Abstract
Endothelium-dependent, nitric oxide (NO)-mediated vasodilation can be impaired by reactive oxygen species (ROS), and this deleterious effect of ROS on NO availability may increase with aging. Endothelial function declines rapidly after menopause, possibly because of loss of circulating estrogen and its antioxidant effects. The purpose of the current study was to determine the role of O2− and H2O2 in regulating flow-induced dilation in coronary arterioles of young (6-mo) and aged (24-mo) intact, ovariectomized (OVX), or OVX + estrogen-treated (OVE) female Fischer 344 rats. Both aging and OVX reduced flow-induced NO production, whereas flow-induced H2O2 production was not altered by age or estrogen status. Flow-induced vasodilation was evaluated before and after treatment with the superoxide dismutase (SOD) mimetic Tempol (100 μM) or the H2O2 scavenger catalase (100 U/ml). Removal of H2O2 with catalase reduced flow-induced dilation in all groups, whereas Tempol diminished vasodilation in intact and OVE, but not OVX, rats. Immunoblot analysis revealed elevated nitrotyrosine with aging and OVX. In young rats, OVX reduced SOD protein while OVE increased SOD in aged rats; catalase protein did not differ in any group. Collectively, these studies suggest that O2− and H2O2 are critical components of flow-induced vasodilation in coronary arterioles from female rats; however, a chronic deficiency of O2− buffering by SOD contributes to impaired flow-induced dilation with aging and loss of estrogen. Furthermore, these data indicate that estrogen replacement restores O2− homeostasis and flow-induced dilation of coronary arterioles, even at an advanced age.
Keywords: superoxide dismutase, hydrogen peroxide, ovariectomy, estrogen replacement
advancing age is a significant risk factor in the development of cardiovascular disease (1); however, striking gender differences exist in the chronological development of heart disease. Women experience relative cardioprotection until menopause, at which point the risk for cardiovascular disease equals or exceeds that of their male counterparts (36). The loss of estrogen at menopause presumably contributes to the increase in cardiovascular disease prevalence in postmenopausal women; however, findings from the Women's Health Initiative (WHI) trial indicate that hormone replacement therapy (HRT) may actually increase the risk for coronary artery disease (28). The mechanisms underlying these contradictory effects of estrogen loss and replacement on cardiovascular disease risk remain unknown. Furthermore, the hormonal shift and accompanying increase in cardiovascular risk that occur at menopause beg the possibility that basic cardiovascular aging processes differ between males and females.
Nitric oxide (NO)-mediated signaling declines with age (2, 19, 45). In contrast, estrogen enhances endothelial nitric oxide synthase (eNOS) expression (30) and nitric oxide synthase (NOS) signaling (44, 47) and may also contribute to NO bioavailability in the vasculature through enhancement of antioxidant function. In mesenteric arterioles of ovariectomized rats, superoxide (O2−) levels were reduced following estrogen treatment (7). Similarly, estrogen supplementation reduced basal O2− production in rat aorta (13). Although the beneficial effects of estrogen in the vasculature have been well documented, most studies have been performed in young animals (7, 29, 42, 48) or in large arteries (13, 16, 17, 27). We have previously demonstrated an age-induced impairment of flow-induced, NO-mediated dilation in coronary arterioles from male and female rats (24, 26). In coronary arterioles from aged male rats, an imbalance in antioxidant defense mechanisms contributes to reduced NO availability (21); however, despite the known antioxidant effects of estrogen (7, 43), the impact of estrogen on reactive oxygen species (ROS) and regulation of NO signaling in the coronary resistance vasculature of aging females remains poorly understood.
ROS have been implicated in the development of age-related endothelial dysfunction (6, 10). Furthermore, elevated hydroxyl radical formation contributes to impairment of flow-induced vasodilation in coronary arterioles from aged male rats (21). Although we have reported an age-related reduction in flow-induced, NO-mediated vasodilation in coronary resistance vessels from female rats (23), the contribution of oxidant stress to this loss of NO-mediated dilation has not been determined. Therefore, we hypothesized that ROS scavenging and estrogen replacement will improve age-related impairments in flow-induced vasodilation in coronary arterioles by increasing NO bioavailability.
METHODS
Animals.
All procedures in this study were approved by the Institutional Animal Care and Use Committee at West Virginia University. All methods fully complied with guidelines set in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, revised 1996). Young (4 mo) and old (22 mo) female Fischer 344 rats were obtained from the National Institute of Aging colony and housed during treatment for 2 mo so that ages at death were 6 and 24 mo, respectively. These rats are sexually mature adult animals at 4 mo, while 24-mo-old rats are senescent, with an ∼50% colony mortality rate at this age. Animals were housed at 23°C with a 12:12-h light-dark cycle and provided water and phytoestrogen-free rat chow ad libitum. Estrous cycles were monitored by daily vaginal smears to determine estrous status at the time of death, and subsequent analysis revealed no correlation between estrous status and vascular reactivity, as previously described (23).
Ovariectomies (OVX) were performed 8 wk before experimentation, as previously described (23). Estrogen replacement (OVE) was performed simultaneous to OVX. Two 0.05-mg 17β-estradiol 60-day slow-release pellets (Innovative Research, Toledo, OH) were implanted subcutaneously.
Microvessel preparation.
Rats were anesthetized (5% isoflurane/O2 balance) and killed by excision of the heart, which was immediately placed in cold, filtered physiological saline solution (PSS, pH 7.4) containing (in mM) 145 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 3.0 MOPS buffer, and 1% BSA. Resistance arterioles (<150 μm) were isolated from the left anterior descending coronary artery distribution as previously described (40). Arterioles were cannulated on pipettes matched for size and resistance in a Lucite chamber that contained PSS equilibrated with room air. In coronary arterioles <150 μm, intraluminal pressure is ∼40–50 mmHg (3), and mean arterial pressure and cardiac output are similar between young and old Fischer-344 rats (9); therefore, mean intraluminal pressure was maintained at 60 cmH2O in arterioles from both young and old rats for all experiments. Arterioles with leaks were discarded. Vessels determined to be free of leaks were warmed to 37°C and allowed to equilibrate for ∼30 min until developing spontaneous tone. Only arterioles that developed and maintained >20% tone were used to assess vasodilatory responses.
Evaluation of vasodilator responses to intraluminal flow.
