.05 compared with nLDL; #P < 0.05 compared with cLDL-treated WT cells.
Fig. 5.
Detection of floating CD31-positive endothelial cells by flow cytometry in whole blood from WT and EndoG KO mice after a single intravenous injection of cLDL. Representative images (A) and quantification (B) are shown; n = 8 per point. FSC-H, forward scatter height. *P < 0.05 compared with nLDL; #P < 0.05 compared with cLDL-treated WT animals.
The inhibition of ERK1/2 or JNK protects endothelial cells from EndoG overexpression and death.
Our previous report showed that MEK-ERK1/2 and JNK-c-jun pathways are involved in the cLDL-induced death of proliferating endothelial cells (2). Because both MAPK and EndoG seem to be responsible for cLDL cytotoxicity, in the current study, their relation was assessed in cLDL-treated HCAECs. Our data showed that cLDL-induced EndoG overexpression was either partially or completely prevented by U-0126 and SP-600125, the inhibitors of MEK and JNK, respectively (Fig. 6A). However, cLDL-induced DNA fragmentation in endothelial cells was more efficiently decreased by MEK inhibitor compared with JNK inhibitor (Fig. 6B). Taken together with our previous publication, these results suggest that in response to cLDL impact to endothelial cells, EndoG overexpression is primarily dependent on the JNK-c-jun mechanism; however, DNA fragmentation and endothelial cell death rely on both MEK-ERK1/2 and JNK-c-jun pathways, which may be tentatively explained by other mechanisms that regulate, for example, nuclear translocation of EndoG or directly cause DNA damage.
Fig. 6.
Inhibition of MAPK pathway prevents EndoG upregulation and cLDL-induced DNA fragmentation. EndoG protein expression was measured by direct cell ELISA (A) and DNA fragmentation was measured by quantitative TUNEL assay (B) in HCAECs treated with cLDL after inhibition of MEK and JNK kinases; n = 3 to 4 per point. OD, optical density. *P < 0.05 compared with nLDL; #P < 0.05 compared with DMSO and cLDL-treated cells.
DISCUSSION
Endothelial injury plays a critical role in the disturbance of vascular homeostasis and significantly contributes to the development of CVD (22, 37). Patients with ESKD are known to be prone to endothelial dysfunction and injury, which may be a key process in their greater predisposition to cardiovascular complications compared with the general population (1, 18, 38). Despite the fact that cLDL is elevated in the plasma of patients with CKD and that experimental uremia-induced atherosclerosis is associated with cLDL, the mechanisms of cLDL-induced endothelial injury and atherosclerosis are not well understood. In the physiological concentrations, which were registered in patients with ESKD (5), cLDL induces both proliferation and injury of endothelial cells (2, 21). The coincidence of the two seemingly controversial processes suggests that there may be a link between them. Indeed, our first study in this direction suggested this (2), and the current study confirmed that cLDL-induced proliferation and cell death are related. The prevention of the cell-cycle drift to S-G2 or mitosis significantly reduced the cell death caused by cLDL. Similar events were also observed in oxidized LDL-treated endothelial cells (2), implicating that this is a potentially universal phenomenon in the pathogenesis of endothelial injury. Cell death associated with proliferation is called “mitotic cell death,” also known as mitotic catastrophe or reproductive or proliferating cell death. The term describes cell death that occurs in the cell-cycle stages that follow the one during which the cell is impacted with an injuring agent (16). Mitotic cell death usually has the morphological features of apoptosis and necrosis and results from DNA damage in vital genes or shows contradictory biological stimuli and inability of the cell to pass a certain checkpoint (16). Mitotic cell death is a common cellular phenomenon that occurs in endothelial cells in response to the number of impacts or treatments (20, 25, 26).
