Abstract
Integrin binding to the extracellular matrix (ECM) activated Rho GTPases, Src, and focal adhesion kinase in intestinal epithelial cells (IEC)-6. Polyamine depletion inhibited activities of Rac1, RhoA, and Cdc42 and thereby migration. However, constitutively active (CA) Rac1 expression abolished the inhibitory effect of polyamine depletion, indicating that polyamines are involved in a process upstream of Rac1. In the present study, we examined the role of polyamines in the regulation of the guanine nucleotide exchange factor, diffuse B-cell lymphoma (Dbl), for Rho GTPases. Polyamine depletion decreased the level as well as the activation of Dbl protein. Dbl knockdown by siRNA altered cytoskeletal structure and decreased Rac1 activity and migration. Cells expressing CA-Dbl increased migration, Rac1 activity, and proliferation. CA-Dbl restored migration in polyamine-depleted cells by activating RhoA, Rac1, and Cdc42. CA-Dbl caused extensive reorganization of the F-actin cortex into stress fibers. Inhibition of Rac1 by NSC23766 significantly decreased migration of vector-transfected cells and CA-Dbl-transfected cells. However, the inhibition of migration was significantly higher in the vector-transfected cells compared with that seen in the CA-Dbl-transfected cells. Dbl localized in the perinuclear region in polyamine-depleted cells, whereas it localized with the stress fibers in control cells. CA-Dbl localized with stress fibers in both the control and polyamine-depleted cells. These results suggest that polyamines regulate the activation of Dbl, a membrane-proximal process upstream of Rac1.
Keywords: putrescine, guanine nucleotide exchange factors, F-actin, intestinal epithelial cell-6, diffuse B-cell lymphoma
the polyamines, spermine, spermidine and their diamine precursor, putrescine, are found in all tissues of most species and are essential for normal functioning of almost all eukaryotic cells (17, 39). These compounds are organic, aliphatic amines, and the amine groups have pKs around 10, so they are cations at physiological pH and bind to proteins, DNA, RNA, and other negatively charged molecules. This binding results in their biological effects. Intracellular polyamine levels are highly regulated by synthesis, catabolism, and reuptake. Levels are primarily dependent on the activity of ornithine decarboxylase (ODC), which catalyzes the first rate-limiting reaction in polyamine biosynthesis, producing putrescine. ODC can be irreversibly inhibited by DL-α-difluoromethylornithine (DFMO), blocking the production of putrescine and inhibiting or reducing any cell functions that require polyamines. If a polyamine or putrescine is supplied exogenously with DFMO, these functions are maintained at normal rates, indicating that all effects are attributable to the depletion of polyamines and not to DFMO per se (17). We have extensively studied the role of polyamines in the regulation of cell proliferation, migration, and apoptosis in intestinal epithelial cells (IEC) (17, 20). In the IEC-6 cell line, DFMO depletes putrescine within 6 h, spermidine after 24 h, and spermine (60%) after 4 days.
The intestinal mucosa is a critical barrier to a wide range of bacteria, toxins, and immunogenic substances within the gut lumen. Cell migration is essential to the maintenance and the repair of that barrier, which can be compromised by a variety of disorders including celiac disease, inflammatory bowel disease, and physical or chemical injury (4, 11, 14). Mucosal repair is highly regulated and consists of two processes; an early phase, termed restitution, depends on cell migration and rapidly seals the wound, restoring the barriers. Cell division, which begins 16–24 h later, replaces the lost cells. We have demonstrated that gastric stress ulcers, corticosterone-induced gastric and duodenal lesions, and hypertonic NaCl damage to the intestinal epithelium are rapidly repaired in rats by a process dependent on polyamines (34–36). This rapid repair was due to cell migration and was accompanied by significant increases in ODC activity. In animals that were given DFMO, repair was almost completely prevented. These studies established the significance of the role of polyamines in cell migration as manifested by mucosal restitution in vivo.
We have used a wounding model employing IEC-6 cells to examine the effects and the mechanism of involvement of polyamines in cell migration in vitro (17). Rho proteins were shown to be essential for endogenous and EGF-induced migration of small-intestinal crypt cells, and these proteins are required elements of a mechanism by which growth factors induce cell migration to restitute intestinal integrity (28, 32). Polyamine depletion decreased the activity of all Rho GTPases (23), but constitutively active RhoA did not restore migration, suggesting that RhoA activity was essential but not sufficient for restoring migration (22). Additional studies showed that Rac1 activity is sufficient for migration in the absence of polyamines because of its ability to activate RhoA and Cdc42 as well as its effects on the additional steps involved in cell migration (23). These finding indicate that polyamines are involved in a process upstream of Rac1 that is between Rac1 and the cell membrane.
The Rho GTPases are important regulators of the actin cytoskeleton, cell polarity, cell-to-cell junctions, and myosin activity, and dysregulation of their activities has been associated with tumor progression. Rho and Rac1 regulate cell-to-cell junctions in a variety of epithelial cells, whereas Cdc42 is required for the establishment of apical-basal polarity (2, 27). A recent report by Xu et al. (38) showed that liver kinase B1 (LKB1), a tumor suppressor, activated ezrin, leading to diffuse B-cell lymphoma (Dbl)-mediated Rho GTPase activation and subsequent brush-border formation. Germline mutation in the gene LKB1, a serine threonine kinase, results in Peutz-Jeghers syndrome, causing intestinal hamartomas and epithelial cancers. In addition, inactivating mutations in the LKB gene have been reported in sporadic human cancers (13). Thus the upstream mechanisms involved in the activation of Rho GTPases might lead to the identification of targets for the treatment and prevention of metastasis of human cancers.