Arterioles were exposed to graded increases in intraluminal flow by adjusting the height of fluid reservoirs in equal and opposite directions, thereby creating a pressure difference across the arterioles without altering intraluminal pressure within the arterioles (22). Diameter measurements were determined in response to pressure differences of 2, 4, 10, 20, 40, and 60 cmH2O, corresponding to physiologically significant flow rates from 5 to 50 nl/s (31). In a subset of vessels of similar diameter to those used to assess functional dilation to flow, red blood cell velocity (Vrbc) was measured at the same pressure differences. Volumetric flow was calculated from mean Vrbc and inner diameter as previously described (25, 31).
To examine the effects of free radical scavenging on flow-induced dilation in coronary arterioles, responses were also assessed in the presence of the cell-permeable superoxide dismutase (SOD) mimetic Tempol [100 μM (32)], the H2O2 scavenger catalase [100 U/ml (12)], or the Cu/Zn SOD inhibitor diethyldithiocarbamate [DETC, 1 mM (11)]. Each pharmacological agent was applied for 20 min before evaluation of the flow response. In some experiments, flow responses in the presence of pharmacological agents were performed after completion of a control response. In instances where a complete control response was not obtained, verification of endothelial responsiveness was assessed before pharmacological intervention by exposing the vessel to a brief period of flow (5 nl/s). The order of interventions was randomized except that a control flow response was always obtained before any pharmacological intervention was performed. Vessels that did not respond to flow under control conditions were discarded.
Fluorescence detection of NO and H2O2.
Vessels were cannulated and allowed to equilibrate for ∼30 min until achieving spontaneous tone. Before fluorescent reagents were added, autofluorescence levels were recorded and were not significantly different between groups (data not shown). Flow-induced production of NO was detected with the cell-permeable fluorescent reagent 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate (DAF, 5 μM) at an excitation wavelength of 490 nm and an emission wavelength of 515 nm. To examine flow-induced production of H2O2, vessels were treated with 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (DCF, 5 μM). DCF fluorescence was visualized at 475 and 515 nm excitation and emission wavelengths, respectively, with a 0.5 neutral density filter (Zeiss) to minimize photo-oxidation. Because albumin may interfere with DAF and DCF fluorescence detection, albumin-free solutions were used for all fluorescence experiments. Intraluminal loading was performed by introducing either DAF or DCF in one perfusion pipette and establishing 20 nl/s flow through the arteriole for 2 min. Flow through the arteriole was then arrested, and the dye was incubated in the arteriole for 5 min. Following incubation, reverse flow was initiated (20 nl/s) and maintained through the vessel for 10 min to wash all dye from the vessel lumen and perfusion pipettes. The arteriole was then allowed to equilibrate for an additional 5 min before a 20-min incubation with vehicle, catalase (100 U/ml), or NG-nitro-l-arginine methyl ester (l-NAME; 10 μM). Fluorescent images were recorded using an Axiovert 40CFL inverted microscope (Zeiss) equipped with an HBO 50 superpressure mercury lamp (Zeiss). At the beginning of each experiment, a baseline image was recorded in the absence of flow. Camera (Axiocam; Zeiss) settings were adjusted to this image and maintained throughout the course of the experiment (34). Focal plane was adjusted to maintain the same user-defined region of interest (ROI) as the vessel dilated to flow. Fluorescent images were captured every 15 s during a 2-min exposure to flow at 7.5, 35, and 50 nl/s for a total of 6 min. At the conclusion of each experiment, vessels were exposed to the NO donor DEA-NONOate (1 μM) or H2O2 (10 mM) to verify DAF or DCF, respectively, was sufficiently loaded through the duration of the experiment.
Fluorescence analysis.
A user-defined segment of the central portion of each vessel (ROI) was analyzed for fluorescence intensity using ImageJ software (National Institutes of Health). Total fluorescence intensity was calculated as average fluorescence intensity per pixel × surface area. Basal fluorescence levels varied among ROIs and in different experiments. Therefore, changes in fluorescence intensity were expressed as [(F − F0)/F0] × 100, where F is fluorescence intensity during flow and F0 is baseline fluorescence intensity (49). Both baseline and flow fluorescence intensity values were measured within the same ROIs in the same focal plane of the vessel.
Evaluation of vasodilator responses to H2O2.
To examine the effects of aging and estrogen status on arteriolar responsiveness to H2O2, concentration-response curves were determined. Isolated microvessels were equilibrated and allowed to develop spontaneous tone. Vasoreactivity to cumulative additions of extraluminal H2O2 (100 nM-10 mM, 3-min stages) were recorded.
Immunoblot analysis.
Coronary arterioles (5 pooled vessels/sample) were dissected and immediately snap-frozen and stored at −80°C until use. Vessels were lysed in 1× sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 6 M urea, 160 mM dithiothreitol, and 0.1% bromphenol blue), and protein concentration was determined using the NanoOrange Protein Quantification Kit (Invitrogen). Samples (10 μg total protein) were subjected to SDS-PAGE (10%) and then transferred to polyvinylidene difluoride membranes. Membranes were blocked for 1 h at room temperature (5% nonfat dry milk in Tris-buffered saline + 0.1% Tween 20) and then incubated overnight at 4°C with catalase (1:6,000; Chemicon), Cu/Zn SOD (1:1,000; Stressgen), nitrotyrosine (NT, 1:500; Abcam), or β-actin (1:4,000; Cell Signaling). After being washed, membranes were incubated with respective horseradish peroxide-conjugated secondary antibody (Cell Signaling) for 1 h at room temperature. Peroxidase activity was determined using SuperSignal West Femto (Pierce), with image analysis performed using ImageJ. Loading differences were normalized by expressing all data as relative densitometric units of the protein of interest vs. β-actin.
Data presentation and analysis.
All data are expressed as means ± SE. Development of spontaneous tone was expressed as the percent constriction relative to maximal diameter and was calculated as follows:
where IDmax is the maximal inner diameter recorded at a pressure of 60 cmH2O under Ca2+-free conditions and IDb is the steady-state baseline diameter. The vasodilator responses to flow and H2O2 are expressed using the following equation:
where IDs is the arteriolar diameter during flow, IDb is the diameter recorded immediately before initiation of the flow- or concentration-diameter curves, and IDmax is the maximal diameter for the arteriole.
Responses to flow and H2O2 were evaluated by ANOVA to detect main effects between (age and estrogen status) or within (flow rate, concentration of H2O2, or inhibitor treatment) groups. Post hoc analyses were performed using Bonferroni's test for pairwise comparisons. Group differences in animal and vessel characteristics were compared by Student's t-test. Differences in fluorescence intensity and protein content were assessed using two-way ANOVA with correction using the general linear model. Statistical significance was defined as P ≤ 0.05.
