Abstract
A significant proportion of heart failure patients develop skeletal muscle wasting and cardiac cachexia, which is associated with a very poor prognosis. Recently, myostatin, a cytokine from the transforming growth factor-β (TGF-β) family and a known strong inhibitor of skeletal muscle growth, has been identified as a direct mediator of skeletal muscle atrophy in mice with heart failure. Myostatin is mainly expressed in skeletal muscle, although basal expression is also detectable in heart and adipose tissue. During pathological loading of the heart, the myocardium produces and secretes myostatin into the circulation where it inhibits skeletal muscle growth. Thus, genetic elimination of myostatin from the heart reduces skeletal muscle atrophy in mice with heart failure, whereas transgenic overexpression of myostatin in the heart is capable of inducing muscle wasting. In addition to its endocrine action on skeletal muscle, cardiac myostatin production also modestly inhibits cardiomyocyte growth under certain circumstances, as well as induces cardiac fibrosis and alterations in ventricular function. Interestingly, heart failure patients show elevated myostatin levels in their serum. To therapeutically influence skeletal muscle wasting, direct inhibition of myostatin was shown to positively impact skeletal muscle mass in heart failure, suggesting a promising strategy for the treatment of cardiac cachexia in the future.
Keywords: cardiac cachexia, activin-a, activin receptor IIB
muscle wasting as part of a syndrome called “cardiac cachexia” is a common complication of chronic heart failure (CHF). Heart failure is defined as deficiency in the capability of the heart to adequately pump blood in response to systemic demands, which results in premature fatigue, dyspnea, and edema. In the Western world, heart failure most commonly develops as a sequel of myocardial infarction or cardiac pressure overload resulting from chronic arterial hypertension. The prevalence of heart failure is rising, and, in the United States alone, more than five million people suffer from this dreadful disease.
In this review, we will briefly discuss cardiac cachexia and different potential mediators, before we focus on myostatin, a cytokine from the transforming growth factor (TGF)-β family that is released from the myocardium and that induces skeletal muscle wasting during heart failure.
Muscle Wasting and Cardiac Cachexia
CHF is associated with significant morbidity and mortality, which was reported to be as high as 50% within four years after diagnosis (22). Weight loss and body wasting, commonly termed as cachexia, are serious complications of many chronic diseases, like cancer, acquired immune deficiency syndrome, chronic obstructive pulmonary disease, and advanced CHF. Cardiac cachexia in CHF is an independent risk factor of mortality (10). Once cardiac cachexia has been diagnosed, the mortality of CHF patients increases dramatically. The 18-months mortality of CHF patients with cachexia was found to be 50% compared with 17% in noncachectic patients (10). Cachectic CHF patients not only show a wasting of muscle mass, but also a loss of total fat mass and a reduction in bone mineral density (11). The prevalence of cardiac cachexia is ∼15%, and up to 68% of CHF patients show wasting of skeletal muscle in the lower extremities without loss of total body weight (10, 48). Until today, there is no common definition of cardiac cachexia. The most useful definition for the clinical practice is suggested by Anker et al. (9) who defined cardiac cachexia as a documented nonedematous weight loss of >6% of the previous normal weight observed over a period of six months.
One proposed mechanism of cardiac cachexia is the development of an anabolic/catabolic imbalance with reduced anabolism and enhanced catabolism because of abnormalities in the neurohormonal systems and the activation of proinflammatory cytokines (8). In this regard, it has been shown that cachectic CHF patients have elevated plasma levels of norepinephrine and epinephrine (8). This aggravated activation of the sympathetic nervous system appears to be sufficient to induce a catabolic state (with an increase in resting metabolic rate) because β-adrenoceptor agonists (like clenbuterol) have been shown to exert a direct cardiac and skeletal myotoxic effect (17, 34, 63, 64, 78). Untreated patients with advanced CHF have a twofold increase of the catabolic hormone cortisol, whereas the anabolic steroid DEAH is decreased (8). Growth hormone (GH) and its anabolic mediator insulin-like growth factor-I (IGF-I) are essential hormones for metabolic homeostasis. An abnormal GH-to-IGF-I ratio in cachectic CHF patients, i.e., elevated serum levels of GH and normal or low IGF-I, suggests a GH resistance in the pathophysiology of cardiac cachexia (19). Abnormal plasma levels of aldosterone and plasma renin activation are also found in CHF patients (8). In animal models, angiotensin II, which is highly abundant in CHF patients, leads to weight loss and muscle wasting through a decrease in IGF-I (21).
The serum concentrations of proinflammatory cytokines like TNF-α, IL-6, and IL-1 are known to be high in patients with cardiac cachexia (8, 45, 82). The degree of previous weight loss is strongly correlated to TNF-α serum levels (8). Furthermore, the serum levels of TNF-α and soluble TNF-α receptors are associated with a poor long- and short-term prognosis (23, 65). Proinflammatory cytokines have pleiotropic biological effects. TNF-α is a key cytokine in the development of catabolism. For example, TNF-α was shown to induce apoptosis and to activate proteasome-dependent protein breakdown in striated muscle (1, 88). It has also been demonstrated that TNF-α impairs endothelial function with a decreased blood and nutrient supply to skeletal muscle, thus reducing exercise endurance (13). In addition, TNF-α inhibits food intake by increasing the plasma level of leptin (99). In this regard, it was suggested that dietary deficiency and malabsorption are also involved in the induction of wasting during heart failure (12).
While the above-mentioned mediators will indeed contribute to the development of muscle wasting and cardiac cachexia during end-stage heart failure, a direct causal link between cardiac dysfunction, activation of neurohormones/cytokines, and muscle atrophy has not yet been established, although it was demonstrated that inhibition of adrenergic signaling by β-blockers or angiotensin-converting enzyme (ACE) inhibition (diminishes the levels of angiotensin II) partially reduces the risk for the development of weight loss in heart failure patients. During ACE inhibition, for example, risk reduction was reported to be 19% (9). While this is significant, clearly other mediators not inhibited by the current treatment regimen need to be identified and therapeutically targeted to inhibit heart failure-associated muscle wasting. Emerging data suggest that such mediators include myostatin and activin-A.
