Abstract
P-glycoprotein (P-gp) mediates efflux of xenobiotics and bacterial toxins from the intestinal mucosa into the lumen. Dysregulation of P-gp has been implicated in inflammatory bowel disease. Certain probiotics have been shown to be effective in treating inflammatory bowel disease. However, direct effects of probiotics on P-gp are not known. Current studies examined the effects of Lactobacilli on P-gp function and expression in intestinal epithelial cells. Caco-2 monolayers and a mouse model of dextran sulfate sodium-induced colitis were utilized. P-gp activity was measured as verapamil-sensitive [3H]digoxin transepithelial flux. Multidrug resistant 1 (MDR1)/P-gp expression was measured by real-time quantitative PCR and immunoblotting. Culture supernatant (CS; 1:10 or 1:50, 24 h) of Lactobacillus acidophilus or Lactobacillus rhamnosus treatment of differentiated Caco-2 monolayers (21 days postplating) increased (∼3-fold) MDR1/P-gp mRNA and protein levels. L. acidophilus or L. rhamnosus CS stimulated P-gp activity (∼2-fold, P < 0.05) via phosphoinositide 3-kinase and ERK1/2 MAPK pathways. In mice, L. acidophilus or L. rhamnosus treatment (3 × 109 colony-forming units) increased mdr1a/P-gp mRNA and protein expression in the ileum and colon (2- to 3-fold). In the dextran sulfate sodium (DSS)-induced colitis model (3% DSS in drinking water for 7 days), the degree of colitis as judged by histological damage and myeloperoxidase activity was reduced by L. acidophilus. L. acidophilus treatment to DSS-treated mice blocked the reduced expression of mdr1a/P-gp mRNA and protein in the distal colon. These findings suggest that Lactobacilli or their soluble factors stimulate P-gp expression and function under normal and inflammatory conditions. These data provide insights into a novel mechanism involving P-gp upregulation in beneficial effects of probiotics in intestinal inflammatory disorders.
Keywords: multidrug resistance 1, Lactobacillus species, phosphoinositide 3-kinase, ERK1/2 kinase, dextran sulfate sodium-induced colitis, intestinal inflammation
intestinal epithelial cells play a major role in providing a barrier between luminal contents (e.g., bacteria, xenobiotics/toxins) and immune cells (37). Disturbance in the integrity of the epithelium leads to development of mucosal inflammation associated with intestinal disorders such as inflammatory bowel disease (IBD) (37, 45). Increasing evidence suggests that P-glycoprotein (P-gp) plays a critical role in protection of the intestinal epithelia by mediating the efflux of drugs/xenobiotics and bacterial toxins from the intestinal mucosa into the gut lumen (18). P-gp, encoded by the multidrug resistance 1 (MDR1) or ABCB1 gene, is a 170-kDa transmembrane protein that is abundantly expressed on the apical surface of intestinal epithelial cells (49). Decreased P-gp function and expression have been implicated in the pathogenesis of IBD (2, 22). Polymorphisms in the human MDR1 gene have been associated with reduced intestinal P-gp expression in patients with ulcerative colitis (UC) and Crohn's disease (1, 28). Furthermore, a decrease in function and expression of P-gp has been shown in an experimental mouse model of dextran sulfate sodium (DSS)-induced colitis (19) and T cell receptor (TCRα) knockout (30) mice. Since P-gp plays an important role in maintaining intestinal epithelial integrity, it is essential to understand the cellular and molecular mechanism(s) regulating P-gp in the intestine.
Recent studies have shown that probiotics are effective in various intestinal disorders, including IBD, infantile or antibiotic-associated diarrhea, and necrotizing enterocolitis (11). Probiotics are live microorganisms that, when administered in adequate amounts, confer health benefit to the host (16). Although their precise mechanism of action is poorly understood, certain probiotics are able to ameliorate the intestinal inflammatory processes (13), counteract the influence of enteropathogens, and stimulate the growth of beneficial bacterial species (46). Certain probiotics are also known to enhance 1) mucin synthesis and secretion (25), 2) cytoprotective heat shock protein levels (48), and 3) cell survival and growth (51, 52). Lactic acid bacteria, particularly Lactobacilli, are among the predominant commensal bacteria in the gut microflora and are most commonly used for prevention and treatment of diarrheal disorders (53). Increasing evidence suggests that probiotics enhance intestinal epithelial homeostasis and integrity (32) in in vitro (12, 32, 41, 42) and in vivo (26, 29, 31) models. However, no studies of the effects of probiotics on P-gp function and expression in the intestine are available. Since P-gp appears to play an important role in protecting the intestinal epithelia, we hypothesized that one of the mechanisms by which probiotics exert their protective effects in intestinal inflammation is modulation of P-gp expression and function. Therefore, the current studies were designed utilizing in vitro and in vivo models to investigate in detail the effects of Lactobacillus sp. on P-gp function and expression.
MATERIALS AND METHODS
Materials
Caco-2 cells were obtained from American Type Culture Collection (Manassas, VA). [3H]digoxin (40 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). Substrate digoxin and the P-gp/MDR1 inhibitor verapamil were obtained from Sigma (St. Louis, MO). The ERK1/2 MAPK inhibitor PD-98059 and the phosphoinositide 3-kinase (PI3K) inhibitor LY-294002 were obtained from Biomol (Plymouth Meeting, PA). Mouse monoclonal MDR1 antibody and goat anti-mouse and goat anti-rabbit antibody conjugated to horseradish peroxidase were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). All other chemicals were of at least reagent grade and were obtained from Sigma Chemical or Fisher Scientific (Pittsburgh, PA).
Bacterial Culture
Lactobacillus acidophilus (4357), Lactobacillus rhamnosus (53103), and Lactobacillus casei (393) were procured from American Type Culture Collection. They were grown overnight, and culture supernatant (CS) was obtained as described previously (40). For all in vitro cell culture studies, CS was diluted in cell culture medium. For in vivo studies, 3 × 109 colony-forming units (CFUs) of live L. acidophilus or L. rhamnosus bacteria were gavaged per mouse (n = 3). This dose was based on previous studies from our laboratory and others showing that live Lactobacillus sp. bacteria was used at 3 × 109 CFUs per animal for in vivo mouse studies (34, 40).