RESULTS
Animal and vessel characteristics.
Body weight, heart weight, and heart weight-to-body weight ratios increased with age. Uterine weight-to-body weight ratio, a biomarker of estrogen status, declined with aging in intact animals and was markedly reduced in both young and old OVX rats. Estrogen replacement significantly increased the uterine weight-to-body weight ratio compared with OVX in both age groups. These data confirm previous work from our laboratory examining the effects of estrogen replacement on estradiol levels and other estrogen biomarkers (23). Advancing age did not alter spontaneous tone in coronary arterioles from intact rats. Similarly, estrogen status did not significantly alter tone within either age group, and maximal diameter was not different in vessels regardless of age or estrogen status (Table 1). Basal tone increased following treatment with Tempol and catalase in both young and aged intact groups.
Table 1.
Animal and vessel characteristics
| Young |
Old |
|||||
|---|---|---|---|---|---|---|
| Intact | OVX | OVE | Intact | OVX | OVE | |
| n | 34 | 21 | 23 | 37 | 18 | 31 |
| Animal characteristics | ||||||
| Body wt, g | 196 ± 4 | 249 ± 4† | 215 ± 3†‡ | 300 ± 4* | 310 ± 6 | 274 ± 4†‡ |
| Heart wt, mg | 538 ± 11 | 623 ± 14† | 653 ± 13† | 766 ± 9* | 780 ± 17 | 794 ± 21 |
| HW/BW | 2.76 ± 0.07 | 2.50 ± 0.04† | 3.04 ± 0.05†‡ | 2.56 ± 0.04* | 2.45 ± 0.05 | 2.96 ± 0.06†‡ |
| Uterine wt, mg | 551 ± 32 | 97 ± 7† | 253 ± 18†‡ | 564 ± 32 | 279 ± 25† | 725 ± 52†‡ |
| UW/BW | 2.73 ± 0.17 | 0.40 ± 0.03† | 1.18 ± 0.08†‡ | 1.91 ± 0.13* | 0.90 ± 0.09† | 2.67 ± 0.20†‡ |
| Vessel characteristics | ||||||
| Maximal diameter, μm | 123 ± 4 | 123 ± 5 | 124 ± 6 | 124 ± 4 | 119 ± 5 | 131 ± 5 |
| Tone, % | ||||||
| Baseline | 25 ± 1 | 24 ± 1 | 25 ± 1 | 27 ± 1 | 29 ± 2 | 29 ± 2 |
| Posttempol | 35 ± 3# | 22 ± 1 | 29 ± 3 | 37 ± 3# | 35 ± 5 | 33 ± 3 |
| Postcatalase | 36 ± 3# | 24 ± 1 | 27 ± 2 | 35 ± 3# | 32 ± 4 | 36 ± 4 |
| Post-DETC | 21 ± 1 | 21 ± 1 | 23 ± 2 | 21 ± 1 | 22 ± 1 | 21 ± 1 |
Data are presented as means ± SE;
n, no. of mice. OVX, ovariectomized; OVE, OVX + estrogen treated; HW/BW, heart weight-to-body weight ratio; UW/BW, urine weight-to-body weight ratio; DETC, diethyldithiocarbamate.
P < 0.05 vs. young intact (*), vs. age-matched intact (†), vs. age-matched OVX (‡), and vs. baseline (#).
Vasodilator responses to intraluminal flow.
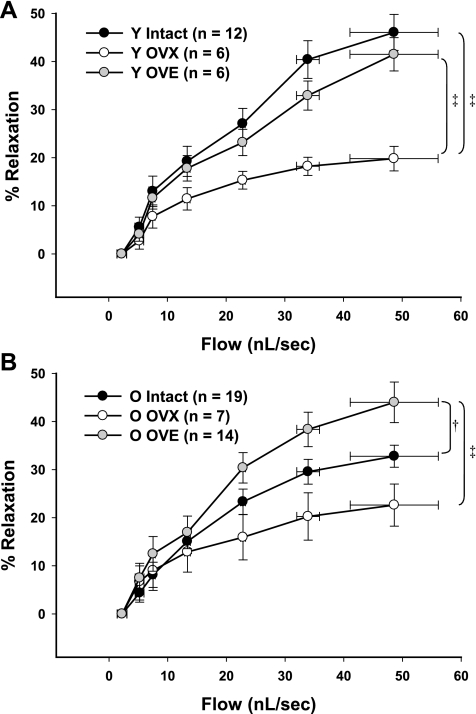
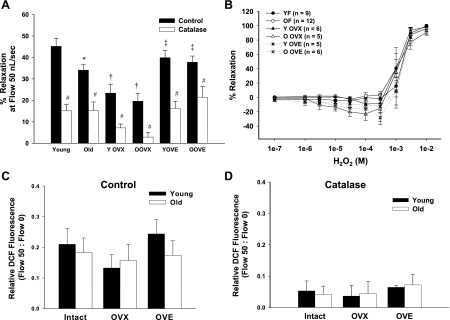
Flow-induced vasodilation was significantly impaired in coronary arterioles from aged compared with young intact rats (P < 0.05; Fig. 1), consistent with previous findings from our laboratory in arterioles from aged male and female rats (24, 26). Maximal dilation in vessels from young intact females reached ∼46% (Fig. 1A) compared with a maximal dilation (∼32%) in vessels from aged intact females (Fig. 1B). OVX reduced flow-induced dilation in arterioles from young rats to a greater extent than in arterioles from old rats, thereby eliminating age-related differences (Fig. 1). Estrogen replacement in young rats restored flow-induced vasodilation to levels similar to those of intact animals (Fig. 1B). In old rats, estrogen replacement increased flow-induced dilation to levels surpassing those of intact rats (Fig. 1B).
Fig. 1.
Flow-induced vasodilation in coronary arterioles from female rats. Aging significantly impaired flow-mediated dilation in arterioles from intact rats (A and B). A: in arterioles from young (Y) females, estrogen restored ovariectomized (OVX)-induced impairment of flow-induced dilation. B: in aged females, estrogen enhanced flow-induced dilation compared with the dilation in arterioles from both intact and OVX rats. OVE, OVX + estrogen treated. Data are presented as means ± SE. ‡P < 0.05 vs. age-matched OVX. †P < 0.05 vs. age-matched intact.
Flow-induced NO production.