Rho GTPases are activated following integrin binding to the extracellular matrix (ECM) and the subsequent activation of Src and focal adhesion kinase (FAK). We have shown that ECM-mediated Src activation is required for the assembly of focal adhesions and Rac1 activation (9). The Rho proteins function as binary switches and cycle between active GTP-bound and inactive GDP-bound states. Rho-mediated signaling is initiated by guanine nucleotide exchange factors (GEFs), which catalyze the release of bound GDP and the binding of GTP. GEFs are members of a large family of Dbl proteins whose functions depend on their ability to interact with and activate Rho GTPases (37). In this study we examine the role of Dbl in mediating the effects of polyamine depletion on the activity of the Rho GTPases and cell migration. A major finding is that polyamine depletion does not interfere with the ability of GEFs to activate the Rho GTPases.
MATERIALS AND METHODS
Antibodies and reagents.
Mouse monoclonal anti-Rac1, mouse monoclonal anti-phospho tyrosine, and mouse monoclonal anti-actin antibodies were obtained from Millipore (Temecula, CA). Rabbit polyclonal Dbl antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Bicinchoninic acid (BCA) and mammalian protein extraction reagents (M-PER) were purchased from Thermo Fisher Scientific (Rockford, IL). Cell culture medium and FBS were obtained from Mediatech (Herndon, VA). Dialyzed FBS and glutathione agarose beads were purchased from Sigma (St. Louis, MO), and trypsin-EDTA, antibiotics, and insulin were from GIBCO (Grand Island, NY). The enhanced chemiluminescence substrate, Western Lightning TM, was purchased from Perkin Elmer Life and Analytical Sciences, (Shelton, CT). DFMO was a gift from ILEX Oncology (San Antonio, TX). All the other reagents used were of the highest analytical grade commercially available.
Cell culture.
The IEC-6 cell line (CRL-1592) was obtained from the American Type Culture Collection (Manassas, VA) and maintained in T-150 flasks in DMEM supplemented with 10% FBS, 10 μg/ml insulin, and 50 μg/ml gentamicin sulfate at 37°C and 10% CO2. Stock cells were passaged once a week, and medium was changed three times a week. Before an experiment, cells were trypsinized, counted using a Beckman Coulter counter, and grown for 3 days in dialyzed FBS in control, DFMO (5 mM), or DFMO plus 10 μM putrescine containing media and were serum starved for 24 h before an experiment. Cell culture plates precoated with fibronectin obtained from BD Bioscience (Bedford, MA) were used throughout the study.
Cell migration assay.
This assay was carried out as described previously (23, 28). Cells grown in control, DFMO, or DFMO plus putrescine-containing media were serum starved for 24 h before the experiment. Six-well plates containing a confluent monolayer of cells were marked in the center (along the diameter) with a marker. A wound was created perpendicular to the mark by scraping the monolayer with a gel-loading microtip. Plates were washed, and the wound area was captured with a charged-coupled device camera system using NIH Image J(Version 1.62) at the intersection of the marked line at 0 h and at desired time point (7 h). The images were captured using a ×20 objective. The wound area covered during migration was measured using NIH Image J software. Cell migration was calculated as wound area covered. Each experiment was carried out in triplicate, and two observations were recorded from each well (n = 6).
Plasmids.
Three Dbl SiRNA oligonucleotide sequences were cloned in to the plasmid vector (pcDNA6.2-GW/EmGFP-MiR) and confirmed by sequencing using appropriate primer pairs. Selected clones for the vector [MiR-LacZ-enhanced green fluorescent protein (EGFP)] and Dbl (MiR-LacZ-Dbl-EGFP) were used to prepare plasmid DNA for the transfection of IEC-6 cells using EndoFree Plasmid Maxi kit from QIAGEN.
Empty vector (pMX-NS-GFP) and constitutively active Dbl (pMX-NS-GFP-CA-Dbl) (CA-Dbl) plasmids obtained from Dr. Yi Zheng were prepared as described above. CA-Dbl lacks NH2-terminal 497 amino acids and contains intact Dbl homology (DH) and pleckstrin homology (PH) domains and retains transforming activity, GEF activity, and cytoskeletal association (7).
Transfections.
Seventy-percent confluent IEC-6 cells were transfected with vector (MiR-LacZ-EGFP)- and Dbl (MiR-LacZ-Dbl-EGFP)-specific siRNA plasmid constructs. Briefly, siRNA plasmid complexes prepared using FUGENE-6-HD transfection reagent following the instructions provided by the manufacturer were added drop wise onto cells in serum-free medium and incubated overnight. Cells were washed with a fresh medium and incubated further for 24 h. About 50% of cells expressed GFP after 24 h incubation. For cell migration studies, a stable cell line expressing Dbl-siRNA is required. Therefore, we subjected cells transfected once (50% cells expressing GFP) to antibiotic selection to eliminate untransfected cells and to propagate cells carrying Dbl-siRNA bearing the blasticidin resistance marker. Cells were trypsinized and seeded at low density in the presence of blasticidin (1.25 μg/ml) to enrich the cells expressing GFP and, thereby, the transfected genes. These cells (85–95% cells expressing GFP) were used for the migration studies and Western blot analysis. Plasmids pMX-IRES-GFP (vector) and pMX-IRES-GFP-CA-Dbl (CA-Dbl) were transfected in IEC-6 cells as described earlier (22, 23). Stable cell line-expressing CA-Dbl was characterized and used in this study.
Preparation of cell lysate.
For Western blot analyses of the various proteins, plates containing cells were placed on an ice bath and washed two times with cold Dulbecco's PBS (DPBS) and harvested in cold cell lysis buffer (M-PER containing protease inhibitor cocktail, 150 mM NaCl, and the phosphatase inhibitors sodium orthovanadate, sodium fluoride, and sodium β-glycerophosphate). The cells were scraped off the plate, and the lysate thus obtained was centrifuged at 10,000 g, for 15 min in the cold. The supernatant obtained was estimated for protein by the BCA method per the manufacturer's protocol. A sample of protein (100 μg) was precipitated with 2% TCA. The precipitate obtained was dissolved in 2× SDS sample buffer, boiled, and used for separation by SDS-PAGE.
Immunoprecipitation.