Cell Culture and Treatment
For flux experiments, Caco-2 cells grown in modified Eagle's medium (40) were plated on 12-well Transwell collagen-coated inserts (43) and used at day 21 postplating. Cells were treated from the apical side with L. acidophilus, L. rhamnosus, or L. casei CS diluted in a ratio of 1:10 or 1:50 in serum-free cell culture medium for 24 h. In separate sets of experiments, cells were pretreated with the specific PI3K inhibitor LY-294002 (50 μM) or ERK1/2 MAPK inhibitor PD-98059 (30 μM) for 1 h and then coincubated with L. acidophilus or L. rhamnosus CS diluted in a ratio of 1:10 in serum-free cell culture medium for another 24 h.
P-gp-Dependent Digoxin Flux Activity
P-gp activity was determined by measurement of verapamil-sensitive [3H]digoxin flux in Caco-2 cells, as previously described (17). Briefly, after treatment, the cell monolayers were washed in the flux buffer. Transport studies were performed in triplicates in the apical-to-basolateral and basolateral-to-apical directions with flux buffer containing [3H]digoxin [1 μCi/ml + 1 μM unlabeled (cold) digoxin] from the apical (200 μl) or basolateral (500 μl) compartments at room temperature in the presence or absence of the P-gp inhibitor verapamil (10 μM). Samples (100 μl) were taken from receiver compartments (initial flux) at 15 min and then at 60 min (final flux). The radioactivity of the receiver samples was determined using a liquid scintillation analyzer (Tri-Carb 1600TR, Packard Instruments, PerkinElmer). The apical-to-basolateral or basolateral-to-apical values of the final flux were subtracted from the initial flux, and digoxin flux activity was expressed as mean basolateral-to-apical and apical-to-basolateral verapamil-sensitive flux ratios (nmol digoxin/45 min).
Transfections
Caco-2 cells were transfected with MDR1 promoter fragment cloned upstream of the luciferase reporter gene in pGL2-basic and β-galactosidase expression vectors by electroporation using the Amaxa Nucleofactor System, as described previously (43). MDR1 promoter activity was expressed in terms of relative luciferase activity normalized to β-galactosidase activity.
Animals
Eight-week-old male C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME) were given drinking water and standard rodent pellets ad libitum. Studies were approved by the Animal Care Committee of the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center.
Experimental Design
L. acidophilus or L. rhamnosus treatment.
Mice were gavaged with L. acidophilus or L. rhamnosus (3 × 109 CFUs, n = 3) or sterile PBS as vehicle for 24 h. Mice were killed after 24 h. Intestines were resected, and mucosa was scraped for RNA and protein extraction.
Induction of colitis.
Four groups of mice (n = 4) were used for the study, as described previously (27). In two of the groups, 3% (wt/vol) DSS (36–50 kDa; MP Biomedicals, Solon, OH) was given orally to mice in drinking distilled water for 7 days, whereas the control groups received distilled water only. In one DSS-induced colitis group, L. acidophilus was administered by oral gavage (3 × 109 CFUs) in sterile PBS along with DSS for the first 2 days (L. acidophilus + DSS). The other DSS-induced colitis group received sterile PBS (DSS). The control groups (without colitis) received L. acidophilus (L. acidophilus) or sterile PBS (control) in a similar manner. Statistical significance was achieved with four mice. Mice were weighed daily and were killed on day 8. The entire colon was dissected out, and length and weight were recorded. Since in the DSS-induced colitis model the distal part of the colon is affected (33), a part (∼2 cm) of it was immediately fixed in 10% neutral buffered formalin for histological studies or in liquid N2 for determination of myeloperoxidase (MPO) activity. Mucosa was scraped from the remaining part of the distal colon for RNA and protein extraction.
MPO Activity
MPO activity in the distal colon was assessed to monitor the degree of inflammation using the method of Krawisz et al. (21) with minor modifications. MPO activity was normalized to the amount of protein in supernatant by Bradford's method (4) and expressed as units per gram of protein.
Real-Time PCR
RNA was extracted from L. acidophilus or L. rhamnosus CS-treated (1:50 dilution) and untreated Caco-2 cells or mouse samples, as described previously by us (52). Human MDR1 (hMDR1) and histone (internal control) were amplified with gene-specific primers, as described previously (2, 40). Mouse mdr1a (mmdr1a, accession no. NM011076) was amplified with gene-specific primers: 5′-GTGGGGGACAGAAACAGAGA-3′ (sense) and 5′-GAACGGTAGACAAGCGATGAG-3′ (anitsense). Mouse histone was amplified as an internal control by utilization of gene-specific primers: 5′-GTACTGTGGCACTCCGTGAA-3′ (sense) and 5′-CAAAAAGGCCAACCAGAAAG-3′ (antisense). Relative levels of hMDR1 or mmdr1a mRNA are expressed as percentage of control normalized to histone.
Western Blotting
L. acidophilus or L. rhamnosus CS-treated and untreated Caco-2 cells or tissue lysates from the scraped mucosa of ileum and colon were prepared as described previously (52). Lysates were run on a 7% gel and then transferred onto a nitrocellulose membrane. Nitrocellulose membranes were incubated with the monoclonal MDR1 antibody (1:400 dilution) in the blocking buffer containing 1× PBS and 1% nonfat dry milk overnight at 4°C. Membranes were then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG antibody (1:2,000 dilution) for 1 h at room temperature and washed for 30 min with agitation, during which the wash buffer was changed every 5 min. Bands were visualized with enhanced chemiluminescence detection reagents.
Statistical Analysis
Results are expressed as means ± SE of three to five independent experiments. Student's t-test or one-way ANOVA with Tukey's test was used for statistical analysis. P < 0.05 or less was considered statistically significant.