Biology and Function of Myostatin
Myostatin (also called gdf-8) is a secreted protein from the TGF-β family and is known as a potent inhibitor of skeletal muscle growth. Among the TGF-β family of genes, myostatin forms a distinct subgroup together with gdf-11, with which it shares 90% amino acid identity in the COOH-terminal domain (41). Myostatin is most abundantly expressed in skeletal muscles where it is expressed higher in fast muscle fibers (with predominant glycolytic metabolism) than in slow muscle fibers (with predominant oxidative metabolism) (49). At a much lower level, the heart and adipose tissue also express myostatin, although expression in these organs can be upregulated under pathological conditions like myocardial infarction (see below) or obesity, respectively (3, 72). Skeletal muscle myostatin is also regulated at the mRNA level and was demonstrated to be upregulated by atrophy stimulation (e.g., muscle unloading) while being downregulated during chronic exercise (e.g., swimming) (2, 18, 29, 36, 49, 66). Besides its expression in heart, skeletal muscle, and adipose tissue, myostatin is also found in serum (98).
Most of the evidence about the function of myostatin is derived from mice in which myostatin expression was either ablated or artificially enhanced. Myostatin knockout mice develop a massive increase (doubling) of skeletal muscle mass (53). This is the result of an increased number and an increased size of the muscle fibers (53). In addition, myostatin also modifies the quality of myofibers because myostatin knockout mice show an increase in the number of fast glycolytic myofibers (these myofibers typically fatigue quickly and use mainly glycolytic metabolism for energy) and a corresponding decrease in slow fibers (these fibers are resistant to fatigue, have more mitochondria, and use predominantly oxidative metabolism for energy) (5, 27). Interestingly, it had been found that, despite the enormous increase in muscle mass and a slight increase in maximal twitch force, the force generated per gram of muscle is reduced by almost 50% in the extensor digitorum longus muscle of myostatin knockout mice (5). Genetic elimination of myostatin in mature mice still leads to an increase in skeletal muscle mass by 25% (90). Mutations in the myostatin gene have been shown to be responsible for the double muscling phenotype in cattle (54). Recently, a loss of function mutation was identified in the myostatin gene in a child with gross muscle hypertrophy, indicating that the function of myostatin to inhibit muscle growth is conserved between mice, cattle, and humans (71). Besides the increased muscularity, the myostatin knockout mice also have reduced body fat with increasing age and are also protected against obesity triggered in genetic models (55). Furthermore, these mice have greater sensitivity for insulin, which might render myostatin an interesting target for treatment of insulin resistance that typically occurs in type II diabetes (52, 93). However, currently, it cannot be discerned whether the favorable metabolic effects in myostatin knockout mice were the consequence of reduced myostatin or occur secondarily to increased muscle mass (52). Overproduction of myostatin in implanted CHO tumor cells induced whole body cachexia, with reduced body weight (approximately −30%) and muscle weights (approximately −50%) and with loss of retroperitoneal fat (98). Overexpression of myostatin in transgenic mice under the control of the muscle creatinin kinase promoter, which resulted in increased myostatin expression in skeletal muscles and heart, led to a significant reduction in lower-limb and forelimb muscle weight (−18 to 24%) and heart weight (67). Interestingly, the effects of myostatin transgene expression were selectively seen in male mice while no reductions in skeletal or cardiac muscle mass were detectable in female myostatin transgenic mice. The reason for the gender difference observed in this study is not known but might involve a mechanism in female mice that is able to override the effects of the myostatin transgene.
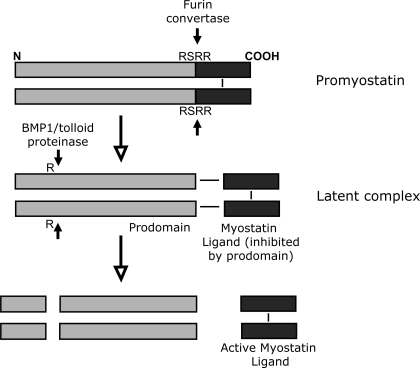
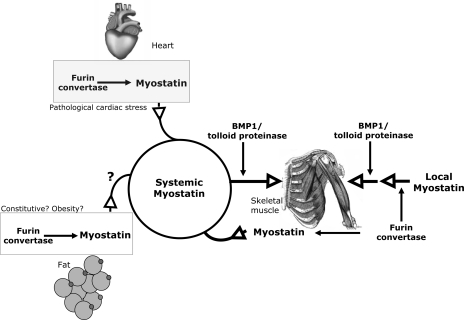
Similar to the other proteins from the TGF-β family, myostatin is produced within the cell as an inactive precursor protein (43, 53). The myostatin precursor, referred to as prepromyostatin, forms a disulfide-linked homodimer after synthesis and translocation to the endoplasmatic reticulum. A first cleavage removes a 24-amino acid signal peptide to generate promyostatin, which is then cleaved by the furin family of protein convertases at an Arg-Ser-Arg-Arg (RSRR) site at amino acids 240–243 to generate an NH2-terminal (27.7 kDa) and a COOH-terminal fragment (12.4 kDa; see Fig. 1) (43, 80). The COOH-terminal fragment exists as a disulfide-linked dimer that stays noncovalently bound to the NH2-terminal fragment (referred to as prodomain; Fig. 1). The COOH-terminal dimer is the biological active myostatin ligand. The noncovalent association of the NH2-terminal prodomain and the COOH-terminal myostatin ligand is described as a latent myostatin complex (Fig. 1) (43, 80). The generation of the latent complex by protein convertases of the furin family was until recently thought to occur constitutively only within the Golgi apparatus of the cell, and, therefore, secretion of myostatin was thought to occur as a latent complex. However, it has recently been demonstrated that myostatin is mainly secreted as uncleaved promyostatin in skeletal myocytes (7). After secretion, this promyostatin is sequestered in the skeletal muscle extracellular space by latent TGF-β-binding protein 3 and covalent attachment to the extracellular matrix. It is converted to latent complex through cleavage by furin protein convertase in the extracellular space (7). Unlike in skeletal muscle, myostatin already exists as a latent complex in serum (32). As a result, there are two different pools of myostatin available that could act to inhibit skeletal muscle growth: one local pool in skeletal muscle that first has to become activated by the furin protein convertase and second the systemic pool of myostatin in serum, which already exists as a latent complex. These two pools with their separate modes of activation are crucial to allow regulation of skeletal muscle growth by myostatin from serum (therefore potentially from heart or adipose tissue) while bypassing the local skeletal muscle myostatin (Fig. 2).
Fig. 1.
Myostatin processing. Top: myostatin is produced within the cell as an inactive precursor protein. Promyostatin forms a disulfide-linked homodimer after synthesis. It is cleaved at an Arg-Ser-Arg-Arg (RSRR) site to generate an NH2-terminal (27.7-kDa) and a COOH-terminal (12.4-kDa) fragment by the furin family of protein convertases. Middle: the COOH-terminal fragment exists as a disulfide-linked dimer that stays noncovalently bound to the NH2-terminal fragment (prodomain). The COOH-terminal dimer is the biological active mature myostatin ligand. The noncovalent association of the NH2-terminal prodomain and the COOH-terminal myostatin ligand is described as latent myostatin complex. Within this complex, the myostatin ligand is inhibited by the myostatin prodomain. Myostatin is activated from the latent complex after cleavage of the prodomain by BMP1/tolloid proteinases. R stands for arginine residue 75. Bottom: the COOH-terminal myostatin dimer is the active ligand that binds to the activin receptor IIB (ActRIIB).
