Abstract
Mice with three amino acid mutations in the calmodulin binding domain of type-2 ryanodine receptor ion channel (Ryr2ADA/ADA mice) have impaired intracellular Ca2+ handling and cardiac hypertrophy with death at an early age. In this report, the role of signaling molecules implicated in cardiac hypertrophy of Ryr2ADA/ADA mice was investigated. Calcineurin A-β (CNA-β) and nuclear factor of activated T cell (NFAT) signaling were monitored in mice carrying either luciferase transgene driven by NFAT-dependent promoter or knockout of CNA-β. NFAT transcriptional activity in Ryr2ADA/ADA hearts was not markedly upregulated at embryonic day 16.5 compared with wild-type but significantly increased at postnatal days 1 and 10. Ablation of CNA-β extended the life span of Ryr2ADA/ADA mice and enhanced cardiac function without improving sarcoplasmic reticulum Ca2+ handling or suppressing the expression of genes implicated in cardiac hypertrophy. Embryonic day 16.5 Ryr2ADA/ADA mice had normal heart weights with no major changes in Akt1 and class II histone deacetylase phosphorylation and myocyte enhancer factor-2 activity. In contrast, phosphorylation levels of Erk1/2, p90 ribosomal S6 kinases (p90RSKs), and GSK-3β were increased in hearts of embryonic day 16.5 homozygous mutant mice. The results indicate that an impaired calmodulin regulation of RyR2 was neither associated with an altered CNA-β/NFAT, class II histone deacetylase (HDAC)/MEF2, nor Akt signaling in embryonic day 16.5 hearts; rather increased Erk1/2 and p90RSK phosphorylation levels likely leading to reduced GSK-3β activity were found to precede development of cardiac hypertrophy in mice expressing dysfunctional ryanodine receptor ion channel.
Keywords: calmodulin, calcineurin/nuclear factor of activated T cell, class II histone deacetylases/myocyte enhancer factor 2, extracellular signal-regulated kinase, glycogen synthetase kinase 3
cardiac hypertrophy and heart failure are complex diseases that develop in response to physiological and pathological stimuli (13, 16, 20, 21, 28, 46). Physiological hypertrophy induced by exercise training is not associated with overt cardiac dysfunction and can be reversed by normal cardiac workloads. Physiological cardiac hypertrophy is thought to be largely controlled through activation of receptor tyrosine kinases that regulate protein synthesis via Akt/PKB signaling. In contrast, pathological hypertrophy occurs in response to prolonged mechanical stress or abnormal neurohormonal activation and has been studied in genetically modified mice subjected to transaortic banding and hormone stimuli. Pathological cardiac growth is triggered via signaling pathways that in many cases are activated by temporal imbalances in cellular Ca2+ concentration.
Pathological cardiac hypertrophy may be triggered via the entry of Ca2+ through canonical transient receptor potential channels (TRPCs) (8, 48) and by hormones that cause Ca2+ release from inositol-1,4,5-trisphosphate (IP3)-sensitive stores (13). Ca2+ plays a critical role in cardiac hypertrophy by binding calmodulin (CaM) and protein kinases C. One proposed pathway involves calcineurin (CN), a Ca2+/CaM-activated serine/threonine phosphatase that dephosphorylates nuclear factor of activated T cell (NFAT), a transcription factor implicated in pathological cardiac hypertrophy (20, 21). A second signaling mechanism involves the activation of Gq/11-coupled receptors that lead to the activation of phospholipase C-β and generation of diacylglycerol, an activator of protein kinases C, and IP3, which binds to its nuclear receptor to cause Ca2+ release in the nucleus and activation of nuclear CaMKIIδB. Phosphorylation of class II histone deacetylases (HDACs) by CaMKII and protein kinases C and D activates myocyte enhancer factor 2 (MEF2) that regulates cardiac gene expression (28).
Ryanodine receptors (RyR2) are large ion channels composed of four 560-kDa RyR subunits and four 12.6-kDa FK506 binding proteins (FKBP12.6). Multiple endogenous effectors that regulate RyR2 include Ca2+, Mg2+, ATP, and associated proteins such as protein kinases, phosphatases, and CaM (14, 15, 29). RyR2s are important for normal cardiac function by releasing Ca2+ from the sarcoplasmic reticulum (SR) required for muscle contraction. Aberrant function of RyR2 has been implicated in cardiac hypertrophy and heart failure. For example, PKA-mediated phosphorylation of RyR2 was reported to cause release of the small FKBP12.6 (calstabin 2), leading to a leaky SR Ca2+ channel, aberrant contractile function, and heart failure in animal models and patients (46). Abnormal SR Ca2+ handling and associated cardiac myopathies were also observed in patients and mice carrying RyR2 mutations associated with catecholaminergic polymorphic ventricular tachycardia (17). We identified three amino acid substitutions in the CaM binding domain of RyR2 (RyR2-W3587A/L3591D/F3603A or RyR2ADA) that eliminate inhibition of RyR2 activity by CaM at both diastolic and systolic Ca2+ concentrations (49). Homozygous mice expressing the CaM-defective mutant form of RyR2 (Ryr2ADA/ADA mice) had increased heart-to-body weight ratios at postnatal day 1 and died within 16 days of birth. Sustained Ca2+ transients revealed abnormal Ca2+ handling in cultured homozygous mutant cardiomyocytes. Biochemical studies indicated upregulation of genes or enzyme activities associated with class II histone deacetylase (class II HDAC)/MEF2 and calcineurin signaling in hearts of postnatal Ryr2ADA/ADA mice.
In the present study, we investigated whether the rapid and pronounced onset of cardiac hypertrophy and heart failure in Ryr2ADA/ADA mice results from early changes in gene expression. We report that neither calcineurin A-β (CNA-β)/NFAT, classII HDAC/MEF2, nor Akt signaling were significantly upregulated in embryonic hearts but that phosphorylation levels of Erk1/2, p90 ribosomal S6 kinases (p90RSKs), and GSK3 were increased in embryonic hearts. The data further indicate that ablation of CNA-β extended the life span and modestly improved cardiac contractility, which suggests that CNA-β and NFAT participate in but are not essential for cardiac hypertrophy in mice expressing a dysfunctional ryanodine receptor.
MATERIALS AND METHODS
Materials.
