Abstract
Defective intestinal epithelial tight junction (TJ) barrier has been shown to be an important pathogenic factor contributing to the development of intestinal inflammation. The expression of occludin is markedly decreased in intestinal permeability disorders, including in Crohn's disease, ulcerative colitis, and celiac disease, suggesting that the decrease in occludin expression may play a role in the increase in intestinal permeability. The purpose of this study was to delineate the involvement of occludin in intestinal epithelial TJ barrier by selective knock down of occludin in in vitro (filter-grown Caco-2 monolayers) and in vivo (recycling perfusion of mouse intestine) intestinal epithelial models. Our results indicated that occludin small-interfering RNA (siRNA) transfection causes an increase in transepithelial flux of various-sized probes, including urea, mannitol, inulin, and dextran, across the Caco-2 monolayers, without affecting the transepithelial resistance. The increase in relative flux rate was progressively greater for larger-sized probes, indicating that occludin depletion has the greatest effect on the flux of large macromolecules. siRNA-induced knock down of occludin in mouse intestine in vivo also caused an increase in intestinal permeability to dextran but did not affect intestinal tissue transepithelial resistance. In conclusion, these results show for the first time that occludin depletion in intestinal epithelial cells in vitro and in vivo leads to a selective or preferential increase in macromolecule flux, suggesting that occludin plays a crucial role in the maintenance of TJ barrier through the large-channel TJ pathway, the pathway responsible for the macromolecule flux.
Keywords: occludin depletion, small-interfering ribonucleic acid, tight junctions, permeability, large-channel pathway
intestinal epithelial tight junctions (TJ) act as an intercellular seal or gate, serving as a structural barrier against paracellular permeation of luminal antigens (23). TJs are the apicalmost intercellular junctions, composed of thin protein complexes that completely encircle the apex of the cell and make contact with TJs of adjacent cells, forming a continuous paracellular seal (3, 23, 40). Defective intestinal epithelial TJ barrier has been postulated to be an important pathogenic mechanism leading to the increase in intestinal antigenic penetration and subsequent development of intestinal inflammation (19, 23, 42). In support of a central role in intestinal inflammation, previous studies in animal models of intestinal inflammation have shown that the enhancement of intestinal epithelial TJ barrier prevents the subsequent development of intestinal inflammation in interleukin (IL)-10-deficient mice and in immune-mediated intestinal inflammation (4, 8). Moreover, clinical studies have also shown that the improvement of intestinal inflammation in patients with active Crohn's disease is associated with therapeutic retightening of intestinal TJ barrier (43). The normalization of intestinal TJ barrier following medical therapy is associated with long-term clinical remission, whereas persistent increase in intestinal permeability portend poor clinical outcome (6, 18, 22). The elucidation of the intracellular processes that lead to the defective intestinal TJ barrier and antigenic penetration is potentially important in developing future therapeutic strategies to prevent the TJ barrier disturbance and to induce retightening of the intestinal TJ barrier.
Occludin, the first transmembrane TJ protein to be identified, is a 65-kDa protein located at the TJ (13). Occludin is a tetraspan protein with two extracellular loops, an NH2- and COOH-terminal cytoplasmic domains (13). Earlier studies have shown occludin to be present in the TJ strands and to have a functional role in TJ barrier (36). However, more recent studies in occludin knockout mice and embryonic stem cells (35) have suggested otherwise. These studies have shown occludin-deficient stem cells to form functional TJs and occludin knockout mice to be viable and to lack any noticeable defect in intestinal epithelial TJ morphology or barrier function (35, 36), suggesting that occludin is not required for the TJ formation or the maintenance of barrier function. An important controversy persists regarding the functional role of occludin in TJ barrier function (13, 36). Previous studies have shown intestinal tissue expression of occludin to be markedly decreased in patients with intestinal permeability disorder, including Crohn's disease, ulcerative colitis, and celiac disease (7, 9, 14, 55), and in animal models of inflammatory bowel disease (11, 14). It has been proposed that the decrease in intestinal occludin expression may be an important mechanism leading to the increase in intestinal epithelial TJ permeability. However, the evidence to support occludin depletion in intestinal TJ barrier function is lacking.
Other families of transmembrane TJ proteins have also been identified, including the claudin family of proteins and junctional adhesion molecules (3, 23, 42). Recent studies have firmly established the involvement of claudins in the formation of TJ “pores,” which serve as the TJ pathway for flux of small solutes and ions (50). It appears that the pore pathway is mainly involved in intestinal ionic and fluid transport and is functional under normal physiological conditions (42, 46). In addition, a nonrestrictive or large-channel pathway also exists, which may be formed in response to TJ-modulating agents and is responsible for the paracellular flux of larger molecules, including bacterial antigens (42, 49, 52). The molecular components that regulate flux through this nonrestrictive, large-channel pathway remain unclear (52). The purpose of this study was to examine the role of occludin in intestinal epithelial TJ barrier function by small-interfering RNA (siRNA)-induced knock down of occludin in an in vitro (consisting of filter-grown Caco-2 monolayers) and in vivo (consisting of recycling perfusion of mouse small intestine) model of intestinal epithelia. Filter-grown Caco-2 cells are a widely used in vitro intestinal epithelial model system for studying the intestinal epithelial TJ barrier function (16, 34, 44). Although derived from a colon carcinoma, when grown to mature monolayers, Caco-2 cells become differentiated and polarized such that their phenotype, morphologically, and functionally resembles the enterocytes lining the small intestine (16, 33). Our data suggest that occludin expression is important for the maintenance of intestinal TJ barrier through the nonrestrictive or large-channel pathway and that occludin depletion leads to a selective or preferential increase in flux of macromolecules.
MATERIALS AND METHODS
Chemicals.
Cell culture medium (DMEM), trypsin, FBS, and related reagents were purchased from Life Technologies. Glutamine, penicillin, streptomycin, and PBS were purchased from Life Technologies-BRL. Anti-occludin, claudin-1, -2, -3, -5, and -8, and anti-β-actin antibodies were obtained from Sigma-Aldrich. Horseradish peroxidase-conjugated secondary antibodies for Western blot analysis were purchased from Invitrogen. Fluorescein isothiocyanate (FITC) and Cy-3 antibodies for immunostaining were purchased from Jackson ImmunoResearch Laboratories. siRNA of occludin and transfection reagents were from Dharmacon (Chicago, IL). All other chemicals were of reagent grade and were purchased from Sigma-Aldrich, VWR, or Fisher Scientific.
Cell cultures.
Caco-2 cells (passage 20) were purchased from the American Type Culture Collection and maintained at 37°C in a culture medium composed of DMEM with 4.5 mg/ml glucose, 50 U/ml penicillin, 50 U/ml streptomycin, 4 mM glutamine, 25 mM HEPES, and 10% FBS. The cells were kept at 37°C in a 5% CO2 environment. Culture medium was changed every 2 days. Caco-2 cells were subcultured after partial digestion with 0.25% trypsin and 0.9 mM EDTA in Ca2+- and Mg2+-free PBS.
siRNA occludin transfection.
