Abstract
AMPK activation during ischemia helps the myocardium to cope with the deficit of energy production. As AMPK activity is considered to be impaired in diabetes, we hypothesized that enhancing AMPK activation during ischemia above physiological levels would protect the ischemic diabetic heart through AMPK activation and subsequent inhibition of mitochondrial permeability transition pore (mPTP) opening. Isolated perfused hearts from normoglycemic Wistar or diabetic Goto-Kakizaki (GK) rats (n ≥ 6/group) were subjected to 35 min of ischemia in the presence of 10, 20, and 40 μM of A-769662, a known activator of AMPK, followed by 120 min of reperfusion with normal buffer. Myocardial infarction and AMPK phosphorylation were assessed. The effect of A-769662 on mPTP opening in adult cardiomyocytes isolated from both strains was also determined. A-769662 at 20 μM reduced infarct size in both Wistar (30.5 ± 2.7 vs. 51.8 ± 3.9% vehicle; P < 0.001) and GK hearts (22.7 ± 3.0 vs. 48.5 ± 4.7% vehicle; P < 0.001). This protection was accompanied by a significant increase in AMPK and GSK-3β phosphorylation. In addition, A-769662 significantly inhibited mPTP opening in both Wistar and GK cardiomyocytes subjected to oxidative stress. We demonstrate that AMPK activation during ischemia via A-769662 reduces myocardial infarct size in both the nondiabetic and diabetic rat heart. Furthermore, this cardioprotective effect appears to be mediated through inhibition of mPTP opening. Our findings suggest that improving AMPK activation during ischemia can be another mechanism for protecting the ischemic heart.
Keywords: diabetes, signal transduction
diabetes, when associated with cardiovascular disease, is responsible for the worsening of clinical outcomes in patients following an acute myocardial infarction (22, 35, 36). The pursuit for novel cardioprotective approaches that are effective in diabetic patients has significantly increased in recent years. In particular, the search for therapeutic strategies that could target diabetes as well as cardiovascular disease has been brought to the limelight. 5′-AMP-activated kinase (AMPK), known to play a key role in regulating both glucose and fatty acid homeostasis and thus controlling whole body energy metabolism, has become one of the strategic cellular targets for the treatment of cardiovascular disease associated with diabetes (27, 30). AMPK inactivation has been linked to diabetes, as demonstrated by Viollet et al. (52), whereby genetic ablation of AMPK α2-catalytic subunit in murine models led to a phenotype of glucose intolerance, insulin resistance, and elevated fatty acid levels. Moreover, glucose uptake and metabolism during myocardial ischemia appear to be affected by impairment of AMPK α2-catalytic subunits in the heart (8, 42, 55, 56). Interestingly, hyperglycemia results in a reduced glucose metabolism and enhanced fatty acid metabolism, in particular in cardiac muscle, an event similar to the metabolic profiles of transgenic models of AMPK. Therefore, it is possible that modulation of AMPK activity in the diabetic heart may improve cardiac function and overcome the increased susceptibility of the diabetic heart to ischemia-reperfusion injury. More recently, Kusmic et al. (34) have reported the improvement of microvascular function in the diabetic murine heart through the upregulation of AMPK signaling in the vasculature, hence suggesting that AMPK activation may be a novel cardiovascular protective kinase.
AMPK, a serine/threonine heterotrimeric kinase, is known to be activated during stress situations whereby energy levels are swiftly depleted, such as exercise, starvation, hypoxia, and ischemia. Upon depletion of energy, AMPK stimulates ATP-producing pathways (e.g., fatty acid oxidation and glycolysis) while inhibiting ATP-consuming pathways (e.g., fatty acid synthesis, cholesterol synthesis, and gluconeogenesis), hence balancing metabolic processes towards cellular energy homeostasis (7, 27).
In the context of ischemia-reperfusion injury, AMPK has been demonstrated to be rapidly activated during ischemia, as part of an innate survival cardiac mechanism (33). Once activated, AMPK stimulates glucose uptake and glycolysis during ischemia in an attempt to restore sufficient ATP to maintain cardiac function. However, it remains undisclosed if AMPK activation is beneficial or harmful for the myocardium. Although initial studies have demonstrated that AMPK activation would lead to cell death in reperfusion due to a reduced cardiac efficiency caused by stimulation of fatty acid oxidation, others suggest that AMPK is essential for myocardial survival during reperfusion injury (for a review, see Refs. 14, 15, 40). In addition, studies by Zarrinpashneh et al. (56) and Carvajal et al. (8), using a murine heart model with genetic ablation of α2-AMPK catalytic subunit, have suggested that cardiac recovery after ischemia-reperfusion injury was not impaired by an absent activation of AMPK heterotrimeric complexes containing α2-subunit.
Several drugs that are cardioprotective against reperfusion injury have been identified as AMPK activity modulators, such as 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; Refs. 39, 40) and metformin (4), as well as adipocytokines such as adiponectin (45) and leptin (46). Furthermore, most pharmacological agents appear to exert their cardioprotective effects through inhibition of the mitochondrial permeability transition pore (mPTP). The mPTP is a nonspecific channel that is thought to span across the outer and inner mitochondrial membranes the formation of which may be induced by calcium or oxidative stress. Opening of the mPTP occurs within the first minutes of reperfusion leading to uncoupled oxidative phosphorylation and mitochondrial swelling with subsequent cardiomyocyte cell death (28, 48).
However, some of the beneficial effects observed after administration of the aforementioned pharmacological agents cannot be exclusively attributed to AMPK activation. For instance, AICAR is known to activate adenosine receptors (5) that are involved in protection against ischemia-reperfusion injury and metformin is known to activate components of the reperfusion injury salvage kinase (RISK) pathway (4), of which AMPK may or may not be a part of.
The necessity for specific AMPK activators has been addressed by the discovery of small molecular activators of AMPK: PT1 by Pang et al. (41) and A-769662 by Cool et al. (11). These small molecules have been shown to be highly specific for AMPK and are able to activate this kinase independently of changes in the AMP-to-ATP ratio. Further studies have demonstrated a potential role for A-769662 as an antidiabetic and antiobesity agent. Obese mice treated chronically with A-769662 had a significant reduction of plasma glucose and body weight as well as an improved lipid profile (11).
On this background, we hypothesized that AMPK activation during ischemia, above physiological levels, will reduce myocardial infarct size in both diabetic and nondiabetic rat hearts. This protective effect may be due to the inhibition of mPTP opening at reperfusion.
EXPERIMENTAL PROCEDURES
Animals.
Adult male Wistar rats (330–400 g) were obtained from Charles River UK (Margate, UK), and male Goto-Kakizaki (GK) diabetic rats (330–400 g) were obtained from an inbred colony from the Biological Services at University College of London. All animals received humane care in accordance with the United Kingdom Animal (Scientific Procedures) Act of 1986 (project license no. 70/7140). The diabetic status of the GK rats was verified by measuring fasting levels of blood glucose and Hb1AC levels as described previously (51).
Materials.
A-769662 (Tocris, Bristol, UK) was dissolved in DMSO and then in Krebs-Henseleit buffer to give a final concentration of 10, 20, or 40 μM. Antibodies for phospho-Akt (Ser 473), phospho-α-AMPK (Thr172), phospho-ERK1/2 (Thr202/Thr204), phospho-GSK-3β (Ser9), phospho-acetyl-CoA carboxylase (Ser79; ACC), total Akt, total α-AMPK, total ERK1/2, total GSK-3β, total ACC, LC3-II, and LC3-I were obtained from Cell Signaling (Hitchin, UK), and α-tubulin was from Abcam (Cambridge, UK) and used in accordance with the manufacturer's instructions. All other reagents were of standard analytical grade.
Isolated perfused heart studies.