[3H]ryanodine was obtained from Perkin Elmer Life Sciences, and protease and phosphatase inhibitor cocktails were from Sigma. HDAC5 rabbit polyclonal antibody was from Biovision. pHDAC4-Ser246/HDAC5-Ser259/HDAC9-Ser220 rabbit polyclonal antibody was from GenScript. GSK-3α rabbit monoclonal antibody and GSK-3α-Ser21 rabbit polyclonal antibody were from Abcam. Erk1/2, p90RSK, Akt, and GSK-3β rabbit monoclonal antibodies were from Cell Signaling Technology. Chemicals were from Sigma-Aldrich unless specified otherwise.
Genetically modified mice.
Ryr2+/ADA mice (49) were mated with CNA-β+/− mice (7), and Ryr2+/ADA and Ryr2+/ADA/CNA-β+/− mice were each backcrossed at least five times to 129svev genetic background. Nine different genotypes of mice were obtained by crossing Ryr2+/ADA/CNA-β+/− mice according to Mendelian Law, out of which the four genotypes investigated were Ryr2+/+/CNA-β+/+ [wild-type (WT)], Ryr2+/+/CNA-β−/−, Ryr2ADA/ADA/CNA-β+/+, and Ryr2ADA/ADA/CNA-β−/−. Transgenic mice carrying the luciferase gene driven by the NFAT-dependent promoter (47) were crossed with Ryr2+/ADA mice (49). Resultant Ryr2+/ADA mice carrying the heterozygous transgene were mated with Ryr2+/ADA mice to obtain three genotypes (Ryr2+/+, Ryr2+/ADA, and Ryr2ADA/ADA) carrying the luciferase transgene. The luciferase reporter gene assay was performed using mice with a hybrid genetic background of 129svev and FVB. All experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Luciferase gene reporter assay.
Whole hearts (ventricles and atria) of 1- and 10-day-old mice and ventricles (hearts without atria) of E16.5 mice were homogenized in 2 mM EDTA, 1% Triton X-100, and 25 mM Tris-phosphate (pH 7.8) in the presence of complete protease inhibitors, using a glass homogenizer. Nonsolubilized material was sedimented at 17,000 g for 20 min. Luciferase activity was measured in replicate with 0.1-ml supernatant and 0.2-ml luciferase reading buffer containing 0.5 mM mono-potassium-D-luciferin salt (Thermo Fisher Scientific), 12.5 mM glycylglycine, 7.5 mM MgCl2, 2.5 mM ATP, and 0.25 mg/ml bovine serum albumin (pH 7.8) using an automated Lumistar Galaxy multiwell plate luminometer (BMG Labtech). Luciferase activity per milligram lysate protein was normalized to WT littermate controls.
Echocardiography.
To determine left ventricular cardiac function, transthoracic M-mode echocardiography was performed on restrained, unanesthetized 1- and 10-day-old mice, using Vevo 770 high resolution imaging system (VisualSonics) with a 40-MHz probe. To restrain mice, they were taped down gently to a warmed mouse board made by Indus Industries for VisualSonics.
Quantitative RT-PCR.
Gene expression was measured by quantitative RT-PCR using the ABI Prism 7700 Sequence Detector (Applied Biosystems) (23). RNA was isolated from left ventricles of 10-day-old mice or both left and right ventricles of mouse embryos with the ABI Prism 6700 Automated Nucleic Acid Workstation according to the manufacturer's protocol. Primers and corresponding fluorogenic probes for β-myosin heavy chain (β-MHC), atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β-actin genes were as described (9). Forward and reverse primers and fluorogenic probes of canonical TRPCs and CNA-α were as follows: TRPC1: 5′-GCATCTTCTGCGAACAGCAA-3′, 5′-GTACCAGAACAGAG CAAAGC-3′ and FaM-5′-TGACACCTTCCACTCGTTCATTGGCAC-3′-TAMRA; TRPC3: 5′-TTACTACCTTGGGGCCAAAG-3′, 5′-TATGGACCAAAACAAAGTCTTG-3′ and FaM-5′-AATCCTGC TTTTACCACGGTTGAAGAAAG-3′-TAMRA; TRPC6: 5′-TACTCCTACTACATTGGCGC-3′, 5′-GATAGCCCAGAACAGTGTCTT-3′, and FaM-5′-CAGAATGAAGCATTCACAACAGTTGAGGAAAG-3′-TAMRA; and CNA-α: 5′-GGAAACCTCGTGTGGATATC-3′, 5′-CTCAATGCAACACTTTCTTCC-3′, and FaM-5′-CCTGCCCTCCTTCATGAGATGTGCT-3′-TAMRA. Relative mRNA levels are expressed as percentage of WT using β-actin as reference.
Preparation of heart homogenates.
Whole hearts (ventricles and atria) of day 1 and day 10 mice and ventricles (hearts without atria) of E16.5 mice were homogenized using a Tekmar Tissumizer for 3 ×7 s at a setting of 13,500 rpm or glass homogenizer, respectively, in 20 mM imidazole (pH 7.0), 0.3 M sucrose, 0.15 M NaCl, protease and phosphatase inhibitor cocktails, 25 mM β-glycerophosphate, 5 mM NaF, and 2.5 mM NaVO4. Homogenates were stored in small aliquots at −80°C. Protein concentrations were determined using bicinchoninic acid assay.
Immunoblot analyses.
Homogenates (20 μg protein/lane) were separated by 10% SDS/PAGE, transferred to nitrocellulose membranes, and probed with rabbit monoclonal or polyclonal antibodies. Western blots were developed using enhanced chemiluminescence and quantified using ImageQuantTL Analysis Software. GAPDH was the loading control.
[3H]ryanodine binding and 45Ca2+ uptake rate.
Intracellular Ca2+ handling activities were measured in heart homogenates as described (49). Maximal number (Bmax) of specific [3H]ryanodine binding sites were determined by incubating homogenates for 4 h at 24°C with a nearly saturating concentration of 20 nM [3H]ryanodine in 20 mM imidazole (pH 7.0), 0.6 M KCl, 0.15 M sucrose, protease inhibitors, and 0.1 mM Ca2+. Nonspecific binding was determined using a 1,000 to 2,000 fold excess of unlabeled ryanodine.
ATP-dependent 45Ca2+ uptake rates by heart homogenates were determined by filtration (49). 45Ca2+ uptake was initiated by placing aliquots of homogenates for 2, 4, and 6 min in 0.15 M KCl, 20 mM imidazole (pH 7.0) containing 5 mM ATP, 8 mM MgCl2, 5 mM K-oxalate, a Ca2+ precipitating agent to increase Ca2+ uptake capacity, 10 μM ruthenium red to inhibit RyR2, 5 mM NaN3 to inhibit mitochondrial Ca2+ uptake, 1 mM EGTA, and Ca2+ and 45Ca2+ to yield a free Ca2+ concentration of 0.4 μM.