Occludin siRNA was obtained from Dharmacon. Caco-2 monolayers were transiently transfected using DharmaFect transfection reagent (Lafayette, CO). Briefly, 5 × 105 cells/filter were seeded in a 12-well Transwell plate and grown to confluency. Caco-2 monolayers were then washed with PBS two times, 0.5 ml Accell medium was added to the apical compartment of each filter, and 1.5 ml were added to the basolateral compartment of each filter. Five nanograms (0.5 nmol) of the occludin siRNA and 2 μl of DharmaFect reagent were preincubated in Accell. After 5 min of incubation, two solutions were mixed, and the mixture was added to the apical compartment of each filter (surface area 1 cm2). The siRNA occludin transfection was carried out for 6 days. The efficiency of silencing was confirmed by Western blot analysis.
Determination of Caco-2 epithelial monolayer resistance and paracellular permeability.
An epithelial voltohmeter (World Precision Instruments, Sarasota, FL) was used for measurements of the transepithelial electrical resistance (TER) of the filter-grown Caco-2 intestinal monolayers as previously reported by us (10, 24). The effect of siRNA occludin transfection on Caco-2 paracellular permeability was determined using the following various-sized paracellular markers: 70 kDa dextran (mol wt = 70,000 g/mol), 10 kDa dextran (mol wt = 10,000 g/mol), inulin (mol wt = 5,000 g/mol), mannitol (mol wt = 182 g/mol), l-glucose (mol wt = 180 g/mol), and urea (mol wt = 60 g/mol). For determination of mucosal-to-serosal flux rates of the paracellular markers, Caco-2-plated filters having epithelial resistance of 350–450 Ω·cm2 were used. Known concentrations (1.5 μM) of 70 kDa dextran, 10 kDa dextran, inulin, mannitol, l-glucose, and urea with their radioactive tracer were added to the apical solution.
Assessment of protein expression by Western blot analysis.
To study the effect of siRNA occludin transfection on protein expression of different TJ protein expression, Caco-2 monolayers were transfected with siRNA occludin for 6 days. At the end of the experimental period, Caco-2 monolayers were immediately rinsed with ice-cold PBS, cells were lysed with lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 500 μM NaF, 2 mM EDTA, 100 μM vanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 40 mM paranitrophenyl phosphate, 1 μg/ml aprotinin, and 1% Triton X-100) and scraped, and the cell lysates were placed in Microfuge tubes. Cell lysates were centrifuged to yield a clear lysate. Supernatant was collected, and protein measurement was performed using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories). Laemmli gel loading buffer was added to the lysate containing 10–20 μg of protein and boiled for 7 min, after which proteins were separated on an SDS-PAGE. Proteins from the gel were transferred to the membrane (Trans-Blot Transfer Medium, Nitrocellulose Membrane; Bio-Rad Laboratories) overnight. The membrane was incubated for 2 h in blocking solution (5% dry milk in TBS-Tween 20 buffer). The membrane was incubated with appropriate primary antibody in blocking solution. After being washed in TBS-1% Tween buffer, the membrane was incubated in appropriate secondary antibody and developed using the Santa Cruz Western Blotting Luminol Reagents (Santa Cruz Biotechnology) on the Kodak BioMax MS film (Fisher Scientific).
Immunostaining of occludin and claudin-2.
Junctional localization of occludin and claudin-2 was assessed by immunofluorescent antibody labeling. At the end of the experimental period, filter-grown Caco-2 monolayers were washed two times in cold PBS and were fixed with 2% paraformaldehyde for 20 min. After being permeabilized with 0.1% Triton X-100 in PBS at room temperature for 20 min, Caco-2 monolayers were then incubated in blocking solution composed of BSA and normal donkey serum in PBS for 1 h. Cells were then labeled with primary antibodies in blocking solution overnight at 4°C. After being washed with PBS, the cells were incubated in FITC and Cy-3-conjugated secondary antibodies for 1 h at room temperature. ProLong Gold antifade reagent (Invitrogen, CA) was used to mount the filters on the cover slips. Immunolocalizations of occludin and claudin-2 were visualized using a Confocal fluorescence microscope (LSM 510; University of New Mexico Imaging Center) equipped with a Hamamatsu digital camera (Hamamatsu Photonics, Hamamatsu, Japan). Images were processed with LSM software (Zeiss, Germany).
RNA isolation and reverse transcription.
Caco-2 cells (5 × 105/filter) were seeded in six-well Transwell permeable inserts and grown to confluency. Filter-grown Caco-2 cells were then treated with appropriate experimental reagents for desired time periods. At the end of the experimental period, cells were washed two times with ice-cold PBS. Total RNA was isolated using the Qiagen RNeasy Kit (Qiagen) according to the manufacturer's protocol. Total RNA concentration was determined by absorbance at 260/280 nm using SpectrraMax 190 (Molecular Devices). The reverse transcription (RT) was carried out using the GeneAmp Gold RNA PCR core kit (Applied Biosystems, Foster City, CA). Two micrograms of total RNA from each sample were reverse transcribed into cDNA in a 40-μl reaction containing 1× RT-PCR buffer, 2.5 mM MgCl2, 250 μM of each dNTP, 20 U RNase inhibitor, 10 mM DTT, 1.25 μM random hexamer, and 30 U multiscribe RT. The RT reactions were performed in a thermocycler (MyCycler; Bio-Rad, Hercules, CA) at 25°C for 10 min, 42°C for 30 min, and 95°C for 5 min.
Quantification of gene expression using real-time PCR.
The real-time PCRs were carried out using the ABI prism 7900 sequence detection system and the Taqman universal PCR master mix kit (Applied Biosystems, Branchburg, NJ) as previously described (1, 2). Each real-time PCR reaction contained 10 μl RT reaction mix, 25 μl 2× Taqman universal PCR master mix, 0.2 μM probe, and 0.6 μM primers. Primer and probe design for the real-time PCR was made with Primer Express version 2 from Applied Biosystems. The sequences of the primers and the probes are shown in Table 1. All runs were performed according to the default PCR protocol (50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 1 min). For each sample, real-time PCR reactions were performed in triplicate, and the average threshold cycle (Ct) was calculated. A standard curve was generated to convert the Ct to copy numbers. Expression of occludin or claudin mRNA was normalized with glyceraldehyde-3-phosphate dehydrogenase mRNA expression. The average copy number of mRNA expression in control samples was set to 1.0.
Table 1.