Male rats (Wistar and GK strains) were anaesthetized with sodium pentobarbital (55 mg/kg) and sodium heparin (300 IU) intraperitoneally. The hearts were rapidly excised into ice-cold buffer, mounted onto a Langendorff perfusion system (ADInstruments, Chalgrove, UK) at constant pressure (70–80 mmHg), and retrogradely perfused with modified Krebs-Henseleit bicarbonate buffer (in mM: 118.5 NaCl, 25.0 NaHCO3, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 1.7 CaCl2, and 11.0 glucose). The pH of the perfusion buffer was maintained at 7.35–7.50 at 37°C by gassing with 95% O2-5% CO2 as previously described (54).
A suture was placed around the left main coronary artery, and the ends were inserted into a pipette tip to form a snare. An intraventricular latex balloon was introduced into the left ventricle, via an incision in the left atrial appendage and inflated to a pressure of 5–10 mmHg, thus allowing permanent monitoring of heart function parameters such as heart rate, coronary flow, left ventricular developed pressure, and rate pressure product. Temperature was constantly measured via a thermo-probe inserted into the pulmonary artery and maintained between 36.5–37.5°C. Hearts were subjected to 35 min of regional ischemia by tightening of the suture, inducing a temporary obstruction of the artery, after which the ligature was released and the myocardial tissue reperfused for 120 min. At the end of the reperfusion period, the suture was retightened and 0.25% Evans blue in saline was infused through the aortic root to delineate the area not at risk and stained blue. Hearts were then frozen at −20°C before being sliced into 2-mm thick transverse sections and incubated with 1% triphenyltetrazolium chloride solution (in phosphate buffer). Triphenyltetrazolium chloride reacts with intracellular dehydrogenases present in viable cells producing a red pigment, hence staining viable myocardium red and infarcted/dead tissue off-white. The slices were transferred onto 10% formalin overnight to further enhance the contrast between different areas and subsequently scanned (Perfect v100Photo; Epson; resolution 600 dpi). Image J was used to measure the percentage of infarcted tissue within the area at risk (I/AAR%). The hearts were randomly assigned to one of the following treatments administered 5 min before regional ischemia and throughout 30 min of ischemia (n ≥ 6 per group; Fig. 1): 1) control: hearts either received 0.01% DMSO or modified Krebs-Henseleit buffer; and 2-4) A-769662: hearts were given 10, 20, or 40 μM of A769662, a known AMPK activator.
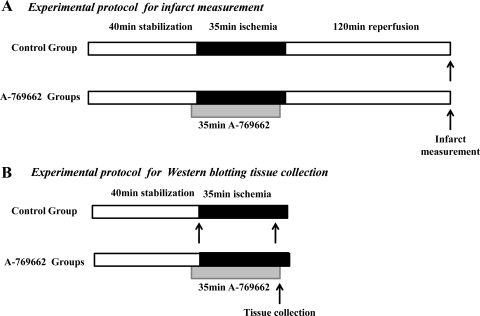
Fig. 1.
Schematic representation of the experimental Langendorff perfusion protocols. A: hearts were randomly assigned to the following groups: control or A-769662. A-769662 was administered in different concentrations (10, 20, or 40 μM) on the last 5 min of stabilization and for 30 min onto to ischemia. At the end of 120 min of reperfusion, hearts were collected for infarct size assessment via triphenyltetrazolium chloride staining. B: hearts were collected for protein analysis via Western blotting at either 40 min of stabilization or at 30 min of ischemia. A-769662-treated hearts were administered 10 or 20 μM of drug before collection (please refer to text for more detail).
For Western blot analysis, Wistar and GK rats (n ≥ 3/group) were subjected to 30 min of regional ischemia accompanied by treatment with 10 or 20 μM of A769662, following which a sample of myocardial tissue from the ischemic myocardium was removed and snap-frozen in liquid nitrogen (Fig. 1).
Western blot analysis.
The tissue samples were homogenized in a lysis buffer containing the following (in mM): 0.1 NaCl, 10 Tris pH 7.6, 1 EDTA, 2 Na pyrophosphate, 2 NaF, 2 β-glycerophosphate, 0.5 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, and Roche cocktail protease inhibitor mixture, and then centrifuged at 14,000 rpm for 5 min at 4 C. Protein content was determined with BCA protein assay reagent kit (Sigma, Dorset, UK). The supernatant was further diluted in sample buffer (100 Tris pH 6.8, 200 mM DTT, 2% SDS, 0.2%, bromophenol blue, and 20% glycerol) and subsequently boiled for 10 min at 100° C.
A total of 45 μg of protein for each sample was loaded into 12.5% acrylamide gels and subsequently transferred onto a nitrocellulose membrane (GE Healthcare, Buckinghamshire, UK). Adequate protein transfer was confirmed by staining with Ponceau red (Sigma) (37).
The phosphorylation states of α-AMPK (phospho-α AMPK Thr172), ACC, Erk1/2 (phospho-Thr202/Thr204), Akt (phospho-Ser473), and GSK-3β (phospho-Ser9) and total α-AMPK, ACC, ERK1/2, Akt, GSK-3β, LC3-I, and LC3-II protein levels were determined for each of the treated groups (n ≥ 3 per group). Equal protein loading was confirmed by α-tubulin probing of membranes. Relative densitometry was determined using the computerized software package ImageJ.
Simulated model of mPTP opening in isolated adult rat ventricular myocytes.
Adult rat cardiomyocytes from both nondiabetic Wistar and diabetic GK rats were isolated by collagenase perfusion as described by Davidson et al. (13). Following isolation, ventricular myocytes were seeded onto sterilized laminin-coated round coverslips (22-mm diameter, borosilicate glass; VWR, Leicestershire, UK) and incubated at 37°C, 90% humidity, and 95%O2-5%CO2 for 45 min in plating medium consisting of medium M199 (PAA; Somerset, UK), supplemented with 2 mg/ml of BSA (VWR), 0.66 mg/ml creatine, 0.662 mg/ml taurine, 0.332 mg/ml carnitine, 50 IU penicillin, and 5 μg/ml streptomycin. Unattached or dead cells were removed by washing the coverslips with plating medium. The adherent cardiomyocytes were supplemented with fresh plating media and used on the same day of isolation.
A well-characterized and highly reproducible model of simulated opening of the mPTP was used to study the effects of A-769662 in isolated adult ventricular myocytes (13, 57). Myocytes were loaded with a cationic fluorescent dye, TMRM, which accumulates in the mitochondria. Exposure of the dye to confocal laser illumination leads to increased oxidative stress within the mitochondria leading to mPTP opening. Subsequent depolarization of mitochondria due to mPTP opening can be monitored by the increase in the intensity of TMRM fluorescent signal (57) because the dye can now exit into the cytosol where it dequenches. The half-time to maximum mitochondrial depolarization was used as an indicative of susceptibility to opening of the mPTP.
Cardiomyocytes were loaded with 3 μM TMRM in imaging buffer (HEPES-buffered saline consisting of 156 mM NaCl, 2 mM CaCl2, 10 mM glucose, 3 mM KCl, 2 mM MgSO47H2O, 1.25 mM KH2PO4, and 10 mM of HEPES pH 7.4 with NaOH) for 15 min at room temperature to allow TMRM accumulation in the mitochondria. TMRM-containing media were gently removed, and fresh imaging buffer containing A-769662 was added 10 min before confocal laser-induced oxidative stress. In parallel experiments, A-769662 was added in the buffer in a similar manner as described above, but the drug was washed out before the cells were subjected to laser-induced oxidative stress.
Cardiomyocytes were randomly assigned to one of the following treatments (n ≥ 5 hearts/group, 50–60 cells imaged/treatment): 1) vehicle: cardiomyocytes of Wistar or GK rats incubated with 0.1% DMSO for 10 min at room temperature; 2-4) A-769662: cardiomyocytes of Wistar or GK rats incubated with 10, 20, or 40 μM of A769662 for 10 min at room temperature; and 5) CsA: cardiomyocytes of Wistar or GK rats incubated with 0.2 μM of cyclosporine A (CsA), a known inhibitor of the mPTP, for 10 min at room temperature (the positive control group) (23).