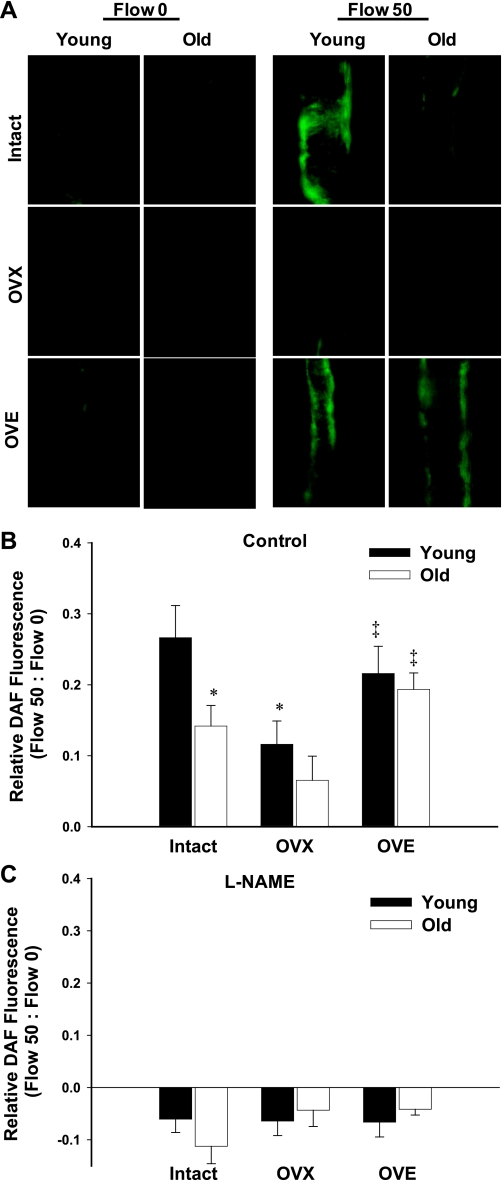
DAF fluorescence microscopy was used to examine flow-induced NO production in isolated coronary arterioles from young and aged females of varying estrogen status. At maximal flow (50 nl/s), DAF fluorescence was significantly reduced in vessels from aged intact rats compared with those from young intact rats (Fig. 2). OVX reduced flow-induced NO availability in arterioles from young but not old rats. In both young and aged rats, estrogen replacement enhanced DAF fluorescence compared with age-matched OVX (Fig. 2B). Hence, age-related differences in flow-induced increases in NO were eliminated in both OVX and OVE rats. l-NAME, a NOS inhibitor, eliminated flow-induced increases in DAF fluorescence in arterioles from all groups, confirming DAF specificity for NO and the contribution of NO to flow-induced vasodilation (Fig. 2C).
Fig. 2.
Flow-induced nitric oxide (NO) production in coronary arterioles as measured by 4-amino-5-methylamino-2′2′-difluorofluorescein diacetate (DAF) fluorescence. A: representative images of vessels from young and old intact, OVX, and OVE rats at maximal flow (50 nl/s). B: DAF fluorescence increased in coronary arterioles from all groups at 50 nl/s flow (n = 6–11 mice/group). NO production in response to flow declined with age in intact females, as evidenced by reduced DAF intensity. OVX reduced NO production in vessels from young females. Estrogen replacement enhanced NO availability in vessels from old rats (A and B). C: treatment with the nitric oxide synthase (NOS) inhibitor NG-nitro-l-arginine methyl ester (l-NAME) inhibited flow-induced NO production in all groups (n = 3/group). Data are presented as means ± SE. *P < 0.05 vs. young intact. ‡P < 0.05 vs. age-matched OVX.
Effect of O2− scavenging on vasodilator responses to intraluminal flow.
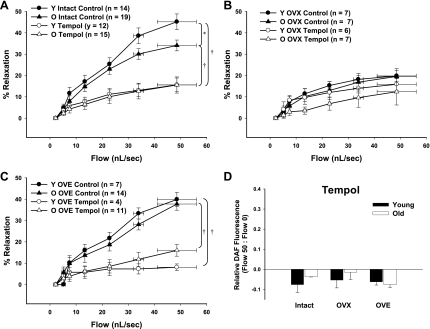
To determine the effects of O2− scavenging on flow-induced dilation in young and old female rats of varying estrogen status, coronary arterioles were incubated with the SOD mimetic Tempol (100 μM) before exposure to flow. Tempol significantly reduced flow-induced dilation in coronary arterioles from intact young and aged females (Fig. 3A). In contrast, treatment with Tempol did not significantly alter flow-induced dilation in either OVX-treated group (Fig. 3B). Similar to vessels from intact rats, flow-induced dilation of arterioles from OVE animals was also impaired by Tempol (Fig. 3C). Similar findings were obtained when coronary arterioles were treated with polyethylene glycol-conjugated SOD (PEG-SOD; data not shown).
Fig. 3.
Flow-induced dilation in coronary arterioles following treatment with the superoxide dismutase (SOD) mimetic Tempol significantly inhibited flow-mediated dilation in vessels from intact and OVE rats (A and C) but did not significantly alter dilation in vessels from OVX rats (P = 0.08; B). D: Tempol similarly blunted flow-induced DAF fluorescence in all groups (n = 3/group). O, old. Data are presented as means ± SE. *P < 0.05 vs. young intact. †P < 0.05 vs. age-matched intact.
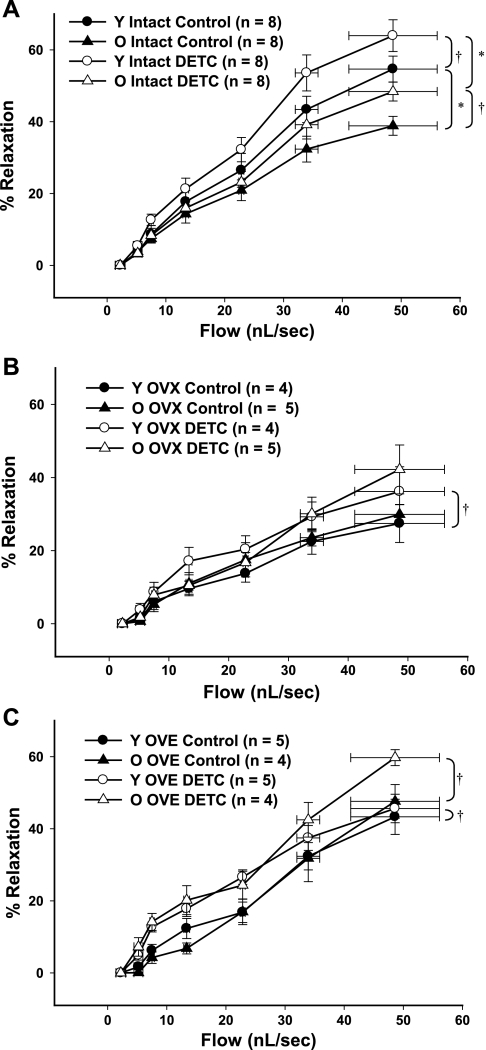
Coronary arterioles were incubated with the Cu/Zn SOD inhibitor DETC (1 mM) to determine the effects of SOD inhibition on flow-induced dilation. In coronary arterioles from young rats, inhibition of O2− scavenging with DETC increased flow-induced dilation, regardless of estrogen status (Fig. 4). In old rats, DETC improved flow-induced vasodilation in coronary arterioles from intact and OVE groups (Fig. 4, A and C), but not in the OVX group (Fig. 4B).
