Abstract
Hydrogen sulfide (H2S) is a gaseous signaling molecule that appears to contribute to the regulation of vascular tone and blood pressure. Multiple potential mechanisms of vascular regulation by H2S exist. Here, we tested the hypothesis that piglet cerebral arteriole smooth muscle cells generate ATP-sensitive K+ (KATP) currents and that H2S induces vasodilation by activating KATP currents. Gas chromatography/mass spectrometry data demonstrated that after placing Na2S, an H2S donor, in solution, it rapidly (1 min) converts to H2S. Patch-clamp electrophysiology indicated that pinacidil (a KATP channel activator), Na2S, and NaHS (another H2S donor) activated K+ currents at physiological steady-state voltage (−50 mV) in isolated cerebral arteriole smooth muscle cells. Glibenclamide, a selective KATP channel inhibitor, fully reversed pinacidil-induced K+ currents and partially reversed (∼58%) H2S-induced K+ currents. Western blot analysis indicated that piglet arterioles expressed inwardly rectifying K+ 6.1 (Kir6.1) channel and sulfonylurea receptor 2B (SUR2B) KATP channel subunits. Pinacidil dilated pressurized (40 mmHg) piglet arterioles, and glibenclamide fully reversed this effect. Na2S also induced reversible and repeatable vasodilation with an EC50 of ∼30 μM, and this effect was partially reversed (∼55%) by glibenclamide. Vasoregulation by H2S was also studied in pressurized resistance-size cerebral arteries of mice with a genetic deletion in the gene encoding SUR2 (SUR2 null). Pinacidil- and H2S-induced vasodilations were smaller in arterioles of SUR2 null mice than in wild-type controls. These data indicate that smooth muscle cell KATP currents control newborn cerebral arteriole contractility and that H2S dilates cerebral arterioles by activating smooth muscle cell KATP channels containing SUR2 subunits.
Keywords: arteriole smooth muscle cells, adenosine 5′-triphosphate-sensitive potassium channels, sulfonylurea receptor 2
hydrogen sulfide (H2S) is endogenously synthesized from l-cysteine by cystathionine β-synthase or cystathionine γ-lyase (13, 44). It is becoming apparent that H2S is a physiologically significant signaling molecule (25, 26, 29). Previous studies have described the cardiovascular effects of exogenous and endogenous H2S in several species. H2S dilates blood vessels, controls blood pressure, and protects the heart from ischemia-reperfusion injury (48). Most studies investigating vasodilation by H2S have been performed in rodents and have examined large arteries, aortic rings, mesenteric arteries, and measured blood pressure (7, 32, 42). This study was designed to examine the regulation of cerebral arteriole contractility by H2S in newborn pigs.
H2S can affect a variety of ion channels and receptors (45). H2S-induced vasorelaxation of systemic arteries and aorta has been demonstrated to occur because of endothelium-dependent dilators, Cl−/HCO3− exchanger, KCNQ channels, and ATP-sensitive K+ (KATP) channels (7, 24, 39, 45). KATP channels are hetero-octameric complexes containing four pore-forming, inwardly rectifying (Kir6.1 or Kir6.2) channel subunits, together with four sulfonylurea receptors (SURs), which are ATP-binding cassette family proteins (43, 45, 46). Molecular diversity exists between tissues regarding their Kir and SUR isoform (SUR1, SUR2A, and SUR2B) expression and channel composition (4). A number of pharmacological studies have provided functional evidence that smooth muscle cell KATP channels regulate the tone of adult cerebral arteries in vitro and in vivo (20, 21, 33). We recently reported that exogenous and endogenous H2S dilates piglet cerebral arterioles in vivo and that this vasodilation is inhibited by glibenclamide, suggesting the involvement of KATP channels (26). Newborn piglet cerebral arteriole smooth muscle cell KATP currents have not been described nor has diameter regulation by these channels been studied in vitro. This study aimed to determine whether newborn arterial smooth muscle cells generate KATP currents. Additional objectives of this study were to determine the effects of H2S on KATP currents and to investigate the contribution of KATP channels to H2S-induced dilation of arterioles isolated from other influences present in the intact brain.
We show that both pinacidil, a KATP channel activator, and H2S activate cerebral arteriole smooth muscle cell KATP currents, leading to vasodilation. Glibenclamide, a plasma membrane and mitochondrial KATP channel inhibitor, reversed pinacidil and H2S-induced K+ current activation and vasodilation. H2S-induced vasodilation was attenuated in cerebral arteries of SUR2 null mice, indicating that plasma-membrane KATP channels mediate this response. These data indicate that exogenous H2S activates arteriole smooth muscle cell plasma-membrane KATP channels containing SUR2, leading to vasodilation.
MATERIALS AND METHODS
Tissue preparation and arteriole smooth muscle cell isolation.
All procedures involving animals were approved by the University of Tennessee Health Science Center Animal Care and Use Committee. Newborn pigs (1–3 days old, 1.5–2.5 kg; Nichols Hog Farm; Olive Branch, MS) were anesthetized with ketamine hydrochloride (33 mg/kg im) and acepromazine (3.3 mg/kg im). Mice with a targeted deletion of exons 14–18 in the Abcc9 gene encoding SUR2 (SUR2 null mice) were generated as previously described (8, 9). Male and female mice (∼20 g) were euthanized with an overdose of pentobarbital sodium (130 mg/kg ip). Brains were removed and placed into oxygenated ice-cold (4°C) physiological saline solution (PSS) of the following composition: (in mM) 112 NaCl, 4.8 KCl, 24 NaHCO3, 1.8 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 10 glucose, which was gassed with 21% O2-5% CO2-74% N2 to pH 7.4. Piglet pial arterioles (100–250 μm in diameter) were dissected from the cerebral cortical surface. Murine posterior cerebral and cerebellar arteries (∼200 μm in diameter) were collected. Arterioles and arteries were cleaned of connective tissue. Individual smooth muscle cells were enzymatically dissociated from cerebral arterioles using a procedure previously described (17). Cells were maintained in ice-cold (4°C) isolation solution and used for experiments within 6 h.