HDAC phosphorylation and MEF2 activity.
HDAC phosphorylation and MEF2 activity were determined in E16.5 heart (without atria) lysates as described (49) using GST-HDAC4 fusion protein (amino acids 419–670) (4) and Active Motif TransAM MEF2 kit (Carlsbad, CA), respectively.
Biochemical assays and data analyses.
Free Ca2+ concentrations were measured using a Ca2+ selective electrode.
Results are expressed as means ± SE. Multiple genotypes were compared by one-way ANOVA followed by Tukey's test. Differences between two genotypes were analyzed using Student's t-test. P < 0.05 was considered significant.
RESULTS
Echocardiography of 1-day-old Ryr2+/+ and Ryr2ADA/ADA mice.
To determine whether the RyR2ADA mutation altered cardiac function in 1-day-old mice, echocardiography was performed on conscious animals. Heart rates of Ryr2ADA/ADA mice (353 beats/min) were significantly slower than heterozygous (445 beats/min) or WT (490 beats/min) mice (Table 1). In agreement with our previous finding, heterozygous ablation does not have a profound effect because binding of CaM to one or two subunits in the heterotetrameric RyR2 mutant channel is sufficient for CaM inhibition (5, 49). Left ventricular end-diastolic and end-systolic dimensions were modestly increased in homozygous mutant mice (Supplemental Fig. S1), and fractional shortening obtained from the two parameters was significantly reduced in homozygous mutant mice (∼65% of WT; Table 1). None of the other left ventricular parameters were significantly different from WT mice (Table 1). The data show that cardiac function is altered in 1-day-old Ryr2ADA/ADA mice, which suggests that cardiac gene remodeling begins before postnatal day 1.
Table 1.
Echocardiography of 1-day-old WT and mutant mice
| Parameters | Ryr2+/+ | Ryr2+/ADA | Ryr2ADA/ADA |
|---|---|---|---|
| HR, beats/min | 490 ± 13 | 445 ± 31 | 353 ± 12* |
| LVEDD, mm | 1.29 ± 0.07 | 1.54 ± 0.09 | 1.61 ± 0.15 |
| LVESD, mm | 0.65 ± 0.08 | 0.76 ± 0.08 | 1.09 ± 0.13 |
| FS, % | 50.0 ± 5.0 | 51.2 ± 3.5 | 33.6 ± 2.3* |
| IVSD, mm | 0.44 ± 0.05 | 0.51 ± 0.03 | 0.48 ± 0.04 |
| IVSS, mm | 0.77 ± 0.12 | 0.82 ± 0.04 | 0.67 ± 0.04 |
| LVPWD, mm | 0.49 ± 0.09 | 0.40 ± 0.01 | 0.50 ± 0.05 |
| LVPWS, mm | 0.75 ± 0.08 | 0.76 ± 0.07 | 0.67 ± 0.04 |
Values are means ± SE; n = 3, 5, and 9 mice for Ryr2+/+, Ryr2+/ADA, and Ryr2ADA/ADA groups, respectively. WT, wild-type; HR, heart rate; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; FS, fractional shortening (LVEDD – LVESD)/LVEDD); IVSD, interventricular septum diastolic thickness; IVSS, interventricular septum systolic thickness; LVPWD, left ventricular posterior wall diastolic thickness; LVPWS, left ventricular posterior wall systolic thickness.
P < 0.05 compared with Ryr2+/+.
RyR2ADA is associated with upregulation of genes in embryonic day 16.5 hearts.
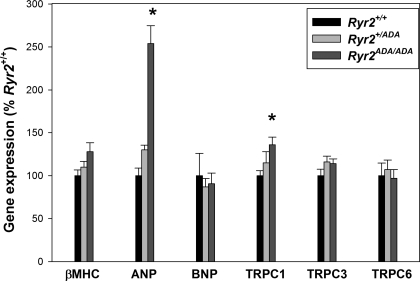
To confirm early changes in cardiac gene expression, quantitative RT-PCR was performed on embryonic day 16.5 (E16.5) Ryr2ADA/ADA hearts. As shown in Fig. 1, ANP mRNA levels increased 2.5-fold in hearts of E16.5 Ryr2ADA/ADA mice compared with WT, whereas expression of two other cardiac hypertrophy associated genes, β-MHC and BNP, was not significantly increased.
Fig. 1.
Quantitative RT-PCR of cardiac hypertrophy associated genes in embryonic day 16.5 (E16.5) hearts of ryanodine receptors (Ryr)2+/+, Ryr2+/ADA, and Ryr2ADA/ADA mice. mRNA levels were measured by quantitative RT-PCR and normalized to those of Ryr2+/+ [wild-type (WT)]. β-MHC, β-myosin heavy chain; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; TRPC, canonical transient receptor potential. Data are means ± SE of 10 samples. *P < 0.05 compared with Ryr2+/+.
Canonical TRPC channels TRPC1, TRPC3, and TRPC6 promote cardiomyocyte hypertrophy and heart failure through the activation of calcineurin/NFAT signaling (8, 24, 31, 33, 40, 48). mRNA expression levels of TRPC1 increased 1.35-fold in Ryr2ADA/ADA hearts, whereas TRPC3 and TRPC6 mRNA levels were unchanged (Fig. 1). At E16.5, heart protein content was unchanged [0.272 ± 0.012 mg for Ryr2ADA/ADA (n = 16) vs. 0.272 ± 0.010 mg for WT (n = 16)]. The results suggest that although embryonic Ryr2ADA/ADA heart protein content, and thus heart weight, was unchanged, there was evidence for gene expression changes as early as E16.5.
Class II HDAC/MEF2 signaling in E16.5 hearts.
