Abstract
Objective:
To compare efficacy of indacaterol to that of fixed-dose combination (FDC) formoterol and budesonide (FOR/BUD) and FDC salmeterol and fluticasone (SAL/FP) for the treatment of chronic obstructive pulmonary disease (COPD) based on the available randomized clinical trials (RCTs).
Methods:
Fifteen placebo-controlled RCTs were included that evaluated: indacaterol 150 μg (n = 5 studies), indacaterol 300 μg (n = 4), FOR/BUD 9/160 μg (n = 2), FOR/BUD 9/320 μg (n = 3), SAL/FP 50/500 μg (n = 5), and SAL/FP 50/250 μg (n = 1). Outcomes of interest were trough forced expiratory volume in 1 second (FEV1), total scores for St. George’s Respiratory Questionnaire (SGRQ), and transition dyspnea index (TDI). All trials were analyzed simultaneously using a Bayesian network meta-analysis and relative treatment effects between all regimens were obtained. Treatment-by-covariate interactions were included where possible to improve the similarity of the trials.
Results:
Indacaterol 150 μg resulted in a higher change from baseline (CFB) in FEV1 at 12 weeks compared to FOR/BUD 9/160 μg (difference in CFB 0.11 L [95% credible intervals: 0.08, 0.13]) and FOR/BUD 9/320 μg (0.09 L [0.06, 0.11]) and was comparable to SAL/FP 50/250 μg (0.02 L [−0.04, 0.08]) and SAL/FP 50/500 μg (0.03 L [0.00, 0.06]). Similar results were observed for indacaterol 300 μg at 12 weeks and indacaterol 150/300 μg at 6 months. Indacaterol 150 μg demonstrated comparable improvement in SGRQ total score at 6 months versus FOR/BUD (both doses), and SAL/FP 50/500 μg (−2.16 point improvement [−4.96, 0.95]). Indacaterol 150 and 300 μg demonstrated comparable TDI scores versus SAL/FP 50/250 μg (0.21 points (−0.57, 0.99); 0.39 [−0.39, 1.17], respectively) and SAL/FP 50/500 μg at 6 months.
Conclusion:
Indacaterol monotherapy is expected to be at least as good as FOR/BUD (9/320 and 9/160 μg) and comparable to SAL/FP (50/250 and 50/500 μg) in terms of lung function. Indacaterol is also expected to be comparable to FOR/BUD (9/320 and 9/160 μg) and SAL/FP 50/500 μg in terms of health status and to SAL/FP (50/250 and 50/500 μg) in terms of breathlessness.
Keywords: COPD, network meta-analysis, indacaterol
Introduction
Chronic obstructive pulmonary disease (COPD) is a disorder characterized by the progressive development of airway obstruction, which manifests as an accelerated decline in lung function, with symptoms such as breathlessness on physical exertion, deteriorating health status, and exacerbations.1
Treatments aim to prevent and control symptoms, reduce exacerbations, improve health status, and increase exercise tolerance. Currently, the Global Initiative for Chronic Obstructive Lung Disease recommend initiation with a short-acting bronchodilator followed by the addition of long-acting bronchodilators as the disease progresses.1 Commonly used bronchodilators include inhaled long-acting β2-agonists (LABAs) (eg, formoterol or salmeterol), the inhaled long-acting anti-cholinergic tiotropium, and oral methylxanthines.1 If a patient with severe disease experiences repeated exacerbations, an inhaled steroid may be added and fixed-dose combinations (FDC) of LABA plus an inhaled steroid, including formoterol/budesonide (FOR/BUD) or salmeterol/fluticasone proprionate (SAL/FP), may be prescribed.1 Despite recommendations, it has been found that a high percentage of patients receive FDCs as a first-line treatment.2
Indacaterol is a novel once-daily inhaled LABA indicated for maintenance bronchodilator treatment of airflow obstruction in adult patients with COPD. The recommended dose is one 150 microgram (μg) capsule once a day, using the Onbrez® Breezhaler® (Novartis) inhaler, increased on medical advice to a maximum dose of one 300 μg capsule once a day.3 In an extensive phase III clinical trial program indacaterol demonstrated superior lung function to LABA monotherapies and was at least as good as LABAs with respect to other outcomes.4–7 Given these findings, and the knowledge of the early use of FDCs, a comparison of indacaterol to FDCs is a relevant clinical question.
In the absence of a head-to-head randomized controlled trial (RCT) for the comparison of interest, the objective of the current study was to indirectly compare the efficacy of indacaterol 150 μg, indacaterol 300 μg, fixed-dose FOR/BUD, and fixed-dose SAL/FP for the treatment of COPD patients based on the currently available RCT evidence by means of a network meta-analysis. Outcomes of interest were lung function measured by trough forced expiratory volume in 1 second (FEV1), health status measured by the St. George’s Respiratory Questionnaire (SGRQ) total score, and breathlessness as assessed by transition dyspnea index (TDI) total score.
Methods
Identification and selection of studies
A systematic literature search was performed using a pre-defined search strategy in MEDLINE® and EMBASE®; study documents for indacaterol studies were provided by Novartis. Search terms included a combination of free-text and thesaurus terms relevant to COPD, indacaterol, salmeterol, formoterol, and RCTs (see Appendix for search strategy). The search strategy was initially performed for the period 1989–2009 and a supplementary search was undertaken for the period 2009–2010 in order to capture the most recent literature.
Two reviewers independently evaluated each identified study against the following predetermined criteria:
Population of interest: adults with COPD.
Interventions: indacaterol 150 μg or 300 μg, fixed dose combinations of FOR/BUD and SAL/FP.
Comparators: comparators included any of the interventions or placebo. Studies that solely evaluated different components of the fixed dose combination separately were excluded.
Outcomes: outcomes of interest included trough FEV1 (reported predose values) at 12 weeks and 6 months, SGRQ total score at 6 months, and TDI total score at 6 months.
Study design: RCTs.
For the studies identified that met the selection criteria, details were extracted on study design, population characteristics, interventions, and the outcomes trough FEV1 at 12 weeks and 6 months, SGRQ total score at 6 months, and TDI total score at 6 months. Only outcomes that were within 2 weeks of the time point of interest were extracted. For each outcome the difference in the change from baseline (CFB) (or difference at follow-up adjusted for baseline) was extracted where reported. In cases where the difference in CFB was not reported, it was calculated by subtracting the CFB in the placebo from the CFB in the active treatment (or the adjusted CFB values). If the CFB values per treatment were not reported they were extracted from figures using the software DigitizIt version 1.5.8. The standard error of the difference in CFB was extracted where available or calculated based on the uncertainty or variation reported (eg, 95% confidence interval or standard deviation). If there was insufficient information to calculate the standard error of the difference, an average standard deviation was calculated from the studies included in each specific analysis and combined with the study-specific sample size to derive the standard error.
Analysis
Bayesian network meta-analysis models were used8–10 to analyze the created data set for the CFB in FEV1 at 12 weeks and at 6 months, the CFB in SGRQ total score at 6 months, and the TDI total score at 6 months, to simultaneously synthesize the results of the included studies and to obtain differences for indacaterol 150 and 300 μg versus FOR/BUD, SAL/FP, and placebo.