H2S measurements using gas chromatography/mass spectrometry.
Detection and quantification of H2S released from Na2S and NaHS was made by comparison to standards with known concentrations of H2S. These standards were made by dilution from water saturated with H2S. Saturated H2S solution (10−1 M at 25°C) was made by placing water in a vial (50% of the volume) with the headspace filled with 100% H2S gas. The vial was vortexed well and the headspace gas replaced with 100% H2S three times at 5-min intervals with vortexing in between. The vial was then left for at least 30 min with intermittent vortexing to ensure equilibration between the gas and liquid. Aliquots of the H2S-saturated water were used to make standard curves. Because the total H2S in the vial and the volume of liquid are known, the mass spectral peak of the headspace gas would be the same as that of a 300-μl sample containing the same molarity as the standard. H2S in 20 μl of the headspace gas was identified and quantified by gas chromatography/mass spectrometry (GC/MS; 5975C series; Agilent Technologies, Santa Clara, CA). Gas chromatography was run on a 0.32 mm × 30 m fused silica HP-PLOT-U column (Agilent Technologies). The gas chromatography was programmed with an initial oven temperature of 45°C, a ramp to 75°C over 30 s, hold for 1 min, ramp to 85°C over 6 s, and hold for 4 min, before returning to 45°C. The carrier gas was H2 at 3.0 ml/min. H2S area counts against molarity (0.37, 0.74, 1.9, 3.7, 5.6, 9.2, and 18.5 μM in 300 μl buffer) were linear and passed through zero/zero (r2 = 0.998). H2S concentrations in Na2S samples were calculated based on the counts from known H2S samples freshly diluted from saturated stock for each experiment.
Different concentrations of Na2S (1, 10, and 100 μM) were made by dissolving Na2S in buffer containing (in mM) 134 NaCl, 6 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose (pH 7.4) at room temperature (25°C). Three hundred microliters of each Na2S sample concentration was placed immediately into a separate 2-ml air-tight vial. To obtain a time course of H2S production, each Na2S sample was vortexed well and kept upside down at 25°C for 1, 10, 20, or 40 min to allow H2S release from Na2S and equilibration of H2S between liquid and headspace gas.
Patch-clamp electrophysiology.
Isolated arteriole smooth muscle cells were placed into an experimental chamber containing Ca2+-free HEPES buffer and allowed to settle for 10 min. Potassium currents were measured using the whole cell patch-clamp configuration (Axopatch 200B and Clampex 9.2; Molecular Devices, Downingtown, PA). To minimize voltage-dependent K+ currents, KATP currents were recorded at a steady membrane potential of −50 mV using a continuous gap-free acquisition protocol. The pipette solution contained (in mM) 10 HEPES, 102 KCl, 38 KOH, 10 EGTA, 1 MgCl2, 1 CaCl2, 0.1 NaATP, 0.1 NaADP, 0.2 NaGTP, and 10 glucose (pH 7.2 with HCl). The calculated free Ca2+ concentration in this solution is 13 nM (WEBMAXC, Stanford University, Stanford, CA). The bath solution contained (in mM) 10 HEPES, 60 KCl, 80 NaCl, 1 MgCl2, 0.1 CaCl2, 1 tetraethylammonium, and 10 glucose (pH 7.4 with KOH) (Fig. 2, C and D) or (in mM) 10 HEPES, 140 KCl, 1 MgCl2, 0.1 CaCl2, 1 tetraethylammonium, and 10 glucose (pH 7.4 with KOH) (Fig. 2A, B and D). Data analysis was performed off-line using Clampfit 9.2 (Molecular Devices). The bath solution was continuously perfused to maintain constant H2S concentrations in the chamber.
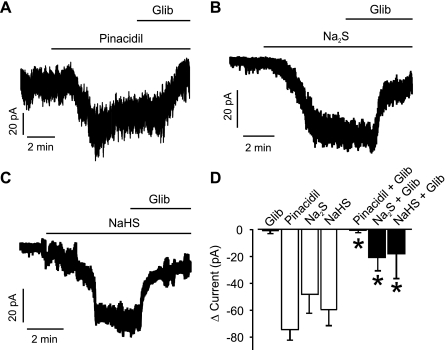
Fig. 2.
Piglet cerebral arteriole smooth muscle cells generate ATP-sensitive K+ (KATP) currents that are activated by H2S. Pinacidil (10 μM, A), Na2S (10 μM, B), and NaHS (20 μM, C) activate glibenclamide (Glib)-sensitive K+ currents. D: mean data: Glib, n = 4; pinacidil and pinacidil + Glib (10 μM), n = 6 for each; Na2S and Na2S + Glib (10 μM), n = 6 for each; and NaHS and NaHS + Glib (20 μM), n = 6 for each. All currents were recorded at a steady holding potential of −50 mV. *P < 0.05 compared with pinacidil, Na2S, or NaHS, respectively.
Pressurized artery diameter measurements.