RESULTS
L. acidophilus and L. rhamnosus CS Increase P-gp Activity in Caco-2 Cells
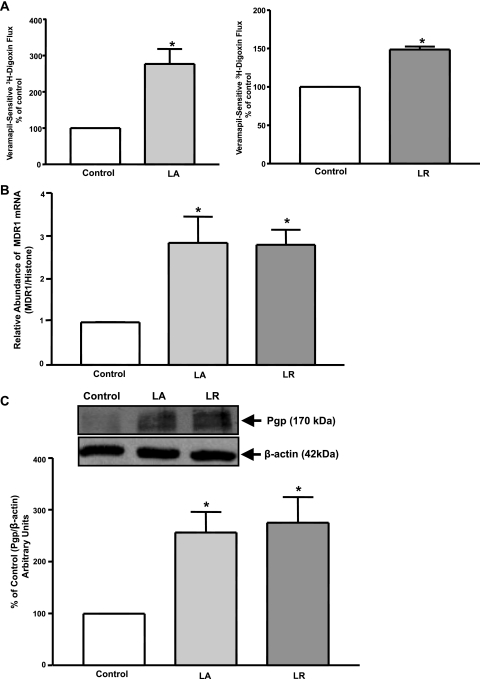
Our previous studies showed that CS of L. acidophilus or L. rhamnosus stimulated expression and activity of Na+/H+ exchanger 3 (NHE3) (39) and DRA (3, 40) in intestinal epithelial cells. To examine the effects of L. acidophilus or L. rhamnosus, Caco-2 monolayers were treated apically with L. acidophilus or L. rhamnosus CS at 1:50 dilution for 24 h, and verapamil-sensitive [3H]digoxin flux was measured. As shown in Fig. 1A, verapamil-sensitive [3H]digoxin flux was significantly increased by L. acidophilus or L. rhamnosus compared with untreated control. However, treatment with another commensal probiotic strain, L. casei CS (1:10 dilution), for 24 h caused no significant alterations in P-gp function in Caco-2 cells (values expressed as percentage of control: 103 ± 20.02 and 100 ± 0.29% for L. casei and control, respectively). These results further indicate that the effects of L. acidophilus and L. rhamnosus on P-gp are specific.
Fig. 1.
Effect of Lactobacillus acidophilus (LA) or Lactobacillus rhamnosus (LR) culture supernatant (CS) on P-glycoprotein (P-gp) activity and expression in Caco-2 cells. Overnight-serum-starved postconfluent Caco-2 cells were treated with a 1:50 dilution of L. acidophilus or L. rhamnosus CS in serum-free cell culture medium for 24 h. A: [3H]digoxin flux (1 μM). Results are expressed as percentage of control. Values are means ± SE of 5 separate experiments performed in triplicate. *P < 0.05 vs. untreated control. B: relative abundance of multidrug resistance 1 (MDR1) mRNA from control and treated Caco-2 cells normalized to histone mRNA (internal control). C: control and treated cell lysates were subjected to 7% SDS-PAGE and then transferred to a nitrocellulose membrane. Blot was probed with anti-MDR1 (P-gp) or anti-β-actin (β-actin) antibody. A representative blot of 3 different experiments is shown. Data were quantified by densitometric analysis and are expressed as percentage of control in arbitrary units. Values are means ± SE of 3 independent experiments performed in triplicate. *P < 0.05 vs. untreated control.
L. acidophilus and L. rhamnosus CS-Mediated Stimulation of P-gp Activity Occurs via Increased MDR1/P-gp Expression
P-gp is encoded by the MDR1 gene in the intestine (49). Therefore, to examine the effect of L. acidophilus or L. rhamnosus CS on MDR1 mRNA, Caco-2 monolayers were treated with L. acidophilus or L. rhamnosus CS (1:50 dilution) for 24 h, and mRNA levels of MDR1 were determined by real-time quantitative PCR. As shown in Fig. 1B, MDR1 mRNA levels were significantly (∼2.5-fold) increased at 24 h. Furthermore, Western blotting showed that L. acidophilus or L. rhamnosus also increased expression of P-gp protein. Densitometric analysis revealed that L. acidophilus or L. rhamnosus CS significantly (∼2.5-fold) enhanced P-gp protein levels in Caco-2 cells (Fig. 1C). Thus this increase in P-gp activity in Caco-2 cells by L. acidophilus or L. rhamnosus was consistent with increased MDR1/P-gp mRNA and protein expression.
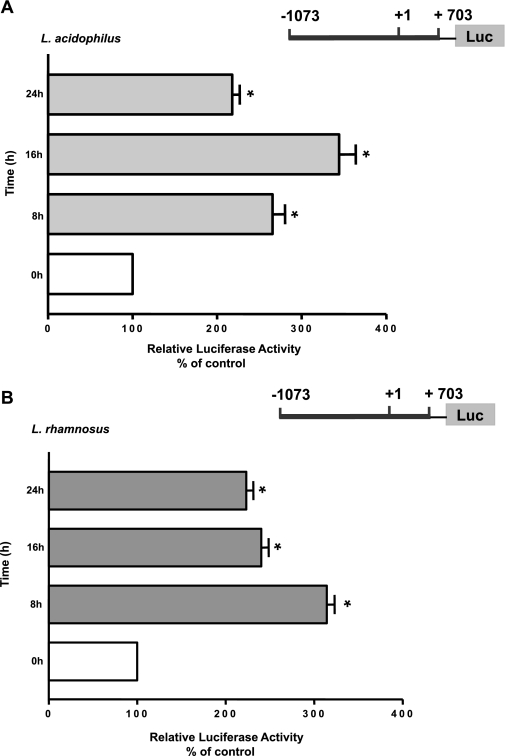
L. acidophilus and L. rhamnosus CS Increase MDR1 Promoter Activity
We next investigated whether the increase in the MDR1 mRNA expression was through a transcriptional mechanism involving increased promoter activity. Caco-2 cells were transfected with the MDR1 promoter reporter plasmid −1073/+703. At 24 h posttransfection, cells were treated with L. acidophilus or L. rhamnosus CS (1:50 dilution) for 8, 16, and 24 h, and MDR1 promoter activity was assessed. A marked increase (>2-fold) in MDR1 promoter activity in response to L. acidophilus or L. rhamnosus CS was noted as early as 8 h and persisted until the 24-h time point (Fig. 2). These results demonstrate that long-term L. acidophilus or L. rhamnosus treatment increases MDR1 expression through a transcriptional mechanism in human intestinal epithelial cells.