Cell extracts (200 μg protein) mixed with 2 μg anti-Dbl antibody and 25 μl protein A/G-agarose were rotated at 4°C for 2–4 h and centrifuged at 2,000 revolution/min for 2 min. Immune complexes were washed twice with lysis buffer, resuspended in 50 μl of sample buffer, boiled for 2 min, and resolved by SDS-PAGE. The levels of total and phosphorylated (pY-Dbl) Dbl were analyzed by Western blot.
Preparation of GST-PAK.
Escherichia coli BL-21DE3 containing glutathione S-transferase tagged p21 activated kinase (GST-PAK) was grown in Luria Broth. Protein expression was induced with isopropylthiogalactoside, and the bacterial pellet was resuspended in a buffer containing 50 mM Tris pH 7.4, 1% Nonidet P-40, 100 mM NaCl, 5 mM MgCl2, and 10% glycerol supplemented with protease inhibitors. The cell suspension was further sonicated and clarified by centrifugation at 10,000 g, for 30 min. The fusion protein was then recovered by the addition of glutathione agarose beads. The quality and quantity of the GST-PAK protein was checked by gel electrophoresis. Protein was stored in the buffer containing 50% glycerol at −20°C for pull-down assays.
Rho GTPase activation assay.
RhoA, Rac1, and Cdc42 activity was determined by pull-down assay as described previously (22, 23). GST-Rho-associated, coiled-coil-containing protein kinase (ROCK) or GST-PAK fusion protein bound with the glutathione agarose beads was mixed with cell lysate (200 μg). The binding was allowed to proceed for 1.5 h at 4°C, the beads were washed with lysis buffer, and the amount of GTP-RhoA, GTP-Rac1, and GTP-Cdc42 bound were analyzed by SDS-PAGE and Western blot using specific antibodies. A sample of cell lysate (10 μg) was loaded simultaneously to determine the respective total protein levels, and Western blots for actin on the same membrane served as loading controls.
Western blotting.
Protein samples (20 μg) were separated by SDS-PAGE and transferred to PVDF membrane. The membranes were then blocked with either 5% BSA or blocking-grade nonfat dry milk made in Tris-buffered saline containing 0.1% Tween 20. Appropriate primary and secondary antibodies were used to detect the proteins of interest by enhanced chemiluminescence detection reagents.
Immunocytochemistry.
Immunostaining for localization studies of proteins was done as described previously (9, 22, 23). Cells were grown on poly-l-lysine-coated glass coverslips placed in 24-well plates. Cells were fixed with 3.7% paraformaldehyde for 15 min, washed twice with DPBS, permeabilized with 0.1% Triton X-100 for 10 min, and washed again with PBS. Blocking was carried out with 2% BSA for 20 min followed by a 2-h incubation with the appropriate primary antibody. The coverslips were washed with PBS, followed by incubation with an appropriate fluorescent dye-conjugated secondary antibody. The coverslips were mounted on glass slides and photographed using a Nikon Diaphot inverted fluorescence microscope with appropriate filters. The images were captured using a ×40 objective.
Statistics.
All data are expressed as means ± SE. Densitometric analysis of Western blots from three different experiments was performed. Analysis of variance and appropriate post hoc testing determined the significance of the differences between means. Values of P < 0.05 were considered significant. Representative blots from three experiments are shown.
RESULTS
Dbl activity and effect on migration.
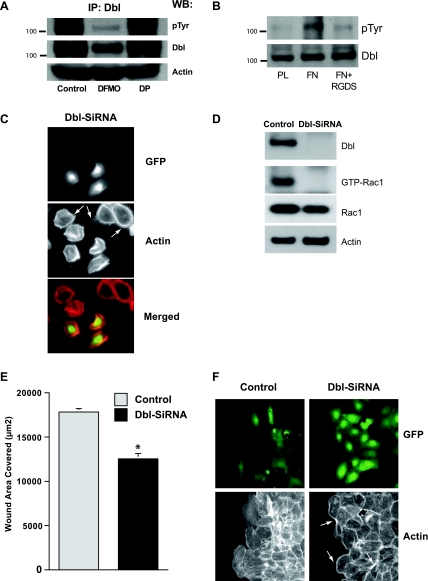
Tyrosine phosphorylation results in the activation of Dbl, and this is almost entirely prevented in cells polyamine depleted by incubating them with 5 mM DFMO (Fig. 1A). DFMO also caused a significant decrease in total Dbl protein. The presence of 10 μM putrescine in the DFMO-containing incubation medium prevented the effects of polyamine depletion on both the activation of Dbl and its cellular content. Because binding to the ECM stimulates cell migration through focal adhesion formation and activation of Rho GTPases (9), we examined the effect of integrin activation on the activity of Dbl. As shown in Fig. 1B, plating IEC-6 cells on fibronectin activated Dbl, whereas no similar activation occurred in cells plated on plastic. Dbl was not activated in cells plated on fibronectin in the presence of the integrin receptor inhibitor Arg-Gly-Asp-Ser (RGDS) peptide. We have previously shown that RGDS peptide significantly blocks integrin β3-phosphorylation and thereby activation in IEC-6 cells (5). Approximately 50% of the cells were transfected in the first round of selection (Fig. 1C). Dbl siRNA-transfected cells had a robust expression of EGFP. Because the Dbl siRNA oligonucleotide sequence was cloned downstream of EGFP, the expression of EGFP indicates Dbl siRNA expression and, thereby, the knockdown of Dbl protein. Cells expressing Dbl siRNA were smaller in size and had a thick actin cortex and lacked lamellipodia (Fig. 1C). These morphological and cytoskeletal alterations were also observed during attachment and spreading of polyamine depleted cells (9, 21). Although actin was also localized in the cortices of the cells lacking Dbl siRNA expression, prominent lamellipodia (arrows) were present in the larger cells lacking EGFP expression (untransfected cells), indicating a role for a Dbl in cell spreading (Fig. 1C). A stable cell line expressing Dbl-SiRNA was established from the cells initially transfected (50%) with Dbl-siRNA by enrichment using antibiotic selection. Stable clones expressing Dbl-siRNA were selected and characterized, and a representative clone was used to examine migration. The expression of Dbl siRNA was also confirmed by the RT-PCR (data not shown). Cells transfected with Dbl-siRNA had decreased levels of GTP-Rac1 (Fig. 1D) and migrated significantly less than the controls (Fig. 1E). Following wounding, GFP-expressing cells from the population transfected with Dbl localized at the migrating edge, whereas those transfected with vector were scattered throughout the monolayer (Fig. 1F). Dbl knockdown caused the formation of thick actin cortices (arrows) and produced cells lacking stress fibers and lamellipodia compared with those expressing normal levels of Dbl (Fig. 1F). These results suggest that Dbl regulates migration via Rho GTPases in IEC-6 cells.