An arteriole segment ∼2 mm in length was cannulated at each end in a temperature-controlled perfusion chamber (Living Systems Instrumentation, Burlington, VT). The chamber was continuously perfused with PSS, equilibrated with a mixture of 21% O2-5% CO2-74% N2, and maintained at 35°C. Arterioles were observed with a charge-coupled device camera attached to an inverted microscope (Nikon TE 200). Arteriole diameter was measured by using the automatic edge-detection function of IonWizard software (Ionoptix; Milton, MA) and digitized at 1 Hz using a personal computer. Steady-state changes in intravascular pressure were achieved by elevating and lowering an attached reservoir and monitored using a pressure transducer. Intraluminal PSS was static during experiments. Tested compounds were applied via chamber perfusion. The presence of intact endothelium was confirmed by observing bradykinin (10 nM)-induced vasodilation.
Western blot analysis.
To determine total protein, arteries were homogenized in a lysis buffer of the following composition: 50 mM Tris·HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 0.1% SDS. Cellular debris was removed by centrifugation at 8,000 rpm for 10 min. Samples were denatured by adding 5× Laemmli buffer containing 2-mercaptoethanol (2%) and boiled for 3 min. Total protein was loaded onto 7.5% SDS-polyacrylamide gels and transferred onto nitrocellulose membranes. Membranes were cut so that the same lysate could be probed for both Kir6.1 and SUR2B proteins. Membranes were incubated either with goat polyclonal anti-Kir6.1 (1:100, Santa Cruz Biotechnology, CA) or rabbit polyclonal anti-SUR2B (BNJ-40, 1:500, a kind gift from Dr. Jonathan Makielski, University of Wisconsin, Ref. 37) primary antibodies overnight at 4°C in Tris-buffered solution (TBS) containing 0.1% Tween 20 (TBS-T) and 5% nonfat dry milk. After being washed with TBS-T, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 2 h, followed by washing with TBS-T. The membranes were then developed using Supersignal West Pico chemiluminescent substrate (Thermo Scientific), and digital images were obtained using a Kodak FX Pro imaging system.
Chemicals.
Glibenclamide and pinacidil were purchased from Tocris (Ellisville, MO). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Data analysis.
Values are given as means ± SE. Student's t-test was used to compare two groups of paired or unpaired data, as appropriate. P < 0.05 was considered significant. Concentration-response curves were fit with a Boltzmann equation to obtain the half-maximal effective concentration (EC50) value.
RESULTS
H2S production from Na2S and reaction time course.
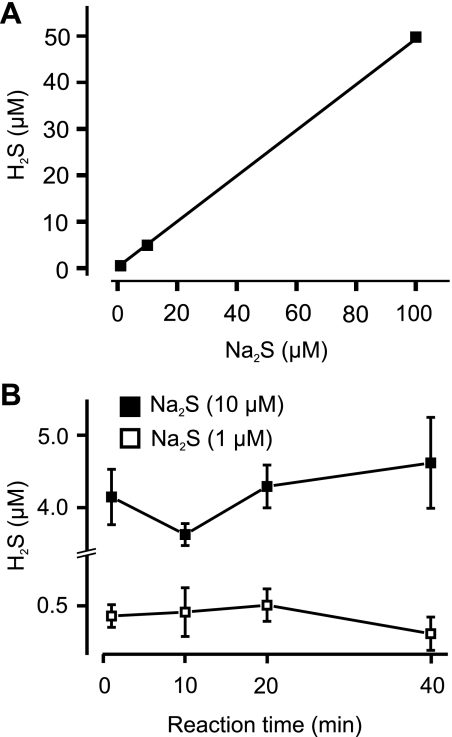
GC/MS was used to measure the amount and rate of H2S generation by Na2S, a H2S donor. With a pH of 7.4 and at a temperature of 25°C, Na2S concentrations of 1, 10, and 100 μM produced ∼0.47, 4.84, and 49.83 μM of gaseous H2S, respectively (Fig. 1A). The regression of H2S concentration versus Na2S concentration was linear (Fig. 1A). After Na2S was dissolved in HEPES-buffered PSS, H2S concentrations in the gas phase reached a maximum level by the first time point (1 min), and there was no significant difference when compared with longer reaction times (10, 20, and 40 min) (Fig. 1B). These data indicate that H2S production from Na2S is very fast with H2S reaching stable levels within 1 min of Na2S addition. Assuming a complete dissociation of Na2S, these data suggest that at pH 7.4 with 1.7 ml gas and 300 μl liquid, gaseous H2S constitutes almost 50% of the total sulfide.
Fig. 1.
Hydrogen sulfide (H2S) production by Na2S determined by gas chromatography/mass spectrometry of headspace gas. A: H2S production by Na2S (1–100 μM) fit with a regression function (n = 9, 12, 9 for each data point). Errors bars are provided for each data point but are small and occluded by the symbols. Mean ± SE values are 0.47 ± 0.04, 4.84 ± 0.43, and 49.83 ± 4.1 μM for 1, 10, 100 μM of Na2S, respectively. Experimental data points were fit with a linear regression function (Y = A + B × X), where X and Y are Na2S and H2S concentrations (in μM), respectively (R2 = 1), and A is the intercept and B the slope, giving A = −0.0843 ± 0.06327 and B = 0.4991 ± 0.0011. B: time course of H2S production from 1 or 10 μM Na2S (n = 3 to 4 for each data point).
H2S activates KATP currents in cerebral arteriole smooth muscle cells.