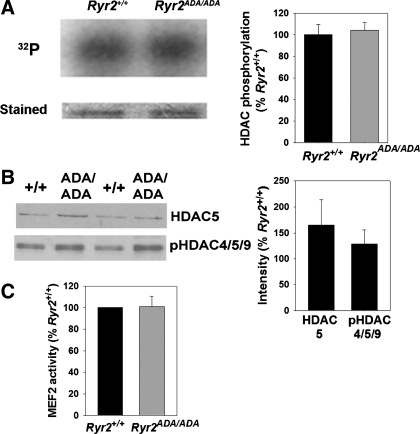
To determine whether class II HDAC/MEF2 signaling was upregulated in E16.5 Ryr2ADA/ADA hearts, in vitro phosphorylation of a recombinant GST-HDAC4 fusion protein with CaMKII and PKC/D phosphorylation sites Ser467 and Ser632 was measured (4). No difference was observed in the phosphorylation of HDAC4 in lysates from homozygous and WT hearts (Fig. 2A). In immunoblots, no significant differences were observed in protein expression level of HDAC5 and phosphorylation level of HDAC4-Ser246/HDAC5-Ser259/HDAC9-Ser220 (Fig. 2B). Moreover, MEF2 transcriptional activity in E16.5 Ryr2ADA/ADA heart lysates was not increased (Fig. 2C). The results suggest that class II HDAC/MEF2 signaling was not upregulated in E16.5 Ryr2ADA/ADA hearts.
Fig. 2.
Histone deacetylase (HDAC) phosphorylation and myocyte enhancer factor 2 (MEF2) activities in hearts of E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. A: [32P] incorporation (left, top) and Coomassie brilliant blue staining (left, bottom) of GST-HDAC4 fusion protein (amino acids 419–670). GST-HDAC4 fusion protein was a substrate for kinase activities of heart lysates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. [32P]radioactivity in GST-HDAC4 SDS-PAGE gel bands was determined by liquid scintillation counting (right). Data are means ± SE of 4 to 5 heart lysates. B: immunoblot analysis of HDAC5 and pHDAC4-Ser246/HDAC5-Ser259/HDAC9-Ser220. Aliquots of heart homogenates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice were separated by 10% SDS/PAGE, transferred to nitrocellulose membranes, and probed with rabbit polyclonal antibodies (left). Intensities of protein bands of E16.5 Ryr2ADA/ADA were normalized by comparing them with respective E16.5 Ryr2+/+ bands. GAPDH was used as loading control. Data are means ± SE of 6 WT and mutant samples each. C: MEF2 activity of heart lysates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice. HDAC4 kinase and MEF2 activities were normalized to those of Ryr2+/+ (WT). Data are means ± SE of experiments with 4 to 5 heart lysates.
NFAT-dependent luciferase activity of E16.5 and postnatal Ryr2ADA/ADA mice.
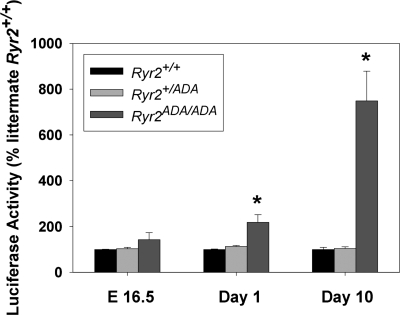
As part of a compensatory response, regulator of calcineurin 1 (Rcan1) is upregulated in hearts expressing a constitutively active form of calcineurin (50). We observed previously an increase in Rcan1 mRNA levels in 7- and 10-day-old but not in 1-day-old mutant hearts, which suggested a progressive increase in calcineurin signaling in postnatal Ryr2ADA/ADA hearts (49). To determine more directly the temporal changes of calcineurin/NFAT signaling, transgenic mice carrying the luciferase gene driven by NFAT-dependent promoter (47) were crossed with Ryr2+/ADA mice to obtain three genotypes (Ryr2+/+, Ryr2+/ADA, and Ryr2ADA/ADA) carrying the luciferase transgene. On days 1 and 10 of age, luciferase activities in homozygous Ryr2ADA/ADA hearts were 2 and 7.5 times higher than in WT hearts, respectively, with a modest but not significant change (1.4 times) at E16.5 (Fig. 3).
Fig. 3.
Nuclear factor of activated T cell (NFAT)-dependent luciferase activity of Ryr2+/+, Ryr2+/ADA, and Ryr2ADA/ADA mice. NFAT-dependent promoter driven luciferase activity was determined in hearts carrying NFAT-luciferase transgene as described in materials and methods. Luciferase activities of E16.5, 1-day-old, and 10-day-old mice were normalized to age-matched WT littermates. Data are means ± SE of 5 to 11 hearts. *P < 0.01 compared with WT at same age.
Knockout of CNA-β from Ryr2ADA/ADA mice.
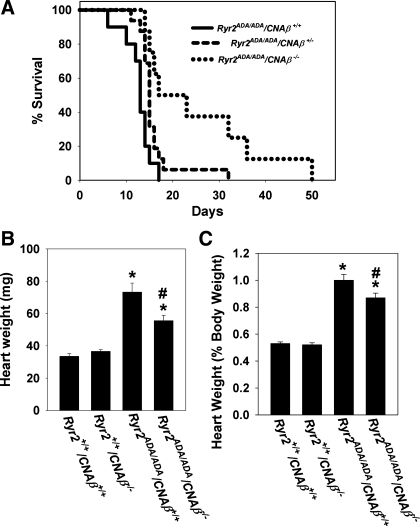
Calcineurin is composed of the 60-kDa catalytic subunit calcineurin A (CNA) and the 19-kDa regulatory subunit calcineurin B (CNB). Two isoforms of CNA, CNA-α and CNA-β, are expressed in cardiac muscle (37). Since previous work showed that catalytic CNA-β has a specific role in regulating hypertrophic growth in hearts (7, 42), Ryr2+/ADA mice were intercrossed with CNA-β knockout mice. Ryr2ADA/ADA/CNA-β+/+ mice died between 6 and 17 days after birth (Fig. 4A) and exhibited an approximately twofold increase in heart weight (Fig. 4B) and heart-to-body weight ratio (Fig. 4C) at day 10 compared with WT mice, as reported previously (49). Homozygous ablation of CNA-β from Ryr2ADA/ADA mice prolonged the life span to between 14 to 50 days (Fig. 4A) and reduced the heart weight (Fig. 4B) and heart-to-body weight ratio (Fig. 4C) by ∼25% and ∼13%, respectively. Heterozygous ablation of CNA-β in Ryr2ADA/ADA mice resulted in a modest (not significant) increase of life span (Fig. 4A) and reduction in heart weight (64.3 ± 2.9 vs. 73.2 ± 5.5 mg for Ryr2ADA/ADA/CNA-β+/+; n = 19–24) and heart-to-body weight ratio (0.91 ± 0.03 vs. 1.00 ± 0.03 for Ryr2ADA/ADA/CNA-β+/+; n = 19–24).
Fig. 4.