Fig. 2.
Effect of L. acidophilus or L. rhamnosus CS on MDR1 promoter activity in Caco-2 cells. Caco-2 cells were transiently transfected with MDR1 luciferase (Luc) promoter construct (−1073/+703) along with pCMVβ vector. Cells were treated with L. acidophilus or L. rhamnosus CS (1:10 dilution) for 24 h in medium containing 1% FBS after 24 h. At 48 h posttransfection, promoter activity was measured by luciferase assay. Values were normalized to β-galactosidase activity to correct for transfection efficiency. Results are expressed as percentage of control: transfected cells treated with L. acidophilus or L. rhamnosus CS vs. untreated cells (control). Values are means ± SE of 4 separate experiments with 3 independent observations for each experiment (n = 12). *P < 0.05 vs. control.
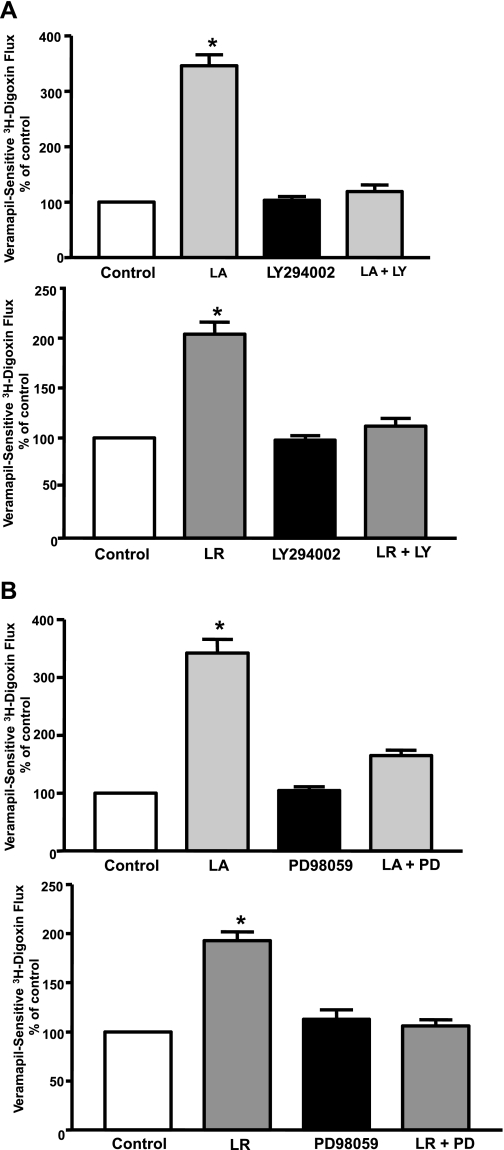
L. acidophilus and L. rhamnosus CS Effects on P-gp Activity in Caco-2 Cells Are PI3K- and ERK1/2 MAPK-Dependent
Previous studies have shown that soluble factors of L. rhamnosus CS prevented cytokine-mediated apoptosis in intestinal epithelial cells in a PI3K-dependent manner (51). Also, ERK1/2 MAPK has been implicated in mediating the protective effects of Lactobacilli in intestinal epithelial cells (42, 48). The specific PI3K inhibitor LY-294002 (50 μM) or the specific ERK1/2 MAPK inhibitor PD-98059 (30 μM) blocked the stimulatory effects of L. acidophilus or L. rhamnosus CS on P-gp activity in Caco-2 cells (Fig. 3), suggesting the involvement of PI3K and ERK1/2 MAPK in L. acidophilus- or L. rhamnosus-stimulated effects.
Fig. 3.
Effect of phosphoinositide 3-kinase (PI3K) or ERK1/2 MAPK inhibitor on L. acidophilus or L. rhamnosus CS-induced stimulation of P-gp activity in Caco-2 cells. Postconfluent Caco-2 cells were pretreated with the specific inhibitor of PI3K, LY-294002 (LY, 50 μM; A), or the specific inhibitor of ERK1/2 MAPK, PD-98059 (PD, 30 μM; B), for 60 min in the serum-free cell culture medium and then coincubated with 1:10 dilution of L. acidophilus or L. rhamnosus CS in serum-free cell culture medium for 24 h, and [3H]digoxin flux (1 μM) was measured. Results are expressed as percentage of control. Values are means ± SE of 4 separate experiments performed in triplicate. *P < 0.05 vs. untreated control.
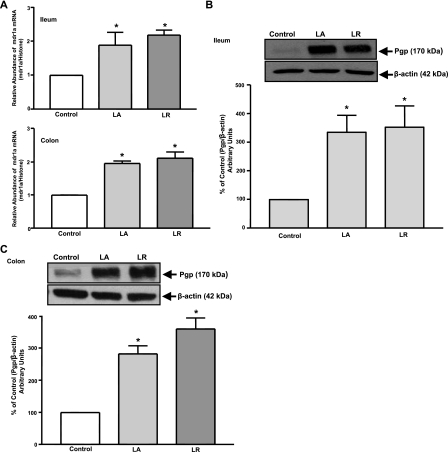
In Vivo Effects of L. acidophilus or L. rhamnosus on P-gp Expression
The in vitro studies are limited in their ability to provide a comprehensive model for assessing the net effect of Lactobacillus sp. on the native intestinal tissue. Therefore, we utilized an in vivo murine model to examine effects of L. acidophilus or L. rhamnosus on P-gp expression in the intestine.