Fig. 4.
A–C: flow-induced dilation in coronary arterioles treated with diethyldithiocarbamate (DETC), an inhibitor of Cu/Zn SOD. DETC increased flow-induced vasodilation in coronary arterioles from all groups of young female rats. In old rats, DETC increased flow-induced vasodilation in intact and OVE groups. Data are presented as means ± SE. *P < 0.05 vs. treatment-matched young. †P < 0.05 vs. age-matched intact.
Effect of O2− scavenging on NO production.
To assess the effect of O2− scavenging on flow-induced increases in NO, DAF fluorescence was assessed in vessels treated with Tempol. Similarly to l-NAME, Tempol eliminated flow-induced increases in DAF fluorescence in vessels from all groups (Fig. 3D), indicating that at least some amount of O2− is required to elicit flow-induced increases of NO in rat coronary arterioles.
Role of H2O2 in flow-induced dilation.
To determine the role of H2O2 in flow-induced dilation of coronary arterioles from young and old female rats of varying estrogen status, coronary arterioles were incubated with the H2O2 scavenger catalase (100 U/ml) before exposure to flow. Treatment with catalase significantly blunted flow-induced vasodilation in all groups, regardless of age or estrogen status (Fig. 5A). To determine whether aging or hormone status alters dilation to authentic H2O2 in coronary arterioles, concentration-response curves to H2O2 (100 nM-10 mM) were assessed. Ten millimolar H2O2 elicited maximal dilation in arterioles from all groups (Fig. 5B). Responsiveness to H2O2 did not differ between groups.
Fig. 5.
Contribution of H2O2 to vasodilation in coronary arterioles of female (F) rats. A: catalase blunted flow-induced dilation in all groups, regardless of age or hormone status (n = 6–12/group). B: H2O2 dilated coronary arterioles concentration dependently similarly in all groups. C: flow-induced H2O2 production in coronary arterioles as measured by DCF fluorescence (n = 3–6/group). DCF fluorescence increased with flow (0–50 nl/s) in coronary arterioles from young and old rats, irrespective of hormone status. D: catalase blunted DCF fluorescence in arterioles from all groups (n = 3/group). Data are presented as means ± SE. *P < 0.05 vs. young intact. †P < 0.05 vs. age-matched intact. ‡P < 0.05 vs. age-matched OVX. #P < 0.05 vs. respective control.
DCF fluorescence was used to assess flow-induced changes in H2O2. Fluorescence increased in response to increasing flow rates in arterioles from all groups (Fig. 5C); however, maximal fluorescence at 50 nl/s flow did not differ between any groups, suggesting that flow-induced H2O2 production does not change significantly with aging or alterations in hormone status (Fig. 5C). Catalase treatment blunted flow-induced increases in DCF fluorescence in arterioles from all groups, demonstrating specificity of increases in dye fluorescence to H2O2 (Fig. 5D).
Immunoblot analysis.
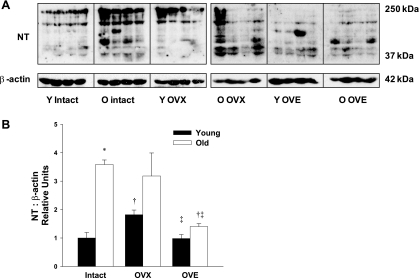
NT, an indicator of peroxynitrite (ONOO−)-induced cell damage, dramatically increased with aging in vessels from old vs. young intact females (Fig. 6). In vessels from young rats, loss of ovarian hormones augmented NT abundance, and this effect was reversed by estrogen replacement. In contrast, OVX did not alter NT levels in arterioles from old rats, and OVE significantly reduced NT levels compared with those in arterioles from both OVX and intact rats (Fig. 6).
Fig. 6.
A: representative immunoblot of nitrotyrosine (NT), a marker of peroxynitrite (ONOO−)-induced cellular damage. B: aging dramatically increased NT protein levels in coronary arterioles from intact females. In young females, OVX elevated NT protein expression, and estrogen treatment restored NT to levels comparable to intact rats. In aged females, estrogen replacement significantly reduced NT compared with intact and OVX rats (n = 6–8/group). Data are presented as means ± SE. *P < 0.05 vs. young intact. †P < 0.05 vs. age-matched intact. ‡P < 0.05 vs. age-matched OVX.
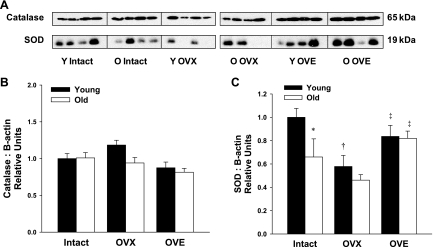
Endogenous catalase protein levels in coronary arterioles of female rats did not vary with age or changes in estrogen status (Fig. 7B). Arteriolar Cu/Zn SOD protein declined with advancing age (P < 0.05). In coronary arterioles from young females, OVX reduced Cu/Zn SOD protein expression, and estrogen replacement restored Cu/Zn SOD levels to those of intact rats (Fig. 7C). In aged females, OVX did not alter Cu/Zn SOD levels, while estrogen replacement enhanced Cu/Zn SOD protein expression compared with vessels from both intact and OVX females (Fig. 7C). Thus, following estrogen replacement, age-related differences in Cu/Zn SOD expression were eliminated.
Fig. 7.
A: representative immunoblots of catalase and SOD protein expression in coronary arterioles from young and aged female rats. B: arteriolar catalase expression did not vary with age or estrogen status (n = 7–8) while SOD protein levels declined with both age and OVX. C: protein expression for catalase and SOD was normalized to the β-actin blot shown in Fig. 6A. In coronary microvessels from aged females, SOD expression increased following estrogen treatment (n = 6–8). Data are presented as means ± SE. *P < 0.05 vs. young intact. †P < 0.05 vs. age-matched intact. ‡P < 0.05 vs. age-matched OVX.