Fig. 2.
Skeletal muscle growth is regulated by the local and the systemic pools of myostatin, which underlie different modes of activation. Systemic myostatin is present in serum as a latent complex. Upon arrival at its target organ (skeletal muscle), it becomes activated through cleavage by BMP1/tolloid proteinases. The heart contributes to the systemic myostatin pool during pathological cardiac stress. Whether fat tissue releases myostatin in the circulation (e.g., in obesity) is currently unknown. The predominant contributor to systemic myostatin is skeletal muscle, although the main local pool of myostatin within skeletal muscle is present extracellularly as promyostatin. This local myostatin is activated in a two-step process that includes first a cleavage by the furin convertase before myostatin activation can be induced by BMP1/tolloid proteinases. With these different mechanisms of activation, it is possible that systemic myostatin regulates skeletal muscle growth while bypassing the high abundant local myostatin.
The mature myostatin ligand (unless otherwise stated referred to simply as myostatin from here on) is released from the latent complex by proteolytic cleavage of the myostatin prodomain by the BMP1/tolloid family of metalloproteinases (like BMP-1, TLL-1, and TLL-2) between Arg-75 and Asp-76 (Fig. 1) (94). BMP-1 and TLL-1 expression were both detected in different skeletal muscles and the heart, and TLL-1 mRNA was even shown to be significantly induced in the tibialis anterior muscle after 3 days of proatrophic hindlimb suspension, while being reduced in expression after 2 days of food deprivation (4). This dynamic regulation would allow for enhanced/reduced release of myostatin in muscle from the latent complex during atrophy stimulation or inhibition of atrophy, respectively. Mice expressing a mutant myostatin prodomain that is resistant to proteolytic cleavage develop severe skeletal muscle hypertrophy similar to that of myostatin knockout mice (39). These data indicate that cleavage of the prodomain is required for myostatin activation and its release from the latent complex. Indeed, it had been demonstrated that the myostatin prodomain inhibits receptor binding and also reporter activation by myostatin (43, 80). In line with this in vitro data, transgenic overexpression of the myostatin prodomain in mouse skeletal muscle results in severe hypermuscularity like in myostatin knockout mice (43, 95). Other proteins that bind myostatin and inhibit its activity include follistatin, FLRG, and GASP-1 (32, 33, 43). Interestingly, transgenic overexpression of follistatin in mouse muscle leads to muscle growth that is more dramatic than in myostatin knockout mice (43). Furthermore, overexpression of follistatin in the myostatin knockout background leads to a further increase in muscle weight, which in total is four times more than in wild-type mice (40). These data indicate that myostatin might have a companion protein in negative regulation of muscle growth that is also bound and inhibited by follistatin and that might use similar signaling mechanisms. Indeed, it is now believed that activin-A is that companion of myostatin, because activin-A also binds follistatin, and muscular overexpression of a mutant form of follistatin that cannot bind activin-A fails to enhance muscle growth in the myostatin knockout background (26). Moreover, heterozygous activin-A knockout mice show increased skeletal muscle weights while overexpression of activin-A induces muscle atrophy (26, 42).
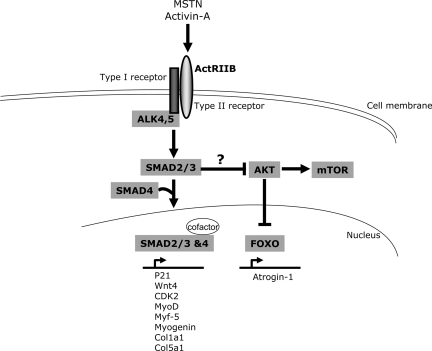
Activin-A, myostatin, and gdf-11 all bind to the activin receptor IIB (ActRIIB) in mice, which is responsible for mediating the growth-inhibiting effects of myostatin on skeletal muscle. Accordingly, transgenic overexpression of a dominant-negative form of ActRIIB results in hypermuscularity in mice similar to myostatin knockout mice (43). Receptor activation and intracellular signaling of myostatin is similar to that of TGF-β1 (Fig. 3). Myostatin first binds to the type II receptor ActRIIB, which then heterodimerizes with either type I receptor ALK 5 or ALK 4. Binding of myostatin to this receptor complex leads to phosphorylation and activation of SMAD2 and SMAD3. These signaling mediators then translocate to the nucleus to regulate gene expression in association with SMAD4, which acts as a co-SMAD (Fig. 3) (84). Although SMADs have intrinsic DNA-binding activity, association with DNA-binding cofactors like p300, TGIF, c-Ski, and Evi-1 add to the complexity of the system (84). Myostatin stimulation also leads to inhibition of the protein kinase B/Akt and the growth-promoting kinase mammalian target of rapamycin (mTOR), which acts immediately downstream of Akt (56, 70, 83, 92). However, the mechanism of SMAD-dependent inactivation of Akt has not been deciphered yet. mTOR is capable of regulating protein synthesis and cell size, but only around 40% of the hypertrophic effects of blocking myostatin can be reversed by anti-mTOR agents (70, 92). Whether Akt inhibition by myostatin also leads to enhanced nuclear localization of forkhead box O1 and thereby increases expression of atrogin-1, an E3 ubiquitin ligase that labels targets for proteosomal degradation, is still a matter of debate. Both in vitro and in vivo studies have demonstrated that myostatin inhibits protein synthesis without increasing proteolysis in C2C12 myotubes or in mouse skeletal muscle (79, 89, 92). Interestingly, recent evidence suggests that satellite cell activation might not be needed for the effects of myostatin inhibition on muscle hypertrophy (6). Together, these data imply that stimulation of protein synthesis is the primary reason for exaggerated muscle growth upon myostatin blockade. Which genes are influenced by myostatin binding to its target cells? It was reported that myostatin inhibits the proliferation of myoblasts and satellite cells by influencing the expression of genes participating in cell cycle regulation like, for example, p21 (upregulation by myostatin), CDK2, or Wnt4 (both repressed by myostatin) (50, 76). Myostatin inhibits myogenic differentiation by downregulating expression of myogenic regulators like MyoD, myogenin, and Myf-5 (35, 38, 68). Moreover, the antifibrotic properties of myostatin inhibition in adult skeletal muscles were shown to be at least partially mediated by downregulating the expression of different collagen genes (e.g., Col1a1 and Col5a1; Fig. 3) (91).