Several previous reports suggested that mitotic cell death may employ caspase-independent mechanisms (27, 33, 34). The current study revealed that cLDL-induced endothelial mitotic cell death is rather caspase independent and associated with the induction of EndoG, one of the major effectors of caspase-independent cell death (33, 45). Both in vitro and ex vivo experiments with EndoG modulation proved that EndoG is causatively involved in the cLDL-induced endothelial cell injury. Furthermore, our in vivo experiments performed in transgenic mice strongly supported the hypothesis that EndoG mediates the cLDL-induced endothelial cell injury. To the best of our knowledge, this is the first report that suggests the causative role of EndoG in mitotic endothelial cell death induced by cLDL or any other modified LDL. Interestingly, Diener and coauthors (17) recently suggested that under normal physiological conditions, EndoG protects endothelial cells from apoptosis and, at the same time, potentiates necrosis. This contradiction with our results can be perhaps explained by the difference in the cell death mechanisms between normal conditions and conditions resulting from cLDL toxicity. Similar contradictory results on the effect of EndoG withdrawal were previously shown in other models (7, 24, 46).
MAPKs seems to be largely responsible for a great number of endothelial cell functions, including cell proliferation, growth arrest, differentiation, and cell death (42). Our previous report suggested that both MEK-ERK1/2 and JNK-c-jun pathways, but not the MAPK p38 pathway, are responsible for mediating mitotic cell death induced by cLDL (2). In the current study, MEK and JNK inhibitors significantly reduced EndoG expression and DNA fragmentation in endothelial cells during cLDL-induced injury. Interestingly, whereas the inhibition of JNK caused a stronger downregulation of EndoG than the inhibition of MEK, the MEK inhibitor was more efficient in protection against cLDL-induced DNA fragmentation compared with the JNK inhibitor. The detected link between JNK and EndoG is in agreement with studies that showed a similar link between EndoG and JNK, but not with ERK1/2 activation in apoptosis (12–14). The higher efficiency of the MEK inhibitor in the prevention of cLDL-induced DNA fragmentation compared with the JNK inhibitor is also in agreement with our previous report that demonstrated a higher efficiency of MEK inhibition above the JNK inhibitor in rescue of the endothelial cells from cLDL toxicity (2). The observed cell protection by MEK-ERK1/2 pathway inhibition is likely resulted from other than EndoG mechanism(s). It is possible that cLDL-induced ERK1/2-mediated TUNEL and cytotoxicity use some known or unknown endonuclease that causes DNA fragmentation. Because ERK1/2 (unlike JNK) participates in scheduled DNA synthesis and cell proliferation (2), it makes DNA even more susceptible for degradation and cells more prone to cell death. Therefore, we may speculate that cLDL-induced DNA fragmentation and endothelial cell toxicity use at least two potential pathways: 1) JNK-c-jun-dependent pathway with EndoG as a major executive endonuclease, and 2) MEK-ERK1/2-dependent pathway with one or more apoptotic endonucleases that cause DNA degradation without great involvement of EndoG (Fig. 7).
Fig. 7.
Schematic of the proposed pathway of cLDL-induced endothelial cell death.
It is important to acknowledge that all described in vitro and in vivo phenomena were observed with the chemically modified cLDL at concentration of 200 μg/ml used in several studies (2, 4, 6). Although in vivo-produced cLDL may differ from an artificially modified one in degree of carbamylation, our previous report suggested that uremic patients have serum cLDL that is equal to the average concentration of 281.5 ± 46.9 μg/ml of chemically modified cLDL (5). Therefore, the used concentration of cLDL is close to the physiologically relevant level observed in chronic uremia. It may be speculated that with a different degree of carbamylation and a longer duration of treatment, cLDL may have alternative pathways of vascular injury.
In conclusion, this study revealed the mechanistic link between MAPK and EndoG in caspase-independent mitotic endothelial cell death induced by cLDL. In the future, this mechanism may be used as a new therapeutic target for the prevention and treatment of CVD associated with chronic uremia.