To test the hypothesis that KATP channels are a target of H2S, K+ currents were measured in isolated newborn piglet arteriole smooth muscle cells using the conventional whole cell patch-clamp configuration. Currents were measured at a physiological steady-state voltage of −50 mV using solutions designed to limit large-conductance Ca2+-activated K+ channel activity. With 140 mM [K+] in the bath solution, pinacidil (10 μM), a KATP channel activator, increased mean inward current by ∼75 pA (Fig. 2, A and D). Glibenclamide (10 μM), a KATP channel inhibitor, did not alter K+ currents when applied alone but abolished pinacidil-activated K+ currents (Fig. 2, A and D). Na2S (10 μM) increased mean inward current by ∼48 pA (Fig. 2, B and D). Subsequent application of glibenclamide reduced mean Na2S-induced K+ currents by ∼28 pA or by ∼58% (Fig. 2, B and D). Similar data were obtained when using NaHS, another H2S-releasing molecule, which activated whole cell K+ currents that were partially inhibited by glibenclamide (20 μM) (Fig. 2, C and D). These data indicate that two different H2S donors activate KATP currents in cerebral arteriole smooth muscle cells.
Piglet cerebral arterioles express Kir6.1 and SUR2B subunits and are regulated by KATP channel modulators.
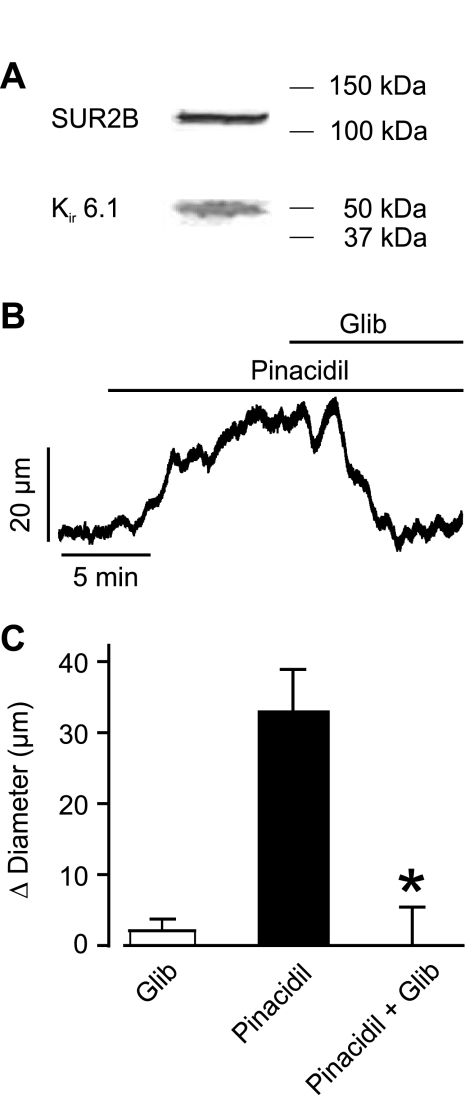
Expression of KATP channel subunits in piglet cerebral arterioles was unclear. Western blot analysis of piglet cerebral arteriole protein lysate revealed expression of both SUR2B (∼135 kDa mol mass) and Kir6.1 (∼51 kDa mol mass) subunits (Fig. 3A). We next studied the myogenic response of piglet cerebral arterioles. At 40 mmHg, arterioles constricted from a mean passive diameter of 239 ± 11 μm to a myogenic diameter of 138 ± 6 μm or by 42% (n = 21). To establish the functional significance of smooth muscle cell KATP channels containing SUR2B and Kir6.1 subunits, diameter responses to pinacidil were measured in myogenic arterioles. Pinacidil (10 μM) increased mean arteriole diameter by ∼33 μm (Fig. 3, B and C). Glibenclamide (10 μM) did not alter arteriole diameter when applied alone but fully reversed pinacidil-induced vasodilation (Fig. 3, B and C). These data indicate that smooth muscle cell KATP channel subunits are expressed and that KATP channels control diameter in piglet cerebral arterioles.
Fig. 3.
KATP current activation induces vasodilation in pressurized (40 mmHg) piglet cerebral arterioles. A: Western blot of piglet cerebral arteriole lysate. The top blot indicates sulfonylurea receptor 2B (SUR2B) at ∼135 kDa, whereas the bottom blot illustrates inwardly rectifying K+ 6.1 (Kir6.1) channel at ∼51 kDa. B: representative trace illustrating Glib (10 μM) reversal of pinacidil (10 μM)-induced vasodilation in a pressurized (40 mmHg) piglet cerebral artery. C: mean data: Glib (10 μM), pinacidil (10 μM), and pinacidil + Glib (10 μM) (n = 6 for each). *P < 0.05 compared with pinacidil.
Glibenclamide attenuates H2S-induced vasodilation in piglet cerebral arterioles.
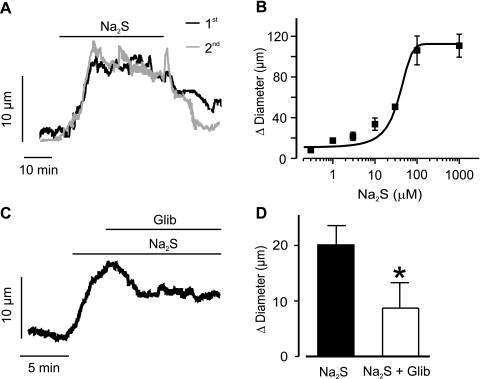
Physiological functions of H2S were studied in pressurized (40 mmHg) piglet cerebral arterioles. Na2S induced a mean vasodilation of ∼20 μm that was reversible upon washout (Fig. 4, A and C). Na2S-induced vasodilation was also repeatable (a second Na2S-induced vasodilation was 107 ± 20% of the vasodilation induced by a prior Na2S application, n = 5, P > 0.05, Fig. 4A). Performing a concentration-response curve to Na2S (0.1–1,000 μM) indicated an EC50 of 30.4 ± 5.3 μM (Fig. 4B). Na2S-induced vasodilation was partially (by ∼55%) reversed by glibenclamide (10 μM) (Fig. 4, C and D). In contrast, glibenclamide did not alter vasodilation induced by isoproterenol, an adrenergic receptor agonist and smooth muscle-specific vasodilator of piglet cerebral arterioles [Δdiameter: isoproterenol (1 μM), 74 ± 10 μm; isoproterenol + glibenclamide (10 μM), 78 ± 9 μm; n = 4, P > 0.05] (22, 28). These data indicate that H2S induces vasodilation via KATP channel-dependent and -independent mechanisms in isolated newborn cerebral arterioles. In contrast, isoproterenol causes vasodilation via KATP channel-independent mechanisms.