Fig. 1.
A: diffuse B-cell lymphoma (Dbl) immunoprecipitation from extracts of serum-starved intestinal epithelial cell (IEC)-6 cells grown in control, difluoromethylornithine (DFMO), or DFMO + putrescine (DP) media was carried out as described in materials and methods. Immunoprecipitated (IP) proteins were resolved by SDS-PAGE and Western blot analysis for the detection of phosphotyrosine (pTyr) and Dbl levels was carried out using specific antibodies. B: serum-starved cells were seeded on plastic- (PL) or fibronectin- (FN) coated plates and allowed to attach for 1.5 h. Cell extracts were resolved by SDS-PAGE, and Western blot analysis for the detection of phosphotyrosine and Dbl levels was carried out using specific antibodies. Representative blots from 3 observations are shown. RGDS, Arg-Gly-Asp-Ser. C: cells transfected with control [MiR-green fluorescent protein (GFP)] and Dbl-SiRNA (MiR-Dbl-GFP) expressing plasmid were fixed, permeabilized, and stained with rhodamine phalloidin. Images were captured for GFP and F-actin. D: confluent monolayers of cells transfected with control and Dbl siRNA were wounded, and cell extracts were analyzed for Dbl, GTP-Rac1, Rac1, and actin as described in materials and methods. E: confluent monolayer of cells transfected with control and Dbl siRNA were wounded, and migration was measured as described in materials and methods. F: 7 h postwounding, monolayers were fixed, permeabilized, and stained with rhodamine phalloidin. Images were captured for the GFP and F-actin. *Significantly different compared with control.
Activation of Rac1 by Dbl.
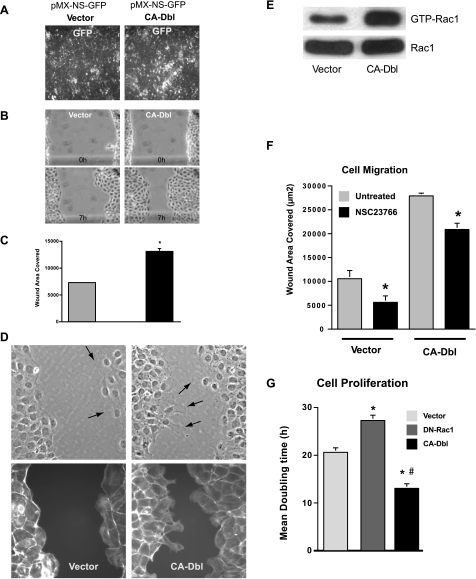
IEC-6 cells were transfected with CA-Dbl or with vector, and the efficiency of transfection was ascertained by the expression of GFP (Fig. 2A). As shown, most cells expressed GFP, indicating a high percentage of transfection. Those cells containing CA-Dbl migrated rapidly compared with the cells transfected with vector alone and were able to cover most of the area of a wound within 7 h (Fig. 2B). As shown in the bar graph, the migration of the cells containing CA-Dbl was approximately twice that of the control vector transfected cells (Fig. 2C). Cells transfected with CA-Dbl had longer lamellipodia at the leading edge compared with those transfected with vector (Fig. 2D, top). F-actin staining also showed that the formation of lamellipodia correlated with the loss of stress fibers and the actin cortex at the migrating edge (Fig. 2D, bottom), which were less evident in the vector cells. Rac1 activity increased significantly in cells transfected with CA-Dbl as evidenced by increased levels of GTP-Rac1 compared with the cells transfected with vector (Fig. 2E). In these two groups, there was little or no difference in total Rac1 protein. As shown in Fig. 2F, inhibition of Rac1 by NSC23766 significantly decreased migration of vector-transfected cells (50% inhibition) compared with those cells transfected with CA-Dbl (25% inhibition). CA-Dbl also decreased the mean doubling time of cells, which is another indication of Rac1 activation (Fig. 2G). In contrast, the mean doubling time of cells transfected with dominant-negative Rac1 (DN-Rac1) was significantly increased.
Fig. 2.
IEC-6 cells transfected with empty vector (Vector) and constitutively active (CA) Dbl grown in control medium were examined for the expression of GFP (A). Serum-starved, vector, and CA-Dbl cells were wounded and photographed at indicated time intervals, and migration was analyzed as described in materials and methods (B and C). Cell monolayers were fixed, permeabilized, and stained with rhodamine phalloidin. Phase contrast images (top) and F-actin staining (bottom) were captured using a ×40 objective (D). Representative images from 3 observations are shown. Wounded, serum-starved, vector, and CA-Dbl cells were lysed in pull-down assay buffer, and GTP-Rac1 levels were measured as described in materials and methods (E). Serum-starved, vector, and CA-Dbl cells incubated with or without NSC23766 (120 μM) were wounded, and migration was analyzed as described in materials and methods (F). IEC-6 cells transfected with empty vector (Vector), dominant-negative (DN) Rac1, and CA Dbl were seeded in control medium. Cell proliferation was measured by counting the cell number, and mean doubling time was calculated (G). Values are means ± SE of triplicates. *Significantly different compared with vector, #significantly different compared with control vector-transfected cells. Representative blots from 3 observations are shown.
Dbl restores migration in polyamine-depleted cells.