Phenotype of 10-day-old Ryr2ADA/ADA/CNA-β−/− mice. A: survival of Ryr2ADA/ADA mice. Mean survival ± SE in days for Ryr2ADA/ADA mice with CNA-β+/+ (12.7 ± 0.9, n = 10; solid line), CNA-β+/− (15.9 ± 1.1, n = 16; dashes), and CNA-β−/− (25.4 ± 4.6, P < 0.01 compared with CNA-β+/+, n = 8; dots). B: heart weights of 4 genotypes of 10-day-old mice. C: heart-to-body weight ratios of 4 genotypes of 10-day-old mice. B and C: data are means ± SE of 15–26 mice. *P < 0.01 compared with Ryr2+/+/CNA-β+/+ mice (WT); #P < 0.01 compared with Ryr2ADA/ADA/CNA-β+/+.
The ventricle function of 10-day-old Ryr2+/+/CNA-β+/+ (WT), Ryr2+/+/CNA-β−/−, Ryr2ADA/ADA/CNA-β+/+, and Ryr2ADA/ADA/CNA-β−/− mice was compared by conscious M-mode echocardiography. The homozygous RyR2ADA mutation decreased the heart rate of Ryr2ADA/ADA/CNA-β+/+ (490 beats/min) and Ryr2ADA/ADA/CNA-β−/− mice (479 beats/min) compared with WT (589 beats/min) (Table 2). In Table 2, Ryr2ADA/ADA/CNA-β+/+ mice had both left ventricular end-diastolic and end-systolic dimensions higher compared with WT, which resulted in significantly reduced fractional shortening (10.3% for Ryr2ADA/ADA/CNA-β+/+, 57% for WT mice). The result indicated dilated cardiomyopathy in Ryr2ADA/ADA/CNA-β+/+ mice.
Table 2.
Echocardiography of 10-day-old WT and mutant mice
|
Ryr2+/+ |
Ryr2ADA/ADA |
|||
|---|---|---|---|---|
| Parameters | CNA-β+/+ | CNA-β−/− | CNA-β+/+ | CNA-β−/− |
| HR, beats/min | 589 ± 21 | 607 ± 18 | 490 ± 18*† | 479 ± 4*† |
| LVEDD, mm | 1.59 ± 0.11 | 1.44 ± 0.06 | 3.28 ± 0.24*† | 2.46 ± 0.17*†‡ |
| LVESD, mm | 0.68 ± 0.06 | 0.67 ± 0.06 | 2.96 ± 0.25*† | 1.94 ± 0.15*†‡ |
| FS, % | 57.0 ± 2.0 | 54.2 ± 2.8 | 10.3 ± 1.3*† | 21.4 ± 0.9*†‡ |
| IVSD, mm | 0.83 ± 0.07 | 0.80 ± 0.07 | 0.84 ± 0.06 | 0.67 ± 0.08 |
| IVSS, mm | 1.21 ± 0.07 | 1.21 ± 0.07 | 0.97 ± 0.06 | 0.90 ± 0.11* |
| LVPWD, mm | 0.95 ± 0.04 | 0.73 ± 0.06 | 0.83 ± 0.08 | 0.81 ± 0.06 |
| LVPWS, mm | 1.28 ± 0.05 | 0.96 ± 0.09* | 0.90 ± 0.09* | 1.03 ± 0.09 |
Values are means ± SE; n = 8, 5, 7, and 4 mice for Ryr2+/+/CNA-β+/+, Ryr2+/+ /CNA-β−/−, Ryr2ADA/ADA/CNA-β+/+, and Ryr2ADA/ADA/CNA-β−/− groups, respectively.
P < 0.05 compared with Ryr2+/+/CNA-β+/+;
P < 0.05 compared with Ryr2+/+/CNA-β−/−;
P < 0.05 compared with Ryr2ADA/ADA/ CNA-β+/+.
Elimination of CNA-β from WT mice (Ryr2+/+/CNA-β−/−; Table 2) caused a moderate decrease in left ventricular posterior wall systolic thickness, without significantly altering the other parameters. By comparison, knockout of CNA-β from Ryr2ADA/ADA mice (Ryr2ADA/ADA/CNA-β−/−; Table 2) significantly reduced left ventricular end-diastolic and end-systolic dimensions. Fractional shortening caused by changes in left ventricular end-diastolic and end-systolic dimensions increased twofold (21.4% for Ryr2ADA/ADA/CNA-β−/− mice, 10.3% for Ryr2ADA/ADA/CNA-β+/+ mice) but was still less than the 57% value for WT. The results indicate that ablation of CNA-β modestly improves cardiac function and thereby likely extends the life span of Ryr2ADA/ADA mice.
Bmax values of [3H]ryanodine binding, which are a measure of RyR2 protein expression, and 45Ca2+ uptake rates, which are a measure of SR Ca2+ pump activity, were determined in 10-day-old heart homogenates of mice targeted for RyR2ADA and CNA-β knockout. Ryr2ADA/ADA/CNA-β+/+ heart homogenates had significantly reduced Bmax values of [3H]ryanodine binding (0.15 compared with 0.35 pmol/mg protein for WT) (Table 3), whereas 45Ca2+ uptake rates of Ryr2ADA/ADA/CNA-β+/+ hearts were not significantly reduced (1.48 compared with 1.75 nmol/mg protein/min for WT). Ablation of CNA-β from Ryr2ADA/ADA mice further reduced Bmax values of [3H]ryanodine binding and 45Ca2+ uptake rates modestly. Similar small changes in [3H]ryanodine binding and Ca2+ uptake activities were also observed in heart homogenates of Ryr2+/+/CNA-β−/− mice compared with WT, which suggests that the effects of deleting CNA-β were not specific to the mutant mice. The results suggest that although ablation of CNA-β from Ryr2ADA/ADA mice improved cardiac function, it did not re-establish RyR expression or SR Ca2+ pump activity to WT levels.
Table 3.
Calcium handling by membrane fractions isolated from 10-day-old hearts of mice double targeted for RyR2ADA and CNA-β
| Genotype | Bmax of [3H]ryanodine Binding, pmol/mg protein | 45Ca2+ Uptake Rate, nmol·mg protein−1·min−1 |
|---|---|---|
| Ryr2+/+ | ||
| CNA-β+/+ (WT) | 0.35 ± 0.04 | 1.75 ± 0.19 |
| CNA-β−/− | 0.29 ± 0.05 | 1.64 ± 0.27 |
| Ryr2ADA/ADA | ||
| CNA-β+/+ | 0.15 ± 0.02* | 1.48 ± 0.22 |
| CNA-β−/− | 0.12 ± 0.01* | 1.38 ± 0.18 |
Values are means ± SE of 5–7 experiments.