Network meta-analyses within the Bayesian framework involve data, a likelihood distribution, a model with parameters, and prior distributions.10 The model relates the data from the individual studies to basic parameters reflecting the (pooled) relative treatment effect of each intervention compared to an overall reference treatment, eg, placebo. Based on these basic parameters, the relative efficacy between each of the competing interventions was obtained. For all endpoints a regression model with a normal likelihood distribution was used.9,10 For each outcome, a fixed and a random effects model was evaluated. The fixed effects model assumes that the differences in true relative treatment effects across studies in the network of evidence are caused only by the differences in treatment comparisons. The random effects model assumes that differences in observed treatment effects across the studies in the network are not only caused by the different treatment comparisons, but that there is also heterogeneity in the relative effects for a particular type of comparison caused by factors that modify that relative treatment effect. A comparison of the fit of the fixed and random effects model to the data based on the residual deviance was used to select a fixed or random effects model.11
With a network meta-analysis, randomization only holds within a trial and not across trials. As a result, there is the risk that patients who were studied in different comparisons are not similar, which leads to consistency violations. In order to minimize confounding bias, treatment by covariate interactions were incorporated in the models.12 Covariates potentially causing bias were selected based on clinical expertise and evaluation of whether these covariates were effect modifiers of any of the treatments under evaluation in individual studies analyzed. The following covariates were included simultaneously where possible and otherwise in separate models where insufficient data were available: 1) Proportion of patients who are current smokers (as opposed to ex-smokers); and 2) Proportion of patients with severe or very severe COPD (as opposed to mild or moderate COPD). Additional analyses were also performed, including study level covariates for age, and sex; which were not presented given the limited impact of the treatment by covariate interactions.
The results of the network meta-analysis provide relative treatment effects of each treatment versus a competing intervention, eg, differences in TDI or the differences in the CFB for FEV1 or SGRQ. In order to transform these relative estimates into absolute expected results with each treatment (eg, TDI or CFB in FEV1 or SGRQ), the relative treatment effects of each regimen relative to placebo were combined with absolute average treatment effect for placebo as a reference.
The Bayesian approach involves a formal combination of a prior probability distribution, with a likelihood distribution for the model parameters to obtain a posterior probability distribution for the estimates of the basic parameters. In order to avoid prior beliefs influencing the results of the model, noninformative prior distributions were used. Prior distributions of the relative treatment effects were normal distributions with mean 0 and a variance of 106. A uniform distribution with range of 0 to 2 was used for the prior distribution of heterogeneity for the random effects models. The posterior distribution can be interpreted in terms of probabilities and permits calculation of the probability that each treatment is best out of those compared given the data at hand; this gives the Bayesian approach an advantage over the frequentist approach.
WinBUGS 1.4.1 statistical software was used for the analyses.13 Summary statistics are presented for the expected absolute and relative treatment effects. In addition to point estimates reflecting the most likely value, 95% credible intervals (95% CrI) reflecting the range of true underlying effects with 95% probability are presented. Furthermore, for each of the endpoints, the probability that indacaterol is better than a certain regimen is presented. Results are presented without adjustment for covariates for the CFB in FEV1 at 12 weeks and 6 months, CFB in SGRQ total score at 6 months, and TDI total score at 6 months. Results with adjustment are discussed for FEV1 at 12 weeks. The inclusion of covariates was explored for SGRQ and TDI, but was not always feasible given the data limitations.
Results
Study selection and characteristics
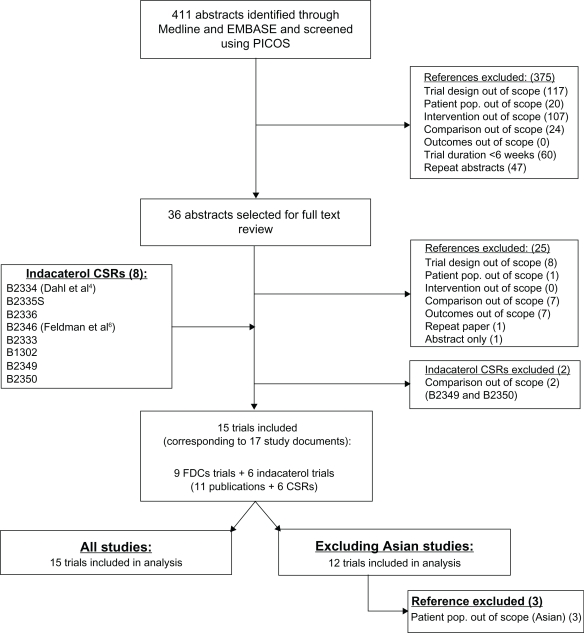
The literature search identified 411 potentially relevant studies (Figure 1). The first review excluded 375 (91%) of these abstracts because of the trial design (117, 28%), intervention (107, 26%), trial duration (60, 15%), duplication (47, 11%), comparator (24, 6%), and population (20, 5%). The full text review of 36 remaining studies excluded 25 (69%) studies, largely because of study design. Overall, 11 studies were identified from the search4,6,14–22 and 4 relevant RCTs for indacaterol were added from its clinical trial program (Novartis studies B2335S,23 B2336,24 B1302,25 and B233326). Data on file were used for studies B233427 and B2346,28 which corresponded to publications by Dahl et al 20104 and Feldman et al 2010,6 respectively.
Figure 1.
Flow diagram of study selection.
Abbreviations: CSR, complete study reports; FDC, fixed-dose combinations; PICOS, patients, interventions, comparators, outcomes, and study design.
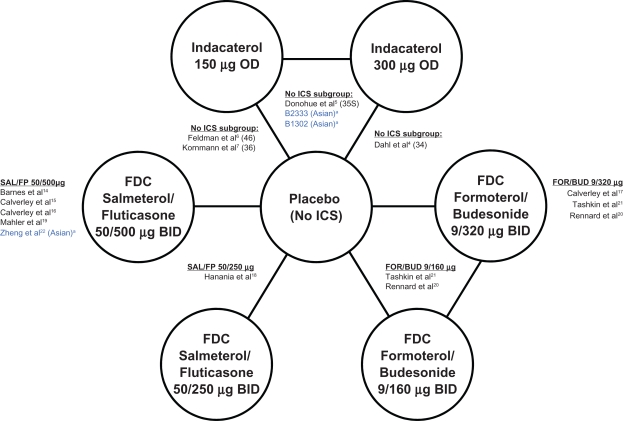
The network of evidence (Figure 2) illustrates that all active therapies were compared to placebo, and that 3 studies directly compared indacaterol 150 μg to indacaterol 300 μg. Study B2334 evaluated indacaterol 300 μg and 600 μg once daily compared to placebo and formoterol 12 μg twice daily over 52 weeks. This was the first pivotal indacaterol registration study, and in addition to data on the 300 μg dose, it provides safety data on the 600 μg dose – a dose that is 2 to 4 times the EU-approved dose. B2335S was an adaptive seamless design study that combined an initial dose-selection phase with a pivotal registration phase and assessed indacaterol 150 μg and 300 μg once daily compared to placebo and open-label tiotropium 18 μg once daily over 26 weeks. B2346 evaluated indacaterol 150 μg once daily compared to placebo over 12 weeks, and was the third indacaterol pivotal registration study (providing the required replicate data for the 150 μg dose), while B2336 compared indacaterol 150 μg once daily to placebo as well as salmeterol 50 μg twice daily over 26 weeks, providing additional data on the 150 μg dose.
Figure 2.
Network of studies.
Note: aStudies included predominantly Asian patients.
Abbreviations: BID, twice daily; FDC, fixed-dose combinations; FOR/BUD, FDC formoterol and budesonide; ICS, inhaled corticosteroids; OD, once daily; SAL/FP, FDC salmeterol and fluticasone proprionate.
Tables 1 and 2 present the details of the study and patient characteristics for the 15 studies included in the analysis. All studies were multicenter placebo-controlled RCTs with a parallel design and included a total of 10,211 adult patients with COPD. The studies included patients ≥40 years of age with FEV1/FVC of ≤0.70 and FEV1 percent predicted <80%, while the indacaterol trials required patients to have a predicted FEV1 of at least 30%. Most studies included patients who were current or ex-smokers with a smoking history of at least 10 years, although some studies included patients with a smoking history of at least 20 pack-years (Hanania et al 2003,18 Mahler et al 2002,19 B2334,4 B2335S,5 B2336,7 and B23466). Three studies included predominantly Asian patients (Zheng et al 2007,22 and studies B130223 and B233324), whereas the remaining studies included mostly Caucasian patients or reported study centers in Europe and North America. Limited information was reported on the comorbidities of the patients, although most studies excluded patients with asthma or other respiratory or pulmonary diseases and other clinically significant diseases that may have affected treatment. Some differences across the studies were observed in baseline FEV1 and health status (as assessed by SGRQ total score), which may have been related to COPD severity.