Fig. 3.
Cellular signal transduction upon stimulation with myostatin (MSTN) or activin-A. MSTN or activin-A bind to the ActRIIB (type II receptor) at the cell membrane. Subsequently, ALK4/5 (type I receptor) gets activated, which subsequently phosphorylates and activates SMAD2 and -3 to translocate to the nuclear compartment in conjunction with SMAD4 (co-SMAD). Although SMADs have intrinsic DNA-binding activity, association with DNA-binding cofactors like p300, TGIF, c-Ski, and Evi-1 enhances their potency to activate transcription. A list of target genes that were demonstrated to be regulated by myostatin is shown under the SMAD complex in the nucleus. The mechanism of SMAD-induced inhibition of protein kinase B (Akt) is currently unknown. Akt induces activation of mammalian target of rapamycin (mTOR, an activator of protein synthesis) and inhibits nuclear translocation of forkhead box O (FOXO) transcription factors that activate expression of atrogin-1 (a promoter of ubiquitin-dependent degradation) in the nucleus.
Because myostatin is such a powerful mediator, its therapeutic manipulation is being tested in the treatment of several disease states. For example, inhibition of myostatin by antibody (clone JA-16 or PF-354) has been shown to ameliorate muscle pathology and to increase strength, especially in younger mice with different forms of muscular dystrophy (16, 59, 61, 86). However, this approach of antibody-based myostatin inhibition was much less effective in a phase I/II trial conducted in adult human subjects with muscular dystrophy, although an insignificant trend toward mildly increased skeletal muscle mass was observed and the myostatin inhibiting antibody (MYO-029) was well tolerated (85). Novel reagents have recently emerged, which block activation of the ActRIIB and therefore inhibit the action of both activin-A and myostatin, which might be more effective (37). Indeed, administration of recombinant ActRIIB as a decoy receptor in mice was able to prevent and reverse cancer-associated cachexia in tumor-bearing mice and even markedly improved mouse survival in this model, although tumor growth itself was not inhibited (97). These results are in itself ground breaking and underscore the need to therapeutically prevent cachexia in different diseases. Increased levels of muscle myostatin and/or serum myostatin have been reported in human patients with human immunodeficiency syndrome, chronic obstructive pulmonary disease, and heart failure, opening up a whole range of different possible applications for ActRIIB inhibition (24, 28, 30, 62).
Cardiac Myostatin in Health and Disease
Proliferating fetal cardiomyocytes (E18) express relatively low levels of myostatin, but its expression (mRNA and protein) rises dramatically until postnatal day 10 and subsequently decreases again toward adulthood (51). Within the ventricular myocardium, myostatin expression is highest in the left ventricle, since a sixfold higher mRNA expression was measured there compared with the right ventricle of 20-day-old piglets (81). Interestingly, cardiac myostatin levels can be reinduced in the adult organism upon increased cardiac load. After myocardial infarction, cardiomyocytes in the peri-infarct area express higher amounts of the protein between 12 hours and 30 days after induction of myocardial ischemia (72). Similar results were obtained eight weeks after myocardial infarction in rats when Lenk and his coworkers (44) observed an increase of myostatin protein (4-fold) and RNA (around 3-fold) in the myocardium. Increased myocardial myostatin was also found in other animal models after pathological stimulation, for example, in a rat model of volume-overload heart failure (around 3-fold upregulation of myostatin mRNA and protein after induction of an aortocaval shunt) and in transgenic mice with cardiac hypertrophy due to chronic overexpression of activated Akt (18.4-fold upregulation of myostatin mRNA) (20, 75). In vitro studies in isolated cardiomyocytes suggest that myostatin mRNA and protein can be directly induced in these cells by mechanical stretch or humoral stimulation with IGF-I, phenylephrine, or angiotensin II, involving intracellular activation of mitogen-activated protein kinases (p38 and/or ERK) and binding of the transcription factor MEF2 within the myostatin promoter region (15, 74, 87). One study failed to report an enhancement of cardiac myostatin mRNA during heart failure progression in dogs due to overpacing but instead detected elevated levels of the related activin-A in moderate and severe heart failure (2- to 3-fold increase) (47). The cause for the deviant result in this study might be that myocardial samples were obtained by biopsy from the septal region within the right ventricle, which reportedly has lower myostatin levels and which is also exposed to different loading conditions compared with the left ventricle. The other studies measured myostatin in the whole heart that is dominated by the higher volume fraction of the left ventricle. Importantly, a recent study by George and colleagues found increased myostatin levels in left ventricular myocardial samples from human patients with ischemic (ICM) or dilated (DCM) cardiomyopathy (24). More myostatin prodomain was detected by Western blot in the myocardium of these patients (2-fold more in DCM, 6-fold more in ICM), although the levels of the full-length myostatin precursor protein (promyostatin) remained unchanged. This indicates that cleavage of the myostatin precursor by the furin protein convertase is enhanced in the diseased human hearts, thus generating more prodomain and active myostatin ligand. In contrast, regardless of the increased cleavage, the myostatin precursor is maintained at a constant level with a self-renewal mechanism, possibly involving a feedback regulatory mechanism by the prodomain or the active COOH-terminal ligand (25).
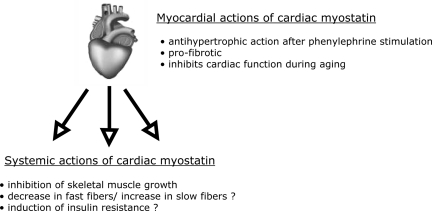
The enhanced myostatin abundance in the heart could either lead to local effects and/or to systemic effects upon release of myostatin from the myocardium into the blood stream (Fig. 4). Local effects of myostatin in the failing human heart are suggested by increased cardiac expression of BMP-1 (which releases and activates myostatin from its latent complex), increased expression of the myostatin receptor ActRIIB, and finally increased SMAD2/3 activation (25). Studies in myostatin knockout mice suggest that myostatin inhibits pathological hypertrophy in male mice (but not in female mice) upon stimulation with the α-adrenergic agonist phenylephrine because these mice show exaggerated hypertrophy after 14 days of phenylephrine infusion (57). Along these lines, systemic blockade of the ActRIIB (which, besides myostatin, is also stimulated by activin-A) also leads to increased myocardial growth during cancer-induced cachexia in mice (97). In contrast, chronic pressure overload (12 wk) through aortic constriction (TAC) did not alter the hypertrophic response in cardiac-specific myostatin knockout mice (31) or in systemic myostatin knockout mice (Heineke and Molkentin, unpublished observation). These data suggest that endogenous myostatin only inhibits cardiac growth in response to select stimuli. One reason could be that myocardial levels of activated myostatin are not high enough to inhibit cardiomyocyte growth under certain conditions. This is supported by the fact that transgenic overexpression of high myostatin levels in the heart leads to inhibition of cardiac growth even at baseline (without any stimulation) (14, 31, 67). During aging, myostatin appears to inhibit cardiac function and induce tissue fibrosis, since 27- to 30-mo-old myostatin knockout mice display less myocardial fibrosis, less ventricular dilation, and improved cardiac function compared with their wild-type counterparts. The improved cardiac function was associated with increased phosphorylation of the sarcoplasmatic protein phospholamban, which leads to increased calcium release in the cytosol during systole (58). Indeed, an increased calcium transient has been detected in adult cardiomyocytes from myostatin knockout mice (69). However, compared with wild-type mice, no change in cardiac function was found in myostatin-deficient mice after chronic pressure overload stimulation (31). Together, all these results suggest modest local effects of myostatin in the heart, which are highly dependent on the stimulus and which include anti-hypertrophic, pro-fibrotic, and inhibitory effects on cardiac function.