P < 0.05 compared with Ryr2+/+/ CNA-β+/+.
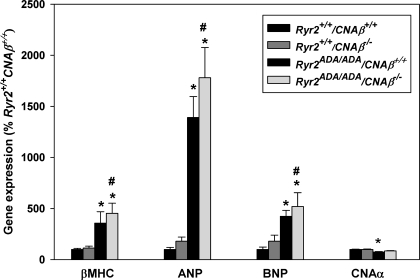
The effects of deleting CNA-β from WT and mutant mice on mRNA levels of several cardiac hypertrophy associated genes were examined. In agreement with our previous finding (49), quantitative RT-PCR showed that mRNA levels for β-MHC, ANP, and BNP were upregulated in 10-day-old Ryr2ADA/ADA/CNA-β+/+ mice compared with WT (Fig. 5). Knockout of CNA-β in 10-day-old WT and Ryr2ADA/ADA mice did not significantly alter expression of β-MHC, ANP, and BNP. mRNA levels of the second cardiac isoform CNA-α were modestly downregulated in Ryr2ADA/ADA/CNA-β+/+, whereas no differences were observed in the expression levels between WT and Ryr2ADA/ADA/CNA-β−/− mice. Thus genes implicated in cardiac hypertrophy were found to be upregulated in Ryr2ADA/ADA mice even in the absence of CNA-β.
Fig. 5.
Quantitative RT-PCR of mRNAs from 10-day-old mice double targeted for RyR2ADA and CNA-β. mRNA levels were measured by quantitative RT-PCR and were normalized to those of Ryr2+/+/CNA-β+/+ (WT). Data are means ± SE of 7–11 left ventricles. *P < 0.05 compared with WT mice; #P < 0.05 compared with Ryr2+/+/CNA-β−/−.
Other signaling pathways associated with cardiac hypertrophy.
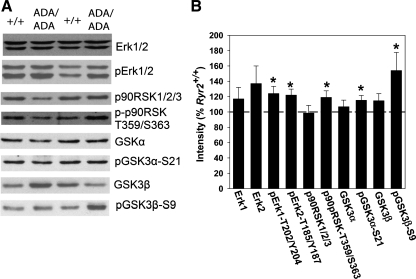
The phosphorylation status of GSK-3α and -β and upstream protein kinases Akt1 and p90RSKs were examined in E16.5 Ryr2ADA/ADA hearts. GSK3 is a serine/threonine kinase with two structurally similar isoforms, α and β. An unusual feature of these kinases is that phosphorylation reduces their activity. Studies with knock-in mice suggest that phosphorylation of GSK-3β-Ser9 leads to pathological cardiac hypertrophy during pressure overload, whereas phosphorylation of GSK-3α-Ser21 has a compensatory role (27). We observed a significant 1.5-fold increase in GSK-3β-Ser9 phosphorylation and a more modest (1.15-fold, significant) increase in GSK-3α-Ser21 phosphorylation in E16.5 Ryr2ADA/ADA hearts compared with WT (Fig. 6, A and B). Total GSK-3β expression levels were unchanged. The results suggest that enhanced phosphorylation of GSK-3β may contribute to increased cardiac growth of postnatal mutant mice.
Fig. 6.
Immunoblot analysis of Erk, p90RSK, and GSK3 in E16.5 heart homogenates. A: aliquots of heart homogenates from E16.5 Ryr2+/+ and Ryr2ADA/ADA mice were separated by 10% SDS/PAGE, transferred to nitrocellulose membranes, and probed with rabbit monoclonal or polyclonal antibodies. B: intensities of protein bands of Ryr2ADA/ADA were normalized by comparing them with respective E16.5 Ryr2+/+ bands. GAPDH was used as loading control. Data are means ± SE of 6–18 WT and mutant samples each. Dotted line indicates 100% (intensity of WT). *P < 0.05 compared with E16.5 Ryr2+/+ (WT).
GSK-3β is phosphorylated and negatively regulated by a multitude of kinases including Akt1 and p90RSKs (13, 19, 41). Of the two Akt isoforms expressed in heart, Akt1 regulates normal growth and Akt2 influences insulin signaling (10–12). Phosphorylation at Thr308 and Ser473 leads to activation of Akt1 (18). Phosphorylation of Thr308 in Ryr2ADA/ADA hearts was slightly downregulated at E16.5 and unchanged at Akt1-Ser473 (not shown). Furthermore, expression levels of pan Akt, or Akt1 alone, were not significantly altered (not shown). The data suggest that Akt1 phosphorylation is not a major early factor contributing to the phosphorylation and inhibition of GSK-3β.
p90RSKs are a group of kinases that regulate diverse cellular functions including cellular growth, survival, and differentiation (1). They lie downstream of Erk1/2 and upstream of GSK-3β (13, 19, 41). Immunoblot analysis indicated increased phosphorylation levels at T202 and Y204 of Erk1, T185 and Y187 of Erk2 and T359 and S363 of p-p90RSK (Fig. 6, A and B), i.e., sites implicated in their activation (1, 35). Total Erk1/2 and p90RSK1/2/3 expression levels were not significantly altered. Thus an early activation of the Erk/p90RSK signaling pathway may have a role in inhibiting GSK-3β activity in E16.5 Ryr2ADA/ADA mice.
DISCUSSION
The present study makes use of several mouse models expressing dysfunctional cardiac ryanodine receptor with and without disruption of CNA-β expression to investigate the role of signaling molecules implicated in cardiac hypertrophy. We report that calcineurin/NFAT signaling, previously thought to have a critical role in pathological cardiac hypertrophy, is not significantly upregulated in embryonic Ryr2ADA/ADA hearts and is not essential for cardiac hypertrophy in homozygous mice expressing a ryanodine receptor with mutations in the CaM binding domain that abrogate CaM binding. In addition, the studies suggest that class II HDAC/MEF2 signaling, another extensively studied signaling mechanism implicated in cardiac hypertrophy, is not upregulated in embryonic mutant hearts. In contrast, the data provide evidence that the Erk/p90RSK signaling pathway likely leading to reduced GSK-3β activity may have a role in promoting the development of cardiac hypertrophy in postnatal Ryr2ADA/ADA mice.