Table 1.
Study characteristics for each study included in the network meta-analysis
| Source | Trial type | Location | Duration | Active treatments | Examples of key comorbidity exclusions | Ethnicityd |
|---|---|---|---|---|---|---|
| Hanania et al18 | RCT, DB, MC | 76 centers; US | 24 weeks | SAL/FP; 50/250 μg; BID | Patients with asthma and significant medical disorders | White: 91%–96% Black: 3%–5% Asian/other: 2%–3% |
| Barnes et al14 | RCT, DB, MC | NR | 13 weeks | SAL/FP; 50/500 μg; BID | NR | White: 99%–100% Asian: 0%–1% |
| Calverley et al15 | RCT, DB, MC | 196 centers; 25 countries in Europe including Russia, Australia, New Zealand, Canada, South Africa | 52 weeks | SAL/FP; 50/500 μg; BID | Patients with other respiratory disorders | NR |
| Calverley et al16 | RCT, DB, MC | 444 centers; 42 countries | 3 years | SAL/FP; 50/500 μg; BID | Patients with asthma, other respiratory disorders, or other diagnosis that may interfere with treatment | United States: 23% Europe: 50%; Asia: 12%–13% Other: 15% |
| Mahler et al19 | RCT, DB, MC | 65 centers; US | 24 weeks | SAL/FP; 50/500 μg; BID | Patients with asthma and/or significant medical disorders (emphysema: 74%–78%) | White: 92%–95%; Black: 4%–6%; Asian/other: 1%–2% |
| Zheng et al22,a | RCT,DB, MC | 12 centers: China | 24 weeks | SAL/FP; 50/500 μg; BID | Patients with other respiratory disorders and/or significant medical disorders | 100% Chinese |
| Calverley et al17 | RCT, DB, MC | 109 centers; 15 countries in Europe, Brazil, South Africa and Asia (China, Malaysia, Taiwan, and Thailand) | 52 weeks | FOR/BUD; 9/320 μg; BID; | Patients with history of asthma, seasonal allergic rhinitis prior the age of 40 years, relevant cardiovascular disorders or significant disorder | NR |
| Rennard et al20 | RCT, DB, DD, MC | 237 centers: US and Mexico | 52 weeks | FOR/BUD; 9/160 μg; BID; FOR/BUD; 9/320 μg; BID | Patients with history of asthma, seasonal allergic rhinitis prior the age of 40 years, relevant cardiovascular disorders or other respiratory tract disorders | White: 92.5%; Black: 2.6%; Asian: 0.2% Other: 4.7% |
| Tashkin et al21 | RCT, DB, DD, MC | 194 centers; US, Czech Republic, The Netherlands, Poland and South Africa | 26 weeks | FOR/BUD; 9/160 μg; BID; FOR/BUD; 9/3200 μg; BID | Patients with history of asthma, seasonal allergic rhinitis before the age of 40 years, any relevant cardiovascular disorders or other respiratory tract disorder | White: 92%–93% Black: 2%–3% Asian: 0%–1% Other: 4%–6% |
| Dahl et al4 (B2334)b,27 Non-ICS | RCT, DB, DD, MC | 240 centers: Europe and Russia, Argentina, Chile, Colombia, Ecuador, Egypt, Israel, Peru, South Korea | 52 weeks | IND; 300 μg; OD | Patients with concomitant pulmonary disease, type 1 diabetes, a history of asthma, or significant conditionc | Caucasian: 92%–94% Black: 0% Asian: 2% Other: 5%–6% |
| Donohu et al5 (B2335S)23 Non-ICS | RCT, PC, DB, DD, MC, 2 stage adaptive seamless | 334 centers: Argentina, Canada, Germany, India, Italy, Korea, Spain, Sweden, Turkey, Taiwan, US | 26 weeks | IND; 150 μg; OD; IND; 300 μg; OD |
Patients with concomitant pulmonary disease, type 1 diabetes, a history of asthma, or significant conditionc | Caucasian: 79%–82% Black: 2%–3% Asian: 13%–19% Other: 0%–1% |
| Kornmann et al7 (B2336)24 Non-ICS | RCT, DB, DD, MC | 142 centers | 26 weeks | IND; 150 μg; OD | Patients with concomitant pulmonary disease, type 1 diabetes, a history of asthma, or significant conditionc | Caucasian: 75%–78% Black: 0% Asian: 16%–17% Other: 7%–8% |
| Feldman et al6 (B2346)28 Non-ICS | RCT, DB, DD, MC | 103 centers: US, Australia/New Zealand, Belgium | 12 weeks | IND; 150 μg; OD | Patients with a history of asthma or any significant pulmonary disease or cardiovascular abnormality | Caucasian: 92%–93% Black: 5%–6%; Asian: 0.5%; Other 2%: |
| B233326,a Non-ICS | RCT, DB, DD, MC | Multiple centers; China and India | 26 weeks | IND; 150 μg; OD; IND; 300 μg; OD |
Patients with concomitant pulmonary disease, a history or asthma, type 1 diabetes, or clinically significant conditionc | Caucasian: 4%–5%; Asian: 95% (Chinese 89%–90%) |
| B130225,a Non-ICS | RCT, DB, MC | 73 centers; Japan, Taiwan, Korea, India, Hong Kong, and Singapore | 12 weeks | IND; 150 μg; OD; IND; 300 μg; OD |
Patients with concomitant pulmonary disease, a history or asthma, type 1 diabetes, or clinically significant conditionc | Asian: 100% (Japanese: 43%–45%; Chinese: 15–17%; Korean: 28%–30%) |
Notes:
These studies included predominantly Asian patients;
This study evaluated indacaterol 600 μg which was not included in the analyses;
The studies genearlly excluded patients that had a ‘clinically signficiant condition’ or ‘significant medical disorder’ that may have interfered with the study results. For example the protocol for study B2334 indicated the following exclusion: patients who, in the judgment of the investigator or the responsible Novartis personnel, had a clinically relevant laboratory abnormality or a clinically significant condition or any condition which in the investigator’s opinion might have compromised patient safety or compliance, interfered with evaluation, or precluded completion of the study;
Ethnicity was reported for all patients in the indacaterol studies regardless of ICS use.
Abbreviations: BID, twice daily; DB, double blind; DD, double dummy; FOR/BUD, fixed-dose formoterol and budesonide; ICS, inhaled corticosteroids; IND, indacaterol; MC, multicenter; NR, not reported; OD, once daily; PC, placebo-controlled; RCT, randomized controlled trial; SAL/FP, fixed-dose salmeterol and fluticasone proprionate.
Table 2.