The cardiac cells were imaged with a confocal microscope DMI3000B (Leica Microsystems, Germany) equipped with a ×40 optical objective (numerical aperture of 1.25, resolution XY 156, Z334) with immersion oil (Type F immersion oil; Leica). The software used to image mitochondria was the Leica Application Suite for Advanced Fluorescence (LAS AF), connecting the confocal laser (Leica TCS SP5) to the microscope.
Confocal images were acquired using xyt (x, y, time) mode at a scan rate of 400 Hz, with a pinhole size of 67.91 μm and a zoom of 1.94 with a line average of 2 and a frame average of 1. Images acquired were in a 512 × 512 format with a real size of 199.74 × 199.74 μm (pixel size of 390.88 × 390.88 nm). The He/Ne laser for excitation was used at an intensity of 40%, which prove to be sufficient to generate reactive oxygen species (ROS) from TMRM loaded into the cells (see Fig. 6C). Image gain was set to 357.5 V, and sequential images were acquires each 1.314-s intervals until mitochondrial depolarization was observed.
Fig. 6.
A-769662 delays mitochondrial permeability transition pore (mPTP) opening in cardiomyocytes from normoglycemic Wistar and diabetic Goto Kakizaki rats. Half-time to induce maximal opening of the mPTP is expressed as percentage of control. A: adult ventricular rat myocytes from normoglycemic or diabetic rat hearts were pretreated for 10 min before laser induced oxidative stress with A-769662 (10, 20, and 40 μM). A-769662 is shown to significantly delay opening of the mPTP in a dose-dependent manner in cardiomyocytes from both strains. B: washing out the drug before the oxidative insult did not abolish this effect. Values are means ± SE. ★P ≤ 0.05 vs. control; ★★P ≤ 0.01 vs. control; ★★★P ≤ 0.001 vs. control; n ≥ 6 for each group; 50–60 cells/treatment. C: representative photos of cardiomyocytes loaded with TMRM before (1), during (2), and after complete depolarization (3).
The results obtained were expressed as percent vehicle group to account for the intrinsic variability of each cellular preparation obtained from different animals. Cell populations that did not demonstrate a significant delay of mPTP opening in the presence of CsA were excluded.
Statistical analysis.
All values are expressed as means ± SE. Myocardial infarct size and times taken to induce global mitochondrial depolarization were analyzed by one-way ANOVA and Newman-Keuls multiple comparison test, using GraphPad Prism v5.0. Differences were considered significant when P < 0.05.
RESULTS
Baseline parameters.
The diabetic status of the GK rats was verified by measuring the nonfasting blood glucose and Hb1AC as described previously (21). The average blood glucose and Hb1AC of the GK rat were 8.8 ± 0.6 mM and 5.6 ± 0.2%, respectively, compared with 5.2 ± 0.3 mM and 4.2 ± 0.2%, respectively, in the Wistar rat (P ≤ 0.05; n ≥ 5/group).
No significant differences could be detected when comparing area of myocardium at risk, body weight, or heart weight of all the animals used for this study, as demonstrated in Table 1. Similarly, cardiac function parameters at baseline, such heart rate, coronary flow, left ventricular developed pressure, and rate pressure product did not reveal any statistically relevant differences between the two rat strains that could have influenced the results (Table 1). Furthermore, no significant differences regarding these functional parameters were recorded amongst groups throughout the experiments (Tables 2, 3, and 4).
Table 1.
Hemodynamical functional parameters at baseline
| BW, g | HW, g | AAR, % | LVDP, mmHg | HR, beats/min | RPP | CF, ml/min | |
|---|---|---|---|---|---|---|---|
| Wistar group | |||||||
| Vehicle | 356.8 ± 3.7 | 1.9 ± 0.1 | 52.1 ± 1.9 | 118.2 ± 14.0 | 278.8 ± 14.3 | 27,925 ± 3,445 | 15.4 ± 0.9 |
| 10 μM A-769662 | 379.9 ± 12.3 | 2.1 ± 0.1 | 49.7 ± 2.0 | 116.5 ± 6.8 | 282.3 ± 11.9 | 30,243 ± 2,725 | 15.0 ± 1.1 |
| 20 μM A-769662 | 388.1 ± 8.8 | 2.0 ± 0.1 | 52.4 ± 2.7 | 123.1 ± 8.7 | 312.2 ± 14.9 | 34,607 ± 2,049 | 16.4 ± 0.9 |
| 40 μM A-769662 | 359.5 ± 4.7 | 1.8 ± 0.1 | 53.5 ± 1.6 | 138.3 ± 14.1 | 293.0 ± 14.2 | 36,345 ± 1,143 | 15.8 ± 0.8 |
| Goto-Kakizaki group | |||||||
| Vehicle | 358.6 ± 5.0 | 1.9 ± 0.1 | 52.7 ± 1.7 | 111.2 ± 15.5 | 266.4 ± 8.9 | 27,858 ± 3,452 | 13.4 ± 0.6 |
| 10 μM A-769662 | 352.2 ± 8.0 | 2.1 ± 0.1 | 48.9 ± 3.2 | 114.8 ± 7.7 | 265.3 ± 22.0 | 27,961 ± 3,629 | 14.8 ± 0.5 |
| 20 μM A-769662 | 338.2 ± 7.1 | 2.20 ± 0.1 | 53.8 ± 2.2 | 132.5 ± 7.2 | 246.3 ± 10.5 | 32,738 ± 2,489 | 16.1 ± 1.2 |
| 40 μM A-769662 | 337.5 ± 7.4 | 2.1 ± 0.1 | 56.0 ± 1.2 | 138.3 ± 17.1 | 274.0 ± 10.4 | 36,643 ± 1,934 | 16.0 ± 0.6 |
Values are means ± SE. AAR, myocardial area at risk; BW, body weight; HW, heart weight; HR, heart rate; CF, coronary flow; LVDP, left ventricular developed pressure; RPP, rate pressure product. P = NS; n ≥ 6 for each group.
Table 2.
Hemodynamic parameters at the end of ischemia
| RPP |
CF, ml/min |
|||
|---|---|---|---|---|
| Treatment | Wistar | Goto-Kakizaki | Wistar | Goto-Kakizaki |
| Vehicle | 19,542 ± 2,497 | 18,635 ± 2,333 | 8.2 ± 0.7 | 8.0 ± 0.7 |
| 10 μM A-769662 | 21,889 ± 3,725 | 19,968 ± 3,919 | 10.4 ± 0.5 | 8.0 ± 0.7 |
| 20 μM A-769662 | 22,717 ± 4,415 | 19,525 ± 2,397 | 10.7 ± 0.8 | 9.7 ± 0.8 |
| 40 μM A-769662 | 20,985 ± 5,936 | 18,323 ± 3,163 | 9.7 ± 0.6 | 10.5 ± 0.9 |
Values are depicted as means ± SE. There were no significant changes among experimental groups. P = NS; n ≥ 6 for each group.
Table 3.
Hemodynamic parameters at the 5 min of reperfusion
| RPP |
CF, ml/min |
|||
|---|---|---|---|---|
| Treatment | Wistar | Goto-Kakizaki | Wistar | Goto-Kakizaki |
| Vehicle | 21,354 ± 2,669 | 18,204 ± 1,614 | 15.4 ± 0.9 | 13.4 ± 0.6 |
| 10 μM A-769662 | 24,403 ± 2,231 | 20,511 ± 1,030 | 15.0 ± 1.1 | 14.8 ± 0.5 |
| 20 μM A-769662 | 20,330 ± 3,122 | 19,971 ± 2,246 | 16.4 ± 0.9 | 16.1 ± 1.2 |
| 40 μM A-769662 | 23,970 ± 3,258 | 25,061 ± 2,198 | 15.8 ± 0.8 | 16.0 ± 0.6 |
Values are means ± SE. There were no significant changes among experimental groups. P = NS; n ≥ 6 for each group.