Fig. 4.
H2S dilates pressurized (40 mmHg) piglet cerebral arterioles by activating KATP currents. A: representative traces illustrating diameter responses to a first and second application of Na2S (10 μM) in the same pressurized piglet cerebral arteriole. B: concentration-dependent vasodilation induced by Na2S in pressurized piglet cerebral arteries (n = 2, 9, 3, 8, 7, 2, and 2 for each). Mean data are fit with a Boltzmann function. C: exemplar trace illustrating that Na2S (1 μM)-induced vasodilation is partially reversed by Glib (10 μM). D: mean data for Na2S (1 μM) and Na2S + Glib (10 μM) (n = 6 for each). *P < 0.05 when compared with Na2S.
Na2S- and pinacidil-induced vasodilation is attenuated in cerebral arteries of SUR2 null mice.
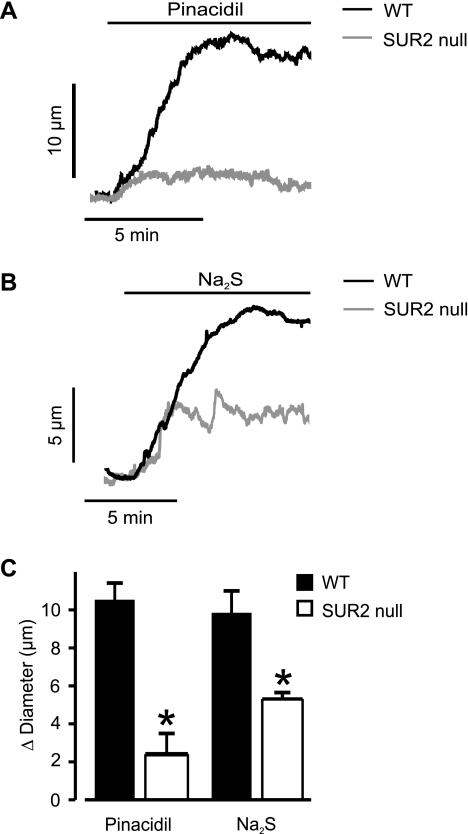
To investigate molecular targets of H2S-induced vasodilation, we used cerebral arteries of SUR2 null mice. Pressurized (60 mmHg) arteries from wild-type and SUR2 null mice developed similar levels of myogenic tone (wild-type, 17 ± 1%, n = 19; and SUR2 null mice, 15 ± 4%, n = 8, P > 0.05). Wild-type and SUR2 null mouse arteries also had similar mean passive diameters of 200 ± 5 (n = 19) and 196 ± 10 μm, respectively, as determined by removal of bath Ca2+ at 60 mmHg (n = 8, P > 0.05). Pinacidil and Na2S dilated pressurized wild-type cerebral arteries by ∼11 and 10 μm, respectively (Fig. 5). In contrast, pinacidil and Na2S dilated pressurized SUR2 null arteries by ∼2 and 5 μm, respectively (Fig. 5). Therefore, mean pinacidil- and Na2S-induced vasodilation was ∼18 and ∼50% of that in wild-type controls. These data indicate that pinacidil- and H2S-induced cerebral artery dilation occurs through plasma membrane KATP channels containing SUR2.
Fig. 5.
Pinacidil- and Na2S-induced vasodilation is attenuated in pressurized (60 mmHg) cerebral arteries of SUR2 null mice. A and B: representative traces illustrating pinacidil (5 μM)- and Na2S (5 μM)-induced vasodilation in cerebral arteries of wild-type (WT) and SUR2 null mice. C: mean data for pinacidil (5 μM; n = 15 for WT, n = 6 for SUR2 null) and Na2S (5 μM; n = 14 for WT, n = 4 for SUR2 null). *P < 0.05 compared with WT mice.
DISCUSSION
Novel findings of this study are as follows: 1) upon dissolution in aqueous medium, Na2S rapidly converts to gaseous H2S; 2) pinacidil- and glibenclamide-sensitive KATP currents are present in newborn piglet cerebral arteriole smooth muscle cells and regulate arteriole contractility; 3) piglet cerebral arterioles express Kir6.1 and SUR2B subunits; 4) exogenous H2S derived from Na2S or NaHS activates KATP currents in isolated piglet cerebral arteriole smooth muscle cells; 5) H2S dilates pressurized piglet cerebral arterioles by activating KATP channels; and 6) pinacidil- and H2S-induced vasodilations are attenuated in cerebral arteries from SUR2 null mice. These data indicate that H2S dilates cerebral arteries and arterioles in part by activating smooth muscle cell KATP channels containing SUR2 subunits.