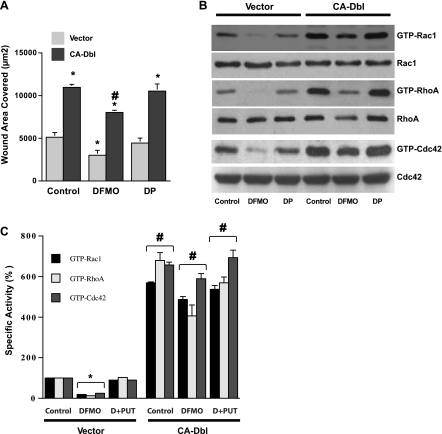
Polyamine depletion by DFMO inhibited cell migration by ∼50%, and this decrease was prevented by the addition of putrescine to the medium (Fig. 3A). Cells transfected with CA-Dbl covered more than twice the wound area of their respective controls transfected with empty vector. Migration of cells incubated in DFMO was restored by transfection with CA-Dbl to levels significantly higher than found in the controls (Fig. 3A). Thus transfection with CA-Dbl more than accounted for the decreased cell migration seen in the polyamine-depleted cells. In control cells containing the empty vector, DFMO and subsequent polyamine depletion decreased the amounts of all active Rho-GTPases (Fig. 3B). Only small amounts of GTP-Rac1 and GTP-Cdc42 were detected compared with their respective controls, whereas no GTP-RhoA was detected. In all three instances, the presence of putrescine along with DFMO prevented almost all, if not all, of the decreases in the levels of active Rho GTPases. There were no apparent changes in the total GTPase proteins in any of the groups.
Fig. 3.
A: serum-starved vector and CA-Dbl cells grown in control, DFMO, or DP (D+PUT) containing medium were wounded, and migration was measured as described in materials and methods. Values are means ± SE of triplicates. *Significantly different compared with control vector. #Significantly different compared with vector in all groups. B: wounded vector and CA-Dbl cells as described above were lysed in pull-down assay buffer, and the levels of total and GTP bound Rac1, RhoA, and Cdc42 were determined by Western blot. Densitometry of the blots is expressed as percentage-specific activity of Rac1, RhoA, and Cdc42 (C). Values are means ± SE of triplicates. *Significantly different compared with control vector. #Significantly different compared with vector in respective treatment groups. Representative blots from 3e observations are shown.
As pointed out above, the cells containing CA-Dbl migrated more rapidly than control cells. This was reflected by the finding that control CA-Dbl cells also contained significantly greater amounts of active Rac1, RhoA, and Cdc42 than the corresponding control cells transfected with vector alone (Fig. 3, B and C). Transfection with CA-Dbl restored active Rho GTPases of cells incubated in DFMO to levels equal to GTP-Rho A or greater than GTP-Rac1 and GTP-Cdc42, those of control cells transfected with empty vector (Fig. 3C). In cells transfected with CA-Dbl, there were no significant differences between levels of any protein in the control group and the group incubated with DFMO and putrescine.
Cellular localization of Dbl.
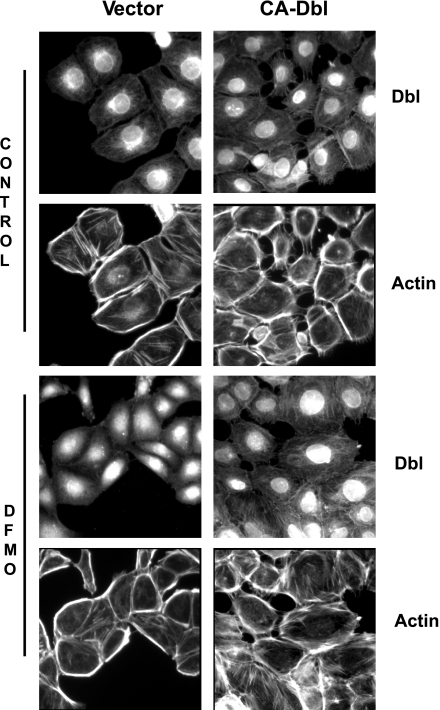
In control cells containing empty vector, Dbl was found primarily in the nuclei with some movement into the Golgi apparatus and the cytoplasm (Fig. 4). Cytoplasmic Dbl appeared to be associated with actin filaments (F-actin). Dbl was strongly associated with F-actin in control cells transfected with CA-Dbl. In these cells, F-actin was much more prominent than in the cells containing vector alone, and in many cells the actin filaments protruded into lamellipodia, which were also quite well developed.
Fig. 4.
Vector and Ca-Dbl cells seeded on poly-lysine-coated glass cover slips were grown in control or DFMO medium as described in materials and methods. Serum-starved cells were washed, fixed, permeabilized, and stained for the detection of Dbl and F-actin as described in materials and methods. Representative images from 3 experiments are shown.
In cells transfected with empty vector and incubated in DFMO, Dbl was confined primarily to the nuclei. That found in the cytoplasm was generally distributed and not localized with F-actin or other cellular structures. The actin found in these cells was primarily part of a heavy actin cortex with few stress fibers being present. This cortical actin distribution is typical of what has been described in cells depleted of polyamines and is indicative of cells that cannot migrate (13). Polyamine-depleted cells that contained CA-Dbl had the appearance of control cells transfected with Dbl (Fig. 4). Dbl was associated with actin stress fibers, which had increased considerably with the disappearance of the heavy actin cortex. The overall effect of CA-Dbl on cells treated with DFMO was to restore F-actin to the organization required for migration.
DISCUSSION
The control of the organization of actin filaments is essential for cell motility, and how this is achieved and regulated is being deciphered. The Rho GTPases, Rac1, RhoA, and Cdc42 regulate actin cytoskeleton structure (1, 4, 12). Rac1 controls the polymerization of actin and the assembly of focal adhesion complexes to produce lamellipodia and membrane ruffles. The activation of RhoA results in the formation of stress fibers, and Cdc42 induces the formation of actin microspikes and filopodia (12). Thus Rac, Rho, and Cdc42 work in concert with each other, creating a complex network for the regulation of the actin cytoskeleton, cell shape, cell-to-cell junction, cell polarity, and cell motility. Each is necessary for these cellular functions, and the activities of each must be coordinated. Polyamine analogs hold promise for cancer therapy because of their effects on growth and apoptosis, and the inhibition of migration in response to polyamine depletion strongly suggests a role in tumor progression and/or metastasis. Thus identifying the key step/s regulated by polyamines during the activation of Rho GTPases may have clinical significance.