Key baseline patient characteristics for each study included in network meta-analysis
| Source | Treatment | Randomized no. | % male | Mean age | % current smokers | % severe or very severe | FEV1 | FVC | BDI | SGRQ |
|---|---|---|---|---|---|---|---|---|---|---|
| Hanania et al18 | Placebo | 185 | 68% | 65 | 47% | 75% | 1.29 (0.43) | NR | 5.7 (NR) | CRDQ = 84.8 |
| SAL/FP 50/250 | 178 | 61% | 63 | 43% | 79% | 1.25 (0.40) | NR | 6.1 (NR) | CRDQ = 84.1 | |
| Barnes et al14 | Placebo | 73 | 74% | 64 | 59% | 19% | 1.68 (0.47) | NR | NR | NR |
| SAL/FP 50/500 | 67 | 82% | 65 | 63% | 33% | 1.67 (0.44) | NR | NR | NR | |
| Calverley et al15 | Placebo | 361 | 75% | 63 | 47% | 66% | 1.27 (0.47) | 2.50 (0.80) | NR | 47.1 (16.5) |
| SAL/FP 50/500 | 358 | 75% | 63 | 52% | 64% | 1.31 (0.53) | 2.54 (0.84) | NR | 47.1 (15.7) | |
| Calverley et al16 | Placebo | 1545 | 76% | 65 | 43% | 68% | 1.12 (0.40) | NR | NR | 49.0 (17.4) |
| SAL/FP 50/500 | 1546 | 75% | 65 | 43% | 68% | 1.12 (0.40) | NR | NR | 48.9 (17.4) | |
| Mahler et al19 | Placebo | 181 | 75% | 64 | 54% | 77% | 1.32 (NR) | NR | 5.6 (NR) | CRDQ = 86.2 |
| SAL/FP 50/500 | 165 | 62% | 62 | 46% | 77% | 1.27 (NR) | NR | 6.2 (NR) | CRDQ = 87.1 | |
| Zheng et ala,22 | Placebo | 148 | 86% | 67 | 23% | 60% | 1.03 (NR) | NR | NR | 44.5 (NR) |
| SAL/FP 50/500 | 297 | 91% | 66 | 21% | 60% | 1.06 (NR) | NR | NR | 44.8 (NR) | |
| Calverley et al17 | Placebo | 256 | 75% | 65 | 30% | 92% | 0.98 (0.33) | NR | NR | 48.0 (18.0) |
| FOR/BUD 9/320 | 254 | 78% | 64 | 33% | 92% | 0.98 (0.33) | NR | NR | 48.0 (19.0) | |
| Rennard et al20 | Placebo | 481 | 65% | 63 | 44% | 81% | 1.10 (0.40) | NR | BCSS: 2.1 | 54.7 (16.1) |
| FOR/BUD 9/160 | 494 | 63% | 64 | 42% | 83% | 1.00 (0.40) | NR | BCSS: 2.2 | 55.7 (16.7) | |
| FOR/BUD 9/320 | 494 | 62% | 63 | 39% | 83% | 1.00 (0.40) | NR | BCSS: 2.2 | 54.6 (17.4) | |
| Tashkin et al21 | Placebo | 300 | 69% | 63 | 40% | 76% | 1.08 (0.38) | NR | BCSS: 2.0 | 55.6 (17.0) |
| FOR/BUD 9/160 | 281 | 64% | 64 | 45% | 82% | 1.04 (0.40) | NR | BCSS: 2.0 | 55.5 (16.3) | |
| FOR/BUD 9/320 | 277 | 68% | 63 | 44% | 82% | 1.04 (0.42) | NR | BCSS: 2.1 | 56.5 (15.8) | |
| B2334;b non-ICS27 | Placebo | 180 | 83% | 64 | 46% | 38% | 1.40 (0.50) | 2.77 (0.81) | 6.7 (2.3) | 43.4 (17.5) |
| IND 300 | 165 | 82% | 64 | 46% | 44% | 1.32 (0.42) | 2.72 (0.75) | 6.5 (2.0) | 46.3 (17.5) | |
| B2335S; non-ICS23 | Placebo | 226 | 60% | 63 | 47% | 32% | 1.39 (0.51) | 2.60 (0.79) | 6.5 (2.4) | 46.7 (17. 5) |
| IND 150 | 240 | 65% | 62 | 52% | 33% | 1.43 (0.53) | 2.65 (0.78) | 6.9 (2.4) | 43.2 (19.1) | |
| IND 300 | 241 | 66% | 63 | 50% | 34% | 1.41 (0.54) | 2.72 (0.84) | 6.7 (2.3) | 44.0 (18.7) | |
| B2336; non-ICS24 | Placebo | 187 | 81% | 65 | 44% | 39% | 1.37 (0.50) | 2.60 (0.78) | 6.7 (2.0) | 42.5 (18.3) |
| IND 150 | 173 | 74% | 63 | 51% | 38% | 1.36 (0.52) | 2.56 (0.88) | 6.9 (2.0) | 42.1 (19.3) | |
| B2346; non-ICS28 | Placebo | 125 | 55% | 64 | 55% | 34% | 1.37 (0.58) | NR | NR | 48.0 (17.3) |
| IND 150 | 144 | 51% | 62 | 58% | 40% | 1.35 (0.60) | NR | NR | 49.2 (20.2) | |
| B2333a; non-ICS26 | Placebo | 113 | 94% | 64 | 27% | 53% | 1.15 (0.39) | 2.68 (0.67) | 6.5 (2.2) | 41.9 (19.6) |
| IND 150 | 116 | 94% | 65 | 27% | 50% | 1.11 (0.37) | 2.64 (0.60) | 6.4 (2.3) | 41.8 (18.1) | |
| IND 300 | 112 | 97% | 65 | 25% | 46% | 1.16 (0.37) | 2.70 (0.64) | 6.7 (2.1) | 42.2 (16.9) | |
| B1302;a non-ICS25 | Placebo | 76 | 94% | 67 | 27% | 35% | 1.20 (0.41) | 2.67 (0.71) | 7.4 (2.5) | 38.6 (17.7) |
| IND 150 | 85 | 96% | 67 | 34% | 31% | 1.31 (0.45) | 2.70 (0.66) | 7.5 (2.1) | 37.8 (18.3) | |
| IND 300 | 87 | 97% | 67 | 36% | 33% | 1.22 (0.41) | 2.61 (0.68) | 7.8 (2.4) | 35.5 (16.2) |
Notes:
These studies included predominantly Asian patients;
this study evaluated indacaterol 600 μg which was not included in the analyses. Data are presented as mean (SD) where available and otherwise indicated by not reported (NR).
Abbreviations: BCSS, Breathless Cough and Sputum Scale Dyspnea Baseline Scores; BDI, Baseline Dyspnea Score; CRDQ, Chronic Respiratory Disease Questionnaire; FEV1, forced expiratory volume in 1 second; FOR/BUD, fixed-dose formoterol and budesonide; FVC, forced vital capacity; IND, indacaterol; SAL/FP, fixed-dose salmeterol and fluticasone proprionate; SGRQ, St. George’s Respiratory Questionnaire total score.
Comparative efficacy
In Table 3 the individual study results for the different endpoints are presented. These study findings were synthesized in 2 series of network meta-analyses: the first analyses included all studies and the second analyses excluded the 3 Asian studies. As patients using background inhaled corticosteroids (ICS) were permitted entry into the indacaterol studies (providing they continued to use ICS at a stable dose and regimen throughout the study), only data for patients not using ICS (‘non-ICS users’) were included in the analyses in order to ensure the patients in the placebo arms of the indacaterol trials were sufficiently similar to those in the FDC studies. Therefore, the analysis was based on unpublished subgroup data provided by Novartis for all indacaterol studies.
Table 3.