In the majority of studies published to date, NaHS has been used as an exogenous H2S donor, with Na2S used to a lesser extent (5). Recently, phase 1 clinical trials in humans have used pharmaceutical grade Na2S for therapeutic treatment of myocardial infarction (31). Here, we used both Na2S and NaHS as H2S donors. These molecules are effective donors that contribute only micromolar Na+ as an additional atom and dilate similarly to H2S gas in vivo (27). For consideration of H2S generated, we used GC/MS of the headspace gas in a sealed vial. With inclusion of a gas phase, the amount dissolved in the liquid will be reduced. In the liquid phase there is dissolved H2S gas, HS− and S2− (H2S↔HS−↔S2−). The quantity of total sulfide in the vial will remain the same, but the reaction will be shifted to the left as H2S diffuses to the gas phase at equilibrium (equal partial pressures of H2S in gas and liquid). The amount in gas and liquid will be determined by the quantity of the gas space relative to the liquid quantity, the pH (lower pH moves reaction left), and temperature (assuming barometric pressure is constant). We used GC/MS to measure H2S in headspace gas to determine both rate of production and levels of H2S generated by Na2S. Our quantitative analysis results indicate that Na2S produces H2S quickly, reaching a maximum level in headspace gas within 1 min of being dissolved in pH 7.4 buffer. Based on these data, in the present study cells or pressurized arteries were continuously perfused with fresh donors during experiments to maintain constant H2S concentrations in experimental chambers. With the assumption that all Na2S was ionized, ∼50% was gaseous H2S at all Na2S concentrations tested between 1 and 100 μM. These measurements are somewhat higher than previous reports for dissolved H2S concentrations at neutral pH (33%) and pH 7.4 (40%) in physiological solutions at 25°C (14, 47). The difference could be explained by the experimental conditions. In these experiments the gas volume was 1,700 μl, the liquid volume was 300 μl, the temperature was constant, and the pH was 7.4. Under these conditions the total sulfide was distributed ∼50% between the liquid and gas.
Earlier studies and reviews have measured a variety of H2S concentrations in blood from different species: ∼10 μM H2S in Wistar rat blood, ∼50 μM H2S in Sprague-Dawley rat blood, and 10–100 μM H2S in human blood (11, 30, 51). Millimolar concentrations of H2S or H2S donors have typically been used to study cardiac and pulmonary protective effects of H2S (6, 23). Our data demonstrate that low micromolar H2S concentrations activate KATP currents in cerebral arteriole smooth muscle cells and induce vasodilation in cerebral arterioles and arteries. We found that 300 μM and 1 mM Na2S induce an alkaline shift in pH from 7.4 to 7.54 and 7.75, respectively, whereas lower concentrations of H2S donors did not alter pH. Therefore, physiological functions of H2S described here cannot be attributed to pH changes. However, such alterations in pH may explain some results in studies where high H2S concentrations have been used.
KATP channels regulate the contractility of a wide variety of smooth muscle cells, including those from coronary, pulmonary, mesenteric, and cerebral arteries, as well as bladder and airway (3, 23, 38). To date, no electrophysiological studies have identified KATP currents in newborn cerebral arteriole smooth muscle cells. Here, we demonstrate that glibenclamide alone did not alter baseline K+ currents or arteriole diameter, indicating that smooth muscle cell KATP channels do not contribute to baseline K+ currents or contractility in pressurized newborn cerebral arterioles. However, pinacidil activated K+ currents and dilated pressurized piglet cerebral arterioles and these effects were abolished by glibenclamide. Therefore, we provide the first direct evidence that KATP channels can generate K+ currents in newborn cerebral arteriole smooth muscle cells and that activation of these currents induces vasodilation.
H2S stimulates KATP currents in rat mesenteric artery and aortic smooth muscle cells and pancreatic β-cells (45). Our data are the first to demonstrate that H2S activates KATP currents in cerebral arteriole smooth muscle cells and dilates cerebral arterioles and arteries. Na2S dilated pressurized cerebral arterioles with an EC50 of ∼30 μM. In contrast, glibenclamide did not alter isoproterenol-induced vasodilation, indicating that KATP channels do not contribute to effects of this smooth muscle-specific vasodilator. Recently, using GC/MS, we measured basal H2S concentrations of ∼600 nM in piglet cortical surface cerebrospinal fluid (26). Dilator concentrations measured here in vitro are near the range we have also measured in cortical surface cerebrospinal fluid during hypercapnia (∼4 μM) (26). Similarly, in vivo dilation of pial arterioles to topical H2S had a threshold of <10 μM (26). Therefore, concentrations of H2S that induced dilation here are consistent with those in vivo.
H2S-induced KATP current activation and vasodilation were only partially reversed by glibenclamide at a concentration that fully blocked pinacidil-induced K+ currents and pinacidil-induced vasodilation. These data suggest that H2S can activate K+ channels other than KATP, an effect that would also contribute to vasodilation. Consistent with this conclusion, 4-aminopyridine, a voltage-gated K+ channel blocker, attenuated relaxation induced by millimolar NaHS in agonist-contracted coronary artery rings (6). XE991, a KCNQ channel blocker, also attenuated NaHS-induced relaxation in aortic rings (39). However, in vivo glibenclamide completely blocked pial arteriole dilation to topical H2S (26). The reasons for greater effectiveness of glibenclamide in blocking dilation to H2S in vivo than in vitro are not known. One explanation for the slightly different glibenclamide sensitivity may be the size of the arterioles studied in vivo (50 μm) and in vitro (200 μm). In addition, the relative contribution of KATP currents to vasodilation may depend on the H2S concentration used. Additional experiments will be required to test these hypotheses.