This coordination is dependent on a large family of proteins termed GEFs. The Dbl family GEFs characterized by a DH domain are responsible for catalyzing nucleotide exchange on the Rho GTPases. A PH domain is located immediately tandem to the DH domain and is responsible for the cellular location of the proteins (7, 37). GEFs are normally inactive, or only partially active, until specific stimuli result in activation, which often occurs by phosphorylation. Phosphorylation of Tyr174 of proto-vav by Src kinases results in its activation (1), and protein kinase C induces the translocation of Tiam1 to the plasma membrane activating it (10). Activity can also be modified by a variety of processes including phosphoinositol phosphate binding to the PH domain (26). Rac1 has been shown to regulate phosphatidylinositol 4,5-bisphosphate production and increases ezrin/radixin/moesin (ERM) activity (31). Both the Rho GTPases and ERM proteins have been shown to regulate remodeling of the actin cytoskeleton (16, 29). Rac and ERM proteins are both required for membrane ruffling and lamellipodial extension. That the membrane structures such as microvilli, membrane ruffles, and cell-to-cell junctions are enriched with ERM proteins suggest that these proteins are involved in the activation of Rac1 (19, 41). Furthermore, Rac-GTP-Rho guanine dissociation inhibitor (Rho-GDI) localizes with integrin activation sites and displaces Rho-GDI from Rac and, thereby, allows the interaction of Rac1 with its effectors via ERM proteins (8). The in vitro interaction between Dbl and ERM proteins suggests a role upstream of the Rho GTPases (30). Yaku et al. (40) found that Dbl activates RhoA, Rac1, and Cdc42 and that Rho-GDI decreased GEF activity of Dbl.
Much of the data elucidating the role of the Rho GTPases in cytoskeletal transformation was the work of Hall's group using NIH 3T3 fibroblasts (12, 25). Our own studies have been conducted in IEC-6. The activities of all three GTPases were decreased significantly by polyamine depletion. Inhibition of each of the Rho GTPases decreased cell migration (23, 28), which corresponds with the findings of Hall and coworkers in fibroblasts (12). In polyamine-depleted cells transfected with constitutively active Rac1, migration was equal to those of vector-transfected controls. Rac1 transfection increased RhoA and Cdc42 activity in both control and polyamine-depleted cells (23). Transfection with constitutively active Cdc42 had effects qualitatively similar to those of Rac1 but quantitatively less. Transfection with constitutively active RhoA did not restore migration in polyamine-depleted cells. These data led to the conclusion that, in the absence of polyamines, an upstream process that is interfered with prevents the activation of the Rho GTPases. From the results cited above, we also concluded that Rac1 is the primary GTPase affected by the depletion of polyamines. Furthermore, polyamine depletion constitutively activated myosin regulatory light chain and inhibited migration. The inhibition of Rho-kinase restored migration of polyamine-depleted cells by stimulating Rac1 activity and reorganizing focal adhesions (3, 24). Lee et al. (15) also reported that myosin II interacts specifically with Dbl and that the inhibition of myosin II ATPase activity released Dbl and activated Rho GTPases (15).
Recently, we have shown that the integrin-β3-induced activation of Src increased tyrosine phosphorylation of Tiam1 and FAK and also stimulated migration (9). Furthermore, inhibition of FAK decreased Tiam1 phosphorylation, suggesting that FAK is required for the phosphorylation of Tiam1 by Src (9). Polyamine depletion decreased the phosphorylation of Tiam1 and FAK, which indicated that polyamines are required for the activation of the Rac1 specific GEF, Tiam1. Our previous reports (9, 33) that the inhibition of Tiam1 by NSC23766 decreased Rac1 activity and migration by 50% and the current data (Fig. 2F) showing the same 50% inhibition of migration suggest that the full activation of Rac1 may require multiple GEFs. The activity of Dbl, the prototype GEF, was greatly reduced by polyamine depletion compared with both control cells and those cells incubated in DFMO plus exogenous putrescine (Fig. 1A). Total amounts of Dbl were also lower in the polyamine-depleted cells. Dbl was activated in cells plated on fibronectin, which is in keeping with previous findings showing that Tiam1 and Rac1 are activated by Src and FAK following incubation on ECM but not on plastic (9). Transfection of IEC-6 cells with Dbl siRNA caused a significant reduction in the activity of Rac1 and cell migration, thus establishing the importance of this GEF in the regulation of Rac1 and mimicking the effects of polyamine depletion on Rac and cell migration (Fig. 1, D–F).
Additional significance of Dbl and indications of its activity in our cell line are indicated by the data shown in Fig. 2. CA-Dbl doubled the rate at which the cells migrated while significantly increasing the activation of Rac1 concomitant with the formation of longer lamellipodia (Fig. 2D, bottom). It is now well established that the biological functions of Dbl family members are dependent on their activation of the Rho GTPases (7, 37). In addition to cytoskeletal remodeling and migration, these actions include the stimulation of cell growth. The cellular transforming activity of Dbl is totally dependent on its ability to activate Rho GTPases (43). Evidence for this dependency includes a number of findings. When DN mutants of Rho GTPases are coexpressed with Dbl family members, transformation is prevented (18, 42). Mutated GEFs that can no longer activate Rho proteins behave as DNs in terms of cell functions (43). An additional indication of this dependency and relationship in IEC-6 cells is shown in Fig. 2G. CA-Dbl decreased the mean doubling time by ∼40%, and, in cells transfected with DN Rac, the doubling time increased significantly (Fig. 2G). Therefore, activated Rac1 is essential for the function of Dbl in IECs.