DISCUSSION
Endothelium-dependent, NO-mediated vasodilation plays a major role in regulation of coronary blood flow (20); however, in the presence of O2−, the beneficial effects of NO are reduced as NO and O2− combine to form ONOO−. Therefore, the purpose of the current study was to determine whether advancing age and declining estrogen alter ROS signaling and NO-mediated dilation of coronary resistance arterioles. We have confirmed our earlier findings that both age and diminished estrogen reduce NO-mediated dilation of coronary arterioles (23), and we now extend those findings by showing that impairment of O2− buffering contributes to this decline in NO-mediated vasodilation. This decline in NO signaling is paralleled by a decrease in Cu/Zn SOD protein and an increase in NT, a marker of cellular oxidative stress, all of which are restored similar to young intact levels following estrogen treatment. Collectively, our data support the conclusion that the decline in NO-mediated dilation that occurs with age or diminished estrogen results, at least in part, from impaired regulation of ROS, and, in particular, O2−. Importantly, our work shows for the first time that the age-related loss of NO-mediated dilation related to heightened oxidant stress can be reversed in females by exogenous estrogen supplementation and improvement of antioxidant defense mechanisms.
The myocardium metabolizes nearly two-thirds of available oxygen in the coronary circulation at rest (39). The heart must therefore rely on increases in flow for sufficient perfusion, and reduction in coronary blood flow, such as occurs with aging, might have detrimental effects on cardiac function. Stepp and colleagues (41a) previously demonstrated resting coronary arteriolar blood flows ranging from 0 to 13 mm/s, which approximates 0–20 nl/s flow rate in the current isolated vessel model. Our data indicate that flow-mediated dilation is significantly impaired with aging at higher flow rates of ∼30–50 nl/s (Fig. 3A).
Hachamovitch et al. (15) reported a 43% reduction in maximal coronary blood flow with aging in conscious male Fischer 344 rats, reflecting a significant loss of coronary flow reserve. Additionally, we have shown that the age-induced impairment of flow occurs predominantly at flow rates corresponding to in vivo flows that would be generated by intense demand or reduced oxygen availability (5). In aged female Wistar-Imamichi rats, coronary blood flow was diminished compared with young rats following NOS inhibition by NG-nitro-l-arginine (14). In contrast, we have previously reported that NOS inhibition with l-NAME impairs flow-mediated dilation in coronary arterioles from young, but not aged, Fischer 344 rats (23). Following estrogen replacement, however, l-NAME impairs flow-induced dilation similarly in both age groups (23). Collectively, these data suggest that both aging and estrogen status alter NOS mediation of NO-dependent flow-mediated dilation.
Previous work in coronary arteries of estrogen receptor (ER)-α knockout mice showed reduced responsiveness to ACh following OVX, with restored endothelium-dependent vasodilation following chronic estrogen treatment (30). Consistent with these reports, OVX blunted flow-induced vasodilation in arterioles from both young and aged females (Fig. 1). OVE restored flow-induced vasodilation in arterioles from young rats (P = 0.44; Fig. 1A) and, notably, enhanced flow-induced dilation in arterioles from old rats to levels similar to that of young intact rats (Fig. 1). Flow-induced NO availability also declined with age in arterioles from intact females (Fig. 2). Removal of ovarian hormones significantly diminished flow-induced NO production in vessels from young but not old females, and estrogen treatment enhanced NO availability in arterioles from old OVX females (Fig. 2). These results are consistent with work performed in cultured endothelial cells, where incubation with estrogen increased NO production (35, 37). Our data suggest that estrogen administered immediately following OVX can enhance endothelial NO availability in coronary arterioles, even at an advanced age.
We hypothesized that ROS scavenging would increase NO availability and improve endothelium-dependent, flow-induced dilation of coronary arterioles, particularly in aged and OVX groups. Contrary to our expectations, we found that the O2− scavenger Tempol blunted flow-induced increases in NO and subsequent vasodilation in arterioles from intact and OVE females (Fig. 3, A and C). We confirmed our Tempol findings with the use of an alternative SOD mimetic, PEG-SOD (data not shown). We also performed experiments using DETC to inhibit endogenous SOD and found that flow-induced vasodilation was augmented in arterioles from all rats except those in the old OVX group (Fig. 4). These results contrast previous reports describing the beneficial effects of exogenous SOD and its analogs on vascular function. In OVE and OVX rats, SOD mimetic manganese(III) tetrakis(1-methyl-4-pyridyl)porphyrin reduced hydroethidine staining in mesenteric arterioles by 75% (7), and, in aged OVX rats, treatment with the SOD mimetic EUK-8 improved cardiac functional recovery following ischemia-reperfusion injury (48). Our data more closely parallel a recent study in aged rats in which skeletal muscle blood flow declined following incubation with Tempol (18). Although supplementation with SOD may diminish O2−, this treatment may also drive formation of H2O2 and ultimately increase intracellular and highly cytotoxic hydroxyl radical (33). It is also possible that acute pharmacological scavenging of O2− leads to a disturbance in NO metabolic pathways, including formation of ONOO−, which may in some vascular beds act as an intermediate NO donor (33). We previously reported that Tempol impaired flow-induced vasodilation in coronary arterioles of male rats (20). This impairment was reversed by treatment with the iron chelator deferoxamine, suggesting that exogenous SOD resulted in excessive production of H2O2 and OH− through activation of Fenton chemistry (20). A similar mechanism may contribute to the impairment of flow-induced dilation in coronary arterioles from young and OVE female rats when the balance between SOD and catalase activity is disrupted by addition of SOD. Future experiments will be needed to address the possibility that acute SOD supplementation and formation of OH− contributes to the impaired flow-induced dilation that occurs with Tempol treatment. Our current data suggest that O2−-derived species are integral in flow-induced signaling in coronary arterioles and that altering endogenous production of these O2−-derived species reduces flow-induced vasodilation.
In contrast, our data suggest that chronic deficiency of SOD in arterioles from aged or OVX rats leads to ONOO−-induced nitrosylation of proteins that are critical to NOS-mediated, flow-induced vasodilation. Restoring endogenous Cu/Zn SOD protein levels with exogenous estrogen reduced NT (Fig. 6) and improved NO-mediated vasodilation (Fig. 1). These results suggest that, although O2−-derived species may be necessary for flow-induced signaling to occur, chronic reduction of the O2−-buffering capacity within the endothelium contributes to the impairment of flow-induced vasodilation in coronary arterioles in aged rats. Further experiments will be necessary to determine whether long-term treatment with SOD mimetics or molecular manipulation of SOD expression can reverse the impairment of flow-induced dilation that occurs with age and/or declining ovarian estrogen.