Fig. 4.
Local and systemic actions of cardiac myostatin are displayed.
Is myostatin released from the heart, when its myocardial abundance is enhanced during pathological stimulation and, even more importantly, does it have systemic effects under these circumstances? A threefold increase in myostatin secretion from isolated Akt transgenic hearts was previously reported (57). To address this question more directly, our group engineered mice with a cardiomyocyte-specific deletion of myostatin (MSTN-CKO). To achieve this, we employed the Cre/Lox system and crossed the cardiac-specific Nkx2.5 Cre mice with mice in which the exon 3 of myostatin (encoding for the COOH-terminal active myostatin ligand) is enclosed by loxp sites (MSTN-flox) (31). The MSTN-CKO mice selectively lacked myostatin mRNA in the heart, whereas myostatin mRNA and its precursor protein were detected at normal levels in skeletal muscle. At baseline, wild-type mice and MSTN-CKO mice at the age of two months had comparable serum levels of the active COOH-terminal myostatin ligand in serum (31). Major differences occurred when these mice were subjected to two weeks of pressure overload stimulation (TAC): while circulating levels of myostatin in wild-type mice rose by about threefold, no increase in serum myostatin was observed in MSTN CKO mice. These data demonstrate that, in the case of pathological loading, the heart releases myostatin into the circulation and thereby generates increased serum levels of the protein. Elevated serum myostatin in wild-type mice was associated with skeletal muscle atrophy, since the quadriceps, gastrocnemius, and soleus muscles all displayed a decreased muscle weight (−10 to 26%). Importantly, however, no such decrease in skeletal muscle weights (i.e., no atrophy) was observed in MSTN-CKO mice. This directly demonstrates that cardiac myostatin is responsible for the induction of muscle atrophy in mouse heart failure induced by pressure overload (31). Whether myostatin from the heart is also responsible for the insulin resistance or the fiber type switching (toward more slow fibers) reported to occur in heart failure was not yet examined, but both effects have been ascribed to myostatin (Fig. 4) (27, 52, 73, 77). Interestingly, elimination of cardiac myostatin did only influence skeletal muscle mass after aortic constriction but not at baseline or after sham operation. This indicates that cardiac myostatin regulates skeletal muscle mass during increased (pathological) cardiac stress but not during normal cardiac development or adult life, when the heart also does not appear to contribute to myostatin serum levels. As a proof of concept, and to see whether indeed myostatin synthesized and released from cardiac myocytes is capable of inducing atrophy in peripheral skeletal muscle (despite the high local abundance of myostatin there), we generated mice that overexpress myostatin specifically in cardiac myocytes under the control of the α-myosin heavy chain promoter. Serum levels of myostatin were increased by three- to fourfold in these transgenic mice compared with nontransgenic littermates (similar to what we observed during pressure overload) and indeed show significantly reduced skeletal muscle weights (minus 13 to 30%, again similar to the reduced muscle weights in wild-type mice in pressure overload-induced heart failure) (31). As would be expected, however, although the heart contributes significantly to serum myostatin during pathological overload, skeletal muscle itself appears to contribute the highest proportion of serum myostatin, since ablation of myostatin specifically in skeletal muscle (by crossing the MSTN-flox mice to mice harboring a skeletal muscle-specific Cre) reduces serum levels by 60–70% (Heineke, Auger-Messier, and Molkentin, unpublished results).
How can even a threefold increase in serum myostatin (like we observe after pressure overload) have an impact on skeletal muscle growth considering the high local concentration of myostatin in skeletal muscle? The likely answer to this question lies in the existence of at least two different pools of myostatin within the mammalian organism (Fig. 2). As already alluded to earlier in this review, the main difference of these two myostatin pools lies in their mode of activation. The main portion of myostatin in skeletal muscle exists as myostatin precursor protein (promyostatin), which is constitutively bound and inhibited by the latent TGF-β-binding protein 3 (LTBP-3) and associates outside skeletal muscle fibers to extracellular matrix (7). Activation of this local (high-abundance) skeletal muscle pool of myostatin requires two steps: first, cleavage at the RSRR site at amino acids 240–243 by furin convertases to generate an NH2-terminal (27.7-kDa) and a COOH-terminal (12.4 kDa) fragment that stay noncovalently associated in a latent complex (also see Fig. 1). Second, the prodomain within this latent complex needs to be cleaved by the BMP1/tolloid family of metalloproteinases to release the active COOH-terminal myostatin ligand in muscle. In contrast, the second pool is the systemic myostatin portion that circulates in the blood stream (7). This second fraction already exists in the latent complex and, when it arrives at its target organ (skeletal muscle), only needs one-step activation through cleavage by the BMP1/tolloid family of metalloproteinases and therefore can bypass the local myostatin pool in skeletal muscle, which needs two steps for activation (Fig. 2). Things are complicated by the fact that skeletal muscle itself is a major contributor to the systemic myostatin pool. Whether and to what extent adipose tissue contributes to systemic myostatin is currently not known (Fig. 2). Although pure speculation at this point, increased adipocyte myostatin production during obesity could contribute to decreased skeletal muscle mass in this setting (52). We have shown that the heart contributes to this second systemic myostatin pool during pathological loading conditions (Fig. 2) (31). In support of this, George and colleagues (25) detected elevated levels of myostatin latent complex in serum of patients with DCM-associated heart failure (25). In support of the myocardium as a contributor to the systemic latent complex, the major myostatin form within the diseased heart was the myostatin prodomain, which is one of the main components of the latent complex found in serum (25). Another, although somewhat less powerful, distinction between local skeletal muscle and systemic myostatin might be represented by the differential regulation of myostatin mRNA transcription: while numerous studies (see above) have demonstrated increased myostatin mRNA in the heart during cardiac disease (which then contributes to elevated systemic myostatin levels), increased myostatin RNA has not been detected locally in skeletal muscle under these circumstances, therefore shifting the balance toward systemic myostatin (25, 44, 46). One study observed elevated myostatin protein in skeletal muscle in infarction-induced heart failure, but no corresponding increase in myostatin mRNA was detected, and thus the myostatin could also be derived from the heart, which displayed increased myostatin mRNA and protein in that study (44).