Cardiac hypertrophy is a complex process involving multiple signaling pathways that appear to initiate during the embryonic life of Ryr2ADA/ADA mice. Echocardiography indicated that chamber dilation of the left ventricle and decreased fractional shortening were less severe in 1-day-old compared with 10-day-old mice. Even though heart weight was unchanged between WT and Ryr2ADA/ADA E16.5 mice, there was evidence for upregulation of genes and enzymatic activities associated with cardiac hypertrophy in embryonic Ryr2ADA/ADA hearts. Thus early changes in gene expression in the embryonic heart may contribute to the rapid and progressive cardiac growth response in postnatal Ryr2ADA/ADA mice.
To test for temporal changes in calcineurin and NFAT signaling, Ryr2ADA/ADA mice were created that carried the luciferase transgene driven by the NFAT-dependent promoter. NFAT-dependent reporter activity is known to respond to stimuli that induce pathological hypertrophy, but not to stimuli associated with physiological cardiac growth (47). In a previous study, the hearts of transgenic mice carrying the luciferase gene driven by NFAT-dependent promoter had a maximal basal NFAT-luciferase activity at postnatal day 3 (47). We observed in hearts of E16.5 Ryr2ADA/ADA mice a small increase in NFAT-luciferase activity, which was significantly upregulated in 1- and 10-day-old Ryr2ADA/ADA mice. Thus an increased calcineurin signaling may have contributed to the rapid progression of cardiac hypertrophy in postnatal Ryr2ADA/ADA mice.
Given that calcineurin/NFAT signaling was reported to be both necessary and sufficient for pathological cardiac hypertrophy (7), it was conceivable that Ryr2ADA/ADA mice lacking CNA-β would show a reduced hypertrophic growth response. Earlier studies demonstrated that CNA-β null mice are viable and do not exhibit increased heart weight when subjected for 14 days to transaortic banding or infusion with angiotensin II or isoproterenol (7). Increases in mRNA and protein levels of CNA-β, but not CNA-α, in response to pressure overload or agonist stimulation, suggested a specific role for CNA-β in the hypertrophic growth response in hearts (42). The present study shows that ablation of CNA-β from Ryr2ADA/ADA mice improved overall cardiac function, albeit to a level less than WT. Moreover, ablation of CNA-β did not compensate for the reduced expression of RyR2 and SR Ca2+ pump-mediated Ca2+ uptake activity in 10-day-old Ryr2ADA/ADA hearts. The data suggest that calcineurin/NFAT signaling contributes but is not essential for the cardiac hypertrophic phenotype in Ryr2ADA/ADA mice.
Our results show that ablation of CNA-β from Ryr2ADA/ADA mice failed to reduce mRNA expression levels of genes implicated in cardiac hypertrophy such as β-MHC, ANP, and BNP. This finding is consistent with the reported elevated ANP and β-MHC mRNA levels in CNA-β null mice subjected for 14 days to angiotensin II infusion (7) and suggests that the increased expression of ANP in E16.5 hearts of Ryr2ADA/ADA mice is independent of CNA-β. One possible explanation is a compensatory upregulation of CNA-α in Ryr2ADA/ADA/CNA-β−/− mice. However, this seems unlikely because luciferase activity measurements showed that NFAT-mediated transcriptional activity was only slightly increased in E16.5 Ryr2ADA/ADA hearts in which the expression level of ANP was upregulated. We also note that to perform reporter measurements with the double targeted Ryr2ADA/ADA/CNA-β−/− littermates would require exceptionally large numbers of embryonic hearts. Another approach could have been to delete both calcineurin isoforms in Ryr2ADA/ADA mice; however, this was expected to cause the early death of the mice (26, 34).
In adult hearts, expression of β-MHC, ANP, and BNP are increased in cardiac hypertrophy. In agreement with an induction of genes for β-MHC, ANP, and BNP in response to pathological stimuli in postnatal hearts, increased mRNA levels of the three genes in hearts of 10-day-old Ryr2ADA/ADA mice were observed. In contrast, only ANP mRNA levels were significantly increased in hearts of embryonic Ryr2ADA/ADA mice. Different onsets for upregulation of the genes could be explained by structural difference in their promoters. Structural and functional analyses have revealed an ANP regulatory region for AP-1 and serum response factor (SRF), whereas β-MHC and BNP expression are regulated through M-CAT elements (22, 44). It will be of interest to evaluate in future experiments the expression levels of these transcription factors in embryonic and postnatal mutant mice.
In pathological cardiac hypertrophy, phosphorylation of class II HDACs by Ca2+/CaM-dependent protein kinase II and protein kinases C and D results in their nuclear export and derepression of MEF2 signaling (3, 6, 28, 45, 51). Previously, we showed that the phosphorylation status of HDAC4 and MEF2 activity increased in heart lysates of 1-day-old Ryr2ADA/ADA mice (49). In the present study, MEF2 activity was not increased in heart lysates of E16.5 Ryr2ADA/ADA mice. Moreover, no increase was observed in the in vitro phosphorylation of a HDAC4 fusion protein, which suggests that Ca2+/CaM-dependent protein kinase II and protein kinases C and D activities were unchanged in E16.5 Ryr2ADA/ADA hearts. We also found that Akt1 phosphorylation, thought to be associated with physiological cardiac hypertrophy, was unchanged in heart homogenates of E16.5 Ryr2ADA/ADA mice. The results suggest that changes in other signaling molecules lead to the early upregulation of genes implicated in cardiac hypertrophy of Ryr2ADA/ADA mice.
Immunoblot analysis suggests that cardiac hypertrophy in Ryr2ADA/ADA mice likely resulted in part from an early increased GSK-3β phosphorylation. A 1.5-fold increase of GSK-3β-Ser9 phosphorylation with an only small (1.15-fold) increase in GSK-3α-Ser21 phosphorylation was observed in E16.5 Ryr2ADA/ADA hearts. By removing a negative control on cardiac hypertrophy, phosphorylation of GSK-3β-Ser9 stimulates pathological cardiac growth response during pressure overload (27). GSK-3β is phosphorylated by a multitude of kinases including Akt and p90RSK (13, 19, 41). Our results suggest that the Erk/p90RSK signaling pathway may have a role in inhibiting GSK-3β activity. Conversely, lack of substantial increase in Akt phosphorylation argued against a major role for Akt1 in inhibiting GSK-3β activity. Phosphorylation of GSK-3β derepresses mediators of cardiac hypertrophy including GATA4 (30) and NFAT (2), which could explain why ablation of CNA-β only marginally affects cardiac hypertrophy of Ryr2ADA/ADA mice. Further studies will be required to determine how a defective SR Ca2+ release regulates signaling pathways leading to the early phosphorylation and inhibition of GSK-3β in embryonic Ryr2ADA/ADA mice.