Table 4.
Hemodinamic parameters at 30 min of reperfusion
| RPP |
CF, ml/min |
|||
|---|---|---|---|---|
| Treatment | Wistar | Goto-Kakizaki | Wistar | Goto-Kakizaki |
| Vehicle | 21,790 ± 2,643 | 20,904 ± 1,626 | 11.8 ± 1.1 | 12.4 ± 0.8 |
| 10 μM A-769662 | 25,216 ± 1,782 | 20,114 ± 3,146 | 12.4 ± 0.9 | 11.5 ± 0.8 |
| 20 μM A-769662 | 21,293 ± 2,736 | 22,537 ± 1,841 | 12.6 ± 1.3 | 12.7 ± 0.8 |
| 40 μM A-769662 | 26,377 ± 4,474 | 23,434 ± 3,034 | 12.0 ± 0.9 | 12.8 ± 0.9 |
Values are means ± SE. There were no significant changes among experimental groups. P = NS; n ≥ 6 for each group.
A-769662 administered during ischemia reduces myocardial infarct size.
This is, to our knowledge, the first study to examine the cardioprotective effects of A-769662, a known AMPK activator, in the Langendorff perfused rat heart. Although there are a few reports demonstrating that A-769662 could protect the murine heart from global ischemia, it was necessary to perform a dose response study using 10, 20, and 40 μM to find a potential cardioprotective dose for the rat heart (nondiabetic and diabetic) (31, 32). The range of concentrations chosen was based on previous published experiments investigating the activation of AMPK purified from heart or muscle extracts (11). A-769662 was administered 5 min before induction of regional ischemia and removed from the perfusion buffer at 30 min of ischemia to both normo- and hyperglycemic rat hearts since the aim of this study was to activate AMPK only during ischemia.
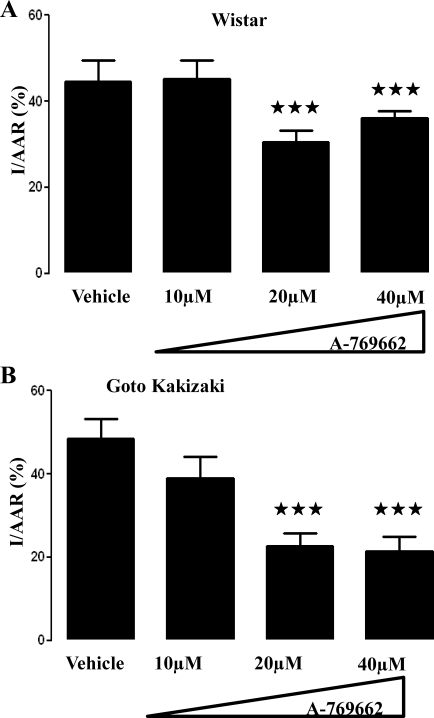
In Wistar rat hearts, 10 μM of A-769662 proved to be insufficient to reduce myocardial; infarction (44.2 ± 3.0 vs. 51.8 ± 3.9% in the vehicle group; P = NS; n ≥ 6). However, protection was achieved in the presence of 20 μM with significant infarct size reduction (30.5 ± 2.6 vs. 51.8 ± 3.9% in the vehicle group; P ≤ 0.001; n ≥ 6). Further increase in the concentration of A-769662 to 40 μM, although still protective (34.2 ± 1.6 vs. 51.8 ± 3.9% in the vehicle group; P ≤ 0.001; n ≥ 6), did not lead to any additional reduction of myocardial injury as assessed by infarct size injury (Fig. 2A).
Fig. 2.
Effect of A-769662 on myocardial infarction in the normoglycemic Wistar (A) and diabetic Goto-Kakizaki (B) rat hearts. Infarct size (I) expressed as percentage of the myocardium at risk (AAR) developed in Langendorff perfused rat hearts subjected to 35 min of regional ischemia and 120 min of reperfusion. Hearts were treated with A-769662 (10, 20, and 40 μM) 5 min before induction of ischemia and for 30 min during ischemia. A-769662 is shown to significantly reduce myocardial infarction in a dose-dependent manner in both non- and diabetic rat hearts. Values are means ± SE. ★★★P ≤ 0.001; n ≥ 6 for each group.
In the diabetic GK rat heart, a similar positive correlation between the concentrations of A-769662 and reduction of myocardial infarction was again observed. Although 10 μM of A-769662 did not confer cardioprotection (39.0 ± 2.2 vs. 48.5 ± 4.7% in the vehicle group; P = NS; n ≥ 6), the presence of 20 μM, during ischemia, significantly reduced myocardial infarction (22.7 ± 3.0 vs. 48.5 ± 4.7% in the vehicle group; P ≤ 0.001; n ≥ 6) As previously observed in the normoglycemic heart, 40 μM of A-769662 also substantially reduced infarct size in the diabetic heart (21.5 ± 3.3 vs. 48.5 ± 4.7% in the vehicle group; P ≤ 0.001; n ≥ 6) without added protection compared with a concentration of 20 μM (Fig. 2B).
Of note, the vehicle (DMSO) did not have any influence on infarct size. No significant differences were registered between control and vehicle groups for both rat strains used (49.8 ± 2.1 vs. 51.8 ± 3.9% in the vehicle group for Wistar hearts; P = NS; n ≥ 6: 39.6 ± 2.2 vs. 48.5 ± 4.7% in the vehicle group for GK hearts; P = NS; n ≥ 6).
A-769662 enhances AMPK activation during ischemia in the diabetic heart.
To verify whether indeed A-769662 activated AMPK in our experimental model, Western blot analysis was used to estimate AMPK activation. The phosphorylation levels of the residue Thr172 of the catalytic α-subunit of AMPK, a requirement for activation of this kinase, were measured.
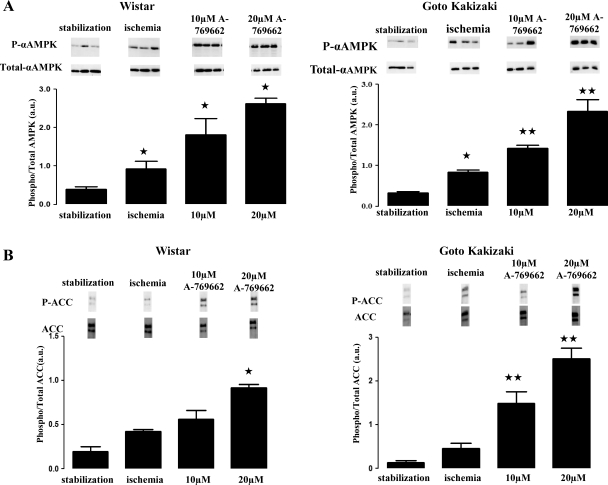
Our data demonstrated an intrinsic increase in AMPK phosphorylation in control normoglycemic hearts at the end of the ischemic period compared with control samples collected at the end of stabilization [0.93 ± 0.06 vs. 0.39 ± 0.06 arbitrary units (au) in the stabilization group; P ≤ 0.05; n = 3/group], as demonstrated in Fig. 3A.
Fig. 3.
Effect of A-769662 on AMPK phosphorylation (P) in the normoglycemic Wistar and diabetic Goto-Kakizaki rat hearts. A: α-AMPK phosphorylation is significantly increased in the hearts perfused with 10 and 20 μM of A-769662 during 30 min of ischemia. B: phosphorylated level of acetyl-CoA carboxylase (ACC; downstream target for AMPK) is increased in the presence of A-76966 in a dose-response manner. Blot images in A and B were obtained from the same gel, respectively. Values represent relative densitometry [in arbitrary units (au)] as means ± SE. ★P ≤ 0.05, ★★P ≤ 0.01, ★★★P ≤ 0.001 vs. stabilization group; n = 3 for each group.