Another level of complexity is added by the fact that activin-A, another TGF-β cytokine that acts much like myostatin by binding to the ActRIIB receptor, is also capable of inducing skeletal muscle wasting and is also elevated in the myocardium after myocardial infarction in rats and increased in serum of heart failure patients (26, 42, 60, 96). Therefore, activin-A, although this has not been formally shown yet as in the case of myostatin, might also be released from the heart during failure and might amplify myostatin's effect on skeletal muscle.
Is inhibition of myostatin feasible as treatment of muscle atrophy in heart failure? In our recent study, we inhibited myostatin through injection of the monoclonal antimyostatin antibody clone JA-16, which inhibits binding of myostatin to its receptor ActRIIB. Treatment was started in preexisting heart failure after TAC, and, compared with treatment with unspecific control antibody, skeletal muscle atrophy could indeed be reversed by six weeks of JA-16 treatment (31). Therefore, inhibition of myostatin during heart failure-induced muscle atrophy could be a valuable approach to increase muscle strength and mobility in these patients. No effect of JA-16 on cardiac function- or heart failure-associated mortality was observed in the study (31), although we noted a modest (but statistically significant) reduction in heart weight upon myostatin inhibition, indicating that cardiac pathology was somewhat improved (Heineke and Molkentin, unpublished observation). Reduction of myostatin levels in heart failure might also be achieved by moderate regular exercise, since Lenk and colleagues (44) observed reduced myostatin levels by regular treadmill exercise in rats after myocardial infarction. Considering that the effects of myostatin in heart failure might be enhanced by activin-A, it would be reasonable to consider blockade of the ActRIIB as a more effective strategy, because this receptor is activated by both cytokines. Indeed, inhibition of ActRIIB activation by a recombinant ActRIIB decoy receptor during cancer cachexia completely reversed muscle wasting and even improved survival in mice with cancer, although tumor growth itself was not changed by the treatment (97). Clearly, therapeutic ActRIIB blockade also needs to be tested in heart failure models.
Concluding Remarks and Future Perspectives
Because of its high prevalence and association with morbidity, muscle wasting with cardiac cachexia is a significant clinical problem. To address it more effectively, a thorough understanding of the underlying molecular pathways is crucial. Increased levels of neuroendocrine mediators like (nor)epinephrine, angiotensin II, or proinflammatory cytokines such as TNF-α, IL-1, and IL-6, corticosteroids, as well as reduced levels of peptide hormones (IGF-I) have been implicated to induce a catabolic state during heart failure. Recently, the TGF-β-related cytokine myostatin was identified as an important mediator of cardiac-induced muscle wasting. In this setting, cardiac myostatin acts in an endocrine fashion to communicate to skeletal muscle and induce atrophy. The reason for the evolutionary conservation of this pathway, which is only active during enhanced cardiac stress, is unknown, although it might restrain unbalanced organ growth and therefore (through a reduction in skeletal muscle mass) limit the circulatory burden imposed on the heart. In this regard, release of myostatin from the myocardium might, as in the case of neurohormones, transiently contribute to the upholding of blood circulation during heart failure. In the long run, however, high levels of myostatin (and activin-A) in patients suffering from cardiac failure are most likely maladaptive, and therapeutic inhibition could lead to improved muscle mass and strength (hence better quality of life for these patients) and perhaps even to improved cardiac function. To translate the mainly animal-based findings highlighted in this review into the clinic, several steps need to be undertaken: first, elevated levels of myostatin and/or activin-A need to be verified in larger cohorts of patients with heart failure, and their usefulness as a biomarker for cardiac cachexia needs to be examined. Second, effective myostatin- and/or ActRIIB-blocking agents that are being developed need to be tested thoroughly in multiple different animal models of heart failure (including larger animal models like pigs) before their safety and effectiveness can be evaluated in clinical studies in patients with heart failure.
GRANTS
This work was funded by the Deutsche Forschungsgemeinschaft through the Cluster of Excellence Rebirth (J. Heineke) and the Klinische Forschergruppe 136 (to J. Heineke). J. D. Molkentin is funded by grants from the National Institute of Health and by the Howard Hughes Medical Institute.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Adams V, Jiang H, Yu J, Mobius-Winkler S, Fiehn E, Linke A, Weigl C, Schuler G, Hambrecht R. Apoptosis in skeletal myocytes of patients with chronic heart failure is associated with exercise intolerance. J Am Coll Cardiol 33: 959–965, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Allen DL, Bandstra ER, Harrison BC, Thorng S, Stodieck LS, Kostenuik PJ, Morony S, Lacey DL, Hammond TG, Leinwand LL, Argraves WS, Bateman TA, Barth JL. Effects of spaceflight on murine skeletal muscle gene expression. J Appl Physiol 9: 359–367, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294: E918–E927, 2008 [DOI] [PubMed] [Google Scholar]
- 4. Allen DL, Greybeck BJ, Hanson AM, Cleary AS, Lindsay SF. Skeletal muscle expression of bone morphogenetic protein-1 and tolloid-like-1 extracellular proteases in different fiber types and in response to unloading, food deprivation and differentiation. J Physiol Sci 60: 343–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci USA 104: 1835–1840, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K, Partridge T. Muscle hypertrophy driven by myostatin blockade does not require stem/precursor-cell activity. Proc Natl Acad Sci USA 106: 7479–7484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson SB, Goldberg AL, Whitman M. Identification of a novel pool of extracellular pro-myostatin in skeletal muscle. J Biol Chem 283: 7027–7035, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation 96: 526–534, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Negassa A, Coats AJ, Afzal R, Poole-Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet 361: 1077–1083, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 349: 1050–1053, 1997 [DOI] [PubMed] [Google Scholar]
- 11. Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole-Wilson PA, Coats AJ. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 20: 683–693, 1999 [DOI] [PubMed] [Google Scholar]