KATP channels are comprised of a Kir6.1/6.2 channel and a SUR1, 2A, or 2B subunit (15). H2S directly activates recombinant KATP channels composed of Kir6.1 and SUR1 subunits by interacting with NH2-terminal cysteine residues (18). However, arterial smooth muscle cell plasma membrane KATP channels are comprised of Kir6.1 and SUR2B subunits, as supported by data here and in previous studies (1, 40). In addition, smooth muscle cells likely employ signaling pathways that are distinct from those present in immortalized cells used to express recombinant proteins. Thus H2S-induced KATP channel activation in arterial smooth muscle cells may occur because of direct or indirect mechanisms (18, 45). KATP channels are located both on the plasma membrane and on mitochondria (12, 16). Glibenclamide inhibits both plasma membrane and mitochondrial KATP channels (49). Therefore, H2S may have activated arteriole smooth muscle cell K+ currents and induced vasodilation by activating plasma membrane and/or mitochondrial KATP channels, particularly since the effects of H2S on both KATP channels and mitochondrial function have been described (10, 35, 41). SUR2B does not appear to be a functional component of arterial smooth muscle cell mitochondrial KATP channels (1). Therefore, to study KATP channels activated by H2S, we measured the functional responses in cerebral arteries of SUR2 null mice. Vascular smooth muscle cell KATP currents are abolished in SUR2 null mice (19). We have previously studied vasoregulation by intravascular pressure and by different KATP channel openers in mesenteric arteries of SUR2 null mice (1), but to our knowledge this study is the first to examine functional responses in SUR2 null cerebral arteries. Data indicate that SUR2 null mice cerebral and mesenteric arteries develop similar levels of myogenic tone to wild-type controls, indicating that KATP channels do not contribute to the myogenic response (1). Here, we show that wild-type and SUR2 null mouse cerebral arteries dilate similarly to removal of bath Ca2+ and have similar passive diameters, consistent with data in mesenteric arteries. These data indicate that SUR2 null arteries do not exhibit generalized alterations in vasodilation. Pinacidil-induced vasodilation was significantly smaller, but not abolished, in SUR2 null cerebral arteries, consistent with data in mesenteric arteries where vasodilation to this KATP channel opener was 10% of that in wild-type controls (1). The mechanism for the residual dilation is unclear but suggests a SUR2-independent target for pinacidil in arterial smooth muscle cells. KATP channel-independent mechanisms proposed to mediate pinacidil-induced vasodilation include stimulation of plasma membrane Ca2+ extrusion, a reduction in contractile apparatus Ca2+ sensitivity, and inhibition of GTP-binding protein-coupled phosphatidylinositol turnover (2, 34). Data with SUR2 null arteries indicate that approximately half of the H2S-induced vasodilation occurs because of activation of arterial smooth muscle cell SUR2-containing KATP channels, results that support our evidence using pharmacological modulators. The H2S-induced vasodilation present in SUR2 null arteries is consistent with our data indicating that although glibenclamide fully blocked pinacidil-induced K+ current activation and vasodilation, H2S-induced K+ current activation and vasodilation were only partially reversed by glibenclamide. These findings indicate that H2S also activates K+ channels other than KATP, leading to vasodilation. Future studies are needed to identify additional K+ channel(s) activated by Na2S and whether Na2S also causes vasodilation through K+ channel-independent mechanisms.
Murine cardiac myocytes express one full-length (130 kDa) and three short (28, 55, and 68 kDa) SUR2 forms (37, 50). Full-length SUR2 contains two nucleotide-binding domains and together with Kir6.1 or -6.2 generate glibenclamide-sensitive KATP channels. All three SUR2 short forms lack nucleotide-binding domain 1. The 55 kDa form together with Kir6.2 generates glibenclamide-insensitive KATP currents in cardiac myocytes. SUR2 null mouse cardiac myocytes lack full-length SUR2 but express the 55 kDa form in addition to the other two short forms (37). It is unclear whether wild-type and SUR2 null mouse cerebral artery smooth muscle cells express SUR2 short forms. However, glibenclamide partially reversed H2S-induced vasodilation in wild-type arteries and H2S-induced vasodilation was attenuated in SUR2 null arteries. Therefore, our data indicate that H2S-induced vasodilation occurs because of the activation of KATP channels containing full-length SUR2B and Kir6.1. Whether SUR2 short forms contribute to the glibenclamide-insensitive component of H2S-induced vasodilation and the H2S-induced vasodilation present in SUR2 null cerebral arteries remains to be determined.
At pH 7.4, H2S is permeable to plasma membranes because its solubility in lipophilic solvents is about fivefold greater than in water (47). H2S dissociates in aqueous solution to make the anionic species HS−. Both H2S and HS− may react with many intracellular targets, including peroxynitrite, peroxide, superoxide, metalloproteins, and cytochrome c (36). Therefore, both H2S and HS− may participate in cerebral arteriole dilation.
In conclusion, our data indicate that functional plasma membrane KATP channels are expressed in newborn piglet cerebral arteriole smooth muscle cells and that H2S activates cerebral arteriole smooth muscle cell Kir6.1- and SUR2B-containing KATP channels, leading to vasodilation. Our data also suggest that K+ channels other than KATP also contribute to H2S-induced vasodilation.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-67061 and HL-94378 (to J. H. Jaggar), HL-34059 and HL42851 (to C. W. Leffler), and HL-0789326 (to E. M. McNally).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Edward S. Umstot for technical assistance with gas chromatography/mass spectrometry analysis.