Live L. acidophilus or L. rhamnosus increases mdr1a expression.
mdr1a mRNA levels were assessed by real-time quantitative RT-PCR in intestinal tissues from mice gavaged with L. acidophilus or L. rhamnosus live bacteria (24 h). Expression of mdr1a mRNA (Fig. 4A) and P-gp protein (Fig. 4, B and C) was significantly (∼2- to 3-fold) increased in L. acidophilus- or L. rhamnosus-treated ileum and colon compared with untreated controls.
Fig. 4.
P-gp expression in response to L. acidophilus or L. rhamnosus [3 × 109 colony-forming units (CFUs)]. A: relative abundance of mdr1a mRNA from ileum and colon of control and treated mice normalized to histone mRNA (internal control). Total lysates extracted from mucosal tissues of ileum (B) and colon (C) of control and treated mice were subjected to 7% SDS-PAGE and then transferred to a nitrocellulose membrane. Blot was probed with anti-mdr1 (P-gp) or anti-β-actin (β-actin) antibody. A representative blot is shown. Data were quantified by densitometric analysis and are expressed as percentage of control in arbitrary units. Values are means ± SE of 3 mice. *P < 0.05 vs. untreated control.
P-gp Expression Is Reduced in Model of DSS-Induced Colitis
To examine whether L. acidophilus could restore the inhibitory effects of DSS on mdr1a/P-gp mRNA and protein expression, mdr1a/P-gp expression was examined in distal colonic tissues of control, DSS (3% DSS), L. acidophilus (3 × 109 CFUs of L. acidophilus bacteria), and L. acidophilus + DSS mice using real-time PCR and immunoblotting.
L. acidophilus reduces clinical disease activity and inflammation in acute DSS-induced colitis.
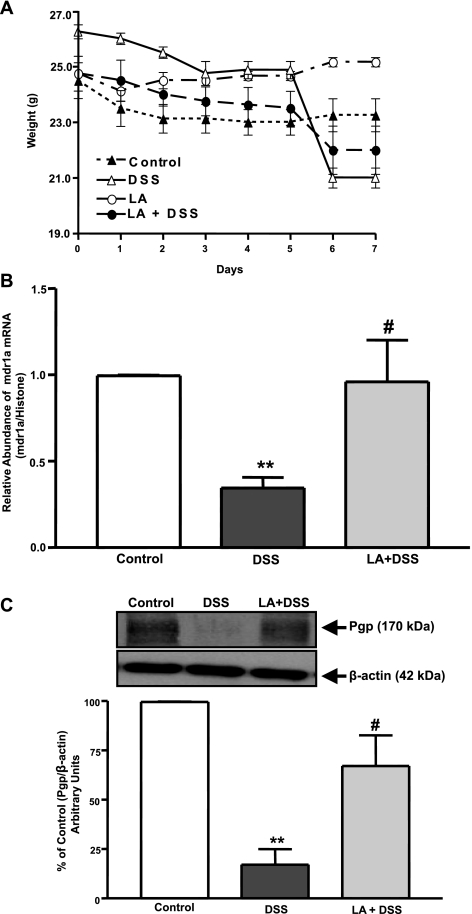
Mice exposed to DSS showed loss of body weight, which was evident after day 5 of DSS treatment and persisted on days 6 and 7 compared with control. Mice treated with L. acidophilus alone did not show any change in body weight. However, in mice treated with L. acidophilus + DSS, L. acidophilus reduced the DSS-induced loss of body weight (Fig. 5A). Also, a loose stool pellet was observed in DSS mice, whereas a solid stool pellet was observed in control, L. acidophilus, and L. acidophilus + DSS mice. A significant increase in colonic MPO activity, an index of neutrophil accumulation, was observed in DSS mice compared with control or L. acidophilus mice. This increase in MPO levels was almost completely blocked by L. acidophilus treatment in L. acidophilus + DSS mice (1.72 ± 0.91, 57.28 ± 1.21, 0.636 ± 0.12, and 2.08 ± 0.76 U/g protein for control, DSS, L. acidophilus, and L. acidophilus + DSS, respectively; P < 0.001, DSS vs. control; P < 0.001, L. acidophilus + DSS vs. DSS). Also, histological studies of the distal colon showed that L. acidophilus caused a reduction in inflammation in L. acidophilus + DSS mice (data not shown). Colonic sections treated with L. acidophilus were similar to control colonic sections. The surface epithelium was completely intact in all sections studied. These results indicate that L. acidophilus exerts its protective effects in the colon of mice with colitis by decreasing inflammation caused by DSS.
Fig. 5.
L. acidophilus attenuates dextran sulfate sodium (DSS)-induced inhibition of P-gp expression in distal colon. A: change in body weight during DSS treatment. Mice were given 3% DSS in drinking water for 7 days. Control mice had only drinking water. L. acidophilus (3 × 109 CFUs) was administered by gavage to control or DSS-treated mice for the first 2 days. Values are means ± SE (n = 4). B: mdr1a expression. Relative abundance of mdr1a mRNA from mucosal tissues of distal colon of control, DSS, or L. acidophilus + DSS mice was normalized to histone mRNA (internal control). C: P-gp expression. Total lysates were subjected to 7% SDS-PAGE and then transferred to a nitrocellulose membrane. Blot was probed with anti-mdr1 (P-gp) or anti-β-actin (β-actin) antibody. A representative blot is shown. Data were quantified by densitometric analysis and expressed as percentage of control in arbitrary units. Values are means ± SE of 4 mice. *P < 0.05 vs. untreated control. #P < 0.05 vs. DSS.
L. acidophilus attenuates decreased mdr1a/P-gp mRNA and protein expression induced by DSS.
DSS caused a significant (∼60–70%) decrease in mdr1a/P-gp mRNA and protein expression in the distal colon. However, the decreased levels of mdr1a/P-gp mRNA and protein were significantly attenuated in L. acidophilus + DSS mice (Fig. 5, B and C). These results suggest that enhanced intestinal epithelial integrity via the modulation of P-gp might be a major contributor to the observed protective and anti-inflammatory effects of L. acidophilus.