GRANTS
This research was supported by American Heart Association South Central Affiliate Grant 0865046F (to E. O. Apostolov); National Heart, Lung, and Blood Institute Grant R21-HL-087405 (to A. G. Basnakian); and Veterans Administration Merit Review grants (to A. G. Basnakian and S. V. Shah).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. US Renal Data System USRDS 2002 Annual Data Report. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 2002 [Google Scholar]
- 2. Apostolov EO, Basnakian AG, Yin X, Ok E, Shah SV. Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am J Physiol Heart Circ Physiol 292: H1836–H1846, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic uremia stimulates LDL carbamylation and atherosclerosis. J Am Soc Nephrol 21: 1852–1857, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apostolov EO, Shah SV, Ok E, Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 27: 826–832, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin Chem 51: 719–728, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Apostolov EO, Shah SV, Ray D, Basnakian AG. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler Thromb Vasc Biol 29: 1622–1630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Apostolov EO, Wang X, Shah SV, Basnakian AG. Role of EndoG in development and cell injury. Cell Death Differ 14: 1971–1974, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Asci G, Basci A, Shah SV, Basnakian A, Toz H, Ozkahya M, Duman S, Ok E. Carbamylated low-density lipoprotein induces proliferation and increases adhesion molecule expression of human coronary artery smooth muscle cells. Nephrology (Carlton) 13: 480–486, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Basnakian AG, Apostolov EO, Yin X, Abiri SO, Stewart AG, Singh AB, Shah SV. Endonuclease G promotes cell death of non-invasive human breast cancer cells. Exp Cell Res 312: 4139–4149, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basnakian AG, Shah SV, Ok E, Altunel E, Apostolov EO. Carbamylated LDL. Adv Clin Chem 51: 25–52, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Basnakian AG, Shah SV, Ok E, Altunel E, Apostolov EO. Carbamylated LDL. Chennai, India: Elsevier, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Boronkai A, Bellyei S, Szigeti A, Pozsgai E, Bognar Z, Sumegi B, Gallyas F., Jr Potentiation of paclitaxel-induced apoptosis by galectin-13 overexpression via activation of Ask-1-p38-MAP kinase and JNK/SAPK pathways and suppression of Akt and ERK1/2 activation in U-937 human macrophage cells. Eur J Cell Biol 88: 753–763, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Chen C, Liu Y, Zheng D. An agonistic monoclonal antibody against DR5 induces ROS production, sustained JNK activation and Endo G release in Jurkat leukemia cells. Cell Res 19: 984–995, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Cheung EC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE 251: PE45, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Collins AJ. Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Darzynkiewicz Z, Juan G, Li X, Gorczyca W, Murakami T, Traganos F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 27: 1–20, 1997 [PubMed] [Google Scholar]
- 17. Diener T, Neuhaus M, Koziel R, Micutkova L, Jansen-Durr P. Role of endonuclease G in senescence-associated cell death of human endothelial cells. Exp Gerontol 45: 638–644, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Gonen B, Goldberg AP, Harter HR, Schonfeld G. Abnormal cell-interactive properties of low-density lipoproteins isolated from patients with chronic renal failure. Metabolism 34: 10–14, 1985 [DOI] [PubMed] [Google Scholar]
- 20. Hari D, Beckett MA, Sukhatme VP, Dhanabal M, Nodzenski E, Lu H, Mauceri HJ, Kufe DW, Weichselbaum RR. Angiostatin induces mitotic cell death of proliferating endothelial cells. Mol Cell Biol Res Commun 3: 277–282, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol 11: 1819–1825, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Horkko S, Huttunen K, Kervinen K, Kesaniemi YA. Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur J Clin Invest 24: 105–113, 1994 [DOI] [PubMed] [Google Scholar]