Reported data in individual studies included in the network meta-analysis
|
FOR/BUD 9/160 μg |
FOR/BUD 9/320 μg |
SAL/FP 50/500 μg |
SAL/FP 50/250 μg |
IND 300 μg |
IND 150 μg |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FEV112 wk | FEV16 m | SGRQ | FEV112 wk | FEV16 m | SGRQ | FEV112 wk | FEV16 m | SGRQ | TDI | FEV112 wk | FEV16 m | TDI | FEV112 wk | FEV16 m | SGRQ | TDI | FEV112 wk | FEV16 m | SGRQ | TDI | ||
| Hanania et al18 | diff | 0.16 | 0.16 | 0.80 | ||||||||||||||||||
| SE | 0.03 | 0.03 | 0.35 | |||||||||||||||||||
| Barnes14 | diff | 0.17 | 0.15 | |||||||||||||||||||
| SE | 0.04 | NR | ||||||||||||||||||||
| Calverley et al15 | diff | 0.13 | 0.16 | −1.00 | ||||||||||||||||||
| SE | NR | NR | NR | |||||||||||||||||||
| Calverley et al16 | diff | −1.71 | ||||||||||||||||||||
| SE | NR | |||||||||||||||||||||
| Mahler et al19 | diff | 0.20 | 0.18 | 1.70 | ||||||||||||||||||
| SE | 0.03 | 0.04 | NR | |||||||||||||||||||
| Zhenga, et al22 | diff | 0.15 | 0.15 | −5.74 | ||||||||||||||||||
| SE | NR | NR | 1.51 | |||||||||||||||||||
| Calverley et al17 | diff | −4.99 | ||||||||||||||||||||
| SE | NR | |||||||||||||||||||||
| Rennard et al20 | diff | 0.07 | 0.07 | 0.09 | 0.08 | |||||||||||||||||
| SE | NR | NR | NR | NR | ||||||||||||||||||
| Tashkin et al21 | diff | 0.06 | 0.05 | −2.95 | 0.08 | 0.08 | −3.12 | |||||||||||||||
| SE | NR | 0.02 | 1.06 | NR | 0.02 | 1.06 | ||||||||||||||||
| Non-ICS: B233427 | diff | 0.18 | 0.18 | −5.38 | 1.44 | |||||||||||||||||
| SE | 0.02 | 0.03 | 1.42 | 0.33 | ||||||||||||||||||
| Non-ICS: B2335S23 | diff | 0.17 | 0.16 | −1.55 | 1.11 | 0.20 | 0.18 | −3.88 | 1.26 | |||||||||||||
| SE | 0.02 | 0.02 | 1.16 | 0.29 | 0.02 | 0.02 | 1.18 | 0.30 | ||||||||||||||
| Non-ICS: B233624 | diff | 0.18 | 0.17 | −6.15 | 0.90 | |||||||||||||||||
| SE | 0.02 | 0.03 | 1.43 | 0.32 | ||||||||||||||||||
| Non-ICS: B234628 | diff | 0.16 | ||||||||||||||||||||
| SE | 0.03 | |||||||||||||||||||||
| Non-ICS: B1302a,26 | diff | 0.20 | 0.17 | |||||||||||||||||||
| SE | 0.02 | 0.02 | ||||||||||||||||||||
| Non-ICS: B2333a,25 | diff | 0.14 | 0.14 | −2.29 | 1.04 | 0.15 | 0.13 | −2.88 | 0.81 | |||||||||||||
| SE | 0.03 | 0.03 | 1.95 | 0.34 | 0.03 | 0.03 | 1.94 | 0.34 | ||||||||||||||
Notes:
Mainly Asian population.
Abbreviations: Diff, difference in change from baseline versus placebo; FEV1, forced expiratory volume in 1 second; FOR/BUD, fixed-dose formoterol and budesonide; ICS, inhaled corticosteroids; IND, indacaterol; m, month; NR, not reported; SAL/FP, fixed-dose salmeterol and fluticasone proprionate; SE, standard error of the difference in change from baseline; SGRQ, St. George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index; wk, week.
Trough FEV1 at 12 weeks and 6 months
All treatments were more efficacious than placebo at 12 weeks and 6 months in terms of trough FEV1 for all analyses without covariates (Table 4). In the analysis including all studies (without covariates), indacaterol 150 μg resulted in higher FEV1 compared to both FOR/BUD 9/160 μg and FOR/BUD 9/320 μg at both time points (see Table 5). Results for indacaterol 300 μg were similar to indacaterol 150 μg, demonstrating a more favorable FEV1 improvement than both doses of FOR/BUD (see Table 6). In comparison to SAL/FP 50/500 μg, indacaterol 150 μg and 300 μg were comparable in terms of FEV1 at both time points. This was also the case for indacaterol 150 μg and 300 μg versus SAL/FP 50/250 μg at 12 weeks and at 6 months. The results were not sensitive to the exclusion of the 3 Asian studies, and only minor differences between the 2 analyses were observed in FEV1 results (≈0.01 L associated with indacaterol 150 μg and 300 μg) in most cases (see Tables 5 and 6).
Table 4.
Results of network meta-analysis: all treatments versus placebo without covariates
| Trough FEV1L difference in CFB (95% CrI) at 12 weeks | Trough FEV1L difference in CFB (95% CrI) at 6 months | SGRQ total score difference in CFB (95% CrI) at 6 months | TDI total score difference (95% CrI) at 6 months | |
|---|---|---|---|---|
| All studies | ||||
| IND 150 μg | 0.17 (0.15, 0.20) | 0.16 (0.13, 0.19) | −4.43 (−6.67, −2.17) | 1.01 (0.65, 1.37) |
| IND 300 μg | 0.17 (0.15, 0.20) | 0.16 (0.13, 0.19) | −3.01 (−5.26, −0.81) | 1.19 (0.83, 1.55) |
| SAL/FP 50/500 μg | 0.14 (0.13, 0.16) | 0.16 (0.13, 0.19) | −2.27 (−4.33, −0.50) | 1.70 (1.11, 2.29) |
| SAL/FP 50/250 μg | 0.16 (0.10, 0.21) | 0.16 (0.10, 0.22) | NR | 0.80 (0.11, 1.49) |
| FOR/BUD 9/320 μg | 0.09 (0.07, 0.11) | 0.08 (0.06, 0.10) | −4.03 (−6.46, −1.60) | NR |
| FOR/BUD 9/160 μg | 0.07 (0.05, 0.09) | 0.06 (0.04, 0.09) | −2.95 (−6.33, 0.40) | NR |
| All studies excluding 3 Asian studies | ||||
| IND 150 μg | 0.18 (0.16, 0.21) | 0.18 (0.14, 0.21) | −4.89 (−7.35, −2.47) | 1.10 (0.67, 1.53) |
| IND 300 μg | 0.17 (0.14, 0.21) | 0.17 (0.14, 0.21) | −3.20 (−5.67, −0.84) | 1.26 (0.83, 1.69) |
| SAL/FP 50/500 μg | 0.14 (0.12, 0.16) | 0.15 (0.12, 0.18) | −1.44 (−3.39, 0.58) | 1.70 (1.10, 2.29) |
| SAL/FP 50/250 μg | 0.16 (0.10, 0.21) | 0.16 (0.10, 0.22) | NR | 0.80 (0.11, 1.49) |
| FOR/BUD 9/320 μg | 0.09 (0.07, 0.11) | 0.08 (0.06, 0.10) | −4.02 (−6.25, −1.80) | NR |
| FOR/BUD 9/160 μg | 0.07 (0.05, 0.09) | 0.06 (0.04, 0.09) | −2.96 (−6.05, 0.13) | NR |
Abbreviations: CFB, change from baseline; CrI, 95% credibility interval; FEV1, forced expiratory volume in 1 second; FOR/BUD, fixed-dose formoterol and budesonide; IND, indacaterol; NR, not reported; SAL/FP, fixed-dose salmeterol and fluticasone proprionate; SGRQ, St. George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
Table 5.