REFERENCES
- 1. Adebiyi A, McNally EM, Jaggar JH. Sulfonylurea receptor-dependent and -independent pathways mediate vasodilation induced by ATP-sensitive K+ channel openers. Mol Pharmacol 74: 736–743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anabuki J, Hori M, Ozaki H, Kato I, Karaki H. Mechanisms of pinacidil-induced vasodilatation. Eur J Pharmacol 190: 373–379, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Bonev AD, Nelson MT. ATP-sensitive potassium channels in smooth muscle cells from guinea pig urinary bladder. Am J Physiol Cell Physiol 264: C1190–C1200, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 29: 312–316, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Med Chem 53: 6275–6286, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY, Huang Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol 53: 94–98, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest 110: 203–208, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chutkow WA, Samuel V, Hansen PA, Pu J, Valdivia CR, Makielski JC, Burant CF. Disruption of Sur2-containing KATP channels enhances insulin-stimulated glucose uptake in skeletal muscle. Proc Natl Acad Sci USA 98: 11760–11764, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elsey DJ, Fowkes RC, Baxter GF. Regulation of cardiovascular cell function by hydrogen sulfide (H2S). Cell Biochem Funct 28: 95–106, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res 84: 973–979, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med 47: 1346–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature 352: 244–247, 1991 [DOI] [PubMed] [Google Scholar]
- 17. Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol 281: C439–C448, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Jiang B, Tang G, Cao K, Wu L, Wang R. Molecular mechanism for H2S-induced activation of KATP channels. Antioxid Redox Signal 12: 1167–1178, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res 98: 682–689, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kitazono T, Faraci FM, Taguchi H, Heistad DD. Role of potassium channels in cerebral blood vessels. Stroke 26: 1713–1723, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Kleppisch T, Nelson MT. ATP-sensitive K+ currents in cerebral arterial smooth muscle: pharmacological and hormonal modulation. Am J Physiol Heart Circ Physiol 269: H1634–H1640, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Knecht KR, Milam S, Wilkinson DA, Fedinec AL, Leffler CW. Time-dependent action of carbon monoxide on the newborn cerebrovascular circulation. Am J Physiol Heart Circ Physiol 299: H70–H75, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kubo S, Doe I, Kurokawa Y, Kawabata A. Hydrogen sulfide causes relaxation in mouse bronchial smooth muscle. J Pharm Sci 104: 392–396, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Lee SW, Cheng Y, Moore PK, Bian JS. Hydrogen sulphide regulates intracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358: 1142–1147, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci USA 104: 17907–17908, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 300: H440–H447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol 300: H440–H447, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol 291: H2897–H2904, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol 100: 1065–1076, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Li L, Hsu A, Moore PK. Actions and interactions of nitric oxide, carbon monoxide and hydrogen sulphide in the cardiovascular system and in inflammation–a tale of three gases! Pharmacol Ther 123: 386–400, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Liu YH, Bian JS. Bicarbonate-dependent effect of hydrogen sulfide on vascular contractility in rat aortic rings. Am J Physiol Cell Physiol 299: C866–C872, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Masuzawa K, Asano M, Matsuda T, Imaizumi Y, Watanabe M. Possible involvement of ATP-sensitive K+ channels in the relaxant response of dog middle cerebral artery to cromakalim. J Pharmacol Exp Ther 255: 818–825, 1990 [PubMed] [Google Scholar]
- 34. Meisheri KD, Swirtz MA, Purohit SS, Cipkus-Dubray LA, Khan SA, Oleynek JJ. Characterization of K+ channel-dependent as well as -independent components of pinacidil-induced vasodilation. J Pharmacol Exp Ther 256: 492–499, 1991 [PubMed] [Google Scholar]
- 35. Pan TT, Feng ZN, Lee SW, Moore PK, Bian JS. Endogenous hydrogen sulfide contributes to the cardioprotection by metabolic inhibition preconditioning in the rat ventricular myocytes. J Mol Cell Cardiol 40: 119–130, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJ. Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491–R511, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Pu JL, Ye B, Kroboth SL, McNally EM, Makielski JC, Shi NQ. Cardiac sulfonylurea receptor short form-based channels confer a glibenclamide-insensitive KATP activity. J Mol Cell Cardiol 44: 188–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 77: 1165–1232, 1997 [DOI] [PubMed] [Google Scholar]
- 39. Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Crean CS, Luft FC, Huang Y, Schubert R, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28: 1875–1882, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Shi W, Cui N, Shi Y, Zhang X, Yang Y, Jiang C. Arginine vasopressin inhibits Kir6.1/SUR2B channel and constricts the mesenteric artery via V1a receptor and protein kinase C. Am J Physiol Regul Integr Comp Physiol 293: R191–R199, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Sivarajah A, McDonald MC, Thiemermann C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 26: 154–161, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Sowmya S, Swathi Y, Yeo AL, Shoon ML, Moore PK, Bhatia M. Hydrogen sulfide: regulatory role on blood pressure in hyperhomocysteinemia. Vascul Pharmacol 53: 138–143, 2010 [DOI] [PubMed] [Google Scholar]
- 43. Standen NB, Quayle JM. K+ channel modulation in arterial smooth muscle. Acta Physiol Scand 164: 549–557, 1998 [DOI] [PubMed] [Google Scholar]
- 44. Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J 206: 267–277, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin Exp Pharmacol Physiol 37: 753–763, 2010 [DOI] [PubMed] [Google Scholar]
- 46. Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol 572: 617–624, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Wang R. Toxic gas, lifesaver. Sci Am 302: 66–71, 2010 [DOI] [PubMed] [Google Scholar]
- 49. Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–362, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ye B, Kroboth SL, Pu JL, Sims JJ, Aggarwal NT, McNally EM, Makielski JC, Shi NQ. Molecular identification and functional characterization of a mitochondrial sulfonylurea receptor 2 splice variant generated by intraexonic splicing. Circ Res 105: 1083–1093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008–6016, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]