However, when normoglycemic hearts were pretreated with 10 μM of A-769662, a significant increase in the phosphorylated status of AMPK above physiological levels was found (1.81 ± 0.43 vs. 0.93 ± 0.06 au in the ischemic group; P ≤ 0.05; n = 3/group), although this concentration was not associated with a reduction in infarct size. When protein samples of hearts treated with the protective concentration of 20 μM were compared with the ischemic group, a significant enhancement of AMPK phosphorylation was detected (2.60 ± 0.14 vs. 0.93 ± 0.06 au in the ischemic group; P ≤ 0.05; n = 3/group).
Similarly for the diabetic heart, upon ischemia, AMPK phosphorylation was significantly increased (0.84 ± 0.05 vs. 0.33 ± 0.03 au in the stabilization group; P ≤ 0.05; n = 3/group), as observed in Fig. 3A. In the presence of 10 μM of A-769662, a significant increase in the phosphorylated status of AMPK above physiological levels was again observed (1.42 ± 0.08 vs. 0.84 ± 0.05 au in the ischemic group; P ≤ 0.001; n = 3/group), although this concentration was not cardioprotective. A treatment of diabetic hearts with the protective concentration of 20 μM of A-769662 resulted in an enhanced phosphorylation of AMPK, compared with what was observed in the ischemic group (2.33 ± 0.28 vs. 0.84 ± 0.05 au in the ischemic group; P ≤ 0.001; n = 3/group). In addition, the level of ACC phosphorylation (a well-known downstream target of AMPK) was also found to be increased following the administration of the AMPK activator in both Wistar (0.42 ± 0.02 au in control ischemia; 0.56 ± 0.02 au for 10 μM and 0.92 ± 0.03 au for 20 μM vs. 0.19 ± 0.07 au in stabilization; P ≤ 0.05; n ≥ 3/group) and GK (0.46 ± 0.12 au in control ischemia; 1.49 ± 0.26 au for 10 μM and 2.50 ± 0.23 au for 20 μM vs. 0.19 ± 0.05 au in stabilization; P ≤ 0.01; n ≥ 3/group) in a concentration-dependent fashion (Fig. 3B).
A-769662 does not modify the expression of the autophagic marker LC3-II/LC3-I during ischemia.
AMPK activation during ischemia has been associated with a controlled autophagic process that may lead to cardioprotection, as autophagy activation in such setting is assumed to be involved in the selective removal of dysfunctional cellular organelles and components (25, 49). To assess the possibility that A-769662 increased autophagy via AMPK, we measured the LC3-II-to-LC3-I ratio, a bona-fide marker of autophagy (25).
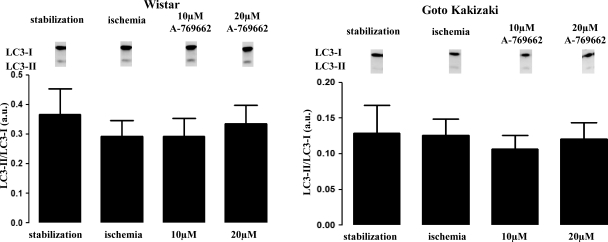
Our data revealed no significant differences in the ratio of LC3-II to LC3-I in both the nondiabetic rat heart (0.37 ± 0.08 au in stabilization vs. 0.29 ± 0.05 au in the end of ischemia; P = NS; n ≥ 3/group) and the diabetic heart (0.13 ± 0.04 au in stabilization vs. 0.12 ± 0.02 au at the end of ischemia; P = NS; n ≥ 3/group; Fig. 4).
Fig. 4.
Effect of A-769662 on the LC3-II-to-LC3-I ratio in the normoglycemic Wistar and diabetic Goto-Kakizaki rat hearts. No significant changes on LC3-II-to-LC3-I ratio, an autophagic marker, were induced by treatment with A-769662 in both rat strains. Blot images were obtained from the same gel. Values represent relative densitometry (in arbitrary units) and are means ± SE.
The presence of A-769662 during ischemia did not cause any significant changes of LC3-II/LC3-I levels in the Wistar rat heart (0.29 ± 0.06 au for 10 μM and 0.33 ± 0.06 au for 20 μM vs. 0.29 ± 0.05 au; P = NS; n ≥ 3/group) or in the GK rat heart (0.11 ± 0.02 au for 10 μM and 0.12 ± 0.02 au for 20 μM vs. 0.12 ± 0.02 au; P = NS; n ≥ 3/group; Fig. 4).
A-769662 does not activate Akt and ERK1/2 during ischemia.
Although the initial characterization of A-769662 demonstrated a high degree of specificity towards activating AMPK (11, 19), recent investigations claimed unspecific activation of other kinases, such as PI3K/Akt (50), which is known to be involved in cardioprotection. Therefore, to clarify this, the levels of Akt and ERK1/2 phosphorylation were also measured in our experiments.
Our data showed that ischemia did not significantly modify Akt activation in both the nondiabetic rat heart (0.22 ± 0.02 au in stabilization vs. 0.17 ± 0.03 au in the end of ischemia; P = NS; n ≥ 3/group) and the diabetic heart (0.34 ± 0.02 au in stabilization vs. 0.26 ± 0.02 au at the end of ischemia; P = NS; n ≥ 3/group; Fig. 5A).
Fig. 5.
Effect of A-769662 on prosurvival kinases Akt and ERK1/2 in the normoglycemic Wistar and diabetic Goto-Kakizaki rat hearts. No significant changes were induced by treatment with A-769662 in the levels of phospho-Akt (A; Ser473) or phosphor-ERK1/2 (B; Thr202/Thr204). Blot images in A and B were obtained from the same gel, respectively. Values represent relative densitometry (in arbitrary units) as means ± SE; n = 3 for each group.
The presence of A-769662 during ischemia did not cause any significant changes of the phosphorylation levels of Akt in the Wistar rat heart (0.25 ± 0.02 au for 10 μM; 0.27 ± 0.01 au for 20 μM vs. 0.17 ± 0.03 au; P = NS, n ≥ 3/group) or in the GK rat heart (0.33 ± 0.05 au for 10 μM and 0.39 ± 0.05 au for 20 μM vs. 0.26 ± 0.02 au; P = NS; n ≥ 3/group; Fig. 5A).
In the Wistar rat heart, ischemia also did not influence the phosphorylation status of ERK1/2 (0.42 ± 0.10 au for ischemic tissue vs. 0.39 ± 0.05 au for heart tissue at stabilization; P = NS; n ≥ 3/group). In addition, the presence of 10 or 20 μM of A-769662 did not correlate with substantial variations in the phosphorylated levels of ERK1/2 (0.49 ± 0.04 au for 10 μM; 0.39 ± 0.02 au for 20 μM vs. 0.42 ± 0.10 au; P = NS; n ≥ 3/group; Fig. 5B).
In the GK rat heart, the phosphorylated status of ERK1/2 remained unchanged with ischemia (0.32 ± 0.03 au for ischemic tissue vs. 0.35 ± 0.04 au for heart tissue at stabilization; P = NS; n ≥ 3/group) or in the presence of A-769662 (0.36 ± 0.01 au for 10 μM and 0.40 ± 0.04 au for 20 μM vs. 0.32 ± 0.03 au; P = NS; n ≥ 3/group) at the end of ischemia (Fig. 5B).
AMPK activation delays mPTP opening in adult rat ventricular myocytes.
In cardiomyocytes from Wistar nondiabetic rat strain, pretreament with 10 μM A-769662 for 10 min did not influence the time taken to induce global mitochondrial membrane depolarization, an indicator of mPTP opening (114.5 ± 5.8 vs. 100% in vehicle groups; P = NS). However, in the presence of 20 and 40 μM of A-769662, a significant delay in opening of the mPTP was observed. (150.8 ± 11.5% for 20 μM and 146.8 ± 15.6% for 40 μM vs. 100% in vehicle-treated cells; P ≤ 0.05; Fig. 6A).