As previously mentioned, constitutively active Rac1 and, to a lesser extent, Cdc42 restored migration in cells depleted of polyamines, whereas RhoA was unable to do so (23). Figure 3A shows that CA-Dbl could duplicate this ability of Rac1. In fact, cells that had been polyamine depleted and transfected with active Dbl migrated significantly faster than the control cells transfected with vector. Polyamine depletion significantly decreased the amount of active Rho GTPases in vector control cells, and levels of all three of these activated Rho GTPases were maintained at normal control levels in polyamine-depleted cells transfected with constitutively active Dbl (Fig. 3, B and C). These findings are identical to those observed when polyamine-depleted cells had been transfected with constitutively active Rac1 (23). Important conclusions from these data are, first, that Dbl is able to activate all three GTPases, and second, the polyamines are not necessary for the binding of GEFs to RhoA, Cdc42, and Rac1.
The PH domain promotes the translocation of the Dbl family proteins to the cytoskeleton and to the plasma membrane where the Rho GTPases are located (6). The various Dbl family GEFs are maintained in the inactive state by a variety of self-regulatory mechanisms. In the case of Dbl, an intermolecular interaction between the NH2 terminus and the PH domain results in autoinhibition and an inactive state. This interaction between the two domains limits the accessibility of the Rho GTPase substrates to the catalytic site of the DH domain. This inhibits GEF function and leads to a characteristic perinuclear localization (6). This perinuclear localization of Dbl is evident in the vector-transfected cells shown in Fig. 4. There is only a weak localization to cytoskeletal elements. In control cells transfected with constitutively active Dbl, the perinuclear localization has disappeared, and Dbl is strongly localized to cytoskeletal elements and the plasma membrane. Numerous stress fibers and lamellipodia present in these cells are specific sites of Dbl localization. In cells depleted of polyamines and transfected with empty vector, Dbl is present primarily in the nuclei. In most cells, any perinuclear localization is obscured by a minor hazy and diffuse presence in the cytoplasm. In these cells there is no colocalization of Dbl to cytoskeletal elements or to the plasma membrane. As previously reported, polyamine depletion results in a heavy actin cortex and few stress fibers (23, 28). This is also obvious in the vector-transfected cells incubated with DFMO (Fig. 4). Cells incubated with DFMO and transfected with constitutively active Dbl, however, did not develop a heavy actin cortex and appeared similar to the control cells containing active Dbl (Fig. 4).
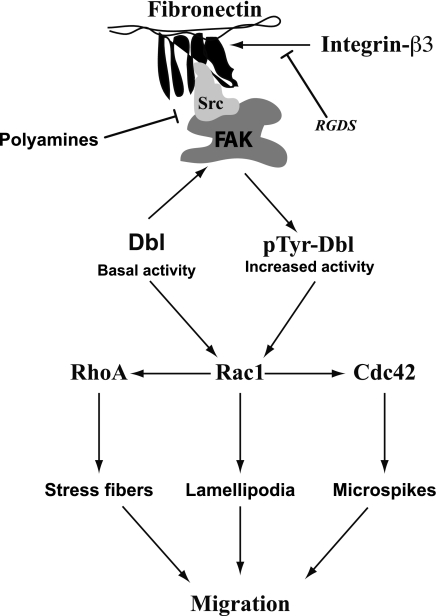
Taken together, our data show that the inactivation of Dbl leads to effects identical to those caused by the depletion of polyamines and that the effects of polyamine depletion are prevented by the activation of Dbl. CA of Dbl requires deletion of the NH2-terminal 497 residues, suggesting that the activation of Dbl requires elimination of autoinhibitory interactions (7). The mechanism by which Dbl is physiologically activated is not known. However, our results clearly indicate that integrin-β3-mediated Src activation increases FAK phosphorylation, which recruits Dbl and facilitates its phosphorylation-eliminating autoinhibition. The absence of polyamines may prevent the formation of the active signaling complex required for the activation of GEFs and, thereby preventing migration (Fig. 5). We have also shown that all three major Rho GTPases are activated when Dbl is present in an active form. The major conclusion from the present data, however, is that polyamine depletion does not alter the ability of GEFs to activate the Rho GTPases. This indicates that the involvement of polyamines in the signaling process that results in cell migration occurs upstream of GEF activation and, therefore, probably involves the interaction of integrins, Src, and FAK (Fig. 5).
Fig. 5.
Schematic representation illustrating the role of polyamines in the regulation of Dbl, a Rho family guanine nucleotide exchange factor, in IEC-6 cells. RGDS is a peptide inhibitor for integrin-β3. FAK, focal adhesion kinase.