Results of network meta-analysis; Indacaterol 150 μg versus alternatives without covariates
|
Trough FEV1L at 12 weeks |
Trough FEV1L at 6 months |
SGRQ total score at 6 months |
TDI total score at 6 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Difference in CFB (95% CrI) | Prob of IND 150 being better | Difference in CFB (95% CrI) | Prob of IND 150 being better | Difference in CFB (95% CrI) | Prob of IND 150 being better | Difference (95% CrI) | Prob of IND 150 being better | |
| All studies | ||||||||
| SAL/FP 50/500μg | 0.03 (0.00, 0.06) | 99% | 0.01 (−0.04, 0.05) | 61% | −2.16 (−4.96, 0.95) | 92% | −0.69 (−1.38, 0.01) | 3% |
| SAL/FP 50/250 μg | 0.02 (−0.04, 0.08) | 72% | 0.00 (−0.07, 0.07) | 51% | NR | NR | 0.21 (−0.57, 0.99) | 70% |
| FOR/BUD 9/320 μg | 0.09 (0.06, 0.11) | >99% | 0.08 (0.05, 0.12) | >99% | −0.39 (−3.69, 2.92) | 60% | NR | NR |
| FOR/BUD 9/160 μg | 0.11 (0.08, 0.13) | >99% | 0.10 (0.06, 0.14) | >99% | −1.48 (−5.51, 2.61) | 78% | NR | NR |
| All studies excluding 3 Asian studies | ||||||||
| SAL/FP 50/500 μg | 0.04 (0.01, 0.08) | 99% | 0.02 (−0.02, 0.07) | 82% | −3.45 (−6.64, −0.39) | 98% | −0.60 (−1.34, 0.14) | 6% |
| SAL/FP 50/250 μg | 0.03 (−0.04, 0.09) | 80% | 0.01 (−0.06, 0.08) | 66% | NR | NR | 0.30 (−0.51, 1.11) | 76% |
| FOR/BUD 9/320 μg | 0.10 (0.06, 0.13) | >99% | 0.10 (0.05, 0.14) | >99% | −0.86 (−4.20, 2.41) | 71% | NR | NR |
| FOR/BUD 9/160 μg | 0.12 (0.08, 0.15) | >99% | 0.11 (0.07, 0.16) | >99% | −1.92 (−5.88, 2.00) | 85% | NR | NR |
Abbreviations: CFB, change from baseline; CrI, 95% credibility interval; FEV1, forced expiratory volume in 1 second; FOR/BUD, fixed-dose formoterol and budesonide; IND, indacaterol; NR, not reported; Prob, probability; SAL/FP, fixed-dose salmeterol and fluticasone proprionate; SGRQ, St. George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
Table 6.
Results of network meta-analysis: indacaterol 300 μg versus alternatives without covariates
|
Trough FEV1L at 12 weeks |
Trough FEV1L at 6 months |
SGRQ total score at 6 months |
TDI total score at 6 months |
|||||
|---|---|---|---|---|---|---|---|---|
| Difference in CFB (95% CrI) | Prob of IND 300 being better | Difference in CFB (95% CrI) | Prob of IND 300 being better | Difference in CFB (95% CrI) | Prob of IND 300 being better | Difference (95% CrI) | Prob of IND 300 being better | |
| All studies | ||||||||
| SAL/FP 50/500μg | 0.03 (0.00, 0.06) | 97% | 0.01 (−0.04, 0.05) | 62% | −0.74 (−3.56, 2.28) | 70% | −0.51 (−1.21, 0.19) | 8% |
| SAL/FP 50/250 μg | 0.02 (−0.05, 0.08) | 70% | 0.00 (−0.07, 0.07) | 52% | NR | NR | 0.39 (−0.39, 1.17) | 84% |
| FOR/BUD 9/320 μg | 0.08 (0.06, 0.11) | >99% | 0.08 (0.05, 0.12) | >99% | 1.02 (−2.30, 4.28) | 26% | NR | NR |
| FOR/BUD 9/160 μg | 0.10 (0.08, 0.13) | >99% | 0.10 (0.06, 0.14) | >99% | −0.06 (−4.12, 3.96) | 51% | NR | NR |
| All studies excluding 3 Asian studies | ||||||||
| SAL/FP 50/500 μg | 0.03 (−0.01, 0.07) | 95% | 0.02 (−0.03, 0.06) | 76% | −1.76 (−4.99, 1.26) | 89% | −0.45 (−1.18, 0.29) | 12% |
| SAL/FP 50/250 μg | 0.02 (−0.05, 0.08) | 70% | 0.01 (−0.06, 0.08) | 61% | NR | NR | 0.46 (−0.35, 1.27) | 86% |
| FOR/BUD 9/320 μg | 0.09 (0.05, 0.12) | >99% | 0.09 (0.05, 0.13) | >99% | 0.81 (−2.50, 4.05) | 30% | NR | NR |
| FOR/BUD 9/160 μg | 0.11 (0.07, 0.14) | >99% | 0.11 (0.06, 0.15) | >99% | −0.23 (−4.21, 3.60) | 55% | NR | NR |
Abbreviations: CFB, change from baseline; CrI, 95% credibility interval; FEV1, forced expiratory volume in 1 second; FOR/BUD, fixed-dose formoterol and budesonide; ICS, inhaled corticosteroids; IND, indacaterol; NR, not reported; Prob, probability; SAL/FP, fixed-dose salmeterol and fluticasone proprionate; SGRQ, St. George’s Respiratory Questionnaire; TDI, Transition Dyspnea Index.
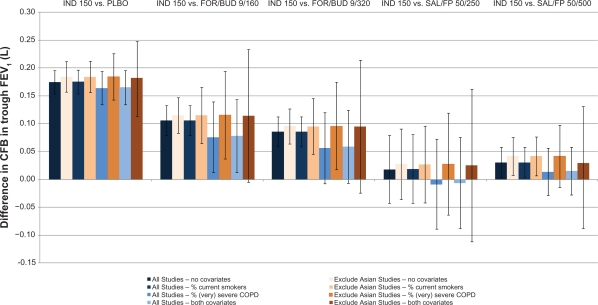
Figure 3 illustrates the impact of adjusting for differences in the proportion of current smokers and patients with severe or very severe COPD on the relative results of indacaterol 150 μg versus the alternatives for FEV1 at 12 weeks for both scenarios (all studies included and 3 Asian studies excluded). Indacaterol 150 μg was more efficacious than FOR/BUD 9/160 μg in most of the scenarios. The increase associated with indacaterol 150 μg in comparison to FOR/BUD 9/320 μg varied from 0.09 L (95% CrI: to −0.02, 0.21) to 0.10 L (95% CrI: 0.02, 0.17) and was most sensitive to the proportion of patients with severe COPD (where the credible internals included zero). Indacaterol 150 μg and 300 μg remained comparable to SAL/FP 50/500 μg. Again, the lowest relative benefits associated with indacaterol were observed when adjusted for severity or both severity and smoking status.
Figure 3.
Impact of adjustment for differences in effect-modifiers across studies: difference in indacaterol 150 μg versus alternatives for CFB in FEV1 at 12 weeks and 95% credible Intervals.
Abbreviations: CFB, change from baseline; FEV1, forced expiratory volume in 1 second; FOR/BUD 9/160, fixed-dose formoterol and budesonide 9/160 μg; FOR/BUD 9/320, fixed-dose formoterol and budesonide 9/320 μg; IND 150, indacaterol 150 μg; PLBO, placebo; SAL/FP 50/250, fixed-dose salmeterol and fluticasone proprionate 50/250 μg; SAL/FP 50/500, fixed-dose salmeterol and fluticasone proprionate 50/500 μg.
SGRQ total score at 6 months
In the scenario with all studies included (without covariates), all active treatments were more efficacious than placebo, with the exception of FOR/BUD 9/160 μg which included zero in the credible intervals (see Table 4). No data were available for SAL/FP 50/250 μg for SGRQ at 6 months. When the 3 Asian studies were excluded from the analysis, SAL/FP 50/500 μg was no longer more efficacious than placebo (as the CrI included zero). Based on the analysis of all studies without covariates, indacaterol 150 μg resulted in comparable improvement in SGRQ total score versus SAL/FP 50/500 μg, FOR/BUD 9/160 μg and FOR/BUD 9/320 μg, showing a trend towards better scores (2.16 points, 1.48 points, and 0.39 points improvement, respectively) (see Table 5). Indacaterol 300 μg resulted in lower scores than indacaterol 150 μg, but remained comparable to the alternative treatments (see Table 6). As with FEV1, excluding the Asian studies had minimal impact on the results and improved the point estimates in favor of indacaterol.