Similarly, in cardiomyocytes from GK diabetic rat strain, 20 μM A-769662 significantly prolonged the time taken to induce global mitochondrial membrane depolarization (indicating mPTP opening; 150.5 ± 11.7 vs. 100% in vehicle group; P ≤ 0.001; Fig. 6A). This protective effect of A-769662 was also present when cells isolated from diabetic animals were pretreated with 40 μM of A-769662 (166.5 ± 12.2 vs. 100% in vehicle-treated cells; P ≤ 0.001).
As previously observed in intact hearts, 10 μM of A-769662 did not exert any cardioprotective effects (126.4 ± 12.7 vs. 100% in vehicle group; P = NS). CsA, a drug known to delay opening of the mPTP and to protect against myocardial ischemia-reperfusion injury (23), significantly increased the time to opening of the mPTP in myocytes from both rat strains (138.9 ± 1.9 vs. 100% in the vehicle group for Wistar hearts; P < 0.05; 143.7 ± 2.2 vs. 100% in the vehicle group for GK hearts; P ≤ 0.05).
Of note, similar effects were observed in both Wistar and GK cardiomyocytes when the AMPK activator A-769662 was removed from the media before the oxidative stress. Hence, we observed that A-769662 treatment significantly delayed opening of the mPTP in a concentration dependent-manner in both the nondiabetic rat strain (126.4 ± 5.5% for 20 μM and 152.8 ± 6.6% for 40 μM vs. 100% in vehicle-treated cells; P ≤ 0.01) and diabetic rat strain (146.8 ± 9.7% for 20 μM and 187.1 ± 26.8% for 40 μM vs. 100% in vehicle-treated cells; P ≤ 0.01; Fig. 6B). As previously observed, in the presence of 10 μM of A-769662, mPTP opening was not significantly delayed (102.9 ± 6.2% for 10 μM vs 100% vehicle-treated nondiabetic cells; 110.4 ± 10.5% for 10 μM vs. 100% in vehicle-treated diabetic cells; P = NS).
A-769662 induces a significant inhibition of GSK-3β during ischemia in the rat heart.
Upon observing that activation of AMPK in the presence of cardioprotective concentrations of A-769662 could delay opening of the mPTP, we decided to investigate the underlying mechanisms of this effect. AMPK is known to phosphorylate GSK-3β, a kinase that can induce cell death in addition to interacting with the voltage-dependent anion chanel and encouraging mPTP formation (12, 18, 29). We therefore assessed the levels of phosphorylation of GSK-3β in cardiac tissue of nondiabetic and diabetic animals in the presence of 10 and 20 μM of A-769662 (Fig. 7).
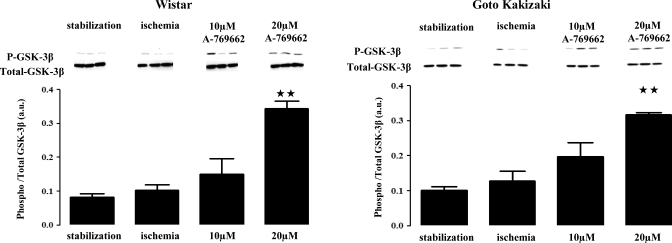
Fig. 7.
Effect of A-769662 on GSK-3β phosphorylation in the normoglycemic Wistar and diabetic Goto-Kakizaki rat hearts; 20 μM of A769662 administered during ischemia increased GSK-3β phosphorylation significantly. (★★P ≤ 0.01 in Wistar and GK hearts; n ≥ 3 /group). Blot images were obtained from the same gel. Values represent relative densitometry (in arbitrary units) and are means ± SE. ★★P ≤ 0.001 vs. stabilization group; n = 3 for each group.
In the normoglycemic rat heart, neither ischemia influenced the phosphorylation status of GSK-3β (0.08 ± 0.10 au in stabilization vs. 0.10 ± 0.02 au in the end of ischemia; P = NS; n ≥ 3/group) nor did 10 μM of A-769662 (0.15 ± 0.04 au for 10 μM vs. 0.10 ± 0.02 au for the ischemic group; P = NS; n ≥ 3/group; Fig. 7). However,treatment of the hearts with 20 μM of A-769662 significantly increased the phosphorylation of GSK-3β on residue Ser9 (0.34 ± 0.01 au for 20 μM vs. 0.10 ± 0.02 au for the ischemic group; P ≤ 0.01; n ≥ 3/group).
In the GK rat heart, a similar trend was observed. Ischemia did not induce any noticeable modifications of the phosphorylated status of this kinase (0.10 ± 0.01 au in stabilization vs. 0.13 ± 0.03 au in the end of ischemia; P = NS, n ≥ 3/group). Furthermore, in the presence of the nonprotective concentration of A-769662, no significant changes regarding phosphorylation of GSK-3β were observed (0.19 ± 0.04 au for 10 μM vs. 0.13 ± 0.03 au for the ischemic group; P = NS; n ≥ 3/group). Interestingly, a significant increment of the phosphorylation levels of GSK-3β can be observed in the presence of 20 μM of A-769662, the cardioprotective dose used in this study (0.32 ± 0.01 au for 20 μM vs. 0.13 ± 0.03 au for the ischemic group; P ≤ 0.01; n ≥ 3/group; Fig. 7).
DISCUSSION
This study showed for the first time that A-769662, a small molecule known to be an AMPK activator, administered at the onset of regional ischemia in a Langendorff perfused rat heart model, significantly reduces myocardial infarction in both normoglycemic and diabetic hearts. This cardioprotective effect was concurrent with an increase in AMPK and GSK-3β phosphorylation above physiological levels at the end of the ischemic period. Of note, significant activation of RISK pathway signaling kinases was not observed during ischemia, either in control or treated hearts, in both normoglycemic and diabetic rat strains. Moreover, in a model of mPTP opening induced by oxidative stress, A-7969662 delayed mitochondrial damage due to increased oxidative stress in cardiomyocytes of both normoglycemic and diabetic rat strains.
The mechanism by which A-769662 activates AMPK is slightly different from the biological activation of this enzyme. In a physiological or pathological condition by which ATP is degraded to AMP and not resynthethized (for example during physical activity or ischemia), the AMP-to-ATP ratio increases and AMP binds to the γ-subunit of AMPK, inducing an allosteric conformation change that allows for the phosphorylation of the residue Thr172 of the α- catalytic subunit of the enzyme by an upstream kinase (LKB1, CamKK, or TAK1). In addition, binding of AMP to AMPK also prevents any phosphatase action (26). AMPK activation by A-769662 is independent of the AMP-to-ATP ratio. Briefly, A-769662 is thought to bind to AMPK, stabilizing the enzyme in a conformation that is resistant to dephosphorylation at Thr-172 (19, 43, 44).
Although A-769662 seems to constitute a valuable tool for the study of AMPK, there are very few investigations assessing the effects of this compound on the myocardium, especially in the setting of ischemia-reperfusion injury. Recently, Kim et al. (31, 32) have observed that A-769662 can reduce myocardial infarction in the murine heart, if given before global ischemia. However, to characterize the cardioprotective effects of A-769662 on the rat myocardium, a range of concentrations (10, 20, or 40 μM) were tested in our study. Of note, the highest concentration used was still bellow the concentrations of A-769662 reported to inhibit proteasomal activity (38) or the H+-ATPase (24) or Na+-K+-ATPase (3).
Importantly, we have demonstrated that A-769662 can protect the ischemic myocardium against myocardial infarction when administered during ischemia. Moreover, although A-769662 has been shown to improve glucose uptake (11, 16), its cardioprotective effects are independent of its hyperglycemic and insulin-sensitizing properties, as protection was demonstrated to be present in isolated rat hearts and adult cardiomyocytes of both nondiabetic and diabetic animals.