Sequences of the primers and probes used for real-time PCR
| Primers | |
|---|---|
| Occludin | 5′-CCCCATCTGACTATGTGGAAAGA-3′ (forward) |
| 5′-AAAACCGCTTGTCATTCACTTTG-3′ (reverse) | |
| FAM 5′-TGACAGTCCCATGGCATACTCTTCCAATG-3′ TAMRA (probe) | |
| Claudin-2 | 5′-CTACTGAGAGGTCTGCCAT-3′ (forward) |
| 5′-GGCACCGACATAAGAACTTG-3′ (reverse) | |
| FAM 5′-CCTAGGATGTAGCCCACAAGTTGC-3′ TAMRA (probe) | |
| GAPDH | 5′-CCACCCATGGCAAATTCC-3′ (forward) |
| 5′-TGGGATTTCCATTGATGACCAG-3′ (reverse) | |
| JOE 5′-TGGCACCGTCAAGGCTGAGAACG-3′ TAMRA (probe) |
Animal surgery and in vivo transfection of occludin siRNA.
The Laboratory Animal Care and Use Committee at the University of New Mexico approved all experimental protocols. Male C57BL/6 mice (9–10 wk) were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were kept two per cage in a temperature-controlled room at 25°C with a 12:12-h light-dark cycle (lights on at 0700). Diet and drinking water were provided ad libitum. Mice were fasted for 24 h before the surgery. Mice were anesthetized with isoflurane (4% for surgical induction, 1% for maintenance) using oxygen as carrier during surgical procedures. Surgical procedures were performed using sterile technique. The abdomen was opened by a midline incision, and a 6-cm intestine segment was isolated at the proximal and distal ends and tied with sutures. siRNA transfection solution (0.5 nmol) containing Accell medium, 2.5 nmol occludin siRNA, and 15 μl transfecting agent DharmaFect (Dharmacon) was introduced in the isolated intestine segment (surface area 6 cm2) for a 1-h transfection period. Control animals underwent sham operation, where the siRNA transfection solution contained Accell medium, 2.5 nmol nontarget siRNA, and 15 μl transfection reagent DharmaFect. The abdominal cavity was covered with moistened gauze. Body temperature was monitored continuously with a rectal probe and maintained at 37.5 ± 0.5°C using a heating pad. After a 1-h transfection period, each end of the intestinal segment was untied, the intestine was placed back in the abdominal cavity, and the abdomen was closed. Three days following transfection, functional studies of intestinal epithelial barrier were performed. The surgery and the in vivo transfection procedures had no effect on the food intake and the body weight of the animals during the experimental period. The average animal weight was averaged between 23 and 25 g during the experimental period.
Epithelial resistance measurement in Ussing chambers.
The small intestinal tissue epithelial resistance was measured using an Ussing chamber 3 days after in vivo occludin siRNA transfection mice were anesthetized with isoflurane. After midline incision of the abdomen, the small intestine was dissected out, and a 1- to 1.5-cm sample was vertically mounted in Ussing chambers that provided an exposed area of 0.126 cm2 as described previously (20) with modifications. The tissues were bathed with Krebs-Ringer bicarbonate solution (in mM: 128 NaCl, 5.1 KCl, 1.4 CaCl2, 1.3 MgCl2, 21 NaHCO3, 1.3 KH2PO4, and 10 NaH2PO4, pH 7.4). Solutions were gassed with 95% O2-5% CO2. After a 15-min equilibration period, the transepithelial electrical voltage and current were measured at 5-min intervals until 30 min using an EVC 4000 Precision V/I clamp device (World Precision Instruments). Epithelial resistance was calculated using Ohm's law (R = V/I). The experiment was repeated a minimum of three times using a different tissue sample each time.
Intestinal permeability measurement.
The intestinal permeability was measured by recycling small intestinal perfusion as previously described (12–14). Three days after occludin siRNA transfection in vivo, mice were anesthetized with isoflurane. After midline incision of the abdomen, a 5-cm siRNA-transfected intestine segment was isolated and cannulated at the proximal and distal ends with 0.76 mm internal diameter polyethylene tubing. Flushing solution (140 mM NaCl and 10 mM HEPES, pH 7.4) warmed to 37°C was first perfused through the intestine at 1 ml/min for 20 min followed by air flush to remove residual contents using a external pump (Bio-Rad Laboratories). This was followed by perfusion of 5 ml perfusate solution (in mM: 85 NaCl, 10 HEPES, 20 sodium ferrocyanide, 5 KCl, and 5 CaCl2, pH 7.4.) containing Texas red-labeled dextran (10 kDa) in a recirculating manner at 0.75 ml/min for 2 h. The abdominal cavity was covered with moistened gauze, body temperature was measured via a rectal thermometer, and temperature was maintained at 37.5 ± 0.5°C using a heating lamp. One-milliliter aliquots of test solution were removed at the beginning and end of the perfusion. After perfusion, the animal was killed, the perfused intestine segment was excised, and the length was measured. The excised intestinal loop was then snap-frozen in optimum cutting temperature compound or used for protein and RNA analysis. Ferrocyanide concentration in the perfusate was measured using colorimetric assay. Texas red-labeled 10 kDa dextran concentration was measured using an excitation wavelength of 595 nm and an emission wavelength of 615 nm in a microplate reader. Probe clearance was calculated as Cprobe = (CiVi − CfVf)/(CavgTL), where Ci represents the measured initial probe concentration, Cf represents the measured final probe concentration, Vi represents the measured initial perfusate volume, Vf was calculated as Vi([ferrocyanide]i/[ferrocyanide]f), Cavg was calculated as (Ci − Cf)/ln(Ci/Cf), T represents hours of perfusion, and L represents the length of the perfused intestine section in centimeters.
Laser capture microdissection of intestinal epithelial cells.
Frozen mouse tissue sections were fixed with 75% ethanol for 30 s, hematoxylin and eosin stained for 20 s, and dehydrated with 75% ethanol for 30 s, 95% ethanol for 30 s, 100% ethanol for 30 s, and xylene for 5 min. After dehydration, sections were air-dried for 5 min. The arcturus PixCell II system (Molecular Devices, Sunnyvale, CA) was used for microdissection and laser capture. The intestinal epithelial cells from the mucosal surface were captured using a 7.5-μM-diameter laser beam typically at 80–100 mV power with pulse duration of 0.5–1.0 ms. On average, about 500 shots were taken per cap, and ∼1,000 enterocytes were obtained per cap. Microdissection caps were inserted in 0.5-ml microcentrifuge tubes containing 350 μl of lysis buffer, and total RNA was isolated.
Statistical analysis.
Results are expressed as means ± SE. Statistical significance of differences between mean values was assessed with Student's t-tests for unpaired data and ANOVA whenever required. All reported significance levels represent two-tailed P values. A P value of <0.05 was used to indicate statistical significance. Each experiment in Caco-2 monolayers was performed in triplicate or quadruplicates (n = 3 or 4), and all experiments were repeated three times to ensure reproducibility. Each experiment in mouse intestine in vivo was performed at least four times.
RESULTS
siRNA-induced silencing of occludin does not affect Caco-2 TER.