Embryonic hearts differ from adult hearts in gene expression profile, cell-cell interactions, and Ca2+ handling (25, 32, 36, 38, 39). Mutant mice lacking RyR2 die at approximately embryonic day 10 with morphological abnormalities in the heart tube (43). Functional RyR2s were detected in cardiomyocytes as early as embryonic days 9 to 10 (36, 38), supporting a role for RyR2 in embryonic heart. Thus a key feature of Ryr2ADA/ADA mice is that they are likely subjected to aberrant SR Ca2+ release at embryonic age earlier than 16.5 days. Neither CNA-β/NFAT nor HDAC4/MEF2 activities were substantially upregulated at embryonic day 16.5 in the mutant mice. Rather, our data suggest that the Erk/p90RSK signaling pathway likely leading to reduced GSK-3β activity may have a role in promoting the development of cardiac hypertrophy in postnatal Ryr2ADA/ADA mice. The identity of additional mechanisms linking impaired SR Ca2+ release to abnormal myocardial growth in Ryr2ADA/ADA mice remains to be established.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-073051.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Jackie Kylander for the echocardiography, Hyung-Suk Kim for RNA analysis, and Johannes Backs and Eric Olson for providing GST-HDAC fusion protein. Present address of N. Yamaguchi: Department of Regenerative Medicine and Cell Biology, Medical University of South Carolina, Charleston, SC 29425.
REFERENCES
- 1. Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9: 747–758, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 99: 907–912, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res 98: 15–24, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Backs J, Song K, Bezprozvannaya S, Chang S, Olsen EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest 116: 1853–1864, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276: 20144–20153, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase IIdelta and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ Res 102: 695–702, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN, Molkentin JD. Impaired cardiac hypertrophic response in calcineurin Abeta-deficient mice. Proc Natl Acad Sci USA 99: 4586–4591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem 281: 33487–33496, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Caron KM, James LR, Kim HS, Knowles J, Uhlir R, Mao L, Hagaman JR, Cascio W, Rockman H, Smithies O. Cardiac hypertrophy and sudden death in mice with a genetically clamped renin transgene. Proc Natl Acad Sci USA 101: 3106–3111, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352, 2001 [DOI] [PubMed] [Google Scholar]
- 12. DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Dorn 2nd GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest 115: 527–537, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77: 699–729, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Frey N, Olson EN. Cardiac hypertrophy: the good, the bad and the ugly. Annu Rev Physiol 65: 45–79, 2003 [DOI] [PubMed] [Google Scholar]
- 17. George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol 42: 34–50, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett 492: 199–203, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hardt SE, Sadoshima J. Glycogen synthase kinase-3β. A novel regulator of cardiac hypertrophy and development. Circ Res 90: 1055–1063, 2002 [DOI] [PubMed] [Google Scholar]
- 20. Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin and NFAT. Genes Dev 17: 2205–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Kariya K, Karns LR, Simpson PC. An enhancer core element mediates stimulation of the rat beta-myosin heavy chain promoter by an alpha 1-adrenergic agonist and activated beta-protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem 269: 3775–3782, 1994 [PubMed] [Google Scholar]
- 23. Kim HS, Lee G, John SWM, Maeda N, Smithies O. Molecular phenotyping for analyzing subtle genetic effects in mice: application to an angiotensinogen gene titration. Proc Natl Acad Sci USA 99: 4602–4607, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116: 3114–3126, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu W, Yasui K, Opthof T, Ishiki R, Lee JK, Kamiya K, Yokota M, Kodama I. Developmental changes of Ca2+ handling in mouse ventricular cells from early embryo to adulthood. Life Sci 71: 1279–1292, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, Lorenz JN, Robbins J, Ventura-Clapier R, Molkentin JD. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J Biol Chem 285: 6716–6724, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA 105: 20900–20905, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKinsey TA. Derepression of pathological cardiac genes by members of the CaM kinase superfamily. Cardiovasc Res 73: 667–677, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium 35: 621–628, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Morisco C, Seta K, Hardt SE, Lee Y, Vatner SF, Sadoshima J. Glycogen synthase kinase 3beta regulates GATA4 in cardiac myocytes. J Biol Chem 276: 28586–28597, 2001 [DOI] [PubMed] [Google Scholar]
- 31. Nakayama H, Wilkin BJ, Bodi I, Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. FASEB J 20: 1660–1670, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nemer M. Genetic insights into normal and abnormal heart development. Cardiovasc Pathol 17: 48–54, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, Mori Y, Nagao T, Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J 25: 5305–5316, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parsons SA, Wilkins BJ, Bueno OF, Molkentin JD. Altered skeletal muscle phenotypes in calcineurin Aalpha and Abeta gene-targeted mice. Mol Cell Biol 23: 4331–4343, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J 10: 885–892, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rapila R, Korhonen T, Tavi P. Excitation-contraction coupling of the mouse embryonic cardiomyocyte. J Gen Physiol 132: 397–405, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483–1521, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J Gen Physiol 130: 133–144, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal, and postnatal development of Ca2+ transients and Ca2+ sparks in rat cardiomyocytes. Cardiovasc Res 58: 535–548, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res 105: 1023–1030, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol 153: S137–S153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA 97: 1196–1201, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J 17: 3309–3316, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Temsah RM, Nemer M. GATA factors and transcriptional regulation of cardiac natriuretic peptide genes. Regul Pept 128: 177–185, 2005 [DOI] [PubMed] [Google Scholar]
- 45. Vega RB, Harrison BC, Meadows E, Roberts CR, Papst PJ, Olson EN, McKinsey TA. Protein kinases C and D mediate agonist-dependent cardiac hypertrophy through nuclear export of histone deacetylase 5. Mol Cell Biol 24: 8374–8385, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wehrens XH, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol 67: 69–98, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Wu X, Eder P, Chang B, Molkentin JD. TRPC channels are necessary mediators of pathologic cardiac hypertrophy. Proc Natl Acad Sci USA 107: 7000–7005, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamaguchi N, Takahashi N, Xu L, Smithies O, Meissner G. Early cardiac hypertrophy in mice with impaired calmodulin regulation of cardiac muscle Ca2+ release channel. J Clin Invest 117: 1344–1353, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS. Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res 87: E61–E68, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIdelta isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J Biol Chem 282: 35078–35087, 2007 [DOI] [PubMed] [Google Scholar]