- 24. Irvine RA, Adachi N, Shibata DK, Cassell GD, Yu K, Karanjawala ZE, Hsieh CL, Lieber MR. Generation and characterization of endonuclease G null mice. Mol Cell Biol 25: 294–302, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li LY, Smulson ME. Induction of apoptosis in proliferating human endothelial cells by the tumor-specific antiangiogenesis agent combretastatin A-4. Cancer Res 58: 4510–4514, 1998 [PubMed] [Google Scholar]
- 26. Kanthou C, Greco O, Stratford A, Cook I, Knight R, Benzakour O, Tozer G. The tubulin-binding agent combretastatin A-4-phosphate arrests endothelial cells in mitosis and induces mitotic cell death. Am J Pathol 165: 1401–1411, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitagawa K. Caspase-Independent Mitotic Death. In: Essentials of Apoptosis: A Guide for Basic and Clinical Research. New York: Humana, 2009, p.635–646 [Google Scholar]
- 28. Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl 78: S102–S107, 2001 [DOI] [PubMed] [Google Scholar]
- 29. Lee BI, Lee DJ, Cho KJ, Kim GW. Early nuclear translocation of endonuclease G and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Neurosci Lett 386: 23–27, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Lincoln DW, 2nd, Larsen AM, Phillips PG, Bove K. Isolation of murine aortic endothelial cells in culture and the effects of sex steroids on their growth. In Vitro Cell Dev Biol Anim 39: 140–145, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Liu PL, Chen YL, Chen YH, Lin SJ, Kou YR. Wood smoke extract induces oxidative stress-mediated caspase-independent apoptosis in human lung endothelial cells: role of AIF and EndoG. Am J Physiol Lung Cell Mol Physiol 289: L739–L749, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Lorenzo HK, Susin SA. Mitochondrial effectors in caspase-independent cell death. FEBS Lett 557: 14–20, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Niikura Y, Dixit A, Scott R, Perkins G, Kitagawa K. BUB1 mediation of caspase-independent mitotic death determines cell fate. J Cell Biol 178: 283–296, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nobel RP. Electrophoresis: separation of plasma lipoproteins in agarose gel. J Lipid Res 9: 693–700, 1968 [PubMed] [Google Scholar]
- 36. Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int 68: 173–178, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Quyyumi AA. Endothelial function in health and disease: new insights into the genesis of cardiovascular disease. Am J Med 105: 32S–39S, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Reis SE, Olson MB, Fried L, Reeser V, Mankad S, Pepine CJ, Kerensky R, Merz CN, Sharaf BL, Sopko G, Rogers WJ, Holubkov R. Mild renal insufficiency is associated with angiographic coronary artery disease in women. Circulation 105: 2826–2829, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Ruiz-Carrillo A, Renaud J. Endonuclease G: a (dG)n X (dC)n-specific DNase from higher eukaryotes. EMBO J 6: 401–407, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 116: 85–97, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Trepanier DJ, Thibert RJ, Draisey TF, Caines PS. Carbamylation of erythrocyte membrane proteins: an in vitro and in vivo study. Clin Biochem 29: 347–355, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkotter C, Schulze-Osthoff K, Roth J. MRP8/MRP14 impairs endothelial integrity and induces a caspase-dependent and -independent cell death program. Blood 109: 2453–2460, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Wang X, Tryndyak V, Apostolov EO, Yin X, Shah SV, Pogribny IP, Basnakian AG. Sensitivity of human prostate cancer cells to chemotherapeutic drugs depends on EndoG expression regulated by promoter methylation. Cancer Lett 270: 132–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin X, Apostolov EO, Shah SV, Wang X, Bogdanov KV, Buzder T, Stewart AG, Basnakian AG. Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J Am Soc Nephrol 18: 2544–2553, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Zanna C, Ghelli A, Porcelli AM, Martinuzzi A, Carelli V, Rugolo M. Caspase-independent death of Leber's hereditary optic neuropathy cybrids is driven by energetic failure and mediated by AIF and Endonuclease G. Apoptosis 10: 997–1007, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Zhang J, Dong M, Li L, Fan Y, Pathre P, Dong J, Lou D, Wells JM, Olivares-Villagomez D, Van Kaer L, Wang X, Xu M. Endonuclease G is required for early embryogenesis and normal apoptosis in mice. Proc Natl Acad Sci USA 100: 15782–15787, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]