TDI total score at 6 months
All treatments were more efficacious than placebo for TDI (see Table 4). Comparative estimates versus FOR/BUD were not possible at 6 months given the lack of data. Comparable results were observed for indacaterol and SAL/FP in the analyses without covariates (see Tables 5 and 6). Indacaterol 150 μg and 300 μg demonstrated slightly higher TDI scores compared to SAL/FP 50/250 μg, with an improvement of 0.21 points and 0.39 points, respectively. However, compared to SAL/FP 50/500 μg, indacaterol 150 μg and 300 μg had slightly lower TDI scores, with point estimates of −0.69 points and −0.51 points, respectively. Consistent results were observed in the scenario without the Asian studies, although the point estimates improved slightly for indacaterol and the CrI widened, since the number of studies included in the analysis was reduced from 6 to 5.
Discussion
The objective of this study was to compare the efficacy of indacaterol 150 μg and 300 μg once daily versus fixed-dose combinations FOR/BUD and SAL/FP twice daily for COPD in terms of trough FEV1, SGRQ total score and TDI total score. In terms of trough FEV1, all treatments were better than placebo. At 12 weeks, indacaterol 150 and 300 μg were more efficacious than FOR/BUD 9/160 μg, at least as efficacious as FOR/BUD 9/320 μg, and comparable to SAL/FP (50/250 and 50/500 μg). Results were consistent at 6 months and therefore both indacaterol doses are expected to be at least comparable to the fixed-dose combinations for this parameter. The probability that the FEV1 was higher for patients receiving indacaterol 150 or 300 than for each active comparator ranged from 51% to 99%. For SGRQ total score at 6 months, results suggest that indacaterol provides a comparable SGRQ improvement to the fixed-dose combinations for FOR/BUD (both doses) and SAL/FP 50/500 μg. In terms of TDI total score at 6 months, the results did support the efficacy of all treatments compared to placebo. Again, results indicate that indacaterol was comparable to both doses of SAL/FP for which data were available. Differences in SGRQ and TDI scores did not reach a clinically meaningful level (eg, less than SGRQ 4 points29 and less than TDI 1 points30), which suggests that indacaterol offers a comparable level of symptom relief to the fixed-dose combinations evaluated. As with previous analyses, improvements in TDI were more pronounced for indacaterol 300 μg compared to indacaterol 150 μg. In a separate analysis of pooled data, this additional improvement with the 300 μg dose was particularly apparent in patients with severe COPD.3
Although RCTs form the basis of the network and allow for the indirect comparisons in the absence of head-to-head comparisons, the key question is whether the trials in the network are sufficiently similar to yield meaningful results. In a network meta-analysis of RCTs involving multiple treatment comparisons, the randomization holds only within the individual trials, and not across trials. If the trials differ among the direct comparisons for study and patient characteristics, and these differences are modifiers of the relative treatment effects, then the estimate of the indirect and mixed comparisons is biased.12
In the indacaterol studies patients were allowed to continue receiving concurrent ICS, which was not the case in the FOR/BUD and SAL/FP studies. To avoid biased estimates of indacaterol versus FOR/BUD and SAL/FP a subgroup of patients who did not receive an ICS in indacaterol studies was evaluated in the network meta-analysis.
Differences were identified in terms of the proportion of males, the average age, the proportion of current smokers, and the proportion of patients with severe or very severe COPD in the indacaterol studies (subgroup) compared to the patients in the other studies. To evaluate the extent of the effect these differences in patient characteristics had on the relative effect estimates, meta-regression models were used. Although it was not feasible to include all of the covariates of interest simultaneously due to the limited amount of data, where possible the proportion of current smokers and the proportion of patients with severe or very severe COPD were included in one model. Results adjusted for the proportion of males and the average age had only a marginal impact on the effect estimates, and are therefore not believed to be a likely source of bias in the unadjusted analysis. Adjustment for smoking status and COPD severity had a greater impact on the relative effect estimates (see Figure 3), but the differences between adjusted and unadjusted models were not greater than the amount of uncertainty in the estimates. As such, adjusted and unadjusted models lead to the same interpretation of the findings. Although the meta-regression analyses suggest that the results of the network meta-analysis are not likely to be greatly affected by similarity and consistency violations, it was not possible to assess the similarity of the studies in terms of all patient characteristics. For example, limited information was presented for the comorbidities of patients across the trials. Therefore, it has to be accepted that with aggregate level data there is the risk of residual confounding bias.
Since the studies did not consistently report the ethnicity of the patients or report subgroup data, it was not feasible to include a covariate to adjust for differences in ethnicity. However, studies included a predominantly Caucasian population, and all studies were combined in the analysis. An additional analysis with 3 Asian studies excluded resulted in similar estimates and suggests that ethnicity is not a factor of importance in the current evidence base.
In conclusion, indacaterol monotherapy (150 μg and 300 μg) (no concomitant ICS) is expected to be at least as good as FOR/BUD (9/320 and 9/160 μg) and comparable to SAL/FP (50/250 and 50/500 μg) with respect to lung function (trough FEV1). Indacaterol monotherapy (150 and 300 μg) is also expected to provide comparable efficacy in terms of health status (SGRQ total score) versus FOR/BUD (9/320 and 9/160 μg) and SAL/FP 50/500 μg, as well as similar improvements in breathlessness (TDI total score) as SAL/FP (50/250 and 50/500 μg).
Acknowledgments
The authors acknowledge the contribution of David Young, Cheryl Lassen, David Lawrence, Cibele Suzuki, Gerardo Machnicki, Joonsu Kim, and Jungdea Kim.
Appendix. Search strategy
The search strategy was applied for the time period from 1989 to 2009 and 2009 to 2010
| No. | Database | Search term |
|---|---|---|
| 1 | MEDLINE | (COPD OR chronic ADJ obstructive ADJ pulmonary ADJ disease OR COAD OR chronic ADJ obstructive ADJ airway ADJ disease OR chronic ADJ obstructive ADJ lung ADJ disease OR chronic ADJ bronchitis OR emphysema).TI,AB. OR Pulmonary-Disease-Chronic-Obstructive#.DE. |
| 2 | MEDLINE | (Formoterol OR eformoterol OR foradil OR oxis OR atimos ADJ modulite OR atock OR perforomist OR salmeterol OR serevent OR tiotropium OR spiriva OR Ba ADJ ‘679’ ADJ BR OR Indacaterol OR onbrez OR arcapta).TI,AB. |
| 3 | MEDLINE | PT = CONTROLLED-CLINICAL-TRIAL OR PT = RANDOMIZED-CONTROLLED-TRIAL OR Clinical-Trials-As-Topic.DE. OR Controlled-Clinical-Trials-As-Topic.DE. OR Randomized-Controlled-Trials-As-Topic.DE. OR Randomized-Controlled-Trials-As-Topic.DE. OR (randomized OR randomized OR randomly OR placebo).TI,AB. OR trial.TI,AB. |
| 4 | MEDLINE | 3 AND HUMAN = YES AND ANIMAL = YES |
| 5 | MEDLINE | 3 AND ANIMAL = YES |
| 6 | MEDLINE | 3 NOT (4 OR 5) |
| 7 | MEDLINE | 1 AND 2 AND 3 AND 6 AND LG = EN AND HUMAN = YES AND ADULT# |
| 8 | EMBASE | (COPD OR chronic ADJ obstructive ADJ pulmonary ADJ disease OR COAD OR chronic ADJ obstructive ADJ airway ADJ disease OR chronic ADJ obstructive ADJ lung ADJ disease OR chronic ADJ bronchitis OR emphysema).TI,AB. |
| 9 | EMBASE | Chronic-Obstructive-Lung-Disease#.DE. |
| 10 | EMBASE | (Formoterol OR eformoterol OR foradil OR oxis OR atimos ADJ modulite OR atock OR perforomist OR salmeterol OR serevent OR tiotropium OR spiriva OR Ba ADJ ‘679’ ADJ BR OR Indacaterol OR onbrez OR arcapta).TI,AB. |
| 11 | EMBASE | Controlled-Clinical-Trial.DE. OR Double-Blind-Procedure.DE. OR Controlled-Clinical-Trial.DE. OR Randomized-Controlled-Trial.DE. OR Randomized-Controlled-Trial.DE. |
| 12 | EMBASE | (randomized OR randomized OR placebo OR randomly).TI,AB. OR trial.TI. |
| 13 | EMBASE | (11 OR 12) AND HUMAN = YES AND ANIMAL = YES |
| 14 | EMBASE | (11 OR 12) AND ANIMAL = YES |
| 15 | EMBASE | (11 OR 12) NOT (13 OR 14) |
| 16 | EMBASE | 8 OR 9 |
| 17 | EMBASE | 16 AND 15 AND 10 AND LG = EN AND HUMAN = YES AND ADULT = YES |
| 18 | MEDLINE and EMBASE [all] | combined sets 7, 17 |
| 19 | MEDLINE and EMBASE [all] | dropped duplicates from 18 |
| 20 | MEDLINE and EMBASE [all] | unique records from 18 |
| 21 | Medline | split set 20 |
| 22 | EMBASE | split set 20 |
Notes: .ab. indicates a search for a term in abstract; .pt. indicates a search for a publication type.