GRANTS
This work was supported by grant DK-052784 from the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). This work was also supported by the Thomas Gerwin endowment.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The contents herein are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1. Aghazadeh BH, Lowry E, Huong XY, Rosen MK. Structural basis for belief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102: 625–633, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116: 457–466, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Bavaria MN, Ray RM, Johnson LR. The phosphorylation state of MRLC is polyamine dependent in intestinal epithelial cells. Am J Physiol Cell Physiol 300: C164–C175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport and inflammation. Gut 52: 439–451, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhattacharya S, Ray RM, Johnson LR. Integrin beta 3- mediated Src activation regulates apoptosis in IEC-6 cells via Akt and STAT3. Biochem J 397: 437–447, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bi F, Balazs D, Zhu K, Salani B, Eva A, Zheng Y. Autoinhibition mechanism of prob-Dbl. Mol Cell Biol 21: 1463–1474, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cerione RA, Zheng Y. The Dbl family of oncogenes. Curr Opin Cell Biol 8: 216–222, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol 4: 232–239, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Elias BC, Bhattacharya S, Ray RM, Johnson LR. Polyamine-dependent activation of Rac 1 is stimulated by focal adhesion-medicated Tiam 1 activation. Cell Adh Migr 4: 1–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming LN, Elliot CM, Buchanan FG, Downes CP, Exton JH. Ca2+/calmodulin dependent protein kinase II regulates Tiam 1 by reversible protein phosphorylation. J Biol Chem 74: 12753–12758, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol 81: 1–44, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Hall A. Small GTP binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol 10: 31–54, 1994 [DOI] [PubMed] [Google Scholar]
- 13. Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, Trimbath JD, Giardiello FM, Gruber SB, Offerhaus GJ, de Rooij FW, Wilson JH, Hansmann A, Möslein G, Royer-Pokora B, Vogel T, Phillips RK, Spigelman AD, Houlston RS. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 12: 3209–3215, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Laukoetter MG, Bruewer M, Nusrat A. Regulation of the intestinal epithelial barrier by the apical junctional complex. Curr Opin Gastroenterol 22: 85–89, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Lee CS, Choi CK, Shin EY, Schwartz MA, Kim EG. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol 190: 663–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCormack SA, Johnson LR. Polyamines and cell migration. J Physiol Pharmacol 52: 327–349, 2001 [PubMed] [Google Scholar]
- 18. Michiels F, Habets GGM, Stam JC, van cler Kammen RA, Collard JG. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375: 338–340, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Oshiro N, Fukata Y, Kaibuchi K. Phosphorylation of moesin by rho-associated kinase (Rho-kinase) plays a crucial role in the formation of microvilli-like structures. J Biol Chem 273: 34663–34666, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Ray RM, Viar MJ, Yuan Q, Johnson LR. Polyamine depletion delays apoptosis of rat intestinal epithelial cells. Am J Physiol Cell Physiol 278: C480–C489, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Ray RM, Viar MJ, McCormack SA, Johnson LR. Focal adhesion kinase signaling is decreased in polyamine-depleted IEC-6 cells. Am J Physiol Cell Physiol 281: C475–C485, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ray RM, Patel A, Viar MJ, McCormack SA, Zheng Y, Tigyi G, Johnson LR. RhoA inactivation inhibits cell migration but does not mediate the effects of polyamine depletion. Gastroenterology 123: 196–205, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Ray RM, McCormack SA, Covington C, Viar MJ, Zheng Y, Johnson LR. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem 278: 13039–13046, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, Johnson LR. Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 292: G983–G995, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Ridley AJ, Hall A. The small GTP binding protein Rho regulates the assembly of focal adhesions and stress fibers in response to growth factors. Cell 70: 401–410, 1992 [DOI] [PubMed] [Google Scholar]
- 26. Russo C, Gao Y, Maucini P, Vauui C, Porotto M, Galasca M, Torrisi MR, Zheng Y, Eva A. Modulation of oncogenic Dbl activity by phosphoinositol phosphate binding to pleckstrin homology domain. J Biol Chem 276: 19524–19531, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci 14: 1129–1142, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Santos MF, McCormack SA, Guo Z, Okolicany J, Zheng Y, Johnson LR, Tigyi G. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Invest 100: 216–225, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, Tsukita S, Takai Y. Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem 272: 23371–23375, 1997 [DOI] [PubMed] [Google Scholar]
- 30. Takahashi K, Sasaki T, Mammoto A, Hotta I, Takaishi K, Imamura H, Nakano K, Kodama A, Takai Y. Interaction of radixin with Rho small G protein GDP/GTP exchange protein Dbl. Oncogene 16: 3279–3284, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Tolias KF, Hartwig JH, Ishihara H, Shibasaki Y, Cantley LC, Carpenter CL. Type I alpha phosphatidylinositol-4-phosphate 5-kinase mediates Rac-dependent actin assembly. Curr Biol 10: 153–156, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Vaidya RJ, Ray RM, Johnson LR. MEKI restores migration of polyamine depleted cells by retention and activation of Rac 1 in the cytoplasm. Am J Physiol Cell Physiol 288: C350–C359, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Vaidya RJ, Ray RM, Johnson LR. Akt-mediated GSK- 3beta inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell Mol Life Sci 63: 2871–2879, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang JY, Johnson LR. Role of ornithine decarboxylase in repair of gastric mucosal stress ulcers. Am J Physiol Gastrointest Liver Physiol 258: G78–G85, 1990 [DOI] [PubMed] [Google Scholar]
- 35. Wang JY, Johnson LR. Gastric and duodenal mucosal ornithine decarboxylase and damage after corticosterone. Am J Physiol Gastrointest Liver Physiol 258: G942–G950, 1990 [DOI] [PubMed] [Google Scholar]
- 36. Wang JY, Viar MJ, Johnson LR. Transglutaminase in response to hypertonic NaCl-induced gastric mucosal injury in rats. Gastroenterology 104: 65–74, 1993 [DOI] [PubMed] [Google Scholar]
- 37. Whitehead IP, Campbell S, Rossman KL, Der CJ. Dbl family proteins. Biochim Biophys Acta 1332: 1–23, 1997 [DOI] [PubMed] [Google Scholar]
- 38. Xu X, Omelchenko T, Hall A. LKB1 tumor suppressor protein regulates actin filament assembly through Rho and its exchange factor Dbl independently of kinase activity (Abstract). BMC Cell Biol 11: 77, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang P, Baylin SB, Luk GD. Polyamines and intestinal growth: absolute requirement for ODC activity in adaptation during lactation. Am J Physiol Gastrointest Liver Physiol 247: G553–G557, 1984 [DOI] [PubMed] [Google Scholar]
- 40. Yaku H, Sasaki T, Takai Y. The Dbl Oncogene product as a GDP/GTP exchange protein for the Rho family: its properties compared with those smgGDS. Biochem Biophys Res Commun 198: 811–817, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Yonemura S, Tsukita S. Direct involvement of ezrin/radixin/moesin (ERM) -binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J Cell Biol 145: 1497–1509, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zheng Y, Olson M, Hall A, Cerione RA, Toksoz D. Direct involvement of the small GTP binding protein Rho in Lbc oncogene function. J Biol Chem 270: 9031–9034, 1995 [DOI] [PubMed] [Google Scholar]
- 43. Zhu K, Debrecini B, Li R, Zheng Y. Identification of Rho GTPase-dependent sites in the DH domain of oncogenic Dbl that are required for transformation. J Biol Chem 275: 25993–26001, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.