- 12. Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med 36: 518–529, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Anker SD, von Haehling S. Inflammatory mediators in chronic heart failure: an overview. Heart 90: 464–470, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Artaza JN, Reisz-Porszasz S, Dow JS, Kloner RA, Tsao J, Bhasin S, Gonzalez-Cadavid NF. Alterations in myostatin expression are associated with changes in cardiac left ventricular mass but not ejection fraction in the mouse. J Endocrinol 194: 63–76, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Bish LT, Morine KJ, Sleeper MM, Sweeney HL. Myostatin is upregulated following stress in an Erk-dependent manner and negatively regulates cardiomyocyte growth in culture and in a mouse model. PLoS One 5: e10230, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418–421, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Burniston JG, Ng Y, Clark WA, Colyer J, Tan LB, Goldspink DF. Myotoxic effects of clenbuterol in the rat heart and soleus muscle. J Appl Physiol 93: 1824–1832, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol Regul Integr Comp Physiol 277: R601–R606, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Cicoira M, Kalra PR, Anker SD. Growth hormone resistance in chronic heart failure and its therapeutic implications. J Card Fail 9: 219–226, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem 277: 22528–22533, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Delafontaine P, Akao M. Angiotensin II as candidate of cardiac cachexia. Curr Opin Clin Nutr Metab Care 9: 220–224, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dickstein K. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: application of natriuretic peptides. Reply. Eur Heart J In press [DOI] [PubMed] [Google Scholar]
- 23. Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A, Cassani G, Visioli O. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation 92: 1479–1486, 1995 [DOI] [PubMed] [Google Scholar]
- 24. George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail 12: 444–453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail 12: 444–453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab 297: E157–E164, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31: 34–40, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, Arver S, Bhasin S. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci USA 95: 14938–14943, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gustafsson T, Osterlund T, Flanagan JN, von Walden F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol 109: 721–727, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Hayot M, Rodriguez J, Vernus B, Carnac G, Jean E, Allen D, Goret L, Obert P, Candau R, Bonnieu A. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol 332: 38–47, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121: 419–425, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. The myostatin propeptide and the follistatin-related gene are inhibitory binding proteins of myostatin in normal serum. J Biol Chem 277: 40735–40741, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Hill JJ, Qiu Y, Hewick RM, Wolfman NM. Regulation of myostatin in vivo by growth and differentiation factor-associated serum protein-1: a novel protein with protease inhibitor and follistatin domains. Mol Endocrinol 17: 1144–1154, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Hyltander A, Daneryd P, Sandstrom R, Korner U, Lundholm K. Beta-adrenoceptor activity and resting energy metabolism in weight losing cancer patients. Eur J Cancer 36: 330–334, 2000 [DOI] [PubMed] [Google Scholar]
- 35. Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G. Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286: 263–275, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Kim JS, Cross JM, Bamman MM. Impact of resistance loading on myostatin expression and cell cycle regulation in young and older men and women. Am J Physiol Endocrinol Metab 288: E1110–E1119, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Kung T, Springer J, Doehner W, Anker SD, von Haehling S. Novel treatment approaches to cachexia and sarcopenia: highlights from the 5th Cachexia Conference. Expert Opin Investig Drugs 19: 579–585, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277: 49831–49840, 2002 [DOI] [PubMed] [Google Scholar]
- 39. Lee SJ. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS One 3: e1628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lee SJ. Quadrupling muscle mass in mice by targeting TGF-beta signaling pathways. PLoS One 2: e789, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20: 61–86, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, Cohn RD, Barton ER. Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24: 1998–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA 98: 9306–9311, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, Schuler G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail 11: 342–348, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323: 236–241, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Lima AR, Martinez PF, Okoshi K, Guizoni DM, Zornoff LA, Campos DH, Oliveira SA, Jr, Bonomo C, Pai-Silva MD, Okoshi MP. Myostatin and follistatin expression in skeletal muscles of rats with chronic heart failure. Int J Exp Pathol 91: 54–62, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mahmoudabady M, Mathieu M, Dewachter L, Hadad I, Ray L, Jespers P, Brimioulle S, Naeije R, McEntee K. Activin-A, transforming growth factor-beta, and myostatin signaling pathway in experimental dilated cardiomyopathy. J Card Fail 14: 703–709, 2008 [DOI] [PubMed] [Google Scholar]
- 48. Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 85: 1364–1373, 1992 [DOI] [PubMed] [Google Scholar]
- 49. Matsakas A, Bozzo C, Cacciani N, Caliaro F, Reggiani C, Mascarello F, Patruno M. Effect of swimming on myostatin expression in white and red gastrocnemius muscle and in cardiac muscle of rats. Exp Physiol 91: 983–994, 2006 [DOI] [PubMed] [Google Scholar]
- 50. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162: 1135–1147, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McKoy G, Bicknell KA, Patel K, Brooks G. Developmental expression of myostatin in cardiomyocytes and its effect on foetal and neonatal rat cardiomyocyte proliferation. Cardiovasc Res 74: 304–312, 2007 [DOI] [PubMed] [Google Scholar]
- 52. McPherron AC. Metabolic Functions of Myostatin and Gdf11. Immunol Endocr Metab Agents Med Chem 10: 217–231, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90, 1997 [DOI] [PubMed] [Google Scholar]
- 54. McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA 94: 12457–12461, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest 109: 595–601, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol 297: C1124–C1132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res 99: 15–24, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morissette MR, Stricker JC, Rosenberg MA, Buranasombati C, Levitan EB, Mittleman MA, Rosenzweig A. Effects of myostatin deletion in aging mice. Aging Cell 8: 573–583, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Murphy KT, Ryall JG, Snell SM, Nair L, Koopman R, Krasney PA, Ibebunjo C, Holden KS, Loria PM, Salatto CT, Lynch GS. Antibody-directed myostatin inhibition improves diaphragm pathology in young but not adult dystrophic mdx mice. Am J Pathol 176: 2425–2434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Oshima Y, Ouchi N, Shimano M, Pimentel DR, Papanicolaou KN, Panse KD, Tsuchida K, Lara-Pezzi E, Lee SJ, Walsh K. Activin A and follistatin-like 3 determine the susceptibility of heart to ischemic injury. Circulation 120: 1606–1615, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Parsons SA, Millay DP, Sargent MA, McNally EM, Molkentin JD. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol 168: 1975–1985, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 42: 461–471, 2010 [DOI] [PubMed] [Google Scholar]