Footnotes
Disclosure
The study was funded by Novartis Pharma AG.
References
- 1.GOLD (Global Initiative for Chronic Obstructive Lung Disease) Global strategy for the diagnosis, management, and prevention of COPD. 2010. http://www.goldcopd.com/Guidelineitem.asp?l1=2&l2=1&intId=989. Accessed February 1, 2010.
- 2.Katz PM, Pegoraro V. L’utilizzo dei corticosteroidi nei pazienti con la broncopneumopatia cronica ostruttiva: aspetti epidemiologici ed economici. Farmeconomia e percorsi terapeutici. 2009;10(4):139–148. [Google Scholar]
- 3.European Medicines Agency Onbrez Breezhaler indacaterol. Doc.Ref.: EMA/625202/2009. 2010 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/001114/WC500053733.pdf. Accessed February 1, 2010. [Google Scholar]
- 4.Dahl R, Chung KF, Buhl R, et al. Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65(6):473–479. doi: 10.1136/thx.2009.125435. [DOI] [PubMed] [Google Scholar]
- 5.Donohue JF, Fogarty C, Lotvall J, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182(2):155–162. doi: 10.1164/rccm.200910-1500OC. [DOI] [PubMed] [Google Scholar]
- 6.Feldman G, Siler T, Prasad N, et al. Efficacy and safety of indacaterol 150 microg once-daily in COPD: a double-blind, randomised, 12-week study. BMC Pulm Med. 2010;10(11):1–9. doi: 10.1186/1471-2466-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornmann O, Dahl R, Centanni S, et al. Once-daily indacaterol vs twice-daily salmeterol for COPD: a placebo-controlled comparison. Eur Respir J. 2011;37:273–279. doi: 10.1183/09031936.00045810. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 10.Jansen J, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11(5):964–966. doi: 10.1111/j.1524-4733.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 11.Dempster AP. The direct use of likelihood for significance testing. Stat Comput. 1997;7:247–252. [Google Scholar]
- 12.Cooper NJ, Sutton AJ, Morris D, Ades AE, Welton NJ. Addressing between-study heterogeneity and inconsistency in mixed treatment comparisons: application to stroke prevention treatments in individuals with non-rheumatic atrial fibrillation. Stat Med. 2009;28:1861–1881. doi: 10.1002/sim.3594. [DOI] [PubMed] [Google Scholar]
- 13.Lunn DJ, Thomas A, Best N, Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 14.Barnes N, Qiu Y, Pavord I, Parker D, Davis P, Zhu J, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173(7):736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- 15.Calverley P, Pauwels R, Vestbo, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 16.Calverley P, Anderson J, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 17.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 18.Hanania N, Darken P, Horstman D, et al. The efficacy and safety of fluticasone propionate (250 mug)/salmeterol (50 mug) combined in the Diskus inhaler for the treatment of COPD. Chest. 2003;124(3):834–843. doi: 10.1378/chest.124.3.834. [DOI] [PubMed] [Google Scholar]
- 19.Mahler D, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(8):1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 20.Rennard SI, Tashkin D, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi: 10.2165/00003495-200969050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tashkin DP, Rennard SI, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000. doi: 10.2165/00003495-200868140-00004. [DOI] [PubMed] [Google Scholar]
- 22.Zheng J, Yang L, Wu Y, et al. The efficacy and safety of combination salmeterol (50 mug)/fluticasone propionate (500 mug) inhalation twice daily via accuhaler in Chinese patients with COPD. Chest. 2007;132(6):1756–1763. doi: 10.1378/chest.06-3009. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal A, Lean H, Lawrence D, Higgins M. Full Clinical Study Report for study number CQAB149B2335S: A 26-week treatment, multicenter, randomized, doubleblind, double dummy, placebo-controlled, adaptive, seamless, parallel-group study to assess the efficacy, safety and tolerability of two doses of indacaterol (selected from 75, 150, 300 and 600 μg o.d.) in patients with chronic obstructive pulmonary disease using blinded formoterol (12 μg b.i.d) and open label tiotropium (18 μg o.d.) as active Controls. Data on file: 2008.
- 24.Luthra A, Kramer B, Swales J, Henley M, Lassen C. Full Clinical Study Report for study number CQAB149B2336: A 26-week treatment, multi center, randomized, double blind, double dummy, placebo controlled, parallel group study to assess the efficacy and safety of indacaterol (150 μg o.d.) in patients with chronic obstructive pulmonary disease, using salmeterol (50 μg b.i.d.) as an active control. Data on file: 2009.
- 25.Hosoe M, Okino N, Maruyama Y, et al. Full Clinical Study Report for study number CQAB149B1302: A 12-week treatment, multi-center, randomized, double blind, placebo-controlled, parallel group study to assess the efficacy, safety and tolerability of Indacaterol (150 and 300 ìg o.d.) in patients with chronic obstructive pulmonary disease (COPD). Data on file: 2010.
- 26.Firth R, Henley M, Kramer B, Lassen C, Yang W, Owen R. Full Clinical Study Report for study number QAB149B2333: A phase III, 26-week multi-center randomized double-blind, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (150 and 300 ìg o.d.) in patients with chronic obstructive pulmonary disease. Data on file: 2010.
- 27.Jack D, Bleasdale P, Berhane I, Higgins M. Full Clinical Study Report for study number CQAB149B2334: A 52-week treatment, multicenter, randomized, double-blind, double dummy, placebo-controlled, parallel-group study to assess the efficacy, safety and tolerability of indacaterol (300 and 600 μg o.d.) in patients with chronic obstructive pulmonary disease, using formoterol (12 μg b.i.d.) as an active control. Data on file: 2008.
- 28.Prasad N, Piggott S, Higgins M, Yu T. Full Clinical Study Report for study number CQAB149B2346: A 12-week treatment, multi-center, randomized, doubleblind, placebo-controlled, parallel-group study to assess the efficacy and safety of indacaterol (150 μg o.d.) in patients with chronic obstructive pulmonary disease. Data on file: 2008.
- 29.Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/copd-200050513. [DOI] [PubMed] [Google Scholar]
- 30.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21(2):267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]