DISCUSSION
Reduced effectiveness of the intestinal epithelial integrity contributes to chronic inflammation and diarrhea in IBD and colitis. P-gp (MDR1) plays an important role in the protection of intestinal epithelial cells by pumping out xenobiotics and bacterial toxins from inside the cells back into the intestinal lumen. Along with P-gp, multidrug resistance protein 2 (MRP2; ABCC2) and breast cancer resistance protein (BCRP, ABCG2) have also been shown to be expressed at the apical membrane of epithelial cells in the intestine (6). Although all these are involved in drug disposition, it appears that MDR1 is important in the maintenance of intestinal epithelial integrity, as mice lacking the mdr1 gene have been shown to develop spontaneous colitis similar to human UC (35). In contrast, mice lacking MRP2 and BCRP did not develop colitis (9, 54). Moreover, several lines of evidence suggest that changes in MDR1/P-gp function and/or expression may contribute to the pathogenesis of inflammatory disorders of the gastrointestinal tract, such as IBD. Recent studies have shown that probiotics can play a role in the prevention and/or treatment of IBD (38). Whether probiotics exert their protective effects by increasing the reduced expression of P-gp in intestinal inflammation is not known. In the present study, we found that CS of L. acidophilus or L. rhamnosus significantly increased P-gp function in intestinal epithelial Caco-2 cells, but L. casei had no effect. These results indicate that the effects of probiotics on the upregulation of P-gp are specific and not a generalized effect of bacteria. The increase in function was consistent with an increase in MDR1/P-gp mRNA and protein levels in Caco-2 cells and occurred via a transcriptional mechanism, as L. acidophilus or L. rhamnosus CS increased MDR1/P-gp promoter activity. Previous studies have shown that the transcription factors involved in mediating the anti-inflammatory effects of Lactobacillus sp. include c-Jun and activator protein (AP)-1 (24) and nuclear factor of activated T-cells (NFAT) (15) transcription factors. Sequence analysis of the MDR1 promoter region revealed multiple potential binding sites for AP-1 and NFAT, suggesting the possible role of these factors in mediating the stimulatory effects of L. acidophilus and L. rhamnosus on P-gp function and expression. However, detailed 5′ deletion and mutagenesis studies are needed to further elucidate the probiotic-responsive region of the MDR1 promoter and will be the subject of future investigations. Although, MRP2 and BCRP are also expressed in Caco-2 cells, the verapamil-sensitive [3H]digoxin flux assay represents a true measurement of P-gp activity for the following reasons: 1) previous studies demonstrated the specificity of digoxin as an efflux substrate for MDR1, but not MRP2 or BCRP, in transepithelial drug transport assays in MDR1-, MRP2-, or BCRP-overexpressed epithelial cell lines (47), and 2) the specific MRP2 inhibitor MK-571 and the specific BCRP inhibitor furnitremorgin C inhibited sulfasalazine transport in Caco-2 cells, while the P-gp inhibitor verapamil failed to inhibit sulfasalazine transport (10).
It has been shown previously that L. acidophilus-mediated regulation of intestinal epithelial homeostasis is dependent on the intimate interaction of bacteria with mammalian cells (41). However, in our studies, L. acidophilus CS also showed a significant stimulation of P-gp function and expression in Caco-2 cells. These results indicate that secreted factors produced by L. acidophilus, rather than the live L. acidophilus bacteria, are effective in increasing MDR1/P-gp function and expression in Caco-2 cells. Several probiotic bacteria, including Lactobacillus GG (LGG or L. rhamnosus), Bifidobacterium sp., and Streptococcus thermophilus, have been shown to secrete soluble or bioactive factors capable of eliciting responses in epithelial cells that contribute to intestinal homeostasis and epithelial integrity (32). Soluble peptides secreted by LGG have been shown to promote cell growth by inducing Akt activation and inhibiting cytokine-induced epithelial cell apoptosis (51). Soluble protein secreted by VSL#3 enhanced epithelial barrier function and resistance to Salmonella invasion in intestinal epithelial cells (26). VSL#3 culture supernatant has also been shown to inhibit NFκB activity and induce expression of cytoprotective heat shock proteins in colonic epithelial cells (36). Some of the identified bioactive molecules secreted by probiotics are polyamines, e.g., spermine and spermidine, and conjugated linoleic acids. Polyamines act as growth promoters and are shown to ameliorate enterpathogenic infection in intestinal epithelial cells (5). Conjugated linoleic acids produced by Lactobacillus have been shown to exert anti-inflammatory effects in Helicobacter pylori-infected gastric epithelial cells (20) by inhibiting NFκB activity and IL-8 expression. Further studies are needed to identify the bioactive factors secreted by L. acidophilus or L. rhamnosus that elicit stimulatory effects on P-gp function and expression in Caco-2 cells. These studies will be of significance, as use of bacterial secreted bioactive molecules, instead of live bacteria, may have therapeutic benefits in IBD patients, where the epithelial barrier integrity is compromised. The administration of live bacteria can further exacerbate inflammation in these compromised patients, who, hence, are at risk for bacteremia and sepsis from live strains.
Previous studies have shown that the protective effects of probiotics are mediated via various intracellular signaling pathways, including PI3K/Akt, MAPK, and NFκB/IκB (36, 41, 42, 48, 51). Previous studies have also shown that treatment of intestinal epithelial cells with probiotic bacteria inhibited the alterations in intestinal epithelial cell integrity caused by stress, infection, or proinflammatory cytokines through PI3K- and MAPK-dependent pathways (12, 23, 26, 42). Furthermore, reversal of alterations in Cl− secretion induced by cytokines, IFNγ and TNFα, was shown to be dependent on ERK, p38, and PI3K activation by the probiotic bacteria S. thermophilus and L. acidophilus in HT29 and Caco-2 cells (42). Recently, we showed that L. acidophilus CS stimulated Cl−/HCO3− (OH−) exchange activity in Caco-2 cells via a PI3K-dependent pathway (3). Consistent with these studies, our current findings demonstrate the involvement of PI3K- and MAPK-dependent pathways in stimulation of P-gp function. Since not much is known about regulation of P-gp in the human intestine, our findings are unique in establishing the role of PI3K and ERK1/2 MAPK in regulating P-gp and provide novel evidence for a possible link of P-gp stimulation by probiotics to increased gut mucosal integrity.