The beneficial outcomes of AMPK activation during ischemia-reperfusion are a controversial issue. AMPK is known to be activated during ischemia, as an innate protective mechanism that allows the cells to manage energy sources (33). Transgenic models of mice lacking functional AMPK have impaired cardiac function, have poor glucose uptake, and are more susceptible to cell death (42). Moreover, deletion of α2-AMPK is associated with alterations in cardiac mitochondrial structure and a decreased maximal mitochondrial respiration, due to inhibition of complex I of the respiratory chain (2). However, some studies, such as that by Folmes et al. (17), have demonstrated that the cardiac expression of a dominant negative mutant of α2-AMPK does not impair function recovery. Similarly, deletion of α2-AMPK in cardiac tissue was shown not to influence cardiac recovery of the murine heart after an ischemic-reperfusion episode (8, 56), thus increasing the controversy around AMPK's role in reperfusion.
Nonetheless, our results support the hypothesis that AMPK activation during ischemia is cardioprotective, as seen by a significant myocardial infarct size reduction when a small activator of AMPK is present during ischemia. The prosurvival pathways associated with the cardioprotective effects of AMPK activation during ischemia above physiological levels remain undisclosed. We can postulate that AMPK activation would result in an increase of glycolysis above physiological levels. The net effect would be an increase of ATP production at the onset of reperfusion, which would preserve cardiac contractility as well as maintain mitochondrial integrity. It worth mentioning that AMPK activation is known to play a significant role in controlling autophagic process (49). Autophagy during ischemia could act as a prosurvival process that can selectively remove dysfunctional cytosolic organelles providing nutrients and energy in stress situations (25). We pursued this hypothesis by assessing the ratio of LC3-II to LC3-I at the end of the ischemic insult in the presence of A-769662. However, we did not observe any noteworthy changes of this value following the drug's administration at any of the time points chosen for the collection of cardiac tissue in both rat strains used. These results suggest that in our experimental setting autophagy does not appear to play a pivotal role in the cardioprotective effects observed in the presence of A-769662 during myocardial ischemia.
To confirm that AMPK activation was involved in the cardioprotection associated with A-769662 administration, we assessed the levels of phosphorylated α-AMPK at the end of the ischemic period in the presence of 10 or 20 μM in the normo- and hyperglycemic rat heart. We found a significantly enhanced AMPK phosphorylation during ischemia, above physiological levels in the presence of A-769662. Importantly, no concurrent activation of the RISK pathway components Akt and ERK1/2 was found. Our results propose a novel approach to cardioprotection against ischemia-reperfusion injury by which it is possible to protect the myocardium during ischemia without activation of known prosurvival kinases before ischemia. It would be of interest for further investigations to assess if these protective effects are cumulative.
The mPTP is a nonspecific pore of the mitochondria that is assumed to span both the outer and the inner mitochondrial membranes, considered to be the final event in reperfusion injury that would trigger cell death. Cardioprotective agents and strategies, such as ischemic preconditioning (1) and postconditioning (28), have been shown to induce protection against reperfusion injury by delaying the opening of the mPTP. Since metformin, an AMPK activator, was previously demonstrated to delay mPTP opening (4, 20), we hypothesized that A-769662 could also induce cardioprotection by delaying mPTP. In this study, we showed for the first time that A-769662 protects both the hyperglycemic and normoglycemic cardiomyocytes against oxidative stress induced mPTP opening. In our experimental model, A-769662 was present before and during oxidative stress while the administration period on the Langendorff system was limited to ischemia. However, interestingly, the effect of the drug on mPTP is manifested even if the drug is washed out before the oxidative insult, thus suggesting that A-769662 activates a signaling pathway that will culminate in the prevention of oxidative stress-induced mPTP opening.
Although the mechanism by which A-769662 activates AMPK has been elucidated, it is still unclear how activation of AMPK would lead to inhibition of the mPTP. Due to AMPK's role in inducing cellular energy preservation, AMPK activation by A-769662 could be involved in delaying opening of the mPTP by maintaining ATP levels. Recently, several studies (9, 53) have claimed that AMPK can also induce antioxidant responses. However, the mPTP assay here described results in opening of the mPTP due to highly localized ROS production in the mitochondria, hence being unlikely for AMPK to trigger an antioxidant response at that level in such short period of time.
Interestingly, although A-769662 stimulates AMPK in the concentrations used for our assays, only in the presence of 20 μM or higher concentrations of A769662 we can observe myocardial infarct size reduction and delay of mPTP opening. This intriguing finding prompted us to assume that a supra-activation of AMPK during ischemia can be protective once a certain phosphorylation threshold of AMPK is overcome. It may also be possible that the mechanism of autoinhibition of AMPK is not abolished in the presence of 10 μM of A769662 but diminished/abolished by increasing concentrations of this activator. Since AMPK is a kinase, it is possible that it may phosphorylate intracellular proteins that hamper opening of the mPTP. In particular, AMPK is known to phosphorylate GSK-3β, a kinase that induces cell death and mPTP opening by interacting with voltage-dependent anion chanel (12, 18, 29). Furthermore, activation of AMPK by a variety of pharmacological agents has been shown to prevent mitochondrial ROS formation through inhibition of GSK-3β (10, 47). In our study, we did not observe any differences in phosphorylation of GSK-3β upon induction of ischemia in either rat strain here used. In the presence of 10 μM of A-769662, there was a slight trend for an increase of phosphorylation of this kinase, albeit nonsignificant in both rat strains. Nonetheless, 20 μM of A-769662, the cardioprotective dose, resulted in a significant increase of phosphorylation of GSK-3β in both the nondiabetic and diabetic rat heart. This inactivation of GSK-3β observed only in the protective concentration of A-769662 may explain why although A-769662 enhances AMPK activity in any concentration used in this study, it fails to be protective in all cases. We may postulate that there is a threshold for the inhibition of GSK-3β that can only be overcome if a significant number of AMPK complexes are activated and that this phenomenon only occurs at concentrations of A-769662 >10 μM.
Our study has some limitations. First, one must be aware that an in vitro perfused heart model cannot accurately reproduce the complex metabolic environment of an in vivo model. In natural conditions, the cardiac tissue mostly metabolizes fatty acids, while in our model glucose is the energetic substrate. Therefore, the Langendorff perfused model was mainly a suitable, basic option to investigate the potential protective effects of AMPK activation during ischemia. Furthermore, this study should be repeated in an in vivo model that would allow for the assessment of the effect of fatty acids at the onset of reperfusion. Indeed, AMPK's controversial role in cardioprotection is highly due to the proposed deleterious effects of its activation during reperfusion in the presence of fatty acids. At the onset of reperfusion, oxygen is reintroduced into the cardiac environment and β-oxidation is significantly enhanced as it leads to a swift restoration of ATP levels in the myocardium. An increase in β-oxidation is accompanied by a decrease of glucose oxidation, which will lead to an excessive production of protons and consequently to the decrease of cardiac function (15). The study of AMPK activation during ischemia in in vivo models would therefore help to clarify this matter. Furthermore, to fully explore the role of AMPK in the cardioprotective mechanisms against myocardial ischemia-reperfusion injury, the experiments here described should be performed in transgenic murine models lacking a fully functional AMPK kinase, and in particular in the setting of diabetes where glucose metabolism is impaired and fatty acid metabolism is enhanced.
In addition, one can investigate if chronic AMPK activation would reduce myocardial injury. It would be also interesting to assess whether activation of AMPK before ischemia would reproduce the results obtained by Lefer and colleagues, where pretreatment with metformin of obese mice led to infarct size reduction (6) and improved cardiac recovery in a heart failure model (21).
In conclusion, we have demonstrated that a specific activator of AMPK, A-769662, administered during myocardial ischemia reduces infarct size in both the diabetic and nondiabetic rat heart. This cardioprotective effect was associated with an enhanced activation of AMPK and increased levels of phosphorylated GSK-3β during ischemia. In addition, A-769662 was shown to significantly reduce the susceptibility of mitochondria from adult ventricular cardiomyocytes to induce mPTP opening upon oxidative stress.