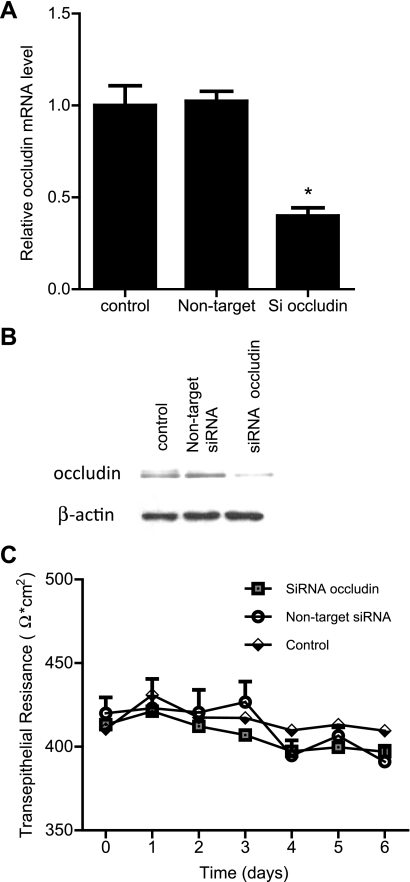
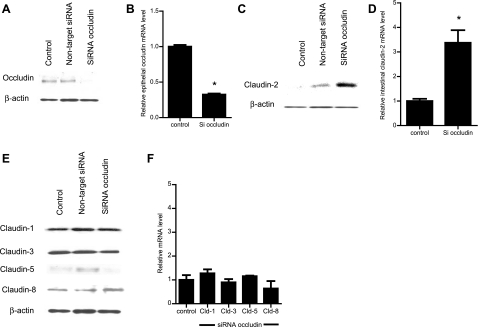
Epithelial electric resistance is a measure of paracellular conductance of ionic species across the epithelial barrier. To examine the role of occludin in the maintenance of Caco-2 TER, occludin depletion was induced by siRNA silencing of occludin in filter-grown Caco-2 monolayers. The occludin siRNA transfection produced a decrease in occludin mRNA level (Fig. 1A) and a near-complete knock down of occludin protein expression (Fig. 1B). The control Caco-2 monolayers achieved a steady-state TER of 350–450 Ω·cm2 when grown on 12-well Transwell filters (Fig. 1C). The siRNA-induced occludin knock down did not have a significant effect on Caco-2 TER (Fig. 1C), suggesting that occludin depletion does not affect ionic conductance.
Fig. 1.
Effect of small-interfering RNA (siRNA)-induced knock down of occludin on filter-grown Caco-2 transepithelial electrical resistance (TER). A: siRNA occludin transfection caused a significant decrease in occludin mRNA levels as measured by real-time PCR (4 days posttransfection). *P < 0.001 vs. control. B: siRNA occludin transfection resulted in a near-complete depletion of occludin expression as assessed by Western blot analysis (6 days posttransfection). C: siRNA-induced knock down of occludin did not affect Caco-2 TER over the 6-day experimental period compared with controls.
Occludin knock down causes an increase in paracellular flux of macromolecules.
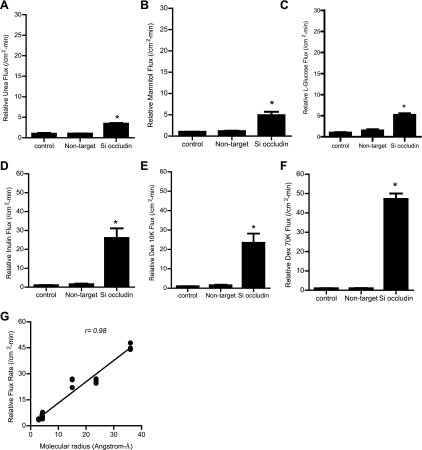
In the following studies, the effect of occludin depletion on transepithelial permeation of increasing molecular size paracellular probes was examined. The effect of occludin depletion on paracellular flux of urea (mol wt = 60 g/mol) (32) and simple sugar probes mannitol (mol wt = 182 g/mol) (31) and l-glucose (mol wt = 180 g/mol) (15) was determined. The siRNA-induced occludin depletion resulted in a three- to- fourfold increase in urea flux and a five- to sixfold increase in mannitol and l-glucose flux rates (Fig. 2, A–C). Next, the effect of occludin knock down on flux of commonly used macromolecular probes inulin (mol wt = 5,000 g/mol) (29) and dextran [mol wt = 10,000 g/mol (27) and 70,000 g/mol (21, 38)] was determined. Occludin knock down resulted in an ∼25-fold increase in inulin and 10 kDa dextran flux rates (Fig. 2, D and E) and a 45-fold increase in 70 kDa dextran flux rate (Fig. 2F). The molecular weight and the published molecular radius of the paracellular markers used in this study are shown in Table 2. To determine the relationship between probe molecular size and increase in flux rates, molecular radii were plotted against the proportional increase in flux rates (Fig. 2G). There was a linear relationship (correlation coefficient, r = 0.98) between increasing molecular size of the probe and the increase in relative flux rates, indicating that occludin depletion causes a proportionally greater increase in the flux rate of larger-sized molecules.
Fig. 2.
Effect of siRNA-induced knock down of occludin on transepithelial flux of increasing-molecular-size paracellular markers across filter-grown Caco-2 monolayers. A: occludin depletion caused a 3- to 4-fold increase in urea flux (means ± SE, n = 6). *P < 0.001 vs. control. B and C: occludin depletion caused a 5- to 6-fold increase in mannitol and l-glucose flux (means ± SE, n = 6). *P < 0.001 vs. control. D and E: occludin depletion caused an ∼25-fold increase in inulin and 10 kDa (K) dextran (Dex) flux (means ± SE, n = 6). *P < 0.0005 vs. control. F: occludin depletion caused an ∼45-fold increase in 70 kDa dextran flux (means ± SE, n = 6). *P < 0.0001 vs. control. G: graph of molecular radius of paracellular markers vs. relative increase in flux rate following occludin siRNA transfection (relative correlation coefficient, r = 0.98).
Table 2.
Molecular weight and molecular radius of selected paracellular markers
| Molecule | Molecular Wt, g/mol | Radius, Å |
|---|---|---|
| Urea | 60 | 2.9 (32) |
| Mannitol | 182 | 4.1 (31) |
| l-Glucose | 180 | 4.3 (16) |
| Inulin | 5,000 | 15 (29) |
| Dextran (10 kDa) | 10,000 | 23.6 (27) |
| Dextran (70 kDa) | 70,000 | 36.0 (21,36) |
Reference nos. in parentheses.
Occludin depletion has differential effect on transmembrane TJ protein expression.