We further validated our in vitro results of Caco-2 cells using an in vivo mouse model. Consistent with the in vitro results, our in vivo data showed that mdr1a/P-gp mRNA and protein expression was significantly increased in the ileum and colon of L. acidophilus- or L. rhamnosus-treated mice. In recent years, probiotics have become the subject of intense investigation, particularly in the context of IBD (44). Their effect appears mainly in maintenance of remission of UC and, to a lesser extent, Crohn's disease (14). Also, it has been shown that administration of a mixture of Lactobacilli and Bifidobacteria prevents the relapse of UC (50). There is strong evidence that probiotics protect epithelial integrity in animal models of intestinal inflammation (12, 26, 31).
Although several studies have implicated a decrease in P-gp function and expression in the pathogenesis of intestinal inflammation (2, 19, 22, 30), no studies directly link the protective effects of probiotics in maintaining epithelial integrity to increased mdr1a/P-gp expression. We, therefore, evaluated the effects of L. acidophilus on mdr1/P-gp expression in a murine model of DSS colitis. In agreement with previous findings (19), our results showed that DSS caused a significant decrease in mdr1a/P-gp mRNA and protein expression. However, L. acidophilus significantly attenuated the inhibitory effects of DSS on mdr1a/P-gp mRNA and protein expression. L. acidophilus also reduced the DSS-induced body weight loss in L. acidophilus + DSS mice. Hematoxylin-eosin-stained sections showed that L. acidophilus was able to significantly reduce the severity of DSS-induced inflammation in distal colonic tissue. We used 3% DSS for 7 days to induce mild colitis in mice, as DSS at high concentrations (4–5%) is known to have a direct toxic effect on epithelial cells that can cause erosions with complete loss of surface epithelium (29). However, in mice treated with 3.5% DSS to mice for 7 days, no loss of surface epithelium of the distal colon was observed (29). Therefore, our studies utilizing 3% DSS suggest that the decrease in mdr1a/P-gp expression is not secondary to loss of surface epithelium. The inhibition of enhanced colonic MPO activity in DSS mice by L. acidophilus reflects a potent intestinal anti-inflammatory effect of L. acidophilus against tissue injury. The intestinal anti-inflammatory/immunomodulatory properties of Lactobacillus sp. have been previously described in experimental models of colitis (7, 8). Our MPO results are in accordance with previous studies of Chen et al. (8) showing >50% (2-fold) reduction in MPO levels by L. acidophilus in Balb/c mice treated for 5 days with 5% DSS. The almost complete prevention of increased MPO levels by L. acidophilus observed in L. acidophilus + DSS mice could be due to the lower concentration of DSS (3%) used in our current studies compared with 5% DSS used by Chen et al. In addition, loose fecal pellets were observed in the DSS mice, but not in any other group, including the L. acidophilus + DSS mice. It seems that prevention of colonic inflammation by L. acidophilus correlates well with the finding of loose fecal pellets in the DSS mice and solid fecal pellets in the L. acidophilus + DSS mice. However, it should be noted that our studies, for the first time, suggest that the beneficial effects of L. acidophilus in the attenuation of intestinal inflammation might occur via the modulation of P-gp involved in the protection of epithelial integrity and maintenance of intestinal homeostasis.
In summary, L. acidophilus and L. rhamnosus CS increased P-gp function and expression in Caco-2 cells. L. acidophilus and L. rhamnosus CS-mediated stimulation of P-gp function occurred via PI3K- and ERK1/2 MAPK-dependent pathways. Expression of mdr1a/P-gp mRNA and protein was also enhanced by L. acidophilus and L. rhamnosus in the ileum and colon of mice. Furthermore, L. acidophilus was effective in attenuating decreased mdr1a/P-gp mRNA and protein levels and inflammation in the distal colon of DSS-treated mice. Our studies suggest that the beneficial effects of L. acidophilus and/or its soluble factors through the stimulation of P-gp may have broader therapeutic implications in treating intestinal inflammatory disorders involving impairment of epithelial integrity.