Oxidant stress has been implicated in the development of endothelial dysfunction (4), and these effects may be exacerbated with advancing age (6). Estrogen, conversely, is correlated with reduced oxidant stress and enhanced antioxidant activity (7, 42). Immunoblot analysis revealed elevated NT with aging and after OVX, which was restored similar to young intact levels following estrogen treatment (Fig. 6). Cu/Zn SOD protein decreased when circulating estrogen was abruptly reduced by OVX in young rats (Fig. 7), likely contributing to increased ONOO− formation and damage to proteins critically involved in NO signaling and NO-mediated vasodilation. In aging female rats, reductions in circulating estrogen may contribute to the age-related decrease observed in Cu/Zn SOD (Fig. 7) and increased abundance of NT (Fig. 6). Previous studies have demonstrated direct transcriptional upregulation of MnSOD and extracellular SOD by estrogen in vascular smooth muscle cells (43), as well as increased Cu/Zn SOD protein in coronary arteries from ERα knockout mice (30). Considered together, these data suggest that estrogen is an important regulator of SOD expression. Furthermore, impaired Cu/Zn SOD due to loss of circulating estrogen may cause long-term endothelial damage, leading to the ultimate decline in NO-mediated vasodilation in coronary arterioles of aged female rats.
Catalase reduced flow-induced dilation in all groups (Fig. 5A). Direct measurements of flow-induced H2O2 production by DCF fluorescence, as well as reactivity to exogenous H2O2, revealed no changes with age (Fig. 5). These data contrast our findings in coronary arterioles from male rats, where a reduction of H2O2 signaling contributes to age-induced impairment of flow-induced vasodilation (21). In vessels from wild-type and ERβ knockout male mice, catalase induced similar impairment of endothelium-mediated vasodilation (26). In contrast, catalase inhibition of flow-induced dilation was similar or greater in arterioles from OVX rats compared with those from age-matched intact or OVE rats (Fig. 5A). This suggests that the relative contribution of H2O2 to flow-induced dilation may increase with removal of circulating estrogen, thereby compensating for the loss of flow-induced NO signaling that occurred in arterioles from OVX rats.
Our study did not identify the source(s) of O2−derived species that contribute to flow-induced signaling in coronary arterioles from female rats. Trott and colleagues (46) recently reported that NAD(P)H-derived H2O2 contributes to ACh-induced dilation in skeletal muscle resistance arteries of young rats but that NAD(P)H-derived O2− reduces endothelium-dependent vasodilation in arteries from old rats. In skeletal muscle arterioles, we have reported an age-related decrease in flow-induced, NO-mediated vasodilation that is accompanied by increased O2− production by uncoupled eNOS (41). Surprisingly, exercise training increased both eNOS-derived NO and O2− signaling in response to intraluminal flow (41). Thus the relative source and contribution of NO- and O2−-derived species to endothelium-dependent vasodilation may be altered by physiological and pathophysiological stress. In order for effective therapies to be developed, future studies will need to investigate the effects of age and alterations of estrogen status on the source of ROS in coronary arterioles.
OVX significantly increased body weight in young rats. This increase in body weight may be attributed, in part, to the loss of the anorexigenic effects of estrogen (38), and it follows that estrogen replacement results in a loss of body weight in both young and aged rats. Because endogenous estrogen levels are initially low in aged intact females, removing the remaining estrogen had an insignificant effect on both body and uterine weight. In young animals, OVX also increased heart weight. Following loss of ovarian hormones, it is likely that the anabolic characteristics of the remaining cortisol-derived androgenic hormones dominate, resulting in a general increase in muscle mass. In both age groups, OVE resulted in increased heart weight-to-body weight ratios compared with OVX and intact animals, primarily because of the decrease in body weight in OVE animals. Current evidence largely supports the beneficial effects of estrogen on cardiovascular function (8), despite WHI reports to the contrary (28). The increased risk for coronary heart disease described in the WHI clinical trials demanded closer investigation of the effects of HRT on the cardiovascular system. Subsequent analysis revealed that timing of HRT might be of utmost importance in determining future cardiovascular risk; in the majority of animal studies emulating HRT, estrogen replacement was initiated immediately following OVX (7, 29, 48). The effects of estrogen on metabolism and body mass may contribute to the varied effects of HRT that depend on timing relative to menopause or OVX. Our data in coronary arterioles support early initiation of HRT (before significant weight gain occurs) to maximize the beneficial effects on NO-mediated signaling and coronary blood flow.
In summary, our data confirm that flow-induced dilation of rat coronary arterioles is highly dependent on NO bioavailability. Direct NO measurements indicate that both age and loss of circulating estrogen due to ovariectomy reduce NO availability in rat coronary arterioles. Additionally, our data demonstrate that age-related impairments of flow-induced NO-mediated dilation may be related to decreases in circulating estrogen. With advancing age or a loss in circulating estrogen, reduced Cu/Zn SOD expression and increased protein nitration correlate with a loss of flow-induced signaling. Furthermore, in young animals, excessive dismutation of O2− by exogenous SOD may inhibit normal NO-mediated signaling, impairing flow-induced dilation. Thus maintenance of carefully regulated NO signaling is critical to healthy flow-induced dilation in the coronary resistance vasculature of female rats. Circulating estrogen appears to be an important regulator of NO signaling in coronary arterioles; thus, estrogen supplementation can improve NO availability, even at an advanced age.
GRANTS
This work was supported by National Institutes of Health Grants HL-077224 and HL-090937 (to J. M. Muller-Delp) and HL-027339 and HL-094447 (to S. J. Mustafa).