- 63. Podbregar M, Voga G. Effect of selective and nonselective beta-blockers on resting energy production rate and total body substrate utilization in chronic heart failure. J Card Fail 8: 369–378, 2002 [DOI] [PubMed] [Google Scholar]
- 64. Poehlman ET, Scheffers J, Gottlieb SS, Fisher ML, Vaitekevicius P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med 121: 860–862, 1994 [DOI] [PubMed] [Google Scholar]
- 65. Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, Niebauer J, Hooper J, Volk HD, Coats AJ, Anker SD. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation 102: 3060–3067, 2000 [DOI] [PubMed] [Google Scholar]
- 66. Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E. Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve 24: 893–899, 2001 [DOI] [PubMed] [Google Scholar]
- 67. Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab 285: E876–E888, 2003 [DOI] [PubMed] [Google Scholar]
- 68. Rios R, Carneiro I, Arce VM, Devesa J. Myostatin is an inhibitor of myogenic differentiation. Am J Physiol Cell Physiol 282: C993–C999, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Rodgers BD, Interlichia JP, Garikipati DK, Mamidi R, Chandra M, Nelson OL, Murry CE, Santana LF. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol 587: 4873–4886, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296: C1248–C1257, 2009 [DOI] [PubMed] [Google Scholar]
- 71. Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004 [DOI] [PubMed] [Google Scholar]
- 72. Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol 180: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 73. Shimizu I, Minamino T, Toko H, Okada S, Ikeda H, Yasuda N, Tateno K, Moriya J, Yokoyama M, Nojima A, Koh GY, Akazawa H, Shiojima I, Kahn CR, Abel ED, Komuro I. Excessive cardiac insulin signaling exacerbates systolic dysfunction induced by pressure overload in rodents. J Clin Invest 120: 1506–1514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shyu KG, Ko WH, Yang WS, Wang BW, Kuan P. Insulin-like growth factor-1 mediates stretch-induced upregulation of myostatin expression in neonatal rat cardiomyocytes. Cardiovasc Res 68: 405–414, 2005 [DOI] [PubMed] [Google Scholar]
- 75. Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest 36: 713–719, 2006 [DOI] [PubMed] [Google Scholar]
- 76. Steelman CA, Recknor JC, Nettleton D, Reecy JM. Transcriptional profiling of myostatin-knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J 20: 580–582, 2006 [DOI] [PubMed] [Google Scholar]
- 77. Strassburg S, Springer J, Anker SD. Muscle wasting in cardiac cachexia. Int J Biochem Cell Biol 37: 1938–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 78. Tan LB, Burniston JG, Clark WA, Ng Y, Goldspink DF. Characterization of adrenoceptor involvement in skeletal and cardiac myotoxicity Induced by sympathomimetic agents: toward a new bioassay for beta-blockers. J Cardiovasc Pharmacol 41: 518–525, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH, Jr, Kull FC, Jr, Gonzalez-Cadavid N. Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 280: E221–E228, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Thies RS, Chen T, Davies MV, Tomkinson KN, Pearson AA, Shakey QA, Wolfman NM. GDF-8 propeptide binds to GDF-8 and antagonizes biological activity by inhibiting GDF-8 receptor binding. Growth Factors 18: 251–259, 2001 [DOI] [PubMed] [Google Scholar]
- 81. Torrado M, Iglesias R, Nespereira B, Mikhailov AT. Identification of candidate genes potentially relevant to chamber-specific remodeling in postnatal ventricular myocardium. J Biomed Biotechnol 2010: 603159, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD). J Am Coll Cardiol 27: 1201–1206, 1996 [DOI] [PubMed] [Google Scholar]
- 83. Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296: C1258–C1270, 2009 [DOI] [PubMed] [Google Scholar]
- 84. Tsuchida K, Nakatani M, Hitachi K, Uezumi A, Sunada Y, Ageta H, Inokuchi K. Activin signaling as an emerging target for therapeutic interventions (Abstract). Cell Commun Signal 7: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63: 561–571, 2008 [DOI] [PubMed] [Google Scholar]
- 86. Wagner KR, McPherron AC, Winik N, Lee SJ. Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52: 832–836, 2002 [DOI] [PubMed] [Google Scholar]
- 87. Wang BW, Chang H, Kuan P, Shyu KG. Angiotensin II activates myostatin expression in cultured rat neonatal cardiomyocytes via p38 MAP kinase and myocyte enhance factor 2 pathway. J Endocrinol 197: 85–93, 2008 [DOI] [PubMed] [Google Scholar]
- 88. Warren RS, Starnes HF, Jr, Gabrilove JL, Oettgen HF, Brennan MF. The acute metabolic effects of tumor necrosis factor administration in humans. Arch Surg 122: 1396–1400, 1987 [DOI] [PubMed] [Google Scholar]
- 89. Welle S, Bhatt K, Pinkert CA. Myofibrillar protein synthesis in myostatin-deficient mice. Am J Physiol Endocrinol Metab 290: E409–E415, 2006 [DOI] [PubMed] [Google Scholar]
- 90. Welle S, Bhatt K, Pinkert CA, Tawil R, Thornton CA. Muscle growth after postdevelopmental myostatin gene knockout. Am J Physiol Endocrinol Metab 292: E985–E991, 2007 [DOI] [PubMed] [Google Scholar]
- 91. Welle S, Cardillo A, Zanche M, Tawil R. Skeletal muscle gene expression after myostatin knockout in mature mice. Physiol Genomics 38: 342–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Welle SL. Myostatin and muscle fiber size. Focus on “Smad2 and 3 transcription factors control muscle mass in adulthood” and “Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size.” Am J Physiol Cell Physiol 296: C1245–C1247, 2009 [DOI] [PubMed] [Google Scholar]
- 93. Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes 58: 1133–1143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, Lee SJ. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci USA 100: 15842–15846, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Yang J, Ratovitski T, Brady JP, Solomon MB, Wells KD, Wall RJ. Expression of myostatin pro domain results in muscular transgenic mice. Mol Reprod Dev 60: 351–361, 2001 [DOI] [PubMed] [Google Scholar]
- 96. Yndestad A, Ueland T, Oie E, Florholmen G, Halvorsen B, Attramadal H, Simonsen S, Froland SS, Gullestad L, Christensen G, Damas JK, Aukrust P. Elevated levels of activin A in heart failure: potential role in myocardial remodeling. Circulation 109: 1379–1385, 2004 [DOI] [PubMed] [Google Scholar]
- 97. Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, Goldberg AL, Han HQ. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142: 531–543, 2010 [DOI] [PubMed] [Google Scholar]
- 98. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488, 2002 [DOI] [PubMed] [Google Scholar]
- 99. Zumbach MS, Boehme MW, Wahl P, Stremmel W, Ziegler R, Nawroth PP. Tumor necrosis factor increases serum leptin levels in humans. J Clin Endocrinol Metab 82: 4080–4082, 1997 [DOI] [PubMed] [Google Scholar]