GRANTS
We thank the British Heart Foundation (Grant RG/03/007) and Medical Research Council (Grant G0700933) for continuing support. M. A. Paiva is the recipient of a fellowship from the Fundação para a Ciência e a Tecnologia (Portuguese Research Council–SFRH/BD/23671/2005).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are thankful for the help and support of Barry Warburton and Jo Emery (University College of London), who were responsible for the GK rat colony.
REFERENCES
- 1.Argaud L, Gateau-Roesch O, Muntean D, Chalabreysse L, Loufouat J, Robert D, Ovize M. Specific inhibition of the mitochondrial permeability transition prevents lethal reperfusion injury. J Mol Cell Cardiol 38: 367–374, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Athea Y, Viollet B, Mateo P, Rousseau D, Novotova M, Garnier A, Vaulont S, Wilding JR, Grynberg A, Veksler V, Hoerter J, Ventura-Clapier R. AMP-activated protein kinase alpha2 deficiency affects cardiac cardiolipin homeostasis and mitochondrial function. Diabetes 56: 786–794, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benziane B, Bjornholm M, Lantier L, Viollet B, Zierath JR, Chibalin AV. AMP-activated protein kinase activator A-769662 is an inhibitor of the Na+-K+-ATPase. Am J Physiol Cell Physiol 297: C1554–C1566, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 103: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Burckhartt B, Yang XM, Tsuchida A, Mullane KM, Downey JM, Cohen MV. Acadesine extends the window of protection afforded by ischemic preconditioning in conscious rabbits. Cardiovasc Res 29: 653–657, 1995 [PubMed] [Google Scholar]
- 6.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 57: 696–705, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29: 18–24, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Carvajal K, Zarrinpashneh E, Szarszoi O, Joubert F, Athea Y, Mateo P, Gillet B, Vaulont S, Viollet B, Bigard X, Bertrand L, Ventura-Clapier R, Hoerter JA. Dual cardiac contractile effects of the α2-AMPK deletion in low-flow ischemia and reperfusion. Am J Physiol Heart Circ Physiol 292: H3136–H3147, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol 27: 2627–2633, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Choi SH, Kim YW, Kim SG. AMPK-mediated GSK3beta inhibition by isoliquiritigenin contributes to protecting mitochondria against iron-catalyzed oxidative stress. Biochem Pharmacol 79: 1352–1362, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, Zhao G, Marsh K, Kym P, Jung P, Camp HS, Frevert E. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3: 403–416, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res 103: 983–991, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson SM, Hausenloy D, Duchen MR, Yellon DM. Signalling via the reperfusion injury signalling kinase (RISK) pathway links closure of the mitochondrial permeability transition pore to cardioprotection. Int J Biochem Cell Biol 38: 414–419, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Dolinsky VW, Dyck JR. Role of AMP-activated protein kinase in healthy and diseased hearts. Am J Physiol Heart Circ Physiol 291: H2557–H2569, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol 574: 95–112, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazakerley DJ, Holman GD, Marley A, James DE, Stockli J, Coster AC. Kinetic evidence for unique regulation of GLUT4 trafficking by insulin and AMP-activated protein kinase activators in L6 myotubes. J Biol Chem 285: 1653–1660, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5′-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol 297: H313–H321, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation 117: 2761–2768, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem 282: 32549–32560, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, Leverve X. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J 382: 877–884, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 104: 403–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339: 229–234, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Halestrap AP, Connern CP, Griffiths EJ, Kerr PM. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischemia/reperfusion injury. Mol Cell Biochem 174: 167–172, 1997 [PubMed] [Google Scholar]
- 24.Hallows KR, Alzamora R, Li H, Gong F, Smolak C, Neumann D, Pastor-Soler NM. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296: C672–C681, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes 32, Suppl 4: S7–S12, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Hausenloy DJ, Duchen MR, Yellon DM. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischemia-reperfusion injury. Cardiovasc Res 60: 617–625, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Javadov S, Rajapurohitam V, Kilic A, Zeidan A, Choi A, Karmazyn M. Anti-hypertrophic effect of NHE-1 inhibition involves GSK-3beta-dependent attenuation of mitochondrial dysfunction. J Mol Cell Cardiol 46: 998–1007, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1: 15–25, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Kim AS, Wright TM, Hu J, Sakamoto K, Young LH. Abstract 3620: A769662 Protects the Heart Against Ischemia-Reperfusion Injury via the AMPK-eNOS Pathway. Circulation 120: S838-a, 2009 [Google Scholar]
- 32.Kim AS, Wright TM, Miller EJ, Sakamoto K, Young LH. Abstract 3892: a small molecule activator of amp-activated protein kinase protects the heart against ischemia-reperfusion injury. Circulation 118: S499, 2008 [Google Scholar]
- 33.Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270: 17513–17520, 1995 [DOI] [PubMed] [Google Scholar]
- 34.Kusmic C, L'Abbate A, Sambuceti G, Drummond G, Barsanti C, Matteucci M, Cao J, Piccolomini F, Cheng J, Abraham NG. Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling. J Cell Biochem 109: 1033–1044, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation 102: 1014–1019, 2000 [DOI] [PubMed] [Google Scholar]
- 36.McGuire DK, Emanuelsson H, Granger CB, Magnus Ohman E, Moliterno DJ, White HD, Ardissino D, Box JW, Califf RM, Topol EJ. Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO-IIb study GUSTO IIb Investigators. Eur Heart J 21: 1750–1758, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol 34: 661–668, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Moreno D, Knecht E, Viollet B, Sanz P. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett 582: 2650–2654, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, Providencia L, Rongen GA, Smits P, Mocanu MM, Yellon DM. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol 53: 373–378, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Paiva MA, Goncalves LM, Providencia LA, Davidson SM, Yellon DM, Mocanu MM. Transitory activation of AMPK at reperfusion protects the ischemic-reperfused rat myocardium against infarction. Cardiovasc Drugs Ther 24: 25–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pang T, Zhang ZS, Gu M, Qiu BY, Yu LF, Cao PR, Shao W, Su MB, Li JY, Nan FJ, Li J. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem 283: 16051–16060, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell RR, III, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest 114: 495–503, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders MJ, Ali ZS, Hegarty BD, Heath R, Snowden MA, Carling D. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem 282: 32539–32548, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Scott JW, van Denderen BJ, Jorgensen SB, Honeyman JE, Steinberg GR, Oakhill JS, Iseli TJ, Koay A, Gooley PR, Stapleton D, Kemp BE. Thienopyridone drugs are selective activators of AMP-activated protein kinase beta1-containing complexes. Chem Biol 15: 1220–1230, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11: 1096–1103, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol 221: 490–497, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol 76: 884–895, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Suleiman MS, Halestrap AP, Griffiths EJ. Mitochondria: a target for myocardial protection. Pharmacol Ther 89: 29–46, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J. AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy 3: 405–407, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Treebak JT, Birk JB, Hansen BF, Olsen GS, Wojtaszewski JF. A-769662 activates AMPK β1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol 297: C1041–C1052, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes 54: 2360–2364, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S. The AMP-activated protein kinase alpha2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111: 91–98, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKα2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res 106: 1117–1128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. J Cardiovasc Pharmacol 46: 817–822, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Xing Y, Musi N, Fujii N, Zou L, Luptak I, Hirshman MF, Goodyear LJ, Tian R. Glucose metabolism and energy homeostasis in mouse hearts overexpressing dominant negative alpha2 subunit of AMP-activated protein kinase. J Biol Chem 278: 28372–28377, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zarrinpashneh E, Carjaval K, Beauloye C, Ginion A, Mateo P, Pouleur AC, Horman S, Vaulont S, Hoerter J, Viollet B, Hue L, Vanoverschelde JL, Bertrand L. Role of the α2-isoform of AMP-activated protein kinase in the metabolic response of the heart to no-flow ischemia. Am J Physiol Heart Circ Physiol 291: H2875–H2883, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]