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. American Heart Association Heart Disease, and Stroke Statistics-2007 Update At-a-Glance. Dallas, TX: American Heart Association, 2006 [Google Scholar]
- 2. Amrani M, Goodwin AT, Gray CC, Yacoub MH. Ageing is associated with reduced basal and stimulated release of nitric oxide by the coronary endothelium. Acta Physiol Scand 157: 79–84, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Cooke CL, Davidge ST. Endothelial-dependent vasodilation is reduced in mesenteric arteries from superoxide dismutase knockout mice. Cardiovasc Res 60: 635–642, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Copp SW, Ferreira LF, Herspring KF, Musch TI, Poole DC. The effects of aging on capillary hemodynamics in contracting rat spinotrapezius muscle. Microvasc Res 77: 113–119, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res 90: 1159–1166, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Dantas AP, Tostes RC, Fortes ZB, Costa SG, Nigro D, Carvalho MH. In vivo evidence for antioxidant potential of estrogen in microvessels of female spontaneously hypertensive rats. Hypertension 39: 405–411, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Das DK. Sexual dimorphism and in the aging heart: role of estrogen, nitric oxide and beyond. J Mol Cell Cardiol 37: 667–669, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Delp MD, Evans MV, Duan C. Effects of aging on cardiac output, regional blood flow, and body composition in Fischer-344 rats. J Appl Physiol 85: 1813–1822, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 100: 1659–1666, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H(2)O(2) production in mouse cerebral arteries. Cardiovasc Res 73: 73–81, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol 126: 1034–1040, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Florian M, Freiman A, Magder S. Treatment with 17-beta-estradiol reduces superoxide production in aorta of ovariectomized rats. Steroids 69: 779–787, 2004 [DOI] [PubMed] [Google Scholar]
- 14. Fukuhara S, Matsushita S, Sakakibara Y. Changes in coronary resistance related to the stages of the female life cycle. Circ J 70: 478–481, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Hachamovitch R, Wicker P, Capasso JM, Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol Heart Circ Physiol 256: H66–H73, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Hamilton CA, Groves S, Carswell HV, Brosnan MJ, Graham D, Dominiczak AF. Estrogen treatment enhances nitric oxide bioavailability in normotensive but not hypertensive rats. Am J Hypertens 19: 859–866, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hayashi T, Fukuto JM, Ignarro LJ, Chaudhuri G. Basal release of nitric oxide from aortic rings is greater in female rabbits than in male rabbits: implications for atherosclerosis. Proc Natl Acad Sci USA 89: 11259–11263, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herspring KF, Ferreira LF, Copp SW, Snyder BS, Poole DC, Musch TI. Effects of antioxidants on contracting spinotrapezius muscle microvascular oxygenation and blood flow in aged rats. J Appl Physiol 105: 1889–1896, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Imaoka Y, Osanai T, Kamada T, Mio Y, Satoh K, Okumura K. Nitric oxide-dependent vasodilator mechanism is not impaired by hypertension but is diminished with aging in the rat aorta. J Cardiovasc Pharmacol 33: 756–761, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM. Role of nitric oxide in the coronary microvascular responses to adenosine and increased metabolic demand. Circulation 91: 1807–1813, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Kang LS, Reyes RA, Muller-Delp JM. Aging impairs flow-induced dilation in coronary arterioles: role of NO and H2O2. Am J Physiol Heart Circ Physiol 297: H1087–H1095, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol 259: H1063–H1070, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Leblanc AJ, Reyes R, Kang LS, Dailey RA, Stallone JN, Moningka NC, Muller-Delp JM. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 297: R1713–R1723, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leblanc AJ, Reyes RA, Muller-Delp JM. Advancing age and loss of ovarian hormones impair flow-induced dilation in the female coronary microvasculature. FASEB J 22: 1142.7, 2008 [Google Scholar]
- 25. LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leblanc AJ, Shipley RD, Kang LS, Muller-Delp JM. Aging impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am J Physiol Heart Circ Physiol 295: H2280–H2288, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via EDHF. J Physiol 577: 945–955, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E, Cushman M. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med 349: 523–534, 2003 [DOI] [PubMed] [Google Scholar]
- 29. McNeill AM, Kim N, Duckles SP, Krause DN, Kontos HA. Chronic estrogen treatment increases levels of endothelial nitric oxide synthase protein in rat cerebral microvessels. Stroke 30: 2186–2190, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Muller-Delp JM, Lubahn DB, Nichol KE, Philips BJ, Price EM, Curran EM, Laughlin MH. Regulation of nitric oxide-dependent vasodilation in coronary arteries of estrogen receptor-α-deficient mice. Am J Physiol Heart Circ Physiol 285: H2150–H2157, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Nurkiewicz TR, Boegehold MA. High salt intake reduces endothelium-dependent dilation of mouse arterioles via superoxide anion generated from nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 292: R1550–R1556, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips SA, Hatoum OA, Gutterman DD. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am J Physiol Heart Circ Physiol 292: H93–H100, 2007 [DOI] [PubMed] [Google Scholar]
- 35. Rathel TR, Leikert JF, Vollmar AM, Dirsch VM. The soy isoflavone genistein induces a late but sustained activation of the endothelial nitric oxide-synthase system in vitro. Br J Pharmacol 144: 394–399, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Sakamoto M, Uen T, Nakamura T, Hashimoto O, Sakata R, Kin M, Ogata R, Kawaguch T, Torimura T, Sata M. Estrogen upregulates nitric oxide synthase expression in cultured rat hepatic sinusoidal endothelial cells. J Hepatol 34: 858–864, 2001 [DOI] [PubMed] [Google Scholar]
- 38. Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293: R2194–R2201, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Sherwood L. Human Physiology: From Cells to Systems. New York, NY: Brooks/Cole, 2001 [Google Scholar]
- 40. Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374–383, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a. Stepp DW, Nishikawa Y, Chilian WM. Regulation of shear stress in the canine coronary microcirculation. Circulation 100: 1555–1561, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol 68: 959–965, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res 93: 170–177, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Thompson LP, Pinkas G, Weiner CP. Chronic 17beta-estradiol replacement increases nitric oxide-mediated vasodilation of guinea pig coronary microcirculation. Circulation 102: 445–451, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Tominaga M, Fujii K, Abe I, Takata Y, Kobayashi K, Fujishima M. Hypertension and ageing impair acetylcholine-induced vasodilation in rats. J Hypertens 12: 259–268, 1994 [PubMed] [Google Scholar]
- 46. Trott DW, Seawright JW, Luttrell MJ, Woodman CR. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, Buralli S, Taddei S, Salvetti A. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation 101: 2258–2263, 2000 [DOI] [PubMed] [Google Scholar]
- 48. Xu Y, Armstrong SJ, Arenas IA, Pehowich DJ, Davidge ST. Cardioprotection by chronic estrogen or superoxide dismutase mimetic treatment in the aged female rat. Am J Physiol Heart Circ Physiol 287: H165–H171, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Zhu L, He P. Platelet-activating factor increases endothelial [Ca2+]i and NO production in individually perfused intact microvessels. Am J Physiol Heart Circ Physiol 288: H2869–H2877, 2005 [DOI] [PubMed] [Google Scholar]