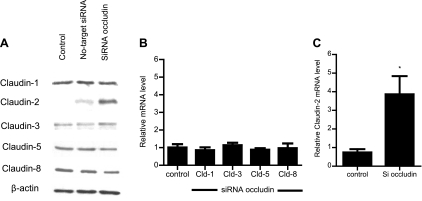
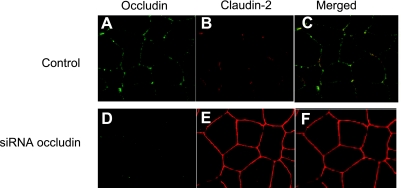
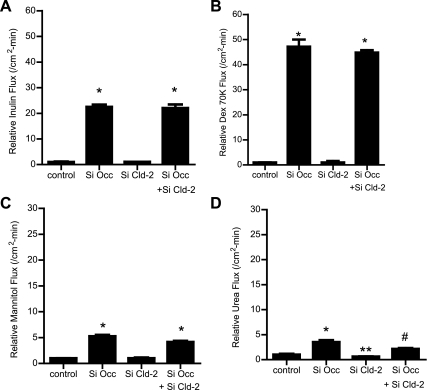
The effect of occludin depletion on expression of other transmembrane TJ proteins, including claudin-1, -2, -3, -5, and -8, was examined. These proteins were selected since they have been implicated in TJ barrier function and are known to be expressed in intestinal epithelial cells (3). The siRNA-induced knock down of occludin did not affect expression of claudin-1, -3, -5, or -8 but caused an increase in claudin-2 expression (Fig. 3A). siRNA-induced knock down of occludin also did not affect claudin-1, -3, -5, or -8 mRNA level (Fig. 3B) but caused an increase in claudin-2 mRNA expression (Fig. 3C), suggesting that the increase in claudin-2 expression was related to the increase in claudin-2 transcription. Next, the effect of occludin depletion on junctional localization of TJ proteins was examined by immunofluorescent antibody labeling. Consistent with the immunoblot studies, siRNA-induced knock down of occludin resulted in a decrease in intensity of occludin staining and an increase in claudin-2 staining at the apical junctions (Fig. 4). In the following studies, the possibility that the occludin depletion-induced increase in Caco-2 paracellular permeability was mediated in part by an increase in claudin-2 expression was examined. In these studies, the effect of siRNA-induced knock down of claudin-2 expression on occludin depletion-induced increase in paracellular permeability was determined. The siRNA-induced silencing of claudin-2 did not affect the occludin depletion-induced increase in inulin or 70 kDa dextran flux (Fig. 5, A and B). Claudin-2 knock down also did not affect the increase in the flux rate of mannitol (Fig. 5C) but inhibited the flux rate of the smaller-sized probe urea (Fig. 5D). [Because of its small molecular radius, urea permeates across the claudin-2-dependent TJ pore (50).] These results suggested that the increase in claudin-2 expression did not play a role in the increase in flux rate of larger-sized molecules.
Fig. 3.
Effect of occludin siRNA on various transmembrane tight junction (TJ) protein and mRNA expression. A: siRNA occludin caused an increase in claudin-2 protein expression but did not affect claudins-1, -3, -5, and -8 protein expression as assessed by Western blot analysis. B: occludin depletion by siRNA transfection in Caco-2 monolayers did not affect the mRNA levels of claudins-1, -3, -5, and -8 as assessed by real-time PCR (4 days posttransfection). C: claudin-2 mRNA levels were significantly increased in occludin siRNA-transfected Caco-2 monolayers (means ± SE, n = 4). *P < 0.001 vs. control.
Fig. 4.
The effect of occludin siRNA transfection on junctional localization of occludin and claudin-2 as determined by immunofluorescent antibody labeling and visualized by confocal microscope. A and B: immunostaining of occludin (green) and claudin-2 (red) in control Caco-2 monolayers. C: colocalization of occludin and claudin-2. D: siRNA-induced knock down of occludin in Caco-2 monolayer resulted in a significant depletion of the junctional localization of occludin. E: siRNA-induced knock down of occludin in Caco-2 monolayer caused an increase in claudin-2 intensity at the junctional localization. F: colocalization of occludin and claudin-2 following occludin siRNA transfection. Magnification, ×40.
Fig. 5.
Effect of siRNA-induced knock down of claudin-2 on transepithelial flux of varying-sized paracellular markers in occludin (Occ)-depleted Caco-2 monolayers. A and B: claudin-2 depletion did not affect the occludin siRNA-induced increase in flux rate of inulin and 70 kDa dextran (means ± SE, n = 4). *P < 0.0001 vs. control. C: claudin-2 depletion did not affect the occludin siRNA-induced increase in flux rate of mannitol (means ± SE, n = 6). *P < 0.0001 vs. control. D: claudin-2 depletion significantly prevented the occludin siRNA-induced increase in flux rate of urea (means ± SE, n = 6). *P < 0.0035 vs. control. **P < 0.0358 vs. control. #P < 0.0358 vs. occludin siRNA transfection.
siRNA-induced knock down of occludin in vivo causes an increase in mouse intestinal permeability.
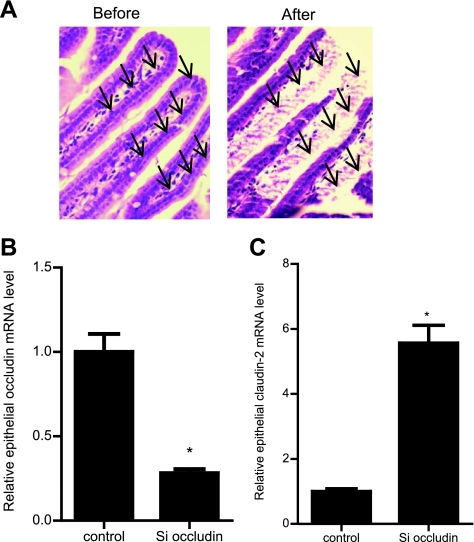
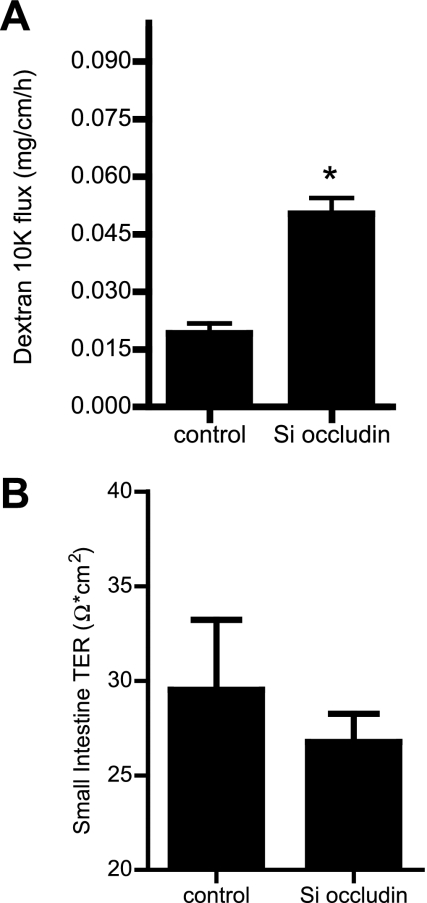
In these studies, we transfected the mouse small intestinal mucosal surface with occludin siRNA in vivo and then, 3 days later (allowing sufficient time to induce occludin depletion), the effect of siRNA transfection on occludin depletion and intestinal permeability was determined. The effect of occludin siRNA transfection on occludin protein and mRNA expression was determined in the resected small intestinal tissue. Occludin siRNA transfection resulted in a near-complete depletion of occludin protein expression by day 3, as assessed by Western blot analysis (Fig. 6A). Occludin siRNA transfection also resulted in a decrease in tissue mRNA expression, as determined by real-time PCR (Fig. 6B). The effect of occludin knock down on the intestinal tissue claudin-2 expression was also examined. Occludin siRNA transfection in vivo caused an increase in intestinal tissue claudin-2 protein and mRNA expression (Fig. 6, C and D). However, occludin silencing did not affect the intestinal tissue expression of claudins-1, -3, -5, and -8 (Fig. 6, E and F). Because intestinal tissue consists of a number of different cell types, in the following studies, a pure population of intestinal epithelial cells was isolated by laser capture microdissection from the intestinal mucosal surface (Fig. 7A), and the effect of occludin siRNA transfection on enterocytes occludin mRNA levels was determined. The occludin siRNA transfection in vivo resulted in a decrease in occludin mRNA level in the intestinal epithelial cells, confirming that occludin siRNA causes a depletion of occludin expression in enterocytes (Fig. 7B). Additionally, occludin depletion in small intestinal tissue caused an increase in the enterocytes claudin-2 mRNA levels (Fig. 7C). The effect of occludin knock down on intestinal permeability was then determined using FITC-labeled dextran (10 kDa) as the macromolecular marker. Occludin knock down resulted in a twofold increase in dextran flux (Fig. 8A). Next, the effect of occludin knock down on tissue TER was determined by mounting intestinal tissue in an Ussing chamber. The siRNA-induced knock down of occludin did not affect intestinal tissue TER (Fig. 8B). These results indicated that siRNA-induced depletion of occludin in enterocytes in vivo also leads to an increase in intestinal permeability to dextran without affecting the tissue TER. [It should be noted that the level of increase in dextran flux in vivo following occludin knock down was similar to those reported in other in vivo models of increase in intestinal permeability (8, 25). In both IL-10-deficient mice and in in vivo tumor necrosis factor-α treatment, the increases in dextran flux rates were 1- to 2-fold compared with the control mice.]