GRANTS
These studies were supported by Crohn's and Colitis Foundation of America Grant 1942 (S. Saksena), the Department of Veterans Affairs, and National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-54016, DK-81858, and P01 DK-067887 (P. K. Dudeja), DK-71596 (W. Alrefai), and DK-74458 (R. K. Gill).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Annese V, Valvano MR, Palmieri O, Latiano A, Bossa F, Andriulli A. Multidrug resistance 1 gene in inflammatory bowel disease: a meta-analysis. World J Gastroenterol 12: 3636–3644, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blokzijl H, Vander Borght S, Bok LI, Libbrecht L, Geuken M, van den Heuvel FA, Dijkstra G, Roskams TA, Moshage H, Jansen PL, Faber KN. Decreased P-glycoprotein (P-gp/MDR1) expression in inflamed human intestinal epithelium is independent of PXR protein levels. Inflamm Bowel Dis 13: 710–720, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Borthakur A, Gill RK, Tyagi S, Koutsouris A, Alrefai WA, Hecht GA, Ramaswamy K, Dudeja PK. The probiotic Lactobacillus acidophilus stimulates chloride/hydroxyl exchange activity in human intestinal epithelial cells. J Nutr 138: 1355–1359, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 5. Broekaert IJ, Nanthakumar NN, Walker WA. Secreted probiotic factors ameliorate enteropathogenic infection in zinc-deficient human Caco2 and T84 cell lines. Pediatr Res 62: 139–144, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chan LM, Lowes S, Hirst BH. The ABCs of drug transport in intestine and liver: efflux proteins limiting drug absorption and bioavailability. Eur J Pharm Sci 21: 25–51, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res 58: 1185–1191, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chen LL, Wang XH, Cui Y, Lian GH, Zhang J, Ouyang CH, Lu FG. Therapeutic effects of four strains of probiotics on experimental colitis in mice. World J Gastroenterol 15: 321–327, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, Wang RW, Yabut J, Hartley DP, Evans DC, Evers R. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2). J Pharmacol Exp Ther 317: 579–589, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Dahan A, Amidon GL. Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am J Physiol Gastrointest Liver Physiol 297: G371–G377, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Doron S, Gorbach SL. Probiotics: their role in the treatment and prevention of disease. Expert Rev Anti Infect Ther 4: 261–275, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol 295: G1025–G1034, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest 39: 429–448, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O'Neil DA, Macfarlane GT. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut 54: 242–249, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galdeano CM, de Leblanc Ade M, Carmuega E, Weill R, Perdigon G. Mechanisms involved in the immunostimulation by probiotic fermented milk. J Dairy Res 76: 446–454, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Gupta V, Garg R. Probiotics. Indian J Med Microbiol 27: 202–209, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Haslam IS, Jones K, Coleman T, Simmons NL. Induction of P-glycoprotein expression and function in human intestinal epithelial cells (T84). Biochem Pharmacol 76: 850–861, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Ho GT, Moodie FM, Satsangi J. Multidrug resistance 1 gene (P-glycoprotein 170): an important determinant in gastrointestinal disease? Gut 52: 759–766, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, Nakamura K, Kobayashi S, Nakashima E. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci 92: 569–576, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Kim JM, Kim JS, Kim YJ, Oh YK, Kim IY, Chee YJ, Han JS, Jung HC. Conjugated linoleic acids produced by Lactobacillus dissociates IKK-γ and Hsp90 complex in Helicobacter pylori-infected gastric epithelial cells. Lab Invest 88: 541–552, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87: 1344–1350, 1984 [PubMed] [Google Scholar]
- 22. Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127: 26–40, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Lievin-Le Moal V, Amsellem R, Servin AL, Coconnier MH. Lactobacillus acidophilus (strain LB) from the resident adult human gastrointestinal microflora exerts activity against brush border damage promoted by a diarrhoeagenic Escherichia coli in human enterocyte-like cells. Gut 50: 803–811, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin YP, Thibodeaux CH, Pena JA, Ferry GD, Versalovic J. Probiotic Lactobacillus reuteri suppress proinflammatory cytokines via c-Jun. Inflamm Bowel Dis 14: 1068–1083, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52: 827–833, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121: 580–591, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Marrero JA, Matkowskyj KA, Yung K, Hecht G, Benya RV. Dextran sulfate sodium-induced murine colitis activates NF-κB and increases galanin-1 receptor expression. Am J Physiol Gastrointest Liver Physiol 278: G797–G804, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther 75: 13–33, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 296: G1140–G1149, 2009 [DOI] [PubMed] [Google Scholar]
- 30. Mizoguchi E, Xavier RJ, Reinecker HC, Uchino H, Bhan AK, Podolsky DK, Mizoguchi A. Colonic epithelial functional phenotype varies with type and phase of experimental colitis. Gastroenterology 125: 148–161, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15: 300–310, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98: 694–702, 1990 [DOI] [PubMed] [Google Scholar]
- 34. Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig Dis Sci 49: 320–327, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Panwala CM, Jones JC, Viney JL. A novel model of inflammatory bowel disease: mice deficient for the multiple drug resistance gene, mdr1a, spontaneously develop colitis. J Immunol 161: 5733–5744, 1998 [PubMed] [Google Scholar]
- 36. Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, Chang EB. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127: 1474–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol Gastrointest Liver Physiol 277: G495–G499, 1999 [DOI] [PubMed] [Google Scholar]
- 38. Prisciandaro L, Geier M, Butler R, Cummins A, Howarth G. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm Bowel Dis 15: 1906–1914, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Raheja G, Singh V, Jhandier M, Boumendjel R, Borthakur A, Gill R, Alrefai W, Ramaswamy K, Malakooti J, Dudeja P. Sodium-hydrogen exchanger 3 (SLC9A3) expression is increased by Lactobacillus species both in in vitro and in vivo models. Gastroenterology 138: S591–S592, 2010 [Google Scholar]
- 40. Raheja G, Singh V, Ma K, Boumendjel R, Borthakur A, Gill RK, Saksena S, Alrefai WA, Ramaswamy K, Dudeja PK. Lactobacillus acidophilus stimulates the expression of SLC26A3 via a transcriptional mechanism. Am J Physiol Gastrointest Liver Physiol 298: G395–G401, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52: 988–997, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-α- and IFN-γ-induced dysfunction in human intestinal epithelial cells. Gastroenterology 130: 731–746, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Saksena S, Singla A, Goyal S, Katyal S, Bansal N, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Mechanisms of transcriptional modulation of the human anion exchanger SLC26A3 gene expression by IFN-γ. Am J Physiol Gastrointest Liver Physiol 298: G159–G166, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 126: 1620–1633, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116: 301–309, 1999 [DOI] [PubMed] [Google Scholar]
- 46. Servin AL. Antagonistic activities of Lactobacilli and Bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28: 405–440, 2004 [DOI] [PubMed] [Google Scholar]
- 47. Taipalensuu J, Tavelin S, Lazorova L, Svensson AC, Artursson P. Exploring the quantitative relationship between the level of MDR1 transcript, protein and function using digoxin as a marker of MDR1-dependent drug efflux activity. Eur J Pharm Sci 21: 69–75, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol 290: C1018–C1030, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84: 7735–7738, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, Matteuzzi D, Campieri M. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther 13: 1103–1108, 1999 [DOI] [PubMed] [Google Scholar]
- 51. Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132: 562–575, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 277: 50959–50965, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care 9: 717–721, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Zaher H, Khan AA, Palandra J, Brayman TG, Yu L, Ware JA. Breast cancer resistance protein (Bcrp/abcg2) is a major determinant of sulfasalazine absorption and elimination in the mouse. Mol Pharmacol 3: 55–61, 2006 [DOI] [PubMed] [Google Scholar]