Fig. 6.
Effect of selective siRNA-induced knock down of occludin in mouse intestine in vivo on mRNA and protein expression of TJ proteins in mouse intestinal tissue. A: occludin siRNA transfection resulted in a near-complete depletion of occludin expression in small intestinal tissue as assessed by Western blot analysis (3 days post-siRNA transfection). B: occludin siRNA transfection caused a significant decrease in occludin mRNA levels as measured by real-time PCR (means ± SE, n = 4). *P < 0.001 vs. control. C: occludin siRNA caused an increase in claudin-2 protein expression in mouse small intestinal tissue as assessed by Western blot analysis. D: claudin-2 mRNA levels were significantly increased in occludin siRNA transfected in mouse intestinal tissue (means ± SE, n = 4). *P < 0.001 vs. control. E: occludin siRNA did not affect claudins-1, -3, -5, and -8 protein expressions in mouse intestinal tissue as assessed by Western blot analysis. F: occludin depletion by siRNA transfection in mouse intestinal tissue did not affect the mRNA levels of claudins (Cld)-1, -3, -5, and -8 as assessed by real-time PCR (4 days posttransfection) (means ± SE, n = 5).
Fig. 7.
Effect of siRNA-induced knock down of occludin on enterocyte epithelial expression of occludin and claudin-2 in vivo. A pure population of villus enterocytes was isolated by laser capture microdissection (LCM). A: before and after LCM image of small intestinal mucosal surface. Arrows indicate the epithelial cells removed by LCM. B: occludin siRNA transfection caused a significant decrease in enterocyte occludin mRNA level as measured by real-time PCR (4 days posttransfection) (means ± SE, n = 4). *P < 0.001 vs. control. C: claudin-2 mRNA level was significantly increased in occludin siRNA-transfected enterocytes (means ± SE, n = 4). *P < 0.0001 vs. control.
Fig. 8.
Effect of occludin siRNA in mouse small intestine permeability in vivo. A: occludin siRNA transfection in mouse intestine caused a significant increase in Texas red-labeled dextran (10 kDa) flux (means ± SE, n = 4). *P < 0.001 vs. control. B: occludin siRNA transfection did not affect the electrical resistance of small intestine tissue mounted on an Ussing chamber (means ± SE, n = 6).
DISCUSSION
An important question persists regarding the role of occludin in intestinal epithelial TJ barrier function. Because occludin levels are decreased in intestinal tissue in patients with intestinal permeability disorders (7, 9, 14, 55), understanding the relevance of occludin depletion on intestinal TJ barrier function has both clinical and scientific relevance. Although earlier in vitro studies in cell culture systems suggested that occludin may be involved in TJ barrier function, more recent studies have called this into question. Most notably, occludin-deficient mice were found to lack any morphological or functional deficiencies in intestinal epithelial barrier function, arguing against a functional role. The major aim of the present study was to examine the role of occludin in intestinal epithelial TJ barrier function by selective knock down of occludin in intestinal epithelial cells both in vitro and in vivo. Our data show that siRNA-induced knock down of occludin in filter-grown Caco-2 monolayers causes a molecular size-dependent increase in paracellular probe flux across the intestinal epithelial TJ barrier. The occludin knock down caused a marked increase in macromolecule flux rates but had only modest effect on flux of smaller-sized probes and even lesser effect on TER (measure of ionic flux). Similarly, occludin knock down in mouse enterocytes in vivo also caused an increase in intestinal flux of the macromolecular probe but did not affect tissue TER. These data suggested that occludin plays an important role in paracellular barrier function to flux of large macromolecules. This is in distinction to claudins, which appear to mainly affect the flux of smaller-sized molecules and ions through a fixed pore size (49, 52).
The functional role of occludin has been debated since its initial discovery by Furuse et al. (13) in 1993, and the controversy regarding its role in TJ barrier function persists (3, 37). In earlier studies, occludin was shown to localize at the TJ strands (12, 13, 17), and a functional role was hypothesized. In support of a functional role in cell-to-cell adhesion, Van Itallie and Anderson (47) showed that expression of occludin conferred an adhesive property to fibroblasts. The occludin-induced increase in fibroblast cell-to-cell contact was inhibited by synthetic peptide containing an amino acid sequence corresponding to the first extracellular loop of occludin, demonstrating that extracellular loop interaction was required for the cell-to-cell contact. The overexpression of occludin in Madin-Darby canine kidney (MDCK) cells resulted in a 32% increase in steady-state TER and an increase in TJ strand number (28), suggesting a functional role in TJ strand formation and in TJ barrier (28). Conversely, Bamforth et al. (5) showed that expression of a dominant mutant occludin construct lacking the NH2-terminus and the extracellular domain resulted in an inhibition of TER development and a marked increase (57-fold increase) in dextran (4 kDa) flux in submandibular gland epithelial cells CSG 120/7 (5). The loss of TJ barrier function was accompanied by a morphological disturbance in TJ strands (5). There have also been a number of studies showing that occludin phosphorylation or endocytosis leads to a loss of TJ barrier function (26, 39, 41). Together, these studies supported a functional role for occludin in TJ barrier.
However, there have also been several compelling studies arguing against a functional role for occludin in TJ barrier function (35, 37, 53). Using embryonic stem cells, Saitou et al. (35) found that occludin-deficient stem cells differentiated into polarized epithelial cells with well-developed TJs and a functional TJ barrier similar to the occludin-expressing wild-type stem cells, indicating that occludin was not required for the development of barrier or fence functions of TJs in embryonic stem cells. Consistent with these findings, occludin-deficient mice were born without detectable alterations in intestinal epithelial TJ morphology as assessed by transmission electron microscopy and freeze fracture analysis (36). Functional studies in occludin-deficient mice also did not reveal any abnormalities in epithelial barrier function in gastric, small intestine, large intestine, or urinary bladder tissue as assessed by TER or mannitol flux studies, prompting the investigators to conclude that “occludin is not required for the formation of TJ strands” and that evidence points “against an essential role of occludin for the barrier formation within the tight junction strand heteropolymer” (37). Similarly, Yu et al. (53) also reported that occludin-deficient MDCK cells were able to attain normal steady-state TER and barrier function to paracellular probes. In combination, these studies provided strong evidence against a functional role for occludin in TJ barrier function.
Does occludin have a role in intestinal TJ barrier? Our present data show that occludin does have an important role in intestinal TJ barrier. Similar to the previous reports by Saitou et al. (35) and Yu et. al (53), in our studies, occludin knock down did not affect Caco-2 TER or mouse intestinal tissue TER. The probe molecular size/flux rate analysis indicated that occludin depletion causes a progressive size-dependent increase in paracellular flux rate, such that the relative flux rate of urea (molecular radius 2.9 Å), mannitol (4.1 Å), inulin (15 Å), 10-kDa dextran (23 Å), and 70-kDa dextran (36 Å) increased by 3- to 4-fold, 5- to 6-fold, ∼25-fold, ∼25-fold, and ∼45-fold, respectively. Thus occludin depletion leads to a proportionally greater increase in flux rate for larger-sized molecules (Fig. 2G). These data show that occludin depletion selectively or preferentially affects the flux rates of macromolecules such as inulin and dextran to a much greater extent than the smaller-sized molecules mannitol and l-glucose. Interestingly, occludin knock down also caused a selective increase in claudin-2 expression. As far as we are aware, this is the first reported study showing that claudin-2 expression was increased when occludin expression was knocked down by siRNA transfection. The possibility that the increase in claudin-2 expression contributed to the increase in macromolecule flux was also considered; however, silencing of claudin-2 expression did not affect the occludin depletion-induced increase in macromolecule flux, indicating that the increase in macromolecule flux was not related to the increase in claudin-2 expression.
Our data suggested that occludin modulates paracellular flux through the nonrestrictive or large-channel TJ pathway, which is responsible for the large molecule flux (51, 52). Watson et al. (52) previously reported the existence of two distinct paracellular pathways that regulate the molecular flux across the intestinal epithelial TJ barrier. Using increasing molecular weight fractions of polyethylene glycol (PEG), they identified a size-restrictive pore pathway responsible for the flux of small-sized PEG fractions. They showed that, in mature Caco-2 and T84 intestinal monolayers, PEG molecular weight fractions with molecular radius <4 Å readily permeated across the TJ barrier. On the other hand, PEG molecules >4 Å in molecular radius were mostly excluded from permeation across the intestinal monolayers. They concluded that TJ pores with fixed pore size of ∼4–4.5 Å in radius were responsible for the flux of PEG molecules having molecular radius <4 Å. They also described the existence of a nonrestrictive pathway that allowed paracellular flux of larger-sized molecules in response to TJ barrier-modulating agents (52). Following treatment with the TJ barrier-disrupting agent EGTA (a Ca2+-binding agent), there was a significant increase in the flux of all PEG fractions across both T84 and Caco-2 monolayers, without regard to molecular size. Interestingly, the increase in flux rates was proportionately much greater for the larger-sized PEG molecules than the smaller PEG molecules. Based on these results, Watson et al. (52) concluded that a high-capacitance size-restrictive pore pathway (∼4–4.5 Å in radius) was normally present in the mature intestinal epithelial monolayers and that a nonrestrictive or large-channel pathway (that allows the flux of larger-sized molecules without restriction to size) was created in response to TJ-disrupting agents (52). In subsequent studies, Van Itallie et al. (49) also reported that the 4-Å pore pathway was present in other epithelial cell systems, including in isolated pig ileum and in high- and low-resistance MDCK cell lines, and suggested that the pore pathway was present in different epithelial cell types regardless of the relative tightness of the epithelial barrier. They also reported that the flux characteristics of the restrictive pore pathway were regulated by the pore composition of claudin proteins (49). Overexpression of claudin-2 caused an increase in the pore number without affecting the pore size, whereas claudin-4 and -8 expression affected the charge selectivity of the pore pathway without affecting the pore number (45, 46, 54). Van Itallie et al. concluded that claudins are “constituents of the pores of radius 4 Å and that pore numbers and pore characteristics (but not pore size) of TJs are determined by differences in claudin expression.” They also reported the existence of a nonrestrictive, size-independent pathway that regulated the flux of larger-sized molecules (49). Together these studies have identified the existence of two distinct TJ paracellular pathways: a claudin-dependent, size-restrictive pore pathway responsible for flux of solutes <4 Å and a nonrestrictive, large-channel pathway responsible for the flux of large-sized molecules, including macromolecules and bacterial antigens. Our data suggest that occludin expression is important for the maintenance of the nonrestrictive, large-channel pathway.
In conclusion, our data provide new insight into the role of occludin in intestinal epithelial TJ barrier function. Our results indicate that occludin depletion in intestinal epithelial cells in vitro and in vivo leads to a molecular size-dependent increase in paracellular flux of larger-sized molecules. These data, combined with previous publications, suggest that claudins have a functional role in the regulation of ionic and small molecule flux through the TJ pores (4 Å in radius) (48), whereas occludin functions in the regulation of flux of large macromolecules, presumably through the nonrestrictive or large-channel pathway. We speculate that, under normal healthy conditions, intestinal epithelial barrier is effective in preventing macromolecular flux but readily allows flux of ions and small molecules through the claudin-regulated pore pathway (48, 51); however, during pathological conditions, occludin depletion leads to the opening of the nonrestrictive pathway, allowing paracellular flux of macromolecules, including bacterial antigens, which leads to an inflammatory response (23, 30). Thus our data suggest a new role for occludin in intestinal TJ barrier regulation.
GRANTS
This research project was supported by a Veterans Affairs (VA) Merit Review grant from the VA Research Service and National Institute of Diabetes and Digestive and Kidney Diseases Grants RO 1-DK-64165 and RO 1-DK-81429.
DISCLOSURES
No conflicts of interest are declared by the authors.
REFERENCES
- 1. Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci 14: 2765–2778, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J Immunol 178: 4641–4649, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 58: 41–48, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bamforth SD, Kniesel U, Wolburg H, Engelhardt B, Risau W. A dominant mutant of occludin disrupts tight junction structure and function. J Cell Sci 112: 1879–1888, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Bitton A, Dobkin PL, Edwardes MD, Sewitch MJ, Meddings JB, Rawal S, Cohen A, Vermeire S, Dufresne L, Franchimont D, Wild GE. Predicting relapse in Crohn's disease: a biopsychosocial model. Gut 57: 1386–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Ciccocioppo R, Finamore A, Ara C, Di Sabatino A, Mengheri E, Corazza GR. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am J Clin Pathol 125: 502–511, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR. Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116: 2682–2694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coeffier M, Gloro R, Boukhettala N, Aziz M, Lecleire S, Vandaele N, Antonietti M, Savoye G, Bole-Feysot C, Dechelotte P, Reimund JM, Ducrotte P. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am J Gastroenterol 105: 1181–1188 [DOI] [PubMed] [Google Scholar]
- 10. Dokladny K, Ye D, Kennedy JC, Moseley PL, Ma TY. Cellular and molecular mechanisms of heat stress-induced up-regulation of occludin protein expression: regulatory role of heat shock factor-1. Am J Pathol 172: 659–670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fries W, Mazzon E, Squarzoni S, Martin A, Martines D, Micali A, Sturniolo GC, Citi S, Longo G. Experimental colitis increases small intestine permeability in the rat. Lab Invest 79: 49–57, 1999 [PubMed] [Google Scholar]
- 12. Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes J Cell Sci 108: 3443–3449, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123: 1777–1788, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermuller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol 281: G216–G228, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Habgood MD. The nature of increased blood-cerebrospinal fluid barrier exchange during CO2 inhalation in newborn and adult rats. Exp Physiol 80: 117–128, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Hidalgo IJ, Raub TJ, Borchardt RT. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96: 736–749, 1989 [PubMed] [Google Scholar]
- 17. Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 110: 1603–1613, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep 1: 410–416, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor. Ann Intern Med 105: 883–885, 1986 [DOI] [PubMed] [Google Scholar]
- 20. Kato M, Akao M, Matsumoto-Ida M, Makiyama T, Iguchi M, Takeda T, Shimizu S, Kita T. The targeting of cyclophilin D by RNAi as a novel cardioprotective therapy: evidence from two-photon imaging. Cardiovasc Res 83: 335–344, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Li G, Simon MJ, Cancel LM, Shi ZD, Ji X, Tarbell JM, Morrison B, 3rd, Fu BM. Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann Biomed Eng 38: 2499–2511, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma TY. Intestinal epithelial barrier dysfunction in Crohn's disease. Proc Soc Exp Biol Med 214: 318–327, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Ma TY, Anderson JM. Tight junctions and the intestinal barrier. In: Physiology of the Gastrointestinal Tract. Burlington, MA: Elsevier, 2006 [Google Scholar]
- 24. Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol 286: G367–G376, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Madsen KL. Inflammatory bowel disease: lessons from the IL-10 gene-deficient mouse. Clin Invest Med 24: 250–257, 2001 [PubMed] [Google Scholar]
- 26. Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, Raleigh DR, Guan Y, Watson AJ, Montrose MH, Turner JR. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol 189: 111–126, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayhan WG. Effect of lipopolysaccharide on the permeability and reactivity of the cerebral microcirculation: role of inducible nitric oxide synthase. Brain Res 792: 353–357, 1998 [DOI] [PubMed] [Google Scholar]
- 28. McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, Rogers RA, Lynch RD, Schneeberger EE. Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298, 1996 [DOI] [PubMed] [Google Scholar]
- 29. Moran SM, Myers BD. Pathophysiology of protracted acute renal failure in man. J Clin Invest 76: 1440–1448, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nazli A, Wang A, Steen O, Prescott D, Lu J, Perdue MH, Soderholm JD, Sherman PM, McKay DM. Enterocyte cytoskeleton changes are crucial for enhanced translocation of nonpathogenic Escherichia coli across metabolically stressed gut epithelia. Infect Immun 74: 192–201, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Page E. Cat heart muscle in vitro. III. The extracellular space. J Gen Physiol 46: 201–213, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Petrucelli RJ, 2nd, Eggena P. Importance of molecular size and hydrogen bonding in vasopressin-stimulated urea transport. Am J Physiol Cell Physiol 243: C27–C34, 1982 [DOI] [PubMed] [Google Scholar]
- 33. Pinto RM, Diez JM, Bosch A. Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses. J Med Virol 44: 310–315, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 447: 171–183, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141: 397–408, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11: 4131–4142, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M. Epithelial transport and barrier function in occludin-deficient mice. Biochim Biophys Acta 1669: 34–42, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Shemesh O, Deen WM, Brenner BM, McNeely E, Myers BD. Effect of colloid volume expansion on glomerular barrier size-selectivity in humans. Kidney Int 29: 916–923, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolae-mediated endocytosis. Mol Biol Cell 16: 3919–3936, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol 17: 283–309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sheth P, Basuroy S, Li C, Naren AP, Rao RK. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278: 49239–49245, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Turner JR. Molecular basis of epithelial barrier regulation: from basic mechanisms to clinical application. Am J Pathol 169: 1901–1909, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Turner JR, Black ED, Ward J, Tse CM, Uchwat FA, Alli HA, Donowitz M, Madara JL, Angle JM. Transepithelial resistance can be regulated by the intestinal brush-border Na+/H+ exchanger NHE3. Am J Physiol Cell Physiol 279: C1918–C1924, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Van Itallie C, Rahner C, Anderson JM. Regulated expression of claudin-4 decreases paracellular conductance through a selective decrease in sodium permeability. J Clin Invest 107: 1319–1327, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Van Itallie CM, Anderson JM. Occludin confers adhesiveness when expressed in fibroblasts. J Cell Sci 110: 1113–1121, 1997 [DOI] [PubMed] [Google Scholar]
- 48. Van Itallie CM, Holmes J, Bridges A, Anderson JM. Claudin-2-dependent changes in noncharged solute flux are mediated by the extracellular domains and require attachment to the PDZ-scaffold. Ann NY Acad Sci 1165: 82–87, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 121: 298–305, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Van Itallie CM, Mitic LL, Anderson JM. Claudin-2 forms homodimers and is a component of a high molecular weight protein complex. J Biol Chem 286: 3442–3450, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G. Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci 118: 5221–5230, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Watson CJ, Rowland M, Warhurst G. Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 281: C388–C397, 2001 [DOI] [PubMed] [Google Scholar]
- 53. Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol 288: C1231–C1241, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta 1778: 709–716, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